Abstract
Metformin has been known to treat type 2 diabetes for decades and is widely prescribed antidiabetic drug. Recently, its anticancer potential has also been discovered. Moreover, metformin has low cost thus it has attained profound research interest. Comprehensing the complexity of the molecular regulatory networks in cancer provides a mode for advancement of research in cancer development and treatment. Metformin targets many pathways that play an important role in cancer cell survival outcome. Here, we described anticancer activity of metformin on the AMPK dependent/independent mechanisms regulating metabolism, oncogene/tumor suppressor signaling pathways together with the issue of clinical studies. We also provided brief overwiev about recently described metformin’s role in cancer immunity. Insight in these complex molecular networks, will simplify application of metformin in clinical trials and contribute to improvement of anti-cancer therapy.
Keywords: Metformin, cancer, AMPK, metabolism, oncogenes, tumor suppressors, immunity
Introduction
French lilac (Galega officinalis), is a herb known for considerable time period (centuries) to decrease the symptoms of diabetes mellitus and the biguanide metformin is derived from this plant [1]. Metformin (N’, N’-dimethylbiguanide), has the greatest efficacy and it is the harmless commonly recommended antidiabetic (in comparison with other biguanides) remedy for type 2 diabetes mellitus (T2DM) [2]. At this time it is concluded that metformin functions by preventing glucose production in the liver [2] and the anti-hyperglycemic action of metformin have been ascribed, in part, to elevated hepatic insulin sensitivity and enlarged glucose uptake into peripheral tissues, such as skeletal muscle and adipose tissues [3].
Metformin has a vast different medical assets. It has successfully been accepted in the administration in polycystic ovarian syndrome (PCOS) and metabolic syndrome [4]. Also metformin may be useful in the treatment of cancer, cardiovascular disease, delay in the aging process and regulation of microbiota [5]. This review will provide an overview of the recent known metabolic effects, influence on oncogenes/tumor suppresors and immunity from AMP-activated protein kinase AMPK-dependent and independent aspect in cancer cells, as well as recent clinical studies examining the ability of metformin in the cancer treatment.
Metformin in the cell
Metformin is a hydrophilic molecule and therefore cannot pass through the cell’s membrane by passive diffusion. The organic cation transporter family (OCT), and specifically the organic cation transporter 1 (OCT1), is responsible for the uptake of metformin into hepatocytes and tumor cells [4]. In 2014 three groups, reported unique and convincing confirmation on the preventing complex I properties by metformin implementing distinct experimental procedure [1]. The suppression of mitochondrial respiration and ATP synthesis by metformin drives to metabolic adaptations striving to retrieve cellular ATP levels [6]. This altered energy status in the cell is detected by a central energy sensor-AMP activated protein kinase (AMPK). AMPK is activated by binding of ADP or AMP molecules to a specific site on its regulatory gamma subunit [7]. Once activated, AMPK stimulates catabolic processes in which ATP is synthesized and inhibits anabolic processes where ATP is consumed and thus the cell adaptes to a state of reduced energy [7,8]. Cancer cells that are not capable to recover from this reduced energy status may encounter apoptosis [9], and the induction of mitochondria-mediated apoptosis by metformin was described in glioma cells in 2007 [1,10]. It is still not defined, whether AMPK plays the role of a major mediator in the action of metformin in cancer cells, therefore the effects of metformin on AMPK-dependent and AMPK-independent mechanisms in cancer cells will be reported in this review.
Metformin in cancer cells, its mode of action remains controversial
Despite extensive research about the molecular events in cancer cells, cancer remains predominant reason for life quality impairment of cancer patients and mortality in the world. Increased enthusiasm in the therapeutic ability of different antidiabetic drugs has appeared after attention that type II diabetes mellitus is linked with increased cancer risk [1].
Further research was prompted after 2 epidemiological reports suggested a link between metformin treatment and a reduced cancer risk and decreased cancer mortality in diabetic patients [11,12]. However, it is important to note that in vitro and in vivo studies tend to overestimate the importance of metformin in cancer therapy, while meta-analyzes of randomized controlled trials do not appear to show a significant effect of metformin on cancer outcome [3].
AMPK-dependent effect of metformin on cancer cells metabolism
Among the first evidences of the AMPK importance in the antiproliferative effect of metformin are studies on ovarian and breast cancer in vitro. Since then, numerous studies have been completed scrutinizing the role of AMPK in the anticancer effect of metformin [13-15].
Although there are still ambiguities in the AMPK activation, direct and indirect mechanisms of AMPK activation can be distinguished [5].
(1) Direct activation of AMPK involves binding AMP/ADP to the gamma regulatory subunit, inducing conformational change and unmasking Thr 172 residue on the catalytic alpha subunit [5].
(2) Indirect activation of AMPK ensues via upstream kinases such as serine threonine kinase-11/LKB1, calmodulin-dependent kinase kinase 2/CAMKK2 and TGF beta-activated kinase-1/TAK [7].
It is thought that activation of the LKB1/AMPK signaling pathway may significantly contribute to the anti-cancer effect of metformin. LKB1, an upstream activator of AMPK, is a known tumor suppressor and mutations in this gene have been associated with Peutz-Jeghers syndrome, an inherited predisposing disorder characterized with development of different cancer types [16].
AMPK-dependent metformin’s role in cancer cells protein metabolism
Mechanistic target of rapamycin mTOR integrates protein synthesis with growth and proliferation of cells depending on availability of nutrients. mTOR is inhibited by AMPK downstream targets Raptor and Tuberous Sclerosis Complex/TSC2 phosphorylation [17]. Subsequent to metformin activation of AMPK it can be assumed decline in pathways regulated by mTOR and prevention of cancer cell proliferation and growth (Figure 1). Several studies confirmed this hypothesis. In human AGS gastric adenocarcinoma cells metformin triggered intrinsic apoptotic response by AMPK stimulation and AKT/mTOR signaling disruption [18]. Another study on human gastric cancer cells reported that AMPK/mTOR pathway dependent inhibition of survivin partly contributes to metformin-induced apoptosis [19]. Study on esophageal squamous cell carcinoma in vivo validated that metformin prevented esophageal carcinogenesis by AMPK/mTOR signaling pathway [20]. Finally there is a remarkably study in breast cancer cells assembling [21] cation-selective transporter expression, the AMPK/mTOR signaling cascade and the antiproliferative role of metformin.
Figure 1.
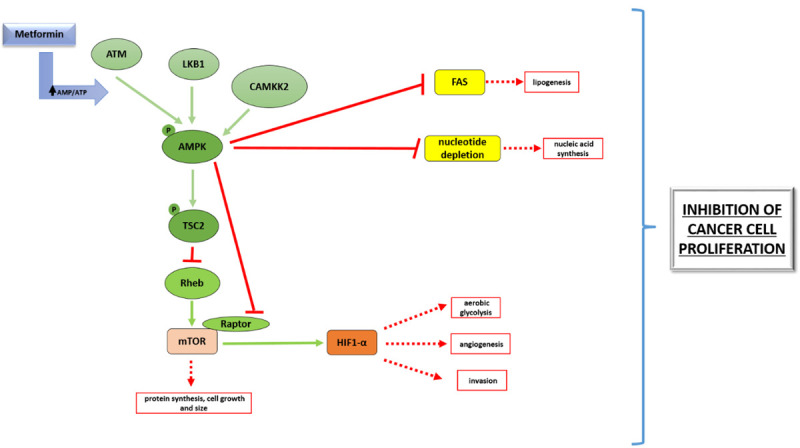
AMPK-dependent effect of metformin on cancer cell metabolism. Key metabolic molecules and their regulation described in review are depicted on Figure 1. Green arrows represent activation, while red lines show inhibitory interaction. Red squared boxes illustrate inhibition of distinct metabolic process.
Apart from metabolic modulations and apoptosis regulation, it is widely accepted that AMPK activation and subsequent mTOR inhibition induces autophagy [22]. Autophagy is highly conserved evolutionary process serving for removement of old proteins and organelles in the cell. Induction of autophagy in cancer cells can have different consequences: it inhibits (cytotoxic autophagy) or promotes (cytoprotective autophagy) cancer cell survival. Metformin in myeloma cell lines induced autophagy and G0/G1 cell cycle arrest by targeting the AMPK/mTOR pathway [23]. It was also shown on melanoma cell line partially AMPK-dependent cytotoxic autophagy activation by metformin [24]. In human hepatocellular carcinoma cells metformin has induced autophagy via the AMPK-mTOR activation, but in this study the role of autophagy (cytotoxic or cytoprotective) was not clearly shown [25]. Contrarily, Chen’s study on NSCLC cell lines implicated that metformin inhibits autophagy by AMPK activation and has sensitized non-small-cell-lung-carcinoma/NSCLC cells to osimertinib. Authors presumed that sustained AMPK activation induced by metformin could impede autophagy in a time-dependent manner. Ben Sahra depicted in prostate cancer cells that combination of 2-deoxy glucose and metformin has supressed autophagy and activated AMPK-dependent apoptosis, recognizing ambiguous role of AMPK modulation in cellular signaling [26].
