Abstract
The median lethal dose of pesticide in acute oral toxicity, used as a conservative index in avian risk assessment, varies by the species with differences of less than one order of magnitude, depending on body size, feeding habit, and metabolic enzyme activity. The profiles of pesticide metabolism in birds with characteristic conjugations are basically common to those in mammals, but less information is available on their relevant enzymes. The higher toxicity of some pesticides in birds than in mammals is due to the lower activity of avian metabolic enzymes. The bioaccumulation in birds is limited for very hydrophobic pesticides resistant to metabolic degradation. Several in silico approaches using the descriptors of a pesticide molecule have recently been employed to estimate the profiles of acute oral toxicity and bioaccumulation.
Keywords: acute oral and dietary toxicity, metabolism, conjugation, enzymology, bioaccumulation, pesticide
Introduction
Many kinds of wild birds inhabit the agricultural and forest regions where pesticides are applied frequently. Birds may either inhale the air contaminated with pesticides or be exposed dermally via skin or during and after the spray application of pesticides. Contact with pesticide deposits on crops and weeds via the feathers may result in the oral uptake of pesticides by preening. Since crops, weeds, and insects are the general food for birds, the oral uptake of pesticide residues therein is most probable.1) When pesticides are applied as a seed dressing, birds may be exposed to pesticides at a higher concentration by ingesting the treated seeds. In the case of a granule formulation, birds mistake the granules for seeds or ingest them as grit.1,2) By taking account of these possible routes of exposure and the feeding habit of birds, the toxicological impact of pesticides should be assessed based on their intrinsic toxicity via direct administration. For this purpose, the competent authority of registration first requires data on the acute oral (a median lethal dose, LD50 in mg/kg)3) and additional dietary (a median lethal concentration, LC50 in ppm)4) toxicity of the pesticide in the standard species, such as the northern bobwhite quail (Colinus virginianus) as an upland game bird and the mallard duck (Anas platrhynchos) as a waterfowl. Generally, it is difficult to extrapolate the toxicity observed in these species to that in another wild species because of differences in body size, feeding habit, and physiology. These differences are supposed to affect the metabolic activity, which finally determines the local concentration of a pesticide at a toxicological target site. Incidentally, when pesticide residues are detected in the feed items of poultry, the data on the transfer of residues to muscle, fat, liver, and eggs should be examined to set appropriate tolerances. The registrant conducts the metabolism and residue studies of the pesticide in laying hens (Gallus gallus domesticus)5) to determine the terminal residues of the pesticide and its major metabolites with the metabolic pathway.
The first part of this review deals with the acute and short-term toxicity as the first tier of avian risk assessment, from the viewpoints of route of exposure, species differences, and bioactivation; it also introduces the progress of their in silico estimation. The possible bioaccumulation of the pesticide via the food web is briefly discussed in relation to its partition coefficient and metabolism. The relevant factors controlling the metabolism, such as the administration route, species differences, and chirality, are summarized in the second part, together with the metabolic profiles of the pesticides by chemical class. Finally, an overview summary is provided, including issues that should be examined for a more refined avian risk assessment.
1. Acute and short-term toxicity
1.1. Routes of exposure
In order to examine the contribution of each exposure route in birds, the inhibition of brain cholinesterase (ChE) activity was conveniently used for northern bobwhite quail kept in an environmentally controlled closed-loop wind tunnel, where the aerial application of methyl parathion (MP) to cotton plants was simulated.6) The inhibition in the untreated birds represents that via all exposure routes. Birds covered with a body bag with only the beak being exposed to air, wearing a neck collar, or fed MP-treated meal worms were used to examine the contribution of inhalation, oral route by preening, or dietary route, respectively. The extent of dermal exposure was estimated by subtracting the contribution of each route above from that of all routes to the inhibition in the untreated birds. Exposure via inhalation and preening was critical only in the early period after aerial application; thereafter, both oral and dermal exposure routes became dominant. The importance of dermal exposure was also reported for brown-headed cowbirds in the semi-field study with the spray application of azinphos-methyl to apple trees.7) Incidentally, standardized guidelines of acute oral toxicity are available, and the pesticide is singly administered either by gelatin capsule or gavage at five to ten doses with a limit of 2000 mg/kg.3) In contrast, an acute dermal toxicity method still is not standardized, and the toxicity seems to depend on the site of application. The dermal toxicity of organochlorine (OC), organophosphorus (OP), and carbamate pesticides was compared among several avian species, when the acetone solution of each pesticide was applied to their foot pads or sparsely feathered breast under their wings.8) Lower LD50 values were obtained for the breast application, possibly due to more absorption of pesticide through the thinner stratum corneum of the breast than that of the foot pads. Incidentally, Hudson et al.9) showed moderate correlation between oral and dermal toxicity (r=0.65) for 20 pesticides applied to the foot pads of mallard ducks. They also introduced the dermal toxicity index, DTI=[LD50 (oral)/LD50 (dermal)]×100. Most of the tested pesticides showed values of less than 100, indicating lower toxicity via the dermal route than via the oral one. This trend was later confirmed for 19 pesticides by using seven avian species, and the DTI value was found to be pesticide-specific and independent of avian species.8)
These studies may show that acute oral toxicity is adequate as a primary index, but the other exposure routes should be kept in mind when assessing the toxic potential of pesticides in the field. Mineau10) analyzed the impact scoring data in the field monitoring of ChE-inhibiting pesticides (n=35) by using a logistic regression model. The 5% hazardous dose of the oral toxicity in the avian species-sensitivity distribution (HD5) was always an important factor for the “kill or no-kill” classification of pesticides. Both the DTI value and Henry’s law constant of a pesticide were necessary for its more precise classification, showing the importance of the dermal and inhalation routes in the field.
1.2. Acute oral toxicity
The short-term dietary toxicity study is another registration requirement. According to the standardized guidelines,4) birds are daily fed a diet homogeneously treated with the pesticide, usually at five concentrations with a limit of 5000 ppm, for five consecutive days. Since there is some difficulty in properly determining the exposure, and the LC50 values weakly correlate with each other among test species, Mineau et al.11) proposed the usage of LD50 instead for assessing the risk of a pesticide. Furthermore, Hilton et al.12) recently conducted a retrospective analysis of 119 pesticides registered between 1998 and 2017 in the USA from the viewpoint of the risk quotient (RQ, a point estimate of exposure divided by LD50 or LC50). The acute oral RQ was greater than the short-term dietary RQ in all but one case, and it could be used as the primary metric in comparison to the US EPA level of concern for avian risk assessment. On the basis of these examinations, acute oral toxicity is considered more appropriate as a conservative index.
In conducting an acute toxicity study, the adequacy of a pen-reared strain as the representative of its wild counterpart should be confirmed at least for standard species in the guidelines. By using northern bobwhite quail, this adequacy was demonstrated by similar brain ChE sensitivities against methyl paraoxon, hepatic microsomal oxidase activity on MP, and the acute oral toxicity of MP.13) Incidentally, the acute oral toxicity data of pesticides are not always available for non-standard species; hence, any kind of extrapolation is highly desired. Since a chemical’s uptake, distribution, and metabolism in mammals are highly related to their body weight, an allometric factor (AF) is generally used to extrapolate LD50. This approach, using the function of (body weight)AF, has been successfully adopted for determining avian acute oral toxicity.14) Regression analysis of the LD50 values in ≥10 avian species calculated the mean AF value to be 1.148 for 36 pesticides.
1.3. Bioactivation
Toxic symptoms of OC pesticides arise from their direct interaction with a target site, for example, axonal voltage–dependent Na channels for DDT and a GABA receptor for cyclodienes.15) OPs and carbamates inhibit acetyl ChE (AChE) at postsynaptic membranes in nervous systems by covalently binding with the AChE serine residue. However, the oxidative bioactivation of the thiophosphoryl moiety to the corresponding phosphoryl (oxon) is indispensable for the exhibition of toxicity of many OPs. Some examples of bioactivation, meaning higher toxicity of a metabolite than of the parent pesticide, are summarized in Table 1. The contribution of metabolism, including abiotic reactions such as hydrolysis and photolysis, to enhancing toxicity should be kept in mind when considering pesticide toxicity.
Table 1. Bioactivation of pesticides.
| Sp.a) | Pesticideb) | LD50c) | Metabolite | LD50 | Transformation | Ratiod) |
|---|---|---|---|---|---|---|
| M | Acephate* | 234 | Methamidophos | 29.5 | hydrolysis | 7.9 |
| M | Carbosulfan | 10 | Carbofuran | 0.76 | hydrolysis | 13 |
| B | Chlorfenapyr* | 34 | AC303,268 | 25 | N-dealkylation | 1.4 |
| M | Chlorothalonil | >2000 | SDS-3701 | 158 | hydrolytic dechlorination | >13 |
| B | Fipronil | 11.3 | MB 46513 | 5.4 | desulfinylation | 2.1 |
| B | Fluopicolide | >1744 | M01 | 1171 | N-debenzylation | >1.5 |
| B | Malathion | 359 | Malaoxon | 43 | oxidative desulfuration | 8.3 |
| M | Trichlorfon* | 36.8 | Dichlorvos | 7.78 | dehydration | 4.7 |
1.4. Species differences
1.4.1. Characteristics of avian anatomy and physiology
Differences in the toxicity of pesticides between birds and mammals are influenced by many things.16) Birds must take in food more rapidly than mammals of similar body size to maintain their higher body temperature (ca. 42°C; mammals, 37°C); as a result, birds may ingest more pesticide from contaminated food. Birds are oviparous animals, and their eggs additionally provide a characteristic excretory route for lipophilic pesticides, which exposes the next generation to the pesticide at an early developmental stage. Furthermore, the relative size of the liver to the body is smaller in birds than in mammals, and avian urine is voided into the cloaca with the possible reabsorption of metabolites from here in addition to the intestine. Finally, the existence of the coccygeal mesenteric vein connecting the hepatic portal vein to the renal one facilitates the transport of the pesticide and its metabolites through the blood stream from the gastrointestinal tract to both the liver and kidneys, which increases the contribution of metabolism in the avian kidney.
1.4.2. Interspecies differences
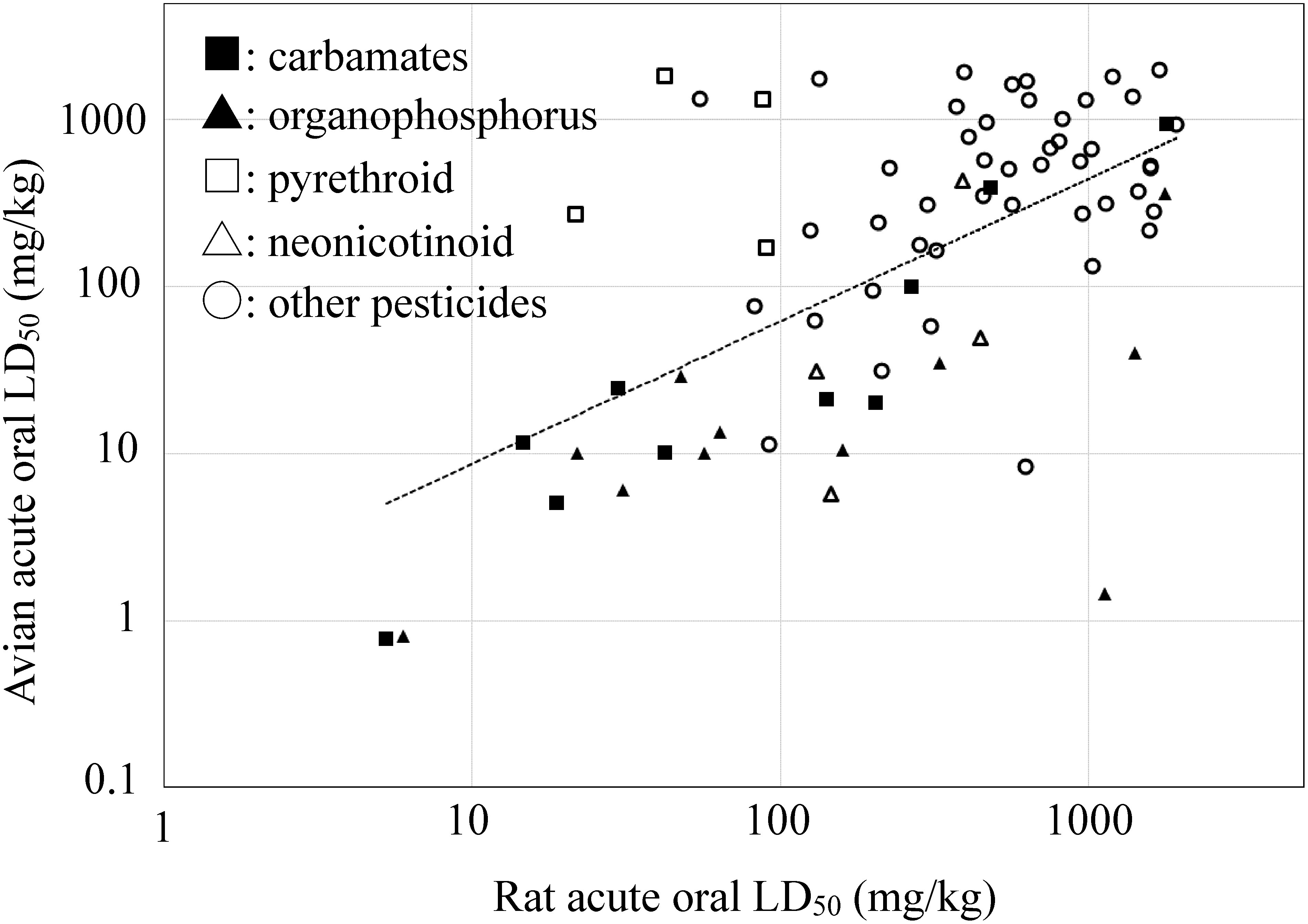
Higher toxicity in birds than mammals has been frequently reported for ChE-inhibiting pesticides, such as OPs and carbamates,16–18) while the opposite is known for pyrethroids.19) These species differences closely relate not only to the enzyme activity of esterases and oxidases16,18,19) but also to the sensitivity at the toxicological target site of each pesticide.20) In order to grasp species differences in acute oral toxicity, we surveyed the literature, European Food Safety Authority (EFSA) regulatory reports,21) and US EPA Reregistration Eligibility Documents.22) Toxicity data are mostly available for several standard avian species and rats. By using the rat and the lowest avian LD50 values in these information, pesticides (n=297; 85 insecticides, 91 fungicides, 121 herbicides) were classified as non-toxic (LD50>2000 mg/kg) in both species (58%), non-toxic only in birds (11%) or rats (6%), and toxic in both species (LD50<2000 mg/kg; 25%, n=74; Fig. 1). The linear regression analysis on pesticides toxic to both species showed a moderate correlation; log LD50 (birds)=0.85 log LD50 (rats)+0.087 (r=0.60). This is consistent with the relationship (r=0.71) reported between mallard ducks and rats for 20 pesticides.9) From the accumulated evidence on species differences, the higher avian toxicity of OPs and carbamates and lower toxicity of pyrethroids were also confirmed (Fig. 1). A slope of less than unity may show a higher toxic sensitivity in birds than in rats. Incidentally, no correlation was observed in acute dermal toxicity between mallard ducks (foot pad application) and rats, most probably due to the difference of structural proteins in the epidermal stratum (α-keratin in mammals and β-keratin in birds).9) When the breast application via its thinner stratum corneum was conducted for seven avian species, the correlation of DTI between birds and rats was found to be good (r=0.94), but it became much lower for pesticides requiring metabolic activation to exhibit toxicity.8)
Fig. 1. Relationship of acute oral LD50 (mg/kg) between birds and rats. The lowest LD50 value of each pesticide on birds was taken from the reported data (see supplemental data).
