Abstract
AIM
To investigate the retinal vascular network alterations in eyes of patients with pterygium.
METHODS
Totally 18 left eyes from 18 female pterygium patients and 18 left eyes from 18 female healthy control subjects were enrolled. Optical coherence tomography angiography (OCTA) images were generated of the superficial retinal layer and deeper retinal layer of the macular retina for each eye. The microvascular (MIR) and macrovascular (MAR) densities were calculated and MIR, MAR, and total microvascular (TMI) density was compared in the healthy control and pterygium groups.
RESULTS
In pterygium group, in the superficial retinal layer, the vascular density in superficial MIR, superior right (SR), inferior right (IR), right (R), superficial central annuli (SC)1, SC2, and SC3 decreased significantly in the macular area (P<0.05). Furthermore, the vascular density in all those decreased regions except R, was significantly and negatively correlated with the disease course (r=-0.6038 to -0.7762, P=0.0008), and the area size of pterygium (r=-0.6043 to -0.9508, P<0.05). For the deeper retinal layer, the density of deep total microvessel (DTMI), deeper MIR, SR, IR, R, DC2, and DC3 decreased significantly in macular area of pterygium patients (P<0.05). Furthermore, the vascular density in all those decreased regions was significantly and negatively correlated with the disease course (r=-0.6901 to -0.7795, P=0.0015), and the area size of pterygium (r=-0.6043 to -0.9563, P<0.05). No statistically significant differences and correlation was found in other region density (|r|<0.47, P>0.05).
CONCLUSION
OCTA findings suggest that pterygium patients present with decreased retinal MIR density, and the major vascular alterations occurr mainly on the bitamporal side. The vascular density of the superficial SC1, SC2, SC3 adjacent to the foveal and deep layer of DC2, DC2 regions, significantly decreased.
Keywords: retinal microvasculature, density, pterygium, optical coherence tomography angiography
INTRODUCTION
Pterygium is a common disease of the ocular surface involving deposition of a triangular mass of fibrovascular tissue from the bulbar conjunctiva to the cornea, usually occurring on the nasal side, and seriously affecting visual acuity[1]. Its pathological mechanism has not been elucidated. Ultraviolet radiation, wind, and dust generally affect the prevalence of pterygium[2] with a incidence up to 39% among people over 50 years old[3]. Pterygium not only affects the patient's appearance, but also leads to a astigmatism, eye irritation, and dry eyes. Furthermore, the recurrence of pterygium can also cause serious complications such as symblepharon and restriction of eyeball movement[3]. Hill and Maske[4] reported that neovascularization might be involved in the occunrrence and development of ptergium, which is further evidenced by Aspiotis et al[5], he reported that the microvascular (MIR) density from 52 ptergium speciments was significantly increased than that of 7 normal conjunctival tissues analyzed by using the immunohistochemistry staining. Jabbarpoor Bonyadi[6] present spectral domain optical coherence tomography (SD-OCT) findings of bilateral photic maculopathy following pterygium excision; the operations were done under local anaesthesia. They think the use of coaxial light for surgical field illumination during entire procedure were predisposing factors to photic maculopathy. Photic maculopathy has been well documented in sun-gazing[7]. Zhou et al[8] found there was a significant increase up in retinal vasculature parameters in macular after cataract surgery. Long-term follow up studies showed that the increase could persist for up to six months. They speculated that the increase in light exposure after intraocular lens (IOL) replacement may be responsible for long-term fundus change. Pterygium that invades the cornea can also block light from entering the refractive media. Whether pterygium can affect the changes of microvessels in the fundus macular area has not been reported at present. Therefore, in-depth studies of pterygium are actively demanded for the prevention and recurrence and disease by throwing light on the its mechanism with great significance.
Misra et al[9] investigated the changes in visual acuity after pterygium surgery, and found that both unaided and best spectacle corrected visual acuity were improved significantly in 1mo postoperatively, and the improvement was persistent and maintained for 3mo postoperatively. The macular area of the human fundus is largely responsible for the human's overall vision, because it is the primary distribution locus for the visual cells and retinal ganglion cells[10]–[11]. It is important to understand the coupling between the macular vascular system and the dense macular neurons. For non-invasive retinal vasculature assessment, in previous studies the researchers used Doppler OCT to monitor blood flow in large vessels, but such method is not sensitive enough to monitor the blood flow of small vessels[12]. To accurately quantify retinal vasculature, optical coherence tomography angiography (OCTA) offers data with good repeatability and reproducibility, which is capable of measuring both macrocirculation and microcirculation[13]. Recently, benefited by the application of OCTA technique as a novel non-invasive measurement. Therefore, in this study, we applied the OCTA technique to investigate the retinal microvasculature alteration in pterygium, and the correlation with the disease course and area size of pterygium.
SUBJECTS AND METHODS
Ethical Approval
This study was approved by the Ethics Committee of Medical School of Nanchang University and performed in accordance with the Declaration of Helsinki principles. Informed consent was obtained from all individual participants included in the study.
In this study, 18 left eyes from 18 female patients with pterygium and 18 left eyes from 18 female healthy controls were enrolled. All subjects were evaluated by a retinal specialist from the First Affiliated Hospital of Nanchang University between 2017 and 2018. The age with all the subjects was between 40 and 48 years old, with average age of 46±10y.
All patients underwent clinical examination and ophthalmological assessment at the first visits. The inclusion criteria included: 1) female patients first diagnosed with primary pterygium occurring in the nasal side in her left eye; 2) head of the pterygium invaded 2.0-7.0 mm toward the corneal limbus; 3) a disease history of 7-20y, with an average of 6.70±5.01y; 4) binocular fixation was good, and the diopter difference between the two eyes not exceed 2 D; 5) intraocular pressure was 11-21 mm Hg (1 mm Hg=0.133 kPa) with normal fundus conditions.
