Abstract
Myasthenia gravis (MG) is an autoimmune condition affecting the neuromuscular junction characterised by weakness and fatiguability, carrying a high morbidity if treatment is delayed. A clear association with thymoma has led to management with thymectomy as a common practice, but MG presenting post-thymectomy has rarely been reported. We present a case of an 82- year-old woman developing fatigue, ptosis and dysarthria 3 months after thymectomy. After a clinical diagnosis of MG was made, she responded well to prompt treatment with prednisolone and pyridostigmine. Her anti-acetylcholine receptor antibody (anti-AChR) subsequently came back positive. Our systematic review reveals that post-thymectomy MG can be categorised as early-onset or late-onset form with differing aetiology, and demonstrated correlation between preoperative anti-AChR titres and post-thymectomy MG. The postulated mechanisms for post-thymectomy MG centre around long-lasting peripheral autoantibodies. Clinicians should actively look for MG symptoms in thymoma patients and measure anti-AChR preoperatively to aid prognostication.
Keywords: neurology, neuromuscular disease, endocrine cancer, cardiothoracic surgery
Background
Myasthenia gravis (MG) is an autoimmune condition in which antibodies bind to the acetyl-choline receptors in the postsynaptic membrane of the neuromuscular junction, characterised clinically by skeletal muscle weakness and fatigability.1 While eminently treatable, MG carries considerable morbidity if there are delays in treatment.1 Thymoma is associated with several autoimmune diseases, but most vitally MG,2 and thymectomy has become an important aspect of MG management. However, the first onset of myasthenic symptoms has been reported in rare cases as late as months to years after thymectomy.3–14 We report an illustrative case that highlights the importance of reviewing thymoma patients for development of MG symptoms, especially in the complex setting of being a distant hospital to that of the original operating team.
Case presentation
An 82-year-old woman with a background history of anterior repair for cystocoele, cataracts and left knee replacement, first presented to the respiratory team in November 2019 with several months of progressive dyspnoea. She was a lifelong non-smoker and had a performance status of 0. Her systemic examination was normal but chest X-ray and later CT chest, abdomen and pelvis confirmed the presence of a 7.0×6.5 cm anterior mediastinal cystic mass. The mass was initially thought to be a haemorrhagic pericardial cyst in the respiratory multidisciplinary meeting. However, the positron emission tomography (PET–CT) demonstrated that the part of the lesion (figure 1) was highly fluorodeoxyglucose (FDG) avid. She was referred to a thoracic surgeon in London, 84 miles from her local hospital, and the histological examination of the resected mass confirmed a type AB thymoma, Masaoka-Koga stage 1 (figure 2). The patient had recovered well after surgery in March 2020 with complete resolution of dyspnoea, which was likely to be secondary to the large size of the thymoma. She was fully mobile and independent within 3 weeks.
Figure 1.
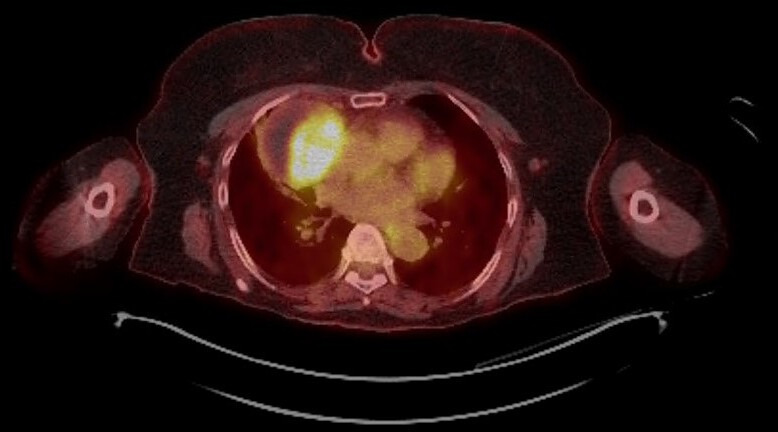
PET–CT showing solid part and rim of mediastinal mass as very fluorodeoxyglucose avid.
