Abstract
Copy number variants (CNVs) were the subject of extensive research in the past years. They are common features of the human genome that play an important role in evolution, contribute to population diversity, development of certain diseases, and influence host–microbiome interactions. CNVs have found application in the molecular diagnosis of many diseases and in non-invasive prenatal care, but their full potential is only emerging. CNVs are expected to have a tremendous impact on screening, diagnosis, prognosis, and monitoring of several disorders, including cancer and cardiovascular disease. Here, we comprehensively review basic definitions of the term CNV, outline mechanisms and factors involved in CNV formation, and discuss their evolutionary and pathological aspects. We suggest a need for better defined distinguishing criteria and boundaries between known types of CNVs.
Keywords: Copy number variants, Structural variation, Human genome, CNV formation, Evolution, Genetic diseases
Copy number variation (CNV) is a general term used to describe a molecular phenomenon in which sequences of the genome are repeated, and the number of repeats varies between individuals of the same species. Biological roles of resulting copy number variants (CNVs) range from seemingly no effect on common variability of physiological traits [1], through morphological variation [2,3], altered metabolic states [4], susceptibility to infectious diseases [5,6], and host–microbiome interactions [[7], [8], [9]], to a substantial contribution to common and rare genetic disorders/syndromes [10]. As such, they have a high potential to contribute to human population diversity [11] and also to micro- and macro-evolutionary processes [12]. In addition to their biological roles, their presence in our genomes may have several technical implications in biomedicine, either as biomarkers for certain pathological processes such as cancer, as biomarkers of environmental exposures such as radiation [13], or even as potential confounding factors when evaluating results of certain genetic diagnostic tests [14]. While it is not yet well described how many genes are absent from human reference genomes, approximately 100 genes were found to be homozygously deleted from the genomes of human individuals without causing apparent phenotypic consequences, likely due to the presence of redundant paralogs, the genes being limited to causing age-related phenotypes or being relevant only under certain environmental or physiological conditions [15]. These findings suggest that the field of pangenomics may open its doors to human pan-genomes [16] in addition to more commonly mentioned bacterial, archaeal, and plant pan-genomes [17]. Much effort has also been made to study the genomes of livestock and domestic animals in the context of CNV-associated, economically important traits [18,19]. These variants overlap genomic regions associated with traits such as feed conversion ratio [20], meat quality [21], milk production [22,23], and animal health [24], thus, may result in significant economic losses due to reduced production and quality of commodities or decreased commercial value of affected animals.
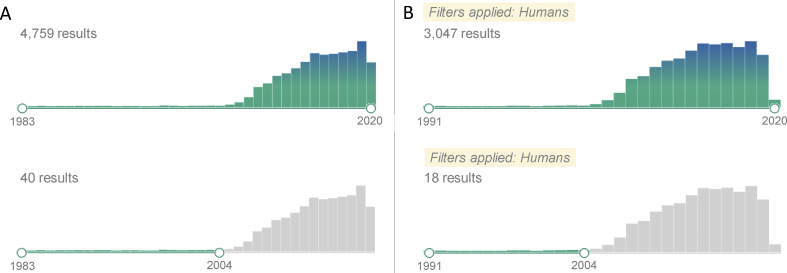
CNVs in the form of large insertions and deletions were reported among the first genetic “mutations” ever [25], well before the description of DNA structure and the birth of molecular biology. When searching PubMed for the term “copy number variation”, the returned list contains 4759 results in a date-range from 1983 to 2020, while limiting the search to “humans” produces 3047 results dated between 1991 and 2020. As visible in publication timelines [Fig. 1], a sharp increase in publication numbers began around the year 2005, partly due to the term “copy number variation” getting widely adopted, but also to the research interest that CNVs started to gain at the time, resulting in rapidly accumulating knowledge in the field. Whether plateauing publication numbers following the year 2014 anticipate a permanent trend is not predictable at the moment, however, this does seem to suggest that CNVs are being readily prepared for routine biomedical applications. One may argue that clinical applications of CNV detection, particularly in the field of oncogenetics and severe congenital anomalies, were among the first in the history of genetic testing and are still in general use. However, considering the technical innovations now allowing population-scale genome-wide CNV screening, together with the still widening spectrum of known biological roles of CNVs, we have yet to take full advantage of CNV detection in routine clinical care [26]. In favor of this point-of-view, official systematic guidelines on the interpretation of CNVs and their classification by clinical impact were issued by the American College of Medical Genetics and Genomics for the first time only in 2019 [27]. Because of their important biological roles, relevant technical impact, and several associated uncertainties, we believe that the field of CNV research and application urgently calls not only for further investigation, but also for regular review of available knowledge and for constant revision of relevant definitions and classifications.
Fig. 1.
Publication records. PubMed search for the term “copy number variation” as of July 24, 2020. Limiting results to the year 2004 reduced the number of entries considerably. Excluded entries are highlighted in grey; (A) no filter applied; (B) applying a built-in species-specific filter for “humans”.
In this review, we outline basic definitions of the term CNV and discuss general perception of copy number variants, along with mechanisms and factors involved in their formation, their evolutionary aspects, and their influence on phenotype and disease.
Definitions, perception, and classification of CNVs
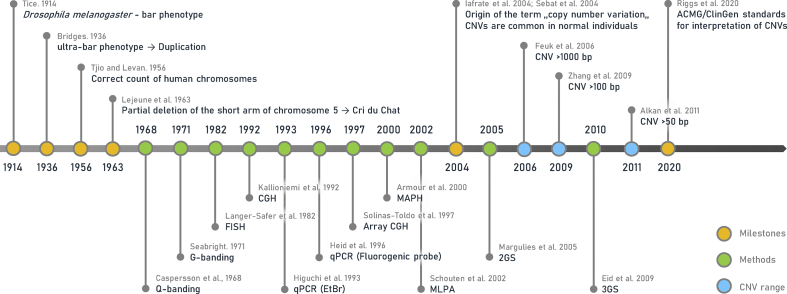
The first association of a CNV with a phenotype reported in a non-human species was the case of a reduced-eye mutant Drosophila melanogaster having the bar eyes phenotype [25] due to a single duplication of the Bar gene [28]. After the “general” human karyotype was established [29], reports of microscopically visible chromosomal aberrations in the human genome emerged as early as the 1960s, when cytogeneticists recognized the genetic background for many disorders, including Cri du chat syndrome associated with a partial deletion of the short arm of chromosome 5 [30]. For decades, due to a limited resolution of microscopy-based cytogenetic methods, reports were mostly limited to large pathogenic abnormalities involving several genes and having prominent adverse effects on physiological processes. Although large structural changes including insertions and deletions were among the first genetic anomalies recognized, the concept of CNV as we perceive it today originates from less than two decades ago [Fig. 2], when large-scale differences between human genomes, previously considered rare mutational events, were shown to be common among normal human individuals, forming a considerable part of our intraspecies physiological variability [31,32]. It is estimated that around 4.8–9.5% of the genome is affected by CNVs [15], a larger fraction than by single nucleotide variants [11].
Fig. 2.
Timeline of CNV research. It includes research milestones (orange) in the context of evolving methods for the assessment of CNVs (green) and the minimal length of variants to be considered as CNVs at a given time (blue). Main trends of CNV research are visible, e.g., the relatively wide time-frame following the first descriptions of large CNVs around the beginning of the 20th century. This nearly century-long phase was mainly about the development of methods, which finally allowed genome-wide, high-resolution CNV detection around the beginning of the 21st century, leading to the recognition of common features of CNVs and a subsequent shift in their definition, allowing shorter and shorter variants to be considered CNVs.