1.4.3. Intra-species differences
The enzyme activities of ChE, carboxylesterase (CaE), and cytochrome P450 (CYP) depend on both the body size and feeding habit of birds.16,23–25) Uridine 5′-diphospho-glucuronosyltransferase (UDPGT) and sulfotransferase (SULT) are the metabolic enzymes participating in the conjugation of various metabolites, and their activity is also dependent on the avian species.26,27) Therefore, these differences may, at least in part, affect the acute oral toxicity in each species. The existing LD50 values in various species exhibit a wide range for each pesticide, pharmaceutical, and industrial chemical, but they mostly differ by less than one order of magnitude. For example, the median factors of differences in the reported LD50 values (number of avian species, chemicals) are calculated to be 2.3 (3, 17),28) 3.1 (20, 24),17) 3.3 (7, 25),8) 3.9 (6, 17),21) and 8.1 (6, 16).29) Since the number of test species is very limited, usually 1 to 3, in the registration of a pesticide, together with the wide range of LD50 values described above, Mineau et al.11) proposed using a species-sensitivity distribution method in the avian risk assessment of a pesticide, by considering the AF value of the body weight. They derived HD5 as a toxicity reference value for each pesticide (n=880) on the basis of the acute oral LD50 data and tentatively identified 34 toxic pesticides as benchmarks, most of which are ChE inhibitors.
1.5. In silico approach to acute toxicity
The prediction of the acute oral LD50 value is very useful, not only for evaluating the potential avian risk of a pesticide candidate prior to its development but also for reasonably designing toxicity studies. In silico methods to describe the toxicity of pesticides have been applied less frequently to birds than to fish. Mazzatorta et al.30) classified 113 chemicals by their acute oral toxicity in northern bobwhite quails, using QSAR analysis with a support vector machine and genetic algorithms. Both the molecular size/shape information and electrostatic properties on its surface were good descriptors relating to their transport process and interactions with a toxicological target site, respectively. Basant et al.31) recently utilized decision tree forest or boost methods with nine descriptors of the molecular size, topology, and electronic properties, and they succeeded in describing the acute oral LD50 values of 131 pesticides in northern bobwhite quails (r2=0.95–0.97). These tree-based QSAR models could also predict LD50 values in the mallard duck, Japanese quail, ring-necked pheasant, and house sparrow at a satisfactory level. Incidentally, several machine learning methods, including those mentioned above, have been applied to classify about 600 chemicals by their short-term dietary toxicity levels (LC50) in the northern bobwhite quail and mallard duck.32) The model using the support vector machine gave the best prediction of LC50 in both species, and the five most important molecular descriptors were related to the electro-topological state of a molecule relating to P and S atoms. Furthermore, the alert substructures for avian toxicity were suggested to be OP and organosulfur moieties, and the reactive anhydrides were suggested to be amidines and aryl bromides.
1.6. Bioaccumulation
Lipophilic pesticides with log Kow ≥3 are susceptible to being accumulated from water, sediment, and soil to fish and sediment/soil-dwellers, both of which may be taken by birds as food.1) Therefore, the bioaccumulation of pesticides by a dietary route, especially via the food web, should be assessed to protect avian species. For example, in a long-term feeding study of OCs using laying hens, high accumulation ratios (AR, the concentration ratio against food) of lindane, HCB, DDT, and dieldrin were observed in the fat (2–17).33) In the case of poultry, the bioaccumulation potential of pesticides can be examined by the metabolism studies required for registration.1) In a feeding study of hydrophobic chlordane in cockerels by a single dose, the physiologically based pharmacokinetic (PBPK) analysis clearly demonstrated that the effective metabolism after rapid absorption to the body results in insignificant accumulation in each organ, but with much slower elimination from fat.34) Feeding studies of beta-cypermethrin in laying hens35) and imidacloprid in Japanese quails36) have also shown that the rapid metabolism of these pesticides results in the insignificant bioaccumulation of the parent insecticides and their metabolites in all tissues and eggs. In contrast, a similar analysis in the feeding study of chlordecone using laying hens showed its slower elimination mainly via bile, with the bioaccumulation in the liver, muscle, and eggs.37) These results clearly show that the metabolism of a pesticide is one important factor in controlling its bioaccumulation. When the compartment model, where the pesticide input to the liver is distributed to each organ via blood flow and eliminated in eggs and excreta, was used in the PBPK analysis, it described more pesticide residues in the fat of broilers than of laying hens.38,39) This difference indicates the importance of the excretory route to eggs.
The potential bioaccumulation of a pesticide can be evaluated by various methods. EFSA has adopted the simple equation, AR=(α * FIR)/k2,1) where FIR is a food intake rate relative to the body weight of an avian species to be assessed. The absorbed fraction of the ingested pesticide (α) and the elimination rate constant (k2) can be obtained in the metabolism study. Alternatively, the structure–activity relationship approach is convenient for estimating the AR value of a pesticide, and the moderate correlation of AR with log Kow (r=ca. 0.5) was reported for poultry and small birds.40) Many kinds of empirical and theoretical models have been developed to estimate the terrestrial bioaccumulation of organic chemicals, including pesticides, using their physico-chemical properties and environmental fate data, as reviewed by Gobas et al.41) The partition coefficient of a pesticide between n-octanol and water (Kow) or air (KOA) is the primary factor for determining the AR value, which has been confirmed recently in the trophic magnification (TM) of persistent organic pollutants (POPs) by stable isotope analysis.42) The tropic positions (TP) of primary producers, prey, and an apex predator in the Canadian urbanized region were estimated from the fraction of 15N in each, as determined by MS, and the 15N isotopic enrichment factor constant. The TM factors were determined from the correlation of TP with the lipid equivalent residue concentrations of POPs in each species. The TM factor was found to be well proportional to the log Kow and log KOA values of each POP (r=–0.99), showing the potential biomagnification of an organic chemical having a log Kow (KOA) of >4–6.
2. Avian metabolic enzymes
The metabolic reactions in birds, phase I (oxidation, reduction, and bond cleavage) and phase II (conjugations), as listed in Table 2, are basically similar to those in mammals.43) In general, the microsomal fraction contains CYPs and UDPGTs, while glutathione-S-transferases (GSTs) and SULTs are localized in the cytosolic fraction, and esterases are distributed in both fractions.
Table 2. Typical metabolic reactions of pesticides in birds.
| Oxidation | Reduction | Hydrolysis (Bond cleavage) | |||
| O1 | Alkyl oxidation/O (N)-dealkylation | R1 | Reductive dehalogenation | H1 | Carboxyl ester |
| O2 | Aryl hydroxylation | R2 | Dehydrohalogenation | H2 | Amide, imide, carbamate, urea |
| O3 | Epoxidation | R3 | Multiple bond (–=N–, –C=C–, C=O) | H3 | (Oxime, thio) Ether |
| O4 | S-oxidation | R4 | Nitro group | H4 | C(N)–C(N, S) bond |
| O5 | Desulfuration (P=S, C–O3H) | R5 | S-reduction | H5 | Phosphoryl (sulfonyl) ester |
| Conjugation | H6 | Dehalogenation/denitration | |||
| C1 | Glucuronidation | C5 | Acetylation | Miscellaneous | |
| C2 | Sulfation | C6 | Methylation | M1 | Rearrangement |
| C3 | Glutathione* | C7 | Other natural products (fatty acids, cholesterol etc.) | M2 | Cyclization |
| C4 | Amino acid | M3 | Hydration/dehydration | ||
*R-X+GSH → R-SG → R-Cys-Gly → R-Cys → R-(N-acetyl-Cys). R, alkyl or aryl; GSH, glutathione; Cys, cysteine; Gly: glycine.
2.1. Oxidases and reductases
CYPs, which catalyze the oxidation of their various moieties, are among the most important enzymes in the metabolism of pesticides. The total amount of CYPs in the avian liver ranges from 0.1 to 0.4 nmol/mg protein, which is lower than that of mammals (0.3–1.5 nmol/mg protein)43–46); therefore, the hepatic CYP activity of birds is generally comparable to or slightly lower than that of mammals and is highly dependent on the substrate.26,43,47) Both the total amount and activity of CYPs also depend on avian species and vary by age, sex, and strain. The age-dependent increase in hepatic enzyme activity was reported for ethoxyresorufin O-deethylase (EROD; CYP1A) and pentoxyresorufin O-depenthylase (PROD; CYP2B) in turkey poults (9–65 days old),48) and for mephobarbital N-demethylase (CYP2B) in chicken embryos (15–18 days old) and chicks (4–9 days old).49) A slight decrease in the aldrin epoxidase (AEase) activity with development was observed in the liver of the Japanese quail (7–35 days old),50) while such a change was insignificant for o-nitroanisole O-demethylase in the chicken.49) Using the liver of broiler chickens (1–56 days old), Hu51) demonstrated that each activity of CYPs (1A, 2H, 2C, 2D, and 3A) is highest just after hatching and, because of the intense lipid metabolism in embryos and thereafter, gradually decreases with development. Less than a twofold difference by sex was reported for aminopyrine demethylase (ADase) activity in the liver and kidneys of chicken and geese, but the hepatic activity differs by a factor of ca. 4 between two strains of chicken.27) Smaller differences by both sex and strain were reported for the hepatic amount and activity of AEase in the Japanese quail.50) The post-mitochondrial subcellular fraction (S9) of the chicken liver exhibited 2–50 times sex differences (male >> female) in the oxidative degradation of eight OPs.52)
The hepatic ADase activity was 1.1–4.8-fold higher than the renal activity in the chicken, turkey, duck, and goose,27) indicating the liver’s greater importance in pesticide metabolism. The alimentary tract may play a role in the metabolism of pesticides when considering its organ weight relative to the liver. Both the amount of CYP and the enzyme activities of EROD and AEase are comparable between the liver and duodenum in each of four avian species, and the weight ratio of the duodenum to the liver ranges from 0.3 to 0.6.44,53) The ADase activity (nmol/hr/mg dry tissue) in the alimentary tract of chicks decreased in the order of duodenum (8)>mid-intestine (6)>rectum (2)>crop (2).27) The activities of EROD and PROD in the small intestine were high, but less than those reported in the liver of the pigeon.54) Incidentally, the feeding habit controls the CYP activity. Fossi et al.25) measured the hepatic AEase activity in four europhagic and three stenophagic avian species collected from the Italian field. They assigned a score to each of six food categories by the percentage of each relative to the total food of birds, and the calculated total score for each avian species was used as an omnivorous index. The AEase activity was directly proportional to this index (r=0.7). By taking account of the EROD and PROD activities, it was found that the narrower the diet range of an avian species, the lower its hepatic CYP activity.25,54)
Enzyme induction is another issue to be considered in either the metabolism or toxicity of pesticides. CYPs can be induced in birds—for example, by β-naphthoflavone, phenobarbital, and 3-methylcholanthrene55)—as reported in mammals. Seven-day dietary exposure to dieldrin at 5 ppm increased the hepatic AEase activity in the Japanese quail twofold.50) No induction of hepatic aniline hydroxylase (AHase) by DDT at 100 ppm was observed in this species, while DDE activity increased by a factor of 2–3.56) Neither ADase nor AHase was induced by DDT, with their activity decreasing in the White Leghorn hen.57) In the case of seven days of dietary exposure to four pyrethroids at 2000 ppm, the organ-dependent effects on the activity of AHase, AEase, and EROD as well as the total amount of CYPs were reported in the Japanese quail.53) Insignificant effects were observed in the liver, but the pyrethroids weakly induced these enzymes in the duodenum. Three dicarboximide fungicides differently induced hepatic CYP activity in this species.53) Through seven days of dietary exposure to iprodione and vinclozolin at 2000 ppm, the activities of AHase, AEase, and EROD increased by a factor of 2–3, while procymidone only induced EROD. The induction of CYPs in the liver of the northern bobwhite quail was examined by the oral administration of several pesticides at 400 ppm once a day for three days.58) Western blot analysis using several antibodies to rat CYPs showed that both vinclozolin and propiconazole increased three- to sixfold the amounts of CYP 1A1/2, 3A, and 4A1, and newly induced 2A1 and 2C11. Interestingly, azole fungicides exhibit a compound-specific effect on hepatic CYPs. Imazalil at 1000 ppm was a weak inducer of AHase in the northern bobwhite quail,59) while the activity of AEase, AHase, and EROD increased up to sixteen-fold with three weeks of dietary exposure to prochloraz at 750 ppm in four avian species.44) In the Japanese quail, ten azole fungicides caused the dual effect of induction and inhibition with the dietary exposure.60) For example, propiconazole increased the hepatic activity of both AHase and EROD, fenarimol only decreased the former, and triadimefon only increased the former.
