Abstract
Background
Methotrexate (MTX) is widely used in chemotherapy but its associated hepatotoxicity is a major complication, limiting its use. This study evaluates possible therapeutic effect of oral alpha-ketoglutarate (AKG) supplementation against MTX-induced hepatotoxicity.
Methods
HepG2 cells were used to evaluate in-vitro cyto-protection conferred by AKG against MTX induced cytotoxicity. For in-vivo animal study, rats were divided into three groups. Group-I served as control. Group-II animals were administered single intraperitoneal injection of MTX (20 mg/kg/body weight), while Group-III received MTX as in group-II followed by oral AKG (2 gm/kg body weight) for 5 days. 99mTc-Mebrofenin hepatobiliary study was performed under a gamma camera to determine real time functional status of rats’ livers. Multiple parameters concerning hepatic mebrofenin uptake and excretion, including Tpeak and T1/2 peak in control and treated animals were determined. Biochemical analysis of the liver homogenate in terms of hepatic enzyme activities in serum, antioxidant status, tissue factor activity, tissue collagen content and histological analysis of the liver tissue were also done.
Results
AKG supplementation significantly reversed MTX induced derangement in activities of serum liver enzymes [ALT and ALP (p = 0.003); AST (p = 0.005)], antioxidant status [LPO and GSH (p = 0.005); CAT (p = 0.004)], tissue factor activity (p = 0.005) and tissue collagen content (p = 0.005). Functional imaging confirmed that hepatic retention and fractional biliary excretion were significantly abnormal in MTX treated group (Tpeak: 234 s ± 40 s; T1/2 peak: 846sec ± 32sec) as compared to AKG supplemented group (Tpeak: 144 s ± 35sec; T1/2peak: 468sec ± 27sec). Hepatic extraction fraction (HEF) was 92.2 ± 1.8%, 48.7 ± 2.6% and 69.8 ± 4.3% in control, MTX and AKG supplemented rats respectively.
Conclusion
99mTc-mebrofenin imaging strongly suggests therapeutic action of AKG in protecting liver damage by MTX in rats. Functional imaging parameters correlated well with biochemical and histopathological findings.
Keywords: Methotrexate, Oxidative stress, Hepatotoxicity, Alpha-ketoglutarate, 99mTc-mebrofenin, Gamma scintigraphy
At a glance commentary
Scientific background on the subject
Methotrexate (MTX) is a chemotherapeutic agent widely used in treatment of various malignancies and autoimmune diseases. However, MTX-induced hepatotoxicity is a major concern, limits its application. We have investigated possible hepatoprotective effect of alphaketoglutarate (AKG), a tricarboxylic acid cycle metabolite and an over-the-counter supplement in alleviating MTX-induced liver toxicity.
What this study adds to the field
99mTc-mebrofenin hepatobilliary scintigraphy is a validated nuclear imaging tool in clinical practice to assess liver function in-vivo. Besides carrying out biochemical and histological assessments, the present study provides quantifiable validation of the ameliorative potential of AKG against MTX induced hepatotoxicity using this functional imaging modality in pre-clinical models.
Methotrexate (MTX) is a chemotherapeutic and immunosuppressive agent widely used to treat inflammatory bowel disease, psoriasis, sarcoidosis, many rheumatologic, dermatologic and hematologic diseases [1]. However, its associated liver toxicity has become one of the major complications of MTX treatment [2]. MTX is known to cause elevations in serum aminotransferase levels and long term therapy has been linked to development of fatty liver disease, fibrosis and even cirrhosis [3]. Although the precise mechanism of MTX induced hepatotoxicity is not yet completely understood, available experimental and clinical evidences suggest that MTX induced hepatic damage may be a consequence of reactive oxygen species (ROS) mediated oxidative stress [4].
Alpha-ketoglutarate (AKG) is an important intermediate in tricarboxylic acid (TCA) cycle and acts as an important nitrogen transporter in metabolic pathways. In United States, it is available as an over-the-counter (OTC) nutritional supplement and is used to improve peak athletic performance, alleviate hyperammonia in sport medicine and for certain chronic debilitating conditions [5]. Studies have elucidated that in response to oxidative stress, TCA cycle enzymes are modulated to diminish production of pro-oxidants NADH and FADH2 with intracellular accumulation of AKG. AKG detoxifies ROS (H2O2- and O2.-) with concomitant formation of succinate, which in turn restores cellular homeostasis and helps cell to cope with oxidative stress [6]. Since endogenously generated AKG is mainly consumed in TCA cycle, exogenous supplementation of AKG is required for using it for nutraceutical purposes or other possible therapeutic interventions [7]. Absorption of AKG occurs in the enterocytes and is a passive process. Unlike glutamate, only 65 percent AKG gets metabolised in enterocytes and the rest becomes available to peripheral tissues [8]. Our group has previously reported bioavailability studies of orally administered AKG in rats. Blood kinetics of orally administered AKG follows a biphasic pattern of clearance, with the first phase being fast and the second phase slower with a t1/2(fast) = 1 h 10 min and t1/2(slow) = 10 h 20 min respectively [9].
