Significance
This work identifies apurinic/apyrimidinic endonuclease1 (APEX1) as a shear stress-sensitive molecule that plays a crucial role in the atherogenic flow-induced endothelial proinflammatory responses. Depletion of endothelial Apex1 in mice ameliorated atherogenesis. We demonstrate that vitexin, a natural flavonoid, can inhibit the activation of APEX1 to protect vascular endothelium against the adverse effects of atherogenic stimuli. Mechanistically, vitexin associates directly with APEX1 to inhibit the atherogenic flow-induced interaction of APEX1 with acetyltransferase p300, its acetylation, and nuclear translocation. The action of APEX1 in the flow-evoked responses is dependent on NF-κB signaling. The translational significance of these findings is that targeting APEX1 with vitexin may represent a potential therapeutic strategy for the treatment endothelial inflammation and the related diseases.
Keywords: hemodynamics, endothelial inflammation, atherogenesis, APEX1, vitexin
Abstract
Vascular endothelial cells are exposed to shear stresses with disturbed vs. laminar flow patterns, which lead to proinflammatory vs. antiinflammatory phenotypes, respectively. Effective treatment against endothelial inflammation and the consequent atherogenesis requires the identification of new therapeutic molecules and the development of drugs targeting these molecules. Using Connectivity Map, we have identified vitexin, a natural flavonoid, as a compound that evokes the gene-expression changes caused by pulsatile shear, which mimics laminar flow with a clear direction, vs. oscillatory shear (OS), which mimics disturbed flow without a clear direction. Treatment with vitexin suppressed the endothelial inflammation induced by OS or tumor necrosis factor-α. Administration of vitexin to mice subjected to carotid partial ligation blocked the disturbed flow-induced endothelial inflammation and neointimal formation. In hyperlipidemic mice, treatment with vitexin ameliorated atherosclerosis. Using SuperPred, we predicted that apurinic/apyrimidinic endonuclease1 (APEX1) may directly interact with vitexin, and we experimentally verified their physical interactions. OS induced APEX1 nuclear translocation, which was inhibited by vitexin. OS promoted the binding of acetyltransferase p300 to APEX1, leading to its acetylation and nuclear translocation. Functionally, knocking down APEX1 with siRNA reversed the OS-induced proinflammatory phenotype, suggesting that APEX1 promotes inflammation by orchestrating the NF-κB pathway. Animal experiments with the partial ligation model indicated that overexpression of APEX1 negated the action of vitexin against endothelial inflammation, and that endothelial-specific deletion of APEX1 ameliorated atherogenesis. We thus propose targeting APEX1 with vitexin as a potential therapeutic strategy to alleviate atherosclerosis.
The focal nature of atherosclerosis suggests a critical role of local hemodynamic microenvironments in atherogenesis (1–3). Disturbed flow with a low and reciprocating shear stress (oscillatory shear, OS) in arterial branches and curvatures up-regulates proinflammatory molecules in vascular endothelial cell (EC), and hence induces the filtration of circulating monocytes into the arterial wall and the migration of smooth muscle cells into the subintimal space, leading to atherosclerosis (4). In contrast, laminar flow with a high and unidirectional shear stress (pulsatile shear, PS) in the straight parts of the arteries elicits an antiinflammatory and atheroprotective effect on the vasculature (2). The distinct gene-expression patterns and phenotypes in ECs provoked by disturbed vs. laminar flows develop before the appearance of any functional outcomes, and they have been suggested as hallmarks of atherosusceptible or atheroresistant endothelium (1). While the importance of endothelial gene expression and phenotype in atherogenesis has been recognized, treatment options for endothelial abnormality are limited. The phase with endothelial phenotypic changes represents a therapeutic window in which the eradication of the disturbed flow-caused endothelial inflammation may lead to a prevention or cure. A systematic understanding of functional connections among the atherogenic phenotypes of endothelium, gene expressions, and drug actions can lead to effective antiatherosclerotic drug screening.
Apurinic/apyrimidinic endonuclease 1/redox factor-1 (APEX1 in human and Apex1 in mouse), which has the ability to cleave at apurinic/apyrimidinic sites in DNA and to activate the DNA binding activity of a number of transcription factors (5), has been implicated in the regulation of vascular EC function (6–9). However, it remains to be determined whether APEX1 plays a role in the hemodynamic regulation of endothelial phenotype. Exogenous overexpression of APEX1 inhibited adhesion of monocytes to ECs activated by tumor necrosis factor-α (TNF-α) (6), and EC-secreted APEX1 could suppress the TNF-α–induced expression of vascular cell adhesion molecule-1 (VCAM1) (7); these findings suggest an antiinflammatory role of APEX1. Knocking down APEX1 by small-interfering RNA (siRNA) promoted the endothelial barrier function (8), and inhibition of nuclear translocation of APEX1 down-regulated the oxidized low-density lipoprotein-stimulated expression of C-C motif chemokine ligand 2 (CCL2) (9): these findings suggest a role of APEX1 in endothelial damage. Thus, the existing evidence reveals that APEX1 plays a complex role in the regulation of endothelial function. Despite its importance, little is known about the exact role of APEX1 in the disturbed flow-induced endothelial inflammation and atherosclerosis.
By using the Connectivity Map (CMap)-based computational systems biology approach to search for compounds that can produce similar gene signature as induced by PS vs. OS, we identified vitexin, a natural flavonoid isolated from the leaf of Crataegus pinnatifida Bunge, which greatly diminishes the endothelial proinflammatory responses. We demonstrated that vitexin associated directly with APEX1 to inhibit the OS-induced interaction of APEX1 with acetyltransferase p300, its acetylation, and nuclear translocation. The action of APEX1 in the OS-regulated EC responses is mechanistically dependent on NF-κB signaling. These findings indicate that suppressing the APEX1/NF-κB pathway represents an approach to prevent endothelial inflammation. In addition, our studies utilizing mice with the intraluminal overexpression and EC-specific depletion of APEX1 provide pivotal evidence demonstrating the proinflammatory role of APEX1 in ECs.
Results
Discovery of Endothelial-Protective Compounds Based on the CMap.
Previously, we presented the gene-expression profiles of human umbilical vein ECs (HUVECs) subjected to PS (12 ± 4 dyn/cm2, 1 Hz) or OS (0.5 ± 4 dyn/cm2, 1 Hz) for four time periods (1, 4, 12, and 24 h) (10). The sequencing data of the cells after 24 h of shearing were analyzed for the degree of similarity of gene expression using the CMap database (https://portals.broadinstitute.org/cmap/) (11) to search for bioactive compounds (among 7,056 treatments) that could simulate the effects of PS vs. OS (Fig. 1A). Based on the “connectivity scores (from −1 to +1),” with positive values denoting the degree of similarity and negative values representing the inverse, we obtained a list of 480 treatments with connectivity scores >0.5.
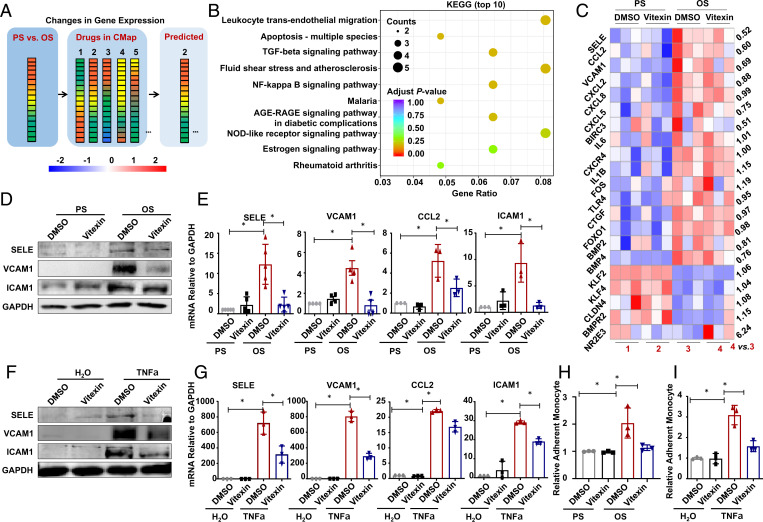
Fig. 1.
