Abstract
Introduction
Albuminuria is a clinical hallmark of diabetic nephropathy (DN). Nevertheless, it is controversial whether pathologic DN lesions exist in individuals with diabetes with normoalbuminuria. We investigated the association between albuminuria levels and the frequency of DN lesions in autopsied diabetic cases from a Japanese community.
Methods
A total of 106 autopsied cases with diabetes mellitus (mean age = 76 years, 43.4% male) who died within 6 years after their last health examination were included in the study. Urinary albumin-creatinine ratio (UACR) levels were divided into the following 3 groups: <30.0, 30.0 to 299.9, and ≥300.0 mg/g. The kidney specimens were evaluated with light microscopy. Glomerular DN lesions were categorized into class 0 to I, IIa, IIb, and III glomerular DN lesions according to the criteria of the Renal Pathology Society. A Cochran-Armitage test was used to evaluate the association between the UACR levels and the presence of class IIa or higher glomerular DN lesions.
Results
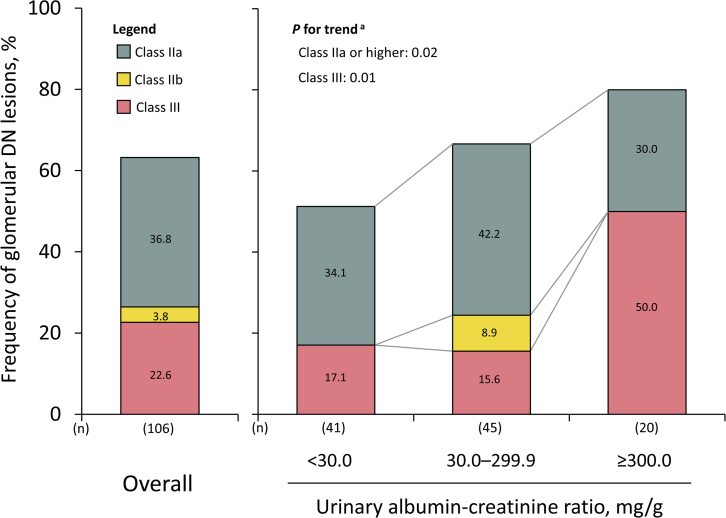
The frequency of class IIa or higher glomerular DN lesions was 63.2% (IIa, 36.8%; IIb, 3.8%; and III, 22.6%) among overall cases. The frequencies increased significantly with higher UACR levels (P for trend = 0.02). The frequency of class IIa or higher glomerular DN lesions was 51.2%, even in individuals with UACR < 30 mg/g.
Conclusion
This study revealed a positive association of the UACR levels with the presence of class IIa or higher glomerular DN lesions, which were also frequently found even in the normal range of UACR levels, among autopsied diabetic cases from a Japanese community.
Keywords: albuminuria, autopsy, community-based study, diabetic nephropathy, morphologic study
Graphical abstract
See Commentary on Page 2939
DN accounts for approximately half of all cases of end-stage kidney disease in many countries.1, 2, 3 The typical clinical course of DN is a period of microalbuminuria followed by overt albuminuria and then a decline in kidney function, and pathologic DN lesions are considered to progress with increasing albuminuria.4 Thus, albuminuria is a clinical hallmark of DN. In contrast, some patients with diabetes have reported a rapid decline in kidney function without overt albuminuria.5
Recently, several hospital-based studies suggested that albuminuria is not necessarily linked with the presence of pathologic DN lesions.6, 7, 8 Clinical studies using kidney biopsy specimens have revealed that patients with impaired kidney function, especially those with a rapid decline of kidney function, had severe DN lesions, despite the absence of microalbuminuria or overt albuminuria.6,7 Nevertheless, the findings from the clinical studies using kidney biopsy specimens are likely to be biased toward severe kidney lesions because kidney biopsies in patients with diabetes tend to be performed in cases of suspected kidney disease other than DN.9 Furthermore, a study using hospital-based autopsied cases revealed that the pathologic DN lesions were present in patients with diabetes before the development of albuminuria.8 In this autopsy study, however, albuminuria was mainly determined by the urinary dipstick test, which is less sensitive and less accurate than quantitative measurement, and could have misclassified the extent of albuminuria. In addition, hospital-based autopsied cases may include clinically specific cases. Although these hospital-based studies suggest that the progression of DN cannot be seen as a continuum of worsening albuminuria followed by kidney dysfunction, these studies include several limitations, as mentioned previously, such as selection bias and differences in the methods used to evaluate the urine findings. Therefore, these findings need to be verified in a community-based autopsy study that includes quantitative measurement of albuminuria.
The Hisayama Study is a community-based longitudinal study of cardiovascular disease in a Japanese population, in which autopsies have been performed among ∼70% of deceased cases in a community to determine the causes of deaths and evaluate pathologic changes in various organs.10,11 The aims of the present study are (i) to evaluate the association between albuminuria levels and pathologic DN lesions and (ii) to determine the frequency of autopsied cases with pathologic DN lesions (i.e., prevalence of pathologic DN) according to albuminuria levels, especially normal albuminuria levels, among autopsied diabetic cases from a Japanese community.