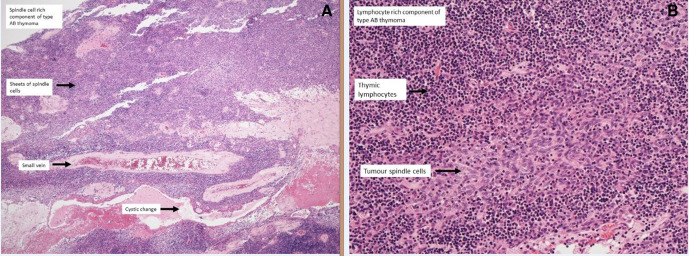
Figure 2.
Histology: (A) Macroscopic section demonstrating type A spindle cell rich area. (B) Microscopic section demonstrating type A spindle cells in lymphocyte rich area.
She was further admitted in May 2020 with 6 weeks of reduced mobility, fatigue and 10 days of intermittent slurred speech and droopy eyelids. There was no dysphagia. She had normal observations except for reduced forced vital capacity at 1.2 L (partially due to poor technique). Neurological examination demonstrated moderate dysarthria, a weak cough and bilateral asymmetrical ptosis. She had mild proximal weakness with Medical Research Council (MRC) grade 4 in shoulder abduction and hip flexion bilaterally, but there was no fasciculation or muscle wasting.
Investigations
She had a reduced vitamin D level (48 nmol/L;≥50 nmol/L), but other blood tests including creatine kinase and thyroid function tests were normal. She had a normal CT brain and MRI of the whole spine. Her anti-acetylcholine receptor antibody was subsequently confirmed as strongly positive (anti-AChR >19 nmol/L; 0–10). Her antimuscle specific tyrosine kinase (anti-MuSK), antinuclear and voltage-gated calcium channel antibodies were negative. A subsequent repeat CT chest and PET-CT did not show recurrence of thymoma or residual thymic tissue.
Treatment
She was later reviewed by a neurologist who diagnosed generalised MG. She improved very quickly after commencing on pyridostigmine and prednisolone. After a short stay in hospital, she was discharged home.
Outcome and follow-up
Her diplopia and dysarthria had resolved by the time of her first follow-up 4 weeks after discharge. The plan from subsequent follow-up appointments was to slowly reduce her prednisolone to reach the lowest effective dose and determine if additional steroid sparing medication is required.
Discussion
MG appearing after removal of thymoma was first reported in 1951,3 and occurs in 1.5%– 28% of thymoma patients without MG preoperatively.7 A detailed search of PUBMED and EMBASE using terms ‘post-thymectomy and myasthenia gravis’ revealed 12 additional studies (figure 3)3–14 on this phenomenon.
Figure 3.
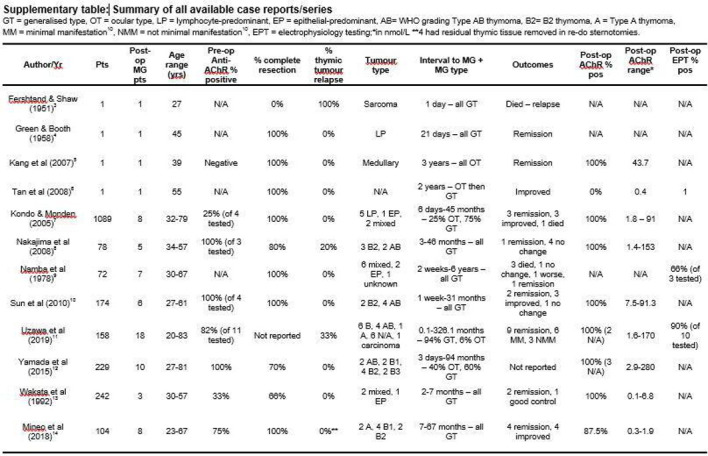
Supplementary table of available literature.