Although it is generally accepted that CNVs are a subtype of structural genome variants, their definition is still somewhat vague. In addition to basic criteria about the repeating nature and numerical variability among individuals, a “considerable” length is usually also required. Earlier studies defined a CNV as a DNA segment larger than 1000 bp [33], followed by ∼100 bp [1], but currently, the size of CNVs is defined from 50 bp [34] to several Mb [35]. Despite CNVs being insertions or deletions, it is also debated in the literature whether a distinction should be made between indels and CNVs based on their size [36]. We did not find suggested criteria for the extent of homology between sequences to be considered CNVs, most likely because commonly used methods other than sequencing are not suitable to determine such a sequence homology. Mechanisms of formation are sometimes considered for distinction criteria, but there is no unambiguous relationship between the length of an insertion or deletion and its mechanism of formation, as models may be shared across different size-ranges [37]. Classification of identified variants is generally a routine procedure with no time or possibility to determine the exact mechanisms behind their formation. A discussion seems justified about whether there is any real reason to restrict the term CNV to variants having a “considerable length” instead of applying it to all variants meeting core criteria with no regard to molecular mechanisms or to the number and length of repeated elements.
For the reasons above, we favor the definition of “copy number variants” used for unbalanced structural rearrangements of the genome, which lead to variability, i.e., relative difference in copy numbers of particular DNA sequences among individuals of the same or distinct populations of a species. In line with this, when considering the definition in the light of the fact that there is no such thing as a “standard genome” [38], the term “copy number variant” is best applied in a general sense to describe variants contributing to “copy number variation” or “copy number variability”, while duplications, insertions, and deletions may be considered as their particular molecular phenotypes. Terms such as “insertion”, “deletion”, “gain” or “loss” are most suitable to describe relative differences: i) against an artificial reference genome when the variant is de novo, or even if it is present in the population and was inherited from an ancestor with no way to determine the original copy number; ii) against a parental genome, when a de novo variant occurs in an offspring; or iii) against a germline genome of the individual, when a de novo somatic variant occurs; in each case with an aim to characterize the type of CNV more precisely.
Complying with these arguments, the list of variants belonging to CNVs can be extended (and is already extended by some authors) with several other variant types. On one end of the spectrum, we find changes involving entire sets of chromosomes and aneuploidies (i.e., numerical abnormalities defined as a loss or gain of an entire chromosome), as well as structural abnormalities represented as non-balanced chromosome rearrangements [39]. The opposite end of the spectrum may range from interspersed and tandem repeats, including microsatellites, minisatellites, and macrosatellites, to few nucleotide insertions and deletions. Perhaps the most common and ancient benign CNV is one that is usually not listed as a CNV at all: the XY sex-determination system in humans and many other species, maintained through a strong balancing selection and determining extensive phenotypic differences between individuals of the same population.
Interspersed repeated sequences are generally composed of low-copy repeats, also called segmental duplications, which have greater than 95% sequence identity, and high-copy repeats, which include endogenous retroviruses, retrotransposons, and other transposable or mobile elements. Retrotransposons encompass long terminal repeat (LTR) and non-LTR retrotransposons, comprising of the well-known short interspersed nuclear elements (SINEs, such as the Alu element) and long interspersed nuclear elements (LINEs, such as L1). Low and high copy number repeats may act as mediators of the formation of other, larger CNVs, apart from being CNVs themselves.
Although the current classification of CNVs is satisfactory, distinguishing criteria and boundaries between different types of variants are not clearly defined, leading to some variants meeting criteria of more than one category at the same time. For core parts of this review, we focus on the conventional concept of CNVs, but where relevant, we discuss variants more difficult to classify.
Mechanisms of CNV formation
One of the main questions in understanding the biology of CNVs is connected to their origin and mechanisms of formation. To date, several different replication and repair mechanisms have been shown to be involved in the development of CNV. Apart from genomic factors, chemical and physical mutagens also drive their formation.
Genomic factors and mechanisms of CNV formation
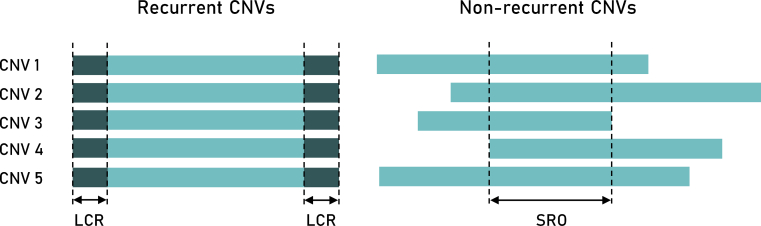
CNVs emerge from different mutational mechanisms, including DNA recombination-, replication- and repair-associated processes. Mechanisms of change in gene copy number have been extensively studied through the analysis of CNV breakpoint junction sequences. Repeated sequences, including low-copy repeats (e.g., segmental duplications) and high-copy repeats (e.g., SINEs, LINEs, and endogenous retroviruses), are enriched in the vicinity of breakpoints, thus represent an important factor for CNV instability [40]. Such sequence motifs play a key role in triggering non-allelic homologous recombination (NAHR), one of the general mechanisms involved in the formation of recurrent CNVs [41]. Recurrent rearrangements share a common size, show clustering of breakpoints, and recur in multiple individuals. On the other hand, non-recurrent rearrangements with scattered breakpoints differ in size, but may share the smallest region of overlap among different patients [Fig. 3] [42] and might be formed by several different mechanisms: i) non-replicative mechanisms, including non-homologous end joining (NHEJ) and microhomology-mediated end joining (MMEJ); or ii) replicative mechanisms such as replication slippage, fork stalling and template switching or microhomology-mediated break-induced replication [41].
Fig. 3.
Recurrent vs. non-recurrent rearrangements. Recurrent CNVs have the same size and common breakpoints enriched in low-copy repeats (LCRs). Non-recurrent CNVs with different sizes may share the smallest region of overlap (SRO). CNVs may occur as inherited or de novo events. Inherited CNVs are not recurrent events but always share the same breakpoints, resulting in a similar phenotypic effect. De novo CNVs are independently arising events that may have recurrent or distinct (non-recurrent) breakpoints. Even CNVs with non-recurrent breakpoints may show overlapping effects, as they disrupt the same SRO.
Short repetitive sequence motifs (e.g., inverted repeats) may adopt non-B DNA structures (e.g., cruciforms) [43] that may result in replication errors inducing CNV formation [40]. Such non-B-DNA forming sequences are also enriched in promoter regions, thus Conrad et al. suggested that the same properties that enable regulation of transcription may also be mutagenic for the formation of CNVs [44]. Consequently, CNVs may influence the evolution of gene regulation.
In addition to accurate replication of DNA during cell division, its equal distribution into daughter cells is essential in genome maintenance, specifically in ensuring the balance of genetic content in eukaryotic cells [45]. However, errors such as nondisjunction, merotelic kinetochore-microtubule attachment, monopolar and multipolar spindle, and telomerase dysfunction may occur during this high-precision process. Nondisjunction is caused by weakened or completely inactive mitotic checkpoints. If a chromosome fails to separate correctly, it results in one daughter cell with a missing copy of the chromosome and the other one having an extra copy. If mitotic checkpoints are completely inactive, it causes the two daughter cells to have a disjoint set of chromosomes. Unbalanced chromosome segregation occurs when one kinetochore is attached to the microtubules emanating from both poles of the spindle [46]. In the case of dysfunction or absence of telomerase, the chromosome ends become uncapped. This condition triggers activation of the NHEJ DNA repair pathway in the cell, leading to a telomere fusion between the two chromosomes and the formation of a dicentric chromosome. Structural aneuploidy may occur by several mechanisms, including incorrect NAHR (the same mechanism that causes CNVs), misalignment of homologous chromosomes and/or unequal crossing over between non-sister chromatids during meiosis [39]. Such structural abnormalities may subsequently affect the development of chromosomal aneuploidies, suggesting a link between them.