Keeping in mind the potent antioxidant properties of AKG along with the fact that it is available OTC, we thought it worthwhile to evaluate its hepatoprotective efficacy against MTX induced hepatic damage in experimental animals. Apart from biochemical and histological assessments, hepatobilliary scintigraphy (HBS) based functional imaging studies have been done to validate the findings in-vivo. HBS is a radionuclide imaging modality utilized for evaluation of hepatocellular function and patency of biliary system. Quantification of functional capacity of liver using HBS has been successfully performed in rats previously [10]. In the present study parameters to assess physiological functioning of hepatocytes in-vivo were obtained using radio-tracer 99mTc-Mebrofenin (99mTc-MEB). This radiotracer has high specificity for hepatocytes (98%), does not undergo metabolism or conjugation, is actively excreted from hepatocytes and shows rapid hepatobiliary transit with no enteric absorption [11]. The physiological parameters studied for measuring hepatic uptake and excretion were (a) Hepatic extraction fraction (HEF): for quantification of uptake, signifying viable parenchymal liver cell mass, (b) Time to half of peak activity (T1/2 peak): for quantification of excretion, signifying hepato-necro-inflammatory activity. The present study therefore utilizes both structural and functional outcome parameters for assessing hepatoprotective ability of AKG.
Materials and methodology
Chemicals
Alpha-ketoglutarate and Methotrexate were purchased from FlukaChemika (Buchs, Switzerland) and IPCA, (Mumbai, India) respectively. 99mTechnetium-Mebrofenin (99mTc-MEB) was supplied by BRIT, BARC (India). HepG2 cells were obtained from National Center for Cell Sciences, Pune, India. MTT (Thiazolyl Tetrazolium Bromide) was procured from Sigma–Aldrich, USA, phosphate buffered saline (PBS) and Hanks Balanced Salt Solution (HBSS) was procured from Hi–Media (Mumbai, India). Biochemical assay kits for detection of Alanine transaminase (ALT), Aspartate transaminase (AST), Alkaline phosphatase (ALP) and Lipid peroxidation were procured from Biovision, India. Assay kits for Catalase (CAT), Glutathione (GSH) were procured from Merck, India. All other chemicals were of analytical grade.
In-vitro studies
Cell culture studies using HepG2 cells
Human hepato-cellular carcinoma cell line (HepG2) was cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 1% Penicillin (100 U/ml) and Streptomycin (50 U/ml). Cell cultures were maintained at 37 °C in a humidified CO2 incubator (Thermo Scientific Steri-cycle) supplemented with 5% CO2 and 95% air stock cultures in the exponential growth phase by sub-culturing (passaging) them in respective growth medium every third day in 25 cm2 tissue culture flask using 0.25% Trypsin–EDTA solution.
MTT assay
Cell viability was observed using tetrazolium dye colorimetric test using MTT; (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay [12]. Individual wells of a cell culture grade 96-well micro-culture plate were seeded with 3 × 103 HepG2 cells and the final volume of growth medium was kept 200μL/well. Plates were incubated in a humidified CO2 incubator at 37 °C overnight. After 24 h of seeding, cells were incubated with various concentrations of methotrexate (MTX) (0.01 μM, 0.1 μM and10μM) with or without the optimized concentration of AKG (5 mM) for 24, 42 and 72 h. All the treatments were given in DMEM. After completion of treatment, DMEM (containing MTX and AKG as per the treatment conditions) was removed gently from each well and growth medium was replenished. Cultures were again incubated till various time points (24–72 h) under similar experimental conditions. At the last 3 h of corresponding time points, cells were incubated with MTT at a final concentration of 0.5 mg/ml (obtained from a stock solution of 5 mg/ml in PBS). After 3 h, the MTT was removed from each well and formazan crystals were dissolved by adding 100 μL of DMSO to each well. The absorbance was read at 570 nm using 630 nm as reference wavelength in microplate reader.
Animal experiments
Animals
Eighteen male Sprague Dawley rats weighing (200–250 g) were obtained from Experimental animal facility of Institute of Nuclear Medicine and Allied Sciences (INMAS), Delhi. Study protocol was approved by the Institutional Animal Ethics Committee (INM/IAEC/2009/06/010). The animals were housed in metabolic cages and were administered food (Hindustan Lever Ltd, Mumbai, India) and water ad libitum. Management and husbandry conditions were identical with 12/12 h light/dark cycle at 21 ± 2 °C.
Drug treatment
Rats were divided into three groups of 6 animals each. Group I served as control; Group II rats were given MTX treatment (single injection of 20 mg/kg/bw; intraperitoneal); Group III rats were given MTX treatment as mentioned above along with AKG supplementation (2 g/kg/body weight; intragastrically using oral gavage for 5 days). After 24 h of last treatment, animals were subjected to 99mTc-Mebrofenin hepatobilliary scintigraphy to assess the functional status in-vivo. After completion of functional imaging, rats from each group were sacrificed and blood samples were collected and serum was separated by centrifugation at 2000×g for 10 min and stored for biochemical estimations. Portions of liver tissue were placed in 10% formalin solution for routine histopathtological examination and one portion was kept in liquid nitrogen for estimation of antioxidant status.
In-vivo functional imaging study: 99mTc-Mebrofenin hepatobilliary scintigraphy
For 99mTc-Mebrofenin hepatobiliary scintigraphy (99mTc-MEB-HBS) in rats, a dual head gamma camera (Symbia, T2, Siemens) was used. Scintigraphy was performed after an overnight fast to minimize effect of food on hepatic blood and bile flow. Animals were sedated with ketamine/Xylazine (50 mg/kg and 5 mg/kg respectively). Once sedated a bolus of 40MBq (in 0.3 ml of saline) of 99mTc-MEB was injected intravenously into rat tail. Anterior images were acquired immediately upon injection for 30min (5sec/frame for 10min and 60sec/frame for 20min).
