Abstract
Ferroptosis is a new form of programmed cell death characterized by iron-dependent accumulation of lipid peroxidation, which plays an important role in cancer biology. Ferroptosis is involved in many biological processes, such as amino acid metabolism, glutathione metabolism, iron metabolism, and lipid metabolism. Iron is an essential trace element in a variety of normal cell processes, such as DNA synthesis and repair, cell respiration, metabolism and signal transduction, etc., and iron metabolism disorder has been considered as one of the metabolic markers of malignant cancer cells. In addition, iron is involved in the regulation of innate and adaptive immune responses, suggesting that targeted regulation of iron metabolism may contribute to anti-tumor immunity and cancer therapy. In this review, the regulatory mechanism of ferroptosis, the interaction between ferroptosis on tumor cell metabolism, and anti-tumor immunity were systematically reviewed. Immunotherapy combined with targeted regulation of iron and iron-dependent regulation of ferroptosis should be the focus of future ferroptosis research.
Keywords: Ferroptosis, cancer, metabolism, lipid peroxidation, anti-tumor immunity
Introduction
The concept of ferroptosis was formally introduced in 2012 [1]. Ferroptosis has been described as an overwhelming mode of cell death triggered by iron-mediated lipid peroxidation. From a biochemical point of view, ferroptosis is characterized by the accumulation of iron-dependent peroxidation that produces lethal levels. Like other cell death patterns, ferroptosis is genetically regulated. Ferroptosis is also accompanied by a range of morphological, biochemical features and is highly associated with multiple intracellular metabolic pathways. Iron metabolism, amino acid metabolism, lipid metabolism, and different metabolic pathways directly affect the occurrence and development of ferroptosis and cells’ sensitivity to this cell death.
During the development and progression of cancer, the metabolism within cancer cells undergoes subtle changes. Cancer cells undergo stronger oxidative metabolism, along with increased dependence and demand for iron in cancer tissue. This undoubtedly makes ferroptosis an ideal target for the treatment of cancer [2]. But does not rule out the exclusion of some cancer cells are indeed sensitive to ferroptosis. However, some cell lines have activity against ferroptosis, because the regulation of ferroptosis is highly relevant and sensitive to cellular metabolism. Simultaneously, classical tumor suppressor genes such as P53 [3] have also been found to be involved in the regulation of ferroptosis, which also suggests the complex correlation between ferroptosis and metabolic pathways. At the same time, ferroptosis is also involved in and regulates other processes related to cancer cells, such as mesenchymalization and metastasis of cancer cells and anti-tumor immunity [4]. Those undoubtedly demonstrate the important role of ferroptosis in cancer. In this review, we list various metabolic activities associated with ferroptosis in the hope of providing ideas and inspiration for further understanding and treatment of cancer.
Characteristics of ferroptosis
Ferroptosis is an iron-dependent mode of regulatory cell death caused by the accumulation of reactive oxygen species in lipids. Unlike other cell death modes, such as necrosis, apoptosis, and autophagy, ferroptosis is mainly manifested by pyknosis of mitochondrial membrane, increased membrane density, blurred, reduced, or disappeared mitochondrial crest, and intact nuclear membrane [5]. In the biochemical aspect, increased iron ion level produces a large number of reactive oxygen species (ROS), decreased glutathione peroxidase 4 (GPX4) activity, and accumulation of lipid metabolites [6]. In terms of molecular mechanism, studies have found that nuclear factor-erythroid 2-related factor 2 (NRF2) plays a renal protective role by inhibiting ferroptosis in folate-induced acute kidney injury mice [7,8].
The mechanism of ferroptosis
Lipid oxidation induces ferroptosis
When the distribution of iron in the body is abnormal, various injuries and diseases will occur. The central role of lipid peroxidation in ferroptosis in driver cells suggests that ferroptosis can be caused by the breakdown of the glutathione (GSH) -glutathione peroxidase 4 (GPX4) antioxidant system [9]. Polyunsaturated fatty acids (PUFAs) are one of the main components of the phospholipid bilayer of cell membranes. They play an important role in maintaining cell membranes’ fluidity, but excessive PUFAs can induce ferroptosis. The main mechanism is that Fe2+ oxidizes excess PUFAs to hydroxyl radicals through the Fenton reaction, and these groups also oxidize PUFAs in a chain reaction way, producing a large number of lipid peroxides and inducing ferroptosis of cells [10]. Lipid peroxidation products of the cell membrane are sources of ROS production, and a large accumulation of lipid ROS will directly trigger ferroptosis, a process that can be prevented by lipophilic antioxidants and iron chelators (Table 1).
Table 1.
