Significance
Global insect declines are profoundly concerning, especially for groups like bees that provide important services to humanity. However, we do not know the extent to which recognized drivers of decline, like pesticides, may produce carryover effects that influence reproduction and population dynamics over time. We reveal that pesticide exposure, both directly to foraging bees and via carryover effects from past exposure, dramatically reduced bee reproduction, which reduced population growth. Carryover effects reduced bee reproduction by 20% beyond current impacts on foraging bees, exacerbating the negative impact on population growth rates. This indicates that bees may require multiple generations to recover from a single pesticide exposure; thus, carryover effects must be considered in risk assessment and conservation management.
Keywords: bee, carryover effects, larva, pesticide, pollinator
Abstract
Pesticides are linked to global insect declines, with impacts on biodiversity and essential ecosystem services. In addition to well-documented direct impacts of pesticides at the current stage or time, potential delayed “carryover” effects from past exposure at a different life stage may augment impacts on individuals and populations. We investigated the effects of current exposure and the carryover effects of past insecticide exposure on the individual vital rates and population growth of the solitary bee, Osmia lignaria. Bees in flight cages freely foraged on wildflowers, some treated with the common insecticide, imidacloprid, in a fully crossed design over 2 y, with insecticide exposure or no exposure in each year. Insecticide exposure directly to foraging adults and via carryover effects from past exposure reduced reproduction. Repeated exposure across 2 y additively impaired individual performance, leading to a nearly fourfold reduction in bee population growth. Exposure to even a single insecticide application can have persistent effects on vital rates and can reduce population growth for multiple generations. Carryover effects had profound implications for population persistence and must be considered in risk assessment, conservation, and management decisions for pollinators to mitigate the effects of insecticide exposure.
Global insect declines threaten biodiversity and associated ecosystem function and services (1–3), and these dramatic declines of many populations have been linked to pesticides (3–6). Substantial growth of global pesticide production (7), as well as the toxicity of applied insecticides (8), emphasize the necessity to understand the mechanisms and magnitude of their impacts on beneficial insects. Studies of these impacts have primarily focused on the effects of pesticide at the time of exposure (9, 10). However, pesticides may substantially affect the performance of individuals and populations long after direct exposure, magnifying their consequences.
Such ecological carryover effects, in which an individual’s past environment or experience impacts current performance, are well documented across taxa and different environmental stressors (11). For example, winter habitat quality influences bird reproduction in the following season (12, 13). For organisms with complex life cycles, stress at one life stage (e.g., larvae) may carry over to affect later life stages (e.g., adults). Because many animals feed extensively as larvae, larval food resources can influence adult performance (14, 15). The maternal environment also affects offspring quality (16, 17) and may profoundly influence the performance of subsequent generations (18, 19). Although not strictly carryover effects, these too have a similar, indirect, delayed impact.
Stress associated with early life stages may be particularly pertinent for insects with complete metamorphosis from larval to adult stages, for which most feeding occurs during the larval stage (20). Furthermore, larvae often have limited mobility and may not be able to escape stressors as easily as adults (21). For example, adult insects commonly move among microsites to moderate temperature, but developing larvae within a nest or attached to a leaf may be unable to escape sun exposure or contaminants within food provisions (22). Carryover effects resulting from larval food environment and temperature conditions are relatively well studied (15, 23, 24), but the effects of other stressors, including pesticides, have been much less explored.
Some research indicates that larval pesticide exposure can have sublethal carryover effects on adults. For example, larval exposure to insecticides has been shown to reduce adult body size in bees (25), butterflies (26), and beetles (27); reduce mating behaviors in adult fruit flies (28); and shorten the lifespan of laboratory-reared adult honeybees (29) and solitary bees (30). However, we lack an understanding of how these effects may influence reproduction and population dynamics over time (31, 32).
Understanding the carryover effects of insecticides and other pesticides is particularly important for pollinators in agroecosystems, where insecticide exposure to bees may limit critical crop pollination services (1, 33, 34). The negative effects of insecticides to foraging bees in these landscapes are well documented; in addition to direct mortality, insecticide exposure can cause sublethal effects, including reduced reproduction and population density, impaired foraging and learning ability, and increased susceptibility to other stressors such as parasites (9, 35, 36), but carryover effects have not been examined.