When considering tandem repeats, both micro-, mini-, and macrosatellites display notable somatic and intergenerational dynamics leading to copy number changes. Microsatellites are typically altered during DNA repair and replication in mitosis mainly due to slippage by strand misalignment, primarily accounting for small-scale instability [47], however, unequal crossing over was also suggested to cause variable copy numbers of microsatellite motifs [48]. In the case of disease-associated repeats, which may gain up to a few thousands of repeat units in affected individuals, the mechanisms of expansion are different and may depend on the repeat sequence, length and location within a genome as well as the organism or cell type. They likely involve transient DNA secondary structures formed by the repetitive tracts as DNA unwinds for replication, transcription, or repair, during which these secondary structures may get excised or incorporated into the DNA, resulting in a reduction or expansion of the repetitive region [47]. These mechanisms are generally involved in expanding copy numbers over certain thresholds at single loci and do not lead to general microsatellite instability. Mechanisms may further be complemented by an impairment of the mismatch repair system leading to general hypermutability of microsatellite loci throughout the genome, called microsatellite instability, most commonly due to a failure to correct common errors during replication of repetitive DNA sequences [49]. While a link was reported between microsatellite instability and telomere shortening suggesting some association between the DNA mismatch repair system and telomere maintenance mechanisms, at least in colorectal cancer [50], telomeres per se may represent atypical mechanisms of CNV generation. Telomeres contain TTAGGG hexanucleotide repeats protecting the genetic content of chromosomes against chromosome shortening during cell division. In an active compensatory mechanism known as telomere length maintenance, telomerase increases copy numbers by adding TTAGGG repeat units to the ends of telomeres [51]. Minisatellites, on the other hand, generally mutate in the germline by complex conversion-like transfer of repeats between alleles. Inter-allelic unequal crossovers also occur between loci, although at low frequency [52].
Environmental factors contributing to the formation of CNVs
The contribution of environmental factors to the origin of CNVs is poorly understood. It was shown that perturbing DNA replication by chemical mutagens (e.g., aphidicolin or hydroxyurea) results in replication stress that induces the formation of CNVs across the human genome. Replication fork delay and subsequent error-prone repair seem to be important mechanisms in the process [53]. Hastings et al. proposed that under stress conditions, the repair of broken replication forks switches from high-fidelity homologous recombinational repair to non-homologous repair promoting CNVs [41]. Physical mutagens such as low-dose ionizing radiation also effectively induce de novo CNV mutations via a replication-dependent mechanism, since radiation-induced DNA strand breaks may collapse the replication fork [13]. It was shown that radiation induces duplications and deletions equally, while chemical mutagens induce copy number losses at a higher frequency compared to gains. On another note, radiation impacts random loci of the genome, while chemical mutagenesis targets specific genomic regions [54].
Costa et al. showed a 1.5-fold increase in the germline CNV mutation burden in offspring of parents accidently exposed to a low dose of ionizing radiation. The mutation rate of de novo CNVs was proven to be a biomarker of parental exposure useful in retrospective studies of human populations exposed to low doses of ionizing radiation [55]. It was also demonstrated that irradiation with laser-driven electrons may induce CNVs in human leukocytes in vitro. These CNVs are usually duplications or amplifications and tend to inversely correlate with chromosome size and gene density. CNVs may persist in the cell population as stable chromosomal changes for several days after exposure [56].
Evolutionary aspects of CNVs
As excessively static genomes are not able to conform to an ever-changing environment, genome plasticity driven by insertion sequence elements, transposons and integrons, together with DNA rearrangements, may determine whether an organism can survive changing conditions and compete for the resources it needs to reproduce, at least in prokaryotes. However, the main players of prokaryote genome plasticity, including CNV-associated mechanisms mentioned in the previous section, are also active in humans. Although changes to the genome, and thus to the phenotype, may threaten the ability to survive, gaining new phenotypes may also enhance chances of surviving even in previously disadvantageous environments [57]. Both are possible scenarios in the context of the extent of change and environmental pressure, which define whether individuals fit the actual environment.
CNVs, like other changes in DNA, create phenotypes in which descendants slightly or largely differ from their predecessors. Small differences in phenotypes are generally considered normal variation, especially when relatively common in the population with small changes in physiology, i.e., benign traits or even common diseases and intolerances. Larger deviations, on the other hand, may be perceived as reproductional losses, malformations, or genetic/genomic disorders, which may result from large CNVs that are both rare and deleterious, as they tend to contain dosage sensitive genes [41]. It was shown that the number of events is steadily decreasing with increased CNV length, so there are many more small (common) CNVs than large (deleterious) ones [58].
CNVs in the light of evolutionary mechanisms
It was reported in several organisms that genes having tissue-specific expression tend to be more variable in copy numbers than widely expressed genes, which might have housekeeping functions [[59], [60], [61]]. The most commonly cited examples of evolutionarily important CNVs in humans fit well into this concept, including the CNV-related evolution of trichromatic vision [62], hemoglobins and myoglobins [63], and olfactory genes [64]. The gene family of amylase, a starch-digesting enzyme, may serve as a multipurpose example in understanding CNV-mediated evolutionary processes, from a mechanistic view of the generation of complex genomic features and novel functions to the explanation of certain adaptation models. The study of Axelsson et al. showed a 7.4-fold average increase of AMY2B copy numbers in dogs relative to wolves. The change correlates to an increase in both expression level and enzyme activity, indicating that duplications of the alpha-amylase locus conferred a selective advantage to early ancestors of modern dogs. An increase in amylase activity allowed them to thrive on a diet rich in starch compared to the carnivorous diet of wolves and constituted a crucial step in early dog domestication [65]. Mammals, in general, produce amylase in their pancreas, while primates, rodents, and lagomorphs also show salivary expression of the enzyme. The GRCh38/hg38 assembly of the human reference genome contains two pancreatic (AMY2A, AMY2B) and three salivary amylase genes (AMY1A, AMY1B, AMY1C), arrayed close to each other on chromosome 1, but AMY1B having an opposite orientation [66]. These genes were reported to be present in highly variable copy numbers in humans, ranging from 2 to 18 copies with an average of 6 copies per person [67]. Average copy numbers of AMY1 were shown to be higher in populations with high-starch diets compared to those with traditionally low-starch diets, suggesting that when starch became a prominent component of the human diet, it led to the positive selection of the AMY1 gene [66]. Evolution of this human multigene family likely involved several steps in primate, and later in human evolutionary lineages, with mechanisms involving unequal, homologous, and inter- and intrachromosomal crossovers [68].
Among typical examples of balancing selection maintaining CNVs in the population, the hemoglobin genes and their association to two severe human diseases, thalassemia and malaria are usually mentioned [69]. While homozygous deletions of one of the α-globin genes cause α-thalassemia on one arm of the balance, heterozygous deletions protect their carriers against malaria on the opposite arm, leading to a correlating frequency of α-thalassemia and malaria prevalence in human populations of malaria-endemic countries [70]. Such balancing selection may shape the distribution of certain CNVs for generations, and they may be further maintained by other selective agents in later generations. For such cases, CNVs involving the immunoregulatory and inflammatory cytokine CCL3L1 may be used as models in which higher copy numbers were found to correlate with a lower risk of HIV infection and AIDS progression [71], but CCL3L1 copy numbers may also be reduced due to their association with other severe diseases such as systemic lupus erythematosus [72], strongly suggesting that certain CNVs may have been subject to highly dynamic and heterogeneous forms of evolutionary pressure [69].
Duality of evolution and disease
The mechanisms by which CNVs contribute to evolution but also cause disease are highly similar and share a common basis. The most commonly mentioned mechanisms are gene duplications or multiplications, which are considered to be essential sources of evolutionary innovation, as redundant gene copies may acquire new functions. If the multiplicated gene is not dosage sensitive, so fitness is not reduced, one of the copies may keep its original function while the other one may escape selective pressure and silently undergo continuous changes by mutation. And although the original copy (or even a functional single-copy gene) may also undergo slight changes over generations, duplicated genes have a tendency to accumulate mutations faster. Some other CNV-related processes acting on disease and evolution [41] include: i) direct influence on the expression of gene products leading to changing levels of a protein, e.g., by changing the copy number of the coding sequence itself, as the gene and its regulatory regions may both be encompassed by the CNV; ii) direct influence on expression levels, or on localization or timing of expression through alteration – including creation or disruption – of regulatory regions when the coding sequence itself is not encompassed by the CNV; or iii) acquisition of new functions by forming new or modified products – or altered localization or timing of expression of pre-existing products – through recombination of functional domains of different genes.