Methods
Study Population and Autopsy
The Hisayama Study was started in 1961 in the town of Hisayama, a suburban community adjacent to Fukuoka City in a metropolitan area of Kyushu Island in southern Japan. To date, 7 prospective cohorts have been established, for which the full-community surveys at baseline were conducted in 1961, 1974, 1983, 1988, 2002 to 2003, 2007 to 2008, and 2012 to 2013 among approximately 70% to 80% of residents aged ≥40 years.10,11 In this study, a 75-g oral glucose tolerance test has been performed at annual health checkups since 1988 to diagnose diabetes mellitus,12 and the UACR was measured at baseline surveys in 2002 to 2003, 2007 to 2008, and 2012 to 2013. In addition, autopsy examinations of deceased people in this town have been performed since 1961. The autopsies were performed at the Department of Pathology, Kyushu University. All autopsied cases were performed in a standard method throughout the study period.
For the present study, we used autopsy specimens obtained from deceased people in the town of Hisayama from July 2002 to November 2017. During this period, a total of 1194 residents died, and 715 of these underwent autopsy examinations. Among them, 513 participants had received the health examinations at a survey in either 2002 to 2003, 2007 to 2008, or 2012 to 2013 within 6 years of death, of whom 131 subjects had diabetes. After excluding 16 autopsied cases without available data of UACR, 5 cases without available kidney specimens, 2 cases with microscopically diagnosed membranous nephropathy, and 2 cases with almost all glomeruli with global glomerular sclerosis, the remaining 106 autopsied diabetic cases were included in the present study (Supplementary Figure S1). The mean period from the last health examination to death was 3.3 (SD = 1.6) years.
Diagnosis of Diabetes Mellitus
Diabetes mellitus was defined as a fasting plasma glucose level ≥ 7.0 mmol/l, 2 hours postloaded or casual plasma glucose level ≥ 11.1 mmol/l, or use of oral glucose-lowering medications or insulin,13 in which plasma glucose levels were measured by the hexokinase method at the health examination. The duration of diabetes mellitus was calculated from the date of the first diagnosis in the past health checkups to the date of the last visit of the survey in either 2002 to 2003, 2007 to 2008, or 2012 to 2013.
Measurement of Albuminuria
We obtained a spot urine sample during the morning of the health examination visit. Urinary creatinine and albumin levels were measured using the turbidimetric immunoassay method. The UACR was calculated by dividing urinary albumin by urinary creatinine concentration. The subjects were divided into 3 groups using UACR levels of 30.0 and 300.0 mg/g as cutoff values.14
Pathologic Examination of the Kidney Tissue and Outcomes
All kidney specimens were fixed in buffered formalin, embedded in paraffin, cut into 2 to 3 μm sections, and stained with hematoxylin and eosin, periodic acid–Schiff, Masson’s trichrome, and periodic acid–methenamine silver. We obtained images of 50 randomly selected glomeruli using light microscopy with a magnification of ×400. The class of glomerular DN lesions was determined based on the classification proposed by the Renal Pathology Society as follows: class IIa glomerular DN lesion, >25% nonsclerotic glomeruli with mesangial expansion smaller than a capillary loop; class IIb glomerular DN lesion, >25% nonsclerotic glomeruli with mesangial expansion larger than a capillary loop; class III glomerular DN lesion, the presence of 1 or more nodular lesions; and class IV glomerular DN lesion, global glomerulosclerosis in >50% of glomeruli with lesions from class I to III glomerular DN lesions.15 In the present study, when no abnormalities were found on light microscopy, the lesion was defined as a class 0 to I glomerular DN lesion owing to the absence of available data based on electron microscopy. No autopsied cases with class IV glomerular DN lesions were included in the present study population after excluding the above-mentioned 2 cases in which almost all glomeruli exhibited global glomerular sclerosis, which could not be evaluated for the presence of glomerular DN lesions or other glomerular diseases. The percentage of global glomerulosclerosis was calculated. Regarding mesangial expansion, each glomerulus was scored as follows: 0, no mesangial expansion; 1, mesangial expansion smaller than a capillary loop; 2, mesangial expansion equivalent to a capillary loop; or 3, mesangial expansion larger than a capillary loop.7,16 The mesangial expansion index was defined as the mean mesangial score for each glomerulus.16 The following other glomerular lesions were evaluated for the presence or absence of nodular lesions, subendothelial space widening, exudative lesions, mesangiolysis, polar vasculosis, and glomerulomegaly.7 The degree of interstitial fibrosis and tubular atrophy (IFTA) and interstitial inflammation was semiquantitatively evaluated every 5% as the percentages of the affected area over the observed cortical area, and the mean values of 3 fields of view at the magnification of ×40 were calculated. The severity of arteriolar hyalinosis was scored as follows: 0, no hyalinosis; 1, partial hyalinosis; 2, hyalinosis covering approximately half of the vessel wall; or 3, hyalinosis covering more than half of the vessel wall and/or hyalinosis covering all layers of the vessels.7,16,17 The arteriolar hyalinosis index was defined as the mean arteriolar hyalinosis scores for each observed vascular pole.16,18 The arterial intima-media ratio was defined as the ratio of intimal thickness to media thickness,7 and the mean values of 3 arteries were calculated.
All pathologic images were captured by 1 investigator (TS) with light microscopy (Leica DM 750; Leica Microsystems, Wetzlar, Germany) and a digital camera (Leica ICC 50 W; Leica Microsystems) throughout the present study. The captured images were saved without any processing or manipulation and distributed to each investigator.
