Abstract
Background
The aim of the present study was to fabricate double layered scaffolds of electrospun polycaprolactone (PCL) and poly(ethylene oxide) (PEO). The electrospun PCL fibers were functionalized with wintergreen oil (WO) as a novel approach to prevent vascular grafts failure due to thrombosis by adjusting biomaterial–blood interactions.
Methods
PCL tubular scaffolds were prepared by electrospinning approach and coated with PEO as a hydrophilic polymer. The single and double layered scaffolds were characterized in terms of their morphological, chemical properties -as well as-hemocompatibility assays (i.e. prothrombin time, hemolysis percentage and platelets adhesion). Moreover, the antioxidant potential of WO-PCL samples were measured by 2,2-diphenyl-1-picrylhydrazyl hydrate (DPPH) free radical assay.
Results
The results demonstrated that incorporation of WO during the electrospinning process decreased the PCL fiber diameter. In addition, the prothrombine time assay shows that WO could be used to lower the electrospun PCL fiber tendency to induce blood clotting. Moreover, SEM observations of platelets adhesion of both single and double layered PCL/PEO scaffolds fiber shows an increase of platelets number, compared with the scaffolds containing WO.
Conclusions
The antioxidant potential and blood compatibility measurements of WO-PCL/PEO samples highlight the approach made so far as an ideal synthetic small size vascular grafts to overcome autogenous grafts shortages and drawbacks.
Keywords: Polycaprolactone (PCL), Poly(ethylene oxide) (PEO), Wintergreen oil (WO), Electrospun vascular grafts, Blood compatibility
Although autologous vascular grafts can provide an effective solution for surgeons to redirect blood flow around the injured or diseased vascular vessels via bypassing two spots within the vessel using natural veins or arteries [[1], [2], [3]], the bypass surgical operation is restricted under certain circumstances including, limited supply, secondary site injury and anatomical - as well as physiological mismatches [4,5]. Today, artificial vascular grafts made of biocompatible materials are often applied to replace autologous grafts [6,7]. Unfortunately, the development of synthetic vascular grafts of small diameter (less than 6 mm) - with functional features similar to the native ones - represents a huge obstacle to overcome the common drawback of traditional bypass vascular surgery [8].
Electrospinning has been highlighted as a fruitful approach to simply draw one dimension (1 D) polymeric fibers of nano/microsize thats directly assembled into 2D (or 3D) mats for different biological applications [[9], [10], [11]]. The electrospun nanofiber exhibit a large surface area and a high porous microstructure which are intrinsically essential for gas and nutrient perfusion - as well as various cellular activities [12]. Aliphatic polyesters, i.e. polyglycolic acid (PGA), polylactic acid (PLA), polycaprolactone (PCL) and their copolymers, have received a special attention as a result of their biocompatibility, mechanical strength and biodegradability [13,14]. However, electrospun polyester vascular scaffolds have been limited due to their lower bioactivity and hydrophobic nature which decrease their capability to interact or integrate with biological systems [15,16]. Hence, bioactive molecules have been conjugated or loaded to improve the blood compatibility of polymeric fiber for vascular grafts application i.e. anticoagulant agents as heparin and pharmaceutical drugs-that appear to be valuable components to promote tissue regeneration process via inducing endothelial precursor cells differentiation or reducing the inflammatory response [17,18].
Natural essential oils (NEOs) are hydrophobic volatile liquids that have a strong aroma because of their high concentration of the aromatic compounds. NEOs are usually extracted as secondary metabolites from the flowers, leaves, roots, fruits and other parts of the plant [19,20]. NEOs have garnered the attention of many researchers for medicinal and health care purposes as effective therapeutic agents due to their unique features including antibacterial, antimicrobial, anti-inflammatory and antioxidant [[21], [22], [23], [24]]. Among them, wintergreen oil (WO) is mainly composed of methyl salicylate (up to 99%), which is responsible for its medicinal activity with minor organics ingredients as α-pinene, myrcene, 3,7-guaiadiene, delta-3-carene, limonene, and delta-cadinene, [25,26]. Despite WO is used topically to reduce inflammations and pains, the lower solubility of WO under physiological conditions and the systematic toxicity of methyl salicylate limit its application [27,28].
Herein, electospun PCL fiber were coated with a hydrophilic layer of PEO as a novel approach to develop multifunctional vascular scaffold. The effects of WO on PCL fiber formation and stability were studied at different concentrations (1, 2 and 3 wt%). The fabricated single/double layered scaffolds were thoroughly characterized in terms of their microstructure properties via Scanning Electron Microscopy (SEM) and Attenuated Total Reflectance Fourier transform infrared spectroscopy (ATR-FTIR). The water uptake and antioxidant capabilities were also measured. Moreover, the blood compatibility of the fabricated single and double layered scaffolds was assessed by testing, prothrombin time, hemolysis percentage and platelets adhesion.
Materials and methods
Materials
PCL pellets (Mw = 80,000 g/mol), poly(ethylene oxide) powder (PEO, MW = 9,00,000 g/mol) and wintergreen oil (WO, Mw = 152.15 g/mol) were purchased from Sigma–Aldrich. Heparin solution (5000 I.U./1 ML) was purchased from Amoun Pharmaceutical Industries Co., APIC, Cairo, Egypt. Chloroform (CHCl3, 99%) and Acetone was supplied by Carlo Erba Reagents. All reagents and solvents were used without any further purification.