Comparative analyses of human and chimpanzee genomes helped us understand the evolutionary significance of CNVs. Approximately one-third of the CNVs observed in the human genome, including some human disease-causing duplications, are not duplicated in chimpanzees [12]. It seems that the evolution of the mammalian genome during primate speciation has led to a genome architecture predisposing some regions to rearrangements, which also resulted in genomic disorders [73]. Genes that have likely evolved under purifying or positive selection for copy number changes have been identified, particularly those with inflammatory response functions such as APOL1, APOL4, CARD18, IL1F7, and IL1F8, which are deleted in chimpanzees. Moreover, a copy number loss of the oncogene TBC1D3 involved in cell proliferation was observed in the chimpanzee compared with the human reference genome [74]. In conclusion, although there is evidence that altered copies of specific genes offer a selective advantage, many variations in copy numbers are disadvantageous as they are involved in the formation and progression of several pathological conditions [41].
Pathological aspects of CNVs
As we mentioned above, many variations of the human genome, including variations in gene copy number, play important roles in human health and disease. Since CNVs often span across a large number of genes and regulatory regions, many of which are biomedically actionable and/or included in the OMIM database, pathogenic CNVs have been found to cause genomic disorders with Mendelian inheritance [75] or to be associated with complex, multifactorial diseases [76] including cancer [77], cardiovascular [78,79], autoimmune [80], neurodevelopmental and neurodegenerative disorders [81,82]. When considering aneuploidies and short tandem repeats (STRs) as CNVs, this list may be extended by aneuploidy-associated syndromes such as Down, Edwards, Patau, Klinefelter, or Turner [83], by repeat expansion disorders, which are typically severe neurodegenerative or neuromuscular conditions [84], and by many types of cancer that involve microsatellite instability [85] or a change in telomere length [86].
Pathomechanisms
CNVs are prevalent in both coding and non-coding regions of the genome. They can directly affect a coding sequence and cause disruption of gene function or alter gene dosage leading to the development of a disorder [87]. However, CNVs that encompass only non-coding regions may also have a functional impact on the human genome by a number of possible mechanisms. It was shown that CNVs may influence tissue-level gene expression through harboring small non-coding RNAs (sncRNAs) [88]. Studies have found that CNV-sncRNAs include miRNA [89], snoRNA [90], piRNA [91], and tRNA [92], and CNVs harboring long non-coding RNAs have also been reported [93]. CNVs may alter chromatin interaction domains, also known as topologically associated domains, which may disrupt spatial organization of the genome and result in pathogenic phenotypes [94].
Expansions of microsatellites – sometimes considered as CNVs – represent additional pathogenic mechanisms such as the formation of extremely stable aggregates of mRNA or protein [95]. Proteins may exert a cumulative direct toxic effect both after canonical translation and non-canonical non-ATG initiated translation [96]. At the RNA level, well-known indirect pathogenic effects act through an expanded RNA-repeat-mediated depletion or activation of regulators of various cellular functions, such as alternative splicing [97]. Telomeres and the highly dynamic nature of their repeat numbers also represent important problems, including their continuous shortening over a lifetime contributing to aging and their escape from shortening in cancerous cell lines [86].
Another conventional, although indirect cause of genomic rearrangement is the unmasking of recessive mutations or functional polymorphisms when a deletion occurs [98]. In contrast, duplication of a normal gene copy on one chromosome may mask a disease-causing mutation on the other chromosome, resulting in a healthy phenotype [99]. Copy number of the SMN2 gene may indirectly modify severity in spinal muscular atrophy (SMA), a disease caused by the homozygous deletion of the SMN1 gene. The gene SMN2 differs from SMN1 in a substitution causing exclusion of exon 7 during splicing, resulting in a truncated and unstable protein for most (∼85%) SMN2 transcripts. Although the absence of SMN2 alone does not cause SMA, its copy number may alter the SMA phenotype: increased SMN2 copy numbers were present in patients with milder (SMA type III) phenotype compared to patients with more severe SMA type I [100]. However, SMN2 copy number is not a definitive modifier of SMA severity, and other modifying factors need to be taken into account for disease characterization [101].
Genomic disorders with Mendelian inheritance
In 1998, the term “genomic disorder” was defined: it refers to disorders caused by structural changes of the human genome. Such DNA rearrangements may lead to the loss or gain of dosage-sensitive gene(s) or disrupt a gene [10]. While in many monogenic diseases, an abnormal phenotype is caused by a point mutation, many of these genomic disorders are known as recombination-based conditions [102]. In addition, similarly to large chromosomal aberrations, smaller CNVs affecting only one exon or even smaller regions are also associated with human diseases. Therefore, depending on the size of the genomic segment involved, its position and genomic context, as well as the number of genes within the rearranged segment together with other risk factors, CNV-associated disorders may be classified as Mendelian diseases, contiguous gene syndromes, chromosomal disorders, or other sporadic or complex traits [73]. Among these, Mendelian genomic disorders may segregate as autosomal recessive, such as nephronophthisis 1, juvenile [103], autosomal dominant, such as Charcot–Marie–Tooth disease type 1A or hereditary neuropathy with liability to pressure palsies (both caused by one of the first identified disease-associated submicroscopic CNVs, i.e., a duplication or a deletion, respectively, which are the reciprocal products of the same non-homologous crossover event) [75], X-linked, such as hemophilia A [104], or even as Y-linked traits, such as azoospermia [105]. Repeat expansion disorders show a mode of inheritance depending on the chromosomal localization of the involved gene. Certain non-Mendelian manifestations are also typical for many of these disorders. These may include anticipation in myotonic dystrophy type 1 [106] or phenotype-modifying potential of certain repeat alleles when in combination with other pathogenic variants, like in the case of the suggested reciprocal interaction between myotonic dystrophy type 2 premutations and congenital myotonia caused by mutations of the CLCN1 gene [107].
Multifactorial diseases
Another example where CNVs may play an important role are complex diseases with multifactorial etiology, caused by the combination of several genetic factors (each one having a low impact on the phenotype alone) and environmental factors. Continuous progress in recent decades has increased our understanding of the pathophysiology of many complex diseases. However, there are still unanswered questions of risk factors or largely unknown genetic background, which prevent us from clearly uncovering and understanding the pathomechanism of such diseases. Therefore, it is assumed that the implication of CNVs in the pathogenesis of complex diseases could explain at least a fraction of the well-known “missing heritability” problem of these complex disorders [108]. Inflammatory bowel disease (IBD) represents a typical example of multifactorial disease. More than 200 IBD associated loci are known, yet the pathogenesis is still unclear [109]. Some authors assume that studying CNVs may shed more light on the mystery of IBD [76,110,111]. Recently, results of Frenkel et al. pointed not only to the important role of CNVs, but also to significant pathways in the pathogenesis of IBD [112]. Even though CNVs are heavily implicated, such large genetic variants are still understudied in IBD and other complex diseases [113]. This is probably due to the limitations of methods suitable for CNV detection at the time when major genome-wide association studies were carried out.
Infectious diseases may also be considered as complex multifactorial diseases as both genetic and environmental variability affect the susceptibility of individuals to infections. It was shown that host CNVs play an important role in clinical phenotypes related to infectious diseases. Examples include variants of α-globin [6] or the CCL3L1 gene [5] as explained in chapter 4 (Evolutionary aspects of CNVs), among others [114].
CNV detection may also find application in the evaluation of microbiome balance through the analysis of CNVs in metagenomes in different body parts. The human microbiome interacts with the host and plays an important role in many host biological processes [7]. Host genomic variations influence the composition of the microbiome, which in turn affects the health of the individual. While numerous studies have been focused on associations between the gut microbiome and specific alleles of the host genome, gene copy number also varies. It was shown, for instance, that duplication of the human AMY1 gene is associated with an increased number of oral Porphyromonas in saliva, which is linked to periodontitis. The gut microbiota of these individuals had an increased abundance of resistant starch-degrading microbes, produced higher levels of short-chain fatty acids, and drove increased adiposity when transferred to germ-free mice [8]. This case demonstrated that even seemingly harmless variants in the host genome may affect the health of individuals.