All pathologic findings except glomerular hypertrophy were evaluated independently by 2 nephrologists (TS and KN), who were blinded to the clinical information, and glomerular hypertrophy was determined as the presence of at least 1 glomerulus > 250 μm by TS. The more severe score from the classification of the glomerular DN lesions by the 2 investigators was used in the analysis. The intraclass correlation coefficient for the classification of glomerular DN lesions between the 2 investigators was 0.84. The mean value of the measurements of the 2 investigators was used to analyze the mesangial expansion index, percentages of global glomerulosclerosis, IFTA, interstitial inflammation, arteriolar hyalinosis index, and arterial intima-media ratio. Other findings were defined as those determined to be present by at least 1 investigator. In the present study, the class of glomerular DN lesions proposed by the Renal Pathology Society was the primary outcome, and the other findings were the secondary outcomes.
Measurement of Risk Factors
Self-administered questionnaires containing the baseline information on smoking habits, medications, and past medical history were completed by each participant and confirmed by well-trained interviewers. At the health examinations, blood pressure (BP) was measured 3 times using an automatic sphygmomanometer with the subject in a seated position after at least 5 minutes of rest. An average of 3 measurements was used for the analysis. Hypertension was defined as systolic BP ≥ 140 mm Hg, diastolic BP ≥ 90 mm Hg, or the use of antihypertensive medications. Body height and weight were measured in light clothing without shoes, and body mass index was calculated as body weight in kilograms divided by body height in meters squared. Hemoglobin A1c was measured as the Japan Diabetes Society value by a latex aggregation immunoassay in 2002 to 2003 and 2007 to 2008 and as the National Glycohemoglobin Standardization Program value by high-performance liquid chromatography in 2012 to 2013. The Japan Diabetes Society values were converted to the National Glycohemoglobin Standardization Program values with the following formula: hemoglobin A1c (%) = 1.02 × hemoglobin A1c (Japan Diabetes Society) (%) + 0.25%.19 Serum total cholesterol was measured enzymatically. Hypercholesterolemia was defined as serum total cholesterol ≥ 5.69 mmol/l or current use of lipid-modifying agents. The estimated glomerular filtration rate was calculated using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation modified by the Japanese coefficient,20 for which serum creatinine levels were measured enzymatically.
Statistical Analyses
Linear trends in the mean values of risk factors, the median values of the index of pathologic kidney lesion, and the frequencies of risk factors and the class of glomerular DN lesion across the UACR levels were tested using a linear regression analysis, a Jonckheere-Terpstra test,21 and a Cochran-Armitage test,21 respectively. A binomial and an ordinal logistic regression analysis were used to evaluate the odds ratios and 95% CIs of the UACR levels on the class of glomerular DN lesions. In the multivariable-adjusted analysis, the risk estimates were adjusted for potential risk factors—namely, age, sex, hemoglobin A1c, duration of diabetes mellitus ≥5 years, systolic BP, use of angiotensin receptor blocker and/or angiotensin-converting enzyme inhibitor, hypercholesterolemia, body mass index, and current smoking. Two-sided P < 0.05 was considered statistically significant in all analyses. SAS version 9.4 (SAS Institute Inc., Cary, NC) was used to perform all statistical analyses.
Ethical Standards
This study was conducted with the approval of the Kyushu University Institutional Review Board for Clinical Research. Written informed consent was obtained from all individuals.
Results
Characteristics of the Autopsied Diabetic Cases Included in the Present Study
In the autopsied cases included in the present study, the mean age at the last health examination was 76 (SD = 9) years, and the proportion of men was 43.4%. The characteristics of the cases at the last health examination according to the UACR levels are found in Table 1. The frequencies of the duration of diabetes mellitus ≥5 years and the mean values of systolic BP increased significantly with increasing UACR levels, whereas the mean values of estimated glomerular filtration rate decreased significantly.
Table 1.
Characteristics of the included autopsied diabetic cases at the last health examination within 6 years before death according to the urinary albumin-creatinine ratio levels
| Variables | Urinary albumin-creatinine ratio, mg/g |
P for trend | ||
|---|---|---|---|---|
| <30.0 |
30.0–299.9 |
≥300.0 |
||
| n = 41 | n = 45 | n = 20 | ||
| Age at the last health examination, yr | 75 (9) | 77 (9) | 78 (10) | 0.17 |
| Age at death, yr | 78 (9) | 80 (9) | 81 (10) | 0.19 |
| Duration from the last health examination to death, yr | 3.2 (1.4) | 3.4 (1.6) | 2.9 (1.6) | 0.68 |
| Male, % | 41.5 | 35.6 | 65.0 | 0.17 |
| Hemoglobin A1c, % | 6.5 (1.1) | 6.7 (1.7) | 6.9 (1.2) | 0.28 |
| Duration of diabetes mellitus ≥5 yr, % | 63.4 | 71.1 | 90.0 | 0.04 |
| Use of diabetic medication, % | 68.3 | 55.6 | 65.0 | 0.60 |
| Use of oral hypoglycemic agent, % | 56.1 | 51.1 | 50.0 | 0.61 |
| Use of insulin, % | 17.1 | 4.4 | 25.0 | 0.76 |
| Systolic blood pressure, mm Hg | 133 (16) | 142 (22) | 150 (20) | <0.001 |
| Diastolic blood pressure, mm Hg | 76 (11) | 78 (10) | 80 (13) | 0.19 |
| Hypertension, % | 70.7 | 84.4 | 80.0 | 0.27 |
| Use of hypertensive medication, % | 56.1 | 64.0 | 70.0 | 0.24 |
| Use of ARB/ACE-I, % | 39.0 | 44.4 | 55.0 | 0.25 |
| Serum total cholesterol, mmol/l | 4.97 (0.93) | 4.71 (0.98) | 4.71 (1.09) | 0.28 |
| Hypercholesterolemia, % | 39.0 | 33.3 | 65.0 | 0.12 |
| Use of lipid-modifying medication, % | 26.9 | 24.4 | 45.0 | 0.23 |
| Body mass index, kg/m2 | 23.0 (4.0) | 23.6 (3.3) | 23.5 (4.1) | 0.57 |
| eGFR, ml/min per 1.73 m2 | 65.6 (13.9) | 60.7 (16.0) | 54.0 (17.4) | 0.007 |
| Current and former smoker, % | 51.2 | 60.0 | 50.0 | 0.90 |
| Current smoker, % | 9.8 | 15.6 | 10.0 | — |
| Former smoker, % | 41.5 | 44.4 | 40.0 | — |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate.