There are also studies not precisely investigating the role of AMPK-mTOR signaling pathway in autophagy induced by metformin. It was revealed that metformin precluded cell tumorigenesis of NIH/3T3 (mouse embrionic fibroblasts cell line) via autophagy-related cell death. Authors did not analyze the role of AMPK-mTOR signaling pathway, instead showing unfolded protein response-mitogen activated protein kinases/UPR-MAPKs dependent autophagy induction by metformin. These findings proposed that autophagic cell death could be recognized as a new mechanism of disposing damaged cells serving as an attractive strategy for discarding potentialy tumorigenic cells [27]. Different than in Chen, study on NSCLC cells demonstrated that metformin has induced cytoprotective autophagy alleviating drug-activated apoptosis in these cells. This study implicated LKB1 (AMPK activator) independent autophagy induction with negligible effects on tumors of diabetic patients [28]. Analysis on human osteosarcoma cell lines delineated that metformin has induced cell cycle arrest, apoptosis and autophagy via reactive oxygen species-c Jun-N terminal kinase/ROS-JNK signaling pathway not clearly investigating AMPK/mTOR signaling pathway and not resolving the role of induced autophagy [29].
Regarding adjuvant therapeutic approach of AMPK/mTOR pathway activation via metformin, enhanced cisplatin antiproliferative effects in cholangiocarcinoma cells was presented [30].
Effect of metformin on glucose, lipid and nucleotide metabolism as a part of AMPK signaling network
Beside from protein metabolism inhibition, activated AMPK suppresses the Warburg effect including as a consequence aerobic glycolysis inhibition and glucose source deprivation for cancer cells. Since metformin is AMPK agonist, Faubert [31] suggested in the study that metformin application abolishes Warburg effect and therefore inhibits tumor growth (Figure 1). Presumed mechanism is that metformin activates AMPK followed by mTOR inihibiton and suppression of HIF-1 alpha transcription factor (hypoxic inducible factor), which is partially activated by mTOR. HIF-1 alpha stimulates the expression of genes involved in glycolysis [32].
Amount of phospholipids and higher fatty acids is elevated in different types of cancer [33], since cancer cells use them for proliferation and growth. Their synthesis is regulated via fatty acid synthase (FAS) enzymes. It has been described that in colorectal cancer metformin-activated AMPK consequently dysregulates lipid metabolism suppressing in vitro cancer stem cells and in vivo on mouse model preventing tumor growth [34]. AMPK activation by metformin downregulated FAS expression in aggresive metastatic cervical cancer and reduced viability in prostate cancer cells [35,36]. Importantly, ex vivo study [37] shown that combination of metformin and salicylate derivatives activates AMPK and synergizes in reducing the survival of prostate and lung cancer through inhibition of de novo lipogenesis (Figure 1).
Elevated DNA synthesis is also specificity of cancer cell metabolism. However there is a few data dealing with metformin control of DNA synthesis. Current studies have shown that metformin depending on AMPK activity hinders de novo synthesis of nucleotides crucial for repair and synthesis of DNA molecules in several breast cancer cell lines [17] and pancreatic ductal cells in vitro [38]. Also, metformin induced nucleotide triphosphate depletion specifically in cancer stem cells [39]. In 5-Fu (5-fluorouracyl) resistant colon cancer cell line metformin preferably altered the DNA damage response and DNA replication but it persist a question of AMPK requirement in this model [40]. We don’t ignore likelihood that noticed metformin alternations of DNA metabolism are mitochondria dependently since Kreb’s cycle disturbation might interfere with DNA synthesis.
Different outcomes of AMPK-dependent metabolic regulation activated by metformin
Quoted studies signify anticancer role of metformin via AMPK activation, promoting changes in protein, glucose, lipid and nucleic acid metabolism hence depriving cancer cells of the essential substrates necessary for their growth and proliferation. A recent research however, reported that pharmacologic inhibition of AMPK curbed proliferation of prostate cancer cell lines [41]. This study hinted that outcome of metformin effect on AMPK activation depends on the cell environment. Namely, activation of AMPK with metformin refers to the glucose concentration in the extracellular environment [42]. For example, AMPK activation by metformin in the presence of a regular glucose concentration in the cellular environment will lead to antiproliferative effects [43] and related result was obtained by metformin and glucose reduction, decreasing the migratory ability of hepatocellular carcinoma cells [44]. Conflictingly, a study has shown that in low glucose environment conditions AMPK activation improves renal cancer cell proliferation [45]. Moreover, an in vivo study showed that in mice on a high-energy starvation metformin reduced tumor growth and away from impact in mice on control diet [46]. A related study posed that AMPK activation, as a result of lower ATP/AMP ratios in tumor microenvironment, promotes cancer cell residue under stressful metabolic conditions [47]. In light of cited study it is not surprisingly that in similar research AMPK activation by metformin promotes survival of dormant ER(+) breast cancer cell population [48].
Important fact to note is a particular outcome of AMPK activation relies not only on the extracellular environment but also on the condition of signaling pathways in the cell, e.g. it is reported that reactivation of AMPK in lung adenocarcinoma cells that do not have its upstream regulator LKB1, protects lung cancer cells from death caused by glucose starvation [49]. Also, cellular condition is described albeit mitochondrial ATP production is reduced by metformin and additionally cancer cell has activity failure AMPK or p53. In afore mentioned situation cancer cells are not producing sufficient energy and lack balance energy consumption finally suggesting energy crisis and cell death, as well as tumor suppression [46,50,51].
Although it is discernible that metformin can restrict anabolic course in cancer cells by activating AMPK, causing numerous AMPK consecutive pathways activation and in the end leading to cell death, it is also evident that during the later stages of malignant tumor development, AMPK activation supports the survival of tumor cells. Conceivable interpretation is that AMPK induction allows the cancer cell to adapt to the metabolic stress that exists intracellular and likewise in the tumor micro-environment.
Since immense importance of AMPK role in autophagy modulation we would suggest that metformin in some conditions activates cytoprotective autophagy as mechanism of tumor cells survival and thus scrutiny in establishing therapeutic strategy of metformin as a single agent in cancer therapy.
Effect of metformin on AMPK-dependent processes in oncogene and tumor suppressor signaling pathways
In addition to metabolic modifications, there are diverse studies proposing that AMPK activation induced by metformin contributes to proliferation and survival regulation by interacting with oncogenes and tumor suppressors.
MAPK pathway
For instance AMPK signaling controls cellular metabolism, whereas the mitogen activated protein kinases-MAPK signaling regulates cellular proliferation, differentiation and survival. Nonetheless, many studies have determined that these specific signaling pathways have sophisticated interplays in physiological and pathological processes [52].
It was shown that metformin inhibited proliferation, promoted apoptosis, reversed and delayed acquired resistance to Epidermal growth factor receptor tyrosin kinase inhibitors/EGFR TKIs in EGFR-mutant lung cancer [53]. These effects of metformin are associated with AMPK activation resulting in suppression of downstream extracellular signal-regulated kinases/Nuclear factor-kappa B-ERK/NF-κB signaling. Customary, mutated kirsten rat sarcoma virus oncogene/KRAS in colorectal cancer determinates excessive glycolysis having as a consequence high ATP production and AMPK inhibition [54]. Therefore AMPK inhibition is associated with decreased response of colorectal cancer cells to anti-EGFR antibody therapy [54]. On the contrary AMPK activation by metformin could suppress the KRAS mutation effect on anti-EGFR antibody resistance in colorectal cancer cells (Figure 2).
Figure 2.
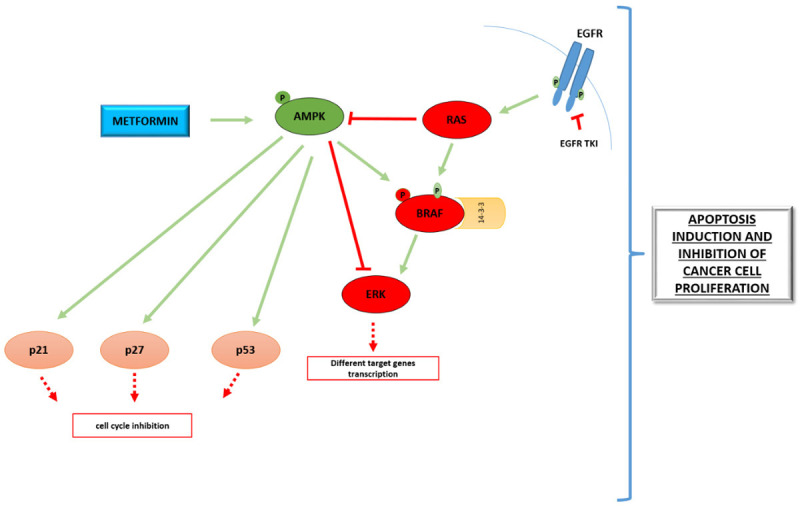
AMPK-dependent effect of metformin on evident oncogenes and tumor suppressor genes. Oncogenic MAPK pathway (red molecules) and tumor suppressors (light pink molecules) described in review are marked. Green arrows represent activation, while red lines show inhibitory interaction. Red squared boxes represent inhibition of pointed signaling regulation.
Metformin may be particularly effective when used in combination with small molecule kinase inhibitors that suppress glycolysis. In melanoma cells, the efficacy of the protein kinase inhibitor v raf murine sarcoma viral oncogenic homologue B1/BRAF has been shown to be limited by the induction of compensatory oxidative phosphorylation [55]. Although there are many oxidative phosphorylation inhibitors known to be toxic in cancer cells, the desirable safety profile of metformin implies this drug may have therapeutic potential [56].
Data from these studies implicated novel and further molecular rationale and preclinical data supporting combination of metformin with EGFR TKIs and small kinase inhibitors to treat cancer patients with mutations in MAPK kinase pathways.
