Abstract
Endometrial cancer (EC) is a highly obesity-driven cancer, with limited treatment options. ONC201 is an imipridone that selectively antagonizes the G protein-coupled receptors dopamine receptor D2 and D3 (DRD2/3) and activates human mitochondrial caseinolytic protease P (ClpP). It is a promising first-in-class small molecule that has been reported to have anti-neoplastic activity in various types of cancer through induction of the integrated stress response (ISR) as well as through stimulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and subsequent induction of apoptosis. ONC201 is being evaluated in Phase II clinical trials for solid tumors and hematological malignancies, including EC. ONC206 is an analog of ONC201 with nanomolar potency in Phase I clinical trials. This study evaluated the anti-tumor efficacy of ONC206 in EC cell lines and the Lkb1fl/flp53fl/fl genetically engineered mouse model of endometrioid EC. ONC206 revealed greater potency than ONC201 in the inhibition of proliferation in EC cell lines, with IC50 concentration ranges of 0.21-0.32 µM for ONC026 versus 2.14-3.53 µM for ONC201. ONC206 induced cellular stress, apoptosis and cell cycle G1 arrest, accompanied by inhibition of the AKT/mTOR/S6 pathways in EC cells. Diet-induced obesity accelerated tumor growth in Lkb1fl/flp53fl/fl mice. ONC206 inhibited EC tumor size and weight in both obese and lean mice after 4 weeks of treatment. Treatment with ONC206 led to a decrease in expression of Ki67, BCL-XL and phosphorylation of S6, as well as an increase in ClpP in endometrial tumors under both obese and lean conditions. Overall, the pre-clinical efficacy of ONC206 is promising and worthy of further exploration in clinical trials for endometrioid EC.
Keywords: ONC206, endometrial cancer, dopamine receptors, apoptosis, obesity
Introduction
Endometrial cancer (EC) is known to be the most commonly diagnosed gynecologic malignancy among women, with an estimated 62,500 new cases and 12,200 deaths projected in the United States in 2020 [1]. As an obesity-driven cancer, the obesity epidemic has directly contributed to the escalating prevalence of EC [1,2]. The majority of women with EC will present with early-stage disease and endometrioid histologic subtype, resulting in an excellent 5-year survival of greater than 85% [3]. However, 10-15% of patients with early-stage disease will experience recurrences. For women with advanced or recurrent EC, overall survival is poor with 5-year survival rates of 10-57% [4,5]. Few effective treatment strategies are available for late-stage and recurrent disease. Therefore, more novel therapies are desperately needed for this disease.
Dopamine receptor D2 (DRD2) is a G protein-coupled receptor that is overexpressed in several cancers, including EC [6,7]. DRD2 has a significant impact on several important signaling pathways that are involved in cell proliferation, apoptosis, angiogenesis, migration and autophagy in cancer cells [6,8-10]. Increasing evidence shows that inhibition of DRD2 through pharmacologic approaches using DRD2 antagonists reduces cancer cell proliferation and induces apoptosis in vitro and in vivo. ONC201 is a first-in-class small molecule DRD2/3 antagonist and human mitochondrial caseinolytic protease P (ClpP) agonist that induces the integrated stress response (ISR) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) pathways in a p53-independent manner, exerting growth inhibitory effects in many types of cancers [11-14]. Our recent study has found that ONC201 exhibited potent antitumor activity in endometrial cancer cell lines and a transgenic mouse of endometrial cancer [15,16]. ONC201 is well tolerated with favorable pharmacokinetics and pharmacodynamics and has been shown to be clinically active in advanced solid tumors, including refractory metastatic EC patients [16,17].
ONC206 is a highly potent, orally bioavailable imipridone that induces ISR and TRAIL by selectively targeting DRD2/3 and ClpP and also exhibits a benign safety profile in pre-clinical models [18-20]. Nanomolar activity has been observed in pre-clinical models of uterine serous carcinoma and glioblastoma [19,21,22]. ONC206 has also demonstrated improved in vivo efficacy relative to ONC201 in uterine serous cancer [23]. Thus, we sought to evaluate the anti-tumorigenic effects of ONC206 in human EC cell lines and an LKB1fl/flp53fl/fl transgenic mouse model of endometrioid EC, using both obese and lean mice.
Methods
Cell culture and reagents
Two EC cell lines, ECC-1 and Ishikawa, were used for our experiments. The Ishikawa cells were grown in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 medium (DMEM/F12) supplemented with 10% fetal bovine serum (FBS). The ECC-1 cells were cultured in RPMI 1640 with 5% FBS. The cells were cultured in humidified 5% CO2 at 37°C. All media included 100 units/ml penicillin and 100 microgram/ml streptomycin. ONC206 was provided by Oncoceutics/Chimerix (Philadelphia, PA). The primary antibodies used in this study were as follows: myeloid leukemia cell differentiation protein (MCL-1), phosphorylated (phos)-S6, pan-S6, phos-AKT, pan-AKT, protein-like endoplasmic reticulum kinase (PERK), binding immunoglobulin protein (Bip), endoplasmic reticulum oxireduction 1 (Ero1), and cyclin dependent kinase 4 and 6 (CDK4 and CKD6) (Cell Signaling Technology, Beverly, MA), Secondary antibodies were horseradish-peroxidase conjugated and acquired from Sigma.
Cell proliferation assay
The ECC-1 and Ishikawa cells (3000-4000 cells/well) were plated in 96-well plates for 24 hours, and then treated with different doses of ONC206 for a period of 72 hours. For comparison, the cells were also treated with the same doses of ONC201 for a period of 72 hours. Following treatments, 5 ul of MTT (5 mg/ml, Sigma) was added to each well for 1 hour. After aspiration of medium, 100 µl DMSO per well was used to terminate the reactions. Absorbance was measured at 490 nm with a plate reader (Tecan, Morrisville, NC). All experiments were performed in triplicate, and the mean of the replicates was plotted. The effect of ONC206 on cell inhibition was calculated as a percentage of control cell growth.
Cell cycle assay
The effect of ONC206 on cell cycle progression was assessed using Cellometer (Nexcelom, Lawrence, MA). The ECC-1 and Ishikawa cells (2.5×105 cells/per well) were cultured with or without ONC206 for 24 hours. The cells were subsequently collected by 0.05% Trypsin (Gibco/Thermo Scientific, Waltham, MA), washed with PBS, and fixed in a 90% methanol solution. On the day of analysis, the cells were re-suspended in RNA A solution for 30 min at 37°C, and then stained with PI staining solution for 10 min in the dark. Cell cycle progression was analyzed by Cellometer and analyzed by the FCS 4 Express Flow Cytometry Software (De Novo Software, Glendale, CA). Each experiment was performed in duplicate and repeated twice.
Apoptosis assay
Apoptotic cells were quantified by the Annexin-V FITC Apoptosis Detection Kit (BioVision, Mountain View, CA). Briefly, ECC-1 and Ishikawa cells were seeded into 6 well plates at 2.5×105 cells/well overnight, and then the cells were treated with different doses of ONC206 for 24 hours. The cells were harvested by 0.25% Trypsin and stained in 100 ul of Annexin-V and PI dual-stain solution for 15 min. The expression of Annexin V was detected by Cellometer, and analyzed by FCS 4 software. Apoptotic cells were expressed as a percentage of the total number of cells stained.
