Abstract
Background
Human epidermal growth factor receptor 2 (HER2) overexpressing malignancies, including breast and gastro-esophageal, are associated with a poor prognosis. The cardiotoxicity of trastuzumab, a HER2-targeting monoclonal antibody, is well established. However, the cardiotoxic effect of pertuzumab, another HER2-directed therapy, is less well documented. The objective of this systematic review and meta-analysis was to determine the risk of cardiac events in patients with HER2-positive cancer who are receiving pertuzumab.
Methods
We performed a systematic review of phase 2 and 3 randomized controlled trials in which the addition of pertuzumab to other standard therapies in patients with stage I-IV HER2-positive cancer was evaluated, and cardiac adverse effects reported. We searched MEDLINE (1946-2020), Embase (1974-2020), and CENTRAL. Two independent reviewers assessed the risk of bias and extracted the data. Risk ratios (RRs) with 95% confidence intervals (CIs) were calculated from the pooled data using the inverse variance method and random-effects models.
Results
Eight randomized controlled trials (8420 patients) were included: 1 was gastro-esophageal; 7 were breast cancer trials. Participants’ median age ranged from 49 to 61.5 years. All participants received trastuzumab and chemotherapy in addition to pertuzumab or placebo. Compared with placebo, pertuzumab increased the risk of clinical heart failure (HF; RR [95% CI]: 1.97 [1.05-3.70]; I2 = 0%). However, pertuzumab had no demonstrable effect on asymptomatic/minimally symptomatic left ventricular systolic dysfunction (RR [95% CI]: 1.19 [0.89-1.61]; I2 = 19%).
Conclusions
Pertuzumab increases the risk of clinical HF, but not asymptomatic/minimally symptomatic left ventricular systolic dysfunction, in HER2-positive cancer patients. Further research into the mechanisms underlying pertuzumab-related HF is needed to understand its clinical spectrum of cardiotoxicity.
Résumé
Introduction
Les tumeurs malignes qui surexpriment le récepteur 2 du facteur de croissance épidermique humain (HER2, de l’anglais Human epidermal growth factor receptor 2), notamment le cancer du sein et le cancer de la jonction gastro-œsophagienne, sont associées à un mauvais pronostic. La cardiotoxicité du trastuzumab, un anticorps monoclonal qui vise le HER2, est bien établie. Toutefois, les effets cardiotoxiques du pertuzumab, un autre traitement qui vise le HER2, sont moins bien démontrés. L’objectif de cette revue systématique et de cette méta-analyse était de déterminer le risque d’événements cardiaques chez les patients atteints d’un cancer HER2 positif qui prennent du pertuzumab.
Méthodes
Nous avons réalisé une revue systématique d’essais comparatifs à répartition aléatoire de phase 2 et de phase 3 lors desquels nous avons évalué l’ajout du pertuzumab à d’autres traitements standards chez les patients atteints d’un cancer HER2 positif de stades I-IV, et signalé les effets indésirables sur le cœur. Nous avons fait des recherches dans MEDLINE (1946-2020), Embase (1974-2020) et CENTRAL. Deux examinateurs indépendants ont évalué le risque de biais et extrait les données. Les données groupées ont permis de calculer les intervalles de confiance (IC) à 95 % des risques relatifs (RR) au moyen de la méthode de la variance inverse et des modèles à effets aléatoires.
Résultats
Nous avons inclus huit essais contrôlés randomisés (8420 patients), soit un qui portait sur le cancer de la jonction gastro-œsophagienne, et sept sur le cancer du sein. L’âge médian des participants se situait entre 49 à 61,5 ans. Tous les participants ont pris le trastuzumab et ont suivi une chimiothérapie en plus de la prise du pertuzumab ou du placebo. Comparativement au placebo, le pertuzumab a fait augmenter le risque de manifestations cliniques de l’insuffisance cardiaque (IC) (RR [IC à 95 %] : 1,97 [1,05-3,70]; I2 = 0 %). Toutefois, le pertuzumab n’a démontré aucun effet sur la dysfonction systolique du ventricule gauche asymptomatique/minimalement symptomatique (RR [IC à 95 %] : 1,19 [0,89-1,61]; I2 = 19 %).
Conclusions
Le pertuzumab fait augmenter le risque de manifestations cliniques de l’IC, mais pais la dysfonction systolique du ventricule gauche asymptomatique/minimalement symptomatique, chez les patients atteints d’un cancer HER2 positif. Des recherches plus approfondies sur les mécanismes sous-jacents à l’IC liée au pertuzumab sont nécessaires pour comprendre son spectre de manifestations cliniques de cardiotoxicité.
Human epidermal growth factor receptor 2 (HER2) is overexpressed in 15%-20% of breast cancers,1 and its presence is associated with poor prognosis.2 A variety of other cancers may also express HER2, including ovarian, cervical, endometrial, salivary gland, gastroesophageal junction, gastric, biliary, pancreatic, colorectal, bladder, head and neck, and non–small cell lung cancers.3, 4, 5, 6 Targeting the HER2 receptor has been associated with improved disease-free survival and overall survival, with 7 anti-HER2 agents currently approved (trastuzumab, pertuzumab, T-DM1, lapatinib, neratinib, fam-trastuzumab deruxtecan-nxki, and tucatinib) for clinical use.7, 8, 9
The first HER2 antibody, trastuzumab, has been in clinical use since 1998, and it is approved for the treatment of HER 2-positive breast, gastric, and gastroesophageal cancer.7 This drug has dramatically changed the natural history of these malignancies.10,11 However, its main adverse effect is cardiac dysfunction, which develops in 8% who are treated with trastuzumab alone, and 29% of those treated with trastuzumab in combination with anthracycline.12,13 The incidence of cardiotoxicity in clinical practice is known to exceed that in clinical trials, especially in older populations and patients with cardiovascular risk factors.14, 15, 16 Therefore, cardiotoxicity is the major dose-limiting toxicity of trastuzumab, potentially leading to its premature and unwanted discontinuation despite its anticancer benefits.
Pertuzumab is a recombinant humanized monoclonal antibody that targets HER2, preventing the dimerization of HER2 with HER3, thus blocking oncogenic signaling.17 It has demonstrated promising efficacy in both metastatic and early breast cancer and is an integral part of HER2-positive breast cancer therapy. The synergistic activity of pertuzumab with trastuzumab has led to the approval of the combination of these 2 drugs with taxane-based chemotherapy (including either paclitaxel or docetaxel) for the first-line treatment of patients with HER2-positive metastatic breast cancer18,19and for the treatment of patients with early-stage HER2-positive breast cancer who are at high risk for recurrence,20,21 for whom it improves invasive disease-free survival22 and pathologic complete response.23
Pertuzumab generally has been considered to be less cardiotoxic than trastuzumab. In an analysis of pooled data from 14 phase II studies (n = 598), which in many cases were nonrandomized, asymptomatic left ventricular systolic dysfunction(LVSD, defined as ≥ 10% reduction in left ventricular ejection fraction [LVEF] to a value of < 50%) occurred in 6.9% of patients receiving pertuzumab as monotherapy and in 6.5% of patients receiving the combination of pertuzumab and trastuzumab. In this study, the incidence rates of symptomatic heart failure (HF) were 0.3% and 1.1%, respectively.24 Since this study, more data on pertuzumab, from randomized, placebo-controlled studies, have been published. However, to our knowledge, these data have not been systematically summarized. We therefore undertook a systematic review, with the objective of evaluating the risk of HF or left ventricular dysfunction associated with pertuzumab use as compared with cancer treatment strategies that did not include pertuzumab.
