Significance
We have identified the presence of cancer cells harboring immune cell–specific surface marker proteins such as CD4 in the tumor microenvironment. Cancer cells acquired not only the T cell marker protein CD4 but also immune regulatory molecules such as CTLA4 by trogocytosis. Unlike other endocytic mechanisms, trogocytosis maintains the cellular localization and functions of the transferred membrane proteins. Therefore, trogocytic transfer of immune regulatory molecules enhances the immunosuppressive functions of cancer cells. This study provides insight into the interactions between cancer cells and tumor-infiltrating immune cells and how they contribute to the development of the immunosuppressive tumor microenvironment.
Keywords: cancer, trogocytosis, tumor-infiltrating lymphocytes, immune regulatory molecules
Abstract
Cancer cells can develop an immunosuppressive tumor microenvironment to control tumor-infiltrating lymphocytes. The underlying mechanisms still remain unclear. Here, we report that mouse and human colon cancer cells acquire lymphocyte membrane proteins including cellular markers such as CD4 and CD45. We observed cell populations harboring both a tumor-specific marker and CD4 in the tumor microenvironment. Sorted cells from these populations were capable of forming organoids, identifying them as cancer cells. Live imaging analysis revealed that lymphocyte membrane proteins were transferred to cancer cells via trogocytosis. As a result of the transfer in vivo, cancer cells also acquired immune regulatory surface proteins such as CTLA4 and Tim3, which suppress activation of immune cells [T. L. Walunas et al., Immunity 1, 405–413 (1994) and L. Monney et al., Nature 415, 536–541 (2002)]. RNA sequencing analysis of ex vivo–cocultured splenocytes with trogocytic cancer cells showed reductions in Th1 activation and natural killer cell signaling pathways compared with the nontrogocytic control. Cancer cell trogocytosis was confirmed in the patient-derived xenograft models of colorectal cancer and head and neck cancer. These findings suggest that cancer cells utilize membrane proteins expressed in lymphocytes, which in turn contribute to the development of the immunosuppressive tumor microenvironment.
Trogocytosis is a process of membrane fragment transfer between two cells in contact. This term trogo is derived from ancient Greek meaning “gnaw.” Previous studies have identified Entamoeba histolytica, Francisella tularensis, microglia, and immune cells as trogocytic (3–7). Indeed, T cells uptake major histocompatibility complex (MHC) class II molecules at the immunological synapse from the encountered, antigen-presenting cells by trogocytosis (3). Consequences of trogocytosis by immune cells in the tumor microenvironment are context dependent. A recent study showed that the efficacy of chimeric antigen receptor T cell therapy was diminished by trogocytosis, since the tumor antigen–MHC class I complex was engulfed by T cells followed by decreased tumor antigen presentation (8). On the other hand, trogocytic neutrophils or macrophages can lead to the death of target tumor cells (7, 9). However, trogocytosis by tumor cells has not yet been investigated.
In this study, we detected cancer cells harboring T cell marker proteins CD4 and CD45 on their surface. The phagocytic potential by cancer cells has been reported, but phagocytosis results in endosomal localization of engulfed targets (10). To maintain the membrane localization of CD4 and CD45, another transfer mechanism is required. The intercellular transfer of P-glycoprotein (P-gp) from P-gp–positive cells to P-gp–negative cells occurred in human and mouse tumor cell lines, implying the possibility that cancer cells transfer membrane proteins from neighboring cells (11). Live imaging analysis of the in vitro–cocultured colon cancer cells and T cells showed that cancer cells transfer those membrane proteins via trogocytosis. It has been confirmed by using the inhibitors for actin polymerization and PI-3Kinase, which are known to be involved in trogocytosis. Trogocytic cancer cells in the tumor microenvironment showed elevation of immune checkpoint inhibitors compared to nontrogocytic cancer cells. Like CD4 and CD45, the T cell–specific negative regulators, such as CTLA4 and Tim3, were detected in trogocytic cells (1, 2). As a result, trogocytic cancer cells acquired immunosuppressive functions. Our findings suggest the underlying mechanisms in how cancer cells develop an immunosuppressive tumor microenvironment in order to escape immune surveillance.
Results
Identification of Cells Harboring Both a Tumor Marker and Lymphocyte Markers in the Tumor Microenvironment.
Recent studies have reported the exchange of cellular materials between cancer cells and immune cells in contact, and it can lead to cell fusion (12). Cancer cells obtain metastatic properties such as epithelial–mesenchymal transition, enhanced cancer stemness, and drug resistancy as a result of cell fusion (13). Since cell fusion or cellular material exchanges between cancer cells and immune cells are more frequent in metastatic cancers, we used metastatic colon cancer cells carrying mutations in Apc, Kras, Tp53, and Smad4 genes with the reporter gene tdTomato (AKPS).