We investigated the carryover effects of insecticide exposure on the performance of the solitary bee species, Osmia lignaria. We conducted an in-field cage experiment in which we exposed foraging adult solitary bees to insecticides (or not) across 2 y. Pesticide exposure to bees is coupled between mothers and offspring because mothers mass–provision resources at the nest (22). Adult females may be exposed to insecticides during foraging and provisioning, which leads to exposure of immature stages through pollen/nectar provisions in the nest. We used the neonicotinoid insecticide imidacloprid, a common systemic insecticide that binds to the nicotinic acetylcholine receptors (nAChRs) in the insect nervous system and poses a high risk to bees (37–39). Using a crossed experiment with past (year 1) and current (year 2) imidacloprid exposure (Fig. 1), we investigated 1) whether past insecticide exposure (earlier in the life cycle) carries over to affect adult foraging and reproduction, 2) whether current insecticide exposure to adults moderates these effects, and 3) how exposure to insecticide across multiple years affects bee population growth rates. The crossed cage design allowed us to partition variation in insecticide exposure between the 2 y and identify carryover effects that may be difficult to detect when not specifically controlled for, especially in real-world landscapes. In addition, this study provides a relatively rare assessment of multiple demographic responses for solitary bees.
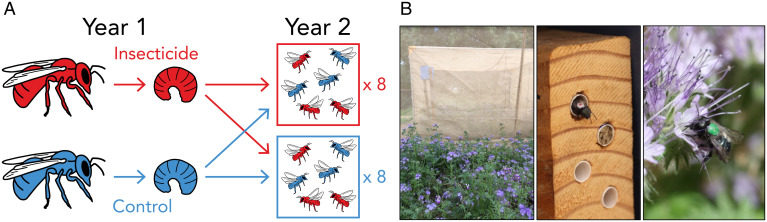
Fig. 1.
Experimental design. (A) Offspring from Stuligross and Williams (44) were released into 16 field cages in a crossed design. Cages in year 2 were treated with (red) or without (blue) imidacloprid and contained foraging female Osmia lignaria with past exposure or without past exposure to imidacloprid in year 1. (B) Flight cage with abundant flower resources (Left), paint-marked female O. lignaria emerging from a nest (Center), and paint-marked female foraging on a Phacelia tanacetifolia flower (Right).
Results
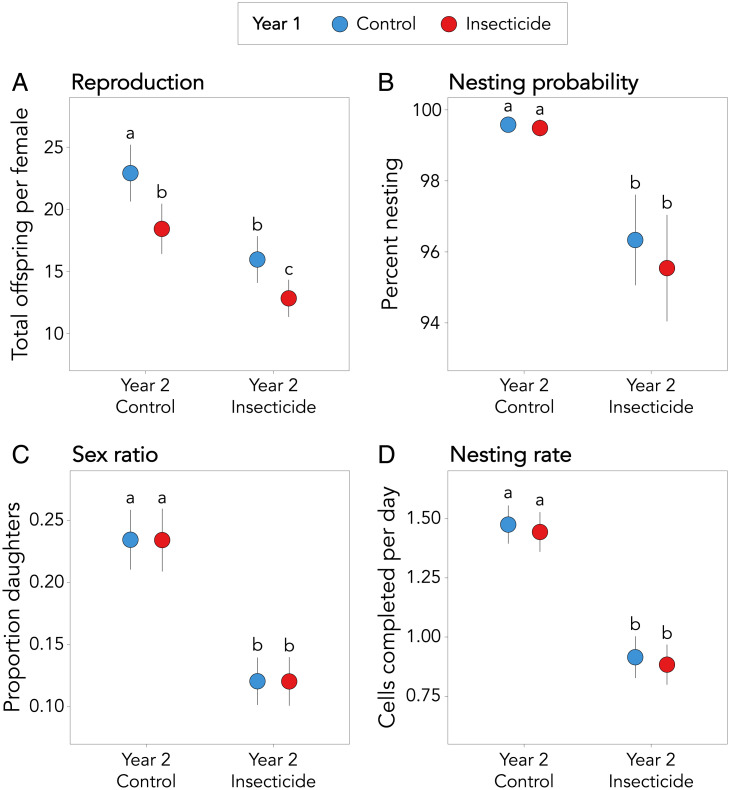
Exposure to insecticide reduced female reproduction, both when exposure was directly to foraging adults and via carryover effects from past exposure. The effects were additive with no interactive effects of exposure between years for any measured response (SI Appendix, Table S1). Of the female bees that initiated nesting, those exposed to imidacloprid as adults (year 2) provisioned 30% fewer offspring than unexposed adults (mean ± SE 14.4 ± 1.5 versus 20.7 ± 1.9, respectively; χ2 = 6.01, P = 0.01; Fig. 2A). Females exposed to imidacloprid in the past (year 1) provisioned 20% fewer offspring compared to individuals with no past exposure (15.6 ± 1.4 versus 19.4 ± 1.6, respectively), indicating a significant carryover effect of insecticide exposure on reproduction (χ2 = 4.68, P = 0.03; Fig. 2A). Together, females exposed to imidacloprid in both years (as larvae and adults) provisioned 44% fewer offspring than females never exposed to insecticide, a difference of ∼10 offspring.