Current knowledge suggests the importance of analyzing CNVs not only in human cells but also in the microbiome. Taxonomic characterization of the human microbiota is often limited to the species level, but each microbial species represents a large collection of strains that may contain considerably different sets or copy numbers of genes resulting in potentially distinct functional features. This intra-species variation is caused by deletion and duplication events, which were shown to be prevalent in the human gut environment, with some species exhibiting CNVs in >20% of their genes. Variability is especially relevant in disease-associated genes involved in important functions such as transport and signaling. Greenblum et al. showed obesity to be associated with higher copy numbers of thioredoxin 1 in Clostridium sp., an increased copy number of an MFS transporter gene in the Roseburia inulinivorans genome cluster, and increased HlyD in Bacteroides uniformis associated with IBD-afflicted individuals. According to the authors, the analysis of species composition alone is not sufficient to capture the true functional potential of the microbiome, as it may fail to capture important functional differences [9]. Hence, the analysis of intra-species variation in microbial communities is crucial.
Cancer
Although cancer may be monogenic or, more typically, multifactorial, oncogenetics tends to be considered as a special field and discussed separately. In cancer genetics, CNVs are divided into two classes based on their size: i) large-scale, also known as chromosome-arm level variants encompassing >25% [115] or ⅓ of the chromosome arm [116]; and ii) focal variants defined as small regions of the genome, usually not more than 3 Mb in size, containing up to a few genes [[117], [118], [119]]. Since 25% of an average human chromosome arm contains more than 15 Mb of DNA, variants ranging from 3 to about 15 Mb do not meet the criteria of either one of the above categories. On the other hand, in the case of 21p or Yp, 25% of total length comprises less than 3 Mb, so a variant at such a location might fall into both classes at the same time. To our knowledge, there is no strict consensus in the literature, so we suggest to classify large-scale variants as >3 Mb, while focal variants should retain their current definition. Both types of CNVs are important in the context of disease, but the relatively small size and low gene content make focal CNVs more suitable for the identification of candidate driver genes. Analysis of CNVs is an important aspect of the molecular diagnosis of cancer. It was shown that recurring deletions are typically overrepresented in tumor suppressor genes and underrepresented in oncogenes [120]. Aberrations in gene copy numbers may reveal therapeutic targets or markers of drug resistance in several types of cancer [121]. High-resolution copy-number profiles of 3131 cancer specimens revealed the CNV landscape of the vast majority of cancer types. An average tumor sample consists of 17% genome amplification and 16% deletion, compared to averages of 0.35% and less than 0.1% in normal samples, respectively. Specific gene families and pathways have been shown to be overrepresented among focal somatic copy-number alterations. The most enriched ones are gene families important in cancer pathogenesis, such as kinases, cell cycle regulators and MYC family members [77], which may represent potential therapeutic targets.
Conclusions
The days when the Mendelian dogma of all our genes being present in two copies could be applied universally have long since passed. Normal and pathogenic CNVs have been described in eukaryotic cells as well as in the microbiome, and their phenotypic associations and causes involving replication, recombination and repair, along with environmental mutagens, are becoming more well-known as research methods evolve. And yet, general awareness of CNVs as common polymorphisms and studies aiming to shed light on their contribution to the missing heritability problem seen in genome-wide association studies are relatively scarce, with SNPs still stealing most of the spotlight [58,122]. If we want to be careful not to “miss the forest for the trees” [123], an integrated approach is advised, taking different forms of polymorphism and gene expression data into account, rather than maintaining a sole focus on SNP analysis [124]. Having assessed recent developments in the field, we are of the view that copy number research deserves more attention as a vital and very interesting aspect of the paradigm shift currently underway in molecular and clinical genetics.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the OP Integrated Infrastructure for the project [ITMS: 313011V578] co-financed by the European Regional Development Fund; the Slovak Research and Development Agency [Grant ID: APVV-18-0319]; and the PANGAIA project H2020-MSCA-RISE-2019 [Grant agreement ID: 872539] funded under H2020-EU.1.3.3. Programme.
Footnotes
Peer review under responsibility of Chang Gung University.
Contributor Information
Jan Radvanszky, Email: jradvanszky@gmail.com.
Bálint Nagy, Email: nagy.balint@med.unideb.hu.
References
- 1.Zhang F., Gu W., Hurles M.E., Lupski J.R. Copy number variation in human health, disease, and evolution. Annu Rev Genom Hum Genet. 2009;10:451–481. doi: 10.1146/annurev.genom.9.081307.164217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright D., Boije H., Meadows J.R., Bed’hom B., Gourichon D., Vieaud A., et al. Copy number variation in Intron 1 of SOX5 causes the Pea-comb phenotype in chickens. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henkel J., Saif R., Jagannathan V., Schmocker C., Zeindler F., Bangerter E., et al. Selection signatures in goats reveal copy number variants underlying breed-defining coat color phenotypes. PLoS Genet. 2019;15 doi: 10.1371/journal.pgen.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder P.J.D., Ramsden D.B., Burnett D., Weickert M.O., Barber T.M. Human amylase gene copy number variation as a determinant of metabolic state. Expet Rev Endocrinol Metabol. 2018;13:193–205. doi: 10.1080/17446651.2018.1499466. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez E., Kulkarni H., Bolivar H., Mangano A., Sanchez R., Catano G., et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–1440. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 6.Harteveld C.L., Higgs D.R. Alpha-thalassaemia. Orphanet J Rare Dis. 2010;5:13. doi: 10.1186/1750-1172-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohajeri M.H., Brummer R.J.M., Rastall R.A., Weersma R.K., Harmsen H.J.M., Faas M., et al. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. 2018;57:1–14. doi: 10.1007/s00394-018-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poole A.C., Goodrich J.K., Youngblut N.D., Luque G.G., Ruaud A., Sutter J.L., et al. Human salivary amylase gene copy number impacts oral and gut microbiomes. Cell Host Microbe. 2019;25:553–564. doi: 10.1016/j.chom.2019.03.001. e7. [DOI] [PubMed] [Google Scholar]
- 9.Greenblum S., Carr R., Borenstein E. Extensive strain-level copy-number variation across human gut microbiome species. Cell. 2015;160:583–594. doi: 10.1016/j.cell.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lupski J.R. Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet. 1998;14:417–422. doi: 10.1016/s0168-9525(98)01555-8. [DOI] [PubMed] [Google Scholar]
- 11.Redon R., Ishikawa S., Fitch K.R., Feuk L., Perry G.H., Andrews T.D., et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Z., Ventura M., She X., Khaitovich P., Graves T., Osoegawa K., et al. A genome-wide comparison of recent chimpanzee and human segmental duplications. Nature. 2005;437:88–93. doi: 10.1038/nature04000. [DOI] [PubMed] [Google Scholar]
- 13.Arlt M.F., Rajendran S., Birkeland S.R., Wilson T.E., Glover T.W. Copy number variants are produced in response to low-dose ionizing radiation in cultured cells. Environ Mol Mutagen. 2014;55:103–113. doi: 10.1002/em.21840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubiritova Z., Gyuraszova M., Nagyova E., Hyblova M., Harsanyova M., Budis J., et al. On the critical evaluation and confirmation of germline sequence variants identified using massively parallel sequencing. J Biotechnol. 2019;298:64–75. doi: 10.1016/j.jbiotec.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Zarrei M., MacDonald J.R., Merico D., Scherer S.W. A copy number variation map of the human genome. Nat Rev Genet. 2015;16:172–183. doi: 10.1038/nrg3871. [DOI] [PubMed] [Google Scholar]