Data are illustrated as the mean values (SD) or percentages.
UACR Levels and Pathologic Findings
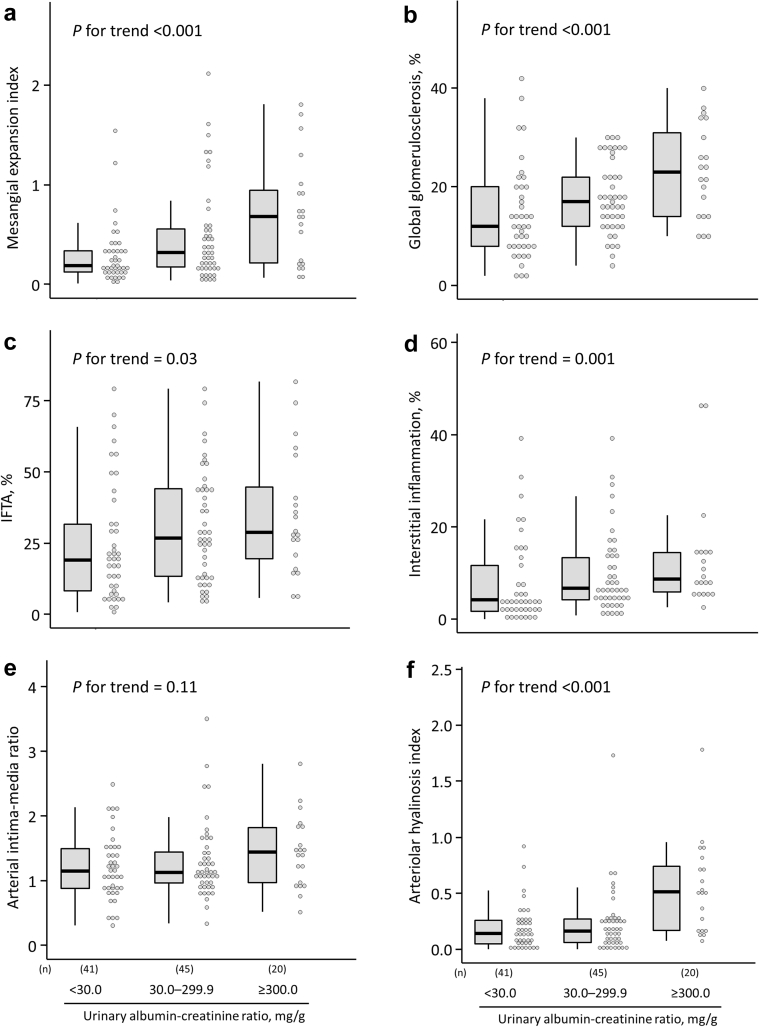
In the overall autopsied diabetic cases, the frequencies of cases with class IIa or higher glomerular DN lesions according to the Renal Pathology Society were as follows: class IIa, 39 (36.8%); class IIb, 4 (3.8%); and class III, 24 (22.6%) (Figure 1). The frequencies of cases with class IIa or higher and class III glomerular DN lesions significantly increased with higher UACR levels in the autopsied diabetic cases (Figure 1; P for trend = 0.02 for class IIa or higher DN lesion; P = 0.01 for class III). The age-, sex-, and multivariable-adjusted odds ratios for the presence of class IIa or higher glomerular DN lesions increased significantly for every 1 increment in log-transformed UACR (Supplementary Table S1). Similarly, in ordinal logistic analysis with the outcome as an ordinal variable for the class of glomerular DN lesions, the odds ratios for the progression of the class of glomerular DN lesions increased significantly as log-transformed UACR levels increased (Supplementary Table S1). With regard to the frequencies of cases with each pathologic DN-related glomerular lesion, higher UACR levels were associated significantly with higher frequencies of cases with nodular lesions and exudative lesions (Table 2). In addition, we addressed the association of UACR levels with the pathologic index for mesangial matrix expansion, glomerulosclerosis, tubulointerstitial damage, and artery/arteriole damage. Consequently, the median values of mesangial expansion index, percentages of global glomerulosclerosis, percentages of IFTA, percentages of interstitial inflammation, and arteriolar hyalinosis index increased significantly with increased UACR levels (Figure 2). Meanwhile, there was no evidence of significant association of UACR levels with the frequencies of cases with the other glomerular lesions, except for nodular lesions and exudative lesions, and median values of the arterial intima-media ratio.
Figure 1.
