Abstract
Objective
To examine if the association between interpregnancy interval (IPI) and pregnancy complications varies by the presence or absence of previous complications.
Design and setting
Population-based longitudinally linked cohort study in Western Australia (WA).
Participants
Mothers who had their first two (n=252 368) and three (n=96 315) consecutive singleton births in WA between 1980 and 2015.
Outcome measures
We estimated absolute risks (AR) of preeclampsia (PE) and gestational diabetes (GDM) for 3–60 months of IPI according to history of each outcome. We modelled IPI using restricted cubic splines and reported adjusted relative risk (RRs) with 95% CI at 3, 6, 12, 24, 36, 48 and 60 months, with 18 months as reference.
Results
Risks of PE and GDM were 9.5%, 2.6% in first pregnancies, with recurrence rates of 19.3% and 41.5% in second pregnancy for PE and GDM, respectively. The AR of GDM ranged from 30% to 43% across the IPI range for mothers with previous GDM compared with 2%–8% for mothers without previous GDM. For mothers with no previous PE, greater risks were observed for IPIs at 3 months (RR 1.24, 95% CI 1.07 to 1.43) and 60 months (RR 1.40, 95% CI 1.29 to 1.53) compared with 18 months. There was insufficient evidence for increased risk of PE at shorter IPIs of <18 months for mothers with previous PE. Shorter IPIs of <18 months were associated with lower risk than at IPIs of 18 months for mothers with no previous GDM.
Conclusions
The associations between IPIs and risk of PE or GDM on subsequent pregnancies are modified by previous experience with these conditions. Mothers with previous complications had higher absolute, but lower RRs than mothers with no previous complications. However, IPI remains a potentially modifiable risk factor for mothers with previous complicated pregnancies.
Keywords: epidemiology, obstetrics, gynaecology, diabetes in pregnancy, hypertension
Strengths and limitations of this study.
Population-based cohort study of mothers who delivered their first two (more than 250 000) and three (96 315) consecutive singlet on births in Western Australia.
Modelling interpregnancy interval (IPI) flexibly allows for risk curve estimations and better clarification of optimal IPI.
Findings from this study provides more clinically applicable information on the association between IPIs and risk of preeclampsia and gestational diabetes based on presence/absence of these complications.
Data set lacks information on pregnancy loss before 20 weeks of gestation.
The possibility of the findings affected by unmeasured confounding is likely.
Introduction
Preeclampsia (PE) and gestational diabetes (GDM) remain the most significant contributors to perinatal and maternal mortalities and morbidities, complicating 2%–10% and 6%–13% of pregnancies worldwide, respectively.1–4 These complications have a higher tendency of recurrence in subsequent pregnancies. Studies have reported recurrence rates of 7%–20% for PE and 30%–70% for GDM, respectively.5–8
Interpregnancy interval (IPI), the length of time between pregnancies, has been identified as a potentially modifiable risk factor for adverse perinatal outcomes, with short and long IPIs found to be associated with adverse outcomes.9–12 Based on these associations, various clinical guidelines and WHO recommend that women wait at least 18–24 months before conceiving another child.13–15
Recently, there has been growing literature on the association between IPIs and recurrence of pregnancy complications.16–18 However, there is currently no recommendation for the optimal interval based on obstetric history, and there is limited evidence to inform such a recommendation.
This study aimed to examine whether the association between IPI and pregnancy complications was modified by obstetric history, specifically PE and GDM. In addition, we estimated the absolute risk of these complications associated with short and long IPIs, to better inform decision-making regarding optimal IPIs.
Materials and methods
Study design
We conducted a population-based, longitudinal cohort study of mothers with at least two consecutive singleton pregnancies in the period between 1980 and 2015 in Western Australia (WA).
Data sources and study population
We obtained maternal, infant and birth information from the Midwives Notification System, a validated database19 that includes >99% of births in WA of at least 20 weeks’ gestation or birth weight of 400 g or more if the gestational age was unknown.20
We sourced hospitalisation records from Hospital Morbidity Data Collection, which includes information on all hospitalisations in the state with International Classification of Diseases (ICD-9/10th revision-Australian Modification (AM)) coded diagnoses.21 Data sources and study protocol have been published elsewhere.12 22 Birth records were probabilistically linked based on maternal information to identify all births to individual women during the study period.
From a total of 487 297 mothers, we sequentially excluded mothers who had multiple births; mothers who had only one pregnancy during the study period; mothers whose children’s birth years were inconsistent with the parity and mothers who had missing gestational age, pregnancy outcomes, age and socioeconomic status (SES). These exclusions resulted in 280 637 eligible mothers with at least two consecutive births who contributed 711 252 pregnancies. Finally, we included 252 368 mothers with their first two (parity 0, 1) and 96 315 mothers with their first three consecutive singleton births (parity 0, 1, 2) in the analytic cohort (online supplemental figure 1).
bmjopen-2020-046962supp001.pdf (757.3KB, pdf)
Exposure
IPI was calculated prior to exclusions as the time between the delivery date of the first eligible birth (that resulted in live birth or stillbirth) during the study period and the estimated conception date of the subsequent pregnancy (date of birth minus gestational age at birth). Gestational age at birth was estimated as the best clinical estimate from dating ultrasounds or last menstrual period when ultrasound was unavailable.
Outcomes
The outcomes of interest were ascertained from midwives notifications and hospital separation data in the state, with the ICD-9 through to ICD-10-AM diagnostic codes consistent with PE (ICD-9/ICD-9-Clinical Modification (CM): 642.4, 642.5, 642.7, ICD-10-AM: O14, O11) and GDM (ICD-9/ICD-9-CM: 648.8, ICD-10-AM: O24.4-).
Covariates
Information on potential confounding factors measured at the birth prior to the interval, and including birth year, maternal age, marital status, parity, race/ethnicity and SES were obtained from hospitalisations and perinatal records. We also included a partner change status, which identifies if a mother changed partner either between first and second or between second and third pregnancies. Race/ethnicity was classified as Caucasian versus non-Caucasian. Marital status was categorised as married, never married, widowed/divorced/separated and unknown.
SES was derived by the Australian Bureau of Statistics as Socio Economic Indexes for Areas (SEIFA) at a geographical area for the maternal residence at the time of birth,23 and categorised into quintiles.
Statistical analysis
Based on existing literature and recent recommendations to represent the potential pathway between IPI and pregnancy outcomes,24 we created a directed acyclic graph (DAG) (online supplemental figure 2, online supplemental figure 3). Covariates fulfilling the minimally sufficient adjustment set were selected. We first tabulated the incidence of each pregnancy complication by IPI (categorised to <6, 6–11, 12–17, 18–23, 24–59 and ≥60 months). We then examined the association between IPI and pregnancy complication (GDM and PE) stratified by the history of each complication using generalised linear models (GLM) fitted using a Poisson distribution with a log link function. We modelled IPI as a continuous variable with a flexible, non-linear approach, restricted cubic splines, with knots placed at 3, 6, 12, 18, 24, 36 and 48 months of IPI. We then estimated the absolute risk of each pregnancy complication in 1-month increments of IPI from 3 to 60 months using postestimation calculations.25 Since the intraclass coefficient was considerably low and the CIs of the estimates were not significantly changed in the multilevel model, the GLM model using SEIFA as a proxy for SES was used.