The inducers and inhibitors of ferroptosis
| Reagents | Target/Funtion | Mechamism | Reference | |
|---|---|---|---|---|
| Inducers | Erastin | System xc- | prevents cystine import, causes GSH depletion | [1] |
| IKE | System xc- | prevents cystine import, causes GSH depletion | [97] | |
| Glutamate | System xc- | high extracellular glutamate leads to prevent cystine import, causes GSH depletion | [98] | |
| IFNG/IFNγ | System xc- | Down-regulates the expression of SLC3A2 and SLC7A11, two subunits of system xc- | [76] | |
| Sorafenib | System xc- | Inhibits system xc- in a non-enzymatic target-dependent manner | [99] | |
| Sulfasalazine | System xc- | Inhibition of nuclear factor κB signaling pathway; inhibition of xc-transporter | [99] | |
| FIN56 | GPX4 | Cause GPX4 depletion | [100] | |
| FINO2 | GPX4 | Inactive GPX4 and oxidizes iron | [101] | |
| Altretamine | GPX4 | Inhibits GPX4 activity, causes ROS accumulation | [102] | |
| JKE-1674/1716 | GPX4 | Inhibits GPX4 activity, causes ROS accumulation | [103] | |
| ML162 | GPX4 | Inhibits GPX4 activity, causes ROS accumulation | [28] | |
| ML210 | GPX4 | Inhibits GPX4 activity, causes ROS accumulation | [104] | |
| RSL3 | GPX4 | Inhibits GPX4 activity, causes ROS accumulation | [28] | |
| Artemisinin | Organic peroxides | ROS manufacturing, disruption of antioxidant defenses by downregulates of GPX4 and GSH causes an imbalance in intracellular oxidative responses | [105] | |
| Artesunate | Organic peroxides | Causes rapid ROS accumulation | [106] | |
| Salinomycin | Ferritinophagy | Increases intracellular iron, causes peroxidation by activating ferritinophagy | [107] | |
| Ironomycin | Ferritinophagy | Increases intracellular iron, causes peroxidation by activating ferritinophagy | [108] | |
| iFSP1 | FSP1 | Inhibit FSP1 activity, cause the accumulation of lipid peroxidation | [109] | |
| BSO | GCLC | Induces GSH depletion | [110] | |
| Doxorubicin | HO-1 | Induces iron overload | [111] | |
| Inhibitors | CoQ10 | Antioxidant/RTA | Prevents lipid peroxidation by the FSP1-catalyzed ubiquinol form | [100] |
| Fer-1 | Antioxidant/RTA | Prevents the accumulation of ROS by reducing activity | [1] | |
| Lip-1 | Antioxidant/RTA | Inhibits lipid peroxidation directly | [112] | |
| Vitamin E | Antioxidant/RTA | Inhibits lipid peroxidation directly | [113] | |
| NAC | Antioxidant/RTA | Suppresses ferroptosis through supplementing GSH | [28] | |
| Ciclopirox | Iron chelator | Reduces intracellular free iron | [1] | |
| Deferiprone | Iron chelator | Reduces intracellular free iron | [114] | |
| Deferoxamine | Iron chelator | Reduces intracellular free iron | [1] | |
| Dexrazoxane | Iron chelator | Reduces intracellular free iron | [115] | |
| Baicalein | ALOXs | Reduces ROS by inhibits ALOXs, and stabilizes GPX4 to protect cells from excessive lipid peroxidation | [116] | |
| AA-861 | ALOXs | Inhibits lipid ALOXs-mediated peroxidation | [117] | |
| CDC | ALOXs | Inhibits lipid ALOXs-mediated peroxidation | [117] | |
| Triacsin C | ACSL4 | Prevents the transfer process of PUFA to the cell membrane, inhibits lipid ACSL4-mediated peroxidation | [118] | |
| Troglitazone | ACSL4 | Prevents the transfer process of PUFA to the cell membrane, inhibits lipid ACSL4-mediated peroxidation | [119] | |
| Rosiglitazone | ACSL4 | Prevents the transfer process of PUFA to the cell membrane, inhibits lipid ACSL4-mediated peroxidation | [118] | |
| DPI | NOXs | Inhibit lipid NOXs-mediated peroxidation | [120] | |
| TMZ | System xc- | Induces system xc-expression | [121] | |
| KI-696 | Keap1 | Binds to Keap1 and prevents it from mediating NRF2 degradation | [122] | |
| Lactate | HCAR1/MCT1 | Promotes PUFAs production | [123] | |
| MUFAs | Lipid | Block PUFA peroxidation | [124] |
Antioxidant defense resists ferroptosis
ROS produced by oxidative stress can directly or indirectly damage intracellular macromolecules’ physiological functions such as proteins, lipids, and nucleic acids, which is the pathophysiological basis of many diseases. Nrf2 is a key factor in the oxidative stress response of cells and controls the cellular antioxidant system in cancer cells [11]. By regulating Keap1 (Kelch Like ECH Associated Protein 1)-Nrf2 through reaction with antioxidant components (antioxidant response element) interaction, regulating antioxidants’ expression and phase alexipharmic alcohol [12-14]. Clinically, cancer and other chronic diseases involved in oxidative and inflammatory stress can be prevented by enhancing the activity of NRF2 [15]. Recent studies have identified the antioxidant enzyme Dehydrogenase (DHODH) localized in mitochondria, which acts independently of FSP1 and GPX4 to resist ferroptosis [16]. It resists ferroptosis by promoting the production of CoQ10.
Unbalanced iron induces ferroptosis
Iron is essential in the accumulation of lipid peroxide and the execution of ferroptosis. Therefore, the amount of intracellular iron affects the sensitivity to ferroptosis. It is well known that intracellular iron metabolism and iron homeostasis are in a state of dynamic equilibrium. The body maintains iron intake, storage, and outflow processes through a complex regulatory network [17]. Transferrin receptor (TFR) and divalent metal-ion transporter-1 (DMT1) regulate extracellular iron intake, while ferroportin (FPN), on the other hand, transfers excess iron from intracellular to extracellular in order to maintain iron homeostasis in the cell [18,19]. In the periphery, transferrin (Trf) has a high affinity with ferric iron (Fe3+), and one Trf molecule can transport 2 Fe3+. Trf transfers Fe3+ to the TFR1 of the cell membrane and then forms Trf- [Fe3+] 2-TFR complex on the on the surface of cell membrane [20]. Basuli et al. [21] showed that this process could be significantly up-regulated in ovarian cancer, breast cancer, and other cancers. Studies have shown that iron transporter proteins (FER-) are found in a variety of cancer cells down-regulation of FPN and up-regulation of TFR1 make cancer cells have a higher demand for iron than non-cancer cells, and such “iron addiction” makes cancer cells more susceptible to the impact of iron overload and ROS accumulation [22-24].