Methods
This systematic review and meta-analysis were performed in accordance with MECIR (Methodological Expectations for Cochrane Intervention Reviews) standards for conducting and reporting intervention reviews,25 and in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.26
Search strategy
We searched the following electronic databases: Ovid MEDLINE (1946 to week 10 of 2020); Ovid Embase (1974 to week 10 of 2020); and the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 4 of 12, accessed February 2020).27 No restrictions based on language or date of publication were used. The MEDLINE and Embase search strategies are available in Supplemental Table S1.
Other sources
We conducted a search of clinical trial registries, including clinicaltrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform. We also searched the electronic abstract databases of the major international congresses’ proceedings (the American Society of Clinical Oncology annual meeting and the San Antonio Breast Cancer Symposium). The lists of references of relevant articles and reviews were scanned.
Study selection
Two reviewers (M.M.A. and A.M.) independently screened the titles, abstracts, and descriptors of identified studies for possible inclusion.
Criteria for considering studies for this review
Types of studies
Eligible papers were abstracts or full-length manuscripts describing phase II or III randomized controlled trials that reported on the addition of pertuzumab to other therapies and reported on the cardiotoxicity of pertuzumab. We excluded nonrandomized trials, phase I randomized trials, and observational studies. When more than one publication was identified from the same clinical trial, we used the most recent or complete report of that trial.
Types of participants
This review included adult patients (age > 18 years) with stage I-IV HER2-positive cancers of any tumour type, in any treatment settings: neoadjuvant, adjuvant, and metastatic.
Types of interventions and comparisons
To assess the cardiotoxic effect of pertuzumab, we considered randomized controlled trials in which use of pertuzumab was the only systematic difference between the 2 arms of the studies.
Types of outcome measures
Each of the following binary outcomes was considered to indicate pertuzumab cardiotoxicity:
-
1.
Asymptomatic/minimally symptomatic LVSD, as defined by a decrease of LVEF to < 50%, or (depending on the threshold implemented in individual studies) by a decrease of LVEF of > 10%-15% from baseline without HF symptoms or in the presence of, at most, mild HF symptoms. The absence of severe HF symptoms was indicated by New York Heart Association (NYHA) class I or II HF symptoms, or LVSD grade 1 or 2 per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 3.0 or 4.0, depending on which version was used in the respective studies). Most of the included trials implemented CTCAE version 3.0, in which grade 1 and 2 LVSD are defined as LVSD that is asymptomatic.28,29
-
2.
HF was considered to be present if participants developed CTCAE grade ≥ 3 LVSD or left ventricular diastolic dysfunction according to CTCAE version 3.0 or 4.0, depending on which version was used in the respective studies,28,29 or New York Heart Association (NYHA) class III/IV symptoms attributed to HF. According to CTCAE version 3.0, congestive HF is present if the patient develops symptomatic LVSD or symptomatic left ventricular diastolic dysfunction.
-
3.
Cardiac death.
-
4.
If adequately reported, we documented the incidence of the following cardiovascular adverse events, to detect any harmful signal that was not previously reported owing to limited data: cardiac ischemia, arrhythmias, QT prolongation, and hypertension.
Data collection and analysis
Data extraction and management
Two authors (M.M.A. and A.M.) independently reviewed the full texts of the potentially related articles and extracted the data from eligible articles. Any disagreement was resolved by discussion and consensus. Data were extracted into a standardized Microsoft Excel spreadsheet.
Assessment of risk of bias in the included studies
For each outcome, dual (M.M.A. and A.M.) independent assessment of risk of bias of the included studies was conducted using the Cochrane Collaboration risk of bias tool (Cochrane’s Handbook, version 5.1.0).30 Disagreements were resolved by discussion. The assessed domains included the sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective outcome reporting. Risk of bias was rated as high/low/unclear (Supplemental Table S2). As this review is concerned with side effects (cardiotoxicity), special interest was given to attrition bias (incomplete outcome data), which was estimated according to the following factors: (i) whether the outcome of interest (cardiotoxicity) was measured in all trial participants; (ii) whether the proportion of missing outcome data was low (< 5%) or high (> 20%); (iii) the difference between the proportions of missing outcome data in the pertuzumab vs placebo arms; and (iv) the reasons the outcome data are missing.
Measures of treatment effect and data synthesis
The outcomes of interest were dichotomous variables reported as number of events in each trial arm. A summary statistic (risk ratio) was calculated, along with 95% confidence intervals (CIs). A P value of ≤ 0.05 was considered statistically significant for the comparison between the groups. The data were pooled in a meta-analysis for each reported outcome if there were 2 or more studies reporting this outcome. For an outcome that could not be pooled in the meta-analysis, it was represented in the narrative results. Absolute effects were calculated using the baseline risk from the placebo arm, where . We then estimated the risk in the pertuzumab group by multiplying the pooled relative risk for pertuzumab by the baseline risk. The absolute risk difference = pertuzumab risk – baseline risk. These estimates were presented with their 95% CIs.
Analysis and pooling of the results were done according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions. All statistical analyses were performed with Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK). We pooled the results of trials using the inverse variance method and the random-effects model. Heterogeneity between studies was evaluated by visual inspection of forest plots, the χ2 with statistical significance set at P < 0.10, and the I2 statistic.31
Subgroup and sensitivity analysis
We prespecified subgroup analyses to compare the risk of cardiotoxicity of pertuzumab in different treatment settings (early vs metastatic cancer), and in different cancers. Three sensitivity analyses were performed. The first was performed using the Peto statistical method, which is proposed to be less biased for rare events. The second and third were to explore the impact of removing studies with T-DM1 or studies that had 2 or more domains of high risk of bias.
Quality of evidence
We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system approach to assess the quality of evidence related to each of the key outcomes (asymptomatic/minimally symptomatic LVSD; HF).30 A ‘’summary of findings’’ table for our main comparisons, including the GRADE level of certainty of evidence, was created using GRADEpro software (McMaster University and Evidence Prime, 2021; gradepro.org).