Identification of vitexin as antiinflammatory compound attenuating the OS- or TNF-α–induced proinflammatory responses in vascular ECs. (A) Schematic depicting a flow diagram of screening potential therapeutic compounds. Gene-expression profiles in cells subjected to PS/OS were analyzed with the CMap database. (B) The transcriptome in ECs pretreated with vitexin or DMSO for 24 h and exposed for 6 h to PS or OS was analyzed by RNA-sequencing, and a subset of genes shown in SI Appendix, Fig. S4B were subjected to KEGG pathway enrichment analysis. Pathways with the 10 lowest adjusted P values are presented. (C) Heatmaps of differentially expressed inflammation-related genes (log2 fold-change > 0.5, P < 0.05 by pairwise comparisons). The fold-changes of group 4 vs. 3 are indicated. (D and E) ECs were pretreated with DMSO or vitexin for 24 h, exposed to PS/OS for 6 h, and the protein and mRNA expressions of the indicated genes were assayed by Western blotting (D) and quantitative RT-PCR (E). (F and G) ECs were pretreated with DMSO or vitexin for 24 h, incubated with TNF-α for 6 h, and the protein and mRNA expressions of the indicated genes were assayed by Western blotting (F) and quantitative RT-PCR (G). (H and I) ECs were pretreated with DMSO or vitexin for 24 h, exposed to PS/OS for 6 h (H) or incubated with TNF-α or the control solvent for 6 h (I), and the Dil (red)-labeled THP-1 monocyte-to-endothelium adhesion assay was performed. Shown are the quantification of relative adherent monocytes. Data are presented as mean ± SEM. *P < 0.05 by two-way ANOVA followed by Tukey’s post hoc test (n ≥ 3).
A literature review on this list using the PubMed/MEDLINE database led to the finding of a c-glycosylated flavone vitexin (SI Appendix, Fig. S1) that has antioxidant, antiinflammatory, antihypertensive, and anticancer activities (12), with a connectivity score of 0.539. Dose–response tests showed that vitexin at 25 ∼200 μmol/L did not affect the EC viability (SI Appendix, Fig. S2). Hence, 50 μmol/L was applied in subsequent experiments to minimize the potential cytotoxic effect of vitexin.
Vitexin Attenuates the OS- or TNF-α–Induced Proinflammatory Responses in ECs.
We performed transcriptional profiling in ECs treated with vitexin or DMSO and subjected to PS/OS exposure. RNA-sequencing analysis showed that 1,168 genes were differentially regulated by OS vs. PS (513 up- and 655 down-regulated, P < 0.05) in the DMSO-treated cells; vitexin decreased the number of differentially expressed genes (DEGs) to 924 (SI Appendix, Fig. S3). In total, there were 2,377 DEGs (P < 0.05) in any of the pairwise comparisons (SI Appendix, Fig. S4A). Importantly, the expression changes of a subset of DEGs in the DMSO-treated cells were attenuated or reversed by vitexin (SI Appendix, Fig. S4B). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis showed that these DEGs were mainly involved in leukocyte transendothelial migration, fluid shear stress and atherosclerosis, NF-κB signaling pathways, and others as presented (Fig. 1B), suggestive of a shear- or vitexin-regulation on endothelial inflammation and barrier function. Since endothelial inflammation plays a central role in the mentioned pathways, we further analyzed the inflammation-related genes (log2 fold-change > 0.5 or < −0.5) and identified proinflammatory adhesion molecules and chemotactic cytokines, such as endothelial selectin E (SELE), CCL2, and VCAM1, which were induced by OS, and the induction was suppressed by vitexin (Fig. 1C), demonstrative of an inhibitory effect of vitexin on proinflammatory gene expression.
Both disturbed flow and TNF-α are implicated in endothelial inflammation and atherosclerosis (13). Application of OS or TNF-α (10 ng/mL) to ECs induced increases in the protein levels of SELE, VCAM1, and intercellular adhesion molecule 1 (ICAM1), and the mRNA levels of SELE, VCAM1, CCL2, and ICAM1. Their inductions were suppressed by vitexin (Fig. 1 D–G and SI Appendix, Fig. S5). The TNF-α–stimulated CCL2 accumulation in the media was also suppressed by vitexin (SI Appendix, Fig. S6). Up-regulation of adhesion molecules and chemotactic cytokines can lead to adhesion of monocytes to endothelium and impairment in endothelial barrier function (14). Application of OS or TNF-α induced an increase in endothelial adhesiveness for THP-1 monocytes, and this increase was attenuated by vitexin (Fig. 1 H and I and SI Appendix, Fig. S7). The TNF-α–promoted endothelial permeability to dextran was also inhibited by vitexin (SI Appendix, Fig. S8A). Reactive oxygen species (ROS) production is associated with endothelial inflammation (15). The OS or TNF-α induction of ROS in ECs was found to be suppressed by vitexin (SI Appendix, Fig. S8B). Altogether, our findings indicate that vitexin protects ECs from the OS- or TNF-α–stimulated endothelial inflammation.
Vitexin Counteracts the Disturbed Flow-Induced Endothelial Inflammation and Neointimal Formation.
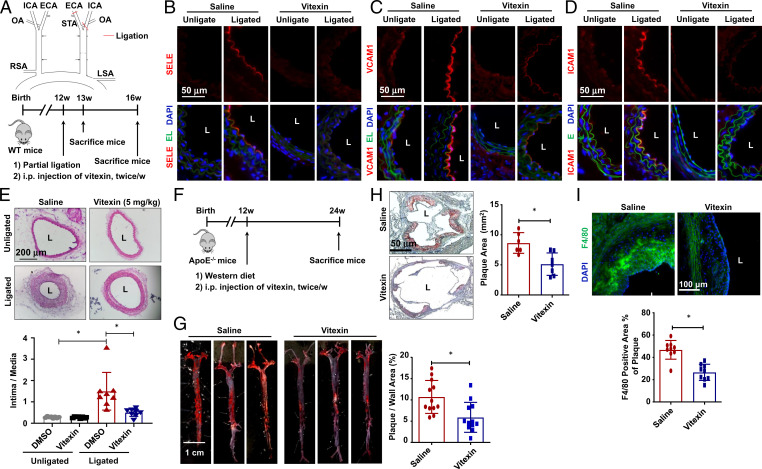
To evaluate the role of vitexin in vivo, partial carotid ligation was conducted in mice to introduce low and oscillatory shear stress in the left common carotid artery (16). In this model the time-averaged wall shear stress in the ligated common carotid artery was reduced to ∼27% of the unligated (16). The mice were subjected to vitexin (5, 10, or 20 mg/kg) or saline (Fig. 2A). Immunofluorescence on sections of ligated (left) or unligated (right) carotid arteries after 1 wk of ligation revealed no apparent endothelial denudation (SI Appendix, Fig. S9). EC expressions of SELE, VCAM1, and ICAM1 were increased in the ligated saline group, indicative of endothelial inflammation. These increases were suppressed by vitexin (Fig. 2 B–D). In mice receiving vitexin or saline for 4 wk, the neointimal thickening induced by partial ligation in mice receiving saline (4 wk) was suppressed by vitexin (4 wk) at all concentrations tested (Fig. 2E and SI Appendix, Fig. S10). Thus, in the following in vivo experiments, the concentration of 5 mg/kg was applied.