Preparation of PCL/PEO double-layered tubular scaffolds
The PCL (6, 8 and 10 wt.%) solutions were prepared by dissolving PCL granules in a mixed solution of acetone chloroform (1:1 v/v). The optimized electrospinning parameters for the PCL solution were carried out to select the favorable conditions to obtain neat PCL nanofibers. The electrospinning process was performed using a homemade apparatus in which PCL solutions were loaded to a plastic syringe with a metallic needle of 0.9 mm diameter and connected to a high-voltage power supply. The selected processing parameters were as following: an applied voltage of 20 kV, a needle-collector distance of 12 cm, while the polymeric solution was fed in using a syringe pump at a flow rate of 1 mL/h. A rotating stainless steel tube of 5 mm diameter was used as a collector to fabricate tubular scaffolds. The WO-PCL nanofiber were obtained under the above processing parameters by the addition of WO to PCL solution before the electrospinning process. Different amount of WO (100, 200, 300 μl) were added to a 10 ml of PCL solution and stirred for 3 h. The samples were named WO1-PCL, WO2-PCL and WO3-PCL. Finally, PEO layer was added to the WO-PCL tubular scaffolds by dipping the whole scaffold into PEO solution of 5% and 10% conc. 2 ml of Heparin (Hep) - as an anticoagulant agent - was mixed with PEO solution. The obtained PCL/PEO double layered scaffolds in absence and presence of Hep were named as WO-PCL/PEO and WO-PCL/PEO-Hep, respectively. The scaffolds were dipped into PEO three times before air drying. The prepared tubular scaffolds were dried at room temperature for solvent traces removal and then stored in the desiccator for further characterizations.
Structural characterization
The microstructure and morphology of elctrospun fiber PCL and double layered PCL-PEO mats were observed via SEM. The prepared samples were coated with a thin layer of gold by sputtering and then the morphology of electrospun mats was determined. The micrographs were taken using a Hitachi S-4100 electron microscope (Tokyo, Japan) at an accelerating voltage of 5 kV and a working distance of 8–10 mm. While the functional groups of neat PCL, PEO and WO, as well as PCL/PEO double layered mats were characterized by ATR-FTIR spectroscopy using Vertex 70 FTIR spectrometer from BrukerOptik GmbH, Germany, equipped with diamond ATR crystal system in the spectral range of 4000–400 cm−1 with the resolution of 4 cm−1.
Water uptake
The obtained single and double layered tubular scaffolds were incubated for 24 h in phosphate buffer solution (PBS) at 37 °C. The degree of water uptake was determined according to the following equation [29]:
| Water uptake (%) = [(Ww– Wd)/ Wd] × 100. |
where Wd is the dry sample weight before immersion in PBS and Ww is the wet weight of the sample after soaking 24 h in the PBS.
Antioxidant assay
Radical scavenging activity was measured against stable 2,2-diphenyl-2-picrylhydrazyl hydrate (DPPH) [30]. The WO1-PCL and WO2-PCL mats were kept in the dark for different time intervals(0.5, 1, 2, 4, 8, 16 and 32 h) at room temperature to react with DPPH solution (0.1 mM in ethanol). The change in colour (from deep violet to light yellow) was measured using UV–visible light spectrophotometer at 517 nm. The experiment was carried out five times for each sample. DPPH free radical scavenging activity was expressed as remaining DPPH % against different time intervals.
Prothrombin test
Prothrombin test (PT), also called International Normalised Ratio test (INR), is a laboratory approach to measure the blood coagulation time [31,32]. In brief, 2.5 ml human blood was taken from a healthy adult and drawn into a sterilized tube containing liquid sodium citrate, as an anticoagulant agent. The samples were spread over a glass slide and then 200 μl of the anticoagulated blood was added on the surface of the samples. The blood coagulation was promoted by adding 20 μl PT liquid (isi = 1.5) and coagulation time was recorded. The data were taken after five measurements were averaged.
| INR = [(PTS / PTb] isi |
Where PTs and PTb represent the blood coagulation time in presence and absence of the samples, respectively.
Hemolysis assay
A fresh blood sample was treated with sodium citrate solution and normal saline, according to a method describe elsewhere [33]. 200 μl of the diluted blood were added to a standard falcon tube containing normal saline and the prepared samples which were cut into equal squares of 4 mm diameter and kept for 1 h at 37 °C. The positive and negative controls were distilled water and saline, respectively. All the samples and the controls were measured in triplicate and mean values were noted. The supernatant was slowly collected after centrifugation at 3000 rpm for 5 min and used to measure the absorbance at 541 nm by a spectrophotometer. The hemolysis percentage was calculated as according to the following relationship.
| Hemolysis (%) = [(O.D.Sample – O.D.Negative)/ (O.D.Positive – O.D.Negative)] × 100. |
where O. DSample is the measured absorbance of the tested sample as well as O.D.Negative and O.D.Positive represents the absorbance of negative and the positive control, respectively.
Platelets adhesion
To investigate the effect of prepared samples on platelets adhesion, a human blood was taken from a donor and then centrifuged at 3000 rpm for 5 min to collect the platelet-rich plasma [34]. Equal volume of platelets rich plasma were seeded on the top of electrospun PCL fiber and double layered tublar scaffolds and incubated at 37 °C for 1 h. The platelet were fixed with 4% glutaraldehyde for 10 min and washed several times with PBS. The samples were coated with gold and visualized using SEM.
Statistical analysis
The results are expressed as the means standard deviations. Statistical analysis was performed using Origin 8.0 (OriginLab, Northampton, MA, 01060 USA). The statistical analysis of the obtained results was evaluated using paired Student's t-test. The differences between groups were considered significant at a significance level of p<0.01.