Results
Search results
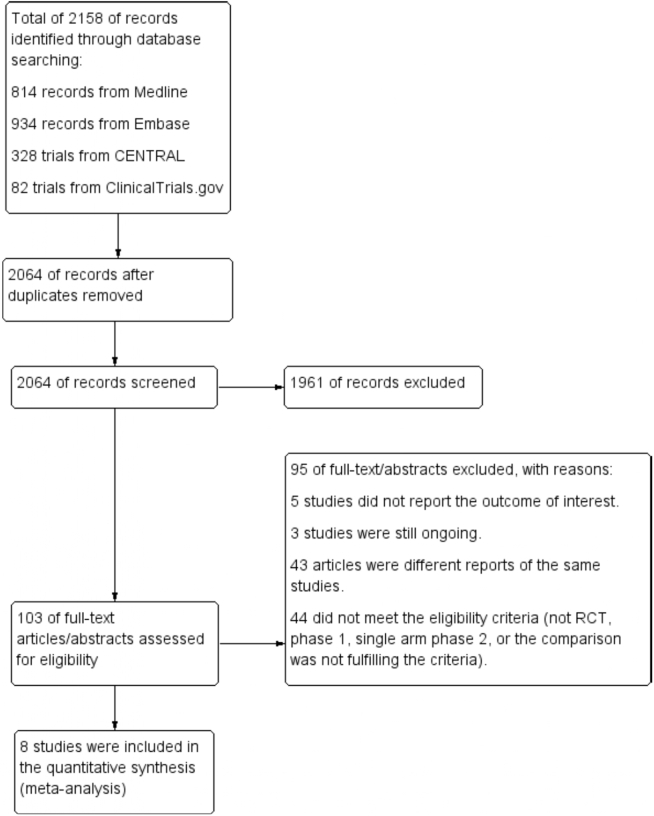
The last search was performed in March 2020. The search yielded 2158 records, of which 2064 were screened after removing duplicates. After screening, we retrieved the full text of 103 potential articles. We excluded 95 articles, for reasons outlined in Figure 1. Ultimately, 8 studies were included in the meta-analysis,22,23,32, 33, 34, 35, 36, 37, 38, 39 There was a high agreement between reviewers (kappa was 0.89).
Figure 1.
Flowchart of the study selection process. RCT, randomized controlled trial.
Brief description of study characteristics
The characteristics of the 8 studies included in the meta-analysis are displayed in Table 1, and a brief description of the excluded studies is shown in Supplemental Table S3. All studies included adult patients (median age range: 49-61.5 years) with HER2-positive malignancy (7 studies included patients with breast cancer, and one study included patients with gastric and gastro-esophageal cancer). The duration of pertuzumab treatment was not consistently reported (5 of 8 studies (63%) reported the median duration, one reported the mean, and 2 did not report on duration). Four studies were in the metastatic setting. Among these, the median (range) number of pertuzumab cycles reported in 2 studies were: 15 (1-68) and 24 (1-96)37,38; one study reported a mean (standard deviation) number of pertuzumab cycles of 13.1 (10.7),35 and one study did not report the pertuzumab duration.33 The median (range) number of pertuzumab cycles was 4 (1-4) in both studies in the neoadjuvant setting,22 and those in the neoadjuvant/adjuvant setting.36 In the study in which patients from all treatment settings (neoadjuvant, adjuvant, and metastatic) were eligible,34 the median (range) number of pertuzumab cycles was 18 (1-65).
Table 1.
Characteristics of studies included in the meta-analysis
| Study/first author (year) |
Study and participant characteristics | Setting | LVEF eligibility | Pertuzumab regimen | Pertuzumab cycles: median (range) |
Concomitant chemotherapy /anthracycline | Cardiac follow-up period | LVEF assessment | HF assessment |
|---|---|---|---|---|---|---|---|---|---|
| NeoSphere/ Gianni 201622 |
Phase: 2 N = 322 Median age: 50 y Cancer type: breast, locally advanced, inflammatory, or early-stage |
Neo-adjuvant | > 55% | 840-mg loading dose, followed by 420 mg every 3 wk | 4 (1–4) | Docetaxel 96.3% in placebo, 95.3% in pertuzumab group received FEC (includes epirubicin) |
2 y | LVEF by ECHO or MUGA every 2 cycles (neoadjuvant), every 2–3 cycles (adjuvant), and then every 6 mo for 2 y | NCI CTCAE V3 LVSD or congestive HF (grade 3 or worse) |
| PHEREXA/ Urruticoechea 201733 |
Phase: 3 N = 446 Median age: 54.5 y Cancer type: breast |
Metastatic | > 50% | 840-mg loading dose in cycle 1, followed by 420 mg maintenance doses once every 3 wk | NR | Capecitabine No information about previous anthracyclines |
< 2 y | NR | NCI CTCAE V3 Symptomatic LVSD (3 patients were classified as NYHA class II, 2 as class III or IV) |
| PERTAIN/ Rimawi 201834 |
Phase: 2 N = 251 Median age: 60 y Cancer type: breast, locally advanced, or metastatic |
Neo-adjuvant, adjuvant, and metastatic | > 50% | Loading dose of 840 mg followed by 420 mg 3 wk | 18 (1–65) | Docetaxel/ paclitaxel anthracycline 27.9% of control and 41.1% of pertuzumab arm |
28 d after the last dose of study drug | NR | NCI CTCAE V4 Grade ≥ 3 adverse events LVSD: One patient with NYHA class I; 2 patients with NYHA class II |
| JACOB/ Tabernero 201835 |
Phase: 3 N = 773 Median age: 61.5 y Cancer type: gastric or gastro-esophageal junction |
Metastatic | > 55% | 840 mg of pertuzumab every 3 wk | Mean (SD): 13.1 (10.7) | Cisplatin, 5-FU, or capecitabine No anthracycline data |
NR | LVEF by ECHO, MUGA, or cardiac MRI at baseline, every 9 wk during chemotherapy treatment and every 12 wk during anti-HER2 treatment | Symptomatic LVSD (LVEF drop plus at least one symptom of probable cardiac failure) |
| PEONY/ Shao 202036 |
Phase: 3 N = 328 Median age: 49 y Cancer type: breast, early or locally advanced |
Neo-adjuvant and adjuvant | > 50% | 840-mg loading dose and 420-mg maintenance | 4 (1–4) | Docetaxel (75 mg/m2). After surgery, patients received 3 cycles of intravenous fluorouracil, epirubicin, and cyclophosphamide | Until disease progression or recurrence or until 5 y after randomization of the last patient, whichever occurr first | LVEF assessed by ECHO (preferred) or MUGA scan | HF (NYHA functional classification III or IV) |
| APHINITY/ Von Minckwitz 201723; Piccart 2019 32 |
Phase: 3 N = 4769 Median age: 51.5 y Cancer type: breast, early-stage |
Adjuvant | > 55% | 840-mg loading dose i.v, followed by 420-mg i.v every 3 wk | NR (84.5% completed 1 year) | 5-fluorouracil, epirubicin or doxorubicin, cyclophosphamide, docetaxel, or paclitaxel; or carboplatin | Median of 74.1 mo | LVEF every 3 mo during treatment, every 6 mo up to mo 36, and yearly thereafter | NYHA class III or IV HF and substantial decrease in LVEF |
| MARIANNE/ Perez 201937 |
Phase: 2 N = 727 Median age: 52 y Cancer type: breast |
Locally advanced (progressive, recurrent) or metastatic | > 50% | 840-mg i.v. on day 1 of cycle 1, then 420-mg i.v. on day 1 of each subsequent 3-wk cycle | 15 (1–68) | None Prior anthracycline in 44.1% of control and 46.3% of pertuzumab arms |