Reactive oxygen species (ROS) assay
The alteration of total production of reactive oxygen species was measured using a DCFH-DA fluorescent dye [24]. The ECC-1 and Ishikawa cells (8000-12,000 cells/well) were seeded in black 96-well plates. After 24 hours, the cells were treated with ONC206 (0.1 μM-5 μM) for 4 hours to induce ROS generation. Cells were incubated with DCFH-DA (20 μM) for 30 minutes. Using a Tecan plate reader, fluorescence was monitored at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. All experiments were performed at least twice to assess for consistency.
Western immunoblotting
The ECC-1 and Ishikawa cells were plated at 2.5×105 cells/well in six well plates in their appropriate media. They were treated for 24 hours with ONC206. Cell lysates were prepared in RIPA buffer (1% NP40, 0.5 sodium deoxycholate and 0.1% SDS) plus PhosStop. The BCA protein assay (Thermo Fisher Scientific, Waltham, MA) was used to determine the protein concentrations. Equal amounts of cell lysates were loaded in 10-12% SDS-PAGE gel and transferred onto a PVDF membrane. The membrane was blocked with 5% nonfat dry milk and then probed by primary antibodies overnight at 4°C. The immunoblots were washed with TBS-T and incubated with the appropriate secondary antibody for 1 hour. Antibody binding was detected by SuperSignal™ West Pico (Thermo Fisher Scientific) and analyzed using ChemiDoc™ Image System (Bio-Rad, Hercules, CA). Each experiment was repeated three times to assess for consistency.
Lkb1fl/flp53fl/fl mouse model of endometrioid EC
The Lkb1fl/flp53fl/fl mouse model is an endometrioid EC mouse model that conditionally knock-outs the tumor suppressor genes, Lkb1 and p53 [25]. Animal protocols for this study were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee (IACUC). To evaluate ONC206’s in vivo effects in obese and lean mice, mice were placed on either a low fat diet (LFD; 10% calories from fat) or a high fat diet (HFD; 60% calories from fat, Research Diets, New Brunswick, NJ), starting at 3 weeks of age. Recombinant adenovirus Ad5-CMV-Cre (AdCre) was purchased from the University of Iowa Transfer Vector Core at a titer of 1011-1012 infectious particles/ml. Intrauterine Ad-Cre injections of Lkb1fl/flp53fl/fl mice were performed at 6-8 weeks of age to induce EC. The LFD (lean) and HFD (obese)-fed mice (N=15 mice per group) were treated with the vehicle (PBS+20%DMSO) or ONC206 (125 mg/kg, weekly, oral gavage), starting 8 weeks after tumor induction. Mice were weighed weekly throughout the study. All mice were euthanized after 4 weeks of ONC206 or vehicle treatment. At sacrifice, endometrial tumors and blood samples were harvested and stored at -80°C until use.
Immunohistochemistry
Five sections of 5-mm-thick paraffin embedded blocks from Lkb1fl/flp53fl/fl mouse tumors were prepared. IHC staining was performed at the IHC Mice Core Facility at UNC-CH. The primary antibodies against Ki-67 (Cell Signaling, Beverly, MA, 1:800), DRD5 (Santa Cruz, Santa Cruz, CA, 1:300), BCL-XL (Cell Signaling, 1:1200) and phos-S6 (Cell Signaling, 1:800) were used in this study. Motic was used to scan the IHC slides. The results of IHC were analyzed by ImagePro software (Vista, CA).
Statistical analysis
Data are reported as the mean ± SD. Statistical tests and graphs were generated using GraphPad Prism 8 software. An unpaired Student’s t test was used for comparisons between groups. Tumor growth in vehicle and ONC206 treatment arms was analyzed by the One-way & Two-way ANOVA test. A P value of <0.05 was considered as a statistically significant difference.
Results
ONC206 inhibits cell proliferation in EC cells and exhibits greater potency compared to ONC201
The effect of ONC201 and ONC206 on EC cell proliferation was assessed by MTT assay. The EC cell lines, ECC-1 and Ishikawa, were treated with different doses of ONC201 and ONC206 for 72 hours. ONC201 and ONC206 inhibited cell proliferation of both EC cell lines in a dose-dependent manner, as demonstrated in Figure 1A. The mean IC50 value of ONC201 for ECC-1 and Ishikawa was 2.14 and 3.53 uM, respectively. The mean IC50 value of ONC 206 for ECC-1 and Ishikawa was 0.21 and 0.32 uM, respectively. These results confirm that ONC206 is a more potent analog of ONC201 in inhibition of cell proliferation in EC cells (P<0.01). Because ONC206 selectively antagonizes DRD2/3 and ONC201 exhibits p53-independent cytotoxicity through TRAIL and death receptor 5 (DR5) induction in uterine serous carcinoma cells [16], we examined whether treatment of ONC206 regulates the expression of DRD2 and DR5 in the ECC-1 and Ishikawa cell lines. Western blotting results showed that ONC206 significantly up-regulated the expression of DRD2 and DR5 proteins after 24 hours of treatment in the ECC-1 and Ishikawa cells (Figure 1B). These results indicate that ONC201 and ONC206 effectively reduces cell proliferation in EC cells through induction of DRD2 and DR5, with ONC206 exhibiting 14-20 times greater potency.
Figure 1.
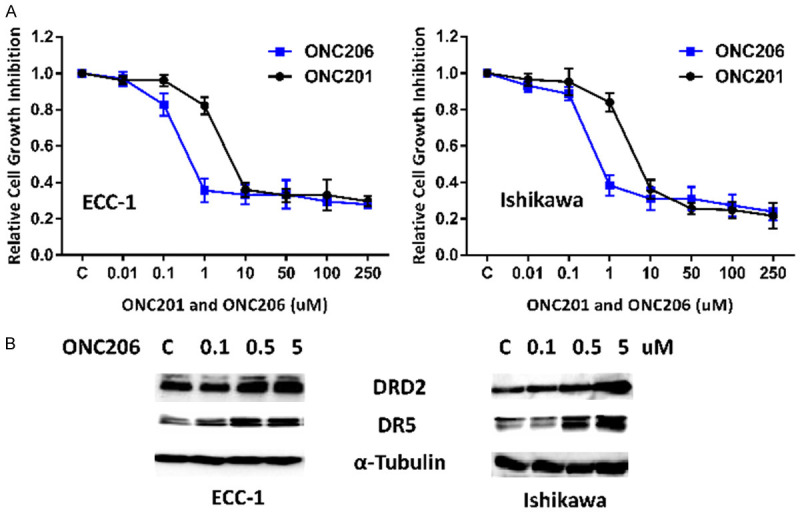
Effect of ONC206 and ONC201 on cell proliferation in EC cells. The ECC-1 and Ishikawa cell lines were cultured in the presence of varying concentrations of ONC206 and ONC201 for 72 hours. Cell proliferation was determined by MTT assay. Based on the IC50 dose comparison, ONC206 demonstrated 14-20 times greater potency when compared to ONC201 (A). Western blotting results indicated that ONC206 induced the expression of DRD2 and DR5 in EC cells after 24 hours of treatment (B).
ONC206 induces cell cycle arrest in EC cells
The cell cycle profile was analyzed after treating the ECC-1 and Ishikawa cell lines with varying doses (0.1-5.0 µM) of ONC206 for 24 hours. As shown in Figure 2A, ONC206 caused G0/G1 cell cycle arrest and reduced S phase and G2 phase in the ECC-1 and Ishikawa EC cell lines in a concentration-dependent manner. G1 arrest increased from 36% in control cells to 57% in the 5 uM ONC206-treated ECC-1 cells and 43% to 57% in the Ishikawa cells. Additionally, cell cycle-related proteins were analyzed by western blotting in the ECC-1 and Ishikawa cell lines treated with ONC206 for 24 hours. The CDK4 and CDK6 proteins are responsible for the progression through G1 and promote G1/S phase transition. We found that ONC206 significantly decreased CDK4 and CDK6 expression in both cell lines (Figure 2B).