Wnt/β-catenin pathway
One of the oncogenic pathways modulated by metformin is Wingless and Int-1 portmanteau-Wnt/β-catenin network pathway. This is an evolutionarily preserved and versatile signaling that is known to participate in physiologic conditions and besides in a wide variety of human diseases. Irregular activation of this pathway generates the accumulation of β-catenin in the nucleus and stimulates the transcription of different oncogenes such as c-Myc and Cyclin D-1 [57].
It was recently shown that metformin can inhibit proto-oncogen activity of β-catenin through non-canonical AMPK/phosphatidylinositol 3-kinases/Protein kinase B-AMPK PI3K/Akt pathway phosphorylation in colorectal cancer cells [58] and to overcome breast cancer cell growth by downregulation of upstream Wnt/β-catenin signaling pathway regulators [59] (Figure 3).
Figure 3.
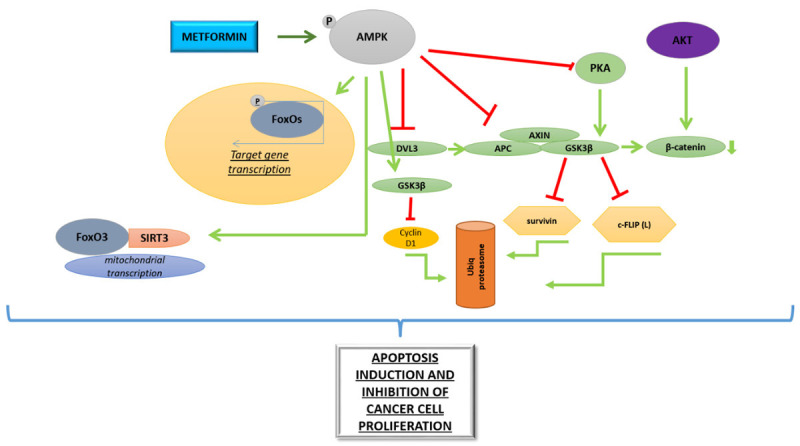
AMPK-dependent effect of metformin on atypical oncogenes and tumor suppressor genes. Green arrows represent induction, while red lines show suppression of phosphorylation or mutual molecules interaction and proper mechanisms.
Compelling study has demonstrated that AMPK activation by metformin can suppress β-catenin-dependent Wnt signaling by cytoplasmic sequestering of β-catenin via AMPK, further reducing cell proliferation in colon carcinoma cells [60].
These studies reported a novel mechanism of antineoplastic effect for metformin, and indicate for the upgrading of new antineoplastic drugs having the AMPK-Wnt/β-catenin pathway as therapeutic goal (Figure 3).
Foxo transcription pathway
Metformin stimulates another crucial pathway in cancer development and progression-tumor suppressor Foxo signaling Forkhead box. FoxO transcription factors are tumor suppressors in human cancers and recent studies have noted that besides their prevailing functions in cell cycle arrest initiation and cell death stimulation FoxOs can also coordinate cancer metabolism [61].
AMPK specifically phosphorylates FoxO3 on six different residues but these phosphorylations do not alter FoxO3 cellular localization, but may control FoxO3 binding on the target genes regulating energy stress [61].
It was recently suggested that metformin inhibits cancer cell growth in different types of cancer: endometrial, hepatocellular cancer cell lines, and in vivo on rat breast carcinoma (Walker-256) by AMPK-FOXO1/3 signaling pathway activation [62-64] (Figure 3).
GSK-3 pathway
Intriguing signaling that cannot been exclusively attributed to oncogenic or tumor suppressor is glycogen synthase kinase 3/GSK-3β pathway.
GSK-3β aside from modulating glycogen metabolism serves as tumor suppressor or oncogene depending on cellular conditions [65].
Group of authors demonstrated in non-small cell lung carcinoma that metformin-induced apoptotic cytotoxicity has promoted survivin and c-FLIP(L) destabilization depending on AMPK/protein kinase A-PKA/GSK-3β-axis [66,67]. Metformin has caused in ovarian cancer cells cyclin D1 degradation via AMPK/GSK3β signaling axis and ubiquitin/proteasome pathway and attained anticancer effect (Figure 3).
Cell cycle regulators
Several studies reported AMPK-dependent effect of metformin on pivotal oncogenes and cell cycle regulators. It has been defined that metformin diminished the level of c-MYC oncogenes in an AMPK-dependent manner in the breast cancer cell line [68]. It is also delineated that AMPK sustained conformation and enhance production of tumor suppressor p53 [69,70]. Metformin potentially through an AMPK activation, endorsed generation of p53, p21CIP1 and p27KIP1 in leukemic cell line and esophageal squamous cell carcinomas [71,72]. Moreover metformin augmented expression of AMPK-dependent cell cycle regulators p21 and p27 in cervical cancer cell line [73] (Figure 2).
The complexity of oncogenic/tumor suppressor regulation proposes multiple molecular levels which can be modified by metformin influence on AMPK thus recommending jointed targeting of important molecular nodes in cancer cells.
AMPK independent effect of metformin on diverse signaling pathways in cancer cell
AMPK-independent processes that may explain the anti-cancer effects of metformin have also been described. These mechanisms include AMPK independent variation of diverse molecular networks in the cancer cell.
Exceptionally, metformin can inhibit the activity of mTOR in an AMPK-independent manner in prostate cancer cell line [74] causing cell cycle arrest persuading expression of a metabolic regulator tumor suppressor Redd1, previously described as a negative regulator of mTOR [74]. Though in glioblastoma metformin-engender AMPK-reliant Redd1 production [1]. Intriguing metformin can constrain mTOR omitting AMPK in a process related to amino acid removal induced starvation [75]. There is evidence provided by experiments on the prostate cancer cell line showing that metformin can inhibit critical cell cycle regulator cyclin D1 [76] and comparably in breast cancer, modulating cell cycle by cell division cycle 42-CDC42 suppression [77].
Numerous studies have demonstrated that metformin increases ROS independently of AMPK and thus far induces apoptosis in osteosarcoma, colon cancer, ovarian cancer, renal cancer, hepatocellular carcinoma and melanoma cell lines [29,70,78-81]. ROS elevation by metformin can also augment transitory decline in colon cancer cell lines proliferation [82], or even ROS dependent cytoprotective autophagy in breast cancer in vitro [83]. Contrary, in vitro study on melanoma [70] concluded that ROS increase induced by metformin stimulated cytotoxic autophagy. Likely, it has been recommended that interference with anti-oxidant hemeoxygenase-1 (HO-1) expression is a potential therapeutic approach to sensitize tumors to chemotherapy and radiotherapy. Results from Do [84] indicate that metformin suppresses HO-1 mRNA and protein expression in human hepatic carcinoma HepG2, cervical cancer HeLa, and non-small-cell lung cancer A549 cells, attenuating rapidly accelerated fibrosarcoma-ERK-nuclear factor erythroid 2-related factor 2/Raf-ERK-Nrf2 signaling-separate of AMPK. Further this study illustrates how metformin curtails MAPK pathway excluding AMPK.
Metformin in cancer cell regulates numerous signaling pathways and there are also studies describing apoptosis activation on AMPK independent manner. A study performed on squamous cell esophageal carcinoma showed that metformin induced apoptosis and autophagy excluding AMPK by interrupting antiapoptotic Stat3 (signal transducer and activator of transcription)/Bcl2 (B-cell lymphoma 2) signaling pathway resulting in reduced tumor growth [85]. Yang has implicated novel role of metformin in induction of endoplasmatic reticulum (ER) dependent apoptosis [86]. The latest study presented evidences that intracellular acidification by metformin in solid tumors triggered the unfolded protein response to induce the global transcriptional repressor (DNA Damage Inducible Transcript 3)/DDIT3, known to block oncogenic Wnt signaling pathway [87]. Further, metformin coordinates glucose metabolism omitting AMPK as blocking glucose uptake in a lung cancer cell line model through direct allosteric inhibition of hexokinase-II [88]. Removal of this energy source causes depolarization of mitochondria and results in activation of apoptosis. A similar finding was observed in the breast cancer cell line [89].
Part of metformin controled AMPK-dependent pathways can also be modified on AMPK independent manner as it is described upper: mTOR inhibition, regulation of cell cycle, MAPK pathway modulation and inhibition of Wnt signaling pathway.
Metformin has also been shown to increase the sensitivity of various types of cancer cells to common chemotherapeutics including cisplatin, paclitaxel, carboplatin, and doxorubicin [90-92]. However, it should not be overlooked that metformin may antagonize the cytotoxic effect of cisplatin in glioma, neuroblastoma, fibrosarcoma and leukemia cell lines [93] via AMPK-independent activation of the Akt signaling pathway in glioma cell line. This question is rather intriguingly since there is the recent study implying that metformin augments the response of CRC (colorectal cancer cell line) cells to cisplatin through ROS-mediated PI3K/Akt signaling pathway [94].
Metformin in cancer clinical studies
Since 2005, when the Evans study appeared, there has been a sharp increase in interest in metformin in the prevention and treatment of cancer. For clearness, the clinical studies finished after 2015 year in this review are presented in tables (Tables 1 and 2). Interest in the use of meformin in the treatment of cancer is not decreasing, thus in 2016, 15 studies that were not completed were started, in 2017-14 studies were started, which are ongoing, and in 2018, 14 studies that were started and still not ended.
Table 1.