The exclusion criteria included: 1) eye surgery or trauma; 2) corneal diseases (including the large cornea, the small cornea, keratoconus, etc.); 3) eye disease or other systemic diseases affecting eye circulation, such as glaucoma, hypertension, diabetes, etc; 4) drug treatment within the past two weeks; 5) fundus examination indicated highly myopic and pathological macular changes (including macular holes, neovascularization, atrophy, etc.); 6) severe cataracts, mblyopia, and patients wearing contact lenses; 7) systemic diseases including mental and central nervous system disorders; 8) pregnancy and lactating women; 9) pseudopterygium and fibromyalgia; 10) pupil dilation and dilation agent sensitive. The demographic characteristics and clinical findings of patients with pterygium and healthy controls were summarized in Table 1.
Table 1. Demographic characteristics and clinical findings of patients with pterygium and healthy controls.
| Condition | Pterygium | Healthy control | t | P a |
| Age (y) | 45±11 | 46±13 | -0.16 | 0.92 |
| Sex (M/F) | 0/18 | 0/18 | N/A | >0.99 |
| Best corrected visual acuity | 0.6±0.5 | 1±1 | 0.35 | 0.04 |
| SE-L (D) | 1.15±1.00 | 1.25±0.75 | 0.19 | 0.83 |
| Astigmatism-L (D) | 2.50±0.75 | 1.25±0.75 | 4.17 | 0.04 |
| SSI | 9±1 | 9±1 | N/A | >0.99 |
| Course of pterygium (y) | 6.70±5.01 | / | / | / |
| Length of pterygium (mm) | 6.25±1.65 | / | / | / |
| Height of pterygium (mm) | 4.01±1.44 | / | / | / |
| Area of pterygium (mm2) | 21.69±11.33 | / | / | / |
| Heart rate (beats/min) | 69±6 | 68±7 | -0.12 | 0.95 |
| Systolic blood pressure (mm Hg) | 128±15 | 127±18 | -0.21 | 0.85 |
| Diastolic blood pressure (mm Hg) | 86±13 | 85±12 | -0.06 | 0.79 |
| Mean intraocular pressure (mm Hg) | 18±3 | 15±5 | -0.25 | 0.72 |
aIndependent t-tests comparing two groups. N/A: Not applicable; L: Left; SE: Spherical equivalent; SSI: Signal strength index.
All subjects underwent complete ophthalmological evaluations, including: 1) Slit lamp examination. Slit lamp microscopy was used to examine the anterior segment of the eye, and to detect ocular inflammation, corneal opacity or severe cataracts with image refraction, and the size of any pterygium, and the scope of corneal limbus invasion. 2) Visual acuity. A logarithmic visual acuity chart was used, and the binocular vision and the best corrected visual acuity was obtained for all the subjects. 3) Intraocular pressure. A TDT tonometer (BiCOM, Long Beach, NY, USA) was used to measure intraocular pressure three times. The intraocular pressure measured in both eyes was less than 21 mm Hg. The difference between the two measurements was ≤3 mm Hg, and the average value was calculated. 4) Dimensional parameters of pterygium include: length (defined as the distance from the corneal limbus to the edge of the pterygium), height (defined as the distance between the relative edge of the pterygium and the corneal limbus), and area (as defined as the surface area of the cornea invaded by pterygium) were calculated using the Image J of the National Institutes of Health, Bethesda, MD, USA (Figure 1)[14]. Corneal diameters were measured with digital calipers from 18 patients. The images were calibrated with pixels/mm (mean=368.12 and standard deviation=9.74 pixels/mm). The accuracy of the measurements per mm was 0.025 mm. Given the possibility that different corneal diameters have different types of effects on the pterygium, three new parameters were defined and calculated from the length, height, and area of the pterygium by using corneal diameters (D). The length, hight, and area of each pterygium were measured 5 times to generate an average.
Figure 1. Illustration of dimensional parameters of pterygium.
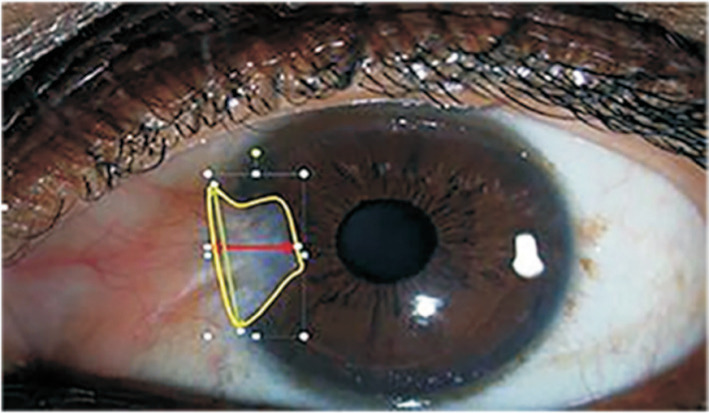
Clinical photographic imaging by using the camera mounted with Zeiss slit lamp and NIH Image J software to measure the size [length (green), height (red), corneal area invasion (yellow)].