Frequency of each class of glomerular diabetic nephropathy lesion in overall autopsied diabetic cases and according to the urinary albumin-to-creatinine ratio levels. aTrends in the frequency of the presence of class IIa or higher glomerular DN lesions across the UACR levels, including trends in the frequency of the presence of class III glomerular DN lesions, were tested by using a Cochran-Armitage test. DN, diabetic nephropathy; UACR, urinary albumin-creatinine ratio.
Table 2.
Frequency of autopsied diabetic cases with glomerular lesions related to diabetic nephropathy according to the UACR levels
| Glomerular lesions | Urinary albumin-creatinine ratio, mg/g |
P for trenda | ||
|---|---|---|---|---|
| <30.0 |
30.0–299.9 |
≥300.0 |
||
| n = 41 | n = 45 | n = 20 | ||
| Nodular lesion, % | 17.1 | 15.6 | 50.0 | 0.01 |
| Subendothelial space widening, % | 36.6 | 35.6 | 40.0 | 0.84 |
| Exudative lesion, % | 12.2 | 17.8 | 55.0 | <0.001 |
| Mesangiolysis, % | 39.0 | 42.2 | 55.0 | 0.27 |
| Polar vasculosis, % | 80.5 | 86.7 | 95.0 | 0.13 |
| Segmental glomerulosclerosis, % | 17.1 | 22.2 | 35.0 | 0.13 |
| Glomerulomegaly, % | 43.9 | 53.3 | 55.0 | 0.35 |
UACR, urinary albumin-creatinine ratio.
Trends in the frequency of each lesion across the UACR levels were tested by using a Cochran-Armitage test.
Figure 2.
Boxplots and dot plots of the extent of the pathologic index for mesangial matrix expansion, glomerulosclerosis, tubulointerstitial damage, and artery/arteriole damage according to urinary albumin-to-creatinine ratio levels. (a) Mesangial expansion index; (b) global glomerulosclerosis; (c) interstitial fibrosis and tubular atrophy; (d) interstitial inflammation; (e) arterial intima-media ratio; and (f) arteriole hyalinosis index. The bold horizontal line represents the median, the box represents the 25th to 75th percentiles, the vertical bar represents the minimum to the maximum except for outliers, and the dots represent individual cases. Trends in the difference across albuminuria levels were tested by using a Jonckheere-Terpstra test. IFTA, interstitial fibrosis and tubular atrophy.
Pathologic Findings in Autopsied Diabetic Cases With Normoalbuminuria
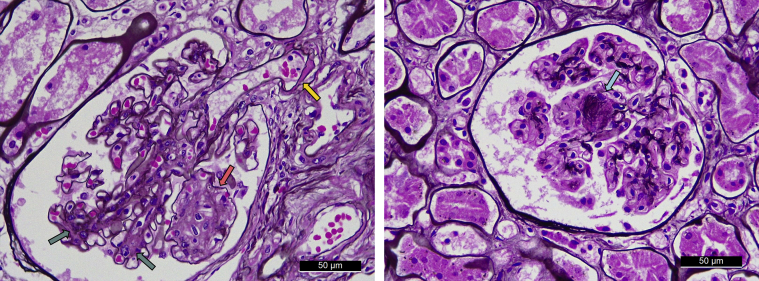
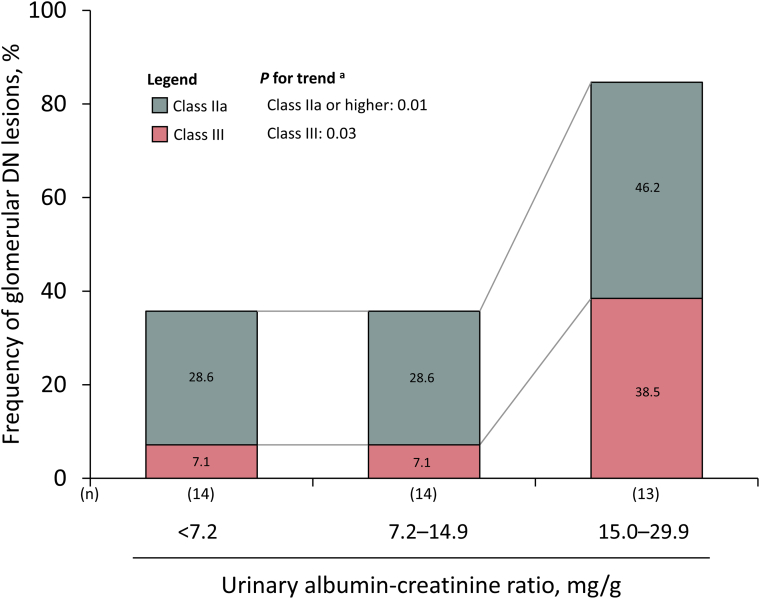
As illustrated in Figure 1, 34.1% of the cases had class IIa glomerular DN lesions, no case had class IIb, and 17.1% had class III among the autopsied diabetic cases with normoalbuminuria defined as UACR < 30.0 mg/g. Figure 3 illustrates the representative histologic findings of DN lesions—namely, mesangial expansion, mesangiolysis, arteriolar hyalinosis, and nodular lesions—identified in autopsied diabetic cases with normoalbuminuria. Similar findings were observed in the sensitivity analyses restricting the following cases (Table 3): (i) cases without the use of an angiotensin receptor blocker and/or angiotensin-converting enzyme inhibitor; (ii) cases with a duration of diabetes mellitus ≥5 years; (iii) cases with persistent normoalbuminuria (or normal proteinuria [negative or trace proteinuria in urinary test strips]) 5 to 6 years before the last health examination; (iv) cases without a current smoking habit; and (v) cases who died within 2 or 3 years after the last health examination. The frequencies of class IIa or higher and class III glomerular DN lesions increased significantly with higher tertile levels of UACR in the group with normoalbuminuria (Figure 4; P for trend = 0.01 for class IIa or higher DN lesions; P = 0.03 for class III).