Scintigraphy data were processed on an inbuilt Pegasys workstation (ADAC laboratory). Region of interest (ROI) were drawn around liver (organ of interest), heart and large mediastinal vessels (blood pool) and over total field of view (total radiotracer activity). Hepatic Extraction Fraction (HEF), a parameter to assess hepatic uptake was calculated based on protocol described earlier and modified for use of 99mTc-MEB-HEF [13]. Further, Tpeak, i.e., time to reach maximum hepatic uptake and T1/2 peak, i.e., time for hepatic uptake to reduce by 50%, were also calculated. For this an additional ROI was drawn excluding large bile ducts and superimposing bowel loops; from which time–activity curve were generated and Tpeak and T1/2 peak were calculated.
Biochemical analysis
Estimation of hepatic enzyme activities in serum
Activities of hepatic intracellular enzymes - Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST) and Alkaline Phosphatase (ALP) in serum were determined by semi-automatic biochemical analyzer (Krish BioMedicals, Delhi, India) using commercially available kits.
Non-enzymatic and enzymatic antioxidant assay
Liver homogenate was prepared in ice cold saline by using tissue homogenizer (IKA-T10 Basic Ultraturrax). The homogenate was then centrifuged in a cooling centrifuge (Thermo Scientific – Heraeus Biofuge stratos) and the supernatant was further used for enzymatic assays. Levels of lipid peroxidation (TBARS), glutathione (GSH) and catalase (CAT) were determined using commercially available kits (Abcam, India).
Tissue factor activity
Tissue factor activity of liver tissues was estimated according to Quick's one stage method using prothrombin time test and expressed in seconds as reported earlier [14].
Tissue collagen measurement
Liver tissues were fixed in 10% formalin and embedded in paraffin wax. Estimation of collagen content was carried out by determination of the binding of Sirius red dye to collagen components and fast green dye to non-collagenous proteins as per previously reported method [15].
Histopathological analysis
Liver tissues were fixed in 10% formalin and processed routinely for paraffin embedding. Standard histopathological procedures were followed to observe any microscopic changes.
Statistical analysis
The results were expressed as mean ± SD or mean ± SEM (in vitro study; n = 3–4 and in vivo study; n = 6). The data were analyzed statistically using GraphPad Prism 8.00 (GraphPad Software, San Diego, California, USA) and Microsoft Excel (Version, 2012). All data test were statistically analyzed by one way ANOVA followed by Tukey's post-hoc test except cell survival test where two-way ANOVA followed by Bonferroni test were used. A value of p < 0.01 was considered significant.
Results
Present study was undertaken to explore the efficacy of AKG to protect against MTX-induced liver damage in HepG2 cells and experimental animals on the basis of cellular, biochemical, histological and in-vivo functional observations.
In vitro studies
Effect on cell viability
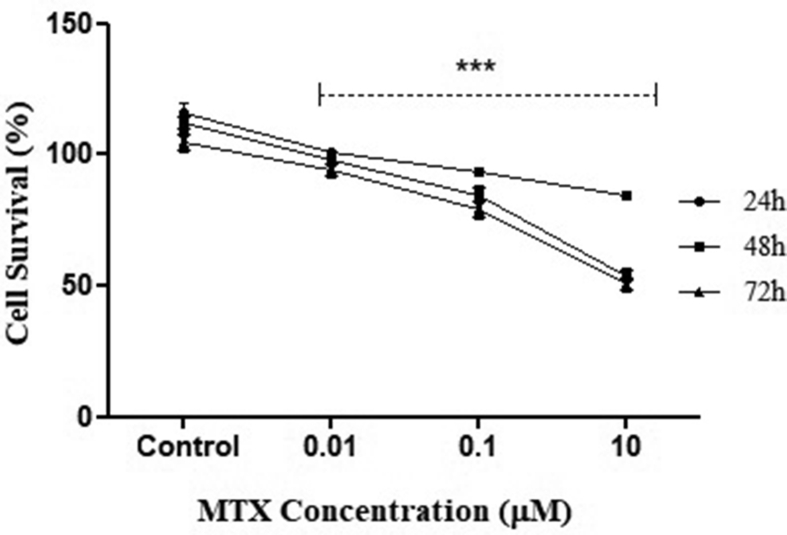
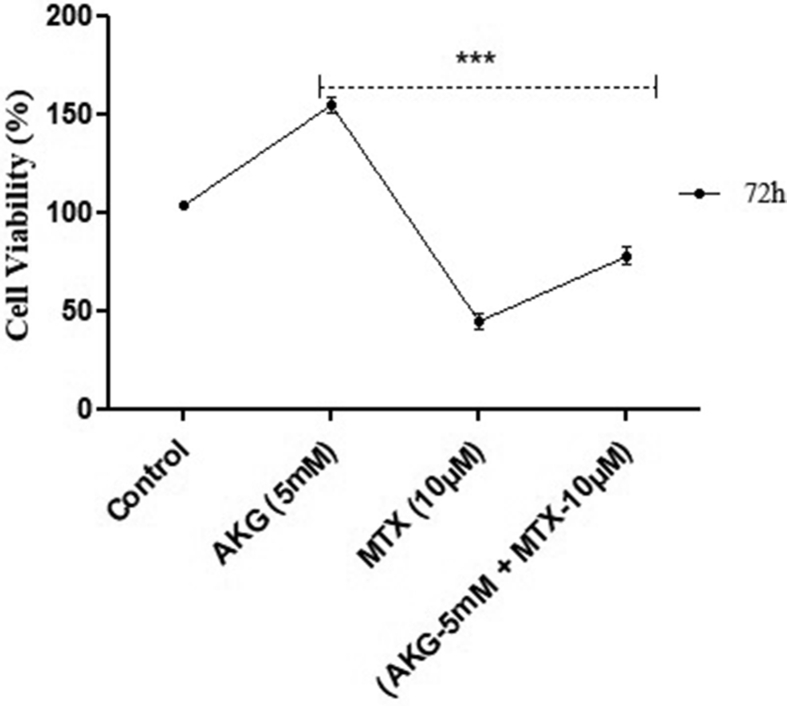
Effect of MTX on metabolic viability of cells was determined. Results indicated that there was no cytotoxic effect at the minimum conc. studied i.e., 0.01 μM MTX, as the metabolic viability of treated cells was similar to control. At the higher conc. of MTX (>0.1 μM) no cytotoxicity was observed till 24 h, but at the end of 72 h, the viability was reduced to 72% (p = 0.006). At further higher conc. of MTX, i.e., 10 μM, cells showed only 48% cell viability at 72 h [p = 0.004; Fig. 1]. For subsequent cytoprotective studies, which involved supplementation of AKG, the highest conc. of MTX (10 μM) was taken. The results indicated that after AKG supplementation along with MTX the viability of cells increased from 48% to 72% at 72 h (p = 0.006 when compared to only MTX treated group) [Fig. 2].