Masked clinical studies completed after 2015
| STUDY | A Randomized Phase II, Double Blind Trial of Standard Chemotherapy With Metformin (vs. Placebo) in Women With Metastatic Breast Cancer Receiving First to Fourth Line Chemotherapy |
|
| |
| NCT NUMBER | NCT01310231 |
| STATUS | Completed |
| PARTICIPANTS | 40 participants, 18-75 years, sex: female |
| PERIOD | February 2011-March 2021 |
| INTERVENTION | Drug: Metformin |
| Metformin vs. placebo 850 mg bid in addition to standard chemotherapy (containing anthracyclines, platinum, taxanes or capecitabine; first or second line). | |
| CANCER TYPE | Metastatic breast cancer |
| OUTCOME | In this population metformin showed no significant effect on RR, PFS or OS. These results do not support the use of metformin with chemotherapy in non-diabetic MBC patients. |
|
| |
| STUDY | Phase II Study of Metformin in a Pre-prostatectomy Prostate Cancer Cohort |
|
| |
| NCT NUMBER | NCT01433913 |
| STATUS | Completed |
| PARTICIPANTS | 20 participants, sex: male |
| PERIOD | Sep 2011-May 2018 |
| INTERVENTION | Metformin hydrochloride vs. placebo both PO 4-12 weeks |
| CANCER TYPE | Adenocarcinoma of the Prostate: Recurrent Prostate Cancer, Stage I Prostate Cancer, Stage IIA Prostate Cancer, Stage IIB Prostate Cancer |
| OUTCOME | No differences between the biomarker expression in the prostatectomy tissue or pre to postintervention changes in serum biomarkers (prostate-specific antigen, insulin, insulin-like growth factor-1, insulin-like growth factor binding protein 3, sex hormone-binding globulin, and testosterone) or tissue biomarkers of proliferation apoptosis, cell cycle regulation, and mTOR inhibition |
|
| |
| STUDY | A Phase II, Randomized, Placebo Controlled Study to Evaluate the Efficacy of the Combination of Gefitinib and Metformin in Patients With Locally Advanced and Metastatic Non-Small-Cell-Lung-Cancer |
|
| |
| NCT NUMBER | NCT01864681 |
| STATUS | Completed |
| PARTICIPANTS | 97 participants, 18-75 years, sex: all |
| PERIOD | May 2013-June 2018 |
| INTERVENTION | Gefitinib + metformin vs. gefitinb + placebo |
| CANCER TYPE | Treatment-naïve stage IIIB-IV with EGFR mutation in NSCLC |
| OUTCOME | incorporation of metformin into standard gefitinib therapy did not prolong PFS or OS in treatment naïve nondiabetic patients with EGFRm NSCLC, neither did it elicit an increase in response to gefitinib |
|
| |
| STUDY | Placebo Controlled Double Blind Crossover Trial of Metformin for Brain Repair in Children With Cranial-Spinal Radiation for MedulloblaStoma |
|
| |
| NCT NUMBER | NCT02040376 |
| STATUS | Completed |
| PARTICIPANTS | 30 participants, 5-21 years, sex: all |
| PERIOD | January 2014-May 2019 |
| INTERVENTION | Metformin and placebo doses will be 500 mg/m2 po daily given in 2 doses for one week and if there are no concerns increased to 1000 mg/m2 po daily given in 2 doses for the rest of the 12 week trial. The investigators will use the closest dose according to body surface area (250-500-750-1000) BID. |
| CANCER TYPE | Brain tumor |
| OUTCOME | Evidence that a clinical trial examining the effects of metformin on cognition and brain structure is feasible in long-term survivors of pediatric brain tumors and that metformin is safe to use and tolerable in this population |
|
| |
| STUDY | A Randomized, Phase II, Double-blind, Placebo-controlled, Multicenter, 2x2 Factorial Design Biomarker Tertiary Prevention Trial of Low-dose Aspirin and Metformin in Stage I-III Colorectal Cancer Patients. The ASAMET Trial |
|
| |
| NCT NUMBER | NCT03047837 |
| STATUS | Unknown |
| PARTICIPANTS | 180 participants, 18-80 years, sex: all |
| PERIOD | March 2017-February 2019 |
| INTERVENTION | Drug: Aspirin (ASA) + Metformin (MET) |
| Arm D (experimental arm) Treatment: active ASA + active MET Dose: 100 mg, 1 tablet daily + 850 mg, 1 tablet twice a day (BID) Duration: 12 months | |
| Drug: ASA | |
| Arm C (experimental arm) Treatment: active ASA + placebo MET Dose: 100 mg, 1 tablet daily + 1 tablet twice a day (BID) Duration: 12 months | |
| Drug: MET | |
| Arm B (experimental arm) Treatment: placebo ASA + active MET Dose: 1 tablet daily + 850 mg, 1 tablet twice a day (BID) Duration: 12 Arm A (control arm) Treatment: placebo ASA + placebo MET Doses: 1 tablet daily + 1 tablet twice a day (BID) Duration: 12 months | |
| CANCER TYPE | Stage I, II, or III primary colorectal cancer |
| OUTCOME | A favorable biomarker modulation by aspirin and metformin may provide important clues for a subsequent phase III adjuvant trial aimed at preventing second primary cancer, delaying recurrence and improving prognosis in patients with CRC. |
|
| |
| STUDY | Effect of Metformin for Deceasing Proliferative Marker in Endometrial Cancer Cells: A Randomized Double Blind Placebo-controlled Trial |
|
| |
| NCT NUMBER | NCT03618472 |
| STATUS | Completed |
| PARTICIPANTS | 50 participants, child, adult,older adult, sex: female |
| PERIOD | August 2018-January 2020 |
| INTERVENTION | Drug: Metformin Hydrochloride 850 MG |
| Regular strength metformin (850 mg/tab) vs. placebo | |
| CANCER TYPE | Endometrial cancer who undergoing complete surgical staging |
| OUTCOME | Metformin administration reduced Ki-67 expression and reduced grade in endometrial tumor when given for 4 weeks before hysterectomy |
Table 2.
Unmasked metformin clinical studies ended after 2015
| STUDY | A Phase II Evaluation of Metformin, Targeting Cancer Stem Cells for the Prevention of Relapse in Patients With Stage IIC/III/IV Ovarian, Fallopian Tube, and Primary Peritoneal Cancer |
|
| |
| NCT NUMBER | NCT01579812 |
| STATUS | Completed |
| PARTICIPANTS | 38 participants, 18-80 years, sex: female |
| PERIOD | April 2012-May 2018 |
| INTERVENTION | Metformin |
| CANCER TYPE | Ovarian, Fallopian Tube, and Primary Peritoneal Cancer |
| OUTCOME | Metformin therapy was associated with better-than-expected overall survival, supporting the use of metformin in phase III studies |
|
| |
| STUDY | Pilot Study of Metformin in Head and Neck Squamous Cell Cancer and Its Effects on Stromal-epithelial Metabolic Uncoupling |
|
| |
| NCT NUMBER | NCT02083692 |
| STATUS | Completed |
| PARTICIPANTS | 50 participants, 18-80 years, sex: all |
| PERIOD | March 2014-October 2016 |
| INTERVENTION | Metformin |
| CANCER TYPE | Head and Neck Squamous Cell Cancer |
| OUTCOME | Metformin treatment may favorably alter the immune TME (tumor microenvironment) in HNSCC independent of HPV status. |
|
| |
| STUDY | Prospective Evaluation of Clinical Safety of Combining Metformin With Anticancer Chemotherapy |
|
| |
| NCT NUMBER | NCT01442870 |
| STATUS | Completed |
| PARTICIPANTS | 105 participants, 18-79 years, sex: all |
| PERIOD | September 2011-May 2017 |
| INTERVENTION | Metformin |
| CANCER TYPE | Solid tumor |
| OUTCOME | This is the largest phase I study of metformin combined with chemotherapy, which suggests that metformin can be given safely with chemotherapy, and offers a platform for future studies. Post-metformin increase in AMPK phosphorylation may potentially explain lack of disease progression in nearly half of our patients. |
Metformin, cancer and immunity
It is of quite relevance to have in mind that human immune system defend us not only from foreign pathogens but also from cancer considering cancer prevention, development, and metastasis [95]. In a cancer growth inflammation is comparable to double-edged sword.
At the onset of tumorigenesis, inflammation carries crucial function in abolishing transformed cells as an essential part of innate immunity, as well presenting cancer antigens for adaptive immunity [95]. Contrarily, chronic inflammation is recognized to raise the prevalence and malignancy in various cancer models [96]. Not long ago it has been described anti-inflammatory function of metformin in certain disease models. It was determined that metformin diminished inception of multiple sclerosis, control-case studies on T2D patients proposed that metformin hampers cardiovascular difficulties [97] and there is also perspective for metformin as treatment in inflammatory skin disorders [98]. So far it was implied that metformin regulates encouraging disease outcome, diminishing pro-inflammatory cytokines amount [99]. These findings have opened a new query focusing metformin as a possible therapeutic in immune-oncology.
The permanent tumor rescue phenomena involves natural killer cells (NK), tumor associated macrophages (TAMs), myeloid derived suppressor cells (MDSCs) and CD8+ cytotoxic T lymphocytes (CTL).
Metformin promotes NK-cells activation
NK cells have crucial function in the innate immune response of the host and are particularly responsive to metastatic cells or certain hematological cancers [100]. The processes by which NK cells detect cancerous cells remain obscure, although CD1d-mediated presentation by APC of glycolipid antigens to NK cells [101] and by major histocompatibility complex class I related chain A (MICA) (a non-classical HLA molecules) [102] has been evidenced. Since AMPK has important role in lipogenic pathways regulation, pretreatment with different pharmacological compounds among them and metformin, appropriately augmented CD1d and MICA mediated NK-cell activation [99]. These data propose that addressing lipogenic cellular metabolism in NK cells may provide an original target of innate immune responses stimulation (Figure 4).