28 d after the last dose of study drug | ECHO (preferred method) or MUGA: at baseline, once on days 15 to 21 of cycle 1, cycle 3, and every third cycle thereafter | NR |
| CLEOPATRA/ Swain 2015; 202038,39 |
Phase: 3 N = 804 Median age: 54 y Cancer type: breast; locally recurrent, unresectable, or metastatic |
Metastatic | > 50% | 840-mg loading dose in cycle 1, followed by 420-mg maintenance doses once every 3 wk | 24 (1–96) | Docetaxel, anthracyclines (40.4% control, 37.3% pertuzumab) | 3 y (however, we used data from their paper reported up to a median of 8 y follow-up) | LVEF at (baseline, every 9 wk during treatment, the time of discontinuation of treatment, every 6 mo in the first y after discontinuation, and annually thereafter for up to 3 y | NCI CTCAE V3 |
APHINITY, Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer; CLEOPATRA, Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer; ECHO, echocardiograph; FEC, fluorouracil, epirubicin, and cyclophosphamide; HER2, human epidermal growth factor receptor 2; HF, heart failure; JACOB, Pertuzumab Plus Trastuzumab and Chemotherapy for HER2-Positive Metastatic Gastric or Gastro-Oesophageal Junction Cancer; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; MARIANNE, Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab With Taxane for Human Epidermal Growth Factor Receptor 2–Positive Advanced Breast Cancer; MRI, magnetic resonance imaging; MUGA, multi-gated acquisition scan; NCI CTCAE V3, National Cancer Institute Common Terminology Criteria for Adverse Events Version 3; NeoSphere, Neoadjuvant Pertuzumab and Trastuzumab in Patients With Locally Advanced, Inflammatory, or Early-Stage HER2-Positive Breast Cancer; NR, not reported; NYHA, New York Heart Association; PEONY, Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia; PERTAIN, First-Line Trastuzumab Plus an Aromatase Inhibitor, With or Without Pertuzumab, in Human Epidermal Growth Factor Receptor 2–Positive and Hormone Receptor–Positive Metastatic or Locally Advanced Breast Cancer; PHEREXA, Randomized Phase III Trial of Trastuzumab Plus Capecitabine With or Without Pertuzumab in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer Who Experienced Disease Progression During or After Trastuzumab-Based Therapy; SD, standard deviation.
In 5 studies (63%), a baseline LVEF < 50% was an exclusion criterion,25,26,28, 29, 30 whereas in the other 3 studies, an LVEF < 55% was an exclusion criterion.22,23,35 All studies excluded patients with history or evidence of poorly controlled hypertension.
In 7 of the 8 studies (88%), the addition of pertuzumab to trastuzumab and chemotherapy was compared with placebo, trastuzumab, and chemotherapy.22,23,33, 34, 35, 36,38 In one study, the combination of pertuzumab and T-DM1 was compared with placebo and T-DM1 alone.37
Six of the 8 studies reported the use of concomitant or previous anthracyclines (NeoSphere [Neoadjuvant Pertuzumab and Trastuzumab in Patients With Locally Advanced, Inflammatory, or Early-Stage HER2-Positive Breast Cancer], APHINITY [Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer], MARIANNE [Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab With Taxane for Human Epidermal Growth Factor Receptor 2–Positive Advanced Breast Cancer], PERTAIN [First-Line Trastuzumab Plus an Aromatase Inhibitor, With or Without Pertuzumab, in Human Epidermal Growth Factor Receptor 2–Positive and Hormone Receptor–Positive Metastatic or Locally Advanced Breast Cancer], CLEOPATRA [Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer], and PEONY [Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia]),22,23,34,36, 37, 38 as a part of adjuvant therapy (3 studies)22,23,36 or in prior exposure in the case of metastatic disease (3 studies).34,37,38 Only one trial (APHINITY), which was the largest, reported adverse events data stratified according to anthracycline use. In this trial, 13 of 15 patients (87%) who had HF in the pertuzumab arm, and 5 of 6 patients (83%) in the placebo arm received anthracyclines. Four trials reported the proportion of participants exposed to anthracycline in each arm. The respective percentages of participants prescribed anthracycline in the pertuzumab vs the placebo arm were 96.3% vs 95.3% in NeoSphere, 46.3% vs 44.1% in MARIANNE, 41.1% vs 27.9% in PERTAIN, and 37.3% vs 40.4% in CLEOPATRA. One study in the metastatic setting (PHEREXA [Randomized Phase III Trial of Trastuzumab Plus Capecitabine With or Without Pertuzumab in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Breast Cancer Who Experienced Disease Progression During or After Trastuzumab-Based Therapy]) reported that all 5 patients who developed HF were exposed to anthracyclines, but it did not mention whether the placebo group was exposed to anthracycline.33 The last study in the metastatic setting (JACOB [Pertuzumab Plus Trastuzumab and Chemotherapy for HER2-Positive Metastatic Gastric or Gastro-Oesophageal Junction Cancer]) did not explicitly report whether participants had anthracycline exposure.35
In all 8 studies (8420 participants), asymptomatic/minimally symptomatic LVSD events were reported. In 7 of 8 studies (7693 participants), HF events were reported. Most studies did not report other outcomes (ie, cardiac death or other cardiovascular adverse effects); however, when given, we have summarized these data.