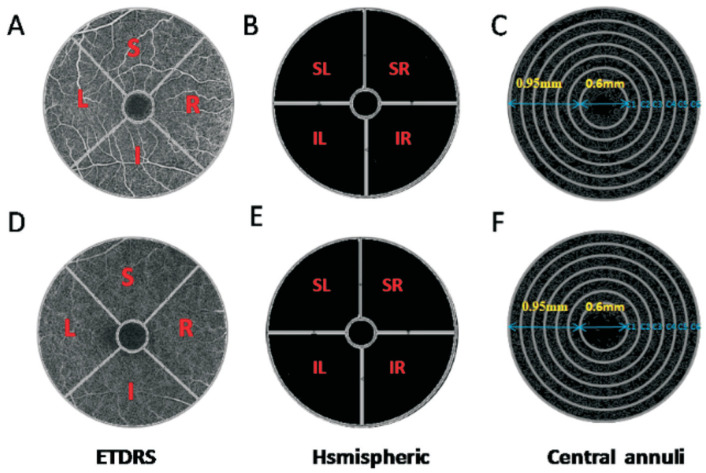
To simultaneously visualize the retinal cross sections and microvasculature, OCTA imaging was performed with the RTVue Avanti XR system (Optovue, Fremont, CA, USA). The scan speed was set to 70 000 A-scans/second, with a central wavelength of 840 nm and bandwidth of 45 nm super light-emitting diode[15]. Imaging was performed using angiographic[16]–[17] repeated B-scans of 6×6-mm2 scan patterns of 216 A-scans (along the X-axis) each at 216 raster positions, focused at the foveal center, and the acquisitiontime was 3.9s. The superficial and deep microvasculature from the retina may be obtained from the automatic fractionation. We acquired 6×6-mm2 OCTA images by a series of 2 volume scans: 1 horizontal and 1 vertical gratings. For each eye, 3-dimensional 6×6-mm2 en-face OCT angiograms were calculated. Motion artifacts were corrected using orthogonal scan alignment algorithm. Density was calculated using SSADA algorithm. The retinal superficial blood flow layer was defined as 3 µm below the inner limiting membrane to 15 µm below the inner plexiform layer, and the retinal deep blood flow layer was defined as 15-70 µm below the inner plexiform layer. The superficial retinal layer consists of ganglion cells and inner plexiform layer, while the deep retinal layer consists of inner nuclear layer and outer plexus layer. These layers contain the entire retinal vascular system[18]. Patients with pterygium invading the pupil and affecting the examination were given point-eye dilatation examination with short-acting dilatation agent. We can better understand the relationship between pterygium and macular vascular density area (Figure 2), and the zoning method for area of macular retinal is shown in Figure 3.
Figure 2. Illustration of the pterygium and macular vascular density area.
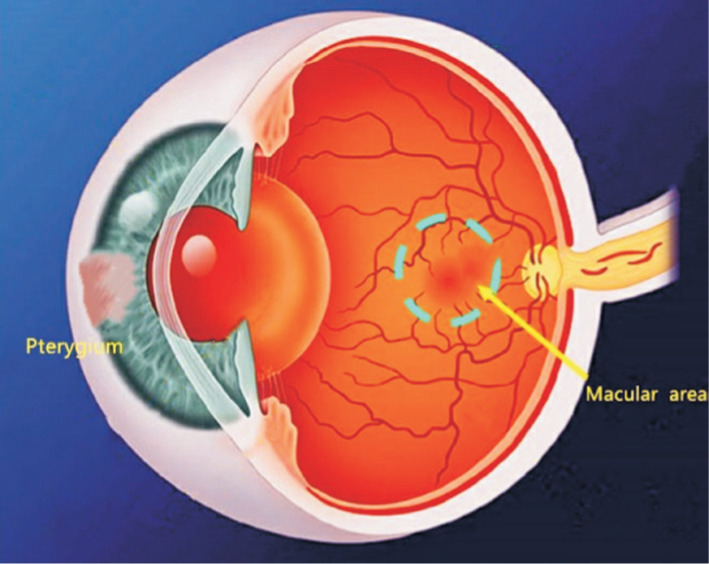
A left corneal limbus pterygium in nasal side and its corresponding macular vascular area.
Figure 3. The 6×6-mm2 OCTA image of the macular region of the retina.
A, D: The original OCTA image of superficial and deep retinal vascular plexus respectively, based on the ETDRS partition method, which divided the image into 4 quadrants of vertical and horizontal regions, followed by R, S, L, and I; B, E: The original image of large retinal vessels in the superficial and deep retina, respectively, based on the hsmispheric partition method, which divided the ring area into 4 quadrants, followed by SR, SL, IL, and IR; C, F: The skeletonized microvessel images captured from the superficial and deep retinal vascular plexus, respectively, based on central annuli partition method after removal of the avascular zone (0.6 mm diameter of the fovea), a circular region of 0.6 to 2.5 mm in diameter is defined as the ring with bandwidth of 0.95 mm. The annular region is divided into 6 thin rings with a bandwidth of 0.16 mm. I: Inferior; IL: Inferior left; IR: Inferior right; L: Left; R: Right; S: Superior; SL: Superior left; SR: Superior right.
Statistical Analysis
All data were analyzed by statistical software packages (StatSoft v7.1; Tulsa, OK, USA), and MedCalc software (v10; MedCalc Software, Mariakerke, Belgium). Continuous variables were calculated as the mean±standard deviation. Univariate analysis of variance (ANOVA) was used to analyze the microvessel density in each area of each group. Minimum significant differences were used to assess the difference between the two using a specific test. P values <0.05 were considered to indicate statistically significant differences. The correlation between macular vascular density and the disease course and size of pterygium area was analyzed using Graphpad prism 7.0, and then SPSS 23.0 (IBM Corp, Armonk, NY, USA) was used to plot the receiver operating characteristic curves (ROC) of microvessel density in the retinal epithelium to differentiate between healthy and diseased subjects.