Fig. 2.
Vitexin counteracts the hemodynamic disturbed flow-induced endothelial inflammation, neointimal formation, and atherosclerosis. (A) (Upper) Schematic diagram of the partial carotid ligation surgery. ECA: external carotid artery; ICA: internal carotid artery; LSA: left subclavian artery; OA: occipital artery; RSA: right subclavian artery; STA: superior thyroid artery. (Lower) Schematic diagram of experimental design in WT mice. (B–E) Representative immunofluorescence staining of SELE (B), VCAM1 (C), and ICAM1 (D), and H&E staining (E) in cross-sections of ligated (the left side) and unligated (the right side) common carotid arteries from mice subjected to vitexin or saline administration for 1 (B–D) or 4 (E) weeks. DAPI: to stain the nucleus; EL: elastic lamina. In E, quantification of the ratios of intima-to-media areas was shown (n = 7). (F) Schematic diagram of the experimental design in ApoE−/− mice. (G and H) Representative Oil red O staining and the quantification of atherosclerosis in whole aorta (n = 12) and cardiac outflow tract (n = 7) of mice receiving vitexin or saline. (I) Representative immunofluorescent staining of F4/80 and the quantification of F4/80+ area in cardiac outflow tract (n = 9). Data are presented as mean ± SEM, *P < 0.05 by Student’s t test or by two-way ANOVA followed by Tukey’s post hoc test. L: lumen.
Vitexin Ameliorates Atherosclerosis.
We used a hyperlipidemia mouse model to test the antiatherogenic effect of vitexin. ApoE−/− mice fed on a Western diet were injected with vitexin or saline (Fig. 2F). The atherosclerotic areas in the aortic tree of vitexin-treated mice were reduced by 55% (Fig. 2G), and the reduction was especially profound in aortic arch (SI Appendix, Fig. S11), where the local fluid shear stress is low and oscillatory (4). Moreover, histological examination of cross-sections of the atherosusceptible cardiac outflow tract showed that atherosclerosis was attenuated by vitexin (Fig. 2H). Circulating monocytes infiltrating into the subcutaneous vascular wall via injured endothelium is a key event in atherogenesis (4). Immunofluorescence of macrophage marker F4/80 in the outflow tract showed a decrease in the ratio of F4/80+ cells to plaque area in the vitexin-treated mice (Fig. 2I), suggesting inhibition of monocyte infiltration by vitexin. No significant differences in serum lipid content, body weight, or blood pressure were observed between the saline and vitexin treatments (SI Appendix, Fig. S12). The results from these experiments indicate that vitexin serves a protective role against endothelial injury and atherosclerosis independent of lipid metabolism.
Vitexin Directly Targets APEX1 in ECs.
To elucidate the mechanisms by which vitexin protects ECs, we searched for molecules that might be directly targeted by vitexin to mediate the functional outcomes. Using the SuperPred web server (https://prediction.charite.de/), a prediction webserver for target prediction of compounds based on the similarity distribution among the targets’ ligands (17), we identified five targets for vitexin: aldose reductase (AKR1B1), DNA polymerase-κ (POLK), runt-related transcription factor 1 (RUNX1), alkaline phosphatase (ALP), and APEX1. Searching vitexin in the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) indicated it as an inhibitor of APEX1 protein based on a quantitative high-throughput screening for small-molecule inhibitors of human APEX1 (18).
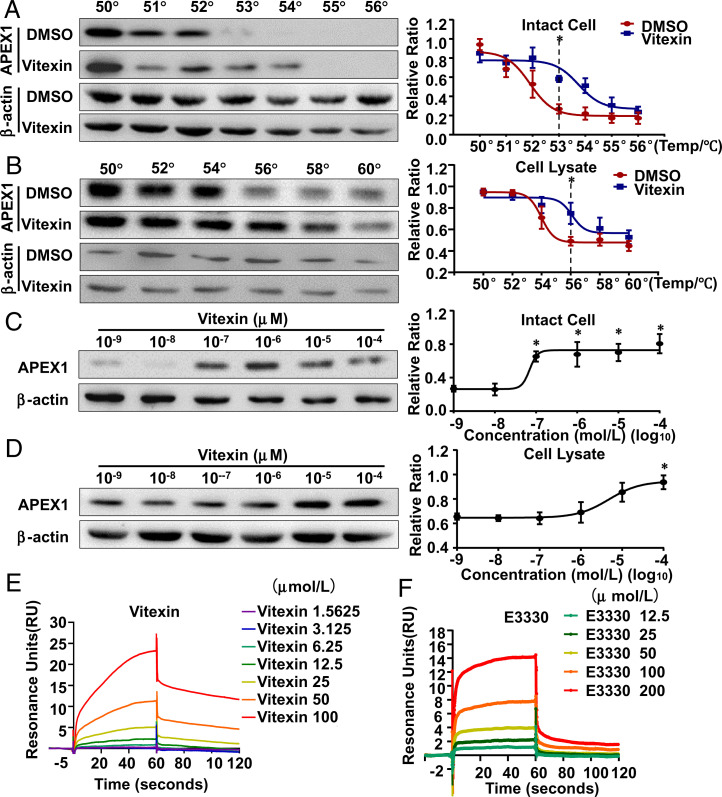
In order to validate APEX1 as a direct target of vitexin in ECs, we implemented the cellular thermal shift assay (CETSA), which monitors drug-target engagement based on the biophysical principle of ligand-induced thermal stabilization of target proteins (19). In this assay, aliquots of DMSO- or vitexin-pretreated intact cells and DMSO- or vitexin-cell lysates mixtures were heated to 50 to 60 °C. Soluble fractions from the treated samples were subjected to Western blotting to quantify APEX1. Compared with DMSO-treated samples, the vitexin-treated samples exhibited a robust thermal stabilization of APEX1 indicative of target engagement, with overall thermal shifts of 2 °C and 4 °C for intact cells and cell lysates, respectively (Fig. 3 A and B), suggesting binding of vitexin to APEX1. The isothermal dose–response (ITDR) reflects protein stabilization in response to a drug-concentration gradient at a single temperature in nondenaturing conditions (20). Because the melting temperatures in the CETSA melt curve assay were 53 °C and 56 °C for intact cells or cell lysates, respectively, we measured the ITDR with vitexin at 53 °C to ensure a nondenaturing condition for both samples. In either intact cells or lysates, APEX1 was stabilized by vitexin in a dose-dependent manner (Fig. 3 C and D).
Fig. 3.
Vitexin directly targets APEX1 in ECs. (A and B) The thermal stabilization effect of vitexin on APEX1 was evaluated by Western blotting and represented as CETSA melt curve. In intact cells (A), *P < 0.05 versus DMSO at 53 °C (n = 5); in cell lysates (B), *P < 0.05 versus DMSO at 56 °C (n = 5). (C) ITDR in intact cells for APEX1 with vitexin or DMSO treatment at 53 °C. *P < 0.05 versus treatment at a concentration of 10 −9 mol/L (n = 5). (D) ITDR in cell lysates for APEX1 with vitexin or DMSO treatment at 56 °C. *P < 0.05 versus treatment at a concentration of 10−9 mol/L (n = 5). In A and B, *P < 0.05 by Student’s t test, and in C and D, *P < 0.05 by one-way ANOVA followed by Tukey’s post hoc test. (E and F) SPR analysis of the interactions between APEX1 and vitexin (E) or E3330 (F). Shown are the real-time sensorgrams for SPR kinetic analysis of the bindings of vitexin (1.5625 to 100 μmol/L) or E3330 (12.5 to 200 μmol/L) to APEX1. Data are presented as mean ± SEM.