Figure 2.
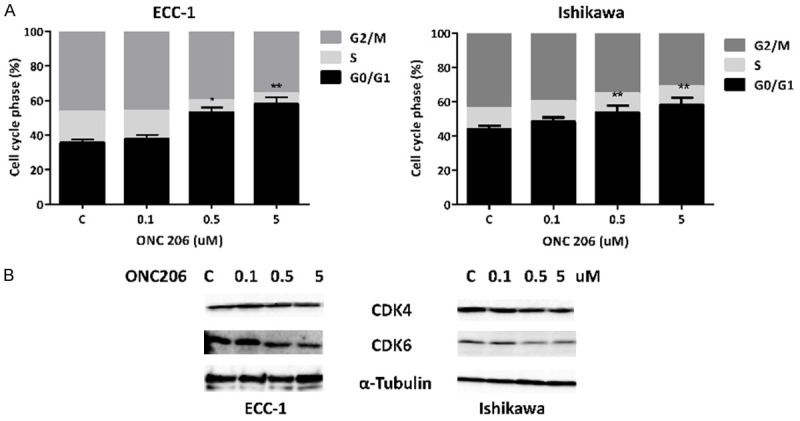
Effect of ONC206 on cell cycle progression in EC cells. The ECC-1 and Ishikawa cells were treated with ONC206 at varying doses for 24 hours. Changes in cell cycle progression were analyzed by Cellometer. ONC206 induced G0/G1 cell cycle arrest and reduced S phase and G2 phase in both EC cell lines (A). Western blotting results showed that ONC206 decreased the expression of CDK4 and CDK6 after 24 hours of treatment (B) (*P<0.05, **P<0.01).
ONC206 induces cellular stress in EC cells
ROS have been known to be a component of the cellular response to stress. In order to determine the presence of oxidative stress as part of the anti-tumorigenic effect of ONC206, we measured cellular ROS products using the DCF-DA assay in both EC cell lines. As shown in Figure 3A, ONC206 (0.1-5.0 uM) robustly induced ROS levels in a concentration-dependent manner in the ECC-1 and Ishikawa cell lines after 4 hours of treatment. Treatment with ONC206 (5 μM) significantly induced ROS production by 0.43 fold in ECC-1 cells and 0.31 fold in Ishikawa cells compared to control (P<0.01). We next detected the changes of endoplasmic reticulum (ER) stress-related markers using western blotting after treatment with ONC206 in the EC cell lines. The results of western blotting showed that ONC206 significantly induced the protein expression of PERK, Bip and Ero1-Lα in a dose dependent manner in ECC-1 and Ishikawa cells (Figure 3B). These results imply that the rise in intracellular ROS levels is involved in the anti-proliferative effects of ONC206 in EC cells.
Figure 3.
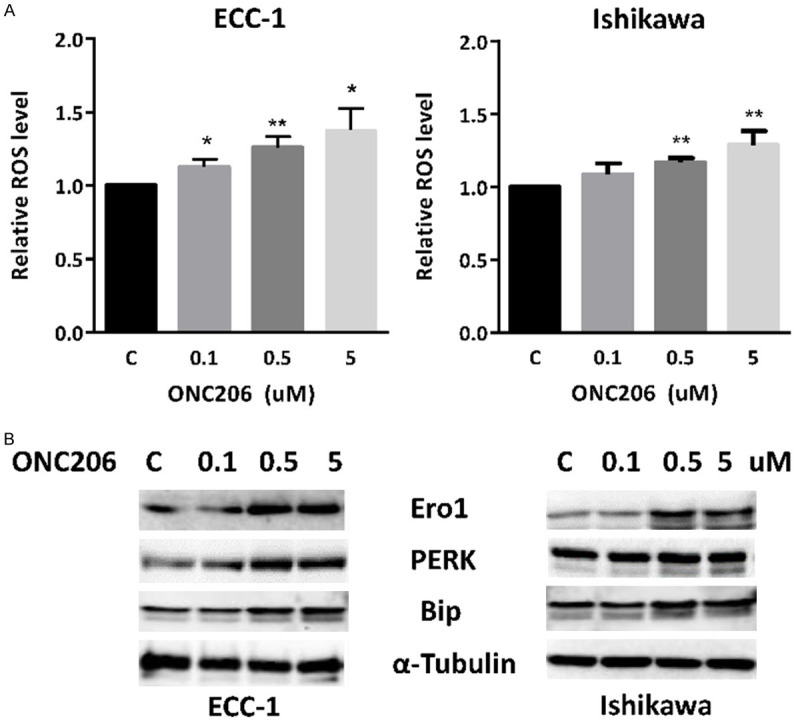
ONC206 induces cellular stress in EC cell lines. The ECC-1 and Ishikawa cell lines were treated with ONC206 at the indicated doses for 4 hours. ROS was assessed by DCFDA assay. ONC206 induced cellular ROS production in a dose-dependent manner in both cell lines (A). Western blotting results showed that ONC206 increased expression of the cellular stress proteins Ero1, Perk and Bip in both EC cell lines after 24 hours of treatment (B). (*P<0.05, **P<0.01).
ONC206 decreases tumor growth in a mouse model of endometrioid EC
To validate the anti-tumorigenic potential of ONC206 in vivo, we used the Lkb1fl/flp53fl/fl mouse model of endometrioid EC under obese and lean conditions [25]. Obese and lean conditions were both assessed in mice, as EC is a highly obesity-driven cancer. The mice were divided into four groups (N=15 mice per group), including LFD (lean) and HFD (obese) groups treated with either ONC206 (125 mg/kg, weekly, oral gavage) or vehicle (PBS+20%DMSO). The initial average body weight of the obese mice when starting treatment with ONC206 was 35.1 gram, while that of the lean mice was only 26.2 gram (Figure 4A, P<0.01), which HFD-fed mice weighing 25.4% more than LFD-fed mice. Tumor weights were significantly increased in HFD-fed mice compared to LFD-fed mice, consistent with our prior work that obesity promotes tumor growth in Lkb1fl/flp53fl/fl mice [15,26]. In the obese mice, tumor weight decreased by 72.0% (P<0.01) with ONC206 treatment when compared with the obese control group. Among the lean mice, tumor weight decreased by 67.3% (P<0.01) after treatment with ONC206 when compared with control-treated animals (Figure 4B). Similarly, ONC206 significantly reduced tumor sizes in obese and lean in Lkb1fl/flp53fl/fl mice after 4 weeks of treatment (Supplementary Figure 1). The mice demonstrated tolerance to ONC206 and showed normal activities during the treatment. Regular weekly measurements yielded no change in body weight.
Figure 4.