RESULTS
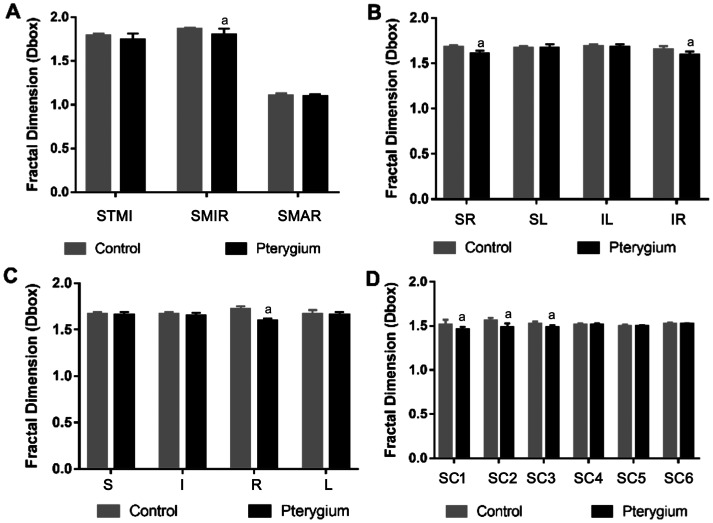
By analysis of the microvessel, macrovascular (MAR) ring, and microvessel density in superficial layer (Figure 4) and deep layer (Figure 5) between the two groups, we found that the vascular density of superficial microvessel (SMIR) in the pterygium group was decreased significantly when compared to the healthy control group (P<0.05; Figure 4A). Similarly, the vascular density of deep total microvessel (DTMI) and deep microvessel (DMIR) also decreased significantly (P<0.05; Figure 5A). However, MAR density was not significantly altered in either layer. Using the hemispheric partition and Early Treatment Diabetic Retinopathy Study (ETDRS) method for comparison, we found that the vascular density of superior right (SR), inferior right (IR), and right (R) in superficial layer was decreased significantly (P<0.05; Figure 4B and 4C), and the vascular density in SR, IR, and R in the deep layer decreased significantly (P<0.05; Figure 5B and 5C) as well. Using the central annuli method for comparison, we found that the vascular density in region of superficial central SC1, SC2, and SC3 in the superficial layer was decreased significantly (P<0.05; Figure 4D). For the deep retinal layers, the vascular density in the regions of deep central (DC)2 and DC3 also decreased significantly (P<0.05; Figure 5D). No statistically significant differences were observed in other regions (P>0.05).
Figure 4. Comparisons of macula retinal vessel density (D box) between pterygium and control subjects in the superficial layer.
Compared with the control group, there were significant differences in the densities of SMIR in macular region of pterygium patients (P<0.05), but no statistically differences in densities in the STMI and SMAR region (all P>0.05). In the pterygium group, the microvessel density in SR, IR, R, SCl, SC2, and SC3 region was significantly decreased compared to the control group (P<0.05). No statistically significant differences was observed in other partitions (P>0.05). aP<0.05, pterygium vs control. SMIR: Superficial microvessels; SMAR: Superficial macrovascula; STMI: Total superficial microvessels; DMAR: Deep macrovascula; IR: Inferior right; R: Right; SR: Superior right; SC: Superficial central annuli.
Figure 5. Comparisons of macula retinal vessel density (D box) between pterygium and control subjects in the deep layer.
Compared with the control group, there were significant differences in the densities of DTMI and DMIR in macular region of pterygium patients (P<0.05), but no statistically differences in densities in DMAR region (all P>0.05). Meanwhile, the microvessel density of the SR, IR, R, DC2, and DC3 region in the pterygium group was significantly altered (P<0.05). No statistically significant differences were observed in other regions. aP<0.05, pterygium vs control. DMIR: Deep microvessel; DTMI: Deep total microvessel; DMAR: Deep macrovascula; I: Inferior; IL: Inferior left; IR: Inferior right; L: Left; R: Right; S: Superior; SL: Superior left; SR: Superior right; DC: Deep central annuli.
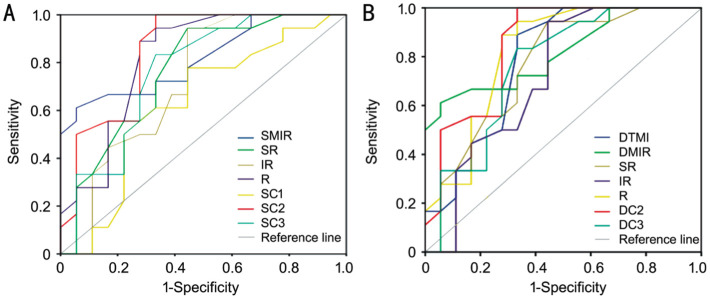
The retinal vessel density measured by OCTA showed the best sensitivity and specificity to differentiate pterygium from healthy control. In the superficial layer, the SC2 density had the highest positive likelihood ratios in the pterygium group, while SC1 showed the lowest negative likelihood ratio (Figure 6). ROC analysis revealed that SC2 had the highest sensitivity and specificity with the area under curve (AUC) 0.85 (95%CI: 0.72-0.98), and SC1 had the lowest sensitivity and specificity with the AUC 0.63 (95%CI: 0.44-0.82; Figure 6). Similarly, in the deep retinal layer, the DC2 density had the highest positive likelihood ratios in the pterygium group, while the IR showed the lowest negative likelihood ratio (Figure 6). ROC analysis revealed that DC2 had the highest sensitivity and specificity with the AUC 0.85 (95%CI: 0.72-0.98), and IR had the lowest sensitivity and specificity with the AUC 0.72 (95%CI: 0.54-0.89; Figure 6).
Figure 6. ROC analysis of microvessel densities in the superficial and deep layers.
A: Representative of ROC obtained with the densities of the superficial layer in pterygium group. The density for largest areas under the AUC of the SC2 was 0.85 (95%CI: 0.72-0.98), and the density for lowest areas under the AUC of SC1 was 0.63 (95%CI: 0.44-0.82). B: Representative of ROC obtained with the density of the deep vessel in the pterygium group. The density for largest areas under the AUC of the DC2 was 0.85 (95%CI: 0.72-0.98), and the density for lowest areas under the AUC of IR was 0.72 (95%CI: 0.54-0.89). ROC: Receiver operating characteristic curve; DMIR: Deep microvessel; SMIR: Superficial microvessels; DTMI: Deep total microvessel; AUC: Area under curve; IR: Inferior right; R: Right; SR: Superior right; SC1, 2: Superficial central annuli 1, 2; DC1, 2: Deep central annuli 1, 2.