The presence of many avian CYP families is going to become clear by the analysis of the gene sequences derived from the DNA clones of, for example, CYP1A, 2H, 2E, and 3A in the chicken.45) More than 20 CYP1-3 genes are known in the chicken, zebra finch, and turkey, and the dominant role of the chicken CYP2C45 is supposed to be for xenobiotic metabolism.61) However, information relevant to its family’s participation in pesticide metabolism seems scarce. Rawal and Coulombe62) examined the metabolism of aflatoxin B1 (AFB1) in the hepatic microsomes of the toxin-susceptible turkey, using the monoclonal antisera against specific CYPs. It was found that the formation of the toxic metabolite, exo-8,9-epoxide of AFB1 (AFBO), was mainly catalyzed by CYP1A5 with the minimal contribution of CYP3A37 (<2%). Furthermore, as the detoxification pathways, CYP1A5 participated in the hydroxylation of the terminal furan ring of AFB1, while CYP3A37 oxidized its cyclopentenone ring. Recently, more CYP families in the liver were found to be involved in AFB1 metabolism in avian species, the turkey and duck (1A1/2, 2A6, 3A4) and the chicken and quail (1A1, 2A6).63) A similar approach to pesticide metabolism should be useful for understanding the role of each CYP in detoxification and/or bioactivation. The regioselectivity of avian CYPs against pesticides has been studied, together with its species differences, by using hepatic microsomes. The isopropyl methine and thiophosphoryl groups of diazinon were almost equally oxidized in the duck and turkey, and the methyl group of the pyrimidinyl ring was less susceptible to oxidation, while the oxon derivative was the main metabolite in the chicken.64) The aromatic carbons of warfarin were more favorably hydoxylated in four avian species than its methylene carbon, and the extent of oxidation decreased in the order of 4′-OH >6-OH >7-/8-OH.65) The S-oxidation of aldicarb to its sulfoxide was the main reaction in the chicken, with the corresponding sulfone as a minor metabolite.66)
Information on avian reductases participating in the metabolism of pesticides is very limited. In the liver and kidney homogenates of the chicken and the English sparrow, flavoprotein nitroreductases catalyzed the reduction of the nitro groups of parathion and EPN with flavin adenine dinucleotide as a cofactor.67) Comparable enzyme activity was observed between the two organs of each species, but with activity in the chicken twice as high as that in the sparrow. No induction of aromatic nitroreductases was reported in Japanese quail fed DDT or DDE at 100 ppm for three weeks, using p-nitrobenzoic acid as a substrate.56) Through the metabolism of AFB1 in the turkey, the aldehyde reductase played a detoxification role, and its presence in the hepatic cytosol was confirmed by western blot analysis.48) The stereo-specific keto reduction of warfarin (S>R) to alcohol in the S-configuration proceeded in the hepatic cytosol of the chicken.68) The complete inhibition of the reduction by menadione suggested the involvement of aldehyde oxidases.
2.2. Esterases
Esterases have been classified conveniently from the viewpoint of their hydrolyzing ability and inhibitors.69) A-esterases such as phosphotriesterases (PTEs) can hydrolyze aromatic esters and are not inhibited by OPs. The structure of the PTE active site is not known, except that of Pseudomonas diminuta, where two zinc cations chelated by at least four histidine residues catalytically hydrolyze OPs. Either the rapid dephosphorylation of the esterase-OP complex70) or no formation of such a tight complex as described above most likely accounts for the lack of inhibition by OPs. In contrast, B-esterases are inhibited by OPs and carbamates, such as ChEs and CaEs, whose active sites are the serine residue in the conserved motif.69)
The hydrolyzing activity against OPs was examined for PTEs in the blood plasma of 14 avian species.18,71) Paraoxon could be hydrolyzed only by the PTEs in the mute swan and Canada goose, with their activities lower than those of 11 mammalian species by two to three orders of magnitude. In the case of the pirimiphos-methyl oxon as a substrate, four avian species had low hydrolytic activity with inter-species differences similar to those above. Much lower PTE activity should result in the higher susceptibility of birds to toxic oxons than that of mammals.72) The PTE activities in the blood plasma and microsomal and cytosolic fractions of the liver and brain were compared between chickens and rabbits, using (RS) O-hexyl O-2,5-dichlorophenyl phosphoramidate (HDCP) as a model substrate.73) The stereo-specificity observed for the PTE (S>R) in rabbit plasma was lost in that of the chicken, with its activity much lower by two orders of magnitude. The stereo-specific activity of the hepatic microsomes (S>R) of the chicken was about fourfold higher than that in the cytosols (S<R). The brain fractions showed comparable activity in both species, but lower than that of the liver by a factor of 2–10 with the same specificity. The same research group next examined the reaction kinetics of this PTE in chicken serum albumin.74) Both HDCP and p-nitrophenyl butyrate were competitively hydrolyzed by this enzyme, which was not inhibited by Ca2+ and EDTA. Therefore, the reaction mechanism of this enzyme would be different from that of the usual PTEs, and it was supposed to be similar to that of ChEs.
The B-esterase activity of birds and mammals was comparable when procaine26) and phenyl acetate (PhA)71) were used as substrates. B-esterases such as CaEs and ChEs in the liver, kidney, and intestine of the duck,75) chicken,76) and quail77) were separated into many isoforms, using polyacrylamide gel electrophoresis (PAGE). Age-dependent enzyme activity was reported especially for CaEs in the liver and plasma of the duck and chicken, with their maxima after hatching.75,78) Similarly to CYPs, B-esterase activity depends on either avian body weight or feeding habit. Bush et al.79) reported in 55 avian species that the hepatic B-esterase activity against PhA and tricelin qualitatively correlates with birds’ body weight and diet range. By measuring the brain AChE and plasma CaE activity of seven avian species, Fossi et al.24) showed good correlation between the brain AChE and plasma CaE activity with not only avian body weight (r=–0.77 and –0.87) but also the omnivorous index (r=0.55 and 0.67), respectively. However, no correlation was observed for either CaE in the hepatic microsomes or butyryl ChE (BChE) in the plasma. In contrast, the total activity of AChE and BChE in plasma highly correlated with the body weight of 20 European raptors (r=–0.71), and the dominant ChE differed among the avian family.80) The involvement of B-esterases has been indirectly demonstrated in the in vitro metabolism of some pesticides by using an inhibitor. The in vitro metabolism of phenmedipham in the blood plasma of the chicken81) and that of cis-cypermethrin in the hepatic microsomes of the Japanese quail82) were greatly suppressed by the addition of OPs or carbaryl.
2.3. Glutathione S-transferases (GST)
GSTs, the homo or heterodimers with each subunit having a molecular weight of 24–28 kDa, catalyze the reaction of glutathione at the electrophilic site of a pesticide and its metabolites,83) as reported for the O-demethylation of many OP pesticides.52,84,85) The intact glutathione conjugate has been detected rarely in the avian metabolism,86,87) due to the successive metabolism by peptidases and N-acetyltransferases finally to form a mercapturic acid conjugate.83) When the herbicide propachlor was orally administered to hens, the cysteine and mercapturic acid conjugated via the reaction of glutathione at the chloromethyl carbon were detected as the main metabolites in the excreta.88)
The GST activity greatly varies by not only a substrate but also a species.89) A comparative study of the GST activity in the hepatic cytosolic fractions of the northern bobwhite quail, rainbow trout, and six mammals was conducted against six substrates.43) Quail showed the lower-to-lowest GST activity of any substrate among the eight species, and 1-chloro-2,4-dinitrobenzene (CDNB) was the best substrate. In vitro metabolism of three OPs by using liver homogenates showed less O-demethylation in the chicken than in mammals.52) Hepatic GST activity against 1,2-dichloro-4-nitrobenzene (DCNB) was reported to be much lower in the turkey than in the rat, but comparable activity against CDNB was observed in both species.90) In the kidney of the Japanese quail, the GST activity against DCNB was comparable to the hepatic activity.91)
GSTs can be conveniently classified into at least seven families in mammals, depending on the physico-chemical, structural, and immunological properties.92) Nine GSTs were isolated from the liver of chicks by using the glutathione affinity chromatography and chromato-focusing, and the molecular weight of each subunit was determined to be 24–27 kDa by SDS-PAGE.93) Furthermore, at least six α-GSTs and three μ-GSTs were identified by the analysis of the amino acid sequences and LC-MS/MS.94) In the case of the turkey, the hepatic cytosolic GSTs were classified into three α-GSTs and one σ-GST by western blot analysis using the respective antibodies against rat and chicken GSTs.90) Another research group reported that the hepatic GSTs of the turkey are heterodimers with optimal activity at a pH of 7.5 and 50°C against CDNB.95) Similar enzymology, but with homodimers, were reported for the hepatic GSTs of the Japanese quail.96,97) Incidentally, AFB1 is more toxic to birds than mammals due to their lack of the ability to metabolize the toxic metabolite AFBO by GST.62,63) The recombinant α- and μ-GSTs prepared by cloning the corresponding genes of the turkey well reproduced their activity against CDNB and DCNB.98) While they could also conjugate AFBO at a comparable reaction rate against DCNB, no activity was observed for the intact GSTs in the hepatic cytosolic fraction. Therefore, the GSTs metabolizing AFBO may be silenced by some regulatory mechanism in birds.
2.4. Other enzymes relevant to conjugation
UDPGTs and SULTs catalyze the glucuronidation and sulfation mainly at the hydroxyl oxygen by using uridine 5′-diphosphoglucuronic acid (UDPGA) and phosphoadenyl phosphosulfate (PAPS) as co-factors, respectively.83) Several pesticides such as carbaryl,99) fenitrothion,140) bifenazate,140) and prothioconazole140) instead undergo conjugation at the nitrogen and sulfur atoms of their metabolites. The hepatic activity of UDPGT and SULT highly depend on both the substrate and species.26,43) As compared with nine mammalian species, four avian species showed comparable or lower UDPGT activity levels against 4-nitrophenol, and lower SULT activity levels were mostly observed against 2-naphthol. In the comparative in vitro metabolism of cypermethrin, using hepatic S9 fractions, the dominant conjugation was the glucuronidation of the hydroxylated insecticide in rats, while the acyl glucuronides of both 3-phenoxybenzoic acid (PBacid) and the acid moiety of the insecticide were mainly formed in the Japanese quail, because of the rapid ester hydrolysis.82)
Among nine avian species, the hepatic activity of these enzymes varies by one to two orders of magnitude.27,100) Furthermore, which enzyme dominantly contributes to the conjugation depends on the avian species. For example, SULT is more involved in the conjugation of phenols than UDPGT in the livers of chickens and ducks,27,101) and the opposite is observed in those of turkeys and Japanese quail.27,102) Either age- or organ-dependent activity was reported for these enzymes. The hepatic enzyme activity, especially SULT, in the chicken was highest just after hatching, and lower thereafter.101) Their activity per dry weight of tissue in chicks decreased in the order of duodenum≒liver>mid-intestine>rectum.27) Incidentally, through a detailed sequence analysis of the UDPGT genes in 43 avian species, Kawai et al.100,103) showed the presence of UDPGT1E and UDPGT2 genes, which are further classified into six and three groups, respectively; feeding habits may affect not only the number of UDPGT1E genes but also the hepatic enzyme activity (carnivorous sp.<omnivorous and herbivorous sp.). A search of the chicken expressed sequence tag database has identified two cDNA clones that represent SULT subfamilies 1B and 1C.104) The recombinant SULTs, having histidine residue as an active site, could catalyze the sulfation of various phenols.
Carboxylic acids, irrespective of pesticides, their metabolites, or natural products, react with coenzyme A (CoA) to form a corresponding thioester.83,105) Acyltransferases (AcTs) catalyze the reaction of this thioester with amino and hydroxy derivatives to form amides and esters, respectively, but the enzymology of avian AcTs has not been extensively investigated. Although the hepatic cytosolic fraction of the rabbit possesses the high activity of acetyltransferases against many substrates, much lower activity in birds has been reported with the exception of some substrates, such as 2-aminofluorene.26,43) The nitro groups of parathion and fenitrothion were reduced and then N-acetylated in the liver and eggs.140) PBacid, the major metabolite of several pyrethroids, is conjugated with glycine, glycylvaline, and N-acetylornithine in mallard ducks and chickens,106,107) and the conjugation profiles are highly dependent on the avian species.108) Another conjugation of the long-chain fatty acid with the hydroxylated metabolite is reported for tebufenozide and bifenthrin.140)
3. Metabolism in birds
Information on the metabolism of many industrial chemicals, drugs, and pesticides by various avian species was accumulated up to the last quarter of the 20th century.108) The requirement for registering the poultry metabolism study by using a radio-labeled pesticide,5) in parallel with recent progress in LC(GC)-MSn, has provided more detailed information regarding avian metabolism. The metabolic pathway of a pesticide is generally examined through the chemical identification of metabolites by co-chromatographies with synthetic standards and/or instrumental analyses. In the case of polar conjugates, the aglycon released by chemical or enzymatic hydrolysis is frequently subjected to identification.