For each outcome, the unadjusted model included the IPI spline terms only, and the adjusted model included covariates measured at birth prior to IPI: birth year, SES, marital status, race/ethnicity and partner change status at recent birth. Maternal age was modelled using restricted cubic splines with 4 knots at the 5th, 35th, 65th and 95th percentiles (ages 18, 24, 29 and 35). We also adjusted for parity (categorised as nulliparous, parity 1 and 2) for the association between IPI and complications to ascertain the sensitivity of our results to higher-order parity (online supplemental table 1). To examine the potential variability of the relationship between IPI and each outcome by the history of complications, we estimated the predicted absolute risk at the values of the following covariates: Caucasian, married, average SES, average maternal age and birth year set to 2010 at birth prior to the IPI. We then plotted the predicted risks with 95% CIs at 1-month increments of IPI for each outcome stratified by the history of complications to illustrate the shapes of the risk curves. For tabulated results, we presented relative risks (RRs) with 95% CIs at 3, 6, 12, 24, 36, 48 and 60 months of IPI, with 18 months as the reference. Robust (sandwich) variance estimation was used to account for non-independence of two or more IPIs per mother.26
Missing data
We carried out a complete case analysis because the proportion of missing data was small (<3%, range 0.04% for maternal age to 1.2% for SES). The majority of missing data were due to lack of availability of information (eg, SES) prior to the year 1997, and we evaluated this bias using sensitivity analyses.
Sensitivity analyses
We conducted a sensitivity analyses to examine the effect of choice of timing of the effect modifier (presence of complication for any previous pregnancy as opposed to complication experienced at the immediate previous pregnancy) by including all mothers with at least two consecutive pregnancies during the study period (online supplemental table 1). We further included a sensitivity analysis restricted to consecutive births after the year 1997 for which more information on potential confounders including paternal age, fertility treatment (assuming that these pregnancies were more likely to be intended), and smoking were available for adjustment (online supplemental table 2).20 We also performed a sensitivity analysis to examine whether our results differed by the timing of covariate adjustment (ie, covariates at birth prior to interval vs at the time of the outcome, online supplemental table 3). All analyses were performed using STATA V.16.1 (Stata Corporation, College Station, Texas, USA). The DAG was created using DAGitty V.2.3.27
Patient and public involvement
Members of the community Healthy Pregnancies Consumer Reference Group provided community and consumer perspectives to this study. This group also provided an insight into issues that affect their pregnancy planning decisions, contextualise results and provided participant experience.
Results
Cohort characteristics
Maternal age at birth of first child peaked between 25 and 29 years. IPIs were more commonly within 24–59 months (31.7%); 4.8% and 7.8% of mothers had IPIs of <6 months and ≥60 months, respectively. The distribution of IPIs was similar for mothers with and without previous complications (table 1).
Table 1.
Maternal characteristics at first pregnancy by previous pregnancy complications, Western Australia 1980–2015
| Characteristics | Total | Preeclampsia | Previous PE | Gestational diabetes | |
| No previous PE | No previous GDM | Previous GDM | |||
| N=252 368 | N=2 28 407 | N=23 961 | N=245 764 | N=6604 | |
| Maternal age, years | |||||
| <20 | 43 473 (17.2) | 38 999 (17.1) | 4474 (18.7) | 43 035 (17.5) | 438 (6.6) |
| 20–24 | 57 209 (22.7) | 51 194 (22.4) | 6015 (25.1) | 56 334 (22.9) | 875 (13.2) |
| 25–29 | 87 480 (34.7) | 79 285 (34.7) | 8195 (34.2) | 85 233 (34.7) | 2247 (34.0) |
| 30–34 | 51 537 (20.4) | 47 291 (20.7) | 4246 (17.7) | 49 332 (20.1) | 2205 (33.4) |
| ≥35 | 12 669 (5.0) | 11 638 (5.1) | 1031 (4.3) | 11 830 (4.8) | 839 (12.7) |
| Time period | |||||
| 1980–1984 | 32 982 (13.1) | 29 087 (12.7) | 3895 (16.3) | 32 940 (13.4) | 42 (0.6) |
| 1985–1989 | 35 703 (14.1) | 31 397 (13.7) | 4306 (18.0) | 35 583 (14.5) | 120 (1.8) |
| 1990–1994 | 36 940 (14.6) | 32 881 (14.4) | 4059 (16.9) | 36 492 (14.8) | 448 (6.8) |
| 1995–1999 | 37 012 (14.7) | 32 715 (14.3) | 4297 (17.9) | 36 070 (14.7) | 942 (14.3) |
| 2000–2004 | 37 260 (14.8) | 33 998 (14.9) | 3262 (13.6) | 36 031 (14.7) | 1229 (18.6) |
| 2005–2009 | 43 151 (17.1) | 40 458 (17.7) | 2693 (11.2) | 41 303 (16.8) | 1848 (28.0) |
| 2010–2015 | 29 320 (11.6) | 27 871 (12.2) | 1449 (6.0) | 27 345 (11.1) | 1975 (29.9) |
| SES in quintiles | |||||
| <20th percentile (Most disadvantaged) | 46 991 (18.6) | 42 087 (18.4) | 4904 (20.5) | 45 883 (18.7) | 1108 (16.8) |
| 20–39th percentile | 51 517 (20.4) | 46 271 (20.3) | 5246 (21.9) | 50 295 (20.5) | 1222 (18.5) |
| 40–59th percentile | 52 503 (20.8) | 47 506 (20.8) | 4997 (20.9) | 51 107 (20.8) | 1396 (21.1) |
| 60–79th percentile | 51 922 (20.6) | 47 140 (20.6) | 4782 (20.0) | 50 462 (20.5) | 1460 (22.1) |
| ≥80th percentile (least disadvantaged) | 49 435 (19.6) | 45 403 (19.9) | 4032 (16.8) | 48 017 (19.5) | 1418 (21.5) |
| Marital status | |||||
| Married | 215 196 (85.3) | 194 800 (85.3) | 20 396 (85.1) | 209 351 (85.2) | 5845 (88.5) |
| Others | 37 172 (14.7) | 33 607 (14.7) | 3565 (14.9) | 36 413 (14.8) | 759 (11.5) |
| Race/ethnicity | |||||
| Caucasian | 219 562 (87.0) | 198 137 (86.7) | 21 425 (89.4) | 214 645 (87.3) | 4917 (74.5) |
| Interpregnancy interval, months | |||||
| <6 | 12 104 (4.8) | 11 006 (4.8) | 1098 (4.6) | 11 780 (4.8) | 324 (4.9) |
| 6–11 | 42 470 (16.8) | 38 678 (16.9) | 3792 (15.8) | 41 267 (16.8) | 1203 (18.2) |
| 12–17 | 55 218 (21.9) | 50 237 (22.0) | 4981 (20.8) | 53 737 (21.9) | 1481 (22.4) |
| 18–23 | 42 934 (17.0) | 38 880 (17.0) | 4054 (16.9) | 41 751 (17.0) | 1183 (17.9) |
| 24–59 | 79 950 (31.7) | 71 980 (31.5) | 7970 (33.3) | 77 890 (31.7) | 2060 (31.2) |
| ≥60 | 19 692 (7.8) | 17 626 (7.7) | 2066 (8.6) | 19 339 (7.9) | 353 (5.3) |
| Partner change * | |||||
| Yes | 15 789 (6.3) | 14 307 (6.3) | 1482 (6.2) | 15 572 (6.3) | 217 (3.3) |
| Smoking | |||||
| Yes | 17 239 (13.6) | 16 062 (13.7) | 1177 (12.7) | 16 705 (13.8) | 534 (9.6) |
| Fertility treatment | |||||
| Yes | 4185 (2.7) | 3872 (2.7) | 313 (2.4) | 3882 (2.6) | 303 (4.9) |
Data are presented in n(%) based on study cohort that consists of first two pregnancies.