The patients reviewed demonstrate varied age and sex distributions, and there was little predominance with regards to histology.8 10 11 The interval between thymectomy and onset of MG was wide, ranging from immediately postoperatively to 326 months,11 with the immediate or intraoperative onset of symptoms being linked to the use of non-depolarising muscle relaxants in the older literature.4 9 At the opposite end of the spectrum, an observed bimodal distribution led to postoperative MG being divided into early-onset (<6 months) and late-onset (>6 months) categories; patients with thymic relapse (one-third of the total) falling entirely into the late-onset category. Similarly, it has been demonstrated that 70% of re-examined patients at postmortem or thoracotomy had no residual tissue9 while, in two other studies, only 20% and 33% of patients had recurrence.8 11 Most studies demonstrate no radiological recurrence in all patients. Our patient demonstrated a mild disease course with their MG, and the literature supports no significant difference in clinical manifestation and prognosis between prethymectomy and post-thymectomy MG.11 One case series from 1978 reports that their mortality rate of 30% did not differ significantly from the contemporary figures of 53% for MG with thymoma, 24% in MG without thymoma or 38% in thymoma without MG.9 Similarly, partial or complete remission was achieved in 40% of post-thymectomy MG patients compared with 26% and 42% of MG with thymoma and MG without thymoma, respectively.9
Anti-AChR is considered highly sensitive and specific for MG, with anti-MuSK present in about 50% of the seronegative patients.1 Nearly all patients with thymoma-associated MG have highly positive anti-AChR titres.8 There were four case series that had complete data on prethymectomy measurement of anti-AChR.7 8 10 11 Of the 407 patients who did not exhibit preoperative myasthenic symptoms, 29 (7.1%) subsequently developed symptoms after surgery, with a sensitivity of 60%–100% and specificity of 73%–85%. Although the titres did not correlate with severity of symptoms,5 7 escalation of levels were seen on the development of MG10 compared with preoperative levels. Therefore, preoperative anti-AChR seropositivity might provide useful prognostic information for selection of a subgroup of these patients for closer monitoring in the recovery phase.14 However, larger prospective studies are required to confirm this finding. Our patient’s preoperative titre was not measured as she was asymptomatic. However, one could not fully exclude the possibility that her highly positive postoperative titre reflected an already present subclinical MG.
Considering the time intervals for onset of postoperative MG may provide a helpful lens when postulating possible causes for delayed presentations.15 16 First, intratumorous T-cell maturation formed autoantigen-specific T-cells, which were exported into the peripheral circulation, contrasting with the normal thymopoiesis seen in the hyperplastic thymus of early-onset MG.15 Second, these thymoma-produced autoantigen-specific T cells could persist in the periphery for as long as 20 years.15 Additionally, it was suggested that thymectomy led to the removal of a downregulatory effect spearheaded by CD4 +T cells, in turn allowing for B cell activity and autoantibody production.12 The post-thymectomy serial measurements stayed high initially and then declined with a 50% reduction by 6 months. This correlated considerably with aforementioned11 bimodal distribution of postoperative MG whereby late-onset was likely to be recurrence, but the early onset peaked at 0–3 months postoperative without a clear cause. We suggest that this group could be explained by the continued high level of autoantigen-specific T cells immediately postoperative,15 which then stimulated subsequent auto-antibodies, triggering autoimmune disease.
Learning points.
To actively look out for myasthenic symptoms in asymptomatic thymoma patients before and after thymectomy with regular follow-up postoperatively so that early detection and treatment of myasthenia gravis can prevent deterioration.
Preoperative completion of anti-acetylcholine receptor antibody levels can be useful for prognostication.
There is a need to investigate late-onset post-thymectomy myasthenia gravis patients for thymoma relapse as a cause of new symptoms.
Acknowledgments
Dr Carolyn MacKinlay, Consultant Respiratory Physician, Great Western Hospital: for her care of the patient throughout her diagnosis, and inpatient admission. Mr Silviu Buderi, Consultant Cardiothoracic Surgeon, Royal Brompton Hospital: for his care of the patient as her operating surgeon. And to both for their involvement and support in writing this case report.