Lipid metabolism associate with ferroptosis
Lipid metabolism is closely related to the sensitivity of cells to ferroptosis. Polyunsaturated fatty acids are a double-edged sword, and their peroxidation may also cause damage to cells. It can be integrated into the membrane by Acyl-CoA Synthetase Long Chain Family Member 4 (ACSK4) and lysophosphatidyl-choline acyltransferase 3 (LPCAT3) [25]. The oxidation of PUFA can be done either by a non-enzymatic free radical chain reaction or enzymatic catalysis. Taking AA as an example [26], ACSL4 catalyzed the linking of CoA to AA to form the intermediate of CoA-AA, which LPCAT3 esterified to form phosphatidylethanolamine (PE-AA) to form arachidonic acid-phosphatidylethanolamine (PE-AA). The resulting PE-AA can be oxidized by LOX in the presence of enzymes or by autooxidation in non-enzymes to form PE-AA-OOH, both of which eventually lead to cell death.
During ferroptosis, the accumulation of lipid peroxides, especially phospholipid peroxides, is considered a symbolic event of ferroptosis [27]. At present, it is generally believed that the ultimate actor of ferroptosis is lipid peroxides, and when excessive accumulation of lipid peroxides causes plasma membrane damage, eventually leading to the occurrence of ferroptosis in cells [10]. Inhibition of GPX4 from a genetic or pharmacological perspective leads to ferroptosis even when cysteine/cysteine supplies are sufficient in cells [5,28]. Studies have shown that lipid peroxides cause cell damage in various ways [29,30]. One is the further decomposition of lipid peroxides into ROS, which further amplifies the lipid peroxidation process. The other is by changing the physical structure of the membrane, such as the thickness and bending degree of the membrane, or by forming holes in the membrane to release harmful substances and disrupt the metabolism in the cell; Third, the byproducts (aldehydes) produced in the process of lipid peroxidation can cause cell damages, such as MDA and 4-HNE.
Amino acid metabolism associate with ferroptosis
Amino acid metabolism is closely related to the regulation of ferroptosis [31]. The entry and exit of amino acids into and out of the cell require specific transporters, cystine/glutamate antiporter System Xc-. The XC - system is a Na - independent reverse transporter that outputs intracellular glutamate and extracellular cystine in a 1:1 ratio [32,33]. Consisting of two subunits connected by a disulfide bond, including member 2 of the heavy chain subunit solute carrier family 3 member 2 (SLC3A2; CD98 or 4F2HC) and light chain subunit solute carrier family 7 member 11 (SLC7A11; Also commonly referred to as XCT). SLC7A11 is a multichannel transmembrane protein that mediates cystine/glutamate antiporter activity in the XC - system [34,35]. SLC3A2 is the chaperone protein that maintains SLC7A11 protein stability and proper membrane localization [36]. Inhibition of the imbalance in amino acid metabolism caused by SystemXC - causes ferroptosis, and glutamate itself can affect the function of SystemXC -. As a substrate of GPX4, GSH participates in the intracellular antioxidant system and is a key factor affecting the occurrence of ferroptosis [37] (Table 2).
Table 2.
Genes involved in ferroptosis
| Gene | Name | Funtion | Ref. | |
|---|---|---|---|---|
| Antioxidant defense | GPX4 | glutathione peroxidase 4 | Reduces lipid ROS with the aid of GSH | [1] |
| FSP1 | ferroptosis suppressor protein 1 | Reduces lipid peroxidation mediated by ubiquinone (CoQ10) | [109,125] | |
| GCH1 | GTP Cyclohydrolase 1 | Prevents lipid peroxidation by its metabolic derivatives BH4/BH2 | [126] | |
| FANCD2 | Fanconi anemia complementation group D2 | Regulats ferroptosis by affacting GPX4 expression | [127] | |
| Peroxidation | ALOXs | lipoxygenases | Directly mediates lipid oxidation | [128] |
| DPP4 | dipeptidyl peptidase 4 | Assists NOXs-mediated oxidation reaction | [129] | |
| NOXs | NADPH oxidases | Transfers electrons through the biofilm and reduces oxygen to superoxide, thus enabling the accumulation of ROS | [1] | |
| POR | NADPH-cytochrome P450 reductase | Promotes lipid peroxidation by Catalyzing the Production of H2O2 | [130] | |
| CyB5R1 | NADH-cytochrome b5 reductase | Promotes lipid peroxidation by Catalyzing the Production of H2O2 | [130] | |
| Iron metabolism | ELAVL1 | embryonic lethal, abnormal vision, Drosophila-like 1 | Selectively active ferrotinophagy as an RNA binding protein | [131] |
| ZFP36 | zinc-finger protein 36 | Selectively inactive ferrotinophagy as an RNA binding protein | [132] | |
| FTMT | mitochondrial ferritin | Stores free iron in mitochondria | [133] | |
| Prominin2 | Prominin2 | Mediates iron ion export by the exocytic form | [134] | |
| HO-1 | heme oxygenase-1 | Elevates intracellular free iron levels by promoting heme degradation | [135] | |
| SLC39A14 | solute carrier family 39 member 14 | Sensitizes cells to ferroptosis by mediating the transport of NTBI | [136] | |
| SLC11A2 | solute carrier family 11 member 2 | Sensitizes cells to ferroptosis by mediating the transport of NTBI | [136] | |
| SLC39A8 | solute carrier family 39 member 8 | Sensitizes cells to ferroptosis by mediating the transport of NTBI | [136] | |