*Measured at second pregnancy.
GDM, gestational diabetes; PE, preeclampsia.
Incident and recurrent risks of pregnancy complications
Risks of PE in first and second pregnancy were 9.5% and 2.4%, respectively, with a recurrence rate of 19.3% at a second pregnancy. The risk of GDM was 2.6% in both first and second pregnancies, with a recurrence rate of 41.5% at second pregnancy (online supplemental table 4).
The lowest incidence at second birth was observed for IPIs of 6–11 months for both PE and GDM. Incidences were relatively higher for IPIs <6 months and ≥24 months (table 2). For both complications, the recurrence risks were generally higher at IPIs <6 months and ≥60 months (online supplemental table 4).
Table 2.
Adjusted relative risk (RRs) and predicted absolute risks (ARs) of pregnancy complications according to interpregnancy interval (IPI) stratified by pregnancy complication at first pregnancy for mothers with their first two consecutive births during the study period (n=252 368 mothers)
| Interpregnancy interval (months): RR, AR and RD (95% CI) | ||||||||
| Outcome | 3 | 6 | 12 | 18 | 24 | 36 | 48 | 60 |
| Preeclampsia (PE) | ||||||||
| Previous PE | ||||||||
| RR (95% CI) | 1.09 (0.94 to 1.25) | 0.99 (0.89 to 1.09) | 0.93 (0.85 to 1.03) | 1 (Reference) | 0.97 (0.90 to 1.05) | 1.04 (0.95 to 1.13) | 1.06 (0.98 to 1.16) | 1.06 (0.98 to 1.15) |
| AR % (95% CI) | 16.3 (13.8 to 18.9) | 14.7 (12.9 to 16.4) | 13.8 (12.3 to 15.3) | 14.8 (13.2 to 16.4) | 14.4 (12.9 to 15.9) | 15.5 (14.0 to 17.0) | 16.0 (14.3 to 17.6) | 15.9 (14.3 to 17.6) |
| RD % (95% CI) | 1.5 (−1.00.6 to 4.1) | −0.1 (−1.7 to 1.5) | −1.0 (−2.5 to 0.4) | Reference | −0.4 (−1.6 to 0.8) | 0.7 (−0.7 to 2.1) | 1.2 (−0.3 to 2.6) | 1.1 (−0.4 to 2.6) |
| No previous PE | ||||||||
| RR (95% CI) | 1.24 (1.07 to 1.43) | 1.00 (0.90 to 1.11) | 0.90 (0.81 to 0.99) | 1 (Reference) | 1.04 (0.96 to 1.13) | 1.23 (1.13 to 1.35) | 1.34 (1.23 to 1.46) | 1.40 (1.29 to 1.53) |
| AR % (95% CI) | 1.5 (1.3 to 1.8) | 1.1 (1.0 to 1.3) | 1.0 (0.9 to 1.1) | 1.1 (1.0 to 1.2) | 1.1 (1.0 to 1.2) | 1.4 (1.3 to 1.5) | 1.6 (1.4 to 1.8) | 1.7 (1.5 to 1.9) |
| RD % (95% CI) | 0.4 (0.2 to 0.7) | 0.1 (−0.1 to 0.2) | −0.1 (−0.2 to 0.01) | Reference | 0.1 (−0.0 to 0.1) | 0.3 (0.2 to 0.4) | 0.5 (0.4 to 0.6) | 0.6 (0.5 to 0.8) |
| Gestational diabetes | ||||||||
| Previous GDM | ||||||||
| RR (95% CI) | 1.11 (0.95 to 1.29) | 0.87 (0.78 to 0.97) | 0.94 (0.85 to 1.04) | 1 (Reference) | 0.96 (0.88 to 1.04) | 1.07 (0.98 to 1.18) | 1.14 (1.05 to 1.25) | 1.18 (1.07 to 1.29) |
| AR % (95% CI) | 39.7 (30.1 to 49.2) | 30.3 (23.5 to 37.1) | 32.6 (24.5 to 40.7) | 35.3 (28.0 to 42.6) | 33.3 (25.4 to 41.2) | 38.6 (31.8 to 45.5) | 41.5 (35.1 to 47.8) | 43.2 (38.3 to 48.2) |
| RD % (95% CI) | 4.4 (−2.6 to 11.3) | −5.0 (−9.0 to −0.9) | −2.7 (−6.7 to 1.3) | Reference | −2.0 (−5.4 to 1.4) | 3.3 (−0.5 to 7.2) | 6.2 (1.9 to 10.5) | 7.9 (2.1 to 13.9) |
| No previous GDM | ||||||||
| RR (95% CI) | 1.00 (0.85 to 1.16) | 0.87 (0.78 to 0.97) | 0.87 (0.79 to 0.96) | 1 (Reference) | 1.20 (1.11 to 1.29) | 1.75 (1.62 to 1.90) | 2.18 (2.01 to 2.35) | 2.58 (2.38 to 2.79) |
| AR % (95% CI) | 3.0 (2.5 to 3.4) | 2.4 (2.2 to 2.7) | 2.3 (2.1 to 2.6) | 2.7 (2.4 to 2.9) | 3.2 (3.0 to 3.5) | 4.9 (4.5 to 5.2) | 6.3 (5.8 to 6.8) | 7.6 (7.0 to 8.3) |
| RD % (95% CI) | 0.3 (−0.2 to 0.8) | −0.2 (−0.5 to 0.1) | −0.3 (−0.6 to −0.1) | Reference | 0.5 (0.4 to 0.9) | 2.2 (1.9 to 2.5) | 3.6 (3.2 to 4.1) | 4.90 (4.4 to 5.6) |
IPI was modelled using restricted cubic splines with knots placed at 3, 6, 12, 18, 24, 36, 48 months of IPI. Models were adjusted for maternal age, socioeconomic status (SES), birth year, ethnicity, marital status at birth prior to IPI and partner change at recent birth with 18 months of IPI as reference. Maternal age was modelled using restricted cubic splines with 4 knots at the 5th, 35th, 65th and 95th percentiles (ages 18, 24, 29 and 35); predicted absolute risks are reported at representative values of covariates: Caucasian, married, average SES, average maternal age (25.1) and birth year in 2010 at birth prior to the IPI.