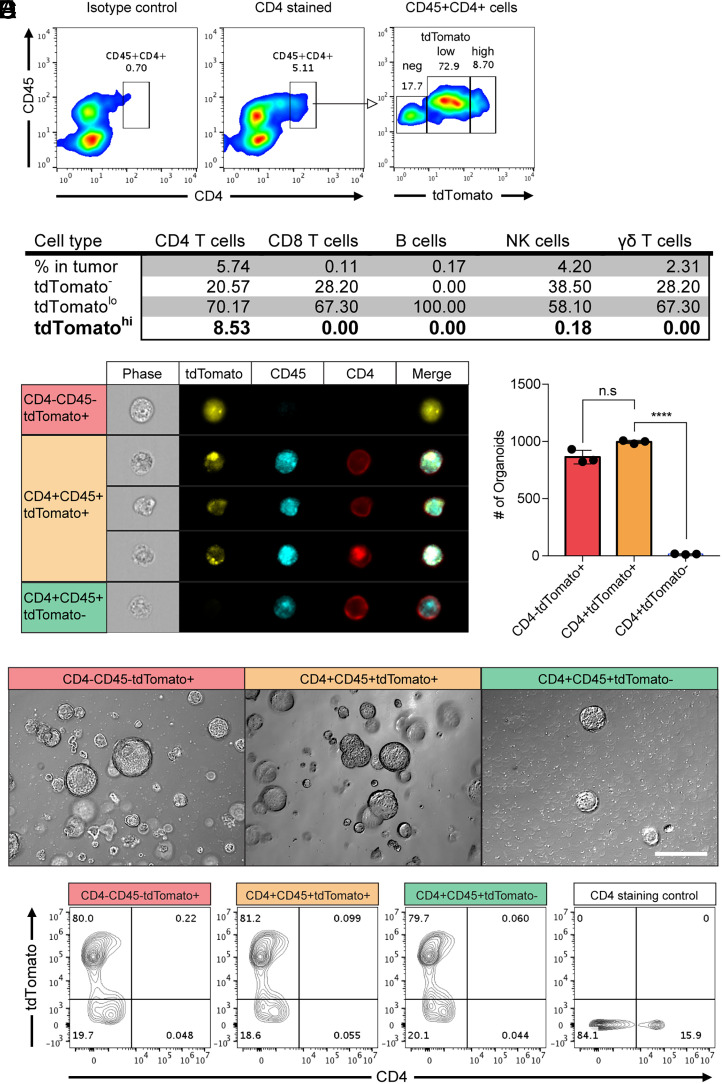
In order to identify the types of cells harboring both cancer and immune cell materials in tumor tissues, we transplanted AKPS organoids into C57BL/6 mice to develop liver metastasized colon cancer and then monitored the AKPS reporter tdTomato in tumor tissues (14). Flow cytometry analysis was performed for single cells isolated from tumor tissues after doublet discrimination by plotting the forward scatter area versus width. This analysis showed a significant amount of hematopoietic, marker-stained cells with tdTomato positivity in the tumor microenvironment (SI Appendix, Fig. S1). The majority of these cells were myeloid cells including dendritic cells, macrophage, monocytes, and myeloid-derived suppressor cells. Myeloid cells are phagocytic and known participants in fusion with cancer cells (12, 15). More than 50% of these cells were tdTomato positive, and about 10% of the cells showed high levels of tdTomato (tdTomatohi) (SI Appendix, Fig. S1A). Increased tdTomato levels were also observed in lymphoid cells, including T cells, B cells, and natural killer (NK) cells. Most of the lymphoid cells showed low levels of tdTomato (tdTomatolo) (SI Appendix, Fig. S1B). Notably, about 10% of CD4 T cells were tdTomatohi as intense as cancer cells, whereas it is not present in other lymphocytes (Fig. 1 A and B). Imaging flow cytometry (amnis) analysis confirmed the presence of these cells as a single cell with cytosolic tdTomato and membranes CD4 and CD45 (Fig. 1C).
Fig. 1.
Colon cancer cells acquired membrane CD4 from CD4-positive T cells in vivo. Colon cancer organoids (AKPS) carrying mutations in Apc, Kras, Tp53, and Smad4 genes with a tdTomato reporter gene were injected into C57BL/6 mice via splenic vein to develop liver metastases. (A) Surface staining of CD4 and CD45 identified the double-positive cell population. Fluorescence intensity of tdTomato in this population is shown. Isotype control: isotype control of anti-mouse CD4 antibody. The numbers indicate the percentile of each population. (B) A summary of the average percentiles of tdTomato-positive, tumor-infiltrated lymphocytes analyzed using flow cytometry. CD45, CD4, CD8, CD19, NK1.1, and γδ-T cell receptor were stained to identify each cell type. tdTomatolo and tdTomatohigh populations are gated as shown in A. (C) Amnis imaging flow cytometry analysis of the expression and staining patterns of tdTomato, CD4, and CD45 in cells from tumor tissues. (D and E) Generation of colon cancer organoids from the sorted cells based on the expression of tdTomato, CD4, and CD45 (CD4+tdTomato+ cells from the CD45+CD4+tdTomatohigh gating) as described in SI Appendix, Fig. S2. The 2,000 sorted cells were plated in 50 μL Matrigel. The numbers of organoid formation on day 7 after plating the sorted cells (D) and representative pictures (E) are shown. n.s, not significant, ****P < 0.0001; two-tailed t test. Error bars indicate mean ± SD.Scale bar: 50 μm. (F) Expanded organoid cells on day 7 in D and E were harvested and stained with anti-mouse CD4 monoclonal antibody. The intensity of tdTomato and CD4 was analyzed. CD4-positive control cells are spleen cells from the C57BL/6 actin-GFP reporter mice. Results are representative of two to five independent experiments.
To identify these cells, we sorted the transplanted cancer cells, tumor-infiltrating CD4 T cells, and those cells having both CD4 and tdTomato based on CD4, CD45, and tdTomato by flow cytometry (SI Appendix, Fig. S2). Those CD4+CD45+tdTomato+ cells were able to form colon cancer organoids like the sorted CD4−CD45−tdTomato+ control cancer cells (Fig. 1 D and E). This identified those CD4+CD45+tdTomato+ cells as colon cancer cells derived from the transplanted organoids. After 7 d of culture, those organoids were no longer CD4 and CD45 positive, whereas tdTomato expression is maintained (Fig. 1F). The presence of CD4 and CD45 on the surface of cancer cells is transient. Thus, cancer cells transiently acquired membrane proteins from tumor-infiltrated CD4 T cells.
Generation of Cancer Cells Harboring Both Tumor and T Cell Markers, tdTomato, and CD4 by In Vitro Organoid Coculture with CD4 T Cells.
Next, we utilized an in vitro organoid coculture system to confirm the transfer of the T cell marker protein CD4 to colon cancer cells. The three-dimensional organoid culture system has limitations in cellular movement of activated CD4 T cells within Matrigel. To increase cellular movement and cell-to-cell (cell–cell) interactions, tdTomato-positive AKPS colon cancer organoids were plated on top of the solidified Matrigel. Then, purified and activated CD4 T cells carrying the green fluorescent protein (GFP) reporter gene were added into the culture (Fig. 2A). Flow cytometry analysis confirmed the presence of CD4 and tdTomato double–positive cells in the coculture system (Fig. 2B). It showed that about 0.5% of cocultured cells were CD4 and tdTomato double positive and one-third of these cells are tdTomato high as control cancer cells. Most of these cells contain the cytosolic T cell reporter GFP as well. This phenomenon was also observed in early-stage colon tumor organoids with a homozygous Apc gene knockout only (A organoids; Fig. 2C) but not in the normal colonic organoids (Fig. 2D). Our data suggest that tumor cells are able to acquire proteins from the contacted CD4 T cells, whereas normal colonic epithelial cells are not.