3.1. In vitro metabolism
In vitro metabolism is easily conducted and useful for grasping the major metabolism profiles in birds, as listed in Table 3. The S9 fraction, microsomes, and cytosols are prepared by the differential centrifugation of the homogenate of the liver, kidney, or intestine in the established manners.82,91,102) The addition of enzyme cofactors such as NADPH, glutathione, UDPGA, and PAPS is sometimes necessary to facilitate the metabolic reactions. Instead of these subcellular fractions, tissue slices67,111) and hepatocytes117,121) can be alternatively used (ex vivo). The metabolism study is usually conducted at 37°C and/or 42°C, which are the typical body temperatures of mammals and birds, respectively. The oxidation of alkyl and/or aryl moieties is frequently observed, as well as the hydrolysis of the ester and amide linkages. Reduction occurs less than oxidation, for example, for the chlorinated alkyl moieties of lindane109) and DDT,111) the nitro group of parathion,67) and the ketone of warfarin.68) O-Glucuronidation of the phenolic oxygen often proceeds in the metabolism, as reported for cypermethrin,82) carbaryl,91) NMC,102) and methoxychlor,112) while conjugations with a sulfate or glutathione are less observed. In the case of PCPMs, the FAB-MS measurement succeeded in directly identifying their glutathione conjugates.86) The concomitant formation of S-methyl glutathione with O-demethylation of tetrachlorvinphos was observed by incubation in the hepatic cytosolic fractions with glutathione, showing that GST catalyzes this reaction.87)
Table 3. In vitro metabolism of pesticides in birds.
| Pesticidea) | Sp.b) | Organc) | °C | Prod) | Sube) | Cofactorf) | MRg) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Lindane | CH | L (c) | 37.5 | na | 3.8† | ± GSH | R2 | 109 |
| Aldrin | CH | L (S9) | 41 | na | na | NADPH | O3 | 110 |
| DDT | FP | L (ts) | 41.5 | 0.1* | 11 | ± GSH | R1/2 | 111 |
| Methoxychlor | JQ | L (ts) | 39 | na* | 5 | none | O1, C1 | 112 |
| PCPMs | CH | L (m) | 37 | 62 | 2.5 | GSH | C3 | 86 |
| Carbaryl | JQ | L,K (m,c,S9) | 37 | 4 | 1 | NADPH, GSH | O1, H2, C1/2/3 | 91 |
| Aldicarb | CH | L (m) | rt | 3 | 50 | NADPH | O4 | 66 |
| Phenmedipham | CH | B | 37 | na | 82 | none | H2 | 81 |
| Trichlorfon | CH | L (c) | 37.5 | na | 73 | none | C3 | 113 |
| Tetrachlorvinphos | CH | L (c) | 37.5 | na | 0.3† | GSH | R2/3, H5, C3 | 87 |
| Diazinon | TU | L (ts) | 37 | 0.4* | 66 | NADPH | O1/5, M3 | 64 |
| Parathion | CH | L,K (ts) | 37 | 10* | 0.5† | NADPH | R4 | 67 |
| Fenitrothion | CH | L (m,c) | 37 | na | 70 | NADPH, GSH | O1/5, H5, C3 | 85 |
| NMC | JQ | L (m,c) | 37 | 10 | 0.5 | UDPGA, PAPS | C1/2 | 102 |
| EPN | CH | L (m) | 37 | 0.5–5 | 0.5† | NADPH | O5, H5 | 114 |
| Chlortoluron | JQ | L,K (S9) | 37 | 4 | 1 | NADPH, GSH | O1 | 91 |
| Diflubenzuron | CH | L (m) | 37 | 3–5 | 21 | NADPH | O2, H2 | 115 |
| Cypermethrin | JQ | L,I (S9),B | 42 | na | 10 | NADPH,UDPGA | O1/2, H1, C1 | 82 |
| Deltamethrin | CH | L (m,c) | 37 | 0.2* | 6.5 | ± NADPH | O1/2, R3, H1 | 116 |
| Fenvalerate | JQ | L (h) | 42 | 1‡ | 1.8 | none | O1/2, H1, M3 | 117 |
| PBacid | CH | L,K,I (m) | 42 | 0.1* | 0.3† | NADPH | O2, H3 | 118,119 |
| Fenproximate | JQ | L (S9) | 25 | na | 0.1† | NADPH | O1/2, M1 | 120 |
| Kresoxim-methyl | CH | L (h) | 37 | 2–5‡ | 0.1† | none | O1/2, H1/3 | 121 |
| Atrazine | CH | L (c) | 37.5 | na | 0.8† | GSH | O1, H6 | 122 |
| Terbutryn | CH | L (m, S9) | 37 | 1–5 | 0.1 | ± NADPH, GSH | O1, H6** | 123 |
| Warfarine | CH | L (m,c) | 41 | 1 | 0.4† | NADPH | O2, R3 | 68 |
a) PCPMs, pentachlorophenyl methyl sulfoxide and sulfone; NMC, 3-methyl-4-nitrophenol (metabolite of fenitrothion); PBacid, 3-phenoxybenzoic acid (metabolite of several pyrethroids). b) Tested species. CH, chicken; FP, feral pigeon; JQ, Japanese quail; TU, turkey. c) L, liver; K, kidney; I, intestine; B, blood plasma. The words in the parentheses mean the microsomal (m), cytosolic (c), post-mitochondrial (S9) fractions, hepatocytes (h), and tissue slices or homogenates (ts), respectively. d) Protein concentration of each fraction in mg/ml; *, in g tissue/ml; ‡, in 106 cells/ml. e) Pesticide concentration in μM. †, in mM. f) NADPH, nicotinamide adenine dinucleotide phosphate; UDPGA, uridine 5′-diphosphoglucuronic acid; GSH, reduced glutathione; PAPS, phosphoadenosyl phosphosulfate. g) Metabolic reactions (see Table 2); **, hydrolytic dethiomethylation. na, not available. r.t., room temperature.
3.2. In vivo metabolism
3.2.1. Route of administration
The oral administration of a pesticide is generally conducted by gavage or intubation, and feeding a diet treated with pesticide is alternatively conducted for a longer period of study. Intravenous administration causes such a rapid uptake of pesticide into the body that the basic profiles can be conveniently obtained for its distribution, metabolism, and elimination. The effect of an administration route on the metabolism of pesticides has not been examined so extensively. When DDT was orally administered at the same dosage to the northern bobwhite quail by feeding or intubation, the latter method increased the absorbed amount of DDT through the gut wall, but the metabolism in the liver was not changed by the administration method.124) Although the metabolic profiles of [benzyl-14C]cypermethrin in the Japanese quail were almost independent of the administration method, by gavage or intraperitoneal injection, lesser elimination of 14C to excreta was observed in the latter, probably due to the rapid distribution of 14C to each organ, especially adipose tissues.125) Kinetic analysis has been applied to the metabolism study of permethrin126) and deltamethrin127) in broilers by different administration methods—intubation into the crop and intraperitoneal injection. In intra-crop application, the rapid absorption of pyrethroids via the digestive system occurred, and the slower elimination rate with the mean absorption time longer than those with injection indicated continuous absorption from the gastrointestinal tract during elimination. The low bioavailability of these pyrethroids (11–22%) was estimated by comparing their concentration curves in serum after the two types of administration.
The dermal route becomes important when birds are directly exposed to pesticides applied aerially. Abou-Donia128,129) compared the fate of [Ph-14C]leptophos in laying hens with a single administration either orally by gavage or dermally to each side of the combs at the same dosage. The two-phase elimination profiles to excreta were observed after administration by both routes, but with the fourfold faster elimination of 14C in the first phase by gavage. Neither the 14C distribution in tissues nor the metabolic profiles were significantly changed by the administration route. The same author’s group has further compared the fate of [Ph-14C]EPN in hens with a single administration either orally by gavage or topically daily to skin at the bird’s neck for 10 days at similar dosages.130,131) Most of the dose (64–74%) was excreted by both administration types with the major 14C residues present in the liver, kidney, and bile. Although the same metabolites were detected, the radioactivity in both non-conjugated and water-soluble fractions by the extraction of the excreta gradually increased as time passed after the single oral administration. Since the corresponding 14C in the topical administration decreased with time, the hydrolysis of conjugated or bound 14C-releasing aglycons was most likely to proceed in the gastrointestinal tract. These results show that both the administration method and route change the profiles of uptake, distribution, and excretion, but the metabolic pathway is basically the same.
3.2.2. Species differences
In the metabolism of kresoxim-methyl using the hepatocytes, though ex vivo, the phenoxy moiety was favorably hydroxylated at the 4-position in the chicken, while the 2-methyl group was the main site of hydroxylation in three mammalian species with more cleavage of the ester and ether linkages.121) Sulfentrazone via oral administration mainly underwent the successive oxidation of the methyl group attached to the hetero ring in rats, goats, and hens, while the reduction of the C=N bond proceeded only in rats.132) In the case of orally administered thiabendazole, the same metabolites, formed via hydroxylation at the 5-position of the benzimidazole moiety followed by sulfation, were detected in the excreta of hens and goats, but more so in goats.133) The main metabolism of fenitrothion in birds is the cleavage of the P-Oaryl linkage, followed by the oxidation of the aryl methyl group and/or conjugation with sulfate on the resulting phenol, and the sulfation markedly proceeded in the Japanese quail, as compared with the hen.85) Furthermore, strain differences in the metabolic profiles were reported for [Ph-14C]diflubenzuron between White Leghorn hens (WL) and Rhode Island Red/Barred Plymouth Rock (RIR/BPR) chickens.115,134) The faster uptake and elimination of 14C were observed in the RIR/BPR strain than in the WL strain, and the major elimination route was different between RIR/BPR (excreta) and WL (eggs). The same metabolites were detected in both strains but with different profiles of the bond cleavage in the benzoylurea bridge connecting the two phenyl groups.
Species differences in conjugations have been extensively studied for pyrethroids and their major metabolite, PBacid. The analysis of metabolites in the excreta after the oral administration of cypermethrin showed different metabolic profiles among the Japanese quail, rat, and mouse.125) Although the total elimination was similar (84–93% of the dose), both the ester cleavage and oxidation at the 4′-position of the α-cyano-3-phenoxybenzyl moiety proceeded in the decreasing order of rat>mouse>quail. The main reactions of the metabolites, PBacid and its 4′-OH derivative, were quite different among the species, that is, the sulfation of 4′-OH-PBacid (rat); the conjugation of PBacid with taurine (mouse); and the specific conjugation of PBacid with ornithine, N-acetylornithine, serine, and glycylvaline (quail). Extensive metabolism studies of PBacid have shown that the main reactions via its oral administration are its conjugation with glycine (dog), glutamic acid (cow), glycylvaline (mallard duck), and ornithine (chicken).106,107) Judging from these limited metabolic data, the main metabolic pathways of pesticides are quite different in birds and mammals, especially for the conjugation of metabolites. Furthermore, the relative contribution of each metabolic reaction seems different, even among birds and their strains.
3.2.3. Isomerism
The biological efficacy and/or toxicity of a pesticide frequently depend on its isomerism, at least in part, due to the stereo-selective metabolism. After the oral administration of [14C]permethrin to laying hens, the trans-isomers more rapidly dissipated from the blood due to the faster hydrolysis of ester than of cis-isomers.135) As a result, more of the hydroxylated intact ester and its conjugates were formed from the cis-isomers, though almost the same 14C residues between the isomers were detected in the excreta. The hydroxylation of the gem-methyl group in the acid moiety proceeded favorably at the cis-position to the carboxyl group, and a similar profile was reported for cypermethrin.136) The elimination kinetics of diniconazole (E-isomerism) by single oral administration to the Japanese quail was analyzed in the blood, heart, liver, and kidney.137) Rapid elimination, with a half-life of 2–5 hr, was observed in any organ for the S-isomer, while the R-isomer dissipated 16–28 times slower in the liver and kidney. The enantio-enrichment of the (-)-cis- and (+)-trans-isomers in the liver and intestine was reported in the oral administration of chlordane to chickens, implying the presence of stereo-selective metabolism.34) The involvement of CYP1A1/2 and CYP2B1 was indicated by inhibition experiments in the metabolism of metalaxyl by using the chicken hepatic LMH cells, and more rapid dissipation of the S-isomer was observed therein.138) These results show the stereo-specific metabolism of pesticides by esterases and oxidases. In the case of methoxychlor, stereo-selective O-demethylation by oxidases was observed in the metabolism by using liver slices.139) The resulting metabolite dominantly had an S-isomerism in the rat and mouse, while the opposite was observed in the Japanese quail and trout. Although information on the stereo-selective reduction of pesticides is very limited, the degradation of HCH in the hepatic cytosolic fraction of laying hens is highly dependent on its isomerism.109) The γ-isomer (lindane) rapidly underwent dehydrochlorination, but the reaction was much slower for α- and δ-isomers, and no metabolism of β-isomers was observed.
3.2.4. Metabolic profiles
The report and evaluation by the Joint FAO/WHO Meeting on Pesticide Residues (JMPR) and Pesticide Specifications (JMPS), available for many pesticides, are useful for understanding their metabolism, mainly in laying hens.140) Based on these information and our literature survey, the metabolic profiles of representative pesticides in each chemical class are listed in Table 4.