GDM, gestational diabetes; RD, risk difference.
Absolute risk of pregnancy complications by IPI and previous complication status
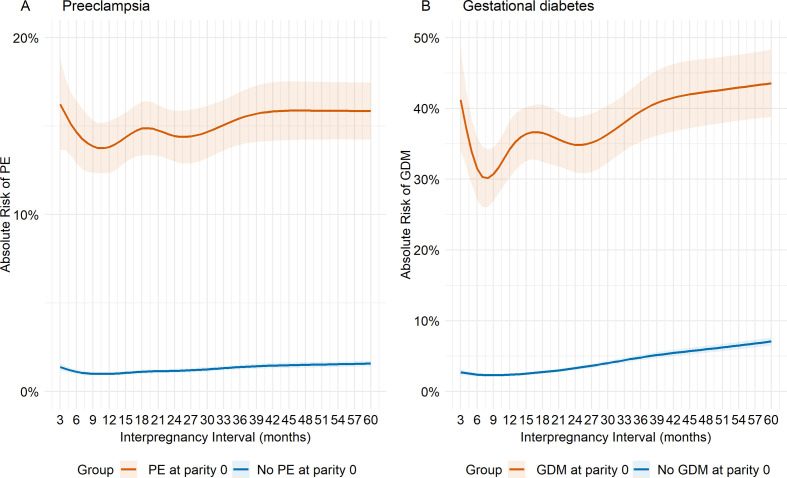
The absolute risks of PE in the second birth were higher for mothers with previous PE than mothers with no previous PE across the IPI continuum (table 2). The absolute risks of PE ranged between 14% and 16% for previous PE and 1%–2% for mothers with no previous PE, with the highest risk at IPI<6 or >60 months and lowest at around 12 months for mothers with previous PE. For mothers with no previous PE, the intervals at which risks were lowest were less clear but appeared to be around 12 months (table 2, figure 1A). The absolute risks of GDM ranged from 30% to 43% for mothers with previous GDM versus 2% to 8% for mothers with no previous GDM. Risks of GDM were smallest at intervals between 6 and 12 months for mothers with and without previous GDM (table 2, figure 1B).
Figure 1.
Predicted absolute risks (95% CIs) at each interpregnancy interval (IPI) from 3 to 60 months according to history for (A) preeclampsia, and (B) gestational diabetes for mothers with first two consecutive pregnancies. Predicted absolute risks are reported at representative values of covariates: Caucasian, married, average socioeconomic status, average maternal age (25.1) and birth year in 2010 at birth prior to the IPI. GDM, gestational diabetes; PE, preeclampsia.
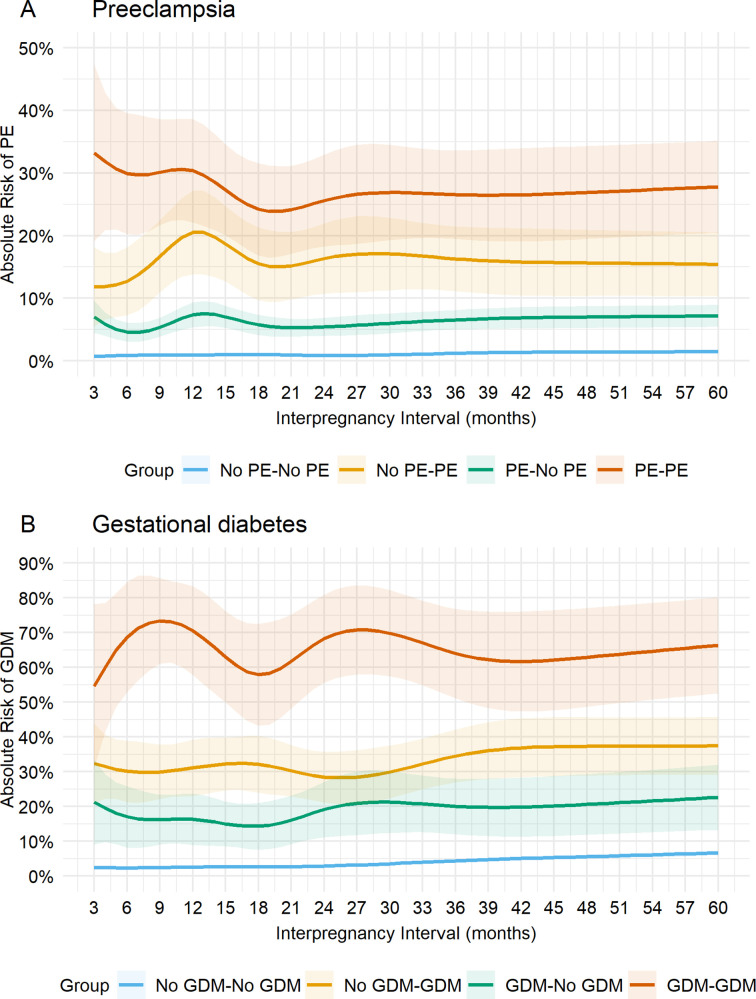
We next estimated the predicted absolute risk of each outcome associated with IPI according to presence or absence of previous complications for the subcohort of mothers with their first three consecutive pregnancies (parity 0, 1, 2), calculated at representative values of each risk factor (table 3, figure 2A, B). The predicted risk of PE for mothers with no PE in their first and second births (No PE–No PE group) ranged between 0.7% and 0.9% for IPIs of <24 months, lowest at around 24 months and increased with IPI afterwards. For mothers with a history of PE in either first or second births, the intervals at which risks were lowest were less clear but appeared to be around 6 months, with elevated risk at 12 months of IPI for both groups. However, the predicted risk of PE was markedly higher for mothers with a history of PE in their recent pregnancy (12%–21% for No PE–PE group) than mothers with PE in their first, but not second birth (5%–7% for PE–No PE group. These risks were even more pronounced in the third birth for mothers who developed PE in their first and second births (24%–33% for PE–PE group) (table 3, figure 2A, online supplemental video 1).