Fig. 2.
CD4+ colon cancer cells were generated by in vitro coculture of cancer organoids with CD4 T cells. (A) A schematic diagram of the two-dimensional in vitro coculture system. Colon cancer organoids and anti-CD3ε/28–activated mouse primary CD4-positive T cells expressing GFP were cocultured for 3 d in vitro at a 1:10 (organoid:T cell) ratio. (B–D) Flow cytometry analysis of the cocultured AKPS (late phase colon cancer), Apc only mutant organoids in A, early-phase colon cancer, and normal colonic organoids with sorted CD4 T cells from actin-GFP reporter mice. CD4+tdTomato+ cells are further gated as CD4+tdTomatohigh (red) and CD4+tdTomatolow (blue), and their GFP intensity is displayed in the right panel entitled as CD4+tdTomato+ in B and C. Normal organoids were generated from the actin-GFP reporter mice to identify green epithelial cells after coculture with CD4 T cells in D. (E) Percentiles of CD4+tdTomatohigh (red in B and C) cells in AKPS, A, and CD4+GFP+ cells in normal organoids were analyzed in the bar graph. *P < 0.05, **P < 0.01; two-tailed t test. Error bars indicate mean ± SD. (F) Confocal microscopy analysis after the coculture. Sorted and activated GFP-positive CD4 T cells were stained with anti-CD4 monoclonal antibody and then cocultured with colon cancer organoids for 3 d. Three inset images are displayed in each panel. The top view is shown on the lower left (blue line square) insets. (Inset) Top (green line square) and right (red line square) side of each panel represent z-stacks in two different orientations. tdTomato, GFP, CD4, and DAPI are displayed as red, green, cyan, and blue, respectively. White boxes indicate region of interest. #1 and #2 white boxes are enlarged in the middle and bottom panels, respectively. CD4 is shown as blue instead of cyan for better visualization in the middle and bottom panels. Results are representative of three independent experiments.
It has been reported that CD4 T cells can obtain membrane proteins from antigen-presenting cells or tumor cells in contact by trogocytosis (3, 8). To focus on cancer cells harboring CD4 and tdTomato, percentiles of CD4+tdTomatohigh cells were analyzed to exclude tdTomato obtained CD4 T cells in the CD4+tdTomatolow cells (Fig. 2 B–E). CD4+tdTomatohigh cells were identified as colon cancer cells above (Fig. 1 D–F). The presence of CD4 and tdTomato double–positive cancer cells was increased in AKPS organoids compared to A organoids, implying the enhanced membrane protein transfer in metastatic colon cancer cells (Fig. 2E).
In order to visualize CD4 and tdTomato double–positive cells in our coculture system, we performed confocal microscopy analysis. After 3 d of coculture, CD4 T cells internalized in AKPS organoids (Fig. 2F). Internalized T cell–derived CD4 and GFP proteins were colocalized with the cancer reporter tdTomato (Fig. 2F, White box #1). CD4 T cells outside of organoids display membrane CD4 and cytosolic GFP, separately (Fig. 2F, White box #2). These results indicate the exchange of membrane and cytoplasmic proteins between colon cancer cells and CD4 T cells as a result of their cell–cell interactions.
Membrane Protein Transfer from T Cells to Cancer Cells via Trogocytosis.
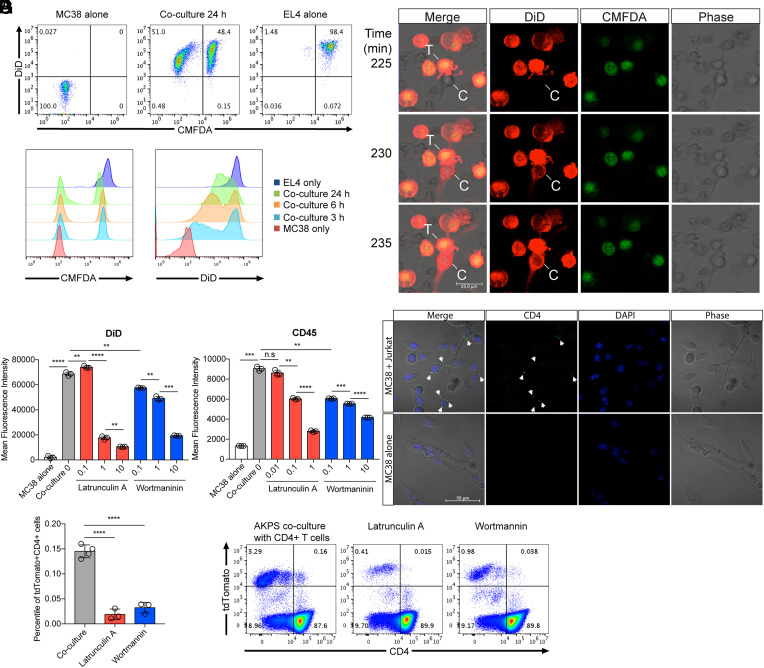
To understand the underlying mechanisms of protein exchanges between cancer cells and T cells, we performed a coculture of mouse colon cancer cells (MC38) and mouse thymocyte cells (EL4). Flow cytometry analysis showed membrane protein transfer from EL4 cells to MC38 cells in a time-dependent manner, whereas cytosolic protein transfer was marginal (Fig. 3A). Live imaging analysis showed the uptake of DiD-stained T cell membrane by colon cancer cells (Fig. 3B and Movie S1). A rapid transfer of DiD signals were detected within 10 min after cancer cells made contact with T cells. High-resolution live imaging during the coculture revealed that part of the cell membrane was transferred (SI Appendix, Fig. S3). These data imply that cancer cells uptake DiD-stained T cell membrane via trogocytosis.