| SLC40A1 | solute carrier family 40 member 1 | Sensitizes cells to ferroptosis by mediating the transport of NTBI | [136] | |
| Trf | transferrin | Extracellularly binds iron | [137] | |
| TfR | transferrin receptor | Mediates Transferrin bound iron import | [138] | |
| CISD1 | CDGSH iron sulfur domain 1 | Increases iron-mediated intramitochondrial lipid peroxidation | [139] | |
| HSPB1 | heat shock protein beta-1 | Inhibits of ferroptosis by affecting iron metabolism | [140] | |
| NFS1 | l-cysteine desulfurase | Inhibits ferroptosis by maintaining iron-sulfur cofactor stability | [141] | |
| Lipid metabolism | LPCAT3 | lysophosphatidyl-choline acyltransferase 3 | Involves in key steps of PUFA synthesis | [25,128] |
| ACSL4 | Long-chain acyl-CoA synthetase-4 | Mediates PUFA insertion into cell membranes | [118] | |
| AMPK | AMP-activated protein kinase | Energy stress-mediated regulation of PUFA synthesis | [142] | |
| ELOVL5 | elongation of very longchain fatty acid protein 5 | Affects the synthesis of PUFAs necessary for ferroptosis | [143] | |
| FADS1 | fatty acid desaturase 1 | Affects the synthesis of PUFAs necessary for ferroptosis | [143] | |
| PPARα | peroxisome proliferator-activated receptor α | Regulates intracellular lipid homeostasis | [144] | |
| ACSL3 | Long-chain acyl-CoA synthetase-3 | Mediates MUFA insertion into cell membranes | [124] | |
| SCD1 | Stearyl-coenzyme A desaturase 1 | Prevents ferroptosis by modulating ACSL4 activity | [123] | |
| RAB7A | member RAS oncogene family 7 | Regulates lipophagy | [145] | |
| Amino acid metabolism | SLC7A11 | solute carrier family 7 member 11 | Transports cysteine by composing the system xc- | [34] |
| SLC3A2 | solute carrier family 3 member 2 | Transports cysteine by composing the system xc- | [34] | |
| mTORC1 | mechanistic tar-get of rapamycin complex 1 | Promotes degradation of SLC7A11 in lysosomes | [146] | |
| mTORC2 | mechanistic target of rapamycin complex2 | Inhibits the activity of the SLC7A11 transporter by phosphorylating serine 26 of SLC7A11 | [147] | |
| CD44v | CD44 variant | Stabilizes system xc- activity | [148,149] | |
| GCLC | glutamylcysteine ligase | Involves in the synthesis of GSH, but also has unconventional anti-ferroptosis activity | [150,151] | |
| CBS | Corticobasal Syndrome | Participates in the rate-limiting step of the transsulfuration pathway | [152] | |
| CGL | coagulation | prevents cellular ferroptosis when cysteine acquisition is limited | [152] | |
| SLC38A1 | solute carrier family 38 member 1 | Mediates glutamate import and sensitizes ferroptosis | [153-155] | |
| SLC1A5 | solute carrier family 1 member 5 | Mediates glutamate import and sensitizes ferroptosis | [153-155] | |
| BECN1 | Beclin 1 | Directly binds to system xc - and blocks its activity | [156] | |
| GLS2 | glutaminase 2 | Prevents ferroptosis by mediating the degradation of glutamate | [153] | |
| Other metabolic pathways | ACSF2 | acyl-CoA synthetase family member 2 | Regulates mitochondria-associated lipid metabolism | [1] |
| VDAC2 | voltage-dependent Anion Channel2 | Iron transporter on mitochondria, affects iron availability | [157] | |
| FXN | Frataxin | Affects the availability of iron as well as some antioxidant enzyme activities by controlling the synthesis of iron-sulfur clusters | [158] | |
| NFE2L2 | nuclear factor erythroid synthase 2 | Affects ferroptosis by affecting the expression of a variety of ferroptosis-associated proteins | [159] | |
| TP53 | tumor protein 53 | Affects ferroptosis by affecting the expression of a variety of ferroptosis-associated proteins | [160] | |
| YAP | Yes-associated protein | Regulates lipid metabolism in the presence of high cell density | [68,161,162] |
Moreover, GSH synthesis is closely related to the metabolism of amino acids. Besides, indole-3-acetone hydrochloride (I3P) inhibits ferroptosis by directly scavenging free radicals and activating antioxidant gene expression programs. Therefore, interleukin-4-inducible 1 (IL4I1) is likely mediated by a local iron-promoting pathway in the metabolism of aromatic amino acids, suggesting that IL4I inhibitors may regulate the death pathway tumor cells [38].
Glutamate and glutamine are important regulators of ferroptosis [23]. Extracellular high concentrations of glutamate can inhibit cystine uptake by inhibiting the activity of SystemXC -, resulting in ferroptosis [18,39]. Notably, outside the cell, reduced glutamate levels protected SystemXC - knockout mice from neurotoxic damage [40]. Therefore, the accumulation of extracellular glutamate may serve as a natural trigger for inducing ferroptosis in the physiological environment. Because ferroptosis is considered an associated cell death [41,42] mechanism in tissue damage, glutaminolysis targeted therapy may effectively treat organ damage mediated by ferroptosis. In fact, in experimental models, inhibition of glutamine breakdown has been shown to reduce heart and kidney injury and cerebral hemorrhage due to ischemia/reperfusion [23,42,43].
Other metabolic pathways associate with ferroptosis
In addition to iron metabolism, lipid metabolism, and amino acid metabolism, factors including Ferropsor suppressor protein 1 (FSP1), NRF2, heat shock proteins (HSPs) also regulate ferroptosis.