Results
Morphology of the obtained PCL nanofibers and PCL/PEO double layered scaffolds
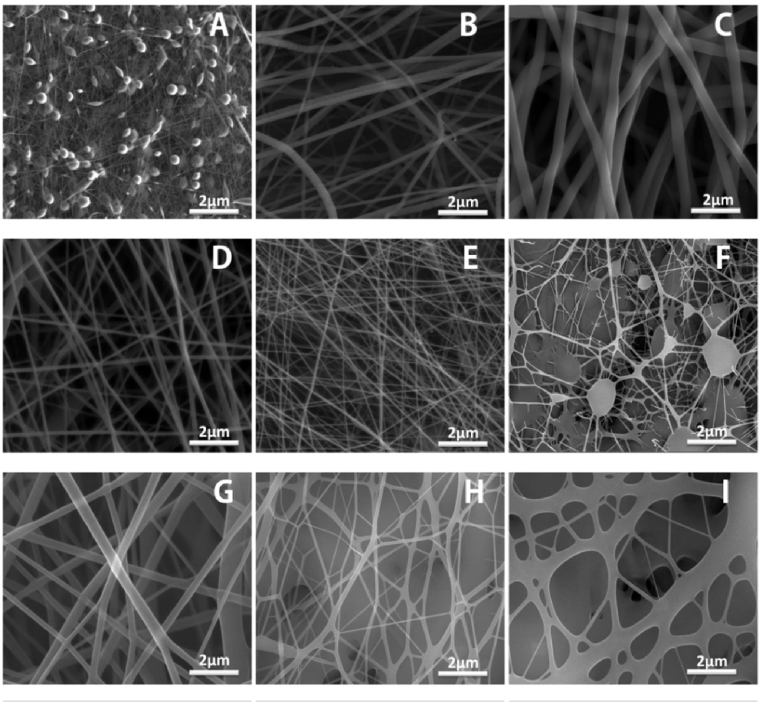
Electrospinning is a highly sensitive method due to the numerous processing parameters that influence the morphology and diameter of obtained fibers. In the current study, PCL solutions concentration. and applied voltage were controlled to select the optimal processing parameters for the fabrication of PCL nanofiber. Fig.(1A–C) shows the obtained PCL fiber under different concentrations. (6, 8 and 10 wt. %) of PCL solution and fixed voltage at 15 kv. The SEM observations indicate that PCL solution with solution conc. lower than 8% result in beads formation. The increase of polymer conc. from 8 to 10 wt. % leads to a significant increase in fiber diameter from 812 ± 103 to 1400 ± 300 nm, respectively. While, the increasing of applied voltage from 17 kv and 20 kv results in a clear decrease in fiber diameter from 244 ± 75 to 127 ± 58 nm, respectively. For WO-PCL fibers, PCL solution conc. was kept at 10% as the addition of WO to 8% PCL solution leads to beads formation, as shown in Fig. (1F). The obtained fiber diameter of 10% PCL solution containing different amounts of 100 and 200 μl of WO were 900 ± 175 nm, 225 ± 40 nm, while the ones containing 300 μl appeared to be fused together, as shown in Fig.(1G–I).
Fig. 1.
Optimization of processing parameters (A, B& C) obtained fibers at 15 kV and distance of 10 cm for solution conc. of 6, 8 &10%, respectively, (D& E) effect of applied voltage 17 and 20 kv, (F) WO1-PCL solution conc. of 8%, (G, H& I) WO1-PCL, WO2-PCL WO3-PCL solution conc. of 10%, respectively.
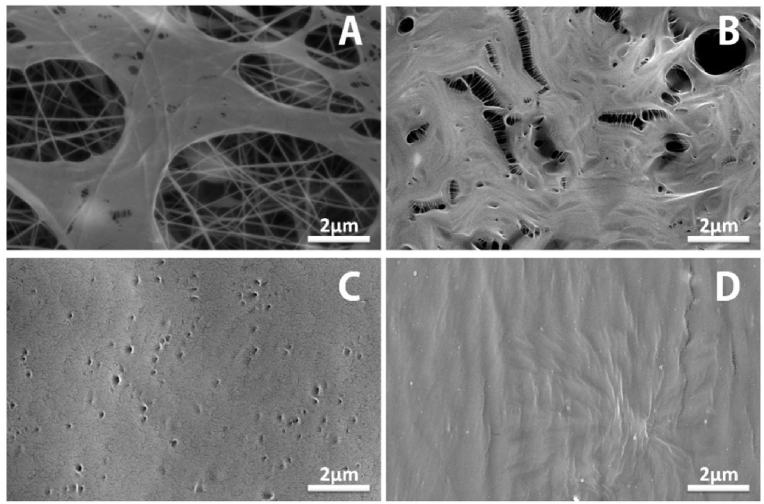
To adapt the coating of PCL electrospun layer, the dip casting was performed at different PEO concentrations. Fig.(2A, B) shows the PCL fiber coated with PEO of 5 and 10 wt%., respectively. The homogenous layers were also observed for PCL/PEO-Hep and WO1-PCL/PEO-Hep, as shown in Fig. (2C, D).
Fig. 2.
SEM micrographs of double layered mats (A) PCL coated PEO solution of 5%, (B) PCL coated PEO solution of 10%, (C) PCL/PEO-Hep and (D) WO1-PCL/PEO-Hep.