Fig. 1.
Effect of varying conc. of MTX on HepG2 cell survival. The graph represents the results of three independent experiments (Mean + SEM), performed in triplicates. ∗∗∗p < 0.01, when compared with control at any given time point (Two way ANOVA followed by Bonferroni test).
Fig. 2.
Effect of AKG on MTX induced toxicity on HepG2 cell viability. The graph represents the results of three independent experiments (Mean + SEM), performed in triplicates. ∗∗∗p < 0.01, comparison within groups (Two way ANOVA followed by Bonferroni test).
Animal experiments
In-vivo functional imaging: 99mTc-Mebrofenin hepatobilliary scintigraphy
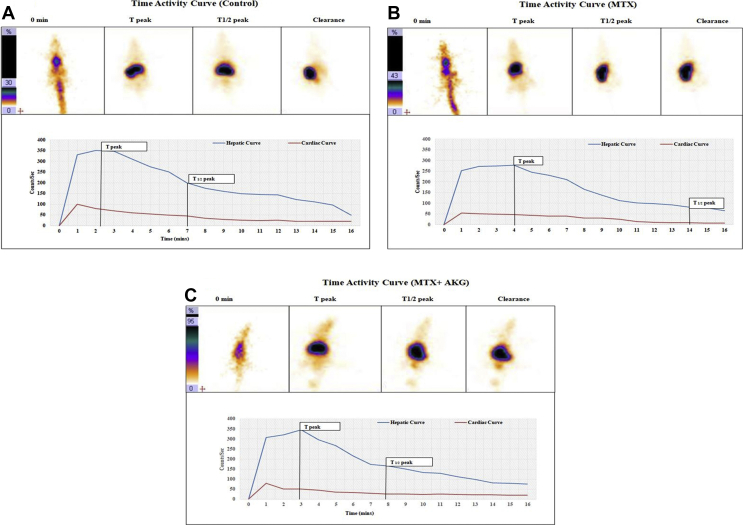
99mTc-Mebrofenin hepatobilliary scintigraphy helped in evaluating the state of liver in terms of preserving hepatocellular functioning after MTX exposure in the absence and presence of AKG supplementation. Results are summarized in Table 1 and Fig. 3(a–c). In control animals (Group-I), 99mTc-Mebrofenin showed prompt hepatic uptake, homogenous and efficient tracer accumulation, with rapid hepatocyte clearance. The Hepatic Extraction Factor (HEF) stood at (92.2 ± 1.8%) with Tpeak (138sec ± 29sec) and T1/2 peak (421sec ± 43sec) for the control group. In contrast, MTX treated group (Group-II) showed significantly deranged 99mTc-MEB handling parameters. The HEF were decreased (48.7 ± 2.6%; p < 0.001), while time required for peak hepatic uptake (Tpeak) was increased to 234sec ± 40sec (p < 0.001) in comparison to control animals. Also, duration for excretion half-life (T1/2peak) were significantly delayed (846 s ± 32sec; p < 0.001). AKG supplemented group (Group-III) showed significantly improved HEF (69.8 ± 4.3%), Tpeak (144sec ± 35sec) and T1/2peak (468sec ± 27sec) when compared to Group-II (p < 0.001).
Table 1.
Determination of hepatic uptake and excretion kinetics in Control, MTX and AKG treatment groups using99m Tc-Mebrofenin hepatobiliary scintigraphy. HEF corresponds to Hepatic Extraction fraction; Tpeak -time to reach maximum hepatic uptake; and T1/2 peak -time for hepatic uptake to reduce by 50%
| 99mTc-Mebrofenin | Control | MTX | MTX + AKG |
|---|---|---|---|
| HEF (%) | 92.2 ± 1.8 | 48.7 ± 2.6∗ | 69.8 ± 4.3$ |
| Tpeak | 138sec ± 29sec | 234sec ± 40sec∗ | 144sec ± 35sec |
| T1/2 peak | 421sec ± 43sec | 846sec ± 32sec∗ | 468sec ± 27sec |
Data is expressed as Mean ± SD; n=6. ∗p < 0.001 when MTX treatment group compared with control; $p < 0.001 when MTX group compared with AKG supplemented group (One way ANOVA followed by Tukey's test).