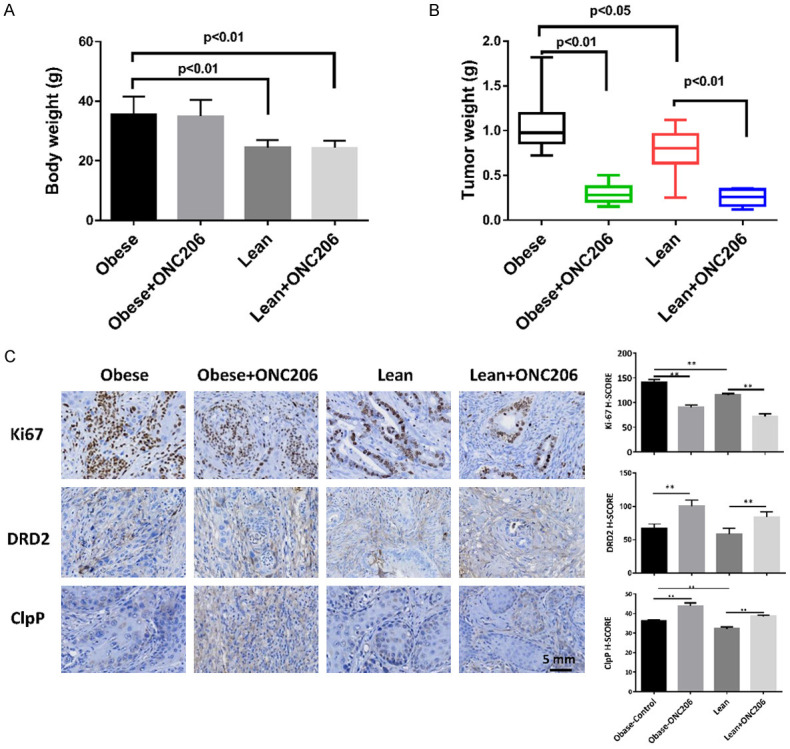
ONC206 significantly decreased tumor weight in the Lkb1fl/flp53fl/fl EC mouse model. Lkb1fl/flp53fl/fl mice were fed a HFD (obese) or LFD (lean) starting at 3 weeks of age. Diet-induced obesity significantly increased the body weights of Lkb1fl/flp53fl/fl mice (A). The mice were treated with ONC206 (125 mg/kg, oral gavage, weekly) or vehicle for 4 weeks, beginning 8 weeks after tumor induction. ONC206 significantly inhibited tumor weight under obese and lean conditions (B). IHC results showed that ONC206 treatment reduced the expression of Ki67 and increased the expression of DRD2 and ClpP in endometrial tumor tissues (C). (*P<0.05, **P<0.01).
After treatment with ONC206 or placebo, the protein expression of Ki-67, ClpP and DRD2 in the endometrial tumors was evaluated by IHC (Figure 4C). The expression of Ki-67 was significantly reduced by 35.8% and 37.9% in the obese and lean groups treated with ONC206 compared with the vehicle-treated mice, respectively (P<0.01). ONC206 induced DRD2 expression in the treated mice on a HFD by 32.1% and those on a LFD by 25.6%. ONC206 treatment increased ClpP expression by 21.1% in the obese group and by 19.7% in the lean group as compared to controls, suggesting that ONC206 is a potent activator of ClpP in vivo.
ONC206 induces apoptosis in vitro and in vivo
The effect of ONC206 on apoptosis was evaluated in EC cells using the Annexin V assay. ECC-1 and Ishikawa cell lines were treated with ONC206 at different concentrations (0.1-5.0 uM) for 24 hours. ONC206 robustly increased the percentage of apoptotic cells in a dose-dependent manner in both cell lines, with a twofold increase in ECC-1 cells (13.8% for 5 uM versus 5.9% for control) and Ishikawa cells (12.2% for 5 uM versus 6.4% for control, P<0.01, Figure 5A). The results of western immunoblotting showed that ONC206 decreased expression of the anti-apoptotic protein, MCL-1 and BCL-XL, after 24 hours of treatment (Figure 5B). To further assess the effect of ONC206 on apoptosis in the Lkb1fl/flp53fl/fl mouse model of EC, IHC was performed to measure the expression of BCL-XL in tumor tissues. The results showed that treatment with ONC206 significantly reduced the expression of BCL-XL in obese and lean mice compared to control mice (Figure 5C). Overall, these results suggest that ONC206 inhibits tumor growth through induction of apoptosis in vitro and in vivo.
Figure 5.
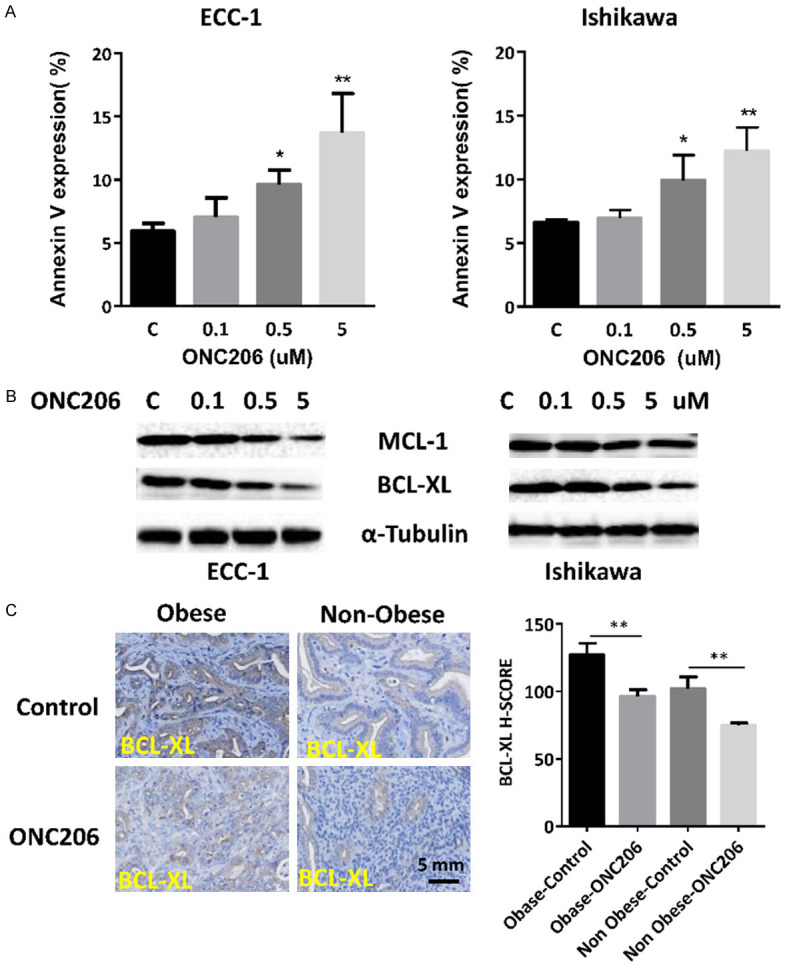
ONC206 induced apoptosis in vitro and in vivo. Cells were treated with ONC206 at the indicated doses for 24 hours and then analyzed for expression of Annexin V by Cellometer. ONC206 increased the expression of Annexin V in a dose-dependent manner in both cell lines (A). Western blotting results showed that ONC206 significantly reduced the expression of MCL-1 and BCL-XL in both cell lines (B). IHC results demonstrated that ONC206 decreased the expression of BCL-XL in endometrial tumor tissues (C). (*P<0.05, **P<0.01).
ONC206 inhibits mTOR/S6 pathway in vitro and in vivo
The effect of ONC206 on the mTOR pathway and downstream signaling targets in both cell lines was assessed using Western immunoblotting. The mTOR protein is a serine-threonine kinase and serves as a central regulator of cell metabolism, growth, proliferation and survival. Ribosomal protein S6 is a downstream target of the mTOR pathway, and phosphorylation of S6 is correlated with increased cell proliferation. Immunoblotting results demonstrate that ONC206 decreased phosphorylation of S6 in ECC-1 and Ishikawa cell lines with increasing drug concentration. Additionally, ONC206 increased phosphorylation of AKT at a dose of 5 uM in both cell lines (Figure 6A). To further confirm the effect of ONC206 on S6 in vivo, we analyzed the ONC206 and vehicle treated endometrial tumors from Lkb1fl/flp53fl/fl mice, via IHC staining for phosphorylated S6. Endometrial tumors from the obese and lean mice treated with ONC206 had significantly reduced phosphorylated S6 (34.5% in the obese group and 37.6% in the lean group), in comparison to vehicle-treated mice (Figure 6B). These results suggest that ONC206 inhibited EC cell proliferation and EC tumor growth through inhibition of mTOR/S6 pathway.