FTIR analysis
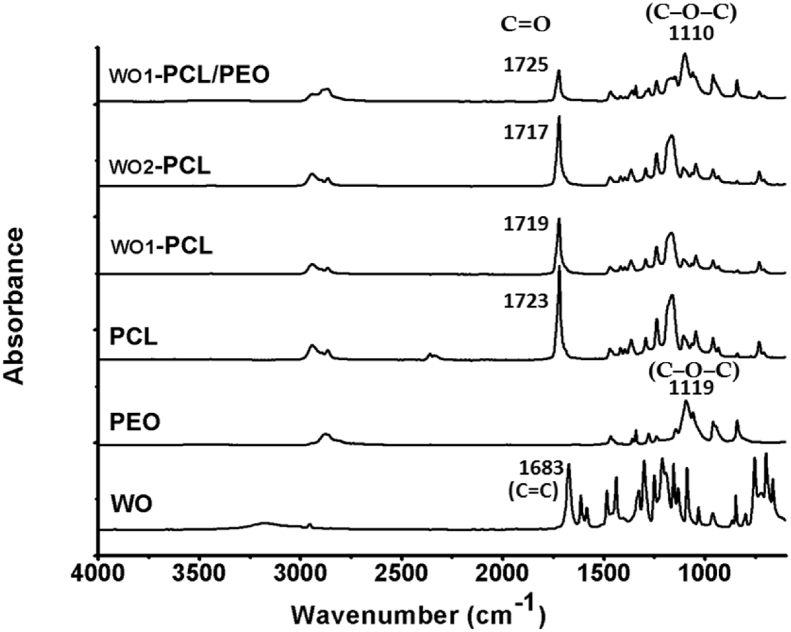
The ATR-FTIR spectroscopy was used to observe the changes in the characteristic absorption bands of PCL due to the incorporation of WO and PEO, as shown in Fig. 3. The spectrum of pure PCL electrospun fiber exhibits two bands at 2940 cm−1 and 2868 cm−1 which are typically due to the symmetric and asymmetric C–H stretching. The sharp band at 1723 cm−1 belongs to stretching vibrations of the ester-carbonyl groups (C=O). The two bands at 1293 cm−1 and 1165 cm−1 are attributed to the C–C and C–O stretching vibrations, respectively. The band at 1238 cm−1 is related to the asymmetric (C–O–C) stretching mode [35,36]. The main component of WO is the methyl ester of salicylic acid which has two functional groups carboxylic acid and phenol [37]. Within the spectrum of WO, the broad band with a high intensity at 3194 cm−1 represents the stretching vibration of the hydroxyl groups which reflect the strong intra and intermolecular H-bonding of phenolic and carboxylic hydroxyl groups. The band at 2916 cm−1 is due to the C–H stretching vibration. The carbonyl stretching (C=O) due to aromatic carboxylic acid was found at 1683 cm−1. The main bands that remain are the aryl ν(C=C) stretches at 1612 cm−1, the asymmetric and symmetric ν(C–O) stretches of the ester groups at 1328 and 1165 cm−1 and the asymmetric stretch for acetates around 1213 cm−1. However, the characteristics bands of PCL were also observed for WO-PCL, the WO carbonyl band at 1683 cm−1 is not observed which could be due to the small amount of WO. Notably, with increasing amount of WO, the intensity of the absorption band assigned to ester band at 1723 cm−1 was changed and shifted to 1719 cm−1 for WO1-PCL and 1717 cm−1 for WO2-PCL that indicate the interaction between PCL and WO. Moreover, the spectra of pure PEO and WO1-PCL/PEO were also investigated for comparison of WO1-PCL and PCL. The absorption bands of PEO were found at 3436 cm−1 (O–H stretch), 2906 cm−1 (C–H stretch), 1464 cm−1 and 1356 cm−1 (CH2 groups in PEO), 1119 cm−1 (C–O–C stretch) [[37], [38], [39]]. For WO1-PCL/PEO, the PEO adsorption on the surface was confirmed by the changes of peak intensity at 1725 and around 1110, corresponding to the carbonyl group of PCL and C–O–C linkage of PEO, respectively [37,38].
Fig. 3.
FTIR spectra of pure WO, PEO and PCL - as well as fabricated WO1-PCL electrospun mats and double layered mats of WO1-PCL/PEO.
Water uptake
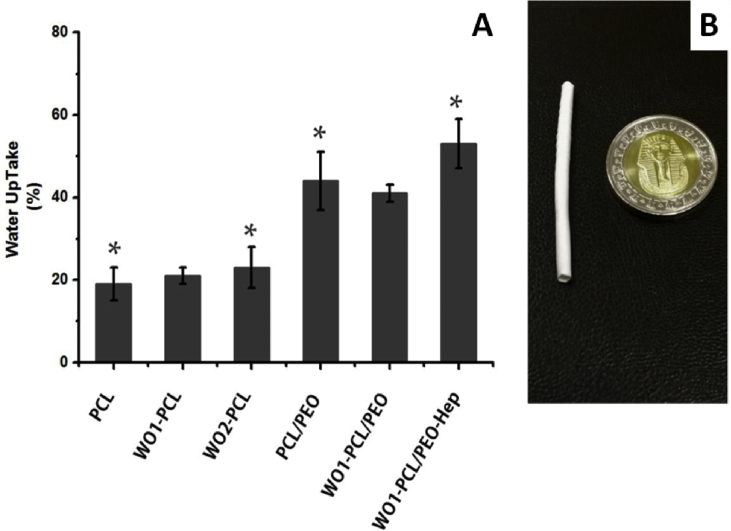
Since, vascular grafts are in direct contact with blood, the water uptake capability is an important aspect for protein absorption and cellular attachment and nutrient diffusion, but high water uptake could result in morphological changes and destruction of the implant. Therefore, water uptake is a debatable issue that should be investigated. Fig.(4A) shows the water uptake of the obtained fiber mats electrospun mats after soaking in PBS for 24 h at 37 °C. As expected, the overall water uptake was increased for PCL/PEO double layered mats due to hydrophilic nature of PEO. The water uptake of PCL fiber was 19% ± 4 and obviously increase to 44% ± 7 for PCL/PEO, 41% ± 2 for WO1-PCL/PEO and 53% ± 6 for WO1-PCL/PEO-Hep. Meanwhile, the water uptake was slightly increased to 21% ± 2 for WO1-PCL and 23% ± 5 for WO2-PCL. According to the above result, both single and double layered PCL fiber scaffolds are not under huge changes upon exposure to water. Moreover, the double layered scaffolds show higher water uptake that would induce diffusion of WO and Hep to the surroundings as well as biodegradation of PCL electrospun fibers.