In 2019, Conrad and Olzmann et al. respective found a new ferroptosis inhibitory protein 1 (FSP1, previously known as mitochondrial apoptosis-inducing factor 2, AIFM2) almost at the same time [44,45]. FSP1 can be used as a biomarker of ferroptosis resistance in a variety of cancer cells. It has NADPH-dependent coenzyme Q oxidoreductase function and can catalyze the regeneration of CoQ10 by NAD(P)H, while CoQ10 can inhibit peroxidation and prevent ferroptosis [46]. The FSP1-CoQ10-NAD(P)H pathway is an independent system that acts synergistically with GPX4 and glutathione to inhibit phospholipid peroxidation and ferroptosis KEAP1-Nrf2-ARE signaling pathway forms a complex oxidative stress response system, and Nrf2 plays a regulatory role in intracellular Fe2+ [6]. Under normal conditions, Nrf2 is inactive, and when exposed to electrophile reagents or reactive oxygen species, it can induce a series of protective proteins to inhibit ferroptosis [47].
Heat shock protein B1 (HSPB1) is also a regulator closely related to ferroptosis [48]. Overexpression of HSP is induced under stress conditions such as heat shock, pH shift, and hypoxia [49]. Phosphorylated HSPB1 inhibits ferroptosis by reducing cellular iron uptake and lipid ROS production. Therefore, inhibition of HSPB1 expression or phosphorylation can increase ferroptosis mediated by Erastin, providing a new research direction for cancer cells to avoid ferroptosis [48].
The interaction between ferroptosis and tumor metabolism
The metabolic specificity of tumor cells confers their particular relationship to ferroptosis. Nowadays, it has been proved that ferroptosis plays an important role in oncogenes and tumor suppressor genes, as well as tumor migration and invasion, tumor microenvironment and immunity. Elucidating the relationship between ferroptosis and these neoplastic events could help to develop better targeted ferroptosis treatment strategies.
Tumor suppressor genes
P53 has been a recognized tumor suppressor since its discovery [47], and it plays an important role in tumor metabolism [50,51]. In 2015, Jiang et al. linked p53 with ferroptosis for the first time [52], indicating that p53 can inhibit SLC7A11 transcription and thus inhibit SystemXC - to absorb cystine (Figure 1), thereby regulating ferroptosis and playing a key role in tumor inhibition. In addition to SLC7A11, some p53 target genes, such as Spermidine/Spermine N1-acetyltransferase 1 (SAT1), prostaglandin peroxidase synthase 2 (PTGS2) and Glutaminase 2 (GLS2) can promote ferroptosis in cells [23,28,53]. Coding mutant p53 protein accumulation within the tumor cells promotes oncogenes’ function or promotes different cell proliferation. The wild-type p53 can suppress abnormal cell proliferation by regulating cell cycle arrest, thus promoting tumor occurrence and development [54,55]. P53 known effects on cell metabolism are complex, involving multiple control nodes [56], through the transcription or transcriptional regulation, activity in various metabolic pathways, such as glycolysis, mitochondrial oxidative phosphorylation, etc. P53 also controls the cancer cells to the fitness of nutrients and oxygen condition. This is the key to survival under metabolically impaired conditions formed in the tumor microenvironment [57].
Figure 1.
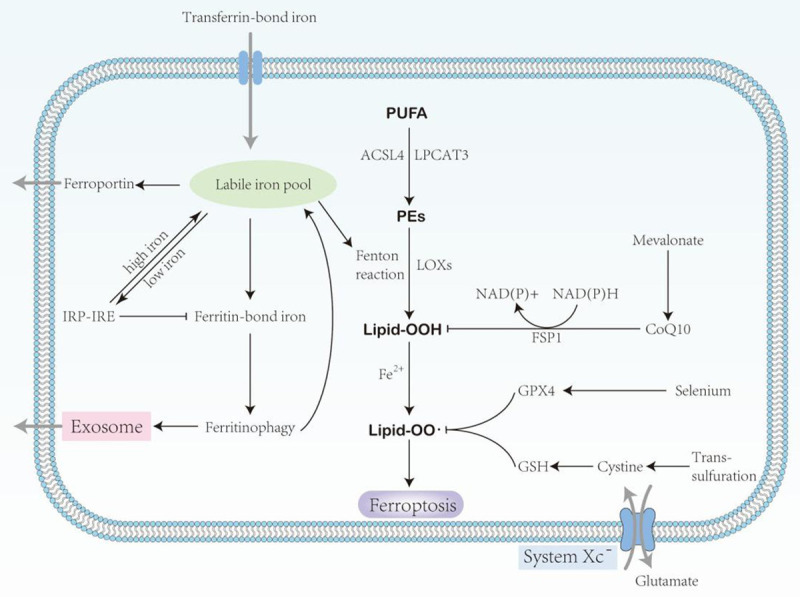
Schematic description of the signaling pathway of ferroptosis. polyunsaturated fatty acids (PUFA); Acyl-CoA synthetase long-chain family member 4 (ACSL4); lysophosphatidylcholine acyltransferase 3 (LPCAT3); phosphatidylethano- lamines (PEs); ferroptosis suppressor protein 1 (FSP1); lipoxygenases (LOXs); glutathione peroxidase 4 (GPX4); Glutathione (GSH).