Risk of bias in the included studies
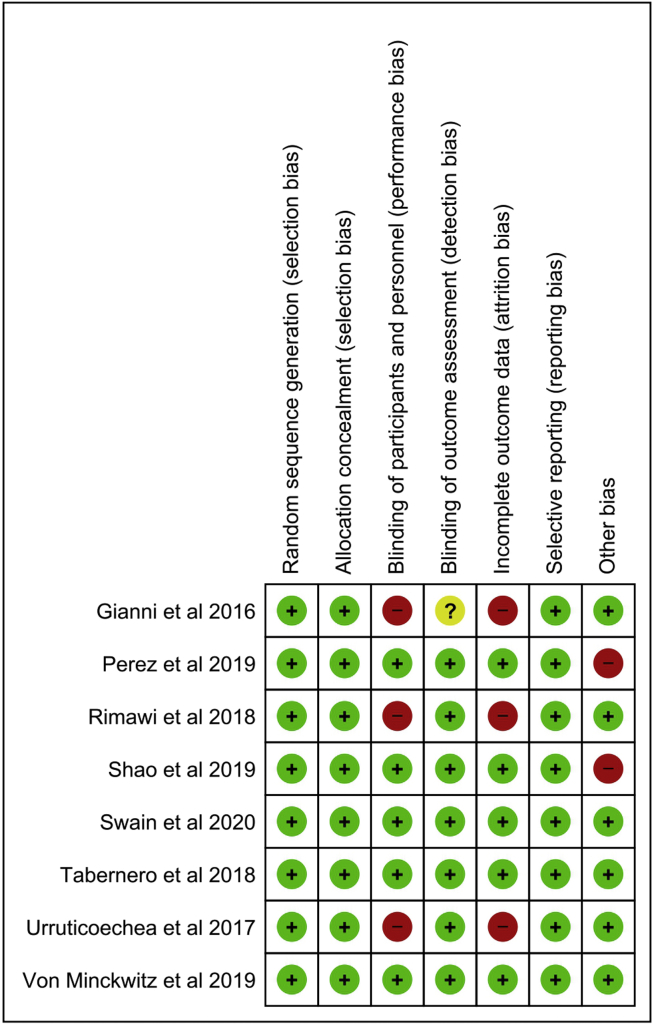
Our assessment of the risk of bias in the individual included studies is shown in Figure 2 (and in Supplemental Table S2; see also Supplemental Figure S1). The domains judged to have low risk of bias in all 8 studies were random-sequence generation, allocation concealment, and selective outcome reporting. The domains judged to have a high risk of bias were blinding of the participants and the personnel (in 3 studies), attrition bias (in 3 studies), and “other bias” related to industry-funded trials (in 2 studies). For the blinding of outcome assessors, 7 studies had low risk of bias, and in 1 study the risk was unclear.
Figure 2.
Assessment of risk of bias in the individual studies. Green (+) symbol = low risk of bias; yellow (?) symbol = unknown; red (–) symbol = high risk of bias.
Effects of pertuzumab
Effect of pertuzumab on asymptomatic/minimally symptomatic LVSD
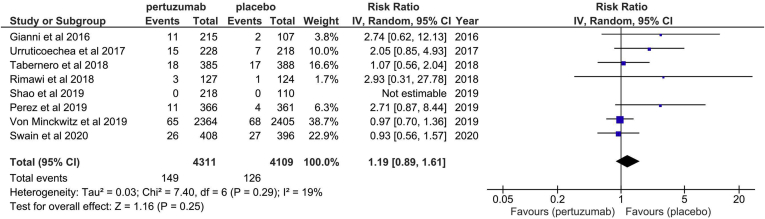
The definition of LVEF reduction was generally consistent among studies (LVEF decline ≥ 10% from baseline and/or below 50%), except for one study37 that defined it as a drop of > 15% from baseline (Table 1). The pooled incidence of asymptomatic/minimally symptomatic LVSD was 3.5% in the pertuzumab group, compared to 3.1% in the placebo group. Pooling the data from 8 studies (8420 participants) showed that the risk of asymptomatic/minimally symptomatic LVSD among patients prescribed pertuzumab was no higher than the effect of placebo (risk ratio [RR]: 1.19; 95% CI: 0.89-1.61; Fig. 3), an absolute risk increase of 6 more patients per 1000 (95% CI, from 3 less, to 19 more patients).
Figure 3.
Forest plot for the effect of pertuzumab on asymptomatic/minimally symptomatic systolic left ventricular dysfunction. CI, confidence interval.
Overall, according to the GRADE system, the quality of the evidence was moderate (Table 2; Supplemental Table S4). Among the studies included, there was only a modest level of heterogeneity (I2 = 19%; P = 0.25).
Table 2.
Summary of findings: pertuzumab compared to control for human epidermal growth factor receptor 2 (HER 2)–positive cancer
| Outcomes | Anticipated absolute effects∗ (95% CI) |
Relative effect (95% CI) |
No of participants (studies) |
Certainty of the evidence (GRADE) |
|
|---|---|---|---|---|---|
| Risk with control | Risk with pertuzumab | ||||
| Asymptomatic/minimally symptomatic LVSD assessed with: Echo, MUGA, or CMR follow-up: range 28 d to 8 y |
31 per 1000 |
37 per 1000 (28 to 50) |
RR 1.19 (0.89 to 1.61) |
8420 (8 RCTs) |
⊕⊕⊕○ MODERATE† |
| Heart failure assessed with clinical follow-up—range: 28 d to 8 y |
4 per 1000 |
8 per 1000 (4 to 14) |
RR 1.97 (1.05 to 3.70) |
7693 (7 RCTs) |
⊕⊕⊕○ MODERATE† |
GRADE Working Group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of the effect. Circle symbols represent the rating for GRADE elements including risk of bias, inconsistency, indirectness, imprecision, publication bias, or other upgrading factors. ⊕: rated not serious; ◯: rated serious (-1) or very serious (-2).
CI, confidence interval; Echo, echocardiography; CMR, cardiac magnetic resonance; GRADE, Grades of Recommendation, Assessment, Development and Evaluation; LVSD, left ventricular systolic dysfunction; MUGA, multi-gated acquisition scan; RCT, randomized controlled trial; RR, risk ratio.
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Imprecise results as the CIs include both no effect and appreciable harm.
Effect of pertuzumab on HF
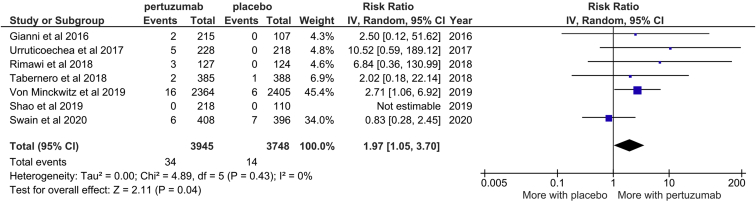
One study did not report the incidence of HF. Pooling the data from the 7 remaining studies (7693 participants) revealed that patients using pertuzumab were at significantly higher risk of HF than patients in the placebo arm (RR 1.97; 95% CI: 1.05-3.70; Fig. 4). This difference represents an absolute risk increase of 4 more patients per 1000 (95% CI: 0 to 10 more per 1000 patients) developing HF while taking pertuzumab, as compared with placebo (the absolute risks in the pertuzumab and placebo groups were 8 per 1000, and 4 per 1000, respectively). Of the 34 HF events across the pooled pertuzumab groups, there was previous/concurrent anthracycline exposure in 13 cases (38%), no anthracycline exposure in 2 cases, and no report regarding anthracycline exposure in the remaining 19 cases. Of the 14 HF events in the placebo group, there was previous/concurrent anthracycline exposure in 4 cases (29%), no anthracycline exposure in 1 case, and no report regarding anthracycline exposure in the remaining 9 cases.