Fig. 3.
99mTc-Mebrofenin functional imaging uptake (Tpeak) and clearance (T1/2 peak) of representative rat each from Control, MTX, and MTX with AKG supplemented groups. (A) Control shows prompt mebrofenin activity clearance from liver. (B) MTX group shows considerably delayed mebrofenin activity clearance from liver. (C) MTX + AKG group shows significant improvement in mebrofenin activity clearance from liver as compared to MTX group.
Biochemical analysis
Estimation of hepatic enzyme activities in serum
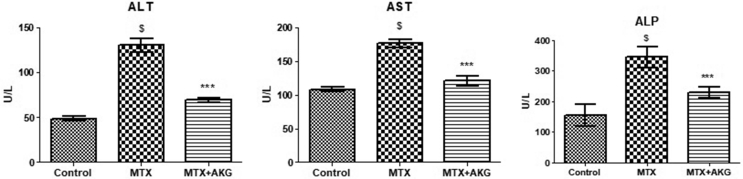
Hepatoprotective effect of AKG against MTX induced liver damage with respect to activities of liver enzymes is shown in Fig. 4. MTX group showed significant elevations in levels of ALT (135 ± 17.7 U/L), AST (188 ± 15.7 U/L) and ALP (389 ± 23.9 U/L) as compared to control group [ALT (43.5 ± 7 U/L; p = 0.005), AST (106.5 ± 21.2 U/L; p = 0.005) and ALP (216 ± 12.5 U/L; p = 0.004)]. Supplementation of AKG significantly reversed elevations of ALT (78.6 ± 12.6 U/L; p = 0.003), AST (115 ± 19.3 U/L; p = 0.005) and ALP (263 ± 19.7 U/L; p = 0.003) in comparison to MTX treated group.
Fig. 4.
Effect of exogenous AKG on liver enzymes (ALT, AST and ALP) in MTX induced hepatotoxicity (n = 6; mean ± SD; p < 0.01). $p < 0.01 when MTX group compared with control; ∗∗∗p < 0.01 when AKG supplemented group compared with MTX (One way ANOVA followed by Tukey's test).
Non-enzymatic and enzymatic antioxidant assay
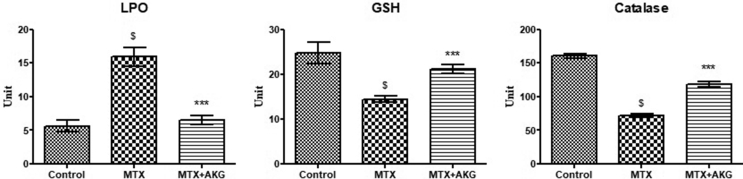
Effect of AKG supplementation on antioxidant status of MTX treated animals is depicted in Fig. 5. Liver TBARS levels were significantly increased in MTX treatment group (16.1 ± 2.9 nmol/mg protein) in comparison to control (6.9 ± 1.1 nmol/mg protein; p = 0.006). AKG supplementation however reversed the MTX induced alterations in TBARS levels (7.1 ± 2.1 nmol/mg protein; p = 0.005). On the other hand, whereas MTX exposure diminished reduced glutathione (GSH) activity (13.7 ± 3.1 μmol/mg protein; p = 0.004) as compared to control (25.9 ± 4.2 μmol/mg protein), AKG supplementation along with MTX treatment significantly restored the GSH levels (19.7 ± 3.1 μmol/mg protein; p = 0.005). A decrease in CAT activity was seen in MTX treated animals (75 ± 9.1 nmol/mg protein; p = 0.002) as compared to controls (160.4 ± 12.1 nmol/mg protein). Co-administration of AKG led to significant increase and restoration of CAT activity towards control value (120 ± 15.2 nmol/mg protein; p = 0.004).
Fig. 5.
Effect of exogenous AKG on Lipid peroxidation, GSH and Catalase in MTX induced hepatotoxicity (n = 6; mean ± SD; p < 0.01). $p < 0.01 when MTX group compared with control; ∗∗∗p < 0.01 when AKG supplemented group compared with MTX (One way ANOVA followed by Tukey's test).
Tissue collagen measurement and tissue factor (TF) activity
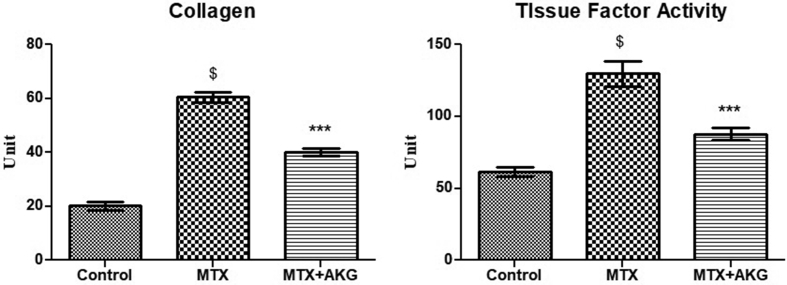
Collagen content of liver tissue were increased from 25 ± 3.0 μg/mg protein (control) to 63.5 ± 6.8 μg/mg protein following MTX treatment (p = 0.005), indicating an enhanced fibrotic activity. On the other hand, increased collagen levels were reversed near to control levels (25 ± 3.0 μg/mg protein) by supplementation of AKG (39 ± 6.2 μg/mg protein; p = 0.005) (Fig. 6).