Table 3.
Adjusted relative risk (RRs) and predicted absolute risks (ARs) of pregnancy complications according to interpregnancy interval (IPI) stratified by pregnancy complications at their first and/or second pregnancy for mothers with their first three consecutive births during the study period (n=96 315 mothers)
| Interpregnancy interval (months): RR, AR and RD (95% CI) | ||||||||
| Outcome | 3 | 6 | 12 | 18 | 24 | 48 | 60 | |
| Preeclampsia | ||||||||
| No PE–No PE | ||||||||
| RR (95% CI) | 0.72 (0.51 to 1.01) | 0.87 (0.71 to 1.08) | 0.94 (0.76 to 1.16) | 1 (Reference) | 0.87 (0.74 to 1.03) | 1.22 (1.03 to 1.43) | 1.41 (1.22 to 1.65) | 1.46 (1.27 to 1.69) |
| AR % (95% CI) | 0.7 (0.47 to 0.93) | 0.9 (0.66 to 1.05) | 0.9 (0.73 to 1.10) | 1.0 (0.79 to 1.17) | 0.9 (0.69 to 1.01) | 1.2 (1.00 to 1.38) | 1.4 (1.15 to 1.62) | 1.4 (1.19 to 1.68) |
| RD % (95% CI) | −0.3 (−0.6 to −0.03) | −0.1 (−0.33 to −0.07) | −0.1 (−0.3 to 0.1) | Reference | −0.1 (−0.3 to 0.02) | 0.2 (0.02 to 0.38) | 0.4 (0.2 to 0.6) | 0.4 (0.2 to 0.6) |
| No PE–PE | ||||||||
| RR (95% CI) | 0.77 (0.45 to 1.32) | 0.83 (0.58 to 1.18) | 1.30 (0.93 to 1.82) | 1 (Reference) | 1.05 (0.80 to 1.37) | 1.05 (0.78 to 1.40) | 1.01 (0.76 to 1.33) | 0.99 (0.75 to 1.31) |
| AR % (95% CI) | 14.9 (6.8 to 23.1) | 15.6 (8.6 to 22.6) | 22.2 (15.2 to 29.3) | 18.4 (11.4 to 25.4) | 18.4 (12.0 to 24.7) | 20.5 (13.1 to 27.9) | 17.2 (11.6 to 22.9) | 16.9 (11.4 to 22.4) |
| RD % (95% CI) | −3.5 (−11.3 to 4.3) | −2.8 (−8.9 to 3.3) | 3.8 (−2.7 to 10.4) | Reference | −0.03 (−5.1 to 5.0) | 2.1 (−3.6 to 7.8) | −1.19 (−6.5 to 4.2) | −1.5 (−6.6 to 3.7) |
| PE–No PE | ||||||||
| RR (95% CI) | 1.21 (0.87 to 1.69) | 0.81 (0.61 to 1.07) | 1.26 (0.96 to 1.65) | 1 (Reference) | 0.95 (0.76 to 1.17) | 1.13 (0.91 to 1.42) | 1.21 (0.97 to 1.49) | 1.23 (1.00 to 1.52) |
| AR % (95% CI) | 6.9 (4.4 to 9.4) | 4.6 (3.1 to 6.1) | 7.3 (5.3 to 9.3) | 5.8 (4.1 to 7.4) | 5.3 (3.9 to 6.7) | 6.4 (4.9 to 8.0) | 6.9 (5.2 to 8.6) | 6.6 (4.8 to 8.5) |
| RD % (95% CI) | 1.2 (−1.3 to 3.6) | −1.2 (−2.7 to 0.4) | 1.6 (−0.3 to 3.4) | Reference | −0.5 (−1.8 to 0.8) | 0.7 (−0.7 to 2.1) | 1.1 (−0.3 to 2.5) | 0.9 (−1.0 to 2.7) |
| PE–PE | ||||||||
| RR (95% CI) | 1.31 (0.92 to 1.89) | 1.20 (0.93 to 1.55) | 1.22 (0.95 to 1.56) | 1 (Reference) | 1.05 (0.86 to 1.29) | 1.08 (0.87 to 1.35) | 1.10 (0.89 to 1.36) | 1.13 (0.92 to 1.39) |
| AR % (95% CI) | 37.2 (21.8 to 52.6) | 30.9 (21.2 to 40.6) | 31.1 (23.0 to 39.3) | 24.1 (16.9 to 31.2) | 27.1 (19.5 to 34.7) | 29.2 (21.0 to 37.4) | 27.9 (20.5 to 35.3) | 28.3 (21.1 to 35.5) |
| RD % (95% CI) | 13.1 (−1.8 to 28.0) | 6.8 (−1.3 to 15.0) | 7.1 (−0.7 to 14.8) | Reference | 3.1 (−3.3 to 9.4) | 5.2 (−2.6 to 12.9) | 3.9 (−2.4 to 10.1) | 4.3 (−1.7 to 10.3) |
| Gestational diabetes | ||||||||
| No GDM–No GDM | ||||||||
| RR (95% CI) | 0.94 (0.73 to 1.21) | 0.90 (0.74 to 1.09) | 0.99 (0.82 to 1.19) | 1 (Reference) | 1.11 (0.97 to 1.27) | 1.71 (1.48 to 1.97) | 2.18 (1.91 to 2.49) | 2.60 (2.29 to 2.95) |
| AR % (95% CI) | 2.6 (1.9 to 3.2) | 2.4 (1.9 to 2.8) | 2.6 (2.2 to 2.9) | 2.5 (2.2 to 2.9) | 2.9 (2.5 to 3.3) | 4.4 (3.9 to 4.9) | 5.7 (5.0 to 6.4) | 7.0 (6.1 to 7.9) |
| RD % (95% CI) | 0.01 (−0.7 to 0.7) | −0.2 (−0.7 to 0.3) | 0.00 (−0.5 to 0.5) | Reference | 0.3 (−0.04 to 0.7) | 1.9 (1.4 to 2.3) | 3.2 (2.6 to 3.8) | 4.5 (3.6 to 5.3) |
| No GDM–GDM | ||||||||
| RR (95% CI) | 1.01 (0.75 to 1.36) | 0.95 (0.75 to 1.19) | 0.97 (0.77 to 1.23) | 1 (Reference) | 0.90 (0.74 to 1.10) | 1.06 (0.88 to 1.29) | 1.14 (0.95 to 1.37) | 1.14 (0.96 to 1.37) |
| AR % (95% CI) | 30.6 (19.6 to 41.6) | 24. (14.6 to 34.7) | 28.5 (20.2 to 36.7) | 32.2 (24.6 to 39.8) | 25.5 (17.9 to 33.2) | 34.9 (27.8 to 41.9) | 38.5 (30.6 to 46.3) | 36.4 (28.6 to 44.2) |
| RD % (95% CI) | −1.6 (−12.7 to 9.5) | −7.6 (−17.5 to 2.4) | −3.7 (−12.5 to 5.0) | Reference | −6.6 (−14.2 to 0.9) | 2.7 (−4.3 to 9.7) | 6.3 (−0.7 to 13.3) | 4.2 (−2.8 to 11.2) |
| GDM–No GDM | ||||||||
| RR (95% CI) | 1.43 (0.84 to 2.44) | 1.17 (0.75 to 1.81) | 1.13 (0.73 to 1.74) | 1 (Reference) | 1.29 (0.92 to 1.82) | 1.37 (0.94 to 1.99) | 1.40 (0.97 to 2.01) | 1.51 (1.06 to 2.16) |
| AR % (95% CI) | 20.7 (11.8 to 29.6) | 27.2 (13.9 to 40.5) | 17.2 (10.6 to 23.8) | 7.8 (4.0 to 11.7) | 19.5 (13.1 to 25.9) | 18.5 (12.9 to 24.1) | 22.1 (14.9 to 29.3) | 17.2 (11.7 to 22.7) |
| RD % (95% CI) | 12.9 (3.7 to 22.1) | 19.4 (5.4 to 33.4) | 9.3 (2.2 to 16.4) | Reference | 11.7 (5.4 to 17.9) | 10.6 (4.9 to 16.3) | 14.3 (7.1 to 21.4) | 9.4 (4.6 to 14.1) |
| GDM–GDM | ||||||||
| RR (95% CI) | 0.94 (0.62 to 1.42) | 1.19 (0.93 to 1.51) | 1.22 (0.97 to 1.54) | 1 (Reference) | 1.18 (0.98 to 1.43) | 1.10 (0.89 to 1.36) | 1.08 (0.88 to 1.33) | 1.15 (0.93 to 1.42) |