Figure 3.
Representative histologic findings of glomerular diabetic nephropathy lesions in autopsied cases with normoalbuminuria. Mesangial expansions (green arrow), mesangiolysis (pink arrow), arteriolar hyalinosis (yellow arrow), and nodular lesions (blue arrow) were observed. Original magnification, ×400.
Table 3.
Sensitivity analyses restricted to several subsets of autopsied diabetic cases with normoalbuminuria for the frequencies of cases with class IIa and class III glomerular diabetic nephropathy lesions
| Subjects | No. of subjects | Class of glomerular diabetic nephropathy |
|
|---|---|---|---|
| IIa, % | III, % | ||
| All autopsied cases with normal albuminuria | 41 | 34.1 | 17.1 |
| Without ARB or ACE-I | 25 | 40.0 | 8.0 |
| Duration of diabetes mellitus ≥5 yr | 26 | 38.5 | 23.1 |
| Persistent normal albuminuria or proteinuria | 23 | 34.8 | 13.0 |
| Noncurrent smoker | 37 | 37.8 | 16.2 |
| Autopsies within 2 yr from the last health examinations | 8 | 37.5 | 12.5 |
| Autopsies within 3 yr from the last health examinations | 20 | 30.0 | 15.0 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
No autopsied cases with normal albuminuria had class IIb glomerular diabetic nephropathy lesions in the present study.
Figure 4.
Frequency of each class of glomerular diabetic nephropathy lesion according to urinary albumin-to-creatinine ratio levels among the autopsied diabetic cases with normoalbuminuria. aTrends in the frequency of the presence of class IIa or higher glomerular DN lesions across the UACR levels, including trends in the frequency of the presence of class III glomerular DN lesions, were tested by using a Cochran-Armitage test. DN, diabetic nephropathy; UACR, urinary albumin-creatinine ratio.
Discussion
This study revealed that 63.2% of the autopsied diabetic cases in a Japanese community had class IIa or higher glomerular DN lesions. In addition, the frequency of cases with class IIa or higher glomerular DN lesions, including nodular and exudative lesions, increased significantly with higher UACR levels. Higher UACR levels were also associated with higher median values for each of the following: mesangial expansion index, percentage of global glomerulosclerosis of IFTA, interstitial inflammation, and arteriolar hyalinosis index. Intriguingly, the frequency of class IIa or higher glomerular DN lesions was >50%, even in diabetic cases with normoalbuminuria. These findings suggest a high frequency of glomerular DN lesions that would be potential therapeutic targets, even in patients with normal albuminuria levels, among autopsied diabetic cases from a Japanese community.
In the present study, elevated UACR levels were significantly associated with glomerular injuries (i.e., class of glomerular DN lesions, mesangial expansion index, global glomerulosclerosis, nodular lesions, and exudative lesions), tubulointerstitial injuries (i.e., IFTA and interstitial inflammation), and arteriolar injuries (i.e., arteriolar hyalinosis index). Studies using kidney biopsy specimens have reported an association between albuminuria levels and pathologic kidney findings.7,22, 23, 24, 25 Moreover, a recent hospital-based autopsy study reported by Klessens et al.8 revealed that cases with albuminuria (i.e., microalbuminuria or proteinuria), which is mainly determined by the urinary dipstick test, had more severe changes in some kidney findings—that is, the percentage of glomeruli with nodular lesions and IFTA—than those without. The underlying mechanisms of albuminuria in DN are considered to be leakage of circulating proteins owing to injury of glomerular components and glomerular hemodynamic abnormalities and impaired reabsorption of proteins in the proximal tubules.26 Therefore, it is rational that the UACR levels were associated with glomerular, tubulointerstitial, and arteriolar injuries in the present study.
In the present study, 51.2% of the autopsied cases had class IIa or higher glomerular DN lesions in autopsied diabetic cases with normoalbuminuria, and this value was not substantially changed in the sensitivity analyses excluding cases that can affect the UACR levels and the class of DN lesions (e.g., cases with use of angiotensin receptor blocker and/or angiotensin-converting enzyme inhibitor). A kidney biopsy–based study did not reveal a significant association between the UACR level and the mesangial volume fraction in the subjects with normoalbuminuria or microalbuminuria.25 Nevertheless, the study had the limitations of small sample size (i.e., <100 subjects) and the possibility of selection bias based on the indications for kidney biopsy. Meanwhile, the above-mentioned autopsy study also found that diabetic cases with normoalbuminuria had histologically proven DN.8 The frequency of the cases with class IIa or higher glomerular DN lesions was similar between the above-mentioned hospital-based autopsy study and our present report. Nevertheless, the above-mentioned autopsy study failed to reveal a significant difference in the frequency in DN lesions between the presence and absence of albuminuria. In contrast, the present study revealed a significant positive association between the UACR levels and the frequency of DN lesions even among cases with normal albuminuria levels. The exact reason for the discrepancy in the findings between these autopsy studies is unclear. Nevertheless, it may be because of differences in the sample size, age at autopsy (i.e., older in the present study), ethnicity (i.e., Asians were more susceptible to DN in a study by Young et al.27), and the methods used to measure albuminuria (i.e., the quantitative measurement used in the present study is more sensitive and accurate, and thus less likely to lead to misclassification). It may be reasonable, however, to suppose that a relatively large number of subjects with class IIa or higher glomerular DN lesions, who are potential targets for DN therapy, exist among autopsied diabetic cases with normoalbuminuria, considering pathologic kidney findings are associated with kidney prognosis7,24 and can be ameliorated by therapeutic interventions.28,29 In particular, some renoprotective agents, such as sodium-glucose cotransporter 2 inhibitors, which have recently been suggested to ameliorate the progression of kidney dysfunction even in patients with normal albuminuria,30 may be available for patients with pathologic DN lesions with normoalbuminuria. Further longitudinal studies will be needed to elucidate the effectiveness of such agents in patients with pathologic DN lesions with normoalbuminuria.