Fig. 4.
Represents water uptake percentage of fabricated PCL and PCL/PEO double layered fibers. The inserted photograph of fabricated tubular scaffolds which was obtained using a rotating mandrel to develop a vessel like structure of electrospun PCL fibers. Data are evaluated as mean values ± SD for three experiments. Differences between the samples were determined using the unpaired t-test (∗p ≤ 0.01).
Antioxidant assay
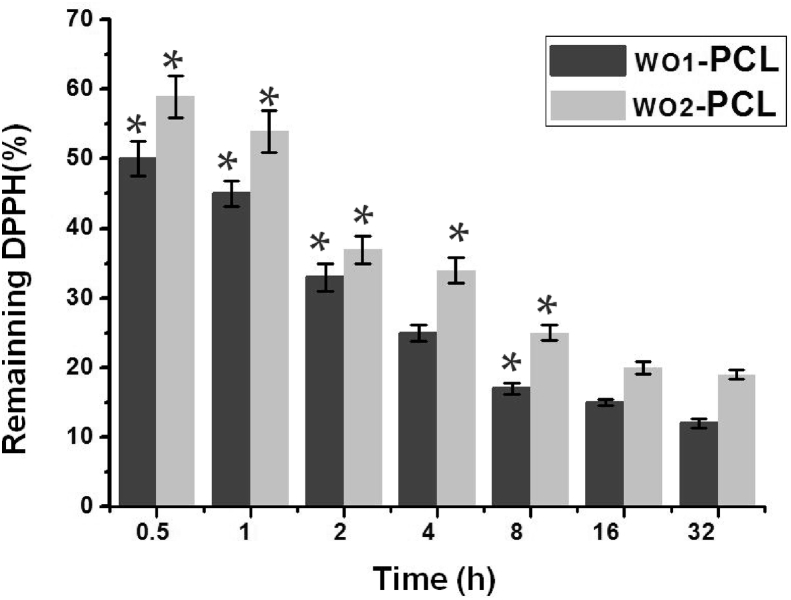
The antioxidant activity is well known to depend on the type and polarity of the extracting solvent, the isolation procedures, and the purity of the active compounds, as well as the assay techniques and substrate used [40,41]. The antioxidant activity of WO-PCL fiber mats were measured by DPPH assay after 0.5, 1, 2, 4, 8, 16 and 32 h, as shown in Fig. 5. The results indicate that the difference between WO1-PCL and WO2-PCL samples was not much. Both of the samples exhibit a stable antioxidant capability after 8 h in which the remaining DPPH % were not slightly changed after 16 and 32 h. This could be attributed to the controlled release of WO form PCL occurs after 8 h.
Fig. 5.
Shows the remaining DPPH % for WO1-PCL and WO2-PCLsamples after different time intervals. The antioxidant potential was calculated and expressed as mean values ± SD for five experiments (p ≤ 0.01).
Hemocompatibility: prothrombin time, hemolysis and platelets adhesion
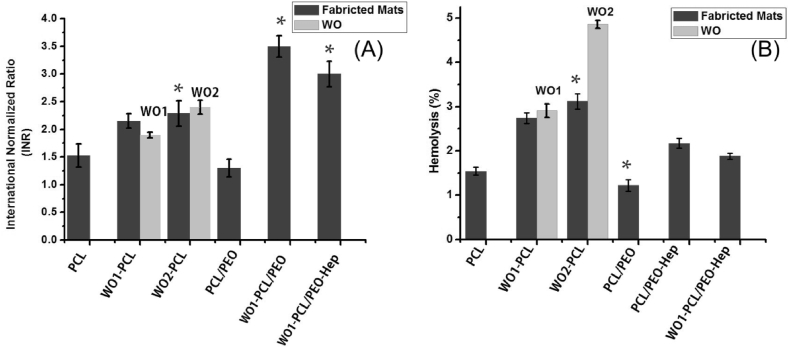
Fig. 6 shows the INR that was applied to measure blood clotting tendency of fabricated samples. The INR for PCL and PCL-PEO were 1.53 ± 0.21 and 1.30 ± 0.16, respectively, which reflect the tendency of electrospun PCL and PEO to induce blood coagulation. While the value for WO1-PCL was 2.15 ± 0.13 and 2.29 ± 0.23 for WO2-PCL. The INR ratio was further increased for PCL/PEO-Hep to 3.5 ± 0.19 and 3 ± 0.23 for WO1-PCL/PEO-Hep. It is important to mention that the INR values of WO were also measured to compare those with WO-PCL fibers as shown in Fig.(6A). The measured ratio for WO1 and WO2 were 1.9 ± 0.05 and 2.4 ± 0.13. The high values of INR (more than 2) highlight the anticoagulation action of heparin and WO. In addition, the results of the H% of fabricated samples are given in Fig. 6. From the data obtained, the H% of neat elctrospun PCL was 1.54 ± 0.9%. However the pure WO samples show a higher H% (2.91 ± 0.15% for WO1 and 4.86 ± 0.09% for WO2), the electrospun WO1-PCL and WO2-PCL exhibit lower values 2.74 ± 0.12% and 3.12 ± 0.17%, respectively. While the hemolytic values for the double layered scaffolds were 1.22 ± 0.13 for PCL/PEO, 2.17 ± 0.11% for PCL/PEO-Hep and 1.88 ± 0.7% for WO1-PCL/PEO-Hep.
Fig. 6.
Represents blood compatibility assay of fabricated single and double layered mats (A) Prothrombin assay and (B) Hemolysis assay. Data were evaluated as mean values ± SD for five measurements (∗p ≤ 0.01).