| AR % (95% CI) | 54.6 (31.1 to 78.1) | 75.5 (61.5 to 89.6) | 77.8 (66.5 to 89.1) | 70.3 (52.9 to 87.7) | 73.7 (64.0 to 83.4) | 79.1 (62.3 to 95.9) | 64.5 (52.0 to 77.1) | 73.9 (55.5 to 92.4) |
| RD % (95% CI) | −3.3 (−12.1 to 5.6) | 5.3 (−8.1 to 18.6) | 7.5 (−4.9 to 19.9) | Reference | 3.4 (−10.3 to 17.1) | 8.7 (−0.1 to 17.6) | −5.8 (−20.3 to 8.9) | 3.6 (−6.9 to 14.2) |
IPI was modelled using restricted cubic splines with knots placed at 3, 6, 12, 18, 24, 36, 48 months of IPI. Models were adjusted for maternal age, socioeconomic status (SES), birth year, ethnicity, marital status at birth prior to IPI and partner change at recent birth with 18 months of IPI as reference. Maternal age was modelled using restricted cubic splines with 4 knots at the 5th, 35th, 65th and 95th percentiles (ages 18, 24, 29 and 35); Predicted absolute risks are reported at representative values of covariates: Caucasian, married, average SES, average maternal age (26.5) and birth year in 2010 at birth prior to the IPI.
GDM, gestational diabetes; PE, preeclampsia; RD, risk difference.
Figure 2.
Predicted absolute risks (95% CIs) at each interpregnancy interval (IPI) from 3 to 60 months according to histories for (A) preeclampsia, and (B) gestational diabetes for mothers with first three consecutive pregnancies. Predicted absolute risks are reported at representative values of covariates: Caucasian, married, average socioeconomic status, average maternal age (26.5) and birth year in 2010 at birth prior to the IPI. GDM, gestational diabetes; PE, preeclampsia.
bmjopen-2020-046962supp002.mp4 (349.4KB, mp4)
Generally, the predicted absolute risk of GDM at third pregnancy differed by mothers’ history of GDM. Absolute risks were relatively lower for mothers without GDM in their first and second pregnancies (2%–7% for No GDM–No GDM group), slightly higher for mothers with pregnancies complicated by GDM during the second but not the first (14%–22% for No GDM–GDM group), and substantially higher for mothers who developed GDM during their first and second pregnancies (55%–70% for GDM–GDM group). For mothers with no history of GDM in both pregnancies (No GDM–No GDM group), risks were minimal at IPI of <18 months, but risks increased consistently with increasing IPI.
For mothers with GDM in first but not second (GDM–No GDM group) and mothers with GDM in their first and second pregnancies (GDM–GDM group), risks were minimal at intervals of approximately 18 months. In contrast, minimal risks were observed at around 24 months for mothers with GDM in their second but not first pregnancy. Interestingly, for most of these groups except mothers with no history of previous GDM (No GDM–No GDM group), risks were higher at IPIs of <6 months (online supplemental video 2).
bmjopen-2020-046962supp003.mp4 (479.2KB, mp4)
RRs of IPI on PE by previous PE status
For mothers with no previous PE at parity 0, there was a ‘J-shaped’ relationship between IPI and PE at parity 1, with greater risk for IPIs at 3 months (RR 1.24, 95% CI 1.07 to 1.43) and 60 months (RR 1.40, 95% CI 1.29 to 1.53) compared with 18 months. However, for mothers with PE at parity 0, there was insufficient evidence for an association between IPI and PE at parity 1, with consistently lower RRs than mothers with no previous PE for all IPIs (table 2).
RRs of IPI on GDM by previous GDM status
There was relatively more evidence that shorter IPIs of less than 18 months were associated with lower risk than at IPIs of 18 months for mothers with no previous GDM. In contrast, adverse associations were more pronounced at longer intervals (RR 1.18, 95% CI 1.07 to 1.29) and (RR 2.58, 95% CI 2.38 to 2.79) at 60 months of IPI for mothers with and without previous GDM, respectively. The J-shaped relationship between IPI and GDM was less clear for mothers with previous GDM than mothers who no previous GDM. These general patterns were also evident in an analysis of mothers with three consecutive pregnancies. The estimates for IPIs longer than 36 months were attenuated for mothers with at least one pregnancy complication (PE or GDM) compared with mothers with no complications in their first and second pregnancies (table 2, figure 1A, B).
Sensitivity analysis
The results of our sensitivity analysis to the choice of timing of the effect modifier (complications for any previous pregnancy as opposed to a complication at the immediate previous pregnancy) were consistent with the main analyses (online supplemental table 1). There was a negligible difference in the associations between IPI and pregnancy complications when we adjusted for additional covariates, including smoking and paternal age (online supplemental table 2). Similarly, we observed a slight difference in the association when we adjusted for variables at the time of the outcome of interest (online supplemental table 3).