We performed surface plasmon resonance (SPR) (21) to identify the kinetic parameters of molecular interactions in real-time to confirm the APEX1–vitexin interaction. A known APEX1 inhibitor, E3330, was employed as a positive control. Resonance reaction units of immobilized human APEX1 recombinant protein and vitexin increased with rising vitexin concentrations, indicative of a dose-dependent interaction between vitexin and APEX1, with an equilibrium dissociation constant (KD) of 2.344 × 10−5 mol/L; E3330 interacted with APEX1 with a KD of 8.921 × 10−5 mol/L (Fig. 3 E and F and SI Appendix, Table S1). Since smaller KD values are indicative of greater ligand-target binding affinity, the results validate the APEX1–vitexin interaction.
Vitexin Inhibits the OS- and TNF-α–Induced APEX1 Translocation.
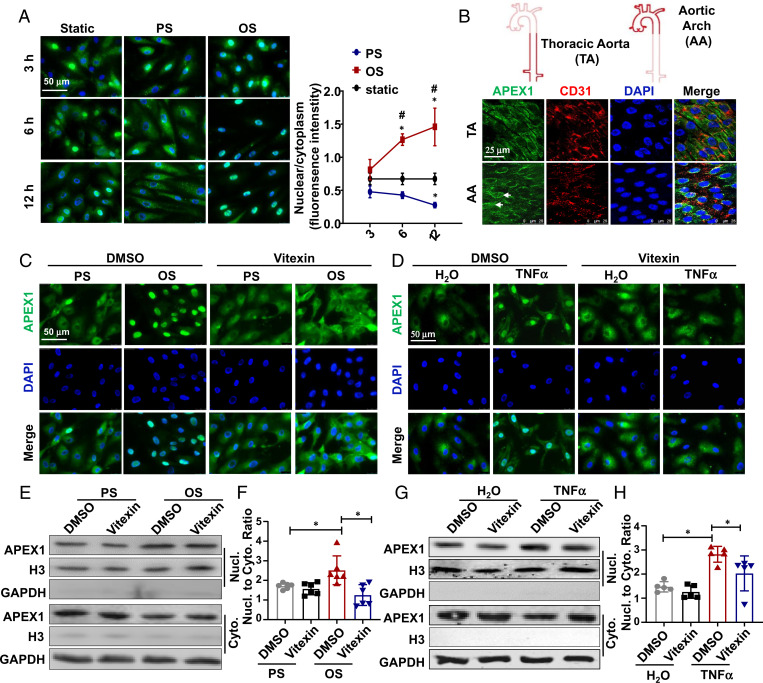
APEX1 exhibits both cytoplasmic and nuclear localizations (22), and it undergoes nuclear translocation in ECs in response to proinflammatory stimuli (9, 23). We found that OS caused a strong, time-dependent nuclear localization of APEX1, in comparison to the heterogeneous distribution of APEX1 within and among cells in the time-matched static controls. In contrast, under PS, APEX1 was mainly located in the cytoplasm (Fig. 4A). These findings were validated in the mouse aorta in vivo: in ECs of the descending thoracic aorta, where the flow is laminar, APEX1 was found predominantly in the cytoplasm, but in ECs of the inner curvature of aortic arch, where disturbed flow occurs, APEX1 exhibited nuclear accumulation (Fig. 4B). TNF-α also induced nuclear translocation of APEX1, and the OS- or TNF-α–induced translocation was blockaded by vitexin (Fig. 4 C and D). The Western blotting results on the localization of APEX1 in the nuclear and cytoplasmic fractions are in line with those of immunofluorescence, as evidenced by the increases in the nuclear-to-cytoplasm ratios of APEX1 signals in the OS- or TNF-α–stimulated cells treated with DMSO and the suppression of these increases by treating these cells with vitexin (Fig. 4 E–H).
Fig. 4.
Vitexin inhibits the OS- or TNF-α–induced nuclear translocation of APEX1. (A) Immunofluorescent staining of APEX1 (green) in ECs exposed to PS/OS or kept in static for time indicated. Nuclei were stained by DAPI. Quantification of the nuclear-to-cytoplasmic APEX1 is shown (Right). (B) En-face staining of APEX1, CD31, and the nuclei (DAPI) in mouse aortic arch (AA) and thoracic aorta (TA). (C and D) Immunofluorescent staining of APEX1in ECs pretreated with DMSO or vitexin for 24 h, and then exposed for 6 h to PS/OS (C) or incubated for 6 h with TNF-α or the vehicle control (D). Images in A to D are n ≥ 3. (E and F) Cells in C or D were subjected to cell fractionation, and accumulation of APEX1 in the nuclear (Nucl.) and cytoplasmic (Cyto.) fractions were analyzed by Western blotting. H3: Histone H3, a nuclear marker. GAPDH serves as a cytoplasmic marker. (G and H) Quantification of E and F, respectively (n = 5–6). Data are presented as mean ± SEM, *P < 0.05 by two-way ANOVA followed by Tukey’s post hoc test.
OS Induces Nuclear Translocation of APEX1 via Promoting Its Acetylation.
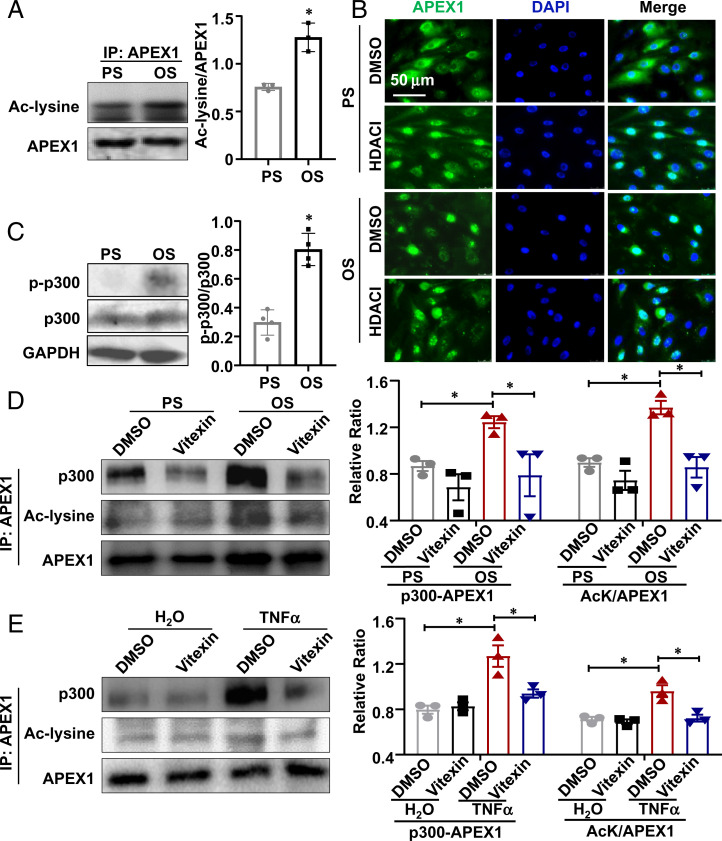
Acetylation at lysine residues of APEX1 promotes its nuclear distribution and binding to its target DNA (24, 25). We measured lysine acetylation of APEX1 in ECs exposed to PS or OS by immunoprecipitation assay. The antiacetylated-lysine antibody detected a higher enrichment of acetylated lysine residues in the APEX1-precipitated immunocomplexes in OS than that in PS (Fig. 5A), suggesting an enhancement of APEX1 acetylation by OS. Pretreating the ECs in PS condition with a deacetylases inhibitor mixture (HDACI) for 24 h increased the percentage of cells exhibiting a predominant APEX1-nuclear distribution, mimicking the effect of OS. No apparent differences were found in OS condition between ECs pretreated with DMSO and HDACI (Fig. 5B). The findings suggest that OS induces nuclear translocation of APEX1 via promoting its acetylation.