We next investigated the correlation among MIR, SR, IR, SC1, SC2, and SC3 in the superficial retinal layer with disease course in pterygium group. In the pterygium group, the correlation coefficient of SMIR density and disease course was -0.7662, and the correlation coefficient of the SR density with disease course was -0.6038. And the correlation coefficient of IR density with disease course was -0.6234, and the correlation coefficient of the SC1 density and disease course was -0.7762. Besides, the correlation coefficient of SC2 density with disease course was -0.7123, and the correlation coefficient of the SC3 density with disease course was -0.6615. No correlation in other region density with disease course was found (|r|<0.47, P>0.05). These results indicated that decreased macular density in superficial layer of MIR, SR, IR, SCI, SC2 and SC3 might be negatively correlated with the disease course, suggesting that the longer the disease course, the lower vascular density in those regions.
Besides, we also analyzed the correlation between the vascular density of superficial retinal and the area size of pterygium. Our analyzed data showed that in the pterygium group, the correlation coefficient of SMIR density with the area size of pterygium was -0.9508, and the correlation coefficient of the SR density with the area size of pterygium was -0.8935. The correlation coefficient of IR density with pterygium area size was -0.9359, and the correlation coefficient of the R density with the area size of pterygium was -0.6043. Besides, the correlation coefficient of SC1, SC2 and SC3 densities with the area size of pterygium was -0.8217, -0.8976, and -0.7757, respectively (Table 2). No correlation in other region density with area size of pterygium was found (|r|<0.47, P>0.05). These results indicated that decreased macular density in superficial layer of MIR, SR, IR, R, SCI, SC2 and SC3 might be negatively correlated with the area size of pterygium, suggesting that the bigger the area size of pterygium, the lower vascular density in those regions.
Table 2. Correlation analysis of density in superficial retinal layer and deep retinal layer with the disease course and the area size of pterygium.
| Parameters | Fractal dimension (Dbox) |
|||||
| Superficial microvessels |
Deep microvessels |
|||||
| r | P | r | P | |||
| Course of disease (y) | SMIR | -0.7662 | 0.0002 | DTMI | -0.7003 | 0.0012 |
| SR | -0.6038 | 0.0080 | DMIR | -0.6901 | 0.0015 | |
| IR | -0.6234 | 0.0057 | SR | -0.7795 | 0.0001 | |
| SC1 | 0.7762 | 0.0002 | IR | 0.7198 | 0.0008 | |
| SC2 | -0.7123 | 0.0009 | R | -0.7608 | 0.0002 | |
| SC3 | -0.6615 | 0.0028 | DC2 | -0.7504 | 0.0003 | |
| DC3 | -0.7195 | 0.0008 | ||||
| Area of pterygium | SMIR | -0.9508 | <0.0001 | DTMI | -0.9563 | <0.0001 |
| SR | -0.8935 | <0.0001 | DMIR | -0.9508 | <0.0001 | |
| IR | -0.9359 | <0.0001 | SR | -0.8935 | <0.0001 | |
| R | -0.6043 | 0.0079 | IR | -0.9359 | <0.0001 | |
| SC1 | -0.8217 | <0.0001 | R | -0.6043 | 0.0079 | |
| SC2 | -0.8976 | <0.0001 | DC2 | -0.8976 | <0.0001 | |
| SC3 | -0.7757 | 0.0002 | DC3 | -0.7757 | <0.0002 | |
DMIR: Deep microvessel; SMIR: Superficial microvessels; DTMI: Deep total microvessel; IR: Inferior right; R: Right; SR: Superior right; SC: Superficial central annuli; DC: Deep central annuli.
Furthermore, in deep retinal layer, we also analyzed the vascular densities of deep retinal (DMIR), and showed that in the pterygium group, the correlation coefficient of DTMI density with disease course was -0.7003, and the correlation coefficient of the DMIR density with disease course was -0.6901. And the correlation coefficient of SR density with disease course was -0.7795, and the correlation coefficient of the IR density with disease course was -0.7198. Besides, the correlation coefficient of R, DC2, and DC3 density with disease course was -0.7608, -7504 and -0.7195, respectively (Table 2). No correlation in other region density with disease course was found (|r|<0.47, P>0.05). These results indicated that decreased macular density in deep layer of microvascular (TMI), MIR, SR, IR, R, C2, and C3 might be negatively correlated to the disease course, suggesting that the longer the disease course, the lower vascular density in those regions.
Besides, we also analyzed the correlation between the vascular density of deep retinal and the area size of pterygium, and showed that in the pterygium group, the correlation coefficient of DTMI density with the area size of pterygium was -0.9563, and the correlation coefficient of the DMIR density with the area size of pterygium was -0.9508. And the correlation coefficient of SR density with pterygium area size was -0.8935, and the correlation coefficient of the IR density with the area size of pterygium was -0.9359. Besides, the correlation coefficient of R, DC2 and DC3 densities with the area size of pterygium was -0.6043, -0.8976, and -0.7757, respectively (Table 2). No correlation in other region density with area size of pterygium was found (|r|<0.47, P>0.05).These results indicated that decreased macular density in deep layer of TMI, MIR, SR, IR, R, C2, and C3 might be negatively correlated to the area size of pterygium, suggesting that the bigger the area size of pterygium, the lower vascular density in those regions.