Fig. 2.
Effects of insecticide exposure on bee performance. (A) Mean number of offspring provisioned per nesting female Osmia lignaria. (B) Percent of female bees that produced at least one offspring. (C) Proportion of daughters produced per nesting female. (D) Mean number of cells completed per day per nesting female bee in 16 field cages exposed to insecticide (red) or unexposed (blue) the previous year (year 1) and/or current year (year 2). Error bars are SEs; letters indicate significant differences (P < 0.05).
Current exposure of adult females to insecticide (year 2) reduced their probability of nesting; adult female bees exposed to imidacloprid were 4% less likely to produce offspring (χ2 = 12.65, P < 0.003; Fig. 2B). Past exposure (larvae and mothers in year 1) did not carry over to affect current nesting probability (χ2 = 0.37, P = 0.55; Fig. 2B).
In addition to direct effects on reproduction, current exposure to insecticide increased the male-biased sex ratios in foraging adults, with a 49% reduction in the proportion of daughters provisioned by imidacloprid-exposed adult females (year 2; χ2 = 13.6, P < 0.001; Fig. 2C). Past exposure (year 1) did not carry over to affect offspring sex ratio (χ2 = 0.0001, P = 0.99; Fig. 2C). Overall, imidacloprid exposure reduced female offspring production by 71%—nesting mothers exposed to imidacloprid in both years provisioned an average of just 1.5 daughters each (Fig. 2 A and C).
One potential mechanism by which current (year 2) exposure reduced offspring production was nesting rate. Imidacloprid exposure to foraging adults slowed nest construction by 38% (0.56 cells/d; χ2 = 17.29, P < 0.0001; Fig. 2D). Past exposure (year 1) did not carry over to affect nesting rate (χ2 = 0.11, P = 0.74; Fig. 2D). Imidacloprid exposure also reduced the total number of days bees spent nesting by 2 d, although this result was not significant (χ2 = 1.6, P = 0.45; SI Appendix, Fig. S1).
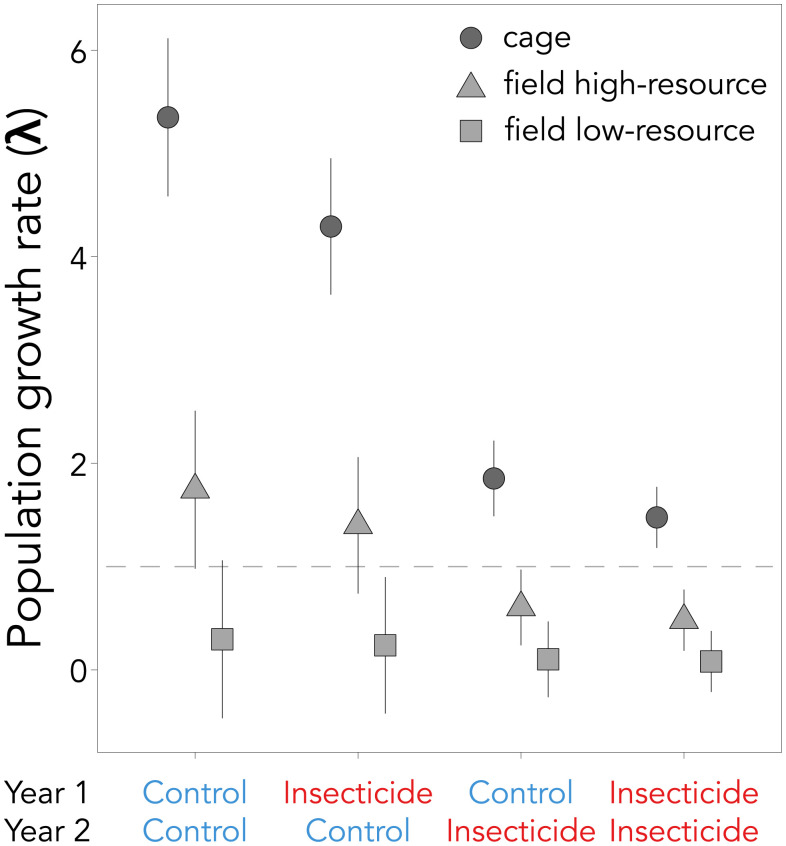
Carryover effects on individual offspring performance also affected population outcomes. Insecticide exposure lowered the growth rate of cage populations, regardless of the exposure timing. Cage populations exposed to imidacloprid in both the past and current year had a population growth rate (λ ± SE) of 1.48 ± 0.30 (Fig. 3). This was 20% lower than exposure just in the current year (year 2; 1.85 ± 0.37), 66% lower than exposure just in the past year (year 1; 4.29 ± 0.66), and 72% lower than no exposure at all (5.35 ± 0.76; Fig. 3). Field population estimates of offspring production from a prior study compared to unexposed cages were 67% lower in the best field environment with abundant floral resources and as much as 94% lower in the low-resource field environment (40). When our corresponding insecticide effects were added on top of the field measures of offspring production to estimate population growth rates, imidacloprid exposure could convert growing populations to declining ones (Fig. 3).