Figure 6.
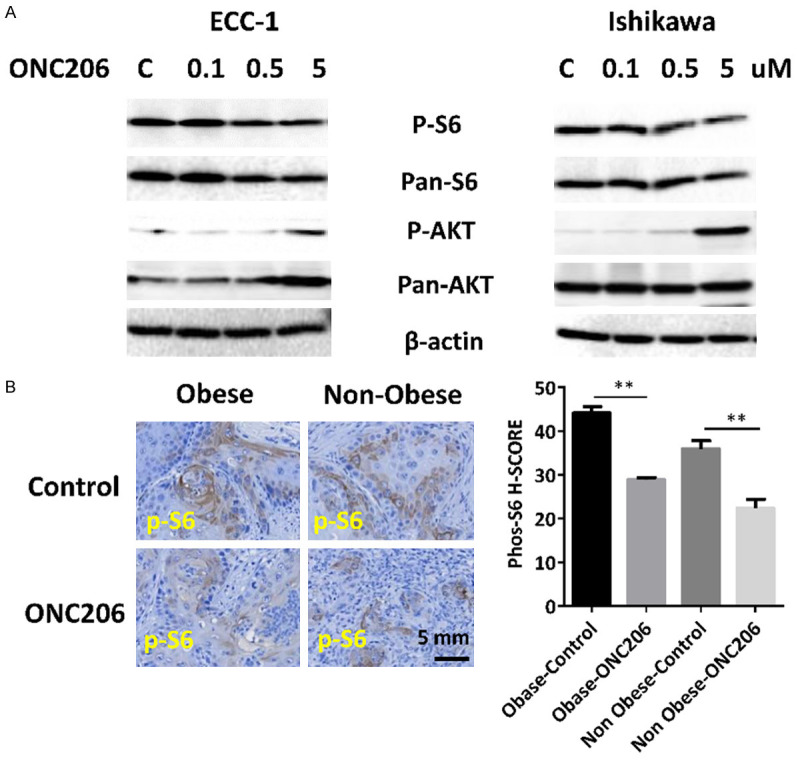
ONC206 inhibited mTOR/S6 pathway in vitro and in vivo. The ECC-1 and Ishikawa cell lines were treated with ONC206 at varying doses for 24 hours. Western blotting results demonstrate that ONC206 decreased phosphorylation of S6 and increased phosphorylation of AKT in both cell lines (A). Treatment with ONC206 for 4 weeks significantly decreased the expression of phosphorylated S6 in endometrial tumor tissues of Lkb1fl/flp53fl/fl mice (B).
Discussion
The incidence of EC is increasing annually, while the number of deaths attributed to EC are also escalating [2]. Between 1987 and 2008, there was a 50% increase in the incidence of EC and a 300% increase in associated deaths [27]. For patients with Stage III or IV disease and for those with recurrent disease, the prognosis remains poor with optimal treatment yet to be defined. Most recently, Matei et al. confirmed the role of cytotoxic therapy in advanced disease, but disease-free survival remains less than 60% just 5 years following adjuvant treatment [28]. For recurrent endometrial cancer, few treatments are approved by the Federal Drug Administration (FDA) and currently include immunotherapies and hormonal agents. Pembrolizumab demonstrated an objective response rate of 57% in the phase II KEYNOTE-158 trial, but only for endometrial tumors with high microsatellite instability, while the combination of megesterol acetate and tamoxifen was associated with only 27% objective response rate in phase II trial [29,30]. More efficacious therapeutic options are needed for patients with advanced and recurrent EC [31,32]. In this study, we delineated the anti-tumorigenic effects of ONC206 in human EC cell lines and a transgenic LKB1fl/flp53fl/fl mouse model of endometrioid EC under obese and lean conditions. We found that ONC206 exhibits its anti-tumorigenic activities via inhibition of the mTOR/S6 pathway as well as induction of apoptosis, cell cycle G1 arrest and cellular stress in EC cells. ONC206 demonstrated anti-tumor efficacy in both obese and lean mice after 4 weeks of treatment, co-incident with a decrease in expression of Ki67, phos-S6 and BCL-XL as well as an increase in ClpP and DRD2 in the EC tumor tissues. Thus, our study provides pre-clinical evidence for the potential benefit of ONC206 as an anti-cancer agent in endometrioid EC.
ONC201 is known to reduce cell proliferation via selectively and competitively antagonizing DRD2 and then inactivating Ras signaling and activating the ISR, ultimately inducing apoptosis and inhibiting cell growth [33-35]. In our previous study of ONC201 in uterine serous carcinoma cell lines, we found that the anti-proliferative activity of ONC201 was involved in induction of apoptosis, independent of p53 via both TRAIL- and mitochondrial-mediated apoptotic pathways in the ARK1 and SPEC-2 uterine serous cancer cell lines [16]. Similar results have been found in solid tumor and hematological malignancies [33,35-39]. ONC206 is a chemically modified derivative of ONC201 with anticipated enhanced efficacy. Recent studies suggested that ONC206 also activated similar signaling pathways as ONC201 including the ISR pathway leading to upregulation of DR5 and TRAIL, ultimately resulting in more potent anti-proliferative activity than ONC201 in glioblastoma and colon cancer cells [18,19]. To clarify the molecular mechanism of ONC206-induced inhibition of cell proliferation, we comprehensively investigated the effects of ONC206 on cell cycle, apoptotic, cellular stress and AKT/mOTR pathways. Inhibition of cell proliferation induced by ONC206 led to G1 cell cycle arrest and an increase in Annexin V expression in the EC cell lines, which was accompanied by increased cellular ROS and inhibited mTOR/S6 pathway inhibition in both cell lines. Similar effects were observed in our Lkb1fl/flp53fl/fl mouse model of endometrioid EC, which demonstrated that ONC206 reduced tumor growth via inhibition of the mTOR/S6 pathways and activation of both cellular stress and apoptotic pathways.
The PI3K/AKT/mTOR pathway is the most significantly altered pathway in EC [40]. Data from The Cancer Genome Atlas Program in 2013 demonstrated alterations in PI3KCA, PIK3R1, AKT1, and PTEN in 59.7%, 33%, 3.2% and 66% of EC cases, respectively [41]. Another important growth regulator is the Ras/MARK pathway, which interacts with the PI3K/AKT/mTOR pathway through RAS proteins, suggesting cooperation between the two pathways to produce functional outcomes [42,43]. In our previous work, ONC201 reduced phosphorylation of AKT and p42/44, while inducing AMPK activation in uterine serous cells in vitro, suggesting that the effect of ONC201 on cell growth may be related to the inhibition of the PI3K/AKT/mTOR and RAS/RAF/MEK pathways. In this study, effect of ONC206 on the mTOR/S6 pathway was assessed in vitro and in vivo. As a downstream target, phosphorylation of the protein S6 is correlated with increased cell proliferation. ONC206 demonstrated inhibition of protein S6 phosphorylation and thus, acts to inhibit cell proliferation and tumor growth.