The strengths of the present study include the use of autopsied cases from a community-based cohort study with a high autopsy rate. In addition, we evaluated 50 glomeruli per subject, which is greater than the number of glomeruli generally available in kidney biopsy specimens. Furthermore, we had accurate information on the quantified values of UACR (rather than urinary test strips), duration of diabetes, medications, and other covariates. Nevertheless, there were also several limitations to the present study. First, although approximately half of the diabetic cases with normoalbuminuria had class IIa or higher glomerular DN lesions in the present study, there was still a possibility of progressing UACR levels (e.g., normoalbuminuria to microalbuminuria) during the maximum 6-year intervals between the last health examination and the autopsy. Nevertheless, the sensitivity analyses restricting autopsied cases to within 2 or 3 years from the last health examinations also found that 45% to 50% of the cases had class IIa or higher glomerular DN lesions. This finding does not entirely rule out the possibility of progressing UACR levels as a limitation, but we believe that our conclusion is reasonable. Second, class I DN lesions could not be determined because we did not evaluate the kidney samples with electron microscopy. Moreover, no case with class IV DN lesions was observed in the present study because we excluded 2 cases in whom global glomerular sclerosis was almost entirely diffuse, such that glomerular DN lesions could not be evaluated. Finally, there is a limit to the generalization of the findings, especially to young or middle-aged people, because most autopsied cases were older people.
Conclusions
We revealed that the frequency of autopsied diabetic cases with class IIa or higher glomerular DN lesions increased significantly with increasing UACR levels, and even among those with normoalbuminuria, the frequency was nearly 50%. The clinical benefit of identifying the group of subjects with DN lesions with normoalbuminuria has not yet been determined. Nevertheless, given that pathologic severity is associated with kidney prognosis, the early diagnosis of DN among diabetic subjects with normoalbuminuria may help delay kidney impairment progression through early therapeutic intervention. Future studies will be needed to investigate biomarkers to identify cases with pathologic DN lesions among diabetic subjects with normoalbuminuria.
Disclosure
The authors declared no competing interests.
Acknowledgments
This study was supported in part by the Grants-in-Aid for Scientific Research B (JP21H03200), C (JP19K07890, JP20K10503, JP20K11020, JP21K07522, JP21K11725, and JP21K10448), and Early Career Scientists (JP18K17925) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by the Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare of Japan (20FA1002); by the Japan Agency for Medical Research and Development (JP21dk0207053); and by The Jikei University Research Fund for Graduate Students. The authors thank the staff of the Division of Health and Welfare of Hisayama for their cooperation in this study. The authors gratefully and sincerely thank Professor Toru Iwaki and colleagues from the Department of Anatomic Pathology and Department of Neuropathology, Graduate School of Medical Sciences, Kyushu University, who provided insight and expertise in analyzing the autopsy findings that greatly assisted the research. The statistical analyses were performed using the computers offered under the category of General Projects by the Research Institute for Information Technology, Kyushu University.
Author Contributions
Study concept and design: TS and TNi. Histopathologic assessment: TS and KN. Clinical data collection: TS, JH, MS, YH, and TNi. Data interpretation: TS, JH, and TNi. Statistical analysis: TS. Study coordination and performance: TNi. All authors contributed relevant intellectual content during manuscript drafting or revision and accepted accountability for the overall work by ensuring that questions on the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Figure S1. Flow diagram of the present study population.
Table S1. Odds ratio for increasing class of glomerular diabetic nephropathy lesions per every 1 increment in the natural log-transformed urinary albumin-creatinine ratio in overall autopsied diabetic cases (n = 106).
STROBE Statement (PDF).
Supplementary Material
Figure S1. Flow diagram of the present study population.
Table S1. Odds ratio for increasing class of glomerular diabetic nephropathy lesions per every 1 increment in the natural log-transformed urinary albumin-creatinine ratio in overall autopsied diabetic cases (n = 106).