Table 4. In vivo metabolism of pesticides in birds.
| Pesticidea) | Period (day)b) | Metabolic profiles | Pesticide | Period (day) | Metabolic profiles | ||||
|---|---|---|---|---|---|---|---|---|---|
| % dosec) | Reactionsd) | Ref. | % dose | Reactions | Ref. | ||||
| Lindane | 4–6 | 44–63, 3–9, 2–4 | R1 | 140 | Carbaryl | 7 (2) | 98, 0.1, <0.1 | O2, H2, C1/2/3 | 99, 140 |
| DDT*† | 1/2–3 | 65–66/5–16, —, — | R1/2 | 142, 143 | Chlorpropham | 7 | 83, <0.1, — | O1/2, H2, C1/2/5 | 140 |
| Methoxychlor | 14 | 77–92, —, — | O1, R1, C1 | 144 | Indoxacarb | 5 | 87–88, 0.3–0.4, <1 | O1, H2/3/4, M2 | 140 |
| Phorate | 5 | 62–66, 0.7–1.5, <1 | O4, H5, C7 | 140 | Diflubenzuron | 1 | 91, 0.8, — | O2, H2 | 140 |
| Tetrachlorvinphos | 7 | 74–77, <1, — | R1/3, H5, C3 | 141 | Methoxyfenozide | 7 | 84–93, <0.1, <0.1 | O1/2, C1 | 140 |
| Fenitrothion** | 7/1 | 93–102, <0.2, — | O1/5, H5, C1/2/3 | 85 | Fenamidone | 14 | >88, —, — | O2, H2/4, C1 | 140 |
| EPN | 21 | 94–100, —, — | O2/5, R4, H5 | 145 | Pinoxaden | 4 | 75, <0.1, <0.1 | O1, H1/3 | 140 |
| MCPA | 7 | 99, 0.1, <0.1 | C4 | 140 | Cycloxidim | 10 | 78, 0.3, 0.1 | O4, H3, M1 | 140 |
| Cypermethrin*** | 1 | 75–87, —, <2 | O1/2, H1, C1/4 | 125 | Clothianidin | 3 | 95, 0.2, — | O1, H2/4, C5/7, M2 | 140 |
| Fluvalinate | 1–3 | 92–94, <0.4, — | O1, H1/3, C1/4 | 146–148 | Kresoxim-methyl | 6 | 72–83, <0.2, <0.1 | O1/2/4, H1/3, C2 | 140 |
| Bifenthrin | 10 | >90, <0.4, <0.8 | O1, H1, C7, M2 | 140 | Tolyfluanid | 1–3 | 84, <0.1, <0.1 | O1, H4, C4 | 140 |
| Fluazifop-p-butyl | 11 | 90–98, —, — | H1/3 | 140 | Chlorfenapyr | 7 | 78–94, —, — | O1/2, R1, H6 | 140 |
| Isofetamid | 14 | 103–116, <0.2, <0.1 | O1/2, H2 | 140 | Thiacloprid | 3 | 75, <0.1, <0.7 | O1/4, H2/4, C4/6, M3 | 140 |
| Fluopicolide | 14 | 82–95, 0.1, <0.2 | O1/2, C2/3 | 140 | Atrazine‡ | 7 | —, —, — | O1, H6 | 149, 150 |
| Benzovindiflupyr | 14 | 88–92, 0.1, <0.1 | O1/2, H4, C1/2 | 140 | Propiconazole | 8–16 | 94–103, <0.6, <0.2 | O1, R3, H4/6, M3 | 140 |
| Flubendiamide | 14 | 62–66, 5–8, — | O1, C1, M2 | 140 | Tiadimefon | 3 | —, —, — | O1, C1/2 | 140 |
| Acetochlor | 7 | 68–82, <0.5, <0.3 | O1, H3, C3/5 | 140 | Cyproconazole | 3–4 | 94–97, 1–2, <1 | O1/2, R3, H4, C2 | 140 |
| Chlorantraniliprole | 14 | 98, 3, — | O1, H2, M2 | 140 | Fludioxonil | 8 | 88–112, —, — | O1/2/4, H4, C1/2 | 140 |
| Aldicarb | 14 (2) | 85, 5, — | O4, H2/3, M3 | 140 | MAB1a | 7 | 64–69, <0.1, — | O1, C7 | 151 |
a) Tested species, laying hens. *, Japanese quail and feral pigeon; **, laying hens and Japanese quail; ***, Japanese quail. b) Oral administration once a day. ‘(2)’ means a twice administration a day. †, intraperitoneal administration. ‡, feeding. c) Distribution (14C) in excreta, eggs, and muscles; —, not available. d) Metabolic reactions listed in Table 2.
Organochlorine (OC) pesticides
Cyclodiene insecticides are metabolized in birds generally by oxidation. The alkenyl bond of aldrin141) and the chlorinated cyclopentyl ring of chlordane34) are rapidly epoxidized, resulting in their short residence in the chicken body. In the case of endosulfan, the primary metabolic routes in the laying hen are S-oxidation and the hydrolytic opening of the 2-oxo-1,3,2-dioxathiolane ring followed by the ether formation from the resulting diol.140) The sulfate derivative was the main metabolite in the eggs, muscle, and fat of laying hens, and its persistent character causes a toxic concern. The reductive dechlorination and/or dehydrochlorination are the main reactions of DDT142,143) and lindane, respectively.140) The hydrophobic character of these insecticides and their metabolites results in their high residues, especially in the fat. Having an analogous chemical structure to DDT, methoxychlor undergoes O-demethylation, followed by glucuronidation.144) The polychlorinated benzene derivatives used as fungicides and herbicides show the different types of metabolism in laying hens. The dechlorination of chlorothalonil proceeds hydrolytically,140) and the replacement of chlorine atoms by glutathione, followed by the successive degradation to form the corresponding cysteine conjugate, was reported for dichlobenil and dicloran.140) Both dicamba and quinclorac undergo O-demethylation and/or glucuronidation instead of dechlorination.140)
Organophosphorus (OP) pesticides
The sulfur atom in the thiophosphoryl moiety is oxidatively desulfurated, while the sulfide is successively oxidized via sulfoxide to sulfone, as reported for phorate and fenamiphos.140) The main reaction of glyphosate and glufosinate is the oxidation of the alkyl moiety (β-oxidation).140) The alkyl group attached to an aromatic ring undergoes successive oxidation for fenitrothion85) and tolclofos-methyl,140) and regioselectively for diazinon.140) Another typical reaction is the cleavage of either the P–OAr or P–OCH3 linkage. The former reaction is catalyzed by esterases, while the latter proceeds via the conjugation with glutathione.87) The resulting phenol is generally conjugated with glucuronic acid (GA) and sulfate, as reported for diazinon140) and fenamiphos,43) respectively, and both conjugates are formed from fenitrothion,85) profenophos,140) methyl parathion,152) and EPN.153) The cleavage of the P–OAr linkage was the dominant metabolic route of EPN in laying hens,145) while O-demethylation and/or desulfuration of leptophos proceeded to a comparable extent of the P–OAr bond cleavage.128) As a reductive metabolism, the conversion of the nitro to amino group is observed for parathion140) and EPN.145)
Acid and ester pesticides, including pyrethroids
β-oxidation to finally form the corresponding phenol is one of the metabolic pathways of the phenoxyacetic acid derivatives and their esters, as reported for 2,4-D,154) while the cleavage of any ether linkage was not observed for fluazifop-P-butyl and haloxyfop.140) The bis adduct of MCPA with ornithine was the major metabolite in the egg yolk and muscle of laying hens.140) In the case of methoprene, the cholesteryl ester of its acid was detected in the chicken liver.140) Many researchers have extensively examined the metabolism of pyrethroids, mainly in laying hens. One common reaction is the hydroxylation of the alkyl carbons in the acid moiety and/or at the 4′-position of the 3-phenoxybenzyl group, followed by conjugation with GA or sulfate, as reported for fenvalerate,117) cypermethrin,125) permethrin,135) lambda-cyhalothrin,140) fenpropathrin,140) and deltamethrin.155) In addition to esterases, the possible involvement of oxidases via the unstable α-hydroxybenzyl intermediate was proposed in the ester hydrolysis of cypermethrin.82) Furthermore, the ether cleavage of PBacid was observed in the metabolism of fenpropathrin,140) fluvalinate,148) and deltamethrin.156) The resulting metabolites generally undergo conjugation with GA, sulfate, and various amino acids, as described in Section 3.2.2. Several types of ornithine conjugates, including bis adduct, were additionally reported for the alcohol moieties of cypermethrin,125) fluvalinate,148) and deltamethrin.156) Two types of a rare conjugation have been reported; one is the conjugation of the acid moiety of fluvalinate with bile acids such as taurochenodeoxycholic acid in the chicken metabolism.146) Another one was reported for bifenthrin, of which, one of the gem-methyl groups was hydroxylated and then conjugated with oleic and palmitic acids.140)
Amide, carbamate, and urea pesticides
These pesticides are basically metabolized via either the cleavage of the N(H)–CO bond, most probably catalyzed by CaEs or ChEs,157,158) or the oxidation of aromatic and/or alkyl moieties, followed by conjugation with GA and/or sulfate, as listed in Table 4. The successive oxidation of the methyl group finally results in O- or N-demethylation. The electrophilic site of the pesticide is conjugated with glutathione, followed by enzymatic conversion to the corresponding cysteine and mercapturic acid conjugates, as observed for acetochlor140) and propachlor.88,159) The electrophilic conjugation with glutathione similarly proceeded at the 5-position of 1-naphthol, the main metabolite of carbaryl, and the 3-position of the benzoyl ring of fluopicolide.140) In the case of methomyl, the main metabolite acetonitrile was conjugated with glutathione to finally form the N-cysteine derivative via rearrangement.160) Other types of conjugation were the N-acetylation of the aniline metabolite of chlorpropham and the conjugation of the benzoic acid metabolite of tolyfluanid with glycine.140) Although either the ring opening or intramolecular cyclization was observed for flubendiamide, indoxacarb, and chlorantriniliprole,140) these reactions were likely to proceed abiotically.
Miscellaneous pesticides
Metabolism profiles similar to those above are generally reported for other pesticides (Table 4). In the case of chlorfenapyr, the debrominated derivative was detected as the minor metabolite in the liver of chickens.140) In addition to the N–O bond cleavage and S-oxidation, cycloxydim underwent the Beckmann rearrangement to form the oxazolyl ring,140) but this is an abiotic reaction. Many kinds of natural acids are conjugated with pesticides and their metabolites, for example glycine for thiacloprid,140) glutamic acid for metaflumizone,140) alanine and acetic acid for propiconazole,140) pyruvic acid for clothianidin,140) fatty acids (palmitic, stearic, and oleic) for tebufenozide,140) oleic and seven other fatty acids for MAB1a,151) and phosphoric acid for flusilazole.140)
Conclusion
Oral uptake is generally the main route of exposure, based on the use pattern of pesticide formulations and avian feeding habits. In the first-tier avian risk assessment of pesticides, acute oral toxicity becomes preferable to short-term dietary toxicity based on its convenience and a more conservative estimation of toxicity. The acute oral toxicity of pesticide varies widely among avian species, depending on body size, feeding habits, and the activity of metabolic enzymes. Furthermore, several field studies have indicated the importance of dermal toxicity for more precise risk assessment of pesticides in the field. From these viewpoints, not only the species-sensitivity distribution approach to acute oral LD50 but also the contribution of acute dermal toxicity had better be considered for refining the avian risk assessment of pesticides exhibiting high toxicity.
In order to monitor the pesticide residues in poultry, if necessary, metabolism study is indispensable not only for identifying relevant metabolites but also for knowing the distribution of a pesticide and its metabolites in the edible commodities of laying hens. The PBPK approach also had better be taken at the same time to obtain kinetic data relevant to the distribution, metabolism, and elimination of the pesticide. Such data are very useful for evaluating the potential toxicity and/or bioaccumulation of pesticide in birds via a dietary route. Since a variety of natural components in addition to GA, sulfate, and glutathione may be conjugated with a pesticide and its metabolites, a thorough identification of major polar metabolites should be conducted, especially for the edible portions of birds. Incidentally, the metabolic information in avian species other than chickens is very limited, except in cases in which higher species-specific toxicity is observed. It is not practical to conduct in vivo metabolism in many species; hence, in vitro and ex vivo studies should be conducted alternatively, using the subcellular fractions of metabolically important organs and/or hepatocytes. As with the accumulation of the relevant data above, the in silico approach to avian toxicity should be further refined by taking account of pesticide metabolism. Concerning metabolic enzymes, their enzymologies except CYPs have not been well examined yet. Not only gene analysis to find out the possible metabolic enzymes but also the classical biochemical approach to isolating and characterizing them is indispensable for knowing species differences in pesticide toxicity in more detail.
Electronic supplementary materials
The online version of this article contains supplementary material (Supplemental data), which is available at https://www.jstage.jst.go.jp/browse/jpestics/.
Supplementary Data
References
- 1).European Food Safety Authority: Risk assessment for birds and mammals. EFSA J. 7(12): 1438, pp. 1–139 (2009). [DOI] [PMC free article] [PubMed]
- 2).L. B. Best and D. L. Fischer: Granular insecticide and birds: Factors to be considered in understanding exposure and reducing risk. Environ. Toxicol. Chem. 11, 1495–1508 (1992). [Google Scholar]
- 3).OECD Guideline for the testing of chemicals 223, Avian acute oral toxicity test (2010). USEPA Ecological effects test guidelines OCSPP 850.2100, Avian acute oral toxicity test (2012).
- 4).OECD Guideline for the testing of chemicals 205, Avian dietary toxicity test (1984). USEPA Ecological effects test guidelines OCSPP 850.2200, Avian dietary toxicity test (2012).
- 5).OECD Guideline for the testing of chemicals 503, Metabolism in livestock (2007). USEPA Residue chemistry test guidelines OPPTS 860.1480, Meat/milk/poultry/eggs (1996).