- 16.Yang X., Lee W.P., Ye K., Lee C. One reference genome is not enough. Genome Biol. 2019;20:849. doi: 10.1186/s13059-019-1717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vernikos G., Medini D., Riley D.R., Tettelin H. Ten years of pan-genome analyses. Curr Opin Microbiol. 2015;23:148–154. doi: 10.1016/j.mib.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Strillacci M.G., Gorla E., Cozzi M.C., Vevey M., Genova F., Scienski K., et al. A copy number variant scan in the autochthonous Valdostana Red Pied cattle breed and comparison with specialized dairy populations. PloS One. 2018;13 doi: 10.1371/journal.pone.0204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y.L., Bosse M., Mullaart E., Groenen M.A.M., Veerkamp R.F., Bouwman A.C. Functional and population genetic features of copy number variations in two dairy cattle populations. BMC Genom. 2020;21:89. doi: 10.1186/s12864-020-6496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strillacci M.G., Gorla E., Ríos-Utrera A., Vega-Murillo V.E., Montaño-Bermudez M., Garcia-Ruiz A., et al. Copy number variation mapping and genomic variation of autochthonous and commercial Turkey populations. Front Genet. 2019;10:982. doi: 10.3389/fgene.2019.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bovo S., Ribani A., Muñoz M., Alves E., Araujo J.P., Bozzi R., et al. Genome-wide detection of copy number variants in European autochthonous and commercial pig breeds by whole-genome sequencing of DNA pools identified breed-characterising copy number states. Anim Genet. 2020;51:541–556. doi: 10.1111/age.12954. [DOI] [PubMed] [Google Scholar]
- 22.Durán Aguilar M., Román Ponce S.I., Ruiz López F.J., González Padilla E., Vásquez Peláez C.G., Bagnato A., et al. Genome-wide association study for milk somatic cell score in holstein cattle using copy number variation as markers. J Anim Breed Genet. 2017;134:49–59. doi: 10.1111/jbg.12238. [DOI] [PubMed] [Google Scholar]
- 23.Di Gerlando R., Sutera A.M., Mastrangelo S., Tolone M., Portolano B., Sottile G., et al. Genome-wide association study between CNVs and milk production traits in Valle del Belice sheep. PloS One. 2019;14 doi: 10.1371/journal.pone.0215204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schurink A., da Silva V.H., Velie B.D., Dibbits B.W., Crooijmans R.P.M.A., Franҫois L., et al. Copy number variations in Friesian horses and genetic risk factors for insect bite hypersensitivity. BMC Genet. 2018;19:49. doi: 10.1186/s12863-018-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tice S.C. A new sex-linked character in drosophila. Biol Bull. 1914;26:221–230. [Google Scholar]
- 26.Pös O., Radvanszky J., Styk J., Pös Z., Buglyó G., Kajsik M., et al. Copy number variation: methods and clinical applications. NATO Adv Sci Inst Ser E Appl Sci. 2021;11:819. [Google Scholar]
- 27.Riggs E.R., Andersen E.F., Cherry A.M., Kantarci S., Kearney H., Patel A., et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen) Genet Med. 2020;22:245–257. doi: 10.1038/s41436-019-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bridges C.B. The bar “gene” a duplication. Science. 1936;83:210–211. doi: 10.1126/science.83.2148.210. [DOI] [PubMed] [Google Scholar]
- 29.Tjio J.H., Levan A. The chromosome number of man. Hereditas. 2010;42:1–6. [Google Scholar]
- 30.Lejeune J., Lafourcade J., Berger R., Vialatte J., Boeswillwald M., Seringe P., et al. 3 cases of partial deletion of the short arm of a 5 chromosome. C R Hebd Seances Acad Sci. 1963;257:3098–3102. [PubMed] [Google Scholar]
- 31.Iafrate A.J., Feuk L., Rivera M.N., Listewnik M.L., Donahoe P.K., Qi Y., et al. Detection of large-scale variation in the human genome. Nat Genet. 2004;36:949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- 32.Sebat J., Lakshmi B., Troge J., Alexander J., Young J., Lundin P., et al. Large-scale copy number polymorphism in the human genome. Science. 2004;305:525–528. doi: 10.1126/science.1098918. [DOI] [PubMed] [Google Scholar]
- 33.Feuk L., Carson A.R., Scherer S.W. Structural variation in the human genome. Nat Rev Genet. 2006;7:85–97. doi: 10.1038/nrg1767. [DOI] [PubMed] [Google Scholar]
- 34.Alkan C., Coe B.P., Eichler E.E. Genome structural variation discovery and genotyping. Nat Rev Genet. 2011;12:363–376. doi: 10.1038/nrg2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonald J.R., Ziman R., Yuen R.K.C., Feuk L., Scherer S.W. The Database of Genomic Variants: a curated collection of structural variation in the human genome. Nucleic Acids Res. 2014;42:D986–D992. doi: 10.1093/nar/gkt958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werdyani S., Yu Y., Skardasi G., Xu J., Shestopaloff K., Xu W., et al. Germline INDELs and CNVs in a cohort of colorectal cancer patients: their characteristics, associations with relapse-free survival time, and potential time-varying effects on the risk of relapse. Cancer Med. 2017;6:1220–1232. doi: 10.1002/cam4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montgomery S.B., Goode D.L., Kvikstad E., Albers C.A., Zhang Z.D., Mu X.J., et al. The origin, evolution, and functional impact of short insertion-deletion variants identified in 179 human genomes. Genome Res. 2013;23:749–761. doi: 10.1101/gr.148718.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballouz S., Dobin A., Gillis J.A. Is it time to change the reference genome? Genome Biol. 2019;20:159. doi: 10.1186/s13059-019-1774-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orr B., Godek K.M., Compton D. Aneuploidy. Curr Biol. 2015;25:R538–R542. doi: 10.1016/j.cub.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L., Zhou W., Zhang L., Zhang F. Genome architecture and its roles in human copy number variation. Genomics Inform. 2014;12:136–144. doi: 10.5808/GI.2014.12.4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hastings P.J., Lupski J.R., Rosenberg S.M., Ira G. Mechanisms of change in gene copy number. Nat Rev Genet. 2009;10:551–564. doi: 10.1038/nrg2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu W., Zhang F., Lupski J.R. Mechanisms for human genomic rearrangements. Pathogenetics. 2008;1:4. doi: 10.1186/1755-8417-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bacolla A., Wells R.D. Non-B DNA conformations, genomic rearrangements, and human disease. J Biol Chem. 2004;279:47411–47414. doi: 10.1074/jbc.R400028200. [DOI] [PubMed] [Google Scholar]
- 44.Conrad D.F., Pinto D., Redon R., Feuk L., Gokcumen O., Zhang Y., et al. Origins and functional impact of copy number variation in the human genome. Nature. 2010;464:704–712. doi: 10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andriani G.A., Vijg J., Montagna C. Mechanisms and consequences of aneuploidy and chromosome instability in the aging brain. Mech Ageing Dev. 2017;161:19–36. doi: 10.1016/j.mad.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gregan J., Polakova S., Zhang L., Tolić-Nørrelykke I.M., Cimini D. Merotelic kinetochore attachment: causes and effects. Trends Cell Biol. 2011;21:374–381. doi: 10.1016/j.tcb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khristich A.N., Mirkin S.M. On the wrong DNA track: molecular mechanisms of repeat-mediated genome instability. J Biol Chem. 2020;295:4134–4170. doi: 10.1074/jbc.REV119.007678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachinski L.L., Czernuszewicz T., Ramagli L.S., Suominen T., Shriver M.D., Udd B., et al. Premutation allele pool in myotonic dystrophy type 2. Neurology. 2009;72:490–497. doi: 10.1212/01.wnl.0000333665.01888.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hause R.J., Pritchard C.C., Shendure J., Salipante S.J. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22:1342–1350. doi: 10.1038/nm.4191. [DOI] [PubMed] [Google Scholar]
- 50.Takagi S., Kinouchi Y., Hiwatashi N., Nagashima F., Chida M., Takahashi S., et al. Relationship between microsatellite instability and telomere shortening in colorectal cancer. Dis Colon Rectum. 2000;43:S12–S17. doi: 10.1007/BF02237220. [DOI] [PubMed] [Google Scholar]
- 51.Witzany G. The viral origins of telomeres and telomerases and their important role in eukaryogenesis and genome maintenance. Biosemiotics. 2008;1:191–206. [Google Scholar]