EMT
EMT refers to the transformation of epithelial cells into mesenchymal cells under certain physiological and pathological conditions. Ferroptosis is known to be sensitive to epithelial-mesenchymal transition (EMT) cells [58]. Studies have confirmed that transcription factors (such as Snail [59-62], Twist [63-65], ZEB [66], etc.) and microRNAs [67] play an important role in EMT. Activation of transcription factors such as YAP1 and WWTR1 (also known as TAZ), which are involved in the Hippo pathway, promotes ferroptosis during growth by regulating ferroptosis-related expression factors as ACSL4, TFRC, EMP1, and AngPTL4 [68]. Viswanathan et al. [69] found that anti-therapy cancer cells undergoing epithelial-mesenchymal transformation (EMT) are more likely to be killed by ferroptosis inducers than non-drug-resistant cancer cells [70-72], which may serve as a starting point for the application of ferroptosis in tumor metabolism. Importantly, through the study of EMT related experimental results, the exaggerated expression in breast cancer cells significantly lower iron deprivation of Fpn get the transfer capacity of EMT marker expression and damaged [73], but Mangmang CSO and others [58] found - SS - Cy7 - Hex/SPION SRFN compound self-assembly mediated ferritin acid, can resist EMT during breast cancer drug resistance, more aggressive and metastasis. Cell adhesion promoters, such as integrin subunits α6 and β4, also protect breast cancer-derived cells from ferroptosis in vitro [74]. Meanwhile, Peng Chen et al. [75] found that β-element combined with cetuximab can inhibit the migration of KRAS mutant CRC cells by inhibiting EMT and inhibiting the growth of KRAS mutant tumor, which provides a new treatment strategy for CRC patients with RAS mutation. In conclusion, EMT plays a key role in the mechanism of ferroptosis in tumor metabolism (Figure 2), but its potential value is unclear.
Figure 2.
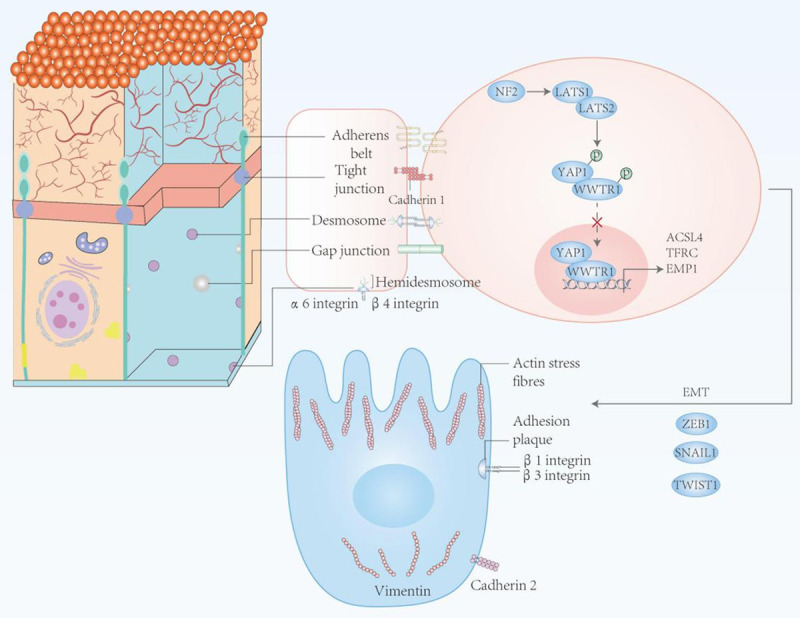
The role of EMT in ferroptosis. In epithelial cells, the intercellular contact portion inhibits ferroptosis through cadherin 1-mediated inhibition of YAP1 transcriptional activity. In contrast, cells in the mesenchymal state are susceptible to ferroptosis due to loss of intercellular contact and activation of factors involved in epithelial to mesenchymal transition (EMT), such as ZEB1, SNAI1, and Twist1.
Ferroptosis and tumor immunity
Current studies have confirmed that ferroptosis plays an important role in cancer immunity. Ferroptosis, as a mode of cell death, is triggered by immune cells as a target. IFNγ released by CD8+ T cells [76] and TGFβ1 released by macrophages [77] can induce ferroptosis in cancer cells by reducing the expression of ferroptosis-related inhibitory proteins. The method of constructing nanoplatforms, such as oxygen-enhanced photodynamic therapy with a nanoplatform of Ferro hemoglobin, successfully induced IFNγ release from T cells, which sensitized cancer cells ferroptosis [78]. The “eat me” signal is an important way for cells to be recognized and remove [79]. Recent studies have found that peroxides produced by ferroptosis on cell membranes can act as “eat me” signals recognized by the immune system and thus removed [80]. This also demonstrates that ferroptosis regulates tumor immunity through peroxidation products. Therefore, it is argued that oxidases like ALOX act as triggers of ferroptosis and modulate immune signaling. Notably, the reduction of GPX4 inhibited the release of proinflammatory mediators such as HETE, LTB4, and thus the oncogenic process [81]. Also, oxidized phospholipids catalyzed by ALOXs can modulate immunity by promoting DC maturation and differentiation of helper T cells [82]. Besides, oxidolipids and lipid droplets are also involved in the regulation of antitumor immune responses. In the tumor microenvironment, dendritic cells accumulate a large amount of peroxidation, leading to antitumor immunodeficiency [83]. Lipid species of the external environment can also affect cancer migration through ferroptosis, and the lymphatic system protects melanoma cells from iron ptosis by increasing ACSL3-dependent MUFAs production, thereby promoting tumor metastasis [84].