Fig. 3.
Population growth rates (±SE) for Osmia lignaria nesting in field cages when exposed to insecticide or unexposed in the previous year (year 1) and/or current year (year 2). Estimates are based on nesting in field cages in the present study (circles) and scaled to open field environments with high-flower resources (triangles) and low-flower resources (squares), as observed in a previous study (40). The horizontal dashed line marks λ = 1 (values above indicate population growth and values below decline).
Discussion
Bee populations in agricultural landscapes often experience insecticide exposure at multiple stages of the life cycle and over multiple generations (41, 42). However, studies to date have generally examined impacts on a single life stage and within 1 y (but see ref. 43). The persistence of pesticide effects from one generation to the next are unknown and have important consequences, such as additive impacts on the dynamics and persistence of populations. We explore these effects to multiple life stages in the same system and on the same individual animals, allowing us to partition the current and carryover effects of chronic insecticide exposure on individual performance and populations to reveal important additive impacts of both. Bees exposed to insecticide, both as nesting adults and in the previous year as developing larvae, provisioned fewer offspring. Insecticide exposure of foraging adults reduced reproduction, reduced the proportion of female offspring produced, delayed nesting onset and cessation, and lowered the rate of nest provisioning; all confirm past research (44). Insecticide exposure from the previous year had an additional negative effect, further reducing reproduction. This carryover effect had a lasting implication for population growth.
Past insecticide exposure reduced bee reproduction regardless of their current exposure as adults. Bees exposed to insecticide as larvae in the past year but not subsequently as adults nonetheless provisioned over 30% fewer offspring than control bees that were never exposed to insecticide. This indicates that even exposure to a single insecticide application could have persistent effects on vital rates and longer lasting transient effects of population dynamics (31). Moreover, because the impacts of insecticides appear to be additive across life stages, repeated exposure may have profound implications for bee population persistence in many agroecosystems, where frequent exposure may lead to population decline (6). This is especially concerning considering the persistence of neonicotinoid insecticides in the environment long after application (39, 45), which we also found in our study (SI Appendix, Table S2).
Importantly, the impacts of larval exposure to imidacloprid were only expressed at the final reproductive output itself. Past insecticide exposure did not affect the subsequent probability of nest initiation, offspring sex ratio, or the rate of nest completion but appeared only in number of offspring produced. This may be the result of minor negative impacts on each of the intermediate variables measured, which add up to reduce the overall reproduction. This delayed observance of effects on later vital rate parameters is precedented in animal and plant systems (46, 47). A particularly striking example in plants found that daytime versus evening pollination of a flowering shrub did not influence seed mass, germination, or seedling emergence in the greenhouse (46). But it led only to significant reductions in seedling emergence in the field the following year (46).
The delayed expression of effects that we observed could help to explain the lack of carryover effects found in some past studies, which have shown no effect of larval insecticide exposure on development time or survival in bees and other insects (26, 48, 49). Our finding emphasizes the importance of considering potential carryover effects of stressors and cautions against interpreting a lack of measured effects on intermediate proxies of vital rates as evidence of no impact on reproduction or population persistence. Studies that have found that no effects of larval neonicotinoid exposure are encouraging but may have missed negative effects that only become evident later.
The biology of O. lignaria, as well as that of other solitary bees (22) and animals that feed their offspring (12), means that effects of past exposure could be through larval exposure to the food provision, as well as exposure of their mothers during provisioning. Offspring fitness outcomes, including changes in sex ratio and insecticide resistance in insects, have been attributed to maternal effects (18). However, it is often difficult to separate maternal effects from offspring environment or genotype (50). Indeed, mother bees exposed to insecticides while foraging in real-world landscapes would similarly pass the exposure onto their offspring through the pollen provisions and nesting materials (21, 45). Our free-flying cage design allows us to study these exposure pathways and separate impacts of exposure to current adults from carryover effects. Although we cannot separate maternal from larval effects, past exposure reduced adult reproduction in our study, demonstrating carryover effects from field-realistic exposure across multiple years that impair individual performance and lower population growth.
Population growth rates for all study treatments were positive, so it is perhaps tempting to dismiss the importance of the carryover effects of insecticides on bee fitness. Growth rates from our study are based on individuals in field cages with unlimited food and nesting resources, protected from other threats such as parasites and predators that they may encounter in an open-field setting. When we applied the insecticide impacts estimated from our cages to field-realistic growth rates, imidacloprid exposure easily converted positive growth to negative growth rates, even in landscapes with abundant floral resources (40). Testing this impact directly is a critical area for future research (35).