Figure 4.
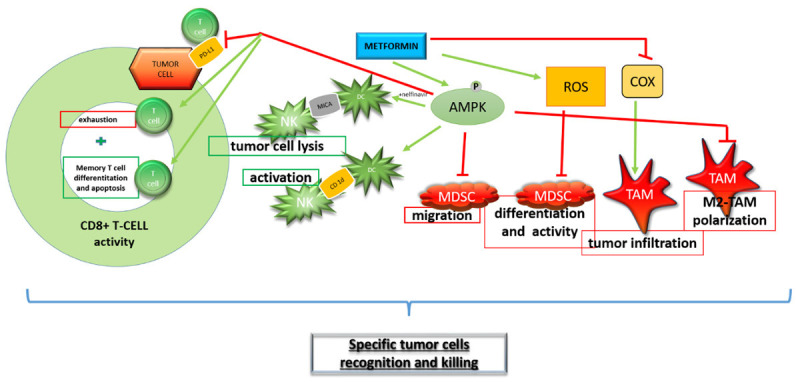
Potential role of metformin in cancer cell killing by immune system regulation. Cells filled red promote tumor survival, while green filled cells are involved in tumor cells killing. Green arrows represent activation of molecules or cells depicted on Figure 4, whereas red lines describes inhibitory interaction between metformin and presented molecules or cells. Red squared boxes show down regulation of specific process by metformin and green squared boxes describe process possibly up-regulated by metformin.
Metformin shifts macrophage polarization
Macrophages can be classified into two main subsets according to their activation state, classically-activated M1 and alternatively-activated M2 [103]. TAMs, for nearly all types of cancer appear the M2-like subset. TAMs make specific cytokines thereupon restricting the antitumor immune response and augmenting tumor growth [103]. Several studies reported that metformin in lung, breast, prostate, osteosarcoma and diverse cancer models in murine alters TAM polarization form M2- to M1-like phenotype generally by switching cytokine production distinct for each subset, preventing the infiltration of TAMs and inducing the metabolic shift from M2 to M1-like TAM phenotype [103] (Figure 4).
TAM polarization has relevance in cancer immunity, thus it could extend a novel benefit for the supplementation of metformin as component of an immunotherapy procedure.
Metformin inhibits MDSCs activity
A significant immunosuppressive cell group that assembly in tumor-bearing patients are MDSCs that can block T cells function and reinforce tumor immune escape. In vitro and in vivo analysis described that metformin curtailed MDSC migration, abolished MDSC inhibitory activity and evoked Th1 and CTL feedback in murine colon cancer CT-26 cell-transplanted mice [99]. Delineated metformin effects are AMPK-dependent, imply inhibition of different AMPK substrates and subsequent restraining of human MDSC mediated immunosuppression (Figure 4).
Effect of metformin on CD8+ CTLs
Cancer cells provoke recurrent T cell receptor-TCR stimulation with tumor antigens and further continue in constantly deterioration of CD8+ CTL capacity to secrete interleukine-2/IL-2, tumor necrosis factor alpha/TNFα, and interferon gamma/IFNγ, subsequently undergoing apoptotic removal in a mechanism accepted as immune exhaustion [104]. Immune exhaustion is characterized by phenotypic modifications in CD8+ CTL cells, simultaneously with the expression of exhaustion markers like programmed cell death protein/PD-, and its high expression is noticed in several types of cancer. This observation is linked with poor prognosis [103]. Accordingly, improved CD8+ CTL activity versus cancer cells could be reached by retraction of PD-L1 impeding signal (Figure 4). Actually, there are several investigations validating significance of metformin in AMPK-dependent manner on PD-L1 levels starting from blocking of PDCD1 gene transcription, PD-L1 phosphorylation and subsequent disintegration in endoplasmic reticulum (ER), decline of membrane-bound PD-L1 in different cancer models [103]. Further, data obtained on various syngeneic animal models propose that fusion of metformin and Anti-CTLA-4 therapy, has the efficiency to improve immunotherapy [103]. Thus, these studies indicate that metformin can reinforce CD8+ CTL feedback by diminishing PD-L1 level (Figure 4).
The effect of metformin in metabolic adjustments of T-cells
It has already been presented metformin’s role in cancer cell metabolism thus recent research explore effect of metformin on metabolic adaptation of T-cells in TME.
Considering that main regulators in energy sensing and mitochondrial function are AMPK and mTOR, well known downstream targets of metformin, there are studies proposing the effect of metformin on AMPK/mTOR axis in metabolic reprogramming of depleted CD8+ CTL in TME. These studies presumed that metformin by metabolic reprogramming favored CD8+ CTL activity, CD8+ memory T-cells generation and shift of T-cell central memory cells (TCM) to CD8+ T cell effector memory cells (TEM) [105]. Also, generation of the continuous antitumor immunity might be supported by negative influence of metformin on Ti-Treg [106].
These functions of metformin could have potential to be addressed for enhancing vaccine efficacy and antitumor immunity.
Possible function of metformin in cancer immunotherapy
Perhaps an original therapeutic concept is immunotherapy that could implement incomparable advantage to certainly amend the treatment of various diseases, including cancer. Current antitumor drugs are generally ambiguous and also harmful to normal cells. Contrary, immunotherapeutics facilitate the immune system to specifically recognize and kill tumor cells. However exhaustion of immune system in TME, imposes request for immune therapy breakthrough. Immunotherapy has the capability with a many recent technological upgrades in physicochemical and molecular biology field to become accepted as cancer treatment.
Form of phototherapy including light and photosensitizing chemical substance, applied in combination with molecular oxygen is referred as photodynamic therapy (PDT). Chemical damage of tumor cells can be obtained if enough oxygen is present to produce ROS by PDT. Likewise, PDT augments the interferon γ (IFN-γ) generation and stimulates the antitumor immunity of T cells. Overlooked consequence of boosted IFN-γ production is important increase of the programmed death-ligand 1 (PD-L1) expression on tumor cell membrane committing inhibition of CD8+ CTL cell function. New research reported structure as a two-in-one nanoplatform (IR775@Met@Lip) designed by packing metformin (Met) and IR775 into a clinically practical liposome. Notably, nanoplatform displayed reduced PD-L1 expression, alleviated T cell exhaustion, conversed tumor hypoxia and successfully abolished both the primary and abscopal tumor growth in bladder and colon cancers, respectively. Aforementioned complex is considerably promising to become a feasible cancer therapy approach [107].
Developing branch of photodynamic therapy (PDT) is photothermal therapy (PTT). This concept proposes utilizing electromagnetic radiation (most often in infrared wavelengths) to move the sensitizer to an excited state where it then releases vibrational energy-heat that kills the targeted cells. Current studies also provided longer wavelength light application, which has less energy and therefore is less detrimental to other cells and tissues. Photothermal therapy (PTT) does not demand oxygen to affect the target cells or tissues contrary to photodynamic therapy.
Group of researchers proposed tumor vaccine vector (TA-Met@MS) comprised of tumor antigen (TA), metformin (Met) and Hollow gold nanospheres (HAuNS) in poly (lactic-co-glycolic acid) (PLGA) microspheres. NIR light-mediated photothermal effect can contribute to a pulsed-release behavior of TA and Met from the microspheres. Primary T cell expansion, contraction and stimulated production of effector T cells at the early immunization stage can be managed by released TA. As a result of AMPK activation controlled by metformin the metabolic scheme in the cells is then shifted from glycolysis into fatty acids oxidation (FAO). FAO can increase T cell survival and assist the differentiation of memory CD8+T cells. For upgrading cancer prevention and therapy this study may give a useful consideration to develop tumor vaccine [108].
Ongoing research in immunotherapy focused on creation the cell membrane-based biomimetic nanoparticles (NPs) constructs, to improve therapeutic and imaging applications. In these constructs the physicochemical properties of the NP are retained, whereas camouflaged complex components of a natural cell membrane should endorse the NPs with many fascinating biological functions. Lately, cancer cells and a variety of cells omitting red blood cells (RBCs), including platelets, immune cells (e.g., macrophages) have been manipulated to restore membrane materials. To support synthetic NPs with multifunctional and sophisticated cell-like functions single cell membrane appliance could be remodeled, by hybrid cell membrane-coating construction. Further, as an encouraging tool to attain multi-modal cancer therapy nano drug co-delivery system has appeared.
To analyse this principle metformin and siRNA Fibrinogen-like protein 1/FGL1 were encapsulated in PLGA to form a basis, following coating with a hybrid biomimetic membrane from macrophages and cancer cells to form a multiple-targeting biomimetic nanoplatform. To promote the endosomal/lysosomal evasion of the encapsulated siRNA for adequate cytosolic siRNA delivery this nanoplatform was created as pH-activated CO2 gas-producing nanoplatform (MC-PLGA@Met-CO2/siFGL1 NPs).
Important synergistic therapeutic efficacy against breast cancer in vitro and in vivo was presented with a PD-L1/programmed death 1 signaling restriction and FGL1 gene silencing. Futhermore, metformin stimulated M1-type differentiation of tumor-related macrophages diminishing tumor hypoxia and certainly developing the microenvironment immunosuppressive for tumor [109].
This results implied plausible position of MC-PLGA@Met-CO2/siFGL1 NPs nanoplatform in breast cancer immunotherapy application.