DISCUSSION
In this study, we used OCTA to investigate the retinal microvasculature alteration in pterygium. To our knowledge, this was the first study to discover and report that the vascular density decreased in the macular area of patients with pterygium, and the major vascular alterations occurred mainly on the bitamporal side. We further found that the decreased vascular density of the macula was negatively correlated to the disease course and to the area size of pterygium. There is quite few technique used in the study of retinal, choroid, and retrobulbar blood circulation[19]–[20]. OCTA has revealed many details of the superficial and deep retinal layers[21]. Many studies have shown that OCTA can be used to diagnose choroidal neovascularization (CNV), age-related macular degeneration (AMD)[22], retinal vein occlusion (RVO)[21], abnormal retinal vessels[23], and even non-permeable AMD[24] and melanocyte tumors. Thus OCTA could be an important technique for studying the progression, retinal pathology, and complications of pterygium by providing scientific evidences on vascular density alteration.
By using the OCTA technique in pterygium, our results showed that vascular densities decreased in the retinal superficial MIR, SR, IR, R, and retinal deep TMI, MIR, SR, IR, R in pterygium patients. The major decreased region was on the bitamporal side of macula. The annuli partition method showed that vascular density of the superficial annuli (SC)1, SC2, SC3 in the foveal and deep layer of C2, C2 regions, significantly decreased. Zhao et al[25] found there was a significant increase in retinal vessel density, a decrease in the foveal avascular zone at the macular area after the cataract surgery. They also found that the retinal vessel density of the parafoveal and perifoveal regions increased significantly at 1wk, 1, and 3mo after the cataract surgery. At 3mo after surgery, there was a mean 6%±11% and 3%±10% increase in vessel density at the parafoveal and perifoveal regions, respectively compared with the baseline, which seems consistent with our data. It has been estimated that a cataract might block 18% to 40% of light at different wave-lengths. Zhao et al[25] thought that the increase density in macular vasculature in cataract patients resulted from the increase of light exposure. Zhou et al[8] found macular vessel density to be significantly increased after cataract surgery. Postoperative inflammatory reactions have been considered as potential pathogenic factors for postoperative fundus change[26]–[27]. But inflammation by itself cannot explain why the increase persists for so long, they thought higher exposure lever of light may lead to angiogenesis by inducing retinal metabolic activation, which could be regarded as postoperative light toxicity, this might also explain the relatively high incidence of AMD found in those IOL eyes[28]. Our data also supported the hypothesis. Pterygium blocked the light exposure from cornea, which may lead to a reduction in retinal activity and metabolic demands[29], leading to a decreased vascular density. Hardarson et al[30] tried to address this question. However, their results were shown uncertain. Pterygium occurs on the nasal side, which blocks refraction and scattering of light from the refractive stroma in this region, therefore mainly affecting the retina on bitamporal side. On the other hand, the decreased light exposure may be associated with the metabolic changes and the unique vascular pattern of the central fovea. Light vision is accomplished by cone cells, which are also in the highest density in the central fovea. The decreased light exposure leads to a decreased activity of cone cells. Furthermore, the consistency of this location also supports the hypothesis that the decreased light exposure may lead to the decreased density in macular blood vessels.
Our data also showed that in the pterygium group, SC2 and DC2 had the highest positive likelihood ratio, and SC1 and IR had the lowest negative likelihood ratio. And we found that the decreased vascular density was negatively correlated with the disease course, and the size of pterygium area. Pterygium is caused by dysfunction of limbal stem cells and a decrease in the number of stem cells, resulting in an active proliferation and remodeling of conjunctival fibroblasts and vascularization of connective tissue. The most significantly pathological alterations of pterygium is the proliferation and degeneration of elastic fibers and collagen fibers. But it is not a simple process of proliferation, but an ever-changing process[31]. This obviously pathological alterations indicated that the longer the disease course of the pterygium, the larger size of the pterygium area would develop, therefore blocking more external sunlight into the bitamporal retina. Aspiotis et al[5] reported that pterygium tissues presented with statistically significant higher density of average count of microvessel, when compared to normal conjunctivae, and the angiogenesis-related factors were highly expressed in pterygium tissue.
Decreased macular vascular density may also affect visual acuity. Hitton et al[32] reported that after cataract surgery, the increased vascular density in macula was beneficial to the eyes. Our results showed that the visual acuity decreased significantly in pterygium when compared to healthy control (Table 1). But pterygium can also cause corneal alteration[33], leading to a decreased visual acuity, although the posterior corneal surface has been shown to compensate for anterior corneal astigmatism[34]. Corneal astigmatism and ocular wavefront aberrations are also found to be related to the area size of the pterygium[35]. Large pterygium can invade the pupillary area and lead to a decrease in visual acuity. Since it is difficult to control these factors, it is not clear whether it is beneficial to the eyes. Surgical removal is still the main therapy for pterygium[36]. After pterygium excision, astigmatism and the wavefront aberration caused by the pterygium on the cornea will be greatly reduced[35]. Visual acuity will also be significantly improved. The decreased vascular density in macula will help to understand the pathophysiological mechanisms involved in the pterygium. This study has some limitations. We did not measure the vascular endothelial growth factor (VEGF). A significant increase of VEGF was reported to occur in pterygium patients[37]. VEGF is mainly produced by fibroblasts. A variety of factors including inflammation, hypoxia, toxic substances, and ultraviolet damage, can affect its expression[38]. The formation of pterygium is accompanied by the growth of activated fibroblasts, the excessive proliferation of extracellular matrix, and inflammation. Whether the production of VEGF will alter the vascular density, we have no answer since no VEGF was tested in the study due to the difficulty in biopsy availabilities. In addition, we also consider that pterygium may lead to low measurements of macular vascular density in healthy eyes, as this can lead to artifact generation, which needs further study.