The magnitude of the effects of insecticide exposure on reproduction was 55% larger when the exposure was directly to adults in the current year compared to when it was through carryover effects of past exposure as larvae. This difference may result from different pesticide sensitivity among life stages. Some studies have found that bee larvae may tolerate higher exposure to neonicotinoids than adults (48, 51). One potential reason for this is that the expression patterns of the nAChRs in the insect nervous system change during development from the larval to adult stage (52). Because bees during early development have fewer structures with nAChRs in the nervous system than adults, the same exposure to imidacloprid may have a weaker effect when an individual is a larva than when it is an adult. This lowered sensitivity early in life may translate to a less dramatic carryover effect on reproduction. The effects of exposure across life stages are nonetheless additive, so both past and current exposure is more than exposure at either stage alone.
Our study reveals that past exposure to environmental stressors such as insecticides, in addition to current exposure, has profound effects, with implications for individual reproduction and population trends. Hundreds of studies have investigated insecticide effects on bees (9, 53), but few quantify exposure across generations or to multiple life stages in the same study (43). In our study, carryover effects of past insecticide exposure were not detected until the final reproductive stage, in which their impacts indicate that populations may take multiple generations to recover from exposure. Furthermore, repeated exposure from 1 y to the next can have additive effects on individuals’ vital rates and, thus, a more detrimental effect on populations. Our results inform pesticide risk assessment and reinforce the importance of preventing insecticide exposure to beneficial insects in landscapes where their effects could substantially reduce population persistence. Future studies to assess multiyear insecticide exposure under field conditions will be important to understand full impacts and inform strategies to mitigate effects of potential exposure.
Materials and Methods
Study System and Experimental Design.
The blue orchard bee O. lignaria is a solitary univoltine species native to North America. It and other Osmia species are widely used as alternative, managed pollinators to honey bees and/or in combination with them in fruit orchards (33, 35). Females nest above ground inside preexisting cavities (e.g., abandoned wood-boring beetle burrows or artificial paper tubes). Nests are constructed as a linear series of brood cells, which are separated by mud partitions. The entire life cycle takes about a year; females mass–provision offspring using pollen and nectar and lay a single egg on or within each provision. Larvae hatch and consume the provision before spinning a cocoon and pupating. Offspring overwinter as adults within their cocoons and emerge the following spring.
We conducted the experiment in 3 × 3 × 1.8 m flight cages at the University of California (UC) Davis Bee Research Facility during the spring of 2019. In each cage, we planted a high-density mix of three common wildflowers: Phacelia tanacetifolia, Phacelia ciliata, and Collinsia heterophylla (SI Appendix, Table S3). These flowers offer high-quality nutrition for offspring, are used by O. lignaria, and bloom during their foraging period (54). When flowers approached full bloom (early May 2019), we released eight newly emerged adult female and 16 male O. lignaria per cage to match their natural, male-biased sex ratio.
Adult bees used in the trials were sourced from different past insecticide exposure backgrounds. In the previous year (2018), we conducted an experiment using the same field cage design (44). In the previous experiment, cages received the same insecticide treatments as the current study, and O. lignaria flying in field cages provisioned offspring in nests. Adult female O. lignaria in the present study were offspring from either imidacloprid-treated cages or control cages with unlimited floral resources from the past study (44). Adult males were offspring only from unexposed control cages, so any effect of past imidacloprid exposure was strictly maternal. Because we sourced all bees from high-resource environments, and males were also from unexposed cages, our findings are likely conservative. The offspring from each past treatment (past exposure versus no past exposure to imidacloprid) were crossed with current imidacloprid treatments (current exposure versus no current exposure) in a reciprocal transplant to enable the differentiation of effects of the current year from those due to past exposure (Fig. 1A). Half of the females released in each cage were randomly assigned from each of two past insecticide exposure treatments, such that each cage had four females with past imidacloprid exposure and four females with no past imidacloprid exposure (Fig. 1A). We individually marked each female to monitor nesting and distinguish between treatments (Fig. 1B). In each cage, we placed a wooden nesting block with 12 predrilled holes, 7.8 mm in diameter and 13 cm in length. We lined each hole with a translucent paper straw, which we removed and replaced as they were filled with nests. We stored all completed nests in the laboratory (40, 44). We provided a consistent mud source for nest construction throughout the trials using moistened soil from each cage.