Fig. 3.
Transfer of T cell membrane proteins to colon cancer cells via trogocytosis. Mouse T cell line EL4 cells were labeled with DiD and CMFDA and membrane and cytosolic cell tracker, respectively. Labeled EL4 cells were cocultured with unlabeled mouse colon cancer cell line MC38 cells. (A) Flow cytometry analysis of the transfer of DiD and CMFDA from EL4 cells to MC38 cells in a time-dependent manner. (B) Time-lapse confocal imaging analysis of membrane protein transfer. T (T cells); EL4, C (colon cancer cells); MC38, time indicates minutes after the addition of the labeled EL4 cells to unlabeled MC38 cells plated chamber wells. EL4-MC38 cell-to-cell contact in the images was detected at about 150 min after the addition of EL4 cells in culture (Movie S1). (C and D) Statistical analyses of the intensity of DiD (C) and surface-stained CD45 (D) in MC38 colon cancer cells after 24 h coculture in the presence of Latrunculin A and Wortmannin, inhibitors of actin polymerization and PI3-Kinase, respectively (unit for inhibitors: uM). (E) Confocal microscopy analysis of CD4 protein transfer from Jurkat T cells to MC38 colon cancer cells. Jurkat cells were stained with Alexa 488–conjugated anti-human CD4 monoclonal antibody and then cocultured with MC38 for 1 h. Jurkat cells were washed out after coculture, and transferred CD4 proteins were monitored. Arrows indicate transferred CD4 proteins. (F and G) Percentiles of cancer and T cell markers tdTomato and CD4 double–positive cells in the AKPS organoid coculture system in the presence of Latrunculin A (1 μM) and Wortmannin (10 μM). Coculture was performed as described in Fig. 2 after the pretreatment of organoids with inhibitors. (C, D, and F) Representative flow cytometry data are displayed in G. n.s, not significant, **P < 0.01, ***P < 0.001, ****P < 0.0001; two-tailed t test. Error bars indicate mean ± SD. Results are representative of at least two independent experiments.
This membrane transfer was abolished in the presence of the actin polymerization inhibitor latrunculin A or the PI-3Kinase inhibitor wortmannin (Fig. 3C and SI Appendix, Fig. S4A). These results were identical when MC38 cells were cocultured with CD45-stained EL4 cells (Fig. 3D and SI Appendix, Fig. S4B). It was further confirmed by MC38 cell coculture with CD4-stained or DiD-stained human Jurkat T cells (Fig. 3E and SI Appendix, Fig. S4 C and D). Lastly, treatment of latrunculin A or wortmannin to AKPS organoids in a coculture with CD4 T cells significantly reduced the presence of CD4 and tdTomato double–positive cells (Fig. 3 F and G). These data suggest that the membrane protein transfer from T cells to cancer cells is actin polymerization and PI-3Kinase signaling dependent. The inhibition of transcription or translation in MC38 and AKPS colon cancer cells during the coculture did not affect membrane protein transfer from T cells (SI Appendix, Fig. S5). This informed us that cytosolic transfer of DNA or RNA from T cells to cancer cells marginally contributed to the transferred protein levels of CD4 or CD45 in cancer cells.
Trogocytosis is defined by the following features compared to other endocytic phenomena: 1) nibbling cell compartments rather than engulfing a whole cell in a short time, 2) maintaining the cell surface localization of the transferred membrane proteins, and 3) inhibition via small molecule inhibitors of actin polymerization– and PI-3Kinase–signaling pathways (4, 8, 16). Based on these, our findings demonstrate cancer cell trogocytosis from the encountered T cells.
The Presence of Immune Regulatory Molecules on the Surface of Trogocytic Cancer Cells In Vivo.
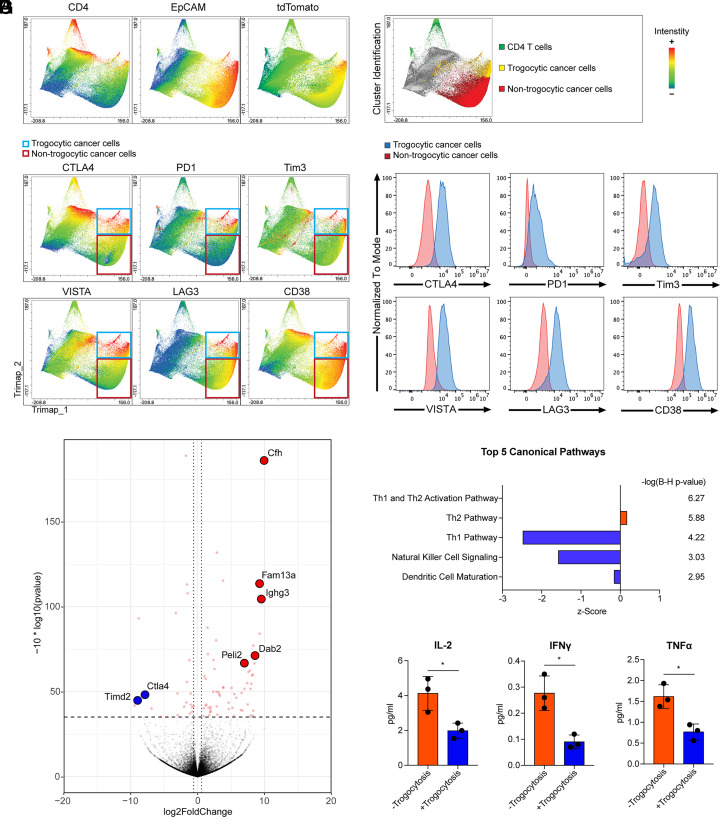
Immunosuppressive properties of the tumor microenvironment are well characterized, for example, elevation of immune checkpoint inhibitors reduce the antitumor immune response (17, 18). Based on the transfer of membrane proteins CD4 and CD45 from T cells to cancer cells, we hypothesized that cancer cells are able to acquire immune checkpoint inhibitors from tumor-infiltrating lymphocytes. To test this, we performed flow cytometry analysis of immune regulatory molecules in colon cancer tissues using the murine liver metastasis model described above in Fig. 1. CD4 T cells and trogocytic and nontrogocytic cancer cells were clustered and identified by the intensity of CD4, the cancer cell marker tdTomato, and epithelial cell marker EpCAM by Trimap analysis (Fig. 4A). Cancer cells harboring tdTomato and EpCAM with CD4 are indicated as trogocytic, and CD4-negative cells are nontrogocytic (Fig. 4B). Trimap analysis showed increased immune regulatory molecules; CTLA4, PD1, Tim3, VISTA, LAG3, and CD38 in trogocytic cancer cells compared to nontrogocytic cancer cells (Fig. 4 C and D). These molecules are mainly expressed in lymphoid and myeloid cells, and particularly, CTLA4 expression is T cell specific (1). The increase of each immune regulatory molecule was further confirmed by single-antibody staining for cancer tissue cells to avoid fluorescence overlap between multiple antibodies in flow cytometry analysis (SI Appendix, Fig. S6). The transfer of these immune regulatory molecules from tumor-infiltrated immune cells may provide immunosuppressive functions to cancer cells.