In summary, this study, by using the OCTA, a convenient and rapid technique to detect the microvessel alteration in the retina macular area in pterygium patients, we first discovered and reported a decreased vascular density in fundus of the pterygium patients. and we further found that the vascular density was negatively correlated to the disease course and the size of the pterygium area. Weather such changes might affect the fundus in long still needs to be verified, more studies are still needed to investigate whether the vascular density of the macula would recover after pterygium is removed by surgery. Pterygium blocked the light exposure from cornea, which may lead to a reduction in retinal activity and metabolic demands leading to a decreased vascular density. Detailed analysis according to non-invasive OCTA techniques could be beneficial towards better to characterize the underlying pathophysiological mechanisms involved in pterygium.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.82160195); Central Government Guides Local Science and Technology Development Foundation (No.20211ZDG02003); Key Research Foundation of Jiangxi Province (No.20181BBG70004; No.20203BBG73059); Excellent Talents Development Project of Jiangxi Province (No.20192BCBL23020).
Conflicts of Interest: Wang F, None; Ge QM, None; Shu HY, None; Liao XL, None; Liang RB, None; Li QY, None; Zhang LJ, None; Gao GP, None; Shao Y, None.
REFERENCES
- 1.Sun NY, Zhang H. Pyroptosis in pterygium pathogenesis. Biosci Rep. 2018;38(3):BSR20180282. doi: 10.1042/BSR20180282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qadi R, AlAmri A, Elnashar M, Sarriyah JF, Alghamdi AH, Fahad Alsolami K, Almalki AM, Alotaibi F. Prevalence of pterygium and associated risk factors in the high-altitude area of Ta'if city, Saudi Arabia. Cureus. 2021;13(1):e12638. doi: 10.7759/cureus.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong H, Cha XP, Wei T, Lin XC, Li X, Li J, Cai N, Li JJ, Su XD, Yang YM, Yu MB, Yuan YS. Prevalence of and risk factors for pterygium in rural adult Chinese populations of the Bai nationality in Dali: the Yunnan Minority Eye Study. Invest Ophthalmol Vis Sci. 2012;53(10):6617–6621. doi: 10.1167/iovs.11-8947. [DOI] [PubMed] [Google Scholar]
- 4.Hill JC, Maske R. Pathogenesis of pterygium. Eye (Lond) 1989;3(Pt 2):218–226. doi: 10.1038/eye.1989.31. [DOI] [PubMed] [Google Scholar]
- 5.Aspiotis M, Tsanou E, Gorezis S, Ioachim E, Skyrlas A, Stefaniotou M, Malamou-Mitsi V. Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye (Lond) 2007;21(8):1095–1101. doi: 10.1038/sj.eye.6702495. [DOI] [PubMed] [Google Scholar]
- 6.Jabbarpoor Bonyadi MH. Bilateral photic maculopathy following pterygium excision: spectral domain optical coherence tomography findings. J Ophthalmic Vis Res. 2016;11(4):436–438. doi: 10.4103/2008-322X.194143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hossein M, Bonyadi J, Soheilian R, Soheilian M, Peyman GA. Spectral-domain optical coherence tomography features of mild and severe acute solar retinopathy. Ophthalmic Surg Lasers Imaging. 2001;42 Online:e84–86. doi: 10.3928/15428877-20110901-05. [DOI] [PubMed] [Google Scholar]
- 8.Zhou YF, Zhou MW, Wang YL, Ben SY, Gao M, Zhang SQ, Liu HY, Sun XD. Short-term changes in retinal vasculature and layer thickness after phacoemulsification surgery. Curr Eye Res. 2020;45(1):31–37. doi: 10.1080/02713683.2019.1649703. [DOI] [PubMed] [Google Scholar]
- 9.Misra S, Craig JP, McGhee CN, Patel DV. A prospective study of pterygium excision and conjunctival autograft with human fibrin tissue adhesive: effects on vision, refraction, and corneal topography. Asia Pac J Ophthalmol (Phila) 2014;3(4):202–206. doi: 10.1097/APO.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 10.Provis JM, Penfold PL, Cornish EE, Sandercoe TM, Madigan MC. Anatomy and development of the macula: specialisation and the vulnerability to macular degeneration. Clin Exp Optom. 2005;88(5):269–281. doi: 10.1111/j.1444-0938.2005.tb06711.x. [DOI] [PubMed] [Google Scholar]
- 11.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292(4):497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 12.Azizi B, Wong T, Wan J, Singer S, Hudson C. The impact of cataract on the quantitative, non-invasive assessment of retinal blood Flow. Acta Ophthalmol. 2012;90(1):e9–e12. doi: 10.1111/j.1755-3768.2011.02223.x. [DOI] [PubMed] [Google Scholar]
- 13.Jia YL, Tan O, Tokayer J, Potsaid B, Wang YM, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanathi M, Goel S, Ganger A, Agarwal T, Dada T, Khokhar S. Corneal tomography and biomechanics in primary pterygium. Int Ophthalmol. 2018;38(2):663–671. doi: 10.1007/s10792-017-0514-6. [DOI] [PubMed] [Google Scholar]
- 15.Durbin MK, An L, Shemonski ND, Soares M, Santos T, Lopes M, Neves C, Cunha-Vaz J. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017;135(4):370–376. doi: 10.1001/jamaophthalmol.2017.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salz DA, de Carlo TE, Adhi M, Moult E, Choi W, Baumal CR, Witkin AJ, Duker JS, Fujimoto JG, Waheed NK. Select features of diabetic retinopathy on swept-source optical coherence tomographic angiography compared with fluorescein angiography and normal eyes. JAMA Ophthalmol. 2016;134(6):644–650. doi: 10.1001/jamaophthalmol.2016.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Wang JH, Jiang H, Yang XL, Feng LM, Hu L, Wang L, Lu F, Shen MX. Retinal microvasculature alteration in high myopia. Invest Ophthalmol Vis Sci. 2016;57(14):6020–6030. doi: 10.1167/iovs.16-19542. [DOI] [PubMed] [Google Scholar]