We suggest that these recently published papers implemented the role of metformin in state-of-the-art technology improvement in cancer immunotherapy.
Conclusion
Metformin is a commonly prescribed anti-diabetic drug showing diverse anticancer effects in vitro and in vivo. The advantage of metformin in cancer therapy has to be validated in human pharmacodynamic, pharmacokinetic and phase II clinical studies especially in non-diabetic patients. The complexity of activated/inhibited signaling pathways in cancer cells during metformin administration in different in vitro/in vivo context may be a limiting factor when using this drug as possibly plain anti-cancer therapy.
Besides considering metformin as a single agent in cancer therapy, metformin might be used in appropriate combinations with targeted therapies taking into account their limited success. Physicians should be aware of possible inhibitory effect of metformin along with conventional chemotherapeutics application.
Recently, novel role of metformin in regulating cancer immunity has emerged. Intriguingly about this function of metformin is modulation of precise AMPK-dependent mechanisms shared between cancer cells and immune cells, but exerting in most instances favorable opposite effect: cancer cell killing and improvement of immune system cells function. Therefore, we imply that original approach in investigation of combined metformin-immunotherapy in cancer treatment could bridge the gap between basic molecular biology research and future clinical studies.
Acknowledgements
The work was supported by The Ministry of education, science and technological development of Republic of Serbia (No. 451-03-9/2021-14/ 200007 and No. 451-03-9/2021-14/200110). We acknowledge the critical discussions and suggestions of Prof Dr. Vladimir Trajkovic during manuscript writing.
Disclosure of conflict of interest
None.
References
- 1.Jara JA, López-Muñoz R. Metformin and cancer: between the bioenergetic disturbances and the antifolate activity. Pharmacol Res. 2015;101:102–108. doi: 10.1016/j.phrs.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 3.Yu X, Mao W, Zhai Y, Tong C, Liu M, Ma L, Yu X, Li S. Anti-tumor activity of metformin: from metabolic and epigenetic perspectives. Oncotarget. 2017;8:5619–5628. doi: 10.18632/oncotarget.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikhlas S, Ahmad M. Metformin: insights into its anticancer potential with special reference to AMPK dependent and independent pathways. Life Sci. 2017;185:53–62. doi: 10.1016/j.lfs.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Pierotti MA, Berrino F, Gariboldi M, Melani C, Mogavero A, Negri T, Pasanisi P, Pilotti S. Targeting metabolism for cancer treatment and prevention: metformin, an old drug with multi-faceted effects. Oncogene. 2013;32:1475–1487. doi: 10.1038/onc.2012.181. [DOI] [PubMed] [Google Scholar]
- 6.Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carling D, Mayer FV, Sanders MJ, Gamblin SJ. AMP-activated protein kinase: nature’s energy sensor. Nat Chem Biol. 2011;7:512–518. doi: 10.1038/nchembio.610. [DOI] [PubMed] [Google Scholar]
- 8.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi A, Kimura F, Yamanaka A, Takebayashi A, Kita N, Takahashi K, Murakami T. Metformin impairs growth of endometrial cancer cells via cell cycle arrest and concomitant autophagy and apoptosis. Cancer Cell Int. 2014;14:53. doi: 10.1186/1475-2867-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isakovic A, Harhaji L, Stevanovic D, Markovic Z, Sumarac-Dumanovic M, Starcevic V, Micic D, Trajkovic V. Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci. 2007;64:1290–1302. doi: 10.1007/s00018-007-7080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 13.Gotlieb WH, Saumet J, Beauchamp MC, Gu J, Lau S, Pollak MN, Bruchim I. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110:246–250. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Shi WY, Xiao D, Wang L, Dong LH, Yan ZX, Shen ZX, Chen SJ, Chen Y, Zhao WL. Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis. 2012;3:e275. doi: 10.1038/cddis.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene. 2007;26:7825–7832. doi: 10.1038/sj.onc.1210594. [DOI] [PubMed] [Google Scholar]
- 17.Pryor R, Cabreiro F. Repurposing metformin: an old drug with new tricks in its binding pockets. Biochem J. 2015;471:307–322. doi: 10.1042/BJ20150497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu CC, Chiang JH, Tsai FJ, Hsu YM, Juan YN, Yang JS, Chiu HY. Metformin triggers the intrinsic apoptotic response in human AGS gastric adenocarcinoma cells by activating AMPK and suppressing mTOR/AKT signaling. Int J Oncol. 2019;54:1271–1281. doi: 10.3892/ijo.2019.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han G, Gong H, Wang Y, Guo S, Liu K. AMPK/mTOR-mediated inhibition of survivin partly contributes to metformin-induced apoptosis in human gastric cancer cell. Cancer Biol Ther. 2015;16:77–87. doi: 10.4161/15384047.2014.987021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan H, Yu X, Zou Z, Zheng W, Deng X, Guo L, Jiang W, Zhan Q, Lu SH. Metformin suppresses the esophageal carcinogenesis in rats treated with NMBzA through inhibiting AMPK/mTOR signaling pathway. Carcinogenesis. 2019;40:669–679. doi: 10.1093/carcin/bgy160. [DOI] [PubMed] [Google Scholar]
- 21.Cai H, Zhang Y, Han TK, Everett RS, Thakker DR. Cation-selective transporters are critical to the AMPK-mediated antiproliferative effects of metformin in human breast cancer cells. Int J Cancer. 2016;138:2281–2292. doi: 10.1002/ijc.29965. [DOI] [PubMed] [Google Scholar]
- 22.Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell Res. 2014;24:42–57. doi: 10.1038/cr.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Xu W, Yan Z, Zhao W, Mi J, Li J, Yan H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J Exp Clin Cancer Res. 2018;37:63. doi: 10.1186/s13046-018-0731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomic T, Botton T, Cerezo M, Robert G, Luciano F, Puissant A, Gounon P, Allegra M, Bertolotto C, Bereder JM, Tartare-Deckert S, Bahadoran P, Auberger P, Ballotti R, Rocchi S. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2:e199. doi: 10.1038/cddis.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao C, Fang L, Zhang H. Metformin induces autophagy via the AMPK-mTOR signaling pathway in human hepatocellular carcinoma cells. 2020; 12:5803–5811. doi: 10.2147/CMAR.S257966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben Sahra I, Tanti JF, Bost F. The combination of metformin and 2-deoxyglucose inhibits autophagy and induces AMPK-dependent apoptosis in prostate cancer cells. Autophagy. 2010;6:670–671. doi: 10.4161/auto.6.5.12434. [DOI] [PubMed] [Google Scholar]
- 27.De Santi M, Baldelli G, Diotallevi A, Galluzzi L, Schiavano GF, Brandi G. Metformin prevents cell tumorigenesis through autophagy-related cell death. Sci Rep. 2019;9:66. doi: 10.1038/s41598-018-37247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao Z, Gaertner S, Morresi-Hauf A, Genzel R, Duell T, Ullrich A, Knyazev PG. Metformin triggers autophagy to attenuate drug-induced apoptosis in NSCLC cells, with minor effects on tumors of diabetic patients. Neoplasia. 2017;19:385–395. doi: 10.1016/j.neo.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Zhou P, Xu K, Chen T, Jiao J, Wei H, Yang X, Xu W, Wan W, Xiao J. Metformin induces cell cycle arrest, apoptosis and autophagy through ROS/JNK signaling pathway in human osteosarcoma. Int J Biol Sci. 2020;16:74–84. doi: 10.7150/ijbs.33787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wandee J, Prawan A, Senggunprai L, Kongpetch S, Tusskorn O, Kukongviriyapan V. Metformin enhances cisplatin induced inhibition of cholangiocarcinoma cells via AMPK-mTOR pathway. Life Sci. 2018;207:172–183. doi: 10.1016/j.lfs.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 31.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, Mamer OA, Avizonis D, DeBerardinis RJ, Siegel PM, Jones RG. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells. Mol Cells. 2013;36:279–287. doi: 10.1007/s10059-013-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 34.Seo Y, Kim J, Park SJ, Park JJ, Cheon JH, Kim WH, Kim TI. Metformin suppresses cancer stem cells through AMPK activation and inhibition of protein prenylation of the mevalonate pathway in colorectal cancer. Cancer (Basel) 2020;12:2554. doi: 10.3390/cancers12092554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 36.Tyszka-Czochara M, Konieczny P, Majka M. Caffeic acid expands anti-tumor effect of metformin in human metastatic cervical carcinoma HTB-34 cells: implications of AMPK activation and impairment of fatty acids de novo biosynthesis. Int J Mol Sci. 2017;18:462. doi: 10.3390/ijms18020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien AJ, Villani LA, Broadfield LA, Houde VP, Galic S, Blandino G, Kemp BE, Tsakiridis T, Muti P, Steinberg GR. Salicylate activates AMPK and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. Biochem J. 2015;469:177–187. doi: 10.1042/BJ20150122. [DOI] [PubMed] [Google Scholar]