Discussion
Principal findings
In this large retrospective cohort, we observed an increased risk of PE for short and long IPIs compared with 18 months, but only for mothers with no previous PE. In addition, adverse associations of IPI with GDM were observed at longer intervals of >36 months for both mothers with and without previous GDM. However, IPIs of less than 18 months were associated with a lower risk of GDM compared with IPI of 18 months in mothers with no previous GDM. Generally, the predicted absolute risks following short or long IPIs for PE and GDM were higher for mothers with previous complications than mothers with no previous pregnancy complications, most notably when the complication was experienced for the more recent birth.
Strengths of the study
This large cohort was sourced from highly reliable population-based perinatal information ascertained from hospital separations and perinatal database. To our knowledge, this is the largest population-based study to examine the non-linear relationships between IPI and pregnancy complications based on previous complication status. Modelling IPI flexibly allows for the estimation of risk curves and better clarification of optimal IPI. Our findings provide more clinically applicable information on the effect of different IPIs on the risk of PE and GDM based on the history of these complications.
Limitations of the data
In interpreting our findings, the following limitations must be considered. First, as we estimated risks at each IPI based on comparing outcomes of different women (between-women), our results might be biased due to unmeasured confounding. Recently, studies that have used within-women (matched designs) have reported substantially attenuated associations between IPI and pregnancy complications, owing to unmeasured or residual confounding.11 12 28 Second, although the information on fecundity was not available, variability in fecundity would be smaller for this cohort, which consisted of mothers who had two or more births. Third, a common limitation of IPI studies, including ours, is that the lack of information on dates of miscarriage and gestational age at miscarriage. Additionally, because it is both unethical and infeasible to randomise IPI to mothers, we cannot rule out the possibility of bias attributable to the observational design employed in our study. Due to small number of events at extremes of IPI for mothers with complications at both of their previous births (PE–PE; GDM–GDM groups), the predicted risks presented should be interpreted cautiously.
Furthermore, our study may have been subject to a certain degree of misclassification as ultrasound confirmed gestations were less common during the earlier periods of our birth cohort. However, results from our sensitivity analyses restricted to the cohort of births later in the study period did not meaningfully change our effect estimates. Finally, our findings should be interpreted as average population risks rather than individual-level risks. We expect individual risks will be more variable than the population averages in our study.
Interpretation
We observed that mothers with previous complications had higher absolute risks for developing recurrent complications as compared with their counterparts, across the IPI continuum. Risks were minimal at IPIs approximately between 6 and 12 months for both complications. In line with a well-documented recurrence effect of PE and GDM,8 18 our results show that mothers who had previous PE or GDM had approximately eight-fold and five-fold increase in absolute risk of PE and GDM in the subsequent pregnancy as compared with mothers with no previous complications, respectively. But most notably the range of absolute risk for mothers with no previous PE and previous PE (12%–15%) and for mothers with no previous GDM and previous GDM (30%–40%) was substantially greater than the observed increase in risk between IPIs (1%–2% for PE and 2%–8% for GDM). That is, the dominant factor contributing to risk was the previous pregnancy complication not the IPI. For mothers with no previous PE, where we observed a relatively larger RRs of short and long IPIs, there was a small increase in absolute risk for both short and long IPIs (~1% for PE and ~5% for GDM). Additionally, for mothers with previous PE or GDM the increased risks were relatively larger across IPI (2% for PE and 8% for GDM), but again the added risk due to IPIs was relatively low as compared with the higher risk of recurrence. This implies that presence of previous pregnancy complications was more important than IPIs in contributing to risk of PE or GDM in subsequent pregnancies.
Previous studies have showed associations between both short and long IPIs and increased risk of pregnancy complications in subsequent pregnancy.11 12 18 29 We showed that, for mothers with no previous complications, IPI is associated with increased risk of complications in subsequent pregnancies. Similarly, consistent with our findings, risk of PE in the second pregnancy increased with increasing IPI for only mothers with no history of PE.16 The observed higher risks at shorter IPIs (<6 months) for mothers with complications in either both or immediately preceding pregnancy can be explained by the maternal depletion hypothesis,30 whereby shorter intervals may not allow sufficient time for recovery from physiological stress at the maternal–fetal interface of a previous pregnancy. The adverse associations observed at longer IPIs for these complications might be attributable to loss of physiologic adaptation, under the hypothesis that the benefits of a previous birth in terms of physiological adaptation are gradually lost.30 Unmeasured variables such as changes in body mass index, pregnancy intention can also confound the association between IPI and pregnancy complications.24 However, results from our sensitivity analysis examining the inclusion of potential confounders (eg, smoking, paternal age, infertility status) did not change our estimates (online supplemental table 2).
Conclusions
This population-based cohort study revealed that the associations between IPI and risk of PE or GDM on subsequent pregnancies varied by presence/absence of these complications in previous pregnancies. The absolute risks following short or long IPIs for both PE and GDM were consistently higher for mothers with the presence of the condition in previous pregnancy. Risk differences varied more across IPIs for mothers with previous pregnancy complications as compared with without the condition in previous pregnancy. However, RRs were higher for mothers without the condition in previous pregnancy. Therefore, if the associations observed in this study reflect true effects, although more pregnancy complications can be prevented by avoiding suboptimal IPIs for women with a history of previous pregnancy complications (because of their higher baseline level of risk), proportionally more pregnancy complications are attributable to suboptimal IPI for mothers without a history of the pregnancy complications (because of their higher RRs).
Supplementary Material
Acknowledgments
The authors would like to thank the Data Linkage Branch (Department of Health Western Australia) as well as the Data Custodian for the Midwives Notification System and Hospital Morbidity Data Collection for providing data for this project.
Footnotes
Twitter: @amanuelpraise, @AnnetteKRegan
Contributors: All authors made a substantial contribution to this study. ATG designed the study, performed the analyses and drafted all sections of the manuscript. All authors contributed to the conceptualisation. All authors provided input on the methodological approach and substantive relevance, contributed to the interpretation of the findings, and reviewed the paper for intellectual content. GP is responsible for the overall content as guarantor. All authors reviewed the drafts of this manuscript and approved the final version for manuscript.
Funding: ATG is a recipient of a Curtin International Postgraduate Research Scholarship (CIPRS). This project was supported with funding from the National Health and Medical Research Council Project Grants #1 099 655 and #1 173 991 (to GFP) and the Research Council of Norway through its Centres of Excellence funding scheme #262 700. AKR is supported by a National Health and Medical Research Council fellowship (GNT1138425).
Disclaimer: The funders had no role in study design, data collection, analysis, interpretation of the results and decision to publish, or preparation of the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Data are available from the Western Australia Department of Health Data Linkage Branch with ethical approval through the Western Australia Department of Health Human Research Ethics Committee.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This research was approved by the Western Australia Department of Health WA Human Research Ethics Committee (reference 2016/51). The Ethics Committee approval was accepted on 14 September 2016.