In addition to peroxidation, damage-associated molecular patterns (DAMPs) of ferroptosis release can modulate immune progression. There has been controversy as to whether ferroptosis is immunogenic. Luliia et al. found that ferroptosis released ATP and HMGB1 in a time-dependent manner and demonstrated that ferroptosis is immunogenic both in vitro and in vivo [85]. The release of DAMPs mediates immunogenic cells’ death and inflammatory responses that promote tumor growth [86,87]. HMGB1, as a member of DAMPs, is released by iron-dead cancer cells and promotes their inflammatory reaction by binding to AGER of macrophages. There is evidence that induction of ferroptosis in cancer cells increases the release of PGE2, an important immunomodulator. And this process may be negatively correlated with GPX4 activity. PGE2, on the one hand, blunts the function of CDC1-type cells and prevents the infiltration of CDC1 cells [88] into the immune microenvironment by NK [89] cells. Besides, PGE2 is active in T cells. Although further studies are needed regarding the downstream signaling of PGE2, it has been demonstrated that PGE2 impairs tumor immune function by acting on the innate immune system. Since the formation of PGE2 is negatively correlated with GPX4 activity, it can be speculated that iron-death-sensitive cell lines can more easily release PGE2 and ensure tumor development by means. It has been shown that PGE2 is released from tumors and neighboring cells during chemotherapy cycles, which is essential for tumor repropagation of cancer stem cells [90].
In recent years, immune checkpoint inhibitors (ICIs) have made effective progress in cancer treatment. Among them, the anti-PD-L1 antibody can promote ferroptosis in cancer cells. The phenomena of ferroptosis induced by ICIs is very similar to cancer cell killed by cytotoxic T cells and macrophages mentioned above. For example, interferon γ released by T cells activates the JAK-STAT1 pathway and downregulates the expression of SLC7A11 and SLC3A2, which leads to the development of ferroptosis in cancer cells. Reduced SLC3A2 names in melanoma patients are consistently associated with enhanced response to ICIs [7]. It can be speculated that the immune checkpoint PD-L1 also palys a role in reducing ferroptosis. But whether anti-PD-L1 antibodies enhance the progress of ferroptosis only remains to be determined because multiple ligands activate the JAK-STAT pathway.
Ferroptosis also acts on immune cells and plays a role in regulating tumor immunity [91]. It was found that CD4+ T cells and CD8+ T cells lacking GPX4 underwent ferroptosis with the rapid accumulation of ROS. This undoubtedly prevents the expansion of T cells, rendering them incompetent for immune activity [92]. In contrast, overexpression of FSP1 and GPX4 in immune cells ensured CD8+ T cells’ immune activity. Interestingly, knockdown of ACSL4, while sparing CD8+ T cells from ferroptosis, impaired their immune activity [93]. There are few studies on B cells, but it has been demonstrated that B cells have a constant sensitivity to ferroptosis [94]. GPX4 activity is unnecessary in the process by which some types of B cells remain immunocompetent. Similar to B cells, M1 and M2 macrophages also have different sensitivities to ferroptosis (Figure 3). In contrast, M1-type cells are more susceptible to induction of ferroptosis, this process regulated by cellular Inos [103]. In addition, ferroptosis pancreatic cancer cells release KRAS-G12D and can be taken up by macrophages, leading to M2 polarization that promotes the tumor phenotype [95]. This strongly demonstrates that ferroptosis can be a promising target to drive immune reprogramming [96]. Existing studies have demonstrated the importance of regulating immune cells in tumor immunity, but the mechanisms regulating ferroptosis in immune cells still need further investigation.
Figure 3.
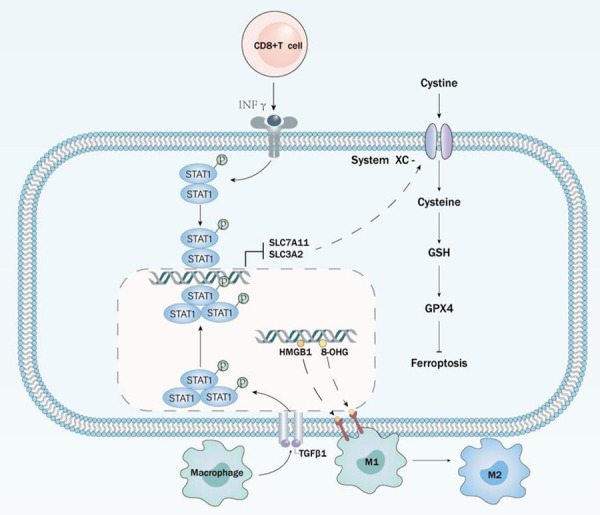
The link between cancer and immunity. Immune cells regulate the ferroptosis sensitivity of cancer cells on the one hand. CD8+T cells and macrophages affect the expression of ferroptosis-related genes in cancer cells by secreting INFγ and TGFβ1, respectively, sensitizing ferroptosis. On the other hand, DAMPs released from iron-dead cells result in M2 polarization of macrophages, leading to immune remodeling.
Conclusion and perspective
As a new type of cell death, ferroptosis is highly closely related to cell metabolism. The metabolic reprogramming of cancer cells, as well as the tumor microenvironment, gives cancer cells different responsiveness from normal tissue cells to ferroptosis. Recent studies have shown that ferroptosis can provide a new research direction for inducing targeted removal of tumor cells and overcoming tumor drug resistance [163,164]. In radiation therapy, induction of ferroptosis can greatly reduce tumor resistance to radiation; while in immunotherapy, the immunogenicity of ferroptosis has been confirmed, and cancer cell ferroptosis is one of the outcomes of cells performing immune killing. However, more clinical studies are needed to prove the value of inducing ferroptosis for existing clinical treatment modalities. At the same time, although the upstream metabolism and signaling pathways of ferroptosis have been explored, it is still inconclusive what the final effector downstream of ferroptosis is, and it is not certain that peroxidation directly leads to ferroptosis. In future studies, it will become the general trend to further elucidate the effector mechanism of ferroptosis, as well as the application of ferroptosis in clinical treatment.