Obesity is well known to increase the risk of EC, complicate clinical management strategies and worsen EC-specific mortality [2,44]. As such, we mimicked the clinically obese state of EC by feeding Lkb1fl/flp53fl/fl mice a HFD (as compared to a control LFD). Treatment with ONC206 in the obese mice appeared to have similar anti-tumor efficacy as that seen in lean mice, with 72% tumor reduction in the obese mice and 67.3% reduction in lean mice. Treatment was well-tolerated in both diet groups. Inhibition of endometrial tumor growth in both obese and lean mice was significantly associated with decreased expression of Ki-67, activated cellular stress and increased ClpP expression as well as induction of apoptosis and inhibition of targets of the mTOR/S6 pathway. These results support that ONC206 exhibits similar anti-tumorigenic mechanisms as compared to ONC201 in vitro and in vivo [16,38,45,46].
Two studies recently reported that ClpP is the molecular target that binds ONC201 in a direct and specific manner; and thus, the activation of ClpP was essential for cell death induced by ONC201 [13,14]. ClpP has an important role in mitochondrial protein quality control by proteolytic activity involved in oxidative phosphorylation and other mitochondrial functions [47]. Hyperactivation of this protease selectively kills cancer cells, independent of p53 status [12,48]. Cancer lethality occurs through selective degradation of the respiratory chain protein substrates, while non-cancer cells remain unaffected, likely resulting in the excellent tolerability seen in multiple mouse models. These findings support earlier reports of reduced oxidative phosphorylation induced by ONC201 [45]. As a result, ONC201 may have the potential to treat chemo-resistant tumors, including in EC, as several reports demonstrate that cancer stem cells and chemo-resistant cells rely heavily on oxidative phosphorylation [49-53]. In this current study, we found that ONC206 induced cellular stress and expression of ClpP in Lkb1fl/flp53fl/fl mice under obese and lean conditions, indicating that ONC206 may trigger cell death via activation of the ClpP pathway in EC. Thus, ClpP activity may serve as a potential biomarker of imipridone response that should be assessed in ongoing clinical trials with ONC201 or ONC206 in EC as well as other cancers [12].
To date, ONC201 is being evaluated for efficacy in several solid tumors and hematological malignancies in multiple clinical trials [14,17,39,54]. NIH has also launched an ONC206 first-in-human study that is currently ongoing. Our pre-clinical data finds that ONC206 demonstrated greater potency in inhibition of cell proliferation in EC cells when compared directly to ONC201 and most importantly, ONC206 was efficacious in reducing tumor growth in the Lkb1fl/flp53fl/fl mouse model of endometrioid EC under obese and lean conditions, without significant toxicities or side effects. Our in vitro and in vivo studies provide strong support and rationale for the investigation of ONC206 in endometrioid EC clinical trials after determination of its clinical safety profile. As part of our future work, we will expand our studies of single agent ONC206 and begin to explore potential therapeutic partners in combination with ONC206 in EC, including both cytotoxic and targeted agents, as novel treatments for obesity driven-EC are so desperately needed.
Acknowledgements
This work is supported by NIH/NCI-R37CA226969 (Bae-Jump).
Disclosure of conflict of interest
VVP and JEA are employees and stockholders of Oncoceutics/Chimerix. Dr. Bae-Jump’s laboratory received ONC201 and ONC206 from Oncoceutics/Chimerix for these studies. No potential conflicts of interest were disclosed by the other authors.
Supporting Information
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J. Clin. Oncol. 2016;34:4225–4230. doi: 10.1200/JCO.2016.69.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGunigal M, Liu J, Kalir T, Chadha M, Gupta V. Survival differences among uterine papillary serous, clear cell and grade 3 endometrioid adenocarcinoma endometrial cancers: a national cancer database analysis. Int J Gynecol Cancer. 2017;27:85–92. doi: 10.1097/IGC.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 4.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, Graham JE. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Wang ZB, Luo C, Mao XY, Li X, Yin JY, Zhang W, Zhou HH, Liu ZQ. The prospective value of dopamine receptors on bio-behavior of tumor. J Cancer. 2019;10:1622–1632. doi: 10.7150/jca.27780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters MAM, Meijer C, Fehrmann RSN, Walenkamp AME, Kema IP, de Vries EGE, Hollema H, Oosting SF. Serotonin and dopamine receptor expression in solid tumours including rare cancers. Pathol Oncol Res. 2020;26:1539–1547. doi: 10.1007/s12253-019-00734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tegowski M, Fan C, Baldwin AS. Thioridazine inhibits self-renewal in breast cancer cells via DRD2-dependent STAT3 inhibition, but induces a G1 arrest independent of DRD2. J Biol Chem. 2018;293:15977–15990. doi: 10.1074/jbc.RA118.003719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jandaghi P, Najafabadi HS, Bauer AS, Papadakis AI, Fassan M, Hall A, Monast A, von Knebel Doeberitz M, Neoptolemos JP, Costello E, Greenhalf W, Scarpa A, Sipos B, Auld D, Lathrop M, Park M, Buchler MW, Strobel O, Hackert T, Giese NA, Zogopoulos G, Sangwan V, Huang S, Riazalhosseini Y, Hoheisel JD. Expression of DRD2 is increased in human pancreatic ductal adenocarcinoma and inhibitors slow tumor growth in mice. Gastroenterology. 2016;151:1218–1231. doi: 10.1053/j.gastro.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 10.Weissenrieder JS, Neighbors JD, Mailman RB, Hohl RJ. Cancer and the dopamine D2 receptor: a pharmacological perspective. J Pharmacol Exp Ther. 2019;370:111–126. doi: 10.1124/jpet.119.256818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen JE, Kline CL, Prabhu VV, Wagner J, Ishizawa J, Madhukar N, Lev A, Baumeister M, Zhou L, Lulla A, Stogniew M, Schalop L, Benes C, Kaufman HL, Pottorf RS, Nallaganchu BR, Olson GL, Al-Mulla F, Duvic M, Wu GS, Dicker DT, Talekar MK, Lim B, Elemento O, Oster W, Bertino J, Flaherty K, Wang ML, Borthakur G, Andreeff M, Stein M, El-Deiry WS. Discovery and clinical introduction of first-in-class imipridone ONC201. Oncotarget. 2016;7:74380–74392. doi: 10.18632/oncotarget.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Dougan DA. The direct molecular target for imipridone ONC201 is finally established. Cancer Cell. 2019;35:707–708. doi: 10.1016/j.ccell.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Ishizawa J, Zarabi SF, Davis RE, Halgas O, Nii T, Jitkova Y, Zhao R, St-Germain J, Heese LE, Egan G, Ruvolo VR, Barghout SH, Nishida Y, Hurren R, Ma W, Gronda M, Link T, Wong K, Mabanglo M, Kojima K, Borthakur G, MacLean N, Ma MCJ, Leber AB, Minden MD, Houry W, Kantarjian H, Stogniew M, Raught B, Pai EF, Schimmer AD, Andreeff M. Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell. 2019;35:721–737. e729. doi: 10.1016/j.ccell.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graves PR, Aponte-Collazo LJ, Fennell EMJ, Graves AC, Hale AE, Dicheva N, Herring LE, Gilbert TSK, East MP, McDonald IM, Lockett MR, Ashamalla H, Moorman NJ, Karanewsky DS, Iwanowicz EJ, Holmuhamedov E, Graves LM. Mitochondrial protease ClpP is a target for the anticancer compounds onc201 and related analogues. ACS Chem Biol. 2019;14:1020–1029. doi: 10.1021/acschembio.9b00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce SR, Fang Z, Yin Y, West L, Asher M, Hao T, Zhang X, Tucker K, Staley A, Fan Y, Sun W, Moore DT, Xu C, Tsai YH, Parker J, Prabhu VV, Allen JE, Lee D, Zhou C, Bae-Jump V. Targeting dopamine receptor D2 as a novel therapeutic strategy in endometrial cancer. J Exp Clin Cancer Res. 2021;40:61. doi: 10.1186/s13046-021-01842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Z, Wang J, Clark LH, Sun W, Yin Y, Kong W, Pierce SR, West L, Sullivan SA, Tran AQ, Prabhu VV, Zhou C, Bae-Jump V. ONC201 demonstrates anti-tumorigenic and anti-metastatic activity in uterine serous carcinoma in vitro. Am J Cancer Res. 2018;8:1551–1563. [PMC free article] [PubMed] [Google Scholar]
- 17.Stein MN, Bertino JR, Kaufman HL, Mayer T, Moss R, Silk A, Chan N, Malhotra J, Rodriguez L, Aisner J, Aiken RD, Haffty BG, DiPaola RS, Saunders T, Zloza A, Damare S, Beckett Y, Yu B, Najmi S, Gabel C, Dickerson S, Zheng L, El-Deiry WS, Allen JE, Stogniew M, Oster W, Mehnert JM. First-in-human clinical trial of oral ONC201 in patients with refractory solid tumors. Clin Cancer Res. 2017;23:4163–4169. doi: 10.1158/1078-0432.CCR-16-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner J, Kline CL, Ralff MD, Lev A, Lulla A, Zhou L, Olson GL, Nallaganchu BR, Benes CH, Allen JE, Prabhu VV, Stogniew M, Oster W, El-Deiry WS. Preclinical evaluation of the imipridone family, analogs of clinical stage anti-cancer small molecule ONC201, reveals potent anti-cancer effects of ONC212. Cell Cycle. 2017;16:1790–1799. doi: 10.1080/15384101.2017.1325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida CT, Zhang Y, Bianchetti E, Shu C, Nguyen TTT, Kleiner G, Sanchez-Quintero MJ, Quinzii CM, Westhoff MA, Karpel-Massler G, Prabhu VV, Allen JE, Siegelin MD. Metabolic reprogramming by Dual AKT/ERK inhibition through imipridones elicits unique vulnerabilities in glioblastoma. Clin Cancer Res. 2018;24:5392–5406. doi: 10.1158/1078-0432.CCR-18-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prabhu V, Morrow S, Kawakibi AR, Jitkova Y, Jung J, Madhukar N, Garnett M, McDermott U, Benes C, Wechsler-Reya R, Elemento O, DeMorrow S, Schimmer A, Stogniew M, Theeler B, Gilbert M, Allen J. EXTH-74. Ind-enabling characterization of dual DRD2- and ClpP-targeting agent ONC206 as the next imipridone for clinical neuro-oncologY. Neuro Oncol. 2020;22:ii103–ii103. [Google Scholar]
- 21.Zhang Y, Huang Y, Yin Y, Fan Y, Sun W, Zhao X, Tucker K, Staley A, Paraghamian S, Hawkins G, Prabhu V, Allen JE, Zhou C, Bae-Jump V. ONC206, an imipridone derivative, induces cell death through activation of the integrated stress response in serous endometrial cancer in vitro. Front Oncol. 2020;10:577141. doi: 10.3389/fonc.2020.577141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhu VV, Morrow S, Rahman Kawakibi A, Zhou L, Ralff M, Ray J, Jhaveri A, Ferrarini I, Lee Y, Parker C, Zhang Y, Borsuk R, Chang WI, Honeyman JN, Tavora F, Carneiro B, Raufi A, Huntington K, Carlsen L, Louie A, Safran H, Seyhan AA, Tarapore RS, Schalop L, Stogniew M, Allen JE, Oster W, El-Deiry WS. ONC201 and imipridones: anti-cancer compounds with clinical efficacy. Neoplasia. 2020;22:725–744. doi: 10.1016/j.neo.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu W, Zhang L, Ferri-Borgogno S, Kwan SY, Lewis KE, Cun HT, Yeung TL, Soliman PT, Tarapore RS, Allen JE, Guan X, Lu KH, Mok SC, Au-Yeung CL. Targeting dopamine receptor D2 by imipridone suppresses uterine serous cancer malignant phenotype. Cancers (Basel) 2020;12:2436. doi: 10.3390/cancers12092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan L, Sheng X, Clark LH, Zhang L, Guo H, Jones HM, Willson AK, Gehrig PA, Zhou C, Bae-Jump VL. Glutaminase inhibitor compound 968 inhibits cell proliferation and sensitizes paclitaxel in ovarian cancer. Am J Transl Res. 2016;8:4265–4277. [PMC free article] [PubMed] [Google Scholar]
- 25.Guo H, Kong W, Zhang L, Han J, Clark LH, Yin Y, Fang Z, Sun W, Wang J, Gilliam TP, Lee D, Makowski L, Zhou C, Bae-Jump VL. Reversal of obesity-driven aggressiveness of endometrial cancer by metformin. Am J Cancer Res. 2019;9:2170–2193. [PMC free article] [PubMed] [Google Scholar]
- 26.Guo H, Kong W, Zhang L, Han J, Clark LH, Yin Y, Fang Z, Sun W, Wang J, Gilliam TP, Lee D, Makowski L, Zhou C, Bae-Jump VL. Reversal of obesity-driven aggressiveness of endometrial cancer by metformin. Am J Cancer Res. 2019;9:2170–2193. [PMC free article] [PubMed] [Google Scholar]
- 27.Makker V, Green AK, Wenham RM, Mutch D, Davidson B, Miller DS. New therapies for advanced, recurrent, and metastatic endometrial cancers. Gynecol Oncol Res Pract. 2017;4:19. doi: 10.1186/s40661-017-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, Moxley KM, Kim YM, Powell MA, O’Malley DM, Spirtos NM, Small W Jr, Tewari KS, Richards WE, Nakayama J, Matulonis UA, Huang HQ, Miller DS. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317–2326. doi: 10.1056/NEJMoa1813181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiorica JV, Brunetto VL, Hanjani P, Lentz SS, Mannel R, Andersen W Gynecologic Oncology Group study. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:10–14. doi: 10.1016/j.ygyno.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH Jr, Italiano A, Kao S, Piha-Paul SA, Delord JP, McWilliams RR, Fabrizio DA, Aurora-Garg D, Xu L, Jin F, Norwood K, Bang YJ. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 31.Lee YC, Lheureux S, Oza AM. Treatment strategies for endometrial cancer: current practice and perspective. Curr Opin Obstet Gynecol. 2017;29:47–58. doi: 10.1097/GCO.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 32.Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer. 2019;19:510–521. doi: 10.1038/s41568-019-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhu VV, Talekar MK, Lulla AR, Kline CLB, Zhou L, Hall J, Van den Heuvel APJ, Dicker DT, Babar J, Grupp SA, Garnett MJ, McDermott U, Benes CH, Pu JJ, Claxton DF, Khan N, Oster W, Allen JE, El-Deiry WS. Single agent and synergistic combinatorial efficacy of first-in-class small molecule imipridone ONC201 in hematological malignancies. Cell Cycle. 