We added new bees periodically as others died to maintain an average of five actively nesting females in each cage. To control for possible effects of timing, we balanced bee additions across treatments, and we also included the release date as a covariate in our analyses. In total, we released 161 bees among all cages (n = 40 untreated in both years, 41 untreated in year 1 and treated in year 2, 37 treated in year 1 and untreated in year 2, and 43 treated in both years). We monitored nesting activity daily for a minimum of 20 min per cage by watching females take foraging trips in and out of their nests; this allowed us to associate each nest with a nesting female. We measured nesting progression daily by temporarily removing the nest straw and marking the nest progress on the outside of the straw (40).
Completed nests were stored in darkness at 22 °C for 6 mo, followed by 4 mo at 6 °C to overwinter. The following spring, we opened all nests to determine the number, sex, and condition of all offspring matched to each mother.
Neonicotinoid Treatments.
We applied a soil drench of the neonicotinoid insecticide imidacloprid (AdmirePro, Bayer Crop Science) 5 wk prior to releasing bees in cages at the maximum label rate (10.5 oz/ac; 767 mL/ha) for herbs and orchard fruit crops. Imidacloprid is the most frequently applied insecticide in California (55) and is widely used across the United States and worldwide (37, 56). AdmirePro is the most common commercial imidacloprid product applied in California (55). Imidacloprid has also been found in Osmia nests in agricultural landscapes (57). To prevent lateral movement of imidacloprid through the soil, we buried eight layers of 4-mm clear plastic sheeting 40 cm into the ground between treated and untreated cages. We measured insecticide exposure based on imidacloprid residues from the pollen provisions within nests, a single male larval provision per cage, which were sent for analysis using a modified QuEChERS protocol (58) using liquid chromatography–mass spectrometry/mass spectrometry analysis at the Cornell University Chemical Ecology Core Facility. All pollen samples from the insecticide-treated cages contained detectable levels of imidacloprid at field-realistic levels (mean ± SE 11.26 ± 2.82 parts per billion [ppb]; SI Appendix, Table S2). None of the pollen samples from untreated control cages contained detectable imidacloprid levels (SI Appendix, Table S2).
Statistical Analysis.
We used a generalized and linear mixed model (GLMM) framework to analyze the effects of past and current insecticide exposure on adult O. lignaria performance. To assess the effects of insecticide exposure on O. lignaria nesting probability, we fit GLMMs with binomial error distribution and logit link. We included past insecticide exposure (year 1 insecticide and year 1 control), current insecticide exposure (year 2 insecticide and year 2 control), and date released in a cage as fixed effects and cage as a random effect. Insecticide exposure did not interact between year 1 and year 2 for any response variable (SI Appendix, Table S1), so we removed the interaction term from final models. We fit a GLMM with negative binomial error distribution (to account for overdispersion) and log link to assess effects of insecticide exposure on total offspring production and a binomial GLMM to assess insecticide effects on offspring sex ratio (proportion female). We assessed differences in nest construction rate (cells per day) and total nesting days using LMMs with normal error distribution. We calculated P values of fixed effects in mixed models using likelihood ratio tests. To determine the population growth rate, we multiplied three vital rates: nesting probability × total offspring × proportion female offspring. We only considered females because they are the demographically important sex; male bees do not contribute to the next-generation offspring production. We calculated SEs for population growth rate means using the delta method (59). To explore the relative impact of insecticide exposure on field populations, we calculated differences in reproduction between our cage study and published field data from high– and low–floral resource landscapes (40) to generate scaling factors that we applied to our cage λ values. We incorporated the same error structure as the cage data and scaled the values according to the effect sizes observed in the cage treatments. We conducted all analyses in R (version 3.6.3).
Supplementary Material
Acknowledgments
We thank Charley Nye, Maureen Page, Abby Primack, Elsa Walsh, Alejandra Ulloa, and Natalie Kopec for field assistance; Maj Rundlöf, Louie Yang, Jay Rosenheim, and Sharon Lawler for suggestions on study design; and Elizabeth Crone for guidance on population analysis. We also thank Elizabeth Crone, Nicholas Dorian, Maj Rundlöf, and Maureen Page for feedback on an earlier version of this manuscript. This study was supported by a UC Davis Jastro Research Award, a UC Davis Graduate Group in Ecology Research Fellowship, and an NSF Graduate Research Fellowship to C.S. The project was also supported in part by NSF grant DEB1354022 to N.M.W. and the UC Davis Department of Entomology through the H. Laidlaw Bee Research Facility and Laidlaw Endowment.
Footnotes
Author contributions: C.S. and N.M.W. designed research; C.S. performed research; C.S. analyzed data; N.M.W. assisted with data interpretation; and C.S. and N.M.W. wrote the paper.
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2109909118/-/DCSupplemental.