- 38.Sinnett-Smith J, Kisfalvi K, Kui R, Rozengurt E. Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: dependence on glucose concentration and role of AMPK. Biochem Biophys Res Commun. 2013;430:352–357. doi: 10.1016/j.bbrc.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janzer A, German NJ, Gonzalez-Herrera KN, Asara JM, Haigis MC, Struhl K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc Natl Acad Sci U S A. 2014;111:10574–10579. doi: 10.1073/pnas.1409844111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SH, Kim SC, Ku JL. Metformin increases chemo-sensitivity via gene downregulation encoding DNA replication proteins in 5-Fu resistant colorectal cancer cells. Oncotarget. 2017;8:56546–56557. doi: 10.18632/oncotarget.17798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan AS, Frigo DE. A spatiotemporal hypothesis for the regulation, role, and targeting of AMPK in prostate cancer. Nat Rev Urol. 2017;14:164–180. doi: 10.1038/nrurol.2016.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato A, Sunayama J, Okada M, Watanabe E, Seino S, Shibuya K, Suzuki K, Narita Y, Shibui S, Kayama T, Kitanaka C. Glioma-initiating cell elimination by metformin activation of FOXO3 via AMPK. Stem Cells Transl Med. 2012;1:811–824. doi: 10.5966/sctm.2012-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 44.Ferretti AC, Hidalgo F, Tonucci FM, Almada E, Pariani A, Larocca MC, Favre C. Metformin and glucose starvation decrease the migratory ability of hepatocellular carcinoma cells: targeting AMPK activation to control migration. Sci Rep. 2019;9:2815. doi: 10.1038/s41598-019-39556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu M, Zhang Z, Wang H, Chen X, Jin C. Activation of AMPK by metformin promotes renal cancer cell proliferation under glucose deprivation through its interaction with PKM2. Int J Biol Sci. 2019;15:617–627. doi: 10.7150/ijbs.29689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer. 2008;15:833–839. doi: 10.1677/ERC-08-0038. [DOI] [PubMed] [Google Scholar]
- 47.Chuang HC, Chou CC, Kulp SK, Chen CS. AMPK as a potential anticancer target-friend or foe? Curr Pharm Des. 2014;20:2607–2618. doi: 10.2174/13816128113199990485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hampsch RA, Wells JD, Traphagen NA, McCleery CF, Fields JL, Shee K, Dillon LM, Pooler DB, Lewis LD, Demidenko E, Huang YH, Marotti JD, Goen AE, Kinlaw WB, Miller TW. AMPK activation by metformin promotes survival of dormant ER(+) breast cancer cells. Clin Cancer Res. 2020;26:3707–3719. doi: 10.1158/1078-0432.CCR-20-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS, Shaw RJ. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 51.Algire C, Amrein L, Bazile M, David S, Zakikhani M, Pollak M. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene. 2011;30:1174–1182. doi: 10.1038/onc.2010.483. [DOI] [PubMed] [Google Scholar]
- 52.Yuan J, Dong X, Yap J, Hu J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J Hematol Oncol. 2020;13:113. doi: 10.1186/s13045-020-00949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Wang T, Hu M, Zhang Y, Chen H, Xu L. Metformin overcomes acquired resistance to EGFR TKIs in EGFR-mutant lung cancer via AMPK/ERK/NF-κB signaling pathway. Front Oncol. 2020;10:1605. doi: 10.3389/fonc.2020.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye H, Liu Y, Wu K, Luo H, Cui L. AMPK activation overcomes anti-EGFR antibody resistance induced by KRAS mutation in colorectal cancer. Cell Commun Signal. 2020;18:115. doi: 10.1186/s12964-020-00584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haq R, Shoag J, Andreu-Perez P, Yokoyama S, Edelman H, Rowe GC, Frederick DT, Hurley AD, Nellore A, Kung AL, Wargo JA, Song JS, Fisher DE, Arany Z, Widlund HR. Oncogenic BRAF regulates oxidative metabolism via PGC1α and MITF. Cancer Cell. 2013;23:302–315. doi: 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pollak M. Targeting oxidative phosphorylation: why, when, and how. Cancer Cell. 2013;23:263–264. doi: 10.1016/j.ccr.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Shang S, Hua F, Hu ZW. The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. 2017;8:33972–33989. doi: 10.18632/oncotarget.15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amable G, Martínez-León E, Picco ME, Di Siervi N, Davio C, Rozengurt E, Rey O. Metformin inhibits β-catenin phosphorylation on Ser-552 through an AMPK/PI3K/Akt pathway in colorectal cancer cells. Int J Biochem Cell Biol. 2019;112:88–94. doi: 10.1016/j.biocel.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Zou YF, Xie CW, Yang SX, Xiong JP. AMPK activators suppress breast cancer cell growth by inhibiting DVL3-facilitated Wnt/β-catenin signaling pathway activity. Mol Med Rep. 2017;15:899–907. doi: 10.3892/mmr.2016.6094. [DOI] [PubMed] [Google Scholar]
- 60.Park SY, Kim D, Kee SH. Metformin-activated AMPK regulates β-catenin to reduce cell proliferation in colon carcinoma RKO cells. Oncol Lett. 2019;17:2695–2702. doi: 10.3892/ol.2019.9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yadav RK, Chauhan AS, Zhuang L, Gan B. FoxO transcription factors in cancer metabolism. Semin Cancer Biol. 2018;50:65–76. doi: 10.1016/j.semcancer.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou J, Hong L, Luo C, Li Z, Zhu Y, Huang T, Zhang Y, Yuan H, Hu Y, Wen T, Zhuang W, Cai B, Zhang X, Huang J, Cheng J. Metformin inhibits estrogen-dependent endometrial cancer cell growth by activating the AMPK-FOXO1 signal pathway. Cancer Sci. 2016;107:1806–1817. doi: 10.1111/cas.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun Y, Tao C, Huang X, He H, Shi H, Zhang Q, Wu H. Metformin induces apoptosis of human hepatocellular carcinoma HepG2 cells by activating an AMPK/p53/miR-23a/FOXA1 pathway. Onco Targets Ther. 2016;9:2845–2853. doi: 10.2147/OTT.S99770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Queiroz EA, Akamine EH, de Carvalho MH, Sampaio SC, Fortes ZB. Metformin reduces the Walker-256 tumor development in obese-MSG rats via AMPK and FOXO3a. Life Sci. 2015;121:78–87. doi: 10.1016/j.lfs.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 65.Mancinelli R, Carpino G, Petrungaro S, Mammola CL, Tomaipitinca L, Filippini A, Facchiano A, Ziparo E, Giampietri C. Multifaceted roles of GSK-3 in cancer and autophagy-related diseases. 2017;2017:4629495. doi: 10.1155/2017/4629495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo Z, Chen W, Wu W, Luo W, Zhu T, Guo G, Zhang L, Wang C, Li M, Shi S. Metformin promotes survivin degradation through AMPK/PKA/GSK-3β-axis in non-small cell lung cancer. J Cell Biochem. 2019 doi: 10.1002/jcb.28470. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 67.Luo Z, Zhu T, Luo W, Lv Y, Zhang L, Wang C, Li M, Wu W, Shi S. Metformin induces apoptotic cytotoxicity depending on AMPK/PKA/GSK-3β-mediated c-FLIP(L) degradation in non-small cell lung cancer. Cancer Manag Res. 2019;11:681–689. doi: 10.2147/CMAR.S178688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blandino G, Valerio M, Cioce M, Mori F, Casadei L, Pulito C, Sacconi A, Biagioni F, Cortese G, Galanti S, Manetti C, Citro G, Muti P, Strano S. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat Commun. 2012;3:865. doi: 10.1038/ncomms1859. [DOI] [PubMed] [Google Scholar]
- 69.He G, Zhang YW, Lee JH, Zeng SX, Wang YV, Luo Z, Dong XC, Viollet B, Wahl GM, Lu H. AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Mol Cell Biol. 2014;34:148–157. doi: 10.1128/MCB.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Janjetovic K, Harhaji-Trajkovic L, Misirkic-Marjanovic M, Vucicevic L, Stevanovic D, Zogovic N, Sumarac-Dumanovic M, Micic D, Trajkovic V. In vitro and in vivo anti-melanoma action of metformin. Eur J Pharmacol. 2011;668:373–382. doi: 10.1016/j.ejphar.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Zhou X, Kuang Y, Liang S, Wang L. Metformin inhibits cell proliferation in SKM-1 cells via AMPK-mediated cell cycle arrest. J Pharmacol Sci. 2019;141:146–152. doi: 10.1016/j.jphs.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Cai X, Hu X, Tan X, Cheng W, Wang Q, Chen X, Guan Y, Chen C, Jing X. Metformin induced AMPK activation, G0/G1 phase cell cycle arrest and the inhibition of growth of esophageal squamous cell carcinomas in vitro and in vivo. PLoS One. 2015;10:e0133349. doi: 10.1371/journal.pone.0133349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim MY, Kim YS, Kim M, Choi MY, Roh GS, Lee DH, Kim HJ, Kang SS, Cho GJ, Shin JK, Choi WS. Metformin inhibits cervical cancer cell proliferation via decreased AMPK O-GlcNAcylation. Anim Cells Syst (Seoul) 2019;23:302–309. doi: 10.1080/19768354.2019.1614092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti JF, Giorgetti-Peraldi S, Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 75.Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ben Sahra I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 77.Athreya AP, Kalari KR, Cairns J, Gaglio AJ, Wills QF, Niu N, Weinshilboum R, Iyer RK, Wang L. Model-based unsupervised learning informs metformin-induced cell-migration inhibition through an AMPK-independent mechanism in breast cancer. Oncotarget. 2017;8:27199–27215. doi: 10.18632/oncotarget.16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan DK, Miskimins WK. Metformin and phenethyl isothiocyanate combined treatment in vitro is cytotoxic to ovarian cancer cultures. J Ovarian Res. 2012;5:19. doi: 10.1186/1757-2215-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khodaei F, Hosseini SM, Omidi M, Hosseini SF, Rezaei M. Cytotoxicity of metformin against HT29 colon cancer cells contributes to mitochondrial Sirt3 upregulation. J Biochem Mol Toxicol. 2021;35:e22662. doi: 10.1002/jbt.22662. [DOI] [PubMed] [Google Scholar]