Fig. 5.
OS induces the acetylation and nuclear translocation of APEX1 via promoting the association between APEX1 and histone acetyltransferase p300. (A) Immunoprecipitation (IP) followed by Western blotting to detect the acetylated APEX1 using a pan-acetyl-lysine antibody (Ac-lysine) in ECs subjected to PS/OS for 6 h. Semiquantification of the blots is shown (Right). *P < 0.05 by Student’s t test (n = 3). (B) Representative immunofluorescence staining of APEX1 and the nuclei (DAPI) in ECs. Cells pretreated with a deacetylase inhibitor mixture (HDACI) for 24 h were exposed to PS/OS for 6 h. (C) Phosphorylation levels of p300 in ECs exposed for 6 h to PS/OS were assessed by Western blotting. Semiquantification of the blots is shown (Right). *P < 0.05 by Student’s t test (n = 3). (D and E) Coimmunoprecipitation followed by Western blotting to detect the association of p300 with APEX1 and the acetylation level of APEX1 in ECs. Cells were pretreated with vitexin for 24 h and were then exposed to PS/OS for 6 h (D) or TNF-α stimulation for hours (E). Semiquantifications of the blots are shown (Lower). Results in B–E are n ≥ 3. *P < 0.05 by Student’s t test. Data are presented as mean ± SEM.
OS Promotes p300 Phosphorylation and Its Association with APEX1.
Rapid increases in phosphorylation and enzymatic activity of histone acyltransferase p300 have been reported in ECs in response to shear stress (26, 27). We found that OS promoted p300 phosphorylation to 2.70-fold of that in PS-treated cells (Fig. 5C). Coimmunoprecipitation experiments further indicated that OS or TNF-α enhanced the binding of p300 to APEX1 compared with PS or the vehicle control, and that their binding was disrupted by vitexin (Fig. 5 D and E). The OS- or TNF-α–promoted APEX1 acetylation was also suppressed by vitexin (Fig. 5 D and E). These data are in keeping with the thesis that OS induces nuclear translocation of APEX1 via promoting its acetylation (Fig. 5 A and B), suggestive of a p300-dependent mechanism of shear-activation of APEX1.
APEX1 Orchestrates the OS-Induced NF-κB Activation.
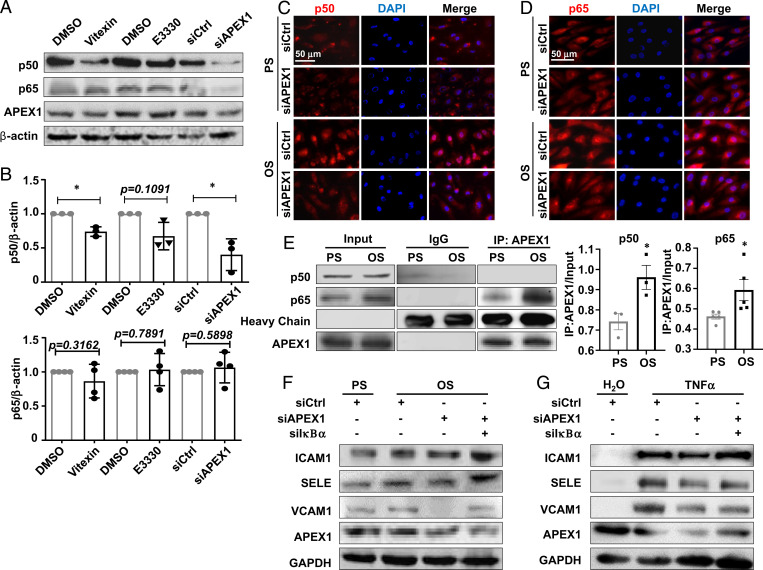
Given that activation of the proinflammatory transcription factor NF-κB was observed in diseased vasculature and in ECs exposed to atheroprone shear stress (4, 28), and that APEX1 inhibition could suppress NF-κB activation (29–31), we hypothesized that the shear-activated APEX1 to promote endothelial inflammation was mediated through potentiation of NF-κB activation. In accord with this hypothesis, inhibition of APEX1 activation by vitexin, E3330 (50 μmol/L), or siRNA-mediated gene silencing down-regulated the expression of p50 NF-κB subunit, but not p65 (Fig. 6 A and B). The cytoplasmic NF-κB forms complexes with their natural inhibitor IκB, and when activated, translocates to the nucleus to bind to the targeted DNA sequences (32). OS promoted a nuclear translocation of p50 and p65; these effects were abolished by APEX1 knockdown (Fig. 6 C and D). We next questioned how APEX1 regulates NF-κB translocation. Coimmunoprecipitation experiments indicated that OS increased the accumulation of p50 and p65 in the anti-APEX1 antibody-precipitated immunocomplexes (Fig. 6E), suggesting enhanced association between APEX1 and p50/p65 in response to OS. To study the role of the APEX1/NF-κB axis in mediating endothelial inflammation, we assessed the expressions of ICAM1, SELE, and VCAM1 in cells with the indicated treatments. Their OS- or TNF-α–induced expressions were found to be suppressed by APEX1 knockdown. Double-knockdown of APEX1 and IκBα abolished the suppressive effect (Fig. 6 F and G). Altogether, these findings support the notion that APEX1 orchestrates the OS-activated NF-κB signaling.
Fig. 6.
APEX1 orchestrates the OS-induced NF-κB activation. (A and B) The expressions of p50, p65, and APEX1 in ECs were assayed by Western blotting. Cells were treated with vitexin, E3330, or DMSO, or transfected with APEX1-specific siRNA (siAPEX1) or the control siRNA (siCL). Quantifications of the blots were shown in B. *P < 0.05 by Student’s t test (n = 3). (C and D) Representative immunofluorescent staining of p50 (C) and p65 (D) in ECs. Cells were transfected with siAPEX1 or siCL and were then exposed to PS/OS for 6 h. (E) Coimmunoprecipitation followed by Western blotting to detect the association of APEX1 with p50 or p65 in ECs exposed to PS/OS for 6 h. IgG was used as an isotype control. (F and G) Western blotting to detect the expressions of ICAM1, VCAM1, SELE, and APEX1 in ECs with the treatments indicated. Cells were transfected with siAPEX1, IκBα-specific siRNA (siIkBα), or siCL, and were then exposed for 6 h to PS/OS (F) or TNF-α versus its control reagent (G). Results in C–G are n ≥ 3. *P < 0.05 by two-way ANOVA followed by Tukey’s post hoc test. Data are presented as mean ± SEM.
Endothelial Overexpression of APEX1 Abolishes the Atheroprotective Effect of Vitexin.