Footnotes
Contributors: LMG, AM and GY cared for the patient on their admission with the acute case as described above. AR analysed the specimen from the patient’s operation and provided a histology report. AM discussed with the patient and analysed the inpatient notes, compiling the case history, and obtained consent for the report including the patient reviewing the manuscript. LG reviewed available literature to analyse existing data and wrote the discussion portion of the report. GY supervised the case report throughout. All authors (LMG, AM, GY and AR) reviewed the manuscript and provided critical feedback.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Gilhus NE. Myasthenia gravis. N Engl J Med Overseas Ed 2016;375:2570–81. 10.1056/NEJMra1602678 [DOI] [PubMed] [Google Scholar]
- 2.Scorsetti M, Leo F, Trama A, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol 2016;99:332–50. 10.1016/j.critrevonc.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 3.Fershtand JB, Shaw RR. Malignant tumor of the thymus gland, myasthenia gravis developing after removal. Ann Intern Med 1951;34:1025–35. 10.7326/0003-4819-34-4-1025 [DOI] [PubMed] [Google Scholar]
- 4.Green RA, Booth CB. The development of myasthenia gravis after removal of thymoma. Am J Med 1958;25:293–302. 10.1016/0002-9343(58)90035-4 [DOI] [PubMed] [Google Scholar]
- 5.Kang S-Y, Lee JS, Choi JC, et al. Myasthenia gravis appearing after thymectomy: a case report and review of the literature. J Clin Neurol 2007;3:158–60. 10.3988/jcn.2007.3.3.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan FU, Kansu T, Akarsu C. Post-thymectomy, seronegative myasthenia gravis. Neuro-Ophthalmology 2008;32:7–12. 10.1080/01658100701818180 [DOI] [Google Scholar]
- 7.Kondo K, Monden Y. Myasthenia gravis appearing after thymectomy for thymoma. Eur J Cardiothorac Surg 2005;28:22–5. 10.1016/j.ejcts.2005.03.039 [DOI] [PubMed] [Google Scholar]
- 8.Nakajima J, Murakawa T, Fukami T, et al. Postthymectomy myasthenia gravis: relationship with thymoma and antiacetylcholine receptor antibody. Ann Thorac Surg 2008;86:941–5. 10.1016/j.athoracsur.2008.04.070 [DOI] [PubMed] [Google Scholar]
- 9.Namba T, Brunner NG, Grob D. Myasthenia gravis in patients with thymoma, with particular reference to onset after thymectomy. Medicine 1978;57:411–34. 10.1097/00005792-197809000-00002 [DOI] [PubMed] [Google Scholar]
- 10.Sun X-gang, Wang Y-li, Liu Y-hai, Sun X, Lui Y, et al. Myasthenia gravis appearing after thymectomy. Journal of Clinical Neuroscience 2011;18:57–60. 10.1016/j.jocn.2010.05.018 [DOI] [PubMed] [Google Scholar]
- 11.Uzawa A, Kanai T, Oda F, et al. Frequency and features of myasthenia gravis developing after thymectomy. Eur J Neurol 2020;27:175–80. 10.1111/ene.14052 [DOI] [PubMed] [Google Scholar]
- 12.Yamada Y, Yoshida S, Iwata T, et al. Risk factors for developing postthymectomy myasthenia gravis in thymoma patients. Ann Thorac Surg 2015;99:1013–9. 10.1016/j.athoracsur.2014.10.068 [DOI] [PubMed] [Google Scholar]
- 13.Wakata N, Fukuya H, Niizuma M, et al. Myasthenia gravis developing after discovery of thymoma. Clin Neurol Neurosurg 1992;94:303–6. 10.1016/0303-8467(92)90178-6 [DOI] [PubMed] [Google Scholar]
- 14.Mineo TC, Tamburrini A, Schillaci O, et al. Onset and evolution of clinically apparent myasthenia gravis after resection of non-myasthenic thymomas. Semin Thorac Cardiovasc Surg 2018;30:222–7. 10.1053/j.semtcvs.2018.02.027 [DOI] [PubMed] [Google Scholar]
- 15.Buckley C, Douek D, Newsom-Davis J, et al. Mature, long-lived CD4+ and CD8+ T cells are generated by the thymoma in myasthenia gravis. Ann Neurol 2001;50:64–72. 10.1002/ana.1017 [DOI] [PubMed] [Google Scholar]
- 16.Hoffacker V, Schultz A, Tiesinga JJ, et al. Thymomas alter the T-cell subset composition in the blood: a potential mechanism for thymoma-associated autoimmune disease. Blood 2000;96:3872–9. 10.1182/blood.V96.12.3872 [DOI] [PubMed] [Google Scholar]