Figure 4.
Forest plot for effect of pertuzumab on heart failure. CI, confidence interval.
According to the GRADE system, the evidence quality was moderate (Table 2; Supplemental Table S5). There was no heterogeneity among the included studies (I2 = 0; P = 0.43).
Of participants who developed HF, recovery was noted in 1 of 2 cases in the pertuzumab arm in NeoSphere (with no HF noted in the placebo arm), 7 of the 15 cases (47%) reported in the pertuzumab arm of APHINITY at the time of trial closure (as compared with 4 of 6 cases in the placebo arm), and 4 (80%) of 5 cases in the pertuzumab arm of the trial by Urruticoechea et al.33 (with no HF noted in the placebo arm). In the trial by Swain et al.,38 reductions in LVEF of ≥ 10% from baseline to an absolute value < 50% occurred in 24 of 394 individuals (6.1%) in the pertuzumab arm and 28 of 378 participants (7.4%) in the control group. These declines reversed in 21 of the 24 affected participants (87.5%) in the pertuzumab group and 22 of the 28 affected individuals (78.6%) in the control group.
Other cardiovascular effects of pertuzumab
Only one study reported cardiac death: there were 2 deaths (1%) in each study arm.23 One study reported incidence rates of hypertension of 5.5% and 4.7% in the pertuzumab and placebo arm, respectively.37 In a substudy of 37 patients in one trial, the effects of pertuzumab on the corrected QT interval and other echocardiographic parameters were evaluated. No clinically relevant changes relative to placebo were identified.40
Cardiotoxicity of pertuzumab in different subgroups
Comparing patients according to treatment settings
There were 4 trials in the metastatic setting, and 3 trials in the adjuvant and/or neoadjuvant setting; one trial included patients from all treatment settings (Supplemental Fig. S2). There was insufficient evidence to suggest differences in the risk of asymptomatic/minimally symptomatic LVSD between those with metastatic vs non-metastatic disease (I2 = 0 for heterogeneity). The respective RRs (95% CI) were 1.30 (0.85-2.03), and 1.25 (0.52-2.99). Although the RR for HF was higher in the non-metastatic group (RR [95% CI]: 2.69 [1.10, 6.59]), as compared with the metastatic group (RR [95% CI]: 1.57 [0.43, 5.77]), there was insufficient evidence to confirm heterogeneity of effect between the subgroups (I2 = 0;Supplemental Fig. S3).
Comparing patients according to type of cancer
The first subgroup included breast cancer (7 studies), and the second included gastric and gastro-esophageal cancers (one study) (Supplemental Figs. S4 and S5). Between the 2 subgroups, there was no evidence of heterogeneity of the effect of pertuzumab for either outcome: asymptomatic/minimally symptomatic LVSD, and HF. The respective I2 values were 0 (P = 0.61 and P = 0.98). For asymptomatic/minimally symptomatic LVSD among breast and gastric/gastro-esophageal cancers, the respective RRs (95% CI) were: 1.29 (0.88-1.90) and 1.07 (0.56-2.04). For HF, the respective RRs (95% CI) in breast and gastric/gastro-esophageal cancer, respectively, were 2.07 (0.93-4.61) and 2.02 (0.18-22.14).
Sensitivity analyses
The Peto analysis (Supplemental Figs. S6 and S7) showed results similar to those of the primary analysis. The respective odds ratios (95% CI) for the asymptomatic/minimally symptomatic LVSD and HF were 1.15 (0.90-1.47) and 2.25 (1.27-3.97).
A sensitivity analysis was performed to explore the impact of excluding the studies that had at least 2 domains with high risk of bias (3 studies) from the analysis. In this sensitivity analysis, pertuzumab did not result in significant risk of asymptomatic/minimally symptomatic LVSD (RR [95% CI] 1.03 (0.80-1.33) of HF (RR [95% CI] 1.63 [0.71-3.73]). In a second sensitivity analysis, we excluded the single study in which TDM-1 was used. Again, pertuzumab had no significant increased risk of asymptomatic/minimally symptomatic LVSD, with a RR (95% CI) of 1.08 (0.84-1.37); this study did not report HF outcomes.
Discussion
In this systematic review and meta-analysis, pertuzumab was associated with an approximately 2-fold increased risk of HF, with no detectable effect on asymptomatic/minimally symptomatic LVSD. These findings are in contrast with those of previous reports that suggest no major risk of cardiotoxicity when pertuzumab is used alone or in combination with other anti-HER2 agents.24,41, 42, 43
In a meta-analysis of cardiotoxicity incidence using data pooled from 598 patients from 14 different studies published in 2012, Lenihan et al. found that patients treated with pertuzumab had a relatively low incidence of asymptomatic left ventricular dysfunction, or symptomatic HF, when pertuzumab was given alone or in combination with either chemotherapy or trastuzumab.24 In this analysis, a decrease in LVEF of ≥ 10% to < 50% occurred in 6.5% of pertuzumab recipients, and HF occurred in 1.1%. In Lenihan’s paper, no data on cardiovascular outcomes from a control group were reported, so the relative risk of adverse cardiovascular outcomes among pertuzumab recipients was not identified.
Since the publication of Lenihan’s analysis, only a few more papers on the efficacy and safety of pertuzumab have been published. In 2013, Valachis et al.41 published a systematic review in which they identified randomized trials of trastuzumab, pertuzumab, and lapatinib up to July 2012. Their study was limited to trials comparing anti-HER2 monotherapy with anti-HER2 combination therapy. They reported no significant difference between these treatment groups with respect to the outcomes of HF (although the numbers of outcome events were low) or asymptomatic/minimally symptomatic LVSD. The respective incidence rates of congestive HF in the groups that received the anti-HER2 therapy in combination and as monotherapy were 0.88% (95% CI: 0.47%-1.64%) and 1.49% (95% CI: 0.98%-2.23%); the respective rates of asymptomatic/minimally symptomatic LVSD were 3.1% (95% CI: 2.2%-4.4%) and 2.9% (95% CI: 2.1%-4.1%).41 Our study represents an important update to, and extension of, the systematic review performed by Valachis et al. With more data and broader study eligibility criteria, we found, in contrast to previous research, that the risk of HF is increased with pertuzumab use.