- 6).C. J. Driver, M. W. Ligotke, P. van Voris, B. D. McVeety, B. J. Greenspan and D. B. Drown: Routes of uptake and their relative contribution to the toxicologic response of northern bobwhite (Colinus virginianus) to an organophosphate pesticide. Environ. Toxicol. Chem. 10, 21–33 (1991). [Google Scholar]
- 7).N. B. Vyas, J. W. Spann, C. S. Hulse, S. Gentry and S. L. Borges: Dermal insecticide residues from birds inhabiting an orchard. Environ. Monit. Assess. 133, 209–214 (2007). [DOI] [PubMed] [Google Scholar]
- 8).P. Mineau: A comprehensive re-analysis of pesticide dermal toxicity data in birds and comparison with the rat. Environ. Toxicol. Pharmacol. 34, 416–427 (2012). [DOI] [PubMed] [Google Scholar]
- 9).R. H. Hudson, M. A. Haegele and R. K. Tucker: Acute oral and percutaneous toxicity of pesticides to mallards: Correlations with mammalian toxicity data. Toxicol. Appl. Pharmacol. 47, 451–460 (1979). [DOI] [PubMed] [Google Scholar]
- 10).P. Mineau: Estimating the probability of bird mortality from pesticide sprays on the basis of the field study record. Environ. Toxicol. Chem. 21, 1497–1506 (2002). [PubMed] [Google Scholar]
- 11).P. Mineau, A. Baril, B. T. Collins, J. Duffe, G. Joerman and R. Luttik: Pesticide acute toxicity reference values for birds. Rev. Environ. Contam. Toxicol. 170, 13–74 (2001). [PubMed] [Google Scholar]
- 12).G. M. Hilton, E. Odenkirchen, M. Panger, G. Waleko, A. Lowit and A. J. Clippinger: Evaluation of the avian acute oral and sub-acute dietary toxicity test for pesticide registration. Regul. Toxicol. Pharmacol. 105, 30–35 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).T. T. Buerger, S. R. Mortensen, R. J. Kendall and M. J. Hooper: Metabolism and acute toxicity of methyl parathion in pen-reared and wild northern bobwhites. Environ. Toxicol. Chem. 13, 1139–1143 (1994). [Google Scholar]
- 14).P. Mineau, B. T. Collins and A. Baril: On the use of scaling factors to improve interspecies extrapolation of acute toxicity in birds. Regul. Toxicol. Pharmacol. 24, 24–29 (1996). [DOI] [PubMed] [Google Scholar]
- 15).A. Mitra, C. Chatterjee and F. B. Mandal: Synthetic chemical pesticides and their effects on birds. Res. J. Environ. Toxicol 5, 81–96 (2011). [Google Scholar]
- 16).C. H. Walker: Pesticides and birds. — Mechanisms of selective toxicity. Agric. Ecosyst. Environ. 9, 211–226 (1983). [Google Scholar]
- 17).E. W. Schafer: The acute oral toxicity of 369 pesticidal, pharmaceutical and other chemicals to wild birds. Toxicol. Appl. Pharmacol. 21, 315–330 (1972). [DOI] [PubMed] [Google Scholar]
- 18).C. J. Brealey, C. H. Walker and B. C. Baldwin: A-Esterase activities in relation to the differential toxicity of pirimiphos-methyl to birds and mammals. Pestic. Sci. 11, 546–554 (1980). [Google Scholar]
- 19).S. P. Bradbury and J. R. Coats: Comparative toxicology of the pyrethroid insecticides. Rev. Environ. Contam. Toxicol. 108, 133–177 (1989). [DOI] [PubMed] [Google Scholar]
- 20).C. Wang and S. D. Murphy: Kinetic Analysis of species difference in acetylcholinesterase sensitivity to organophosphate insecticides. Toxicol. Appl. Pharmacol. 66, 409–419 (1982). [DOI] [PubMed] [Google Scholar]
- 21).European Food Safety Authority: Conclusion regarding the peer review of the pesticide risk assessment of the active substance. “Publications, conclusion on pesticides” http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1831-4732 (Accessed 20 May, 2021).
- 22).US Environmental Protection Agency: “Reregistration Eligibility Document.” https://iaspub.epa.gov/apex/pesticides/f?p=chemicalsearch:1 (Accessed 20 May, 2021).
- 23).H. M. Thompson: Avian serum esterases: Species and temporal variations and their possible consequences. Chem. Biol. Interact. 87, 329–338 (1993). [DOI] [PubMed] [Google Scholar]
- 24).M. C. Fossi, L. Lari and S. Casini: Interspecies variation of “B” esterases in birds: The influence of size and feeding habits. Arch. Environ. Contam. Toxicol. 31, 525–532 (1996). [DOI] [PubMed] [Google Scholar]
- 25).M. C. Fossi, A. Massi, L. Lari, L. Marsili, S. Focardi, C. Leonzio and A. Renzoni: Interspecies differences in mixed function oxidase activity in birds: Relationship between feeding habits, detoxication activities and organochlorine accumulation. Environ. Pollut. 90, 15–24 (1995). [DOI] [PubMed] [Google Scholar]
- 26).C. R. Short, W. Flory, L. C. Hsieh, T. Aranas, S.-P. Ou and J. Weissinger: Comparison of hepatic drug metabolizing enzyme acticities in several agricultural species. Comp. Biochem. Physiol. 91C, 419–424 (1988). [DOI] [PubMed] [Google Scholar]
- 27).A. L. Bartlet and L. M. Kirinya: Activities of mixed function oxidases, UDP-glucuronyl transferase and sulphate conjugation enzymes in Galliformes and anseriformes. Q. J. Exp. Physiol. 61, 105–119 (1976). [DOI] [PubMed] [Google Scholar]
- 28).E. W. Schafer Jr., R. B. Brunton, N. F. Lockyer and J. W. De Grazio: Comparative toxicity of seventeen pesticides to the quelea, house sparrow, and red-winged blackbird. Toxicol. Appl. Pharmacol. 26, 154–157 (1973). [DOI] [PubMed] [Google Scholar]
- 29).R. K. Tucker and M. A. Haegele: Comparative acute oral toxicity of pesticides to six species of birds. Toxicol. Appl. Pharmacol. 20, 57–65 (1971). [DOI] [PubMed] [Google Scholar]
- 30).P. Mazzatorta, M. T. D. Cronin and E. Benfenati: A QSAR study of avian oral toxicity using support vector machines and genetic algorithms. QSAR Comb. Sci. 25, 616–628 (2006). [Google Scholar]
- 31).N. Basant, S. Gupta and K. P. Singh: Predicting toxicities of diverse chemical pesticides in multiple avian species using tree-based QSAR approaches for regulatory purposes. J. Chem. Inf. Model. 55, 1337–1348 (2015). [DOI] [PubMed] [Google Scholar]
- 32).C. Zhang, F. Cheng, L. Sun, S. Zhuang, W. Li, G. Liu, P. W. Lee and Y. Tang: In silico prediction of chemical toxicity on avian species using chemical category approaches. Chemosphere 122, 280–287 (2015). [DOI] [PubMed] [Google Scholar]
- 33).C. A. Kan and L. G. M. Th. Tuinstra: Accumulation and excretion of certain organochlorine Insecticides in broiler breeder hens. J. Agric. Food Chem. 24, 775–778 (1976). [DOI] [PubMed] [Google Scholar]
- 34).Z. Lu, M. Xue, G. Shen, K. Li, X. Li, X. Wang and S. Tao: Accumulation dynamics of chlordanes and their enantiomers in cockerels (Gallus gallus) after oral exposure. Environ. Sci. Technol. 45, 7928–7935 (2011). [DOI] [PubMed] [Google Scholar]
- 35).X. Liu, P. Wang, C. Liu, Y. Liang, Z. Zhou and D. Liu: Absorption, distribution, metabolism, and in vitro digestion of beta-cypermethrin in laying hens. J. Agric. Food Chem. 65, 7647–7652 (2017). [DOI] [PubMed] [Google Scholar]
- 36).T. G. Bean, M. S. Gross, N. K. Karouna-Renier, P. F. P. Henry, S. L. Schultz, M. L. Hladik, K. M. Kuivila and B. A. Rattner: Toxicokinetics ofimidacloprid-coated wheat seeds in Japanese quail (Coturnix japonica) and an evaluation of hazard. Environ. Sci. Technol. 53, 3888–3897 (2019). [DOI] [PubMed] [Google Scholar]
- 37).C. Jondreville, A. Fournier, M. Mahieu, C. Feidt, H. Archimède and G. Rychen: Kinetic study of chlordecone orally given to laying hens (Gallus domesticus). Chemosphere 114, 275–281 (2014). [DOI] [PubMed] [Google Scholar]
- 38).D. J. MacLachlan: Physiologically based pharmacokinetic (PBPK) model for residues of lipophilic pesticides in poultry. Food Addit. Contam. 27A, 302–314 (2010). [DOI] [PubMed] [Google Scholar]
- 39).D. J. MacLachlan: Transfer of fat-soluble pesticides from contaminated feed to poultry tissues and eggs. Br. Poult. Sci. 49, 290–298 (2008). [DOI] [PubMed] [Google Scholar]
- 40).C. T. Garten Jr. and J. R. Trabalka: Evaluation of models for predicting terrestrial food chain behavior of xenobiotics. Environ. Sci. Technol. 17, 590–595 (1983). [DOI] [PubMed] [Google Scholar]
- 41).F. A. P. C. Gobas, L. P. Burkhard, W. J. Doucette, K. G. Sappington, E. M. J. Verbruggen, B. K. Hope, M. A. Bonnell, J. A. Arnot and J. V. Tarazona: Review of existing terrestrial bioaccumulation models and terrestrial bioaccumulation modeling needs for organic chemicals. Integr. Environ. Assess. Manag. 12, 123–134 (2016). [DOI] [PubMed] [Google Scholar]
- 42).K. M. Fremlin, J. E. Elliott, D. J. Green, K. G. Drouillard, T. Harner, A. Eng and F. A. P. C. Gobas: Trophic magnification of legacy persistent organic pollutants in an urban terrestrial food web. Sci. Total Environ. 714, 14 (2020). [DOI] [PubMed] [Google Scholar]
- 43).Z. Gregus, J. B. Watkins, T. N. Thompson, M. J. Harvey, K. Rozman and C. D. Klaassen: Hepatic phase I and phase II biotransformations in quail and trout: Comparison to other species commonly used in toxicity testing. Toxicol. Appl. Pharmacol. 67, 430–441 (1983). [DOI] [PubMed] [Google Scholar]
- 44).J. L. Rivière, J. Bach and G. Grolleau: Effects of prochloraz on drug metabolism in the Japanese quail, grey partridge, chicken, and pheasant. Arch. Environ. Contam. Toxicol. 14, 299–306 (1985). [DOI] [PubMed] [Google Scholar]
- 45).R. P. Gupta and M. B. Abou-Donia: Cytochrome P450 enzymes in chickens: Characteristics and induction by xenobiotics. Comp. Biochem. Physiol. 121C, 73–83 (1998). [DOI] [PubMed] [Google Scholar]
- 46).R. R. Dalvi, V. A. Nunn and J. Juskevich: Stusies on comparative drug metabolism by hepatic cytochrome P-450-containing microsomal enzymes in quail, ducks, geese, chickens, turkeys and rats. Comp. Biochem. Physiol. 87C, 421–424 (1987). [DOI] [PubMed] [Google Scholar]
- 47).C. H. Walker, C. J. Brealey, M. I. Mackness and G. Johnston: Toxicity of pesticides to birds; The enzymic factor. Biochem. Soc. Trans. 9, 741–745 (1991). [DOI] [PubMed] [Google Scholar]
- 48).P. J. Klein, T. R. van Vleet, J. O. Hall and R. A. Coulombe Jr.: Biochemical factors underlying the age-related sensitivity of turkeys to aflatoxin B1. Comp. Biochem. Physiol. 132C, 193–201 (2002). [DOI] [PubMed] [Google Scholar]
- 49).C. F. Strittmatter and F. T. Umberger: Oxidative enzyme components of avian liver microsomes changes during embryonic development and the effects of phenobarbital administration. Biochim. Biophys. Acta 180, 18–27 (1969). [DOI] [PubMed] [Google Scholar]
- 50).J. W. Gillett and G. H. Arscott: Microsomal epoxidation in Japanese quail: Induction by dietary dieldrin. Comp. Biochem. Physiol. 30, 589–600 (1969). [DOI] [PubMed] [Google Scholar]
- 51).S. X. Hu: Effect of age on hepatic cytochrome P450 of Ross 708 broiler chickens. Poult. Sci. 92, 1283–1291 (2013). [DOI] [PubMed] [Google Scholar]
- 52).S. L. N. Rao and W. P. McKinley: Metabolism of organophosphorus insecticides by liver homogenates from different species. Can. J. Biochem. 47, 1155–1159 (1969). [DOI] [PubMed] [Google Scholar]
- 53).J. L. Riviere, J. Bach and G. Grolleau: Effect of pyrethroid insecticides and N-(3,5-dichlorophenyl) dicarboximide fungicides on microsomal drug-metabolizing enzymes in the Japanese quail (Coturnix coturnix). Bull. Environ. Contam. Toxicol. 31, 479–485 (1983). [DOI] [PubMed] [Google Scholar]
- 54).T. Liukkonen-Anttila, H. Honkanen, P. Peltokangas, O. Pelkonen and E. Hohtola: Cytochrome P450 enzyme activity in five herbivorous, non-passerine bird species. Comp. Biochem. Physiol. 134C, 69–77 (2003). [DOI] [PubMed] [Google Scholar]
- 55).C. H. Walker: Avian forms of cytochrome P450. Comp. Biochem. Physiol. 121C, 65–72 (1998). [DOI] [PubMed] [Google Scholar]
- 56).P. J. Bunyan, M. G. Townsend and A. Taylor: Pesticide-induced changes in hepatic microsomal enzyme systems. Some effects of 1,1-di(p-chlorophenyl)-2,2,2-trichloroethane (DDT) and 1,1-di(p-chlorophenyl)-2,2-dichloroethylene (DDE) in the rat and Japanese quail. Chem. Biol. Interact. 5, 13–26 (1972). [DOI] [PubMed] [Google Scholar]