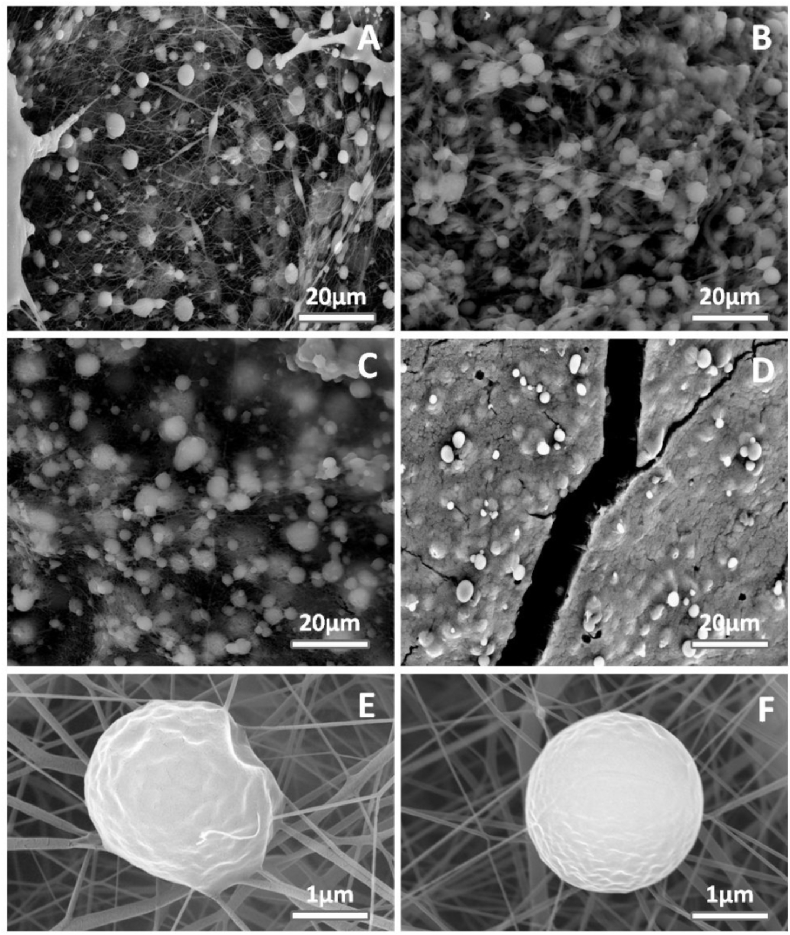
The platelets adhesion after incubation with platelet-rich plasma for 2 h at 37 °C was observed by using SEM images, as shown in Fig. 7. In comparison to neat PCL, the double layered PCL/PEO mats resulted in an increase of platelets deposition which could be attributed to the hydrophobic nature of the PCL surface and hydrophilic nature of PEO, as shown in Fig.(7A, B). Remarkably, the number of adhered platelets were reduced with the addition WO (WO1-PCL/PEO) and further decreased by the WO1-PCL/PEO-Hep surface. Fig.(7E, F) show the discoid morphology of platelets on the PCL and (WO1-PCL/PEO-Hep) surfaces at higher magnification.
Fig. 7.
SEM micrograph of adhered plated on the surface of (A) Electrospun PCL mats,(B) PCL/PEO mats,(C) WO1-PCL/PEO mats, (D) WO1-PCL/PEO-Hep, (E) Higher magnification of adhered platelet on the surface of PCL/PEO and (F) adhered platelet on WO1-PCL/PEO.
Discussion
The ideal vascular scaffolds should be biocompatible and biodegradable as well as able to integrate within the host vascular tissue. Moreover, vascular grafts should allow blood flow without any physical or chemical interactions with blood components (i.e., blood cells or platelets) to avoid thrombosis that is commonly associated with currently used natural and synthetic grafts, especially for small-size blood vessel implants [4,7,42]. Electrospinning has been introduced to simply draw polymeric fibers of ultrafine diameter and extremely large surface area, which mimic the natural extracellular matrix for biomedical application due to their capability to promoted cell adhesion, migration and proliferation [9,11,15]. Over the last decade, electrospun polymeric fibers of aliphatic polyesters have received special attention, because of their biocompatibility, mechanical strength and biodegradability such as poly(caprolactone) (PCL) etc [16]. Although, electrospun mats have been successfully used in tissue engineering, wound dressing and drug delivery systems. The applicability of electrospun fibers as a vascular grafts is often restricted because of their hydrophobic nature and their tendency to induce blood coagulation. To overcome the above description, we introduced a facial approach to improve performance of PCL fibers via coating with PEO and the addition of WO as a natural oil to offer a range of biological benefits. The result within this study demonstrates the capability of WO to improve the blood clotting tendency and hemolytic abilities of electrospun PCL vascular scaffolds. For instance, the prothrombin time test shows that the INR value of PCL and PCL-PEO were lower than 2, which reflect the tendency of electrospun PCL and PEO to induce blood clotting. On the other hand, the values for the sample containing WO were higher than 2 which indicates capability of WO to delay blood clotting process [42]. These result were supported with H% test which indicate that the fabricated PCL and PCL/PEO with and without WO or Hep are classified as non-hemolyic materials and meet the essential requirements for vascular grafts due to their hemolytic percentages which are lower than 5% [43]. Moreover, it is well known that platelet adhesion and aggregation are one of the essential key parameters to activate the coagulation pathway. The release of platelet factor from the active platelets can activate pro-thrombin to result in coagulation. Therefore, the adhesion of platelets on the vascular scaffolds can be used to determine the anticoagulant potential and blood compatibility of the vascular scaffolds [44]. Remarkably, the number of adhered platelets were reduced with the addition WO (WO1-PCL/PEO) and further decreased by the WO1-PCL/PEO-Hep surface, as shown in Fig. 7 which reflect the ability of WO to decrease the tendency of electrospun PCL to induce blood clotting via delaying platelets adhesion on the surface of vascular grafts.