We found a surprising discordance between the effect of pertuzumab on asymptomatic/minimally symptomatic LVSD and its effect on HF risk. One possible explanation is that pertuzumab may lead to predominantly diastolic, rather than systolic, left ventricular dysfunction, hence resulting in HF with preserved ejection fraction. In one observational study, diastolic dysfunction developed frequently after the initiation of trastuzumab.44 However, the biologic mechanisms underlying this observation are unknown, and no direct evidence was found in the included papers to confirm this hypothesis. In the largest trial included in our meta-analysis (APHINITY), although there was an excess of moderate–severe HF with impaired systolic left ventricular function in the pertuzumab group, there was no difference in the rate of asymptomatic/minimally symptomatic LVSD between the pertuzumab and placebo groups. This unexpected finding warrants further research to understand how pertuzumab leads to an excess of HF but with no excess of asymptomatic/minimally symptomatic HF with reduced LVEF. Furthermore, this finding highlights the importance of clinical outcomes over surrogate outcomes because surrogate outcomes may not always correlate with clinical events, and clinical events are ultimately what most affects patients’ well-being.
The definition of HF varied between the studies—some have included only patients with NYHA class III-IV, whereas others have also included NYHA class I-II, and some did not explicitly mention NYHA class, but used grade 3 or more HF according to the National Cancer Institute (CTCAE). Consequently, this variability may have led to an underestimate of the effect of HF, since not all degrees of NYHA class were included. The other concern is that patients with cancer can have symptoms that could erroneously be attributed to HF, and this might lead to overestimation of HF events. On the other hand, although there was one study that differed in the definition of asymptomatic/minimally symptomatic LVSD, this study was excluded in the sensitivity analysis for TDM-1, with no significant change in the effect estimate.
The event rates reported in the studies we identified were lower than those that have been reported previously in trastuzumab studies. Although we found that pertuzumab (generally used in combination with trastuzumab) was associated with an absolute risk of 8 HF cases per 1000 patients, the HF risk reported in other studies of trastuzumab monotherapy was between 24 and 30 cases per 1000 patients).14,45 Similarly, the incidence of asymptomatic/minimally symptomatic LVSD in the control group treated only with trastuzumab was 3.1% in our study, a proportion much lower than that previously reported—7.66% to 11.2%—in different meta-analyses.45,46 The difference may be because the patients in the population represented in our pooled data are highly selected and at low cardiac risk.
Strengths and limitations
Our study represents the largest pooled experience from randomized clinical trials of pertuzumab. However, this review has several limitations. First, the outcomes of our analysis were not the primary endpoints of the included studies; in fact, they were reported as adverse events. Second, not all cardiac adverse effects of interest were reported in the trials (e, cardiac death), restricting our analysis to 2 main outcomes. We are uncertain whether other cardiac outcomes or indicators of cardiac function, such as diastolic echocardiographic parameters, differed between pertuzumab recipients and controls. Finally, although we specifically included randomized trials, as this study design minimizes the risk of bias and confounding, a number of other phase II nonrandomized trials evaluating pertuzumab monotherapy have also provided data about cardiotoxicity but were not included.
Implications
Our findings suggest that surveillance for HF may be important in individuals treated with pertuzumab, and that LVEF alone may not be sufficient to identify those at risk of developing HF. Other echocardiographic tools, including global longitudinal strain and diastolic function assessment, and/or monitoring of cardiac biomarkers, such as troponin and N-terminal prohormone of brain natriuretic peptide (NTproBNP), have shown promise in the early detection of cardiotoxicity.47,48 Further research is needed to determine their value in identifying those who will develop HF related to pertuzumab use.
Conclusion
In patients with HER2 overexpressing cancers who took part in clinical trials and underwent frequent cardiac monitoring, pertuzumab use was associated with an increased risk of HF but was not associated with an increased risk of asymptomatic LVSD. The incidence of HF was very low in these trials; therefore, despite the increased risk of HF, we do not recommend against using pertuzumab in eligible patients with low cardiac risk.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported has adhered to relevant ethical guidelines.
See page 1381 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.06.019.
Supplementary Material
References
- 1.Wolff A.C., Hammond M.E., Hicks D.G., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 2.Slamon D.J., Godolphin W., Jones L.A., et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Menard S., Casalini P., Campiglio M., et al. HER2 overexpression in various tumor types, focussing on its relationship to the development of invasive breast cancer. Ann Oncol. 2001;12(suppl. I):S15–S19. doi: 10.1093/annonc/12.suppl_1.s15. [DOI] [PubMed] [Google Scholar]
- 4.Scholl S., Beuzeboc P., Pouillart P. Targeting HER2 in other tumor types. Ann Oncol. 2001;12(suppl 1):S81–S87. doi: 10.1093/annonc/12.suppl_1.s81. [DOI] [PubMed] [Google Scholar]
- 5.Corbo V., Beghelli S., Bersani S., et al. Pancreatic endocrine tumours: mutational and immunohistochemical survey of protein kinases reveals alterations in targetable kinases in cancer cell lines and rare primaries. Ann Oncol. 2012;23:127–134. doi: 10.1093/annonc/mdr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siena S., Sartore-Bianchi A., Marsoni S., et al. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann Oncol. 2018;29:1108–1119. doi: 10.1093/annonc/mdy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan M., Parker B.A., Schwab R., Kurzrock R. HER2 aberrations in cancer: implications for therapy. Cancer Treat Rev. 2014;40:770–780. doi: 10.1016/j.ctrv.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 8.US Food & Drug Administration FDA approves fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2-positive-breast-cancer Available at:
- 9.US Food & Drug Administration FDA approves tucatinib for patients with HER2-positive metastatic breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tucatinib-patients-her2-positive-metastatic-breast-cancer Available at:
- 10.Slamon D.J., Leyland-Jones B., Shak S., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Eng J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 11.Romond E.H., Perez E.A., Bryant J., et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Eng J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 12.Sparano J.A. Cardiac toxicity of trastuzumab (Herceptin): implications for the design of adjuvant trials. Semin Oncol. 2001;28:20–27. doi: 10.1016/s0093-7754(01)90189-7. [DOI] [PubMed] [Google Scholar]