- 57).J. L. Sell, K. L. Davison and R. L. Puyear: Aniline hydroxylase, N-demethylase, and cytochrome P450 in liver microsomes of hens fed DDT and dieldrin. J. Agric. Food Chem. 19, 58–60 (1971). [DOI] [PubMed] [Google Scholar]
- 58).M. J. J. Ronis, M. Ingelman-Sundberg and T. M. Badger: Induction, suppression and inhibition of multiple hepatic cytochrome P450 isozymes in the male rat and bobwhite quail (Colinus virginianus) by ergosterol biosynthesis inhibiting fungicides (EBIFs). Biochem. Pharmacol. 48, 1953–1965 (1994). [DOI] [PubMed] [Google Scholar]
- 59).K. Lavrijsen, J. van Houdt, D. van Dyck, W. Meuldermans and J. Heykants: Effect of the fungicide imazalil on hepatic microsomal cytochrome P-450 and cytochrome P-450 dependent activities in the bobwhite quail (Colinus virginianus). Pestic. Sci. 29, 47–56 (1990). [Google Scholar]
- 60).J.-L. Rivière, P. Leroux, J. Bach and M. Gredt: Effect of some ergosterol biosynthesis inhibiting fungicides on sterols and cytochrome P-450 from the Japanese quail Coturnix coturnix. Pestic. Sci. 15, 317–323 (1984). [Google Scholar]
- 61).K. P. Watanabe, Y. K. Kawai, Y. Ikenaka, M. Kawata, S. Ikushiro, T. Sakaki and M. Ishizuka: Avian cytochrome P450 (CYP) 1-3 family genes: Isoforms, evolutionary relationships, and mRNA expression in chicken liver. PLoS One 8, e75689 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).S. Rawal and R. A. Coulombe Jr.: Metabolism of aflatoxin B1 in Turkey liver microsomes: The relative roles of cytochromes P450 1A5 and 3A37. Toxicol. Appl. Pharmacol. 254, 349–354 (2011). [DOI] [PubMed] [Google Scholar]
- 63).J. Deng, L. Zhao, N.-Y. Zhang, N. A. Karrow, C. S. Krumm, D.-S. Qi and L.-H. Sun: Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. Rev. Mutat. Res. 778, 79–89 (2018). [DOI] [PubMed] [Google Scholar]
- 64).A. F. Machin, H. Rogers, A. J. Cross, M. P. Quick, L. C. Howells and N. F. Janes: Metabolic aspects of the toxicology of diazinon I. Hepatic metabolism in the sheep, cow, pig, guinea-pig, rat, turkey, chicken and duck. Pestic. Sci. 6, 461–473 (1975). [Google Scholar]
- 65).K. P. Watanabe, A. Saengtienchai, K. D. Tanaka, Y. Ikenaka and M. Ishizuka: Comparison of warfarin sensitivity between rat and bird species. Comp. Biochem. Physiol. 152C, 114–119 (2010). [DOI] [PubMed] [Google Scholar]
- 66).C. Montesissa, M. de Liguoro, M. Amorena, A. Lucisano and S. Carli: In vitro comparison of aldicarb oxidation in various food-producing animal species. Vet. Hum. Toxicol. 37, 333–336 (1995). [PubMed] [Google Scholar]
- 67).M. Hitchcock and S. D. Murphy: Enzymatic reduction of O,O-(4-nitrophenyl) phosphorothioate, O,O-diethyl O-(4-nitrophenyl) phosphate, and O-ethyl O-(4-nitrophenyl)benzene thiophosphonate by tissues from mammals, birds, and fishes. Biochem. Pharmacol. 16, 1801–1811 (1967). [DOI] [PubMed] [Google Scholar]
- 68).A. Saengtienchai, Y. Ikenaka, K. Watanabe, T. Ishida and M. Ishizuka: Comparative metabolism of warfarin in rats and chickens. Poult. Sci. 90, 2775–2781 (2011). [DOI] [PubMed] [Google Scholar]
- 69).E. Vilanova and M. A. Sogorb: The role of phosphotriesterases in the detoxication of organophosphorus compounds. Crit. Rev. Toxicol. 29, 21–57 (1999). [DOI] [PubMed] [Google Scholar]
- 70).C. H. Walker and M. I. Mackness: “A” esterases and their role in regulating the toxicity of organophosphates. Arch. Toxicol. 60, 30–33 (1987). [DOI] [PubMed] [Google Scholar]
- 71).M. I. Mackness, H. M. Thopson, A. R. Hardy and C. H. Walker: Distinction between ‘A’-esterases and arylesterases. Implications for esterase classification. Biochem. J. 245, 293–296 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).M. A. Sogorb and E. Vilanova: Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol. Lett. 128, 215–228 (2002). [DOI] [PubMed] [Google Scholar]
- 73).N. Díaz-Alejo, A. Monroy, E. Vilanova, J. L. Vicedo and M. A. Sogorb: A stereospecific phosphotriesterase in hen liver and brain. Chem. Biol. Interact. 108, 187–196 (1998). [DOI] [PubMed] [Google Scholar]
- 74).M. A. Sogorb, A. Monroy and E. Vilanova: Chicken serum albumin hydrolyzes dichlorophenyl phosphoramidates by a mechanism based on transient phosphorylation. Chem. Res. Toxicol. 11, 1141–1446 (1998). [DOI] [PubMed] [Google Scholar]
- 75).R. S. Holmes and C. J. Masters: The ontogeny of pig and duck esterases. Biochim. Biophys. Acta 159, 81–93 (1968). [DOI] [PubMed] [Google Scholar]
- 76).M. Veini, O. E. Tsitsiloni, S. M. Martini and A. A. Haritos: Multiple esterases molecular forms of soluble in the digestive system of the developing chicken. Comp. Biochem. Physiol. 83B, 775–781 (1986). [DOI] [PubMed] [Google Scholar]
- 77).O. E. Tsitsiloni, M. Veini and A. A. Haritos: Esterases in quail (Coturnix coturnix). Physicochemical and developmental aspects. Comp. Biochem. Physiol. 106B, 1009–1014 (1993). [DOI] [PubMed] [Google Scholar]
- 78).M. Farage-Elawar: Development of esterase activities in the chicken before and after hatching. Neurotoxicol. Teratol. 13, 147–152 (1991). [DOI] [PubMed] [Google Scholar]
- 79).F. M. Bush, J. R. Price and J. I. Townsend: Avian hepatic esterases, pesticides and diet. Comp. Biochem. Physiol. 44B, 1137–1151 (1973). [DOI] [PubMed] [Google Scholar]
- 80).C. Roy, G. Grolleau, S. Chamoulaud and J.-L. Rivière: Plasma B-esterase activities in European raptors. J. Wildl. Dis. 41, 184–208 (2005). [DOI] [PubMed] [Google Scholar]
- 81).B. R. Sonawane and C. O. Knowles: Comparative metabolism of two carbanilate herbicides (EP-475 and phenmedipham) in rats. Pestic. Biochem. Physiol. 1, 472–482 (1971). [Google Scholar]
- 82).R. Edwards, P. Millburn and D. H. Hutson: Factors influencing the selective toxicity of cis- and trans-cypermethrin in rainbow trout, frog, mouse and quail: Biotransformation in liver, plasma, brain and intestine. Pestic. Sci. 21, 1–21 (1987). [Google Scholar]
- 83).T. Katagi: Bioconcentration, bioaccumulation, and metabolism of pesticides in aquatic organisms. Rev. Environ. Contam. Toxicol. 204, 1–132 (2010). [DOI] [PubMed] [Google Scholar]
- 84).M. H. Akhtar and T. S. Foster: Fate of tetrachlorvinphos and its isomer in soluble fraction (105000g) from goose and turkey liver homogenates. J. Agric. Food Chem. 28, 693–697 (1980). [DOI] [PubMed] [Google Scholar]
- 85).K. Mihara, Y. Misaki and J. Miyamoto: Metabolism of fenitrothion in birds. Pestic. Sci. 4, 175–185 (1979). [Google Scholar]
- 86).D. M. Dulik, J. K. Huwe, J. E. Bakke, M. S. Connors and C. Fenselau: Conjugation of polychlorinated agrochemical sulphoxides and sulphones by glutathione. Xenobiotica 22, 325–334 (1992). [DOI] [PubMed] [Google Scholar]
- 87).M. H. Akhtar and T. S. Foster: Metabolism of tetrachlorvinphos by the soluble fraction (105000g) from chicken liver homogenates. J. Agric. Food Chem. 25, 1017–1022 (1977). [DOI] [PubMed] [Google Scholar]
- 88).J. E. Bakke and G. L. Larsen: Metabolism of 2-chloro-N-isopropylacetanilide in chickens. Chemosphere 14, 1749–1754 (1985). [Google Scholar]
- 89).J. G. Wit: Conjugations with glutathione distribution of glutathione S-aryl transferase in wild birds. Eur. J. Pharmacol. 5, 100–102 (1968). [DOI] [PubMed] [Google Scholar]
- 90).P. J. Klein, R. Buckner, J. Kelly and R. A. Coulombe Jr.: Biochemical basis for the extreme sensitivity of turkeys to aflatoxin B1. Toxicol. Appl. Pharmacol. 165, 45–52 (2000). [DOI] [PubMed] [Google Scholar]
- 91).R. Hinderer and R. E. Menzer: Enzyme activities and cytochrome P-450 levels of some Japanese quail tissues with respect to their metabolism of several pesticides. Pestic. Biochem. Physiol. 6, 161–169 (1976). [Google Scholar]
- 92).D. L. Eaton and T. K. Bammler: Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol. Sci. 49, 156–164 (1999). [DOI] [PubMed] [Google Scholar]
- 93).L.-H. Chang, L.-F. Chuang, C.-P. Tsai, C.-P. D. Tu and M. F. Tam: Characterization of glutathione S-transferases from day-old chick livers. Biochemistry 29, 744–750 (1990). [DOI] [PubMed] [Google Scholar]
- 94).C.-H. Hsieh, L.-F. Li, S.-P. Tsai and M. F. Tam: Characterization and cloning of avian-hepatic glutathione S-transferases. Biochem. J. 343, 87–93 (1999). [PMC free article] [PubMed] [Google Scholar]
- 95).E. Akkemik, P. Taser, A. Bayindir, H. Budak and M. Ciftci: Purification and characterization of glutathione S-transferase from turkey liver and inhibition effects of some metal ions on enzyme activity. Environ. Toxicol. Pharmacol. 34, 888–894 (2012). [DOI] [PubMed] [Google Scholar]
- 96).Y. Temel, U. M. Koçyigit, M. Ş. Taysı, F. Gökalp, M. B. Gürdere, Y. Budak, M. Ceylan, İ. Gülçin and M. Çiftci: Purification of glutathione S-transferase enzyme from quail liver tissue and inhibition effects of (3aR,4S,7R,7aS)-2-(4-((E)-3-(aryl)acryloyl)phenyl)-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione derivatives on the enzyme activity. J. Biochem. Mol. Toxicol. 32, e22034 (2018). [DOI] [PubMed] [Google Scholar]
- 97).B. M. Ahmed, Y. Temel and M. Çiftci: Purification and characterization glutathione S-transferase enzyme from quail (Coturnix, coturnix japonica) heart and investigation the effect of some metal ions on enzyme activity. Cumhuriyet Sci. J. 40, 802–812 (2019). [Google Scholar]
- 98).J. E. Kim, B. R. Bunderson, A. Croasdell and R. A. Coulombe Jr.: Functional characterization of alpha-class glutathione S-transferases from the turkey (Meleagris gallopavo). Toxicol. Sci. 124, 45–53 (2011). [DOI] [PubMed] [Google Scholar]
- 99).G. D. Paulson, R. G. Zaylskie, M. V. Zehr, C. E. Portnoy and V. J. Feil: Metabolites of carbaryl (1-naphthyl methylcarbamate) in chicken urine. J. Agric. Food Chem. 18, 110–115 (1970). [DOI] [PubMed] [Google Scholar]
- 100).Y. K. Kawai, S. Shinya, Y. Ikenaka, A. Saengtienchai, T. Kondo, W. S. Darwish, S. M. M. Nakayama, H. Mizukawa and M. Ishizuka: Characterization of function and genetic feature of UDPglucuronosyltransferase in avian species. Comp. Biochem. Physiol. 217C, 5–14 (2019). [DOI] [PubMed] [Google Scholar]
- 101).S. X. Hu: Age-related change of hepatic uridine diphosphate glucuronosyltransferase and sulfotransferase activities in male chickens and pigs. J. Vet. Pharmacol. Ther. 40, 270–278 (2016). [DOI] [PubMed] [Google Scholar]
- 102).C.-H. Lee, M. Kamijima, C. Li, S. Taneda, A. K. Suzuki and T. Nakajima: 3-Methyl-4-nitrophenol metabolism by uridine diphosphate glucuronosyltransferase and sulfotransferase in liver microsomes of mice, rats, and Japanese Quail (Coturnix japonica). Environ. Toxicol. Chem. 26, 1873–1878 (2007). [DOI] [PubMed] [Google Scholar]
- 103).Y. K. Kawai, Y. Ikenaka, M. Ishizuka and A. Kubota: The evolution of UDP-glycosyl/glucuronosyltransferase 1E (UGT1E) genes in bird lineages is linked to feeding habits but UGT2 genes is not. PLoS One 13, e0205266 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104).L. A. Wilson, G. E. Reyns, V. M. Darras and M. W. H. Coughtrie: cDNA cloning, functional expression, and characterization of chicken sulfotransferases belonging to the SULT1B and SULT1C families. Arch. Biochem. Biophys. 428, 64–72 (2004). [DOI] [PubMed] [Google Scholar]
- 105).M. Darnell and L. Weidolf: Metabolism of xenobiotic carboxylic acids: Focus on coenzyme A conjugation, reactivity, and interference with lipid metabolism. Chem. Res. Toxicol. 26, 1139–1155 (2013). [DOI] [PubMed] [Google Scholar]
- 106).K. R. Huckle, I. J. G. Climie, D. H. Hutson and P. Millburn: Dipeptide conjugation of 3-phenoxybenzoic acid in the mallard duck. Drug Metab. Dispos. 9, 147–149 (1981). [PubMed] [Google Scholar]
- 107).K. R. Huckle, G. Stoydin, D. H. Hutson and P. Millburn: Formation of an N-acetylornithine conjugate of 3-phenoxybenzoic acid in the chicken. Drug Metab. Dispos. 10, 523–528 (1982). [PubMed] [Google Scholar]