Fig. 4.
Trogocytic cancer cells acquired immune regulatory molecules in the tumor microenvironment. Trimap plot analysis of cell type marker proteins (CD4, EpCAM, and tdTomato) and immune regulatory molecules in liver metastasized colon cancer tissues are shown. Metastatic cancer cells were generated using AKPS organoids as described in Fig. 1. (A) CD4 T cells and cancer cells were identified by T cell marker CD4, epithelial cell marker EpCAM, and tumor cell reporter tdTomato. (B) The Trimap algorithm clustered tumor tissue cells based on the expression patterns of analyzed proteins. CD4+ T cells, trogocytic cancer cells (CD4+EpCAM+tdTomato+), and nontrogocytic cancer cells (CD4-EpCAM+tdTomato+) were identified and highlighted as green, yellow, and red, respectively. (C) Intensities of immune regulatory molecules are displayed in the Trimap plot. Trogocytic and nontrogocytic cancer cells were indicated by blue and red rectangules, respectively. (D) Histogram analysis of the levels of immune regulatory molecules in trogocytic cancer cells compared to nontrogocytic cancer cells. The majority cluster of trogocytic cancer cells was compared to one of the nontrogocytic cancer cell clusters with the identical intensity of tdTomato and EpCAM. (E) Liver-metastasized colon cancer cells were generated by splenic injection of dissociated AKPS organoids as described above. Cancer cells acquired CD4 and CD45 T cell markers (+Trogocytosis) and control cancer cells (−Trogocytosis) were sorted as described in SI Appendix, Fig. S2. Sorted cells were ex vivo cocultured with C57BL/6 splenocytes for 5 d at a ratio of 1 organoid to 50 splenocytes. A volcano plot analysis of the bulk RNA-seq data using the cocultured +Trogocytosis samples compared to −Trogocytosis samples is shown. Cfh (log-fold change; 9.95, q-value; 1.83E-15), Fam13a (9.22, 9.94E-09), Ighg3 (9.43, 5.85E-08), Dab2 (8.52, 1.51E-04), Peli2 (7.07, 1.61E-04), Timd2 (−8.80, 0.0012), and Ctla4 (−7.71, 0.0057) are highlighted. (F) Ingenuity pathway analysis of the RNA-seq data. Enriched top five canonical pathways are shown. Statistics of each canonical pathway are displayed as Benjamini–Hochberg P value. (G) Quantitative analysis of cytokine expression in ex vivo–cocultured samples by enzyme-linked immunosorbent assay (Eve Technology). *P < 0.05; two-tailed t test. Error bars indicate mean ± SD. Results are representative of three independent experiments. RNA-seq data used four biological replicates.
Acquired Immune Regulatory Functions of Colon Cancer Cells after Trogocytic Transfer of Surface Proteins from Tumor-Infiltrated Hematopoietic Cells.
In order to test whether the trogocytic transfer of immune regulatory molecules to cancer cells leads to immune suppressive functions, trogocytic and nontrogocytic cancer cells were sorted as described above in Fig. 1 (Fig. 1 D and E, SI Appendix, Fig. S2). Then, the sorted cells were cocultured with splenocytes from syngeneic C57BL/6 mice. Bulk RNA sequencing (RNA-seq) analysis of the cocultured cells showed a significant increase of Cfh, Fam13a, Ighg3, Dab2, and Peli2 expression in the trogocytic cancer cell cocultured splenocytes (Fig. 4E). These genes are involved in regulatory T cell–mediated immunosuppression, negative regulation of T cell activation, inhibition of NK cell maturation, and suppression of toll-like receptor signaling in antigen-presenting cells (19–22). On the other hand, Ctla4 and Timd2 expression was reduced in the presence of trogocytic cancer cells (Fig. 4E, GSE186692). Expression of these genes are increased by T cell activation, suggesting that trogocytic cancer cells reduced T cell activation during coculture (1, 23). Likewise, ingenuity pathway analysis of the bulk RNA-seq data indicated the Th1 and Th2 activation pathway as the most enriched canonical pathway (Fig. 4F). Particularly, decreased activation of the Th1 pathway, NK cell signaling, and dendritic cell maturation are observed in trogocytic cancer cell cocultured splenocytes. It has been confirmed by quantitative cytokine analysis for the cultured supernatants showing reduction of IL-2, TNF-α, and IFN-γ (Fig. 4G). These results suggest that trogocytosis potentiates immunosuppressive properties of cancer cells in contact with tumor-infiltrated lymphocytes.
Trogocytic Cancer Cells in Human Colorectal Cancer and Head and Neck Cancer.