STROBE Statement (PDF)
References
- 1.Alicic R.Z., Rooney M.T., Tuttle K.R. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jha V., Garcia-Garcia G., Iseki K., et al. Chronic kidney disease: global dimension and perspectives [published correction appears in Lancet. 2013;382:208] Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 3.Masakane I., Nakai S., Ogata S., et al. Annual dialysis data report 2014, JSDT Renal Data Registry (JRDR) Ren Replace Ther. 2017;3:18. doi: 10.1186/s41100-017-0097-8. [DOI] [Google Scholar]
- 4.Mogensen C.E., Christensen C.K., Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(suppl 2):64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- 5.Krolewski A.S., Gohda T., Niewczas M.A. Progressive renal decline as the major feature of diabetic nephropathy in type 1 diabetes. Clin Exp Nephrol. 2014;18:571–583. doi: 10.1007/s10157-013-0900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu M., Furuichi K., Yokoyama H., et al. Kidney lesions in diabetic patients with normoalbuminuric renal insufficiency. Clin Exp Nephrol. 2014;18:305–312. doi: 10.1007/s10157-013-0870-0. [DOI] [PubMed] [Google Scholar]
- 7.Furuichi K., Yuzawa Y., Shimizu M., et al. Nationwide multicentre kidney biopsy study of Japanese patients with type 2 diabetes. Nephrol Dial Transplant. 2018;33:138–148. doi: 10.1093/ndt/gfw417. [DOI] [PubMed] [Google Scholar]
- 8.Klessens C.Q., Woutman T.D., Veraar K.A., et al. An autopsy study suggests that diabetic nephropathy is underdiagnosed. Kidney Int. 2016;90:149–156. doi: 10.1016/j.kint.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez Suarez M.L., Thomas D.B., Barisoni L., Fornoni A. Diabetic nephropathy: is it time yet for routine kidney biopsy? World J Diabetes. 2013;4:245–255. doi: 10.4239/wjd.v4.i6.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hata J., Ninomiya T., Hirakawa Y., et al. Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961-2009) Circulation. 2013;128:1198–1205. doi: 10.1161/CIRCULATIONAHA.113.002424. [DOI] [PubMed] [Google Scholar]
- 11.Ninomiya T. Japanese legacy cohort studies: the Hisayama Study. J Epidemiol. 2018;28:444–451. doi: 10.2188/jea.JE20180150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohmura T., Ueda K., Kiyohara Y., et al. Prevalence of type 2 (non-insulin-dependent) diabetes mellitus and impaired glucose tolerance in the Japanese general population: the Hisayama Study. Diabetologia. 1993;36:1198–1203. doi: 10.1007/BF00401066. [DOI] [PubMed] [Google Scholar]
- 13.Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Levey A.S., de Jong P.E., Coresh J., et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report [published correction appears in Kidney Int. 2011;80:1000] [published correction appears in Kidney Int. 2011;80:1000] Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 15.Tervaert T.W., Mooyaart A.L., Amann K., et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki T., Tsuboi N., Okabayashi Y., et al. Synergistic impact of diabetes and hypertension on the progression and distribution of glomerular histopathological lesions. Am J Hypertens. 2019;32:900–908. doi: 10.1093/ajh/hpz059. [DOI] [PubMed] [Google Scholar]
- 17.Bader H., Meyer D.S. The size of the juxtaglomerular apparatus in diabetic glomerulosclerosis and its correlation with arteriolosclerosis and arterial hypertension: a morphometric light microscopic study on human renal biopsies. Clin Nephrol. 1977;8:308–311. [PubMed] [Google Scholar]
- 18.Kubo M., Kiyohara Y., Kato I., et al. Risk factors for renal glomerular and vascular changes in an autopsy-based population survey: the Hisayama Study. Kidney Int. 2003;63:1508–1515. doi: 10.1046/j.1523-1755.2003.00886.x. [DOI] [PubMed] [Google Scholar]
- 19.Kashiwagi A., Kasuga M., Araki E., et al. International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horio M., Imai E., Yasuda Y., Watanabe T., Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32–38. doi: 10.1053/j.ajkd.2010.02.344. [DOI] [PubMed] [Google Scholar]
- 21.SAS/STAT 15.2® user’s guide. The FREQ procedure. SAS documentation. https://documentation.sas.com/api/docsets/statug/15.2/content/freq.pdf?locale=en#nameddest=statug_freq_details77 Published November 6, 2020. Accessed August 10. 2021.
- 22.Chavers B.M., Bilous R.W., Ellis E.N., Steffes M.W., Mauer S.M. Glomerular lesions and urinary albumin excretion in type I diabetes without overt proteinuria. N Engl J Med. 1989;320:966–970. doi: 10.1056/NEJM198904133201503. [DOI] [PubMed] [Google Scholar]
- 23.Fioretto P., Steffes M.W., Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes. 1994;43:1358–1364. doi: 10.2337/diab.43.11.1358. [DOI] [PubMed] [Google Scholar]
- 24.Fufaa G.D., Weil E.J., Lemley K.V., et al. Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clin J Am Soc Nephrol. 2016;11:254–261. doi: 10.2215/CJN.05760515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriya T., Moriya R., Yajima Y., Steffes M.W., Mauer M. Urinary albumin as an indicator of diabetic nephropathy lesions in Japanese type 2 diabetic patients. Nephron. 2002;91:292–299. doi: 10.1159/000058407. [DOI] [PubMed] [Google Scholar]
- 26.Jefferson J.A., Shankland S.J., Pichler R.H. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74:22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- 27.Young B.A., Maynard C., Boyko E.J. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26:2392–2399. doi: 10.2337/diacare.26.8.2392. [DOI] [PubMed] [Google Scholar]
- 28.Fioretto P., Steffes M.W., Sutherland D.E., Goetz F.C., Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 29.Pichaiwong W., Hudkins K.L., Wietecha T., et al. Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J Am Soc Nephrol. 2013;24:1088–1102. doi: 10.1681/ASN.2012050445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wanner C., Inzucchi S.E., Lachin J.M., et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.