Conclusions
Tubular vascular grafts of multiple functions were prepared by combining electrospinning and dip coating approaches. The obtained electrospun PCL fibers functionalized by WO and Hep were added to PEO as an anticoagulant agent. The hydrophicity of the double layered PCL/PEO mats was improved upon comparing with single layered PCL mats. The double layered grafts exhibited a good blood compatibility in terms of their non hemolytic features and tendency to delay blood clotting processes - as well as antioxidant activity. However, it is important to highlight that the amount of WO should be controlled to avoid side effects. In summary, the fabricated PCL/PEO mats may be considered as a multifunctional conduit for small-diameter (less than 6 mm) and are valuable for further assessment - such as in-vitro cell culture and in-vivo studies.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Research Center, Cairo, Egypt and also Physics Department, Faculty of Science, Helwan University, Cairo, Egypt.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Acar C., Ramsheyi A., Pagny J.Y., Jebara V., Barrier P., Fabiani J.N., et al. The radial artery for coronary artery bypass grafting: clinical and angiographic results at five years. J Thorac Cardiovasc Surg. 1998;116:981–989. doi: 10.1016/S0022-5223(98)70050-9. [DOI] [PubMed] [Google Scholar]
- 2.Spray T.L., Roberts W.C. Changes in saphenous veins used as aortocoronary bypass grafts. Am Heart J. 1977;94:500–516. doi: 10.1016/s0002-8703(77)80046-x. [DOI] [PubMed] [Google Scholar]
- 3.Cox J.L., Chiasson D.A., Gotlieb A.I. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34:45–68. doi: 10.1016/0033-0620(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura G., Hibino N., Ikada Y., Kurosawa H., Shin'oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24:2303–2308. doi: 10.1016/s0142-9612(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 5.Hoenig M.R., Campbell G.R., Rolfe B.E., Campbell J.H. Tissue-engineered blood vessels: alternative to autologous grafts? Arterioscler Thromb Vasc Biol. 2005;25:1128–1134. doi: 10.1161/01.ATV.0000158996.03867.72. [DOI] [PubMed] [Google Scholar]
- 6.Kurobe H., Maxfield M.W., Breuer C.K., Shinoka T. Concise review: tissue-engineered vascular grafts for cardiac surgery: past, present, and future. Stem Cells Transl Med. 2012;1:566–571. doi: 10.5966/sctm.2012-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X., Lin P., Yao Q., Chen C. Development of small-diameter vascular grafts. World J Surg. 2007;31:682–689. doi: 10.1007/s00268-006-0731-z. [DOI] [PubMed] [Google Scholar]
- 8.Isenberg B.C., Williams C., Tranquillo R.T. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 9.Li D., Xia Y. Electrospinning of nanofibers: reinventing the wheel? Adv Mater Res. 2004;16:1151–1170. [Google Scholar]
- 10.Doshi J., Reneker D.H. Electrospinning process and applications of electrospun fibers. J Electrosat. 1995;35:151–160. [Google Scholar]
- 11.Schröder H.C., Tolba E., Diehl-Seifert B., Wang X., Müller W.E. Electrospinning of bioactive wound-healing nets. Prog Mol Subcell Biol. 2017:259–290. doi: 10.1007/978-3-319-51284-6_8. [DOI] [PubMed] [Google Scholar]
- 12.Pham Q.P., Sharma U., Mikos A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng Regen Med. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 13.Teo W.E., Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006;17:R89–R106. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 14.Müller W.E., Tolba E., Schröder H.C., Diehl-Seifert B., Link T., Wang X. Biosilica-loaded poly (ε-caprolactone) nanofibers mats provide a morphogenetically active surface scaffold for the growth and mineralization of the osteoclast-related SaOS-2 cells. Biotechnol J. 2014;9:1312–1321. doi: 10.1002/biot.201400277. [DOI] [PubMed] [Google Scholar]
- 15.Ignatova M., Manolova N., Markova N., Rashkov I. Electrospun non-woven nanofibrous hybrid mats based on chitosan and PLA for wound-dressing applications. Macromol Biosci. 2009;9:102–111. doi: 10.1002/mabi.200800189. [DOI] [PubMed] [Google Scholar]
- 16.Zhan J., Singh A., Zhang Z., Huang L., Elisseeff J.H. Multifunctional aliphatic polyester nanofibers for tissue engineering. Biomatter. 2012;2:202–212. doi: 10.4161/biom.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye L., Wu X., Duan H.Y., Geng X., Chen B., Gu Y.Q., et al. The in vitro and in vivo biocompatibility evaluation of heparin–poly (ε-caprolactone) conjugate for vascular tissue engineering scaffolds. J Biomed Mater Res. 2012;100:3251–3258. doi: 10.1002/jbm.a.34270. [DOI] [PubMed] [Google Scholar]