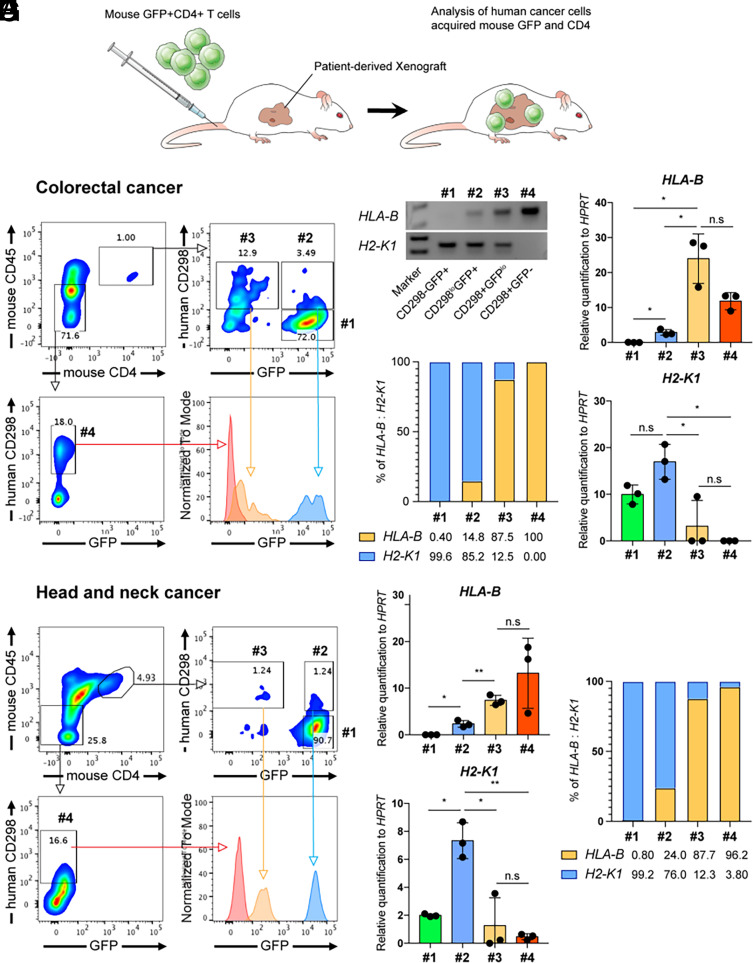
In order to test our observation using human cancer cells, we utilized patient-derived xenograft (PDX) models. Purified mouse GFP-positive CD4 T cells were infused into immune-deficient mice bearing a human colon cancer PDX intravenously to test whether human cancer cells are able to acquire mouse CD4 and CD45 (Fig. 5A). Two independent, patient-derived colorectal cancer samples were tested. Consistent with the previous results in the murine metastasis model of colon cancer, about 15% of mouse CD4 and CD45 double–positive cells were positive for the human CD298 in the tumor microenvironment (Fig. 5B). These cells were further divided into two populations, human CD298+mouse GFPlow and human CD298lowmouse GFP+. The intensity of mouse membrane proteins CD4 and CD45 in human CD298+mouse GFPlow cells were similar to tumor-infiltrated mouse CD4 T cells, while their cytosolic protein GFP intensity was lower (Fig. 5B). Genomic DNA PCR analysis for human and mouse MHC class I genes showed that the human CD298+mouse GFPlow population contains 87.5% of human HLA-B and 12.5% of mouse H2-K1, suggesting the majority of cells in this population are human cancer cells. On the other hand, the human CD298lowmouse GFP+ population showed 14.8% of human and 85.2% of mouse MHC class I genomic DNA ratio, suggesting most of these cells are mouse T cells (Fig. 5 C–E). These results confirmed the presence of cancer cells acquired CD4 and CD45 from tumor-infiltrated T cells (human CD298+mouse GFPlow) as well as trogocytic T cells (human CD298lowmouse GFP+ cells) in the tumor microenvironment (8). The presence of human cancer cells with mouse CD4 and CD45 was also detected in head and neck cancer PDX models mice as well (Fig. 5 F–H). Caco-2 cell line–derived human colon cancer organoids were able to acquire membrane proteins including CD45 from human Jurkat T cells after coculture (SI Appendix, Fig. S7). Collectively, these results confirmed the presence of trogocytic cancer cells in humans.
Fig. 5.
Human colorectal and head and neck cancer cells obtained murine CD4 and CD45 in the model of PDX. (A) A schematic diagram of murine CD4 T cell infusion into patient-derived cancer xenograft mice. Mouse CD4 T cells were purified with magnetic-activated cell sorting. Purified CD4 T cells were stimulated with anti-CD3 and anti-CD28 antibodies for 3 d and then rested for 4 d in the presence of human IL-2 (30 U/mL). Rested mouse CD4 T cells were injected intravenously into human colon cancer or head and neck cancer bearing NSG mice. (B) 2 wk after T cell infusion, colon cancer tissues were dissociated and analyzed by flow cytometry for human cancer marker CD298 and mouse T cell markers CD4, CD45, and GFP. Tumor-infiltrated mouse CD4 T cells (#1, CD298−GFP+), control cancer cells (#4, CD298+GFP−), and cells possessing both markers for human and mouse (#2 and #3) are gated, and their GFP levels are analyzed in the histogram. The numbers indicate the percentile of each population. (C) Genomic DNA PCR analysis of the sorted cells from #1 to 4 gated populations. Agarose gel pictures of the PCR products, human HLA-B gene (237 bp), and mouse H2-K1 gene (242 bp) are shown. Marker bands are 200 bp and 300 bp (bp; base pair). (D) Relative quantification of HLA-B and H2-K1 PCR to HPRT (or Hprt) using compatible primers for human HPRT and mouse Hprt genes. (E) Percentiles of human and mouse MHC molecules in #1 to 4 gated cells are presented based on the genomic DNA PCR quantification in D. The numbers indicate the percentiles of HLA-B or H2-K1. (F–H) Flow cytometry and quantitative genomic DNA PCR analyses of human head and neck cancer tissues as described above. n.s, not significant, *P < 0.05, **P < 0.01; two-tailed t test. Error bars indicate mean ± SD. Results are representative of two independent experiments. Two independent patient-derived colorectal cancer samples were tested.