Data Availability
Source data for study analysis have been deposited in the Dryad Digital Repository (https://doi.org/10.25338/B8GK8Q). All other study data are included in the article and/or SI Appendix.
References
- 1.Chagnon M., et al. , Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. Int. 22, 119–134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner D. L., Grames E. M., Forister M. L., Berenbaum M. R., Stopak D., Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. U.S.A. 118, e2023989118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sánchez-Bayo F., Wyckhuys K. A. G., Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 232, 8–27 (2019). [Google Scholar]
- 4.Goulson D., Nicholls E., Botías C., Rotheray E. L., Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Potts S. G., et al. , Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Woodcock B. A., et al. , Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat. Commun. 7, 12459 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilman D., et al. , Forecasting agriculturally driven global environmental change. Science 292, 281–284 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Schulz R., Bub S., Petschick L. L., Stehle S., Wolfram J., Applied pesticide toxicity shifts toward plants and invertebrates, even in GM crops. Science 372, 81–84 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Müller C., Impacts of sublethal insecticide exposure on insects — Facts and knowledge gaps. Basic Appl. Ecol. 30, 1–10 (2018). [Google Scholar]
- 10.Wu-Smart J., Spivak M., Sub-lethal effects of dietary neonicotinoid insecticide exposure on honey bee queen fecundity and colony development. Sci. Rep. 6, 32108 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor C. M., Norris D. R., Crossin G. T., Cooke S. J., Biological carryover effects: Linking common concepts and mechanisms in ecology and evolution. Ecosphere 5, art28 (2014). [Google Scholar]
- 12.Norris D. R., Marra P. P., Kyser T. K., Sherry T. W., Ratcliffe L. M., Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc. Biol. Sci. 271, 59–64 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robb G. N., et al. , Winter feeding of birds increases productivity in the subsequent breeding season. Biol. Lett. 4, 220–223 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chelgren N. D., Rosenberg D. K., Heppell S. S., Gitelman A. I., Carryover aquatic effects on survival of metamorphic frogs during pond emigration. Ecol. Appl. 16, 250–261 (2006). [DOI] [PubMed] [Google Scholar]
- 15.De Block M., Stoks R., Fitness effects from egg to reproduction: Bridging the life history transition. Ecology 86, 185–197 (2005). [Google Scholar]
- 16.Mousseau T. A., Fox C. W., The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Moore M. P., Whiteman H. H., Martin R. A., A mother’s legacy: The strength of maternal effects in animal populations. Ecol. Lett. 22, 1620–1628 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Mousseau T. A., Dingle H., Maternal effects in insect life histories. Annu. Rev. Entomol. 36, 511–534 (1991). [Google Scholar]
- 19.Tran T. T., Janssens L., Dinh K. V., Stoks R., Transgenerational interactions between pesticide exposure and warming in a vector mosquito. Evol. Appl. 11, 906–917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boggs C. L., Understanding insect life histories and senescence through a resource allocation lens. Funct. Ecol. 23, 27–37 (2009). [Google Scholar]
- 21.Sgolastra F., et al. , Pesticide exposure assessment paradigm for solitary bees. Environ. Entomol. 48, 22–35 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Danforth B. N., Minckley R. L., Neff J. L., Fawcett F., The Solitary Bees: Biology, Evolution, Conservation (Princeton University Press, 2019). [Google Scholar]
- 23.Metcalfe N. B., Monaghan P., Compensation for a bad start: Grow now, pay later? Trends Ecol. Evol. 16, 254–260 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Galarza J. A., et al. , Evaluating responses to temperature during pre-metamorphosis and carry-over effects at post-metamorphosis in the wood tiger moth (Arctia plantaginis). Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20190295 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wintermantel D., et al. , Field-level clothianidin exposure affects bumblebees but generally not their pathogens. Nat. Commun. 9, 5446 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olaya-Arenas P., Hauri K., Scharf M. E., Kaplan I., Larval pesticide exposure impacts monarch butterfly performance. Sci. Rep. 10, 14490 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller T., Gesing M. A., Segeler M., Müller C., Sublethal insecticide exposure of an herbivore alters the response of its predator. Environ. Pollut. 247, 39–45 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Young H. K., Denecke S. M., Robin C., Fournier-Level A., Sublethal larval exposure to imidacloprid impacts adult behaviour in Drosophila melanogaster. J. Evol. Biol. 33, 151–164 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Tsvetkov N., et al. , Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 356, 1395–1397 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Anderson N. L., Harmon-Threatt A. N., Chronic contact with realistic soil concentrations of imidacloprid affects the mass, immature development speed, and adult longevity of solitary bees. Sci. Rep. 9, 3724 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckerman A., Benton T. G., Ranta E., Kaitala V., Lundberg P., Population dynamic consequences of delayed life-history effects. Trends Ecol. Evol. 17, 263–269 (2002). [Google Scholar]