- 52.Jeffreys A.J., Neil D.L., Neumann R. Repeat instability at human minisatellites arising from meiotic recombination. EMBO J. 1998;17:4147–4157. doi: 10.1093/emboj/17.14.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arlt M.F., Wilson T.E., Glover T.W. Replication stress and mechanisms of CNV formation. Curr Opin Genet Dev. 2012;22:204–210. doi: 10.1016/j.gde.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hovhannisyan G., Harutyunyan T., Aroutiounian R., Liehr T. DNA copy number variations as markers of mutagenic impact. Int J Mol Sci. 2019;20:4723. doi: 10.3390/ijms20194723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costa E.O.A., Pinto I.P., Gonçalves M.W., da Silva J.F., Oliveira L.G., da Cruz A.S., et al. Small de novo CNVs as biomarkers of parental exposure to low doses of ionizing radiation of caesium-137. Sci Rep. 2018;8:5914. doi: 10.1038/s41598-018-23813-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harutyunyan T., Hovhannisyan G., Sargsyan A., Grigoryan B., Al-Rikabi A.H., Weise A., et al. Analysis of copy number variations induced by ultrashort electron beam radiation in human leukocytes in vitro. Mol Cytogenet. 2019;12:18. doi: 10.1186/s13039-019-0433-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bennett P.M. Genome plasticity: insertion sequence elements, transposons and integrons, and DNA rearrangement. Methods Mol Biol. 2004;266:71–113. doi: 10.1385/1-59259-763-7:071. [DOI] [PubMed] [Google Scholar]
- 58.Shen H., Li J., Zhang J., Xu C., Jiang Y., Wu Z., et al. Comprehensive characterization of human genome variation by high coverage whole-genome sequencing of forty four Caucasians. PloS One. 2013;8 doi: 10.1371/journal.pone.0059494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dopman E.B., Hartl D.L. A portrait of copy-number polymorphism in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2007;104:19920–19925. doi: 10.1073/pnas.0709888104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henrichsen C.N., Vinckenbosch N., Zöllner S., Chaignat E., Pradervand S., Schütz F., et al. Segmental copy number variation shapes tissue transcriptomes. Nat Genet. 2009;41:424–429. doi: 10.1038/ng.345. [DOI] [PubMed] [Google Scholar]
- 61.Keel B.N., Lindholm-Perry A.K., Snelling W.M. Evolutionary and functional features of copy number variation in the cattle genome. Front Genet. 2016;7:207. doi: 10.3389/fgene.2016.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilad Y., Wiebe V., Przeworski M., Lancet D., Pääbo S. Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biol. 2004;5:e148. doi: 10.1371/journal.pbio.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Storz J.F. Gene duplication and evolutionary innovations in hemoglobin-oxygen transport. Physiology. 2016;31:223–232. doi: 10.1152/physiol.00060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young J.M., Endicott R.M., Parghi S.S., Walker M., Kidd J.M., Trask B.J. Extensive copy-number variation of the human olfactory receptor gene family. Am J Hum Genet. 2008;83:228–242. doi: 10.1016/j.ajhg.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Axelsson E., Ratnakumar A., Arendt M.L., Maqbool K., Webster M.T., Perloski M., et al. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature. 2013;495:360–364. doi: 10.1038/nature11837. [DOI] [PubMed] [Google Scholar]
- 66.Perry G.H., Dominy N.J., Claw K.G., Lee A.S., Fiegler H., Redon R., et al. Diet and the evolution of human amylase gene copy number variation. Nat Genet. 2007;39:1256–1260. doi: 10.1038/ng2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carpenter D., Mitchell L.M., Armour J.A.L. Copy number variation of human AMY1 is a minor contributor to variation in salivary amylase expression and activity. Hum Genom. 2017;11:2. doi: 10.1186/s40246-017-0097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groot P.C., Mager W.H., Henriquez N.V., Pronk J.C., Arwert F., Planta R.J., et al. Evolution of the human α-amylase multigene family through unequal, homologous, and inter- and intrachromosomal crossovers. Genomics. 1990;8:97–105. doi: 10.1016/0888-7543(90)90230-r. [DOI] [PubMed] [Google Scholar]
- 69.Perry G.H. The evolutionary significance of copy number variation in the human genome. Cytogenet Genome Res. 2008;123:283–287. doi: 10.1159/000184719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farashi S., Harteveld C.L. Molecular basis of α-thalassemia. Blood Cells Mol Dis. 2018;70:43–53. doi: 10.1016/j.bcmd.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Liu S., Yao L., Ding D., Zhu H. CCL3L1 copy number variation and susceptibility to HIV-1 infection: a meta-analysis. PloS One. 2010;5 doi: 10.1371/journal.pone.0015778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mamtani M., Rovin B., Brey R., Camargo J.F., Kulkarni H., Herrera M., et al. CCL3L1 gene-containing segmental duplications and polymorphisms in CCR5 affect risk of systemic lupus erythaematosus. Ann Rheum Dis. 2008;67:1076–1083. doi: 10.1136/ard.2007.078048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stankiewicz P., Lupski J.R. Genome architecture, rearrangements and genomic disorders. Trends Genet. 2002;18:74–82. doi: 10.1016/s0168-9525(02)02592-1. [DOI] [PubMed] [Google Scholar]
- 74.Perry G.H., Yang F., Marques-Bonet T., Murphy C., Fitzgerald T., Lee A.S., et al. Copy number variation and evolution in humans and chimpanzees. Genome Res. 2008;18:1698–1710. doi: 10.1101/gr.082016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lupski J.R., de Oca-Luna R.M., Slaugenhaupt S., Pentao L., Guzzetta V., Trask B.J., et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66:219–232. doi: 10.1016/0092-8674(91)90613-4. [DOI] [PubMed] [Google Scholar]
- 76.Fellermann K., Stange D.E., Schaeffeler E., Schmalzl H., Wehkamp J., Bevins C.L., et al. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number Predisposes to crohn disease of the colon. Am J Hum Genet. 2006;79:439–448. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beroukhim R., Mermel C.H., Porter D., Wei G., Raychaudhuri S., Donovan J., et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fahed A.C., Gelb B.D., Seidman J.G., Seidman C.E. Genetics of congenital heart disease: the glass half empty. Circ Res. 2013;112:707–720. doi: 10.1161/CIRCRESAHA.112.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zekavat S.M., Ruotsalainen S., Handsaker R.E., Alver M., Bloom J., Poterba T., et al. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun. 2018;9:2606. doi: 10.1038/s41467-018-04668-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olsson L.M., Holmdahl R. Copy number variation in autoimmunity-importance hidden in complexity? Eur J Immunol. 2012;42:1969–1976. doi: 10.1002/eji.201242601. [DOI] [PubMed] [Google Scholar]
- 81.Lee J.A., Lupski J.R. Genomic rearrangements and gene copy-number alterations as a cause of nervous system disorders. Neuron. 2006;52:103–121. doi: 10.1016/j.neuron.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 82.Sekar A., Bialas A.R., de Rivera H., Davis A., Hammond T.R., Kamitaki N., et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin C.L., Kirkpatrick B.E., Ledbetter D.H. Copy number variants, aneuploidies, and human disease. Clin Perinatol. 2015;42:227–242. doi: 10.1016/j.clp.2015.03.001. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vittori A., Breda C., Repici M., Orth M., Roos R.A.C., Outeiro T.F., et al. Copy-number variation of the neuronal glucose transporter gene SLC2A3 and age of onset in Huntington's disease. Hum Mol Genet. 2014;23:3129–3137. doi: 10.1093/hmg/ddu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cortes-Ciriano I., Lee S., Park W.Y., Kim T.M., Park P.J. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun. 2017;8:15180. doi: 10.1038/ncomms15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shammas M.A. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. 2011;14:28–34. doi: 10.1097/MCO.0b013e32834121b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nowakowska B. Clinical interpretation of copy number variants in the human genome. J Appl Genet. 