References
- 1.Buckley BS, Harreiter J, Damm P, et al. Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabet Med 2012;29:844–54. 10.1111/j.1464-5491.2011.03541.x [DOI] [PubMed] [Google Scholar]
- 2.Gillon TER, Pels A, von Dadelszen P, et al. Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS One 2014;9:e113715. 10.1371/journal.pone.0113715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence RL, Wall CR, Bloomfield FH. Prevalence of gestational diabetes according to commonly used data sources: an observational study. BMC Pregnancy Childbirth 2019;19:1–9. 10.1186/s12884-019-2521-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:391–403. 10.1016/j.bpobgyn.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 5.Hernández-Díaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ 2009;338:b2255. 10.1136/bmj.b2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald SD, Best C, Lam K. The recurrence risk of severe de novo pre-eclampsia in singleton pregnancies: a population-based cohort. BJOG 2009;116:1578–84. 10.1111/j.1471-0528.2009.02317.x [DOI] [PubMed] [Google Scholar]
- 7.Major CA, deVeciana M, Weeks J, et al. Recurrence of gestational diabetes: who is at risk? Am J Obstet Gynecol 1998;179:1038–42. 10.1016/S0002-9378(98)70211-X [DOI] [PubMed] [Google Scholar]
- 8.Getahun D, Fassett MJ, Jacobsen SJ. Gestational diabetes: risk of recurrence in subsequent pregnancies. Am J Obstet Gynecol 2010;203:467.e1–467.e6. 10.1016/j.ajog.2010.05.032 [DOI] [PubMed] [Google Scholar]
- 9.Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Effects of birth spacing on maternal health: a systematic review. Am J Obstet Gynecol 2007;196:297–308. 10.1016/j.ajog.2006.05.055 [DOI] [PubMed] [Google Scholar]
- 10.Ball SJ, Pereira G, Jacoby P, et al. Re-evaluation of link between interpregnancy interval and adverse birth outcomes: retrospective cohort study matching two intervals per mother. BMJ 2014;349:g4333. 10.1136/bmj.g4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebremedhin AT, Regan AK, Ball S, et al. Effect of interpregnancy interval on gestational diabetes: a retrospective matched cohort study. Ann Epidemiol 2019;39:e33:33–8. 10.1016/j.annepidem.2019.09.004 [DOI] [PubMed] [Google Scholar]
- 12.Gebremedhin AT, Regan AK, Ball S, et al. Interpregnancy interval and hypertensive disorders of pregnancy: a population-based cohort study. Paediatr Perinat Epidemiol 2021;35:404-414. 10.1111/ppe.12668 [DOI] [PubMed] [Google Scholar]
- 13.WHO . Report of a WHO technical consultation on birth spacing. Geneva Switzerland: World Health Organization, 2005. [Google Scholar]
- 14.Royal College of Obstetricians and Gynaecologists . Postpartum family planning (best practice paper No. 1). United Kingdom: RCOG, 2015. [Google Scholar]
- 15.ACOG Committee opinion no. 736: optimizing postpartum care. Obstet Gynecol 2018;131:e140–50. 10.1097/AOG.0000000000002633 [DOI] [PubMed] [Google Scholar]
- 16.Trogstad LI, Eskild A, Magnus P, et al. Changing paternity and time since last pregnancy; the impact on pre-eclampsia risk. A study of 547 238 women with and without previous pre-eclampsia. Int J Epidemiol 2001;30:1317–22. 10.1093/ije/30.6.1317 [DOI] [PubMed] [Google Scholar]
- 17.Basso O, Weinberg CR, Baird DD, et al. Subfecundity as a correlate of preeclampsia: a study within the Danish national birth cohort. Am J Epidemiol 2003;157:195–202. 10.1093/aje/kwf194 [DOI] [PubMed] [Google Scholar]
- 18.Mostello D, Kallogjeri D, Tungsiripat R, et al. Recurrence of preeclampsia: effects of gestational age at delivery of the first pregnancy, body mass index, paternity, and interval between births. Am J Obstet Gynecol 2008;199:55.e1–55.e7. 10.1016/j.ajog.2007.11.058 [DOI] [PubMed] [Google Scholar]
- 19.Downey F. A validation study of the Western Australian Midwives’ Notification System. 2005 data. Perth: Department of Health, Western Australia, 2007. [Google Scholar]
- 20.Department of Health Western Australia . Midwives notification system, 2017. Available: http://ww2.health.wa.gov.au/Articles/J_M/Midwives-Notification-System [Accessed 28 Nov 2017].
- 21.Department of Health WA . Hospital Morbdiity data system reference manual. Perth, Australia: Health Data Collections Branch, Health Information Centre, 2004. [Google Scholar]
- 22.Marinovich ML, Regan AK, Gissler M, et al. Developing evidence-based recommendations for optimal interpregnancy intervals in high-income countries: protocol for an international cohort study. BMJ Open 2019;9:e027941. 10.1136/bmjopen-2018-027941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Australian Bureau of Statistics . Socio-Economic indexes for areas. Australian Bureau of statistics, 2013. Available: http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa [Accessed 28 Nov 2017].
- 24.Hutcheon JA, Moskosky S, Ananth CV, et al. Good practices for the design, analysis, and interpretation of observational studies on birth spacing and perinatal health outcomes. Paediatr Perinat Epidemiol 2019;33:O15-O24. 10.1111/ppe.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orsini N, Greenland S. A procedure to Tabulate and plot results after flexible modeling of a quantitative covariate. Stata J 2011;11:1–29. 10.1177/1536867X1101100101 [DOI] [Google Scholar]
- 26.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 27.Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology 2011;22:745. 10.1097/EDE.0b013e318225c2be [DOI] [PubMed] [Google Scholar]
- 28.Hanley GE, Hutcheon JA, Kinniburgh BA, et al. Interpregnancy interval and adverse pregnancy outcomes: an analysis of successive pregnancies. Obstet Gynecol 2017;129:408–15. 10.1097/AOG.0000000000001891 [DOI] [PubMed] [Google Scholar]
- 29.Basso O, Christensen K, Olsen J. Higher risk of pre-eclampsia after change of partner. An effect of longer interpregnancy intervals? Epidemiology 2001;12:624–9. 10.1097/00001648-200111000-00008 [DOI] [PubMed] [Google Scholar]
- 30.Miller JE. Birth intervals and perinatal health: an investigation of three hypotheses. Fam Plann Perspect 1991;23:62–70. 10.2307/2135451 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-046962supp001.pdf (757.3KB, pdf)
bmjopen-2020-046962supp002.mp4 (349.4KB, mp4)
bmjopen-2020-046962supp003.mp4 (479.2KB, mp4)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Data are available from the Western Australia Department of Health Data Linkage Branch with ethical approval through the Western Australia Department of Health Human Research Ethics Committee.