2018;17:468–478. doi: 10.1080/15384101.2017.1403689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen JE, Krigsfeld G, Patel L, Mayes PA, Dicker DT, Wu GS, El-Deiry WS. Identification of TRAIL-inducing compounds highlights small molecule ONC201/TIC10 as a unique anti-cancer agent that activates the TRAIL pathway. Mol Cancer. 2015;14:99. doi: 10.1186/s12943-015-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen JE, Krigsfeld G, Mayes PA, Patel L, Dicker DT, Patel AS, Dolloff NG, Messaris E, Scata KA, Wang W, Zhou JY, Wu GS, El-Deiry WS. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5:171ra117. doi: 10.1126/scitranslmed.3004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kline CLB, Ralff MD, Lulla AR, Wagner JM, Abbosh PH, Dicker DT, Allen JE, El-Deiry WS. Role of dopamine receptors in the anticancer activity of ONC201. Neoplasia. 2018;20:80–91. doi: 10.1016/j.neo.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni X, Zhang X, Hu CH, Langridge T, Tarapore RS, Allen JE, Oster W, Duvic M. ONC201 selectively induces apoptosis in cutaneous T-cell lymphoma cells via activating pro-apoptotic integrated stress response and inactivating JAK/STAT and NF-kappaB pathways. Oncotarget. 2017;8:61761–61776. doi: 10.18632/oncotarget.18688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ralff MD, Kline CLB, Kucukkase OC, Wagner J, Lim B, Dicker DT, Prabhu VV, Oster W, El-Deiry WS. ONC201 demonstrates antitumor effects in both triple-negative and non-triple-negative breast cancers through TRAIL-dependent and TRAIL-independent mechanisms. Mol Cancer Ther. 2017;16:1290–1298. doi: 10.1158/1535-7163.MCT-17-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein MN, Malhotra J, Tarapore RS, Malhotra U, Silk AW, Chan N, Rodriguez L, Aisner J, Aiken RD, Mayer T, Haffty BG, Newman JH, Aspromonte SM, Bommareddy PK, Estupinian R, Chesson CB, Sadimin ET, Li S, Medina DJ, Saunders T, Frankel M, Kareddula A, Damare S, Wesolowsky E, Gabel C, El-Deiry WS, Prabhu VV, Allen JE, Stogniew M, Oster W, Bertino JR, Libutti SK, Mehnert JM, Zloza A. Safety and enhanced immunostimulatory activity of the DRD2 antagonist ONC201 in advanced solid tumor patients with weekly oral administration. J Immunother Cancer. 2019;7:136. doi: 10.1186/s40425-019-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Husseinzadeh N, Husseinzadeh HD. mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: a critical review. Gynecol Oncol. 2014;133:375–381. doi: 10.1016/j.ygyno.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 41.Travaglino A, Raffone A, Gencarelli A, Mollo A, Guida M, Insabato L, Santoro A, Zannoni GF, Zullo F. TCGA classification of endometrial cancer: the place of carcinosarcoma. Pathol Oncol Res. 2020;26:2067–2073. doi: 10.1007/s12253-020-00829-9. [DOI] [PubMed] [Google Scholar]
- 42.Husseinzadeh N, Davenport SM. Role of toll-like receptors in cervical, endometrial and ovarian cancers: a review. Gynecol Oncol. 2014;135:359–363. doi: 10.1016/j.ygyno.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Nishiyama A, Matsuyama M, Wang Z, Yuan Y. The (pro)renin receptor: a novel biomarker and potential therapeutic target for various cancers. Cell Commun Signal. 2020;18:39. doi: 10.1186/s12964-020-0531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papatla K, Huang M, Slomovitz B. The obese endometrial cancer patient: how do we effectively improve morbidity and mortality in this patient population? Ann Oncol. 2016;27:1988–1994. doi: 10.1093/annonc/mdw310. [DOI] [PubMed] [Google Scholar]
- 45.Greer YE, Porat-Shliom N, Nagashima K, Stuelten C, Crooks D, Koparde VN, Gilbert SF, Islam C, Ubaldini A, Ji Y, Gattinoni L, Soheilian F, Wang X, Hafner M, Shetty J, Tran B, Jailwala P, Cam M, Lang M, Voeller D, Reinhold WC, Rajapakse V, Pommier Y, Weigert R, Linehan WM, Lipkowitz S. ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget. 2018;9:18454–18479. doi: 10.18632/oncotarget.24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lev A, Lulla AR, Ross BC, Ralff MD, Makhov PB, Dicker DT, El-Deiry WS. ONC201 targets AR and AR-V7 signaling, reduces PSA, and synergizes with everolimus in prostate cancer. Mol Cancer Res. 2018;16:754–766. doi: 10.1158/1541-7786.MCR-17-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nouri K, Feng Y, Schimmer AD. Mitochondrial ClpP serine protease-biological function and emerging target for cancer therapy. Cell Death Dis. 2020;11:841. doi: 10.1038/s41419-020-03062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cole A, Wang Z, Coyaud E, Voisin V, Gronda M, Jitkova Y, Mattson R, Hurren R, Babovic S, Maclean N, Restall I, Wang X, Jeyaraju DV, Sukhai MA, Prabha S, Bashir S, Ramakrishnan A, Leung E, Qia YH, Zhang N, Combes KR, Ketela T, Lin F, Houry WA, Aman A, Al-Awar R, Zheng W, Wienholds E, Xu CJ, Dick J, Wang JC, Moffat J, Minden MD, Eaves CJ, Bader GD, Hao Z, Kornblau SM, Raught B, Schimmer AD. Inhibition of the mitochondrial protease ClpP as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell. 2015;27:864–876. doi: 10.1016/j.ccell.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farge T, Saland E, de Toni F, Aroua N, Hosseini M, Perry R, Bosc C, Sugita M, Stuani L, Fraisse M, Scotland S, Larrue C, Boutzen H, Feliu V, Nicolau-Travers ML, Cassant-Sourdy S, Broin N, David M, Serhan N, Sarry A, Tavitian S, Kaoma T, Vallar L, Iacovoni J, Linares LK, Montersino C, Castellano R, Griessinger E, Collette Y, Duchamp O, Barreira Y, Hirsch P, Palama T, Gales L, Delhommeau F, Garmy-Susini BH, Portais JC, Vergez F, Selak M, Danet-Desnoyers G, Carroll M, Recher C, Sarry JE. Chemotherapy-resistant human acute myeloid leukemia cells are not enriched for leukemic stem cells but require oxidative metabolism. Cancer Discov. 2017;7:716–735. doi: 10.1158/2159-8290.CD-16-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuntz EM, Baquero P, Michie AM, Dunn K, Tardito S, Holyoake TL, Helgason GV, Gottlieb E. Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat Med. 2017;23:1234–1240. doi: 10.1038/nm.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z, Cai L, Good L, Tu BP, Hatanpaa KJ, Mickey BE, Mates JM, Pascual JM, Maher EA, Malloy CR, Deberardinis RJ, Bachoo RM. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O’Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Jordan CT. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, Kost-Alimova M, Muller F, Colla S, Nezi L, Genovese G, Deem AK, Kapoor A, Yao W, Brunetto E, Kang Y, Yuan M, Asara JM, Wang YA, Heffernan TP, Kimmelman AC, Wang H, Fleming JB, Cantley LC, DePinho RA, Draetta GF. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McSweeney KR, Gadanec LK, Qaradakhi T, Gammune TM, Kubatka P, Caprnda M, Fedotova J, Radonak J, Kruzliak P, Zulli A. Imipridone enhances vascular relaxation via FOXO1 pathway. Clin Exp Pharmacol Physiol. 2020;47:1816–1823. doi: 10.1111/1440-1681.13377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