2017;58:449–457. doi: 10.1007/s13353-017-0407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumaran M., Krishnan P., Cass C.E., Hubaux R., Lam W., Yasui Y., et al. Breast cancer associated germline structural variants harboring small noncoding RNAs impact post-transcriptional gene regulation. Sci Rep. 2018;8:7529. doi: 10.1038/s41598-018-25801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marcinkowska M., Szymanski M., Krzyzosiak W.J., Kozlowski P. Copy number variation of microRNA genes in the human genome. BMC Genom. 2011;12:183. doi: 10.1186/1471-2164-12-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sahoo T., del Gaudio D., German J.R., Shinawi M., Peters S.U., Person R.E., et al. Prader-Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat Genet. 2008;40:719–721. doi: 10.1038/ng.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gould D.W., Lukic S., Chen K.C. Selective constraint on copy number variation in human piwi-interacting RNA Loci. PloS One. 2012;7 doi: 10.1371/journal.pone.0046611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iben J.R., Maraia R.J. tRNA gene copy number variation in humans. Gene. 2014;536:376–384. doi: 10.1016/j.gene.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu H., Gu X., Wang G., Huang Y., Ju S., Huang J., et al. Copy number variations primed lncRNAs deregulation contribute to poor prognosis in colorectal cancer. Aging. 2019;11:6089–6108. doi: 10.18632/aging.102168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lupiáñez D.G., Kraft K., Heinrich V., Krawitz P., Brancati F., Klopocki E., et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161:1012–1025. doi: 10.1016/j.cell.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Conlon E.G., Manley J.L. RNA-binding proteins in neurodegeneration: mechanisms in aggregate. Genes Dev. 2017;31:1509–1528. doi: 10.1101/gad.304055.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pearson CE. Repeat Associated Non-ATG Translation Initiation: One DNA, Two Transcripts, Seven Reading Frames, Potentially Nine Toxic Entities! PLoS Genet 2011;7:e1002018. [DOI] [PMC free article] [PubMed]
- 97.Radvanszky J., Kadasi L. Molecular genetic basis of myotonic dystrophy. eLS, vol. 72. Chichester, UK: John Wiley & Sons, Ltd; 2001 p. 490. [Google Scholar]
- 98.Stankiewicz P., Lupski J.R. Structural variation in the human genome and its role in disease. Annu Rev Med. 2010;61:437–455. doi: 10.1146/annurev-med-100708-204735. [DOI] [PubMed] [Google Scholar]
- 99.Beckmann J.S., Estivill X., Antonarakis S.E. Copy number variants and genetic traits: closer to the resolution of phenotypic to genotypic variability. Nat Rev Genet. 2007;8:639–646. doi: 10.1038/nrg2149. [DOI] [PubMed] [Google Scholar]
- 100.Kolb S.J., Kissel J.T. Spinal muscular atrophy. Neurol Clin. 2015;33:831–846. doi: 10.1016/j.ncl.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wirth B., Karakaya M., Kye M.J., Mendoza-Ferreira N. Twenty-five years of spinal muscular atrophy research: from phenotype to genotype to therapy, and what comes next. Annu Rev Genom Hum Genet. 2020;21:231–261. doi: 10.1146/annurev-genom-102319-103602. [DOI] [PubMed] [Google Scholar]
- 102.Shaffer L.G., Lupski J.R. Molecular mechanisms for constitutional chromosomal rearrangements in humans. Annu Rev Genet. 2000;34:297–329. doi: 10.1146/annurev.genet.34.1.297. [DOI] [PubMed] [Google Scholar]
- 103.Konrad M., Saunier S., Heidet L., Silbermann F., Benessy F., Calado J., et al. Large homozygous deletions of the 2q13 region are a major cause of juvenile nephronophthisis. Hum Mol Genet. 1996;5:367–371. doi: 10.1093/hmg/5.3.367. [DOI] [PubMed] [Google Scholar]
- 104.Gitschier J., Wood W.I., Tuddenham E.G., Shuman M.A., Goralka T.M., Chen E.Y., et al. Detection and sequence of mutations in the factor VIII gene of haemophiliacs. Nature. 1985;315:427–430. doi: 10.1038/315427a0. [DOI] [PubMed] [Google Scholar]
- 105.Kuroda-Kawaguchi T., Skaletsky H., Brown L.G., Minx P.J., Cordum H.S., Waterston R.H., et al. The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men. Nat Genet. 2001;29:279–286. doi: 10.1038/ng757. [DOI] [PubMed] [Google Scholar]
- 106.Brook J.D., McCurrach M.E., Harley H.G., Buckler A.J., Church D., Aburatani H., et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3’ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 107.Radvanszky J., Surovy M., Polak E., Kadasi L. Uninterrupted CCTG tracts in the myotonic dystrophy type 2 associated locus. Neuromuscul Disord. 2013;23:591–598. doi: 10.1016/j.nmd.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 108.Manolio T.A., Collins F.S., Cox N.J., Goldstein D.B., Hindorff L.A., Hunter D.J., et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Lange K.M., Moutsianas L., Lee J.C., Lamb C.A., Luo Y., Kennedy N.A., et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017;49:256–261. doi: 10.1038/ng.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCarroll S.A., Huett A., Kuballa P., Chilewski S.D., Landry A., Goyette P., et al. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn's disease. Nat Genet. 2008;40:1107–1112. doi: 10.1038/ng.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saadati H.R., Wittig M., Helbig I., Häsler R., Anderson C.A., Mathew C.G., et al. Genome-wide rare copy number variation screening in ulcerative colitis identifies potential susceptibility loci. BMC Med Genet. 2016;17:26. doi: 10.1186/s12881-016-0289-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frenkel S., Bernstein C.N., Sargent M., Kuang Q., Jiang W., Wei J., et al. Genome-wide analysis identifies rare copy number variations associated with inflammatory bowel disease. PloS One. 2019;14 doi: 10.1371/journal.pone.0217846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ellinghaus D., Bethune J., Petersen B.S., Franke A. The genetics of Crohn's disease and ulcerative colitis-status quo and beyond. Scand J Gastroenterol. 2015;50:13–23. doi: 10.3109/00365521.2014.990507. [DOI] [PubMed] [Google Scholar]
- 114.Hollox E.J., Hoh B.P. Human gene copy number variation and infectious disease. Hum Genet. 2014;133:1217–1233. doi: 10.1007/s00439-014-1457-x. [DOI] [PubMed] [Google Scholar]
- 115.Koboldt D.C., Zhang Q., Larson D.E., Shen D., McLellan M.D., Lin L., et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Serin Harmanci A., Harmanci A.O., Zhou X. CaSpER identifies and visualizes CNV events by integrative analysis of single-cell or bulk RNA-sequencing data. Nat Commun. 2020;11:89. doi: 10.1038/s41467-019-13779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leary R.J., Lin J.C., Cummins J., Boca S., Wood L.D., Parsons D.W., et al. Integrated analysis of homozygous deletions, focal amplifications, and sequence alterations in breast and colorectal cancers. Proc Natl Acad Sci U S A. 2008;105:16224–16229. doi: 10.1073/pnas.0808041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brosens R.P.M., Haan J.C., Carvalho B., Rustenburg F., Grabsch H., Quirke P., et al. Candidate driver genes in focal chromosomal aberrations of stage II colon cancer. J Pathol. 2010;221:411–424. doi: 10.1002/path.2724. [DOI] [PubMed] [Google Scholar]
- 119.Krijgsman O., Carvalho B., Meijer G.A., Steenbergen R.D.M., Ylstra B. Focal chromosomal copy number aberrations in cancer-Needles in a genome haystack. Biochim Biophys Acta. 2014;1843:2698–2704. doi: 10.1016/j.bbamcr.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 120.Zhang L., Yuan Y., Lu K.H., Zhang L. Identification of recurrent focal copy number variations and their putative targeted driver genes in ovarian cancer. BMC Bioinf. 2016;17:222. doi: 10.1186/s12859-016-1085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peng H., Lu L., Zhou Z., Liu J., Zhang D., Nan K., et al. CNV detection from circulating tumor DNA in late stage non-small cell lung cancer patients. Genes. 2019;10:926. doi: 10.3390/genes10110926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagao Y. Copy number variations play important roles in heredity of common diseases: a novel method to calculate heritability of a polymorphism. Sci Rep. 2015;5:17156. doi: 10.1038/srep17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pollex R.L., Hegele R.A. Copy number variation in the human genome and its implications for cardiovascular disease. Circulation. 2007;115:3130–3138. doi: 10.1161/CIRCULATIONAHA.106.677591. [DOI] [PubMed] [Google Scholar]
- 124.Momtaz R., Ghanem N.M., El-Makky N.M., Ismail M.A. Integrated analysis of SNP, CNV and gene expression data in genetic association studies. Clin Genet. 2018;93:557–566. doi: 10.1111/cge.13092. [DOI] [PubMed] [Google Scholar]