Fig. 6.
Effect of exogenous AKG on collagen content and liver Tissue Factor activity in MTX induced hepatotoxicity (n = 6; mean ± SD; p < 0.01). $p < 0.01 when MTX group compared with control; ∗∗∗p < 0.01 when AKG supplemented group compared with MTX (One way ANOVA followed by Tukey's test).
Tissue factor activity was also measured by using prothrombin time test. TF activity of MTX group (133 ± 7.2s) was significantly increased when compared to control group (70.8 ± 6.2s; p = 0.007). Co-administration of AKG resulted in significant improvement in TF activity (90.6 ± 12.3s; p = 0.005) [Fig. 6].
Histological analysis
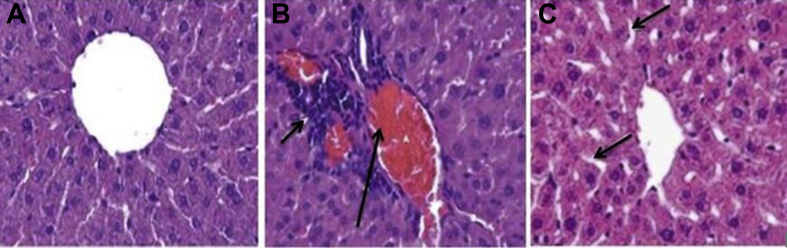
Control rats had normal histological pattern of liver [Fig. 7A], whereas MTX-treated rats showed various histological changes corresponding to liver injury characterized by degenerated hepatocytes, vascular congestion in sinusoids, dilatation of sinusoids, and increased number of activated Kupffer cells [Fig. 7B]. AKG supplementation markedly diminished tissue damage induced by MTX treatment [Fig. 7C].
Fig. 7.
Effect of exogenous AKG on histopathological evaluation of rat livers in MTX induced hepatotoxicity. (A) Control; arrows indicate hepatocytes (B) MTX treated group; arrows indicating severe congestion and sinusoidal enlargement (C) MTX + AKG supplemented group; arrows indicate mild cytoplasmic vacuolation. Scale Bars = 25 μm in a,b and c.
Discussion
Alpha-ketoglutarate (AKG) is a key intermediate in the Krebs's cycle, which serves as a source of energy for the cells. Studies have shown that around 50% of the exogenously added AKG is converted to energy, whereas the rest of it is involved in anabolic effects like synthesis of amino acids [16]. There is a growing body of literature appreciating the role of exogenous AKG in detoxification of reactive oxygen species (ROS) mediated oxidative stress by neutralizing ROS in non-enzymatic/NADPH independent fashion with concomitant formation of succinate and CO2 [7]. The present study evaluates the role of AKG in protecting against methotrexate induced hepatotoxicity owing to its ROS scavenging properties. Besides in vitro studies, gamma scintigraphy imaging was done to validate the findings in vivo.
Our in vitro studies on HepG2 cells showed a significant increase in cell viability in MTX treated group after supplementing them with AKG in comparison to only MTX treated group. This may be because AKG also plays an indirect but a very crucial role through G protein-coupled receptors, in modulating cell signaling and metabolism, which leads to enhanced cell proliferation [17]. In addition, AKG by its ammonia scavenging properties increases cell viability and allowing the cells to proliferate for a longer period of time [18]. Our cell culture results with MTX corroborate well with the findings of Yiang et al. (2014) on dose dependent toxicity exhibited by MTX in Hep3B hepatocellular carcinoma cells [19].Hepatocellular functioning predominately includes physiological processes involved in uptake, metabolism, conjugation and excretion of various endogenous and exogenous substances, in which various transporters mediate an important role. Hepatobiliary scintigraphy (HBS), utilizing 99Tc-Mebrofenin (99mTc-MEB) as radiotracer, is a novel molecular nuclear imaging modality, which can measure multiple aspects of aforementioned hepatocellular functions in real time [20]. Once injected, 99Tc-MEB circulates in the serum in albumin bound form. Subsequently, mebrofenin is taken up by hepatocytes via salt and organic anion transporters (OATP 1&2), and without undergoing biotransformation gets excreted from hepatocytes via multidrug resistance protein (MRP 2&3) [21]. Although 99Tc-Mebrofenin doesn't undergo biotransformation, the transport mechanism resembles transport of various endogenous and exogenous substances such as hormones, bilirubin, toxins and drugs. 99Tc-MEB hepatobiliary scintigraphy, therefore is a true representative of physiological hepatocellular functioning [22]. Results of our in-vivo scintigraphy experiments using 99Tc-MEB clearly demonstrated protection conferred by AKG against MTX induced hepatic damage. Earlier studies have also reported significant co-relation between hepatobiliary scintigraphy with hepatic biochemical and histopathological parameters [23].
ALT and AST are the most commonly used markers of hepatocellular injury and death. Elevated levels of serum ALT, AST and ALP in MTX treated rats was probably due to damage caused to plasma membrane of hepatic parenchymal cells. Methotrexate is known to binds to hydrofolic reductase enzyme, which blocks the conversion of folic acid to folinic acid, thereby inhibiting synthesis of some amino acids and nucleic acids [24]. This process interferes with the function of hepatic parenchymal cells by damaging their organelles and plasma membrane leading to leakage of hepatic enzymes such as ALT and AST [25]. Higher level of these enzymes in serum represents the extent of damage. Co-supplementation of AKG along with MTX, however significantly prevented the enzyme elevations, indicating hepatoprotective ability of AKG.