- 32.Köhler H.-R., Triebskorn R., Wildlife ecotoxicology of pesticides: Can we track effects to the population level and beyond? Science 341, 759–765 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Klein A.-M., et al. , Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 274, 303–313 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanley D. A., et al. , Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature 528, 548–550 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rundlöf M., et al. , Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Stanley D. A., Raine N. E., Chronic exposure to a neonicotinoid pesticide alters the interactions between bumblebees and wild plants. Funct. Ecol. 30, 1132–1139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeschke P., Nauen R., Schindler M., Elbert A., Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 59, 2897–2908 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Craddock H. A., Huang D., Turner P. C., Quirós-Alcalá L., Payne-Sturges D. C., Trends in neonicotinoid pesticide residues in food and water in the United States, 1999-2015. Environ. Health 18, 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goulson D., An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987 (2013). [Google Scholar]
- 40.Williams N. M., Kremen C., Resource distributions among habitats determine solitary bee offspring production in a mosaic landscape. Ecol. Appl. 17, 910–921 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Mullin C. A., et al. , High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS One 5, e9754 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goulson D., Thompson J., Croombs A., Rapid rise in toxic load for bees revealed by analysis of pesticide use in Great Britain. PeerJ 6, e5255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willis Chan D. S., Raine N. E., Population decline in a ground-nesting solitary squash bee (Eucera pruinosa) following exposure to a neonicotinoid insecticide treated crop (Cucurbita pepo). Sci. Rep. 11, 4241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stuligross C., Williams N. M., Pesticide and resource stressors additively impair wild bee reproduction. Proc. Biol. Sci. 287, 20201390 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willis Chan D. S., Prosser R. S., Rodríguez-Gil J. L., Raine N. E., Assessment of risk to hoary squash bees (Peponapis pruinosa) and other ground-nesting bees from systemic insecticides in agricultural soil. Sci. Rep. 9, 11870 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrera C. M., Flower-to-seedling consequences of different pollination regimes in an insect-pollinated shrub. Ecology 81, 15–29 (2000). [Google Scholar]
- 47.Jackson N. J., Stewart K. M., Wisdom M. J., Clark D. A., Rowland M. M., Demographic performance of a large herbivore: Effects of winter nutrition and weather. Ecosphere 12, e03328 (2021). [Google Scholar]
- 48.Nicholls E., Fowler R., Niven J. E., Gilbert J. D., Goulson D., Larval exposure to field-realistic concentrations of clothianidin has no effect on development rate, over-winter survival or adult metabolic rate in a solitary bee, Osmia bicornis. PeerJ 5, e3417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strobl V., et al. , No impact of neonicotinoids on male solitary bees Osmia cornuta under semi-field conditions. Physiol. Entomol. 46, 105–109 (2021). [Google Scholar]
- 50.Wolf J. B., Wade M. J., What are maternal effects (and what are they not)? Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1107–1115 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang E.-C., Chang H.-C., Wu W.-Y., Chen Y.-W., Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS One 7, e49472 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dupuis J., Louis T., Gauthier M., Raymond V., Insights from honeybee (Apis mellifera) and fly (Drosophila melanogaster) nicotinic acetylcholine receptors: From genes to behavioral functions. Neurosci. Biobehav. Rev. 36, 1553–1564 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Lundin O., Rundlöf M., Smith H. G., Fries I., Bommarco R., Neonicotinoid insecticides and their impacts on bees: A systematic review of research approaches and identification of knowledge gaps. PLoS One 10, e0136928 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyle N. K., et al. , Wildflower plantings promote blue orchard bee, Osmia lignaria (Hymenoptera: Megachilidae), reproduction in California almond orchards. Ecol. Evol. 10, 3189–3199 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.California Department of Pesticide Regulation, Data from "2018 Pesticide use report." CalPIP. https://calpip.cdpr.ca.gov/main.cfm. Accessed 1 May 2021.
- 56.Bass C., Denholm I., Williamson M. S., Nauen R., The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 121, 78–87 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Centrella M., et al. , Diet diversity and pesticide risk mediate the negative effects of land use change on solitary bee offspring production. J. Appl. Ecol. 57, 1031–1042 (2020). [Google Scholar]
- 58.Anastassiades M., Lehotay S. J., Štajnbaher D., Schenck F. J., Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 86, 412–431 (2003). [PubMed] [Google Scholar]
- 59.Williams B. K., Nichols J. D., Conroy M. J., Analysis and Management of Animal Populations (Academic Press, 2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data for study analysis have been deposited in the Dryad Digital Repository (https://doi.org/10.25338/B8GK8Q). All other study data are included in the article and/or SI Appendix.