Discussion
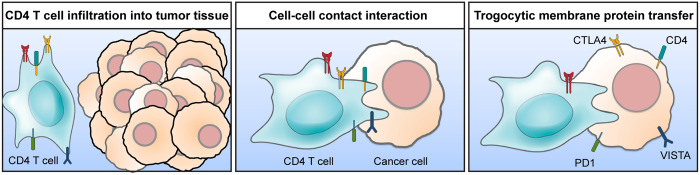
In this study, we showed the transfer of membrane proteins from tumor-infiltrating lymphocytes to colon cancer cells, such as hematopoietic cell marker proteins CD4 and CD45 via trogocytosis (Fig. 6). Importantly, trogocytic cancer cells in the tumor microenvironment acquired immune regulatory molecules. An ex vivo coculture of trogocytic cancer cells with splenocytes from the syngeneic mice reduced antitumor immune responses compared to nontrogocytic cancer cells. Taken together, we have revealed the following: first, trogocytosis by cancer cells and second, trogocytic cancer cells acquire immune regulatory molecules from tumor-infiltrating lymphocytes.
Fig. 6.
Schematic illustration. Hematopoietic cells including CD4 T cells infiltrate into tumor tissues. Tumor-infiltrating hematopoietic cells interact with cancer cells in the tumor microenvironment. During the cell–cell contact interaction, cancer cells acquire membrane proteins from the contacted hematopoietic cells by trogocytosis. Unlike phagocytosis or entosis, which internalize endocytosed materials into the cytosol, trogocytosis maintains cell surface localization of the transferred membrane proteins. Trogocytic transfer of immune modulatory proteins, such as CTLA4, LAG3, PD1, Tim3, VISTA, and CD38, contributes to develop the immunosuppressive tumor microenvironment.
Unlike phagocytosis, trogocytosis transfers membrane proteins from a donor cell surface to cancer cell surfaces, which elicits functional changes in particular, immunosuppression in the tumor microenvironment. Our study focused on trogocytic transfer from CD4 T cells to cancer cells based on the high frequency of cells harboring both CD4 and the tumor reporter protein tdTomato. CD4 T cells are the most abundant lymphocytes in the tumor microenvironment of the murine metastatic colon cancer model (Fig. 1B and SI Appendix, Fig. S1B). Increased numbers of tumor-infiltrated CD4 T cells were observed in human colorectal cancer patient samples compared to normal tissues, and these CD4 T cells are mainly suppressive regulatory T cells (17). Regulatory T cells in human colon tumor tissues express immunosuppressive molecules, CTLA4, PD1, Tim3, and LAG3, which are transferred molecules to colon cancer cells via trogocytosis in the murine model system (Fig. 4) (17, 24). These findings suggest regulatory T cells as a potent participant in colon cancer trogocytosis. Frequencies of myeloid lineage cells harboring tdTomato imply the possibility that cancer cells acquire immune modulatory proteins from tumor-infiltrated myeloid cells as well (SI Appendix, Fig. S1A). Considering the heterogeneity and various differentiation properties of cancer cells, further characterization of trogocytic cancer cells in the tumor microenvironment needs to be addressed to answer what cancer cell types are trogocytic and which hematopoietic cells are involved in trogocytosis.
Trogocytosis of cancer cells in contact with T cells were visualized by live imaging analysis in vitro. Rapid transfer of membrane patches from T cells to cancer cells was observed within 10 min. Previous studies have reported that costimulatory molecules in the immunological synapse are transferred from antigen-presenting cells to T cells by trogocytosis followed by sustained T cell activation (25, 26). This implies the possibility of immune-stimulatory molecule transfer to trogocytic cancer cells. Indeed, costimulaotory ligands B7.1 and B7.2 molecules were detected in trogocytic mouse colon cancer cells, which acquired CTLA4 and the MHC class II molecule as well (SI Appendix, Fig. S8 A and B). PD-L1 levels were also increased in trogocytic cancer cells compared to nontrogocytic cancer cells (SI Appendix, Fig. S8C). While trogocytic cancer cells acquired both immune stimulatory and inhibitory molecules, an in vitro coculture of the sorted trogocytic cancer cells with immune cells showed enhanced immunosuppression compared to nontrogocytic cancer cells (Fig. 4). Furthermore, CTLA4 binds to B7.1 and B7.2 with 100-fold increased affinity compared to the costimulatory receptor CD28 to inhibit T cell activation (27). These data suggest that trogocytosis-mediated transferred proteins will be determined by the properties of the microenvironment. For instance, in the developed tumor microenvironment with increased expression of immune-regulatory proteins, cancer cells are more likely to acquire these suppressive proteins including immune checkpoint inhibitors. Further investigation is needed to test whether early phase cancer cells acquire immune-stimulatory proteins at the initiation of tumorigenesis.
In colorectal cancer, our data showed sequential elevation of the number of trogocytic cells among early phase colon cancer cells with a mutation in the Apc gene and late phase colon cancer cells with mutations in Apc, Kras, Tp53, and Smad4 genes in vitro. There was no detectable trogocytosis with normal colonic epithelial cells. Indeed, about 80% of colorectal cancer patients have mutations in the APC gene, and other types of cancer commonly have mutations in KRAS and TP53 genes (28). Future investigation will address the relevance between these oncogenic mutations and cancer cell trogocytosis during the progression of malignant colorectal cancer.
Here, we showed that cancer cells acquired membrane proteins from tumor-infiltrating T cells by trogocytosis. Although trogocytosis of amoeba, neuronal cells, and hematopoietic cells has been reported, the underlying molecular mechanisms remain unknown (29). Hwang et al. have demonstrated that CD8 T cell trogocytosis is adhesion molecule ICAM1 dependent (30). Our study is a report of cancer cell trogocytosis, and we showed immune regulatory functions of trogocytic cancer cells ex vivo. Identification of the molecular mechanisms of trogocytosis in each type of cancer will provide insight into new therapeutic approaches targeting cancer diseases.
Materials and Methods
In Vivo Tumor Models.
Apc−/−KrasG12D/+Tp53−/−Smad4−/−tdTomato+ (AKPS) organoids were digested by TrypLE Express Enzyme (1×), phenol red (Gibco, 12605010) at 37 °C for 30 min. A total of 300,000 isolated cells in 200 μL phosphate-buffered saline were injected into the C57BL/6 mice via the splenic vein (14). Liver-metastasized colon cancer cells were harvested 3 to 4 wk after transplantation.