Thiobarbituric acid reactive substances (TBARS) have generally been used as an important biomarker of oxidative stress [26]. MTX induced lipid peroxidation as seen from raised TBARS may have resulted from cellular and intra-cellular membrane lysis owing to free radical species damaging the unsaturated lipid membranes, leading to hepatocytic necrosis and thereby affecting the hepatic antioxidant enzymes, namely catalase and reduced glutathione (GSH). Catalase needs nicotinamide adenine dinucleotide phosphate (NADPH) for it to be in an active form. Under oxidative stress conditions, this NADPH gets oxidized, thereby negatively affecting catalase activity. AKG offer protection against oxidative damage by participating in the non-enzymatic, NADPH independent oxidative decarboxylation in the hydrogen peroxide decomposition process. Moreover, AKG is a precursor to glutamine, which in turn is a precursor to glutathione, a natural intra- and extracellular antioxidant essential for the activity of other GSH dependent antioxidant enzymes [27]. This might be the reason why supplementation of AKG with MTX was probably able to restore activities of antioxidant enzymes, namely, catalase and glutathione towards normal levels. Exogenously administered AKG is known to improve redox homeostasis and protects the cell from free radical mediated oxidative stress by acting as a ubiquitous collector of amino (-NH2) groups, detoxifying ammonia and suppressing the generation of free radicals [28]. This may have led to an observed decrease in lipid peroxidation after supplementation of AKG along with MTX.
Another observation with respect to protective efficacy of AKG involved measurement of tissue factor (TF) activity in liver tissue. Tissue factor, also known as thromboplastin is an important coagulation factor that initiates extrinsic blood coagulation. Variations in TF activity occurs even in slight alterations in bodily mechanisms [29]. An increase in liver TF activity was seen in MTX treated group, which was significantly reversed by AKG supplementation. A significant decline in TF activity in AKG supplemented group probably indicates faster healing of liver tissue post-MTX exposure, as compared to when only MTX was given without any AKG supplementation. Further, over-expression of hepatic tissue collagen content in MTX treatment group suggests fibrinolytic activity, which disturbs the functioning of normal liver cells. AKG supplementation significantly decreased the hepatic collagen content to control levels. The results of the present study indicate that AKG can act as a potential therapeutic agent in treating methotrexate induced hepatotoxicity.
Conclusion
The results, especially 99mTc-mebrofenin functional imaging strongly suggests a possible therapeutic action of AKG in protecting liver damage by methotrexate. Apart from other generally assessed liver biomarkers, 99mTc-mebrofenin hepatobiliary scintigraphy has probably provided for the first time a real time in-vivo quantifiable validation of the ameliorative potential of AKG in mitigating MTX induced hepatotoxicity. This technique could be used to design and develop other hepatoprotective agents in future. Results of this study have helped us in a) proposing a new role for AKG as a supplement in neutralizing hepatotoxic effects of MTX therapy, b) initiating dose-fixation studies, and will c) subsequently form the basis of carrying out Phase-I/II trials to validate the findings in humans.
Conflicts of interest
The authors declare that there are no personal or financial conflicts of interest with any individual or organization.
Acknowledgement
The authors acknowledge the technical inputs provided by Dr. Ravi Soni, Sci ‘E’, INMAS in presenting results of in vitro cellular studies. Financial assistance provided to the first author by University Grants Commission, New Delhi, India is also duly acknowledged.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Wu J.J., Schiff K.R. Sarcoidosis. Am Fam Physician. 2004;70:312–322. [PubMed] [Google Scholar]
- 2.Uraz S., Tahan V., Aygun C., Eren F., Unluguzel G., Yuksel M., et al. Role of ursodeoxycholic acid in prevention of methotrexate-induced liver toxicity. Dig Dis Sci. 2008;53:1071–1077. doi: 10.1007/s10620-007-9949-3. [DOI] [PubMed] [Google Scholar]
- 3.West S.G. Methotrexate hepatotoxicity. Rheum Dis Clin N Am. 1997;23:883–915. doi: 10.1016/s0889-857x(05)70365-3. [DOI] [PubMed] [Google Scholar]
- 4.Cetinkaya A., Bulbuloglu E., Kurutas E.B., Kantarceken B. N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med Sci Monit. 2006;12:BR274–BR278. [PubMed] [Google Scholar]
- 5.Liu Y., Lange R., Langanky J., Hamma T., Yang B., Steinacker J.M. Improved training tolerance by supplementation with α-Keto acids in untrained young adults: a randomized, double blind, placebo-controlled trial. J Int Soc Sport Nutr. 2012;9:37. doi: 10.1186/1550-2783-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokołowska M., Oleszek A., Włodek L. Protective effect of alpha-keto acids on the oxidative hemolysis. Pol J Pharmacol. 1999;51:429–434. [PubMed] [Google Scholar]