- 108).H. P. Pan and J. R. Fouts: Drug metabolism in birds. Drug Metab. Rev. 7, 1–253 (1978). [DOI] [PubMed] [Google Scholar]
- 109).T. S. Foster and J. G. Sana: The in vitro metabolism of lindane by an enzyme preparation from chicken liver. J. Environ. Sci. Health 13B, 25–45 (1978). [DOI] [PubMed] [Google Scholar]
- 110).N. Furusawa and Y. Morita: In vitro hepatic biotransformation of aldrin and dieldrin in food-producing animals. Acta Vet. Hung. 49, 349–353 (2001). [DOI] [PubMed] [Google Scholar]
- 111).P. J. Bunyan, J. M. J. Page and A. Taylor: In vitro metabolism of p,p′DDT in pigeon liver. Nature 210, 1048–1049 (1966). [DOI] [PubMed] [Google Scholar]
- 112).K. Ohyama, S. Maki, K. Sato and Y. Kato: In vitro metabolism of [14C]methoxychlor in rat, mouse, Japanese quail and rainbow trout in precision-cut liver slices. Xenobiotica 34, 741–754 (2004). [DOI] [PubMed] [Google Scholar]
- 113).M. H. Akhtar: Fate of trichlorfon in buffer and soluble fraction (105000g) from cow and chicken liver homogenates. J. Agric. Food Chem. 30, 551–554 (1982). [DOI] [PubMed] [Google Scholar]
- 114).J. M. Lasker, D. G. Graham and M. B. Abou-Donia: Differential metabolism of O-ethyl O-4-nitrophenyl phenylphosphonothioate by rat and chicken hepatic microsomes. Biochem. Pharmacol. 31, 1961–1967 (1982). [DOI] [PubMed] [Google Scholar]
- 115).J. C. Opdycke, R. W. Miller and R. E. Menzer: In vivo and liver microsomal metabolism of diflubenzuron by two breeds of chickens. J. Agric. Food Chem. 30, 1227–1233 (1982). [DOI] [PubMed] [Google Scholar]
- 116).M. H. Akhtar: Metabolism of deltamethrin by cow and chicken Liver enzyme preparations. J. Agric. Food Chem. 32, 258–262 (1984). [Google Scholar]
- 117).M. M. Mumtaz and R. E. Menzer: Comparative metabolism and fate of fenvalerate in Japanese quail (Coturnix coturnix japonica) and rats (Rattus norwegicus). J. Agric. Food Chem. 34, 929–936 (1986). [Google Scholar]
- 118).M. H. Akhtar and S. Mahadevan: Diphenyl ether cleavage of 3-phenoxybenzoic acid by chicken kidney microsomal preparations. Drug Metab. Dispos. 20, 356–359 (1992). [PubMed] [Google Scholar]
- 119).M. H. Akhtar, S. Mahadevan and F. Russell: Cleavage of 3-phenoxybenzoic acid by chicken microsomal preparations. J. Environ. Sci. Health 28B, 527–543 (1993). [DOI] [PubMed] [Google Scholar]
- 120).K. Motoba, H. Nishizawa, T. Suzuki, H. Hamaguchi, M. Uchida and S. Funayama: Species-specific detoxification metabolism of fenpyroximate, a potent acaricide. Pestic. Biochem. Physiol. 67, 73–84 (2000). [Google Scholar]
- 121).F. Salmon and W. Kohl: Use of fresh and cryopreserved hepatocytes to study the metabolism of pesticides in food-producing animals and rats. Xenobiotica 26, 803–811 (1996). [DOI] [PubMed] [Google Scholar]
- 122).T. S. Foster, S. U. Khan and M. H. Akhtar: Metabolism of atrazine by the soluble fraction (105000g) from chicken liver homogenates. J. Agric. Food Chem. 27, 300–302 (1979). [DOI] [PubMed] [Google Scholar]
- 123).N. H. Adams, P. E. Levi and E. Hodgson: In vitro studies of the metabolism of atrazine, simazine, and terbutryn in several vertebrate species. J. Agric. Food Chem. 38, 1411–1417 (1990). [Google Scholar]
- 124).J. P. Sullivan and P. F. Scanlon: A comparison between intubation and food addition as routes of oral exposure for northern bobwhites to DDT insecticide. J. Wildl. Dis. 27, 435–440 (1991). [DOI] [PubMed] [Google Scholar]
- 125).R. Edwards, P. Millburn and D. H. Hutson: Comparative metabolism and disposition of [14C-benzyl] cypermethrin in quail, rat and mouse. Pestic. Sci. 30, 159–181 (1990). [Google Scholar]
- 126).T. Gögebakan and G. Eraslan: Single-dose toxicokinetics of permethrin in broiler chickens. Br. Poult. Sci. 56, 605–611 (2015). [DOI] [PubMed] [Google Scholar]
- 127).R. Hüyük and G. Eraslan: Toxicokinetics of the broad-spectrum pyrethroid insecticide deltamethrin in broiler chickens. Br. Poult. Sci. 58, 95–99 (2017). [DOI] [PubMed] [Google Scholar]
- 128).M. B. Abou-Donia: Metabolism and pharmacokinetics of a single oral dose of O-4-bromo-2,5-dichlorophenyl O-methyl phenylphosphonoyjioate (leptophos) in hens. Toxicol. Appl. Pharmacol. 55, 131–145 (1980). [DOI] [PubMed] [Google Scholar]
- 129).M. B. Abou-donia: Pharmacokinetics and metabolism of a topically applied dose of O-4-bromo-2,5-dichlorophenyl O-methyl phenylphosphonothioate in hens. Toxicol. Appl. Pharmacol. 51, 311–328 (1979). [DOI] [PubMed] [Google Scholar]
- 130).M. B. Abou-Donia, H. M. Abdel-Kader and S. A. Abou-Donia: Tissue distribution, elimination, and metabolism of O-ethyl O-4-nitrophenyl phenylphosphonothioate in hens following daily dermal doses. J. Am. Coll. Toxicol. 2, 391–404 (1983). [Google Scholar]
- 131).M. B. Abou-Donia, B. L. Reichert and M. A. Ashry: The absorption, distribution, excretion, and metabolism of a single oral dose of O-ethyl O-4-nitrophenyl phenylphosphonothioate in hens. Toxicol. Appl. Pharmacol. 70, 18–28 (1983). [DOI] [PubMed] [Google Scholar]
- 132).L. Y. Leung, J. W. Lyga and R. A. Robinson: Metabolism and distribution of the experimental triazolone herbicide F6285 [1-[2,4-dichloro-5-[N-(methylsulfonyl)amino]phenyl]-l,4-dihydro-3-methyl-4-(difluoromethyl)-5H-triazol-5-one] in the rat, goat, and hen. J. Agric. Food Chem. 39, 1509–1514 (1991). [Google Scholar]
- 133).A. C. Chukwudebe, P. G. Wislocki, D. R. Sanson, T. D. J. Halls and W. J. A. VandenHeuvel: Metabolism of thiabendazole in laying hen and lactating goats. J. Agric. Food Chem. 42, 2964–2969 (1994). [Google Scholar]
- 134).J. C. Opdycke and R. E. Menzer: Pharmacokinetics of diflubenzuron in two types of chickens. J. Toxicol. Environ. Health 13, 721–733 (1984). [DOI] [PubMed] [Google Scholar]
- 135).L. C. Gaughan, R. A. Robinson and J. E. Casida: Distribution and metabolic fate of trans- and cis-permethrin in laying hens. J. Agric. Food Chem. 26, 1374–1380 (1978). [DOI] [PubMed] [Google Scholar]
- 136).M. H. Akhtar, R. M. G. Hamilton and H. L. Trenholm: Excretion, distribution and depletion of [14C]cypermethrin and cis and trans Isomers of 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid administered orally to laying hens. Pestic. Sci. 20, 53–73 (1987). [Google Scholar]
- 137).J. H. Chen, H. L. Wang, B. Y. Guo, P. Xu and J. Z. Li: The enantioselective pharmacokinetics metabolism of diniconazole in quail (Coturnix coturnixs japonica). Chirality 25, 910–916 (2013). [DOI] [PubMed] [Google Scholar]
- 138).W. Xie and F. Yang: CYP450 enzyme-specific enantioselective species-specific response for metalaxyl in in vitro hepatic cells. Ecotoxicol. Environ. Saf. 149, 10–18 (2018). [DOI] [PubMed] [Google Scholar]
- 139).K. Ohyama: “Comparative In Vitro Metabolism of [14C]methoxychlor in Vertebrate Species Using Precision-cut Liver Slice Technique. In Environmental Fate and Safety Management of Agrochemicals,” eds. by J. M. Clark and H. Ohkawa, ACS Symp. Ser. 899, Chapt. 16, American Chemical Society, Washington D. C., pp. 185–194, 2005.
- 140).Food and Agriculture Organization: “List of Pesticides evaluated by JMPR and JMPS” in http://www.fao.org/agriculture/crops/thematic-sitemap/theme/pests/lpe/jp/ (Accessed 20 May, 2021).
- 141).N. Furusawa: Distribution of aldrin and its epoxide (dieldrin) in egg-forming TISSUES and eggs of laying hens following an oral application. J. Environ. Sci. Health 37B, 123–129 (2002). [DOI] [PubMed] [Google Scholar]
- 142).M. M. Ahmed and C. H. Walker: The metabolism of DDT in vivo by the Japanese quail (Coturnix coturnix japonica). Pestic. Biochem. Physiol. 10, 40–48 (1979). [Google Scholar]
- 143).M. S. Sidra and C. H. Walker: The metabolism of p,p′-DDT by the feral pigeon (Columba livia). Pestic. Biochem. Physiol. 14, 62–71 (1980). [Google Scholar]
- 144).K. L. Davison, C. H. Lamoureux and V. J. Feil: Methoxychlor metabolism in chickens. J. Agric. Food Chem. 32, 900–908 (1984). [Google Scholar]
- 145).R. L. Chrzanowski and A. G. Jelinek: Metabolism of phenyl carbon-14-labeled O-ethyl O-(4-nitrophenyl) phenylphosphonothioate in the rat and in hens at toxic and subtoxic Dose levels. J. Agric. Food Chem. 29, 580–587 (1981). [DOI] [PubMed] [Google Scholar]
- 146).L. E. Staiger, G. B. Quistad, S. K. Duddy and D. A. Schooley: Metabolism of fluvalinate by laying hens. J. Agric. Food Chem. 30, 901–906 (1982). [DOI] [PubMed] [Google Scholar]
- 147).G. B. Quistad, L. E. Staiger and D. A. Schooley: Xenobiotic conjugation: a novel role for bile acids. Nature 296, 462–464 (1982). [DOI] [PubMed] [Google Scholar]
- 148).G. B. Quistad, A. L. Saunders, W. S. Skinner, K. D. Collier, D. H. Sakai and C. C. Reuter: O-Dephenylation and conjugation with benzoylornithine. New metabolic pathways for 3-phenoxybenzoic acid in chickens. Drug Metab. Dispos. 16, 818–822 (1988). [PubMed] [Google Scholar]
- 149).T. S. Foster and S. U. Khan: Metabolism of atrazine by the chicken. J. Agric. Food Chem. 24, 566–570 (1976). [DOI] [PubMed] [Google Scholar]
- 150).S. U. Khan and T. S. Foster: Residues of atrazine (2-chloro-4-ethylamino-6-isopropylamino-s-triazine) and its metabolites in chicken tissues. J. Agric. Food Chem. 24, 768–771 (1976). [DOI] [PubMed] [Google Scholar]
- 151).C. L. Wrzesinski, M. Mushtaq, T. Faidley, N. Johnson, B. Arison and L. S. Crouch: Metabolism of 3H/14C-labeled 4″-deoxy-4″-epimethylaminoavermectin B1a benzoate in chickens. Identification of novel fatty ccid conjugates of 4″-deoxy-4″-epimethylaminoavermectin B1a. Drug Metab. Dispos. 26, 786–794 (1998). [PubMed] [Google Scholar]
- 152).A. W. Abu-Qare, A. A. Abdel-Rahman, H. Ahmad, A. M. Kishk and M. B. Abou-Donia: Absorption, distribution, metabolism and excretion of daily oral doses of [14C]methyl parathion in hens. Toxicol. Lett. 125, 1–10 (2001). [DOI] [PubMed] [Google Scholar]
- 153).M. B. Abou-Donia, Y. M. Hernandez, N. S. Ahmed and S. A. Abou-Donia: Distribution and metabolism of O-ethyl O-4-nitrophenyl phenylphosphonothioate after a single oral dose in one-week old chicks. Arch. Toxicol. 54, 83–96 (1983). [DOI] [PubMed] [Google Scholar]
- 154).D. E. Barnekow, A. W. Hamburg, V. Puvanesarajah and M. Guo: Metabolism of 2,4-dichlorophenoxyacetic acid in laying hens and lactating goats. J. Agric. Food Chem. 49, 156–163 (2001). [DOI] [PubMed] [Google Scholar]
- 155).M. H. Akhtar, R. M. G. Hamilton and H. L. Trenholm: Metabolism, distribution, and excretion of deltamethrin by leghorn hens. J. Agric. Food Chem. 33, 610–617 (1985). [Google Scholar]
- 156).M. H. Akhtar, S. Mahadevan and A. Paquet: Comparative metabolism of deltamethrin and 3-phenoxybenzoic acid in chickens. J. Environ. Sci. Health 29B, 369–394 (1994). [DOI] [PubMed] [Google Scholar]
- 157).E. Weitnauer, A. Robitzki and P. G. Layer: Aryl acylamidase activity exhibited by butyrylcholinesterase is higher in chick than in horse, but much lower than in fetal calf serum. Neurosci. Lett. 254, 153–156 (1998). [DOI] [PubMed] [Google Scholar]
- 158).M. K. Ross, T. M. Streit, K. L. Herring and S. Xie: Carboxylesterases: Dual roles in lipid and pesticide metabolism. J. Pestic. Sci. 35, 257–264 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159).K. L. Davison, J. E. Bakke and G. L. Larsen: A kidney perfusion method for metabolism studies with chickens using propachlor as a model. Xenobiotica 18, 323–329 (1988). [DOI] [PubMed] [Google Scholar]
- 160).R. W. Reiser, R. F. Dietrich, T. K. S. Djanegara, A. J. Fogiel, W. G. Payne, D. L. Ryan and W. T. Zimmerman: Identification of a novel animal metabolite of methomyl insecticide. J. Agric. Food Chem. 45, 2309–2313 (1997). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