- 18.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31(5):377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-López M, Sales-Sanz M, Rebolleda G, Casas-Llera P, González-Gordaliza C, Jarrín E, Muñoz-Negrete FJ. Retrobulbar ocular blood flow changes after orbital decompression in Graves' ophthalmopathy measured by color Doppler imaging. Invest Ophthalmol Vis Sci. 2011;52(8):5612–5617. doi: 10.1167/iovs.10-6907. [DOI] [PubMed] [Google Scholar]
- 20.Wu YJ, Wei X, Xiao MY, Xiong W. Orbital decompression surgery and horse chestnut seed extract improved superior orbital vein blood flow in patients with thyroid-associated ophthalmopathy. Int J Ophthalmol. 2016;9(6):869–875. doi: 10.18240/ijo.2016.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mastropasqua R, di Antonio L, Di Staso S, Agnifili L, di Gregorio A, Ciancaglini M, Mastropasqua L. Optical coherence tomography angiography in retinal vascular diseases and choroidal neovascularization. J Ophthalmol. 2015;2015:343515. doi: 10.1155/2015/343515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marduel R. Angio OCT, dye less angiography, a new approach of age related macular degeneration (ARMD) Adv Ophthalmol Vis Syst. 2015;2(2):00034. [Google Scholar]
- 23.Sharma P, Sridhar J, Rayess N, Maguire JI. Optical coherence tomography angiography (OCT-A) of type 2 retinal arteriovenous malformation. Can J Ophthalmol. 2015;50(5):e93–e96. doi: 10.1016/j.jcjo.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz DM, Fingler J, Kim DY, Zawadzki RJ, Morse LS, Park SS, Fraser SE, Werner JS. Phase-variance optical coherence tomography: a technique for noninvasive angiography. Ophthalmology. 2014;121(1):180–187. doi: 10.1016/j.ophtha.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Z, Wen W, Jiang C, Lu Y. Changes in macular vasculature after uncomplicated phacoemulsification surgery: optical coherence tomography angiography study. J Cataract Refract Surg. 2018;44(4):453–458. doi: 10.1016/j.jcrs.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Kurt A, Kılıç R. The effects of uncomplicated cataract surgery on retinal layer thickness. J Ophthalmol. 2018;2018:7218639. doi: 10.1155/2018/7218639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pilotto E, Leonardi F, Stefanon G, Longhin E, Torresin T, Deganello D, Cavarzeran F, Miglionico G, Parrozzani R, Midena E. Early retinal and choroidal OCT and OCT angiography signs of inflammation after uncomplicated cataract surgery. Br J Ophthalmol. 2019;103(7):1001–1007. doi: 10.1136/bjophthalmol-2018-312461. [DOI] [PubMed] [Google Scholar]
- 28.Yılmaz T, Karci AA, Yilmaz İ, Yılmaz A, Yıldırım Y, Sakalar YB. Long-term changes in subfoveal choroidal thickness after cataract surgery. Med Sci Monit. 2016;22:1566–1570. doi: 10.12659/MSM.898714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao FP, Cai SJ, Huang Z, Ding PS, Du CX. Optical coherence tomography angiography in pinguecula and pterygium. Cornea. 2020;39(1):99–103. doi: 10.1097/ICO.0000000000002114. [DOI] [PubMed] [Google Scholar]
- 30.Hardarson SH, Basit S, Jonsdottir TE, Eysteinsson T, Halldorsson GH, Karlsson RA, Beach JM, Benediktsson JA, Stefansson E. Oxygen saturation in human retinal vessels is higher in dark than in light. Invest Ophthalmol Vis Sci. 2009;50(5):2308–2311. doi: 10.1167/iovs.08-2576. [DOI] [PubMed] [Google Scholar]
- 31.Zhao DD, Zhao HX, He Y, Yang Y, Du Y, Zhang MX. The inhibitive effects of proteasome inhibitor MG-132 on pterygium fibroblasts in vitro and the potential key regulators involved. Life Sci. 2021;270:119088. doi: 10.1016/j.lfs.2021.119088. [DOI] [PubMed] [Google Scholar]
- 32.Hilton EJ, Hosking SL, Gherghel D, Embleton S, Cunliffe IA. Beneficial effects of small-incision cataract surgery in patients demonstrating reduced ocular blood flow characteristics. Eye (Lond) 2005;19(6):670–675. doi: 10.1038/sj.eye.6701620. [DOI] [PubMed] [Google Scholar]
- 33.Zhang LM, Lu Y, Gong L. Pterygium is related to short axial length. Cornea. 2020;39(2):140–145. doi: 10.1097/ICO.0000000000002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levinger E, Sorkin N, Sella S, Trivizki O, Lapira M, Keren S. Posterior corneal surface changes after pterygium excision surgery. Cornea. 2020;39(7):823–826. doi: 10.1097/ICO.0000000000002325. [DOI] [PubMed] [Google Scholar]
- 35.Tang Y, Qian D, Wei L, Du Y, Qiu X, Lu Y, Zhu X. Influences of the three-dimensional parameters of pterygium on corneal astigmatism and the intraocular lens power calculation. Sci Rep. 2020;10(1):5017. doi: 10.1038/s41598-020-61959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuzzi R, Tridico F. How to minimize pterygium recurrence rates: clinical perspectives. Clin Ophthalmol. 2018;12:2347–2362. doi: 10.2147/OPTH.S186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu C, Song YY, Wang XR, Lai ZG, Li CY, Wan PX, Xu N, Huang DP, Liu YZ, Wang ZC. The key role of VEGF in the cross talk between pterygium and dry eye and its clinical significance. Ophthalmic Res. 2020;63(3):320–331. doi: 10.1159/000503636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mudaliar S, Hupfeld C, Chao DL. SGLT2 inhibitor-induced low-grade ketonemia ameliorates retinal hypoxia in diabetic retinopathy-A novel hypothesis. J Clin Endocrinol Metab. 2021;106(5):1235–1244. doi: 10.1210/clinem/dgab050. [DOI] [PubMed] [Google Scholar]