- 80.Zhao Y, Luo Q, Mo J, Li J, Ye D, Ao Z, Chen L, Liu J. Metformin in combination with JS-K inhibits growth of renal cell carcinoma cells via reactive oxygen species activation and inducing DNA breaks. J Cancer. 2020;11:3701–3712. doi: 10.7150/jca.36372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park D. Metformin induces oxidative stress-mediated apoptosis without the blockade of glycolysis in H4IIE hepatocellular carcinoma cells. Biol Pharm Bull. 2019;42:2002–2008. doi: 10.1248/bpb.b19-00474. [DOI] [PubMed] [Google Scholar]
- 82.Mogavero A, Maiorana MV, Zanutto S, Varinelli L, Bozzi F, Belfiore A, Volpi CC, Gloghini A, Pierotti MA, Gariboldi M. Metformin transiently inhibits colorectal cancer cell proliferation as a result of either AMPK activation or increased ROS production. Sci Rep. 2017;7:15992. doi: 10.1038/s41598-017-16149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tan M, Wu A, Liao N, Liu M, Guo Q, Yi J, Wang T, Huang Y, Qiu B, Zhou W. Inhibiting ROS-TFE3-dependent autophagy enhances the therapeutic response to metformin in breast cancer. Free Radic Res. 2018;52:872–886. doi: 10.1080/10715762.2018.1485075. [DOI] [PubMed] [Google Scholar]
- 84.Do MT, Kim HG, Khanal T, Choi JH, Kim DH, Jeong TC, Jeong HG. Metformin inhibits heme oxygenase-1 expression in cancer cells through inactivation of Raf-ERK-Nrf2 signaling and AMPK-independent pathways. Toxicol Appl Pharmacol. 2013;271:229–238. doi: 10.1016/j.taap.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 85.Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin W, Ke J, Huang J, Yeung SC, Zhang H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5:e1088. doi: 10.1038/cddis.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang J, Wei J, Wu Y, Wang Z, Guo Y, Lee P, Li X. Metformin induces ER stress-dependent apoptosis through miR-708-5p/NNAT pathway in prostate cancer. Oncogenesis. 2015;4:e158. doi: 10.1038/oncsis.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Melnik S, Dvornikov D, Müller-Decker K, Depner S, Stannek P, Meister M, Warth A, Thomas M, Muley T, Risch A, Plass C. Cancer cell specific inhibition of Wnt/β-catenin signaling by forced intracellular acidification. Cell Discov. 2018;4:37. doi: 10.1038/s41421-018-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salani B, Marini C, Rio AD, Ravera S, Massollo M, Orengo AM, Amaro A, Passalacqua M, Maffioli S, Pfeffer U, Cordera R, Maggi D, Sambuceti G. Metformin impairs glucose consumption and survival in Calu-1 cells by direct inhibition of hexokinase-II. Sci Rep. 2013;3:2070. doi: 10.1038/srep02070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marini C, Salani B, Massollo M, Amaro A, Esposito AI, Orengo AM, Capitanio S, Emionite L, Riondato M, Bottoni G, Massara C, Boccardo S, Fabbi M, Campi C, Ravera S, Angelini G, Morbelli S, Cilli M, Cordera R, Truini M, Maggi D, Pfeffer U, Sambuceti G. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle. 2013;12:3490–3499. doi: 10.4161/cc.26461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong L, Zhou Q, Zhang Z, Zhu Y, Duan T, Feng Y. Metformin sensitizes endometrial cancer cells to chemotherapy by repressing glyoxalase I expression. J Obstet Gynaecol Res. 2012;38:1077–1085. doi: 10.1111/j.1447-0756.2011.01839.x. [DOI] [PubMed] [Google Scholar]
- 91.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lengyel E, Litchfield LM, Mitra AK, Nieman KM, Mukherjee A, Zhang Y, Johnson A, Bradaric M, Lee W, Romero IL. Metformin inhibits ovarian cancer growth and increases sensitivity to paclitaxel in mouse models. Am J Obstet Gynecol. 2015;212:479.e1–479.e10. doi: 10.1016/j.ajog.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Janjetovic K, Vucicevic L, Misirkic M, Vilimanovich U, Tovilovic G, Zogovic N, Nikolic Z, Jovanovic S, Bumbasirevic V, Trajkovic V, Harhaji-Trajkovic L. Metformin reduces cisplatin-mediated apoptotic death of cancer cells through AMPK-independent activation of Akt. Eur J Pharmacol. 2011;651:41–50. doi: 10.1016/j.ejphar.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 94.Zhang P, Zhao S, Lu X, Shi Z, Liu H, Zhu B. Metformin enhances the sensitivity of colorectal cancer cells to cisplatin through ROS-mediated PI3K/Akt signaling pathway. Gene. 2020;745:144623. doi: 10.1016/j.gene.2020.144623. [DOI] [PubMed] [Google Scholar]
- 95.Göbel A, Dell’Endice S, Jaschke N, Pählig S, Shahid A, Hofbauer LC, Rachner TD. The role of inflammation in breast and prostate cancer metastasis to bone. Int J Mol Sci. 2021;22:5078. doi: 10.3390/ijms22105078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roe K. An inflammation classification system using cytokine parameters. Scand J Immunol. 2021;93:e12970. doi: 10.1111/sji.12970. [DOI] [PubMed] [Google Scholar]
- 97.Triggle CR, Ding H. Cardiovascular impact of drugs used in the treatment of diabetes. Ther Adv Chronic Dis. 2014;5:245–268. doi: 10.1177/2040622314546125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang JE, Choi MS. A molecular perspective on the potential benefits of metformin for the treatment of inflammatory skin disorders. Int J Mol Sci. 2020;21:8960. doi: 10.3390/ijms21238960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim K, Yang WH, Jung YS, Cha JH. A new aspect of an old friend: the beneficial effect of metformin on anti-tumor immunity. BMB Rep. 2020;53:512–520. doi: 10.5483/BMBRep.2020.53.10.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Souza-Fonseca-Guimaraes F, Cursons J, Huntington ND. The emergence of natural killer cells as a major target in cancer immunotherapy. Trends Immunol. 2019;40:142–158. doi: 10.1016/j.it.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 101.Webb TJ, Carey GB, East JE, Sun W, Bollino DR, Kimball AS, Brutkiewicz RR. Alterations in cellular metabolism modulate CD1d-mediated NKT-cell responses. Pathog Dis. 2016;74:ftw055. doi: 10.1093/femspd/ftw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xia C, He Z, Liang S, Chen R, Xu W, Yang J, Xiao G, Jiang S. Metformin combined with nelfinavir induces SIRT3/mROS-dependent autophagy in human cervical cancer cells and xenograft in nude mice. Eur J Pharmacol. 2019;848:62–69. doi: 10.1016/j.ejphar.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 103.Ma R, Yi B, Riker AI, Xi Y. Metformin and cancer immunity. Acta Pharmacol Sin. 2020;41:1403–1409. doi: 10.1038/s41401-020-00508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ren D, Qin G, Zhao J, Sun Y, Zhang B, Li D, Wang B, Jin X, Wu H. Metformin activates the STING/IRF3/IFN-β pathway by inhibiting AKT phosphorylation in pancreatic cancer. Am J Cancer Res. 2020;10:2851–2864. [PMC free article] [PubMed] [Google Scholar]
- 105.Pereira FV, Melo ACL, Low JS, de Castro ÍA, Braga TT, Almeida DC, Batista de Lima AGU, Hiyane MI, Correa-Costa M, Andrade-Oliveira V, Origassa CST, Pereira RM, Kaech SM, Rodrigues EG, Câmara NOS. Metformin exerts antitumor activity via induction of multiple death pathways in tumor cells and activation of a protective immune response. Oncotarget. 2018;9:25808–25825. doi: 10.18632/oncotarget.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kunisada Y, Eikawa S, Tomonobu N, Domae S, Uehara T, Hori S, Furusawa Y, Hase K, Sasaki A, Udono H. Attenuation of CD4(+)CD25(+) regulatory T cells in the tumor microenvironment by metformin, a type 2 diabetes drug. EBioMedicine. 2017;25:154–164. doi: 10.1016/j.ebiom.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiong W, Qi L, Jiang N, Zhao Q, Chen L, Jiang X, Li Y, Zhou Z, Shen J. Metformin liposome-mediated PD-L1 downregulation for amplifying the photodynamic immunotherapy efficacy. ACS Appl Mater Interfaces. 2021;13:8026–8041. doi: 10.1021/acsami.0c21743. [DOI] [PubMed] [Google Scholar]
- 108.Luo L, Li X, Zhang J, Zhu C, Jiang M, Luo Z, Qin B, Wang Y, Chen B, Du Y, Lou Y, You J. Enhanced immune memory through a constant photothermal-metabolism regulation for cancer prevention and treatment. Biomaterials. 2021;270:120678. doi: 10.1016/j.biomaterials.2021.120678. [DOI] [PubMed] [Google Scholar]
- 109.Gong C, Yu X, Zhang W, Han L, Wang R, Wang Y, Gao S. Regulating the immunosuppressive tumor microenvironment to enhance breast cancer immunotherapy using pH-responsive hybrid membrane-coated nanoparticles. J Nanobiotechnology. 2021;19:58. doi: 10.1186/s12951-021-00805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