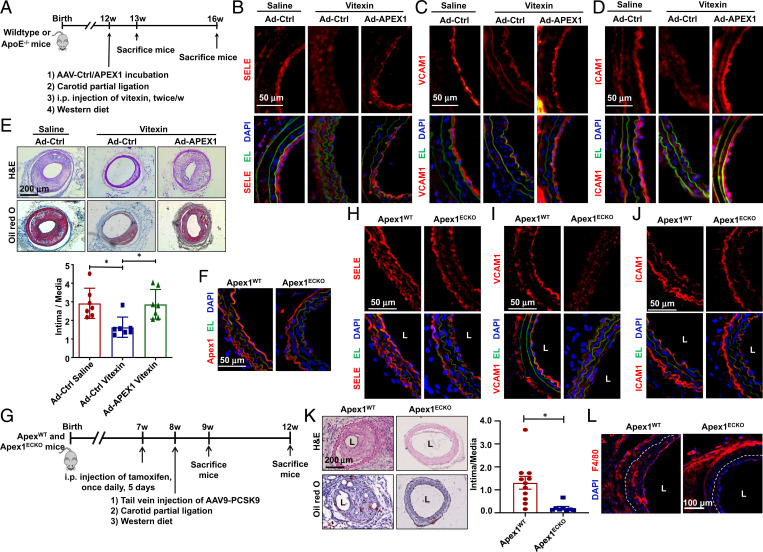
To assess the function of APEX1 in vivo, we performed partial carotid ligation in WT or ApoE−/− mice, in which the left common carotid arteries were subjected to a local intraluminal incubation with adenovirus expressing APEX1 (Ad-APEX1) or its control virus (Ad-CL) before ligation. The operated WT and ApoE−/− mice were fed on a chow or a Western diet, respectively. After surgery, the mice were subjected to intraperitoneal injection of vitexin or saline twice a week for 1 or 4 wk. At 1 wk after ligation in the WT mice, carotid arteries were harvested for assessing the expressions of proinflammatory markers in the endothelia. The carotid arteries from ApoE−/− mice receiving vitexin for 4 wk were used to analyze the disturbed flow-acceleration of atherosclerosis (Fig. 7A). In mice receiving Ad-CL, vitexin inhibited the partial ligation-induced expression of SELE, VCAM1, and ICAM1 in the ECs. Intraluminal overexpression of APEX1 de-suppressed the expressions of these proinflammatory genes, abrogating the inhibitory effect of vitexin against endothelial inflammation (Fig. 7 B–D and SI Appendix, Fig. S13). In the Ad-CL–treated mice, vitexin decreased the intima-to-media ratio by 44% in comparison to the saline group, suggesting the alleviation of neointimal thickening. Intraluminal application of Ad-APEX1 abolished this ameliorative effect (Fig. 7E). Mice treated with Ad-APEX1 exhibited markedly enhanced formation of atherosclerosis, compared with the mice with Ad-CL (SI Appendix, Fig. S14). Collectively, our results suggest that endothelial overexpression of APEX1 could block the atheroprotective effect of vitexin.
Fig. 7.
Endothelial Apex1 contributes to the disturbed flow-accelerated atherogenesis. (A) Schematic diagram of experimental design. (B–D) Representative immunofluorescence staining of SELE (B), VCAM1 (C), and ICAM1 (D) in cross-sections of common carotid arteries from mice received vitexin or saline administration and were subjected to local intraluminal incubation with adenovirus-expressing APEX1 (Ad-APEX1) or control virus (Ad-CL) followed by partial ligation. Arterial specimens were harvested at 1-wk after the ligation (n = 7). DAPI: to stain the nucleus; EL: elastic lamina. (E) Representative H&E and Oil red O staining and quantification of atherosclerosis in common carotid arteries from mice with treatments as described in A to D. Arterial specimens were harvested at 4 wk after ligation (n = 7). Data are presented as mean ± SEM, *P < 0.05 by the Kruskal–Wallis test followed by Dunn’s test. (F) Representative immunofluorescent staining of APEX1 in carotid arteries from Apex1WT or Apex1ECKO mice receiving tamoxifen to verify Apex1-depletion. (G) Schematic diagram of experimental design. (H–J) Representative immunofluorescence staining of SELE (H), VCAM1 (I), and ICAM1 (J) in cross-sections of the left common carotid arteries from the Apex1WT or Apex1ECKO mice. Arterial specimens were harvested at 1-wk after ligation. (K) Representative H&E and Oil red O staining and quantification of neointima in the left common carotid arteries from the Apex1WT or Apex1ECKO mice. (L) Representative immunofluorescent staining of F4/80 indicative of monocyte infiltration in the left common carotid arteries from the Apex1WT or Apex1ECKO mice. Arterial specimens in K and L were harvested at 4-wk after ligation (n = 7–11). *P < 0.05 by the Mann–Whitney test. L: lumen.
Endothelial-Specific Deletion of Apex1 Ameliorates the Disturbed Flow-Accelerated Atherosclerosis.
To further assess the role of endothelial Apex1 in atherosclerosis, we generated inducible EC-specific Apex1-deletion (Apex1ECKO) and the control (Apex1WT) mice (SI Appendix, Fig. S15A). Immunostaining of the arterial cross-sections from the Apex1ECKO mice receiving tamoxifen (1.5 mg daily for 5 d) confirmed that Apex1 expression was undetectable in the ECs (Fig. 7F). The body weight, serum lipid level, and blood pressure were comparable between the Apex1WT and Apex1ECKO mice (SI Appendix, Fig. S15 B–D). These mice were injected once with adeno-associated-virus-9 (AAV9)-overexpressing PCSK9 (AAV9-PCSK9) via the tail vein and were fed on a Western diet. Partial ligation of the left carotid artery was performed (Fig. 7G). At 1-wk postsurgery, depletion of Apex1 was found to ameliorate the disturbed flow-induced EC expressions of SELE, VCAM1, and ICAM1 (Fig. 7 H–J). At 4-wk postligation, the Apex1WT mice exhibited marked neointimal formation, which was markedly inhibited in the Apex1ECKO mice (Fig. 7K). Scattered deposition of lipid shown by Oil red O staining and monocyte infiltration indicated by F4/80 signals were observed in the Apex1WT mice, but these changes were largely reduced or undetectable in the Apex1ECKO mice (Fig. 7 K and L). Taken together, these findings suggest that endothelial Apex1 positively contributes to atherogenesis.
Discussion
APEX1 plays a central role in the repair of oxidized and alkylated bases in mammalian genomes by acting as a nuclease in the base excision repair pathway (33). It not only functions as a redox effector for several redox-sensitive transcription factors, but also as a direct transacting factor (34). The role of APEX1 in vascular inflammation and atherosclerosis remains elusive. There is evidence that the expression levels of Apex1 in plasma and EC/macrophage were highly correlated with vascular and systemic inflammation in ApoE−/− mice and that ApoE−/− mice fed on a Western diet exhibited significantly increased their expression of APEX1 in macrophages and foam cells (35, 36), suggesting a positive correlation between APEX1 expression and atherosclerosis. However, there were also contradictory in vitro findings showing that the intracellular and secretory APEX1 might play an antiinflammatory role in vascular ECs (6, 7). Studies on animals with endothelial-specific depletion of APEX1 may provide critical insights to resolve this issue. Data obtained from the present study, by using mice with EC-specific Apex1 deletion and with intraluminal overexpression of APEX1, support the notion that endothelial APEX1 plays a proatherogenic rather than an antiatherogenic role, in the context of flow-regulation of endothelial function. We discovered a role for APEX1 in promoting the atheroprone phenotypes of vascular endothelium under disturbed flow. Mechanistically, we found that the OS-induced expressions of proinflammatory molecules (SELE, VCAM1, and ICAM1) were mediated by APEX1 through the NF-κB pathway. Our findings demonstrate that the APEX1-mediated endothelial inflammation and dysfunction serve as critical pathophysiological signatures and mechanisms in the disturbed flow-stimulated atherosusceptible endothelium.