- 13.Riccio G., Coppola C., Piscopo G., et al. Trastuzumab and target-therapy side effects: Is still valid to differentiate anthracycline Type I from Type II cardiomyopathies? Hum Vaccin Immunother. 2016;12:1124–1131. doi: 10.1080/21645515.2015.1125056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantarro S., Rossi M., Bonifazi M., et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern Emerg Med. 2016;11:123–140. doi: 10.1007/s11739-015-1362-x. [DOI] [PubMed] [Google Scholar]
- 15.Chen J., Long J.B., Hurria A., et al. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am College Cardiol. 2012;60:2504–2512. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 16.Serrano C., Cortes J., De Mattos-Arruda L., et al. Trastuzumab-related cardiotoxicity in the elderly: a role for cardiovascular risk factors. Ann Oncol. 2012;23:897–902. doi: 10.1093/annonc/mdr348. [DOI] [PubMed] [Google Scholar]
- 17.Chung C., Lam M.S. Pertuzumab for the treatment of human epidermal growth factor receptor type 2-positive metastatic breast cancer. Am J Health Syst Pharm. 2013;70:1579–1587. doi: 10.2146/ajhp120735. [DOI] [PubMed] [Google Scholar]
- 18.Blumenthal G.M., Scher N.S., Cortazar P., et al. First FDA approval of dual anti-HER2 regimen: pertuzumab in combination with trastuzumab and docetaxel for HER2-positive metastatic breast cancer. Clin Cancer Res. 2013;19:4911–4916. doi: 10.1158/1078-0432.CCR-13-1212. [DOI] [PubMed] [Google Scholar]
- 19.Baselga J., Cortés J., Kim S.-B., et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann-La Roche Ltd Pertuzumab (Prejeta) monograph. https://www.rochecanada.com/content/dam/rochexx/roche-ca/products/ConsumerInformation/MonographsandPublicAdvisories/Perjeta/Perjeta_PM_E.pdf Available at:
- 21.Howie L.J., Scher N.S., Amiri-Kordestani L., et al. FDA approval summary: pertuzumab for adjuvant treatment of HER2-positive early breast cancer. Clin Cancer Res. 2019;25:2949–2955. doi: 10.1158/1078-0432.CCR-18-3003. [DOI] [PubMed] [Google Scholar]
- 22.Gianni L., Pienkowski T., Im Y.H., et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomized trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 23.von Minckwitz G., Procter M., de Azambuja E., et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenihan D., Suter T., Brammer M., et al. Pooled analysis of cardiac safety in patients with cancer treated with pertuzumab. Ann Oncol. 2012;23:791–800. doi: 10.1093/annonc/mdr294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J.P.T., Lasserson T., Chandler J., et al. Cochrane; London: 2019. Methodological Expectations of Cochrane Intervention Reviews.https://community.cochrane.org/mecir-manual/key-points-and-introduction/versions-and-changes-mecir Available at: [Google Scholar]
- 26.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cochrane Library Cochrane central register of controlled trials (CENTRAL) https://www.cochranelibrary.com/central Available at:
- 28.National Cancer Institute Common terminology criteria for adverse events (CTCAE), version 3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf Available at:
- 29.National Cancer Institute Common terminology criteria for adverse events (CTCAE), version 4.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40 Available at:
- 30.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Available at: https://training.cochrane.org/handbook/archive/v5.1/. Accessed January 31, 2019.
- 31.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccart M, Procter M, Fumagalli D, et al. Interim overall survival analysis of APHINITY (BIG 4-11): a randomized, multicenter, double-blind, placebo-controlled trial comparing chemotherapy plus trastuzumab plus pertuzumab vs chemotherapy plus trastuzumab plus placebo as adjuvant therapy in patients with operable HER2-positive early breast cancer. 2019 San Antonio Breast Cancer Symposium. Abstract GS1-04. Available at: https://cancerres.aacrjournals.org/content/80/4_Supplement/GS1-04. Accessed April 30, 2020.
- 33.Urruticoechea A., Rizwanullah M., Im S.A., et al. Randomized phase iii trial of trastuzumab plus capecitabine with or without pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who experienced disease progression during or after trastuzumab-based therapy. J Clin Oncol. 2017;35:3030–3038. doi: 10.1200/JCO.2016.70.6267. [DOI] [PubMed] [Google Scholar]
- 34.Rimawi M., Ferrero J.M., de la Haba-Rodriguez J., et al. First-line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2-positive and hormone receptor-positive metastatic or locally advanced breast cancer (PERTAIN): a randomized, open-label phase ii trial. J Clin Oncol. 2018;36:2826–2835. doi: 10.1200/JCO.2017.76.7863. [DOI] [PubMed] [Google Scholar]
- 35.Tabernero J., Hoff P.M., Shen L., et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372–1384. doi: 10.1016/S1470-2045(18)30481-9. [DOI] [PubMed] [Google Scholar]
- 36.Shao Z., Pang D., Yang H., et al. Efficacy, safety, and tolerability of pertuzumab, trastuzumab, and docetaxel for patients with early or locally advanced ERBB2-positive breast cancer in Asia: the PEONY phase 3 randomized clinical trial. JAMA Oncol. 2020;6 doi: 10.1001/jamaoncol.2019.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez E.A., Barrios C., Eiermann W., et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab with taxane for human epidermal growth factor receptor 2-positive advanced breast cancer: final results from MARIANNE. Cancer. 2019;125:3974–3984. doi: 10.1002/cncr.32392. [DOI] [PubMed] [Google Scholar]
- 38.Swain S.M., Baselga J., Kim S.B., et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swain S.M., Miles D., Kim S.B., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 40.Garg A., Li J., Clark E., et al. Exposure-response analysis of pertuzumab in HER2-positive metastatic breast cancer: absence of effect on QTc prolongation and other ECG parameters. Cancer Chemother Pharmacol. 2013;72:1133–1141. doi: 10.1007/s00280-013-2279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valachis A., Nearchou A., Polyzos N.P., Lind P. Cardiac toxicity in breast cancer patients treated with dual HER2 blockade. Int J Cancer. 2013;133:2245–2252. doi: 10.1002/ijc.28234. [DOI] [PubMed] [Google Scholar]
- 42.Sendur M.A., Aksoy S., Altundag K. Pertuzumab in HER2-positive breast cancer. Curr Med Res Opin. 2012;28 doi: 10.1185/03007995.2012.728132. 1709-176. [DOI] [PubMed] [Google Scholar]
- 43.Swain S.M., Ewer M.S., Cortés J., et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase iii study. Oncologist. 2013;18:257–264. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao L., Cai G., Chang C., et al. Diastolic dysfunction occurs early in HER2-positive breast cancer patients treated concurrently with radiation therapy and trastuzumab. Oncologist. 2015;20:605–614. doi: 10.1634/theoncologist.2014-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moja L., Tagliabue L., Balduzzi S., et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;2012:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen T., Xu T., Li Y., et al. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer Treat Rev. 2011;37:312–320. doi: 10.1016/j.ctrv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Plana J.C., Thavendiranathan P., Bucciarelli-Ducci C., Lancellotti P. Multi-modality imaging in the assessment of cardiovascular toxicity in the cancer patient. JACC Cardiovasc Imaging. 2018;11:1173–1186. doi: 10.1016/j.jcmg.2018.06.003. [correction 2019;12:224] [DOI] [PubMed] [Google Scholar]
- 48.Tan L.L., Lyon A.R. Role of biomarkers in prediction of cardiotoxicity during cancer treatment. Curr Treat Options Cardiovasc Med. 2018;20:55. doi: 10.1007/s11936-018-0641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.