Patient-derived colorectal cancer line number one and two (CRC01 and CRC02) and head and neck cancer line number 26 (HN26) were implanted to NOD-scid IL2Rgammanull (NSG) mice (Jackson Lab, 005557) subcutaneously. A total of 2 mm3 solid tumor tissues were implanted with Matrigel (Corning, 356231) bilaterally. When the tumor size reached about 1 cm in diameter, 106 purified mouse CD4 T were injected intravenously. CD4 T cells were isolated from splenocytes of Actin-GFP reporter mice using EasySep Mouse CD4 Positive Selection Kit II (Stemcell, 18952). Isolated CD4 T cells were stimulated with the plate-coated 2.5 μg/mL anti-mouse CD3ε antibody (eBioscience, 16–0031-85, clone: 145–2C11) and 5 μg/mL anti-mouse CD28 antibody (eBioscience, 16–0281-85, clone: 37.51) for 3 d in the presence of 50 U/mL human IL-2. Activated CD4 T cells were rested for 4 d in the presence of 30 U/mL human IL-2. Magnetic beads were removed before T cell infusion into mice. A total of 2 to 3 wk after CD4 T cell infusion, mice were killed for harvesting tumor tissues.
Supplementary Material
Acknowledgments
We thank Gouzel Tokmulina and Ewa Menet for the fluorescence-activated cell sorting. This work was supported by NIH Grant RO1 CA168670-01 (awarded to A.L.M.B.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2110241118/-/DCSupplemental.
Data Availability
The RNA-seq data reported in this study have been deposited in the Gene Expression Omnibus (GSE186692) (31). All other data are included in the article and supporting information.
References
- 1.Huang J.-F., et al. , TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science 286, 952–954 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Ralston K. S., et al. , Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature 508, 526–530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steele S., Radlinski L., Taft-Benz S., Brunton J., Kawula T. H., Trogocytosis-associated cell to cell spread of intracellular bacterial pathogens. eLife 5, e10625 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinhard L., et al. , Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 9, 1228 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matlung H. L., et al. , Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 23, 3946–3959.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Hamieh M., et al. , CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568, 112–116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velmurugan R., Challa D. K., Ram S., Ober R. J., Ward E. S., Macrophage-mediated trogocytosis leads to death of antibody-opsonized tumor cells. Mol. Cancer Ther. 15, 1879–1889 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonnessen-Murray C. A., et al. , Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J. Cell Biol. 218, 3827–3844 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levchenko A., et al. , Intercellular transfer of P-glycoprotein mediates acquired multidrug resistance in tumor cells. Proc. Natl. Acad. Sci. U.S.A. 102, 1933–1938 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walunas T. L., et al. , CTLA-4 can function as a negative regulator of T cell activation. Immunity 1, 405–413 (1994). [DOI] [PubMed] [Google Scholar]
- 11.Monney L., et al. , Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415, 536–541 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Gast C. E., et al. , Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 4, eaat7828 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shabo I., et al. , Roles of cell fusion, hybridization and polyploid cell formation in cancer metastasis. World J. Clin. Oncol. 11, 121–135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Rourke K. P., et al. , Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat. Biotechnol. 35, 577–582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laberge G. S., Duvall E., Haedicke K., Pawelek J., Leukocyte–cancer cell fusion—Genesis of a deadly journey. Cells 8, 170 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li K.-J., et al. , Trogocytosis between non-immune cells for cell clearance, and among immune-related cells for modulating immune responses and autoimmunity. Int. J. Mol. Sci. 22, 2236 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toor S. M., et al. , Immune checkpoints in circulating and tumor-infiltrating CD4+ T cell subsets in colorectal cancer patients. Front. Immunol. 10, 2936 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ElTanbouly M. A., et al. , VISTA is a checkpoint regulator for naïve T cell quiescence and peripheral tolerance. Science 367, eaay0524 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang M., et al. , The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat. Immunol. 12, 1002–1009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang R., Liu Q., Li T., Liao Q., Zhao Y., Role of the complement system in the tumor microenvironment. Cancer Cell Int. 19, 300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim N., et al. , Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nat. Commun. 11, 2285 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figliuolo da Paz V., Ghishan F. K., Kiela P. R., Emerging roles of disabled homolog 2 (DAB2) in immune regulation. Front. Immunol. 11, 580302 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumanogoh A., et al. , Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature 419, 629–633 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Betts G., et al. , Suppression of tumour-specific CD4+ T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut 61, 1163–1171 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He T., Zong S., Wu X., Wei Y., Xiang J., CD4+ T cell acquisition of the bystander pMHC I colocalizing in the same immunological synapse comprising pMHC II and costimulatory CD40, CD54, CD80, OX40L, and 41BBL. Biochem. Biophys. Res. Commun. 362, 822–828 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Osborne D. G., Wetzel S. A., Trogocytosis results in sustained intracellular signaling in CD4(+) T cells. J. Immunol. 189, 4728–4739 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin J. H., et al. , Positive conversion of negative signaling of CTLA4 potentiates antitumor efficacy of adoptive T-cell therapy in murine tumor models. Blood 119, 5678–5687 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Fodde R., Smits R., Clevers H., APC, signal transduction and genetic instability in colorectal cancer. Nat. Rev. Cancer 1, 55–67 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Dance A., Core concept: Cells nibble one another via the under-appreciated process of trogocytosis. Proc. Natl. Acad. Sci. U.S.A. 116, 17608–17610 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang I., Shen X., Sprent J., Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: Distinct roles for CD54 and B7 molecules. Proc. Natl. Acad. Sci. U.S.A. 100, 6670–6675 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.J. H. Shin, J. Lim, A. Bothwell, Colon cancer cells acquire immune regulatory molecules from tumor-infiltrating lymphocytes by trogocytosis. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE186692. Deposited 27 October 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data reported in this study have been deposited in the Gene Expression Omnibus (GSE186692) (31). All other data are included in the article and supporting information.