By using CMap-based drug screening and expression profiling, we have demonstrated the effects of vitexin on shear-sensitive gene expression. Subsequent functional and mechanistic investigations revealed that vitexin alleviated the endothelial proinflammatory responses to OS and the proinflammatory cytokine TNF-α. Furthermore, in vivo administration of vitexin attenuated the disturbed flow-induced neointimal formation and atherosclerosis. There have been reports on the inhibition of proinflammatory cytokines and ROS production in ECs by vitexin (37, 38) and the use of vitexin in protecting against myocardial dysfunction including cardiac hypertrophy, myocardial infarction, and ischemia/reperfusion injury (39–41), but these studies did not identify the molecules directly targeted by vitexin, nor the comprehensive mechanistic pathways in ECs. It is to be emphasized that the present study is unique in reporting the antiatherogenic effects of vitexin.
In developing potential drug molecules, a determinant of the quality and effectiveness is their ability to engage the intracellular specific targets (42). Our results identified vitexin as a direct inhibitor of APEX1 in ECs through utilization of a target engagement assay (CETSA) coupled with biophysical characterization (SPR). Using CETSA, we found that addition of vitexin stabilized the thermally induced aggregation of APEX1. We applied the SPR assay for the kinetic analysis of the interactions of vitexin with APEX1. The SPR method was also employed to interrogate the action of an established APEX1 inhibitory compound, E3330, and the results demonstrated an interaction of E3330 with APEX1. The pharmacological characterization links the endothelial protective activity of vitexin with the proinflammatory role of APEX1, thus providing a theoretical basis for the utilization of vitexin in antiinflammatory therapy in atherosclerosis. Prior to this work, the coupling of CETSA and SPR had been applied to identifying antimyocardial fibrosis compounds (43), but not to antiatherosclerotic drug screening.
APEX1 can be posttranslationally modified via acetylation on its critical lysine residues, such as K6, K7, K27, K31, K32, and K35 (44, 45). Acetylation of APEX1 prevents its proteolysis and enhances the activity (46, 47). Another major finding of the present study is the demonstration that OS and TNF-α induce nuclear translocation of APEX1, and the induction is very likely through enhancing the binding of acetyltransferase p300 to APEX1 to lead to its acetylation. Our experiments reveal that the association between p300 and APEX1, as well as the subsequent acetylation and nuclear translocation, could be disrupted by vitexin. It is to be noted that exclusive nuclear accumulation of acetylated APEX1 has been found in lung fibroblasts and lung adenocarcinoma cells (48). In kidney cells, treatment with histone deacetylases inhibitor trichostatin A caused a nuclear-to-cytoplasmic translocation of APEX1 (44). These phenomena have not been documented in ECs prior to the present study. Although the underlying mechanisms regarding the enzymes responsible for catalyzing APEX1 acetylation or deacetylation remain to be elucidated, one possibility is the increased binding of APEX1 by acetyltransferases, such as p300 (34). Despite that the mechanotransduction of shear stress in ECs is still not completely understood (4, 49), p300 was indicated a mechano-sensitive protein, as shear-regulated changes in its phosphorylation or acetyltransferase activity has been shown in ECs (26, 50). Our study resolved the questions of whether and how p300 responds to distinct shear patterns by showing that OS (in contrast to PS) elevated p300 phosphorylation and promoted its binding to APEX1 in ECs. However, there is also the possibility that OS may promote APEX1 acetylation via p300-independeent pathways, since direct interaction between APEX1 and HDAC1 has been found and this interaction facilitates the recruitment of HDAC1 to the promoter regions of the APEX1-targeted genes (51).
In summary, our results established that inhibition of endothelial APEX1 by vitexin is an approach for antiinflammation therapies that target the disturbed flow-induced atheroprone EC phenotype and the associated atherogenesis. We propose a mechanism that disturbed shear stress promotes phosphorylation of p300 to induce its binding to APEX1 to lead to the acetylation of APEX1, and that the acetylated APEX1 forms complexes with p50 and p65 NF-κB, translocates into the nucleus, and facilitates the NF-κB signaling to activate the expressions of proinflammatory molecules. This mechanism suggests a critical role of APEX1 in mediating the regulation of endothelial function/dysfunction by hemodynamic forces. We have demonstrated an antiinflammatory role of vitexin on vasculature and identified APEX-1 as one of its directly targeted molecules within EC. Further investigations on discovery of potential multiple direct targets of vitexin and functional pathways downstream of APEX1 might provide valuable insights into the pharmacodynamics and validate vitexin as a drug for the prevention of arteriosclerotic cardiovascular diseases.
Materials and Methods
An extended section with detailed information on materials, reagents, and procedures is provided in SI Appendix. HUVECs were isolated from umbilical cords from healthy patients after full-term deliveries. De-identified umbilical cords were obtained with the agreement of the patients and approved by the Peking University People’s Hospital Medical Ethics Committee (2015PHB024). All animal studies were performed in accordance with the approved protocol of the Animal Care and Use Committee of Peking University and approved by the Ethics Committee of Peking University Health Science Center (LA2019262).
Supplementary Material
Acknowledgments
This work was funded by the National Natural Science Foundation of China (Projects 91939302, 81974052, 91949112, and 81921001) and US NIH (Grants HL-108735 and HL-196579).
Footnotes
Reviewers: M.G., Charité University Hospital; and K.Y., University of Tokyo.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2115158118/-/DCSupplemental.
Data Availability
All the raw sequencing data have been deposited at National Center for Biotechnology Information Sequence Read Archive, https://www.ncbi.nlm.nih.gov/sra (BioProject ID PRJNA772796). All other study data are included in main text and SI Appendix.