- 18.Del Gaudio C., Ercolani E., Galloni P., Santilli F., Baiguera S., Polizzi L., et al. Aspirinloaded electrospun poly (ε-caprolactone) tubular scaffolds: potential small-diameter vascular grafts for thrombosis prevention. J Mater Sci Mater Med. 2013;24:523–532. doi: 10.1007/s10856-012-4803-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee M.S., Choi J., Posadzki P., Ernst E. Aromatherapy for health care: an overview of systematic reviews. Maturitas. 2012;71:257–260. doi: 10.1016/j.maturitas.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Edris A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res – Int J Devoted Pharmacol Toxicol Eval Nat Prod Deriv. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 21.Stea S., Beraudi A., De Pasquale D. Essential oils for complementary treatment of surgical patients: state of the art. Evid Based Complement Alternat Med. 2014;2014:726341. doi: 10.1155/2014/726341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chouhan S., Sharma K., Guleria S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines. 2017;4:58. doi: 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalemba D., Kunicka A. Antibacterial and antifungal properties of essential oils current medicinal chemistry. Curr Med Chem. 2003;10:813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- 24.Harkenthal M., Reichling J., Geiss H.K., Saller R. Comparative study on the in vitro antibacterial activity of Australian tea tree oil, cajuput oil, niaouli oil, manuka oil, kanuka oil, and eucalyptus oil. Pharmazie. 1999;54:460–463. [PubMed] [Google Scholar]
- 25.Ranyaphi R.A., Warjri A.G., Mao A.A. 2002. Gaultheria fragmentissima wall, an important wild medicinal plant. In Regional seminar on the role of biodiversity and environmental strategies in North East India; pp. 158–161. [Google Scholar]
- 26.Jänicke C., Grünwald J., Brendler T. Handbuch phytotherapie: indikationen - anwendungen - wirksamkeit - präparate. WVG Wissenschaftliche VerlagsGesellschaft. 2003 [Google Scholar]
- 27.Zhang D., Liu R., Sun L., Huang C., Wang C., Zhang D.M., et al. Antiinflammatory activity of methyl salicylate glycosides isolated from Gaultheria yunnanensis (Franch.) Rehder. Molecules. 2011;16:3875–3884. doi: 10.3390/molecules16053875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brubacher J.R., Hoffman R.S. Salicylism from topical salicylates: review of the literature. J Toxicol Clin Toxicol. 1996;34:431–436. doi: 10.3109/15563659609013814. [DOI] [PubMed] [Google Scholar]
- 29.Natu M.V., de Sousa H.C., Gil M.H. Effects of drug solubility, state and loading on controlled release in bicomponent electrospun fibers. Int J Pharm. 2010;397:50–58. doi: 10.1016/j.ijpharm.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 30.Brand-Williams W., Cuvelier M.E., Berset C.L. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 31.Khandwekar A.P., Patil D.P., Shouche Y., Doble M. Surface engineering of polycaprolactone by biomacromolecules and their blood compatibility. J Biomater Appl. 2011;26:227–252. doi: 10.1177/0885328210367442. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Wang Y., Ye J., Yuan J., Xiao Y. Fabrication of poly (ε-caprolactone)/keratin nanofibrous mats as a potential scaffold for vascular tissue engineering. Mat Sci Eng C-Mater. 2016;68:177–183. doi: 10.1016/j.msec.2016.05.117. [DOI] [PubMed] [Google Scholar]
- 33.Bhaskar N., Padmavathy N., Jain S., Bose S., Basu B. Modulated in vitro biocompatibility of a unique cross-linked salicylic acid–poly (ε-caprolactone)-based biodegradable polymer. Acs Appl Mater Inter. 2016;8:29721–29733. doi: 10.1021/acsami.6b10711. [DOI] [PubMed] [Google Scholar]
- 34.Li C., Wang F., Ge P., Mao Y., Wang L. Anti-acute thrombogenic surface using coaxial electrospraying coating for vascular graft application. Mater Lett. 2017;205:15–19. [Google Scholar]
- 35.Elzein T., Nasser-Eddine M., Delaite C., Bistac S., Dumas P. FTIR study of polycaprolactone chain organization at interfaces. J Colloid Interface Sci. 2004;273:381–387. doi: 10.1016/j.jcis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Ghasemi-Mobarakeh L., Prabhakaran M.P., Morshed M., Nasr-Esfahani M.H., Ramakrishna S. Bio-functionalized PCL nanofibrous scaffolds for nerve tissue engineering. Mater Sci Eng C. 2010;30:1129–1136. [Google Scholar]
- 37.Wu Y.W., Sun S.Q., Zhou Q., Tao J.X., Noda I. Volatility-dependent 2D IR correlation analysis of traditional Chinese medicine ‘Red Flower Oil’preparation from different manufacturers. J Mol Struct. 2008;882:107–115. [Google Scholar]
- 38.Morlat S., Gardette J.L. Phototransformation of water-soluble polymers. I: photo-and thermooxidation of poly (ethylene oxide) in solid state. Polymer. 2001;42:6071–6079. [Google Scholar]
- 39.Jayaramudu T., Ko H.U., Kim H.C., Kim J.W., Choi E.S., Kim J. Adhesion properties of poly (ethylene oxide)-lignin blend for nanocellulose composites. Compos B Eng. 2019;156:43–50. [Google Scholar]
- 40.Benites R.S., Formagio A.S., Argandoña E.J., Volobuff C.R., Trevizan L.N., Vieira M.C., et al. Contents of constituents and antioxidant activity of seed and pulp extracts of Annona coriacea and Annona sylvatica. Braz J Biol. 2015;75:685–691. doi: 10.1590/1519-6984.21313. [DOI] [PubMed] [Google Scholar]
- 41.Meyer A.S., Heinonen M., Frankel E.N. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem. 1998;61:71–75. [Google Scholar]
- 42.Teotia R.S., Dahe G.J., Bellare J. In-situ coating of 2-methacryloyloxyethyl phosphorylcholine polymer on polysulfone hollow fiber membranes for hemodialysis. Adv Sci Lett. 2014;20:1191–1197. [Google Scholar]
- 43.Gao J., Guo H., Zhao L., Zhao X., Wang L. Water-stability and biological behavior of electrospun collagen/PEO fibers by environmental friendly crosslinking. Fibers Polym. 2017;18:1496–1503. [Google Scholar]
- 44.Yuan W., Feng Y., Wang H., Yang D., An B., Zhang W., et al. Hemocompatible surface of electrospun nanofibrous scaffolds by ATRP modification. Mater Sci Eng CMater Biol Appl. 2013;33:3644–3651. doi: 10.1016/j.msec.2013.04.048. [DOI] [PubMed] [Google Scholar]