- 7.Zdzisińska B., Żurek A., Kandefer-Szerszeń M. Alpha-ketoglutarate as a molecule with pleiotropic activity: well-known and novel possibilities of therapeutic use. Arch Immunol Ther Exp. 2017;65:21–36. doi: 10.1007/s00005-016-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dąbek M., Kruszewska D., Filip R., Hotowy A., Pierzynowski Ł., Wojtasz-Pająk A., et al. alpha-Ketoglutarate (AKG) absorption from pig intestine and plasma pharmacokinetics. J Anim Physiol Anim Nutr. 2005;89:419–426. doi: 10.1111/j.1439-0396.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- 9.Mittal G., Singh T., Kumar N., Bhatnagar A., Tripathi R.P., Tulsawani R., et al. Radiolabeling and dose fixation study of oral alpha-ketoglutarate as a cyanide antidote in healthy human volunteers. Clin Toxicol. 2010;48:509–515. doi: 10.3109/15563650.2010.496371. [DOI] [PubMed] [Google Scholar]
- 10.Malhi H., Bhargava K.K., Afriyie M.O., Volenberg I., Schilsky M.L., Palestro C.J., et al. 99mTc-mebrofenin scintigraphy for evaluating liver disease in a rat model of Wilson's disease. J Nucl Med. 2002;43:246–252. [PubMed] [Google Scholar]
- 11.Krishnamurthy S., Krishnamurthy G.T. Technetium-99m-iminodiacetic acid organic anions: review of biokinetics and clinical application in hepatology. Hepatology. 1989;9:139–153. doi: 10.1002/hep.1840090123. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Veteläinen R.L., Vliet A.V., Gulik T.M.V., Bennink R.J., Bruin K.d. Hepatobiliary function assessed by 99m Tc-mebrofenin cholescintigraphy in the evaluation of severity of steatosis in a rat model. Eur J Nucl Med Mol Imag. 2006;33:1107–1114. doi: 10.1007/s00259-006-0125-3. [DOI] [PubMed] [Google Scholar]
- 14.Talstad I. An analysis of the one-stage prothrombin time. Pathophysiol Haemostasis Thrombosis. 1985;15:310–317. doi: 10.1159/000215165. [DOI] [PubMed] [Google Scholar]
- 15.López-De León A., Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem. 1985;33:737–743. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- 16.Harrison A.P., Pierzynowski S.G. Biological effects of 2-oxoglutarate with particular emphasis on the regulation of protein, mineral and lipid absorption/metabolism, muscle performance, kidney function, bone formation and cancerogenesis, all viewed from a healthy ageing perspective state of the art-review article. J Physiol Pharmacol. 2008;59:91–106. [PubMed] [Google Scholar]
- 17.Cai X., Zhu C., Xu Y., Jing Y., Yuan Y., Wang L., et al. Alpha-ketoglutarate promotes skeletal muscle hypertrophy and protein synthesis through Akt/mTOR signaling pathways. Sci Rep. 2016;6:26802. doi: 10.1038/srep26802. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Nilsang S., Kumar A., Rakshit S.K. Effect of alpha-ketoglutarate on monoclonal antibody production of hybridoma cell lines in serum-free and serum-containing medium. Appl Biochem Biotechnol. 2008;151:489–501. doi: 10.1007/s12010-008-8225-0. [DOI] [PubMed] [Google Scholar]
- 19.Yiang G.T., Chou P.L., Hung Y.T., Chen J.N., Chang W.J., Yu Y.L., et al. Vitamin C enhances anticancer activity in methotrexate-treated Hep3B hepatocellular carcinoma cells. Oncol Rep. 2014;32:1057–1063. doi: 10.3892/or.2014.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Graaf W., van Lienden K.P., van Gulik T.M., Bennink R.J. 99mTc-mebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy. J Nucl Med. 2010;51:229–236. doi: 10.2967/jnumed.109.069724. [DOI] [PubMed] [Google Scholar]
- 21.Sun P., Wang C., Liu Q., Meng Q., Zhang A., Huo X., et al. OATP and MRP2-mediated hepatic uptake and biliary excretion of eprosartan in rat and human. Pharmacol Rep. 2014;66:311–319. doi: 10.1016/j.pharep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Ekman M., Fjälling M., Friman S., Carlson S., Volkmann R. Liver uptake function measured by IODIDA clearance rate in liver transplant patients and healthy volunteers. Nucl Med Commun. 1996;17:235–242. doi: 10.1097/00006231-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Krishnamurthy G.T., Krishnamurthy S. Cholescintigraphic measurement of liver function: how is it different from other methods? Eur J Nucl Med Mol. 2006;33:1103–1106. doi: 10.1007/s00259-006-0182-7. [DOI] [PubMed] [Google Scholar]
- 24.Tian H., Cronstein B.N. Understanding the mechanisms of action of methotrexate. Bull NYU Hosp Jt Dis. 2007;65:168–173. [PubMed] [Google Scholar]
- 25.McGill M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016;15:817–828. doi: 10.17179/excli2016-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bezerra F.J., Rezende A.A., Rodrigues S.J., Almeida M.d. Thiobarbituric acid reactive substances as an index of lipid peroxidation in sevoflurane-treated rats. Rev Bras Anestesiol. 2004;54:640–649. doi: 10.1590/s0034-70942004000500004. [DOI] [PubMed] [Google Scholar]
- 27.Meister A., Anderson M.E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 28.Liu S., He L., Yao K. The antioxidative function of alpha-ketoglutarate and its applications. Biomed Res Int BioMed. 2018;2018:3408467. doi: 10.1155/2018/3408467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butenas S., Orfeo T., Mann K.G. Tissue factor in coagulation: which? where? when? Arterioscler Thromb Vasc Biol. 2009;29:1989–1996. doi: 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]