References
- 1.Davies P. F., Endothelial transcriptome profiles in vivo in complex arterial flow fields. Ann. Biomed. Eng. 36, 563–570 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Chien S., Effects of disturbed flow on endothelial cells. Ann. Biomed. Eng. 36, 554–562 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando J., Yamamoto K., Vascular mechanobiology: Endothelial cell responses to fluid shear stress. Circ. J. 73, 1983–1992 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Chiu J. J., Chien S., Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 91, 327–387 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M., Wilson D. M. 3rd, Human apurinic/apyrimidinic endonuclease 1. Antioxid. Redox Signal. 20, 678–707 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim C. S., et al. , Apurinic/apyrimidinic endonuclease1/redox factor-1 inhibits monocyte adhesion in endothelial cells. Cardiovasc. Res. 69, 520–526 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Park M. S., et al. , Secreted APE1/Ref-1 inhibits TNF-α-stimulated endothelial inflammation via thiol-disulfide exchange in TNF receptor. Sci. Rep. 6, 23015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uddin M. A., Akhter M. S., Siejka A., Catravas J. D., Barabutis N., P53 supports endothelial barrier function via APE1/Ref1 suppression. Immunobiology 224, 532–538 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B., Guan D., Cui Z. J., Wang X., Shen X., Thioredoxin 1 downregulates MCP-1 secretion and expression in human endothelial cells by suppressing nuclear translocation of activator protein 1 and redox factor-1. Am. J. Physiol. Cell Physiol. 298, C1170–C1179 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Huang T. S., et al. , LINC00341 exerts an anti-inflammatory effect on endothelial cells by repressing VCAM1. Physiol. Genomics 49, 339–345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb J., et al. , The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science 313, 1929–1935 (2006). [DOI] [PubMed] [Google Scholar]
- 12.He M., et al. , A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 115, 74–85 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Gimbrone M. A. Jr., García-Cardeña G., Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pichavaram P., Mani A. M., Singh N. K., Rao G. N., Cholesterol crystals promote endothelial cell and monocyte interactions via H2O2-mediated PP2A inhibition, NFκB activation and ICAM1 and VCAM1 expression. Redox Biol. 24, 101180 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X., et al. , Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 481, 63–70 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Nam D., et al. , Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 297, H1535–H1543 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickel J., et al. , SuperPred: Update on drug classification and target prediction. Nucleic Acids Res. 42, W26−W31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rai G., et al. , “Small molecule inhibitors of the human apurinic/apyrimidinic endonuclease 1 (APE1)” in Probe Reports from the NIH Molecular Libraries Program (National Center for Biotechnology Information, Bethesda, MD, 2010). [Google Scholar]
- 19.Martinez Molina D., et al. , Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Dziekan J. M., et al. , Identifying purine nucleoside phosphorylase as the target of quinine using cellular thermal shift assay. Sci. Transl. Med. 11, eaau3174 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Karlsson R., Michaelsson A., Mattsson L., Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J. Immunol. Methods 145, 229–240 (1991). [DOI] [PubMed] [Google Scholar]
- 22.Choi S., Joo H. K., Jeon B. H., Dynamic regulation of APE1/Ref-1 as a therapeutic target protein. Chonnam Med. J. 52, 75–80 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angkeow P., et al. , Redox factor-1: An extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 9, 717–725 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Roychoudhury S., et al. , Endogenous oxidized DNA bases and APE1 regulate the formation of G-quadruplex structures in the genome. Proc. Natl. Acad. Sci. U.S.A. 117, 11409–11420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lirussi L., et al. , Nucleolar accumulation of APE1 depends on charged lysine residues that undergo acetylation upon genotoxic stress and modulate its BER activity in cells. Mol. Biol. Cell 23, 4079–4096 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takai J., et al. , Laminar shear stress upregulates endothelial Ca2+-activated K+ channels KCa2.3 and KCa3.1 via a Ca2+/calmodulin-dependent protein kinase kinase/Akt/p300 cascade. Am. J. Physiol. Heart Circ. Physiol. 305, H484–H493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G., et al. , Laminar shear stress alters endothelial KCa2.3 expression in H9c2 cells partially via regulating the PI3K/Akt/p300 axis. Int. J. Mol. Med. 43, 1289–1298 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voelkl J., et al. , SGK1 induces vascular smooth muscle cell calcification through NF-κB signaling. J. Clin. Invest. 128, 3024–3040 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sardar Pasha S. P. B., et al. , Ref-1/APE1 inhibition with novel small molecules blocks ocular neovascularization. J. Pharmacol. Exp. Ther. 367, 108–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ando K., et al. , A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 36, 4327–4336 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H. M., et al. , Apurinic/apyrimidinic endonuclease 1 is a key modulator of keratinocyte inflammatory responses. J. Immunol. 183, 6839−6848 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Wang T., Zhang X., Li J. J., The role of NF-kappaB in the regulation of cell stress responses. Int. Immunopharmacol. 2, 1509–1520 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Kuninger D. T., Izumi T., Papaconstantinou J., Mitra S., Human AP-endonuclease 1 and hnRNP-L interact with a nCaRE-like repressor element in the AP-endonuclease 1 promoter. Nucleic Acids Res. 30, 823–829 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhakat K. K., Mantha A. K., Mitra S., Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein. Antioxid. Redox Signal. 11, 621–638 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y. R., et al. , Plasma APE1/Ref-1 correlates with atherosclerotic inflammation in ApoE−/− mice. Biomedicines 8, E366 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai J., et al. , Role of redox factor-1 in hyperhomocysteinemia-accelerated atherosclerosis. Free Radic. Biol. Med. 41, 1566–1577 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Cao H., Wang X., Zhang B., Ren M., The protective effect of vitexinin septic encephalopathy by reducing leukocyte-endothelial adhesion and inflammatory response. Ann. Palliat. Med. 9, 2079–2089 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Zhang S., et al. , Vitexin alleviates ox-LDL-mediated endothelial injury by inducing autophagy via AMPK signaling activation. Mol. Immunol. 85, 214–221 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Lu C. C., et al. , Vitexin protects against cardiac hypertrophy via inhibiting calcineurin and CaMKII signaling pathways. Naunyn Schmiedebergs Arch. Pharmacol. 386, 747–755 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Ashokkumar R., Jamuna S., Sakeena Sadullah M. S., Niranjali Devaraj S., Vitexin protects isoproterenol induced post myocardial injury by modulating hipposignaling and ER stress responses. Biochem. Biophys. Res. Commun. 496, 731–737 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Dong L., Fan Y., Shao X., Chen Z., Vitexin protects against myocardial ischemia/reperfusion injury in Langendorff-perfused rat hearts by attenuating inflammatory response and apoptosis. Food Chem. Toxicol. 49, 3211−3216 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Page B. D. G., et al. , Targeted NUDT5 inhibitors block hormone signaling in breast cancer cells. Nat. Commun. 9, 250 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan Y. J., et al. , Protocatechualdehyde reduces myocardial fibrosis by directly targeting conformational dynamics of collagen. Eur. J. Pharmacol. 855, 183–191 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Choi S., et al. , Histone deacetylases inhibitor trichostatin A modulates the extracellular release of APE1/Ref-1. Biochem. Biophys. Res. Commun. 435, 403–407 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Busso C. S., Lake M. W., Izumi T., Posttranslational modification of mammalian AP endonuclease (APE1). Cell. Mol. Life Sci. 67, 3609–3620 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhakat K. K., et al. , Regulation of limited N-terminal proteolysis of APE1 in tumor via acetylation and its role in cell proliferation. Oncotarget 7, 22590–22604 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sengupta S., et al. , Elevated level of acetylation of APE1 in tumor cells modulates DNA damage repair. Oncotarget 7, 75197–75209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roychoudhury S., et al. , Human apurinic/apyrimidinic endonuclease (APE1) is acetylated at DNA damage sites in chromatin, and acetylation modulates its DNA repair activity. Mol. Cell. Biol. 37, e00401-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto K., Nogimori Y., Imamura H., Ando J., Shear stress activates mitochondrial oxidative phosphorylation by reducing plasma membrane cholesterol in vascular endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 117, 33660–33667 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen W., Bacanamwo M., Harrison D. G., Activation of p300 histone acetyltransferase activity is an early endothelial response to laminar shear stress and is essential for stimulation of endothelial nitric-oxide synthase mRNA transcription. J. Biol. Chem. 283, 16293–16298 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhakat K. K., Izumi T., Yang S. H., Hazra T. K., Mitra S., Role of acetylated human AP-endonuclease (APE1/Ref-1) in regulation of the parathyroid hormone gene. EMBO J. 22, 6299–6309 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the raw sequencing data have been deposited at National Center for Biotechnology Information Sequence Read Archive, https://www.ncbi.nlm.nih.gov/sra (BioProject ID PRJNA772796). All other study data are included in main text and SI Appendix.