Acknowledgements
This project was supported by the Administration of Traditional Chinese Medicine of Guangdong Province (20201180); Administration of Traditional Chinese Medicine of Guangdong Province (20211223); Science and Technology Special Project of Zhanjiang (2019A01009); Basic and Applied Basic Research Program of Guangdong Province (2019A1515110201); Program of Department of Natural Resources of Guangdong Province (No. GDNRC [2020]038 and [2021]53); Fund of Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (ZJW-2019-007). Discipline Construction Project of Guangdong Medical University (4SG21004G).
Disclosure of conflict of interest
None.
References
- 1.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu G, Wang H, Li X, Huang R, Luo L. Recent progress on targeting ferroptosis for cancer therapy. Biochem Pharmacol. 2021:114584. doi: 10.1016/j.bcp.2021.114584. [DOI] [PubMed] [Google Scholar]
- 3.Wang SJ, Li D, Ou Y, Jiang L, Chen Y, Zhao Y, Gu W. Acetylation is crucial for p53-mediated ferroptosis and tumor suppression. Cell Rep. 2016;17:366–373. doi: 10.1016/j.celrep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19:405–414. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 5.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O’Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin D, Kim EH, Lee J, Roh JL. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018;129:454–462. doi: 10.1016/j.freeradbiomed.2018.10.426. [DOI] [PubMed] [Google Scholar]
- 7.Stallons LJ, Whitaker RM, Schnellmann RG. Suppressed mitochondrial biogenesis in folic acid-induced acute kidney injury and early fibrosis. Toxicol Lett. 2014;224:326–332. doi: 10.1016/j.toxlet.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aparicio-Trejo OE, Reyes-Fermín LM, Briones-Herrera A, Tapia E, León-Contreras JC, Hernández-Pando R, Sánchez-Lozada LG, Pedraza-Chaverri J. Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid. Free Radic Biol Med. 2019;130:379–396. doi: 10.1016/j.freeradbiomed.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Yu C, Kang R, Tang D. Iron Metabolism in Ferroptosis. Front Cell Dev Biol. 2020;8:590226. doi: 10.3389/fcell.2020.590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 15.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, Koppula P, Wu S, Zhuang L, Fang B, Poyurovsky MV, Olszewski K, Gan B. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–590. doi: 10.1038/s41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 18.Schweichel JU, Merker HJ. The morphology of various types of cell death in prenatal tissues. Teratology. 1973;7:253–266. doi: 10.1002/tera.1420070306. [DOI] [PubMed] [Google Scholar]
- 19.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 20.Beguin Y, Aapro M, Ludwig H, Mizzen L, Osterborg A. Epidemiological and nonclinical studies investigating effects of iron in carcinogenesis--a critical review. Crit Rev Oncol Hematol. 2014;89:1–15. doi: 10.1016/j.critrevonc.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Basuli D, Tesfay L, Deng Z, Paul B, Yamamoto Y, Ning G, Xian W, McKeon F, Lynch M, Crum CP, Hegde P, Brewer M, Wang X, Miller LD, Dyment N, Torti FM, Torti SV. Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene. 2017;36:4089–4099. doi: 10.1038/onc.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trujillo-Alonso V, Pratt EC, Zong H, Lara-Martinez A, Kaittanis C, Rabie MO, Longo V, Becker MW, Roboz GJ, Grimm J, Guzman ML. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat Nanotechnol. 2019;14:616–622. doi: 10.1038/s41565-019-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, Baer CE, Dixon SJ, Mercurio AM. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell. 2019;51:575–586. e4. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, Superti-Furga G, Stockwell BR. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell Death. ACS Chem Biol. 2015;10:1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayır H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Herde K, Krysko DV. Ferroptosis: oxidized PEs trigger death. Nat Chem Biol. 2017;13:4–5. doi: 10.1038/nchembio.2261. [DOI] [PubMed] [Google Scholar]
- 28.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 2017;482:419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng H, Stockwell BR. Unsolved mysteries: how does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16:e2006203. doi: 10.1371/journal.pbio.2006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angeli JPF, Shah R, Pratt DA, Conrad M. Ferroptosis inhibition: mechanisms and opportunities. Trends Pharmacol Sci. 2017;38:489–498. doi: 10.1016/j.tips.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem. 1986;261:2256–2263. [PubMed] [Google Scholar]
- 33.Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (-): cystine supplier and beyond. Amino Acids. 2012;42:231–246. doi: 10.1007/s00726-011-0867-5. [DOI] [PubMed] [Google Scholar]
- 34.Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 35.Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond) 2018;38:12. doi: 10.1186/s40880-018-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura E, Sato M, Yang H, Miyagawa F, Harasaki M, Tomita K, Matsuoka S, Noma A, Iwai K, Minato N. 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J Biol Chem. 1999;274:3009–3016. doi: 10.1074/jbc.274.5.3009. [DOI] [PubMed] [Google Scholar]
- 37.Maiorino M, Conrad M, Ursini F. GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxid Redox Signal. 2018;29:61–74. doi: 10.1089/ars.2017.7115. [DOI] [PubMed] [Google Scholar]
- 38.Zeitler L, Fiore A, Meyer C, Russier M, Zanella G, Suppmann S, Gargaro M, Sidhu SS, Seshagiri S, Ohnmacht C, Köcher T, Fallarino F, Linkermann A, Murray PJ. Anti-ferroptotic mechanism of IL4i1-mediated amino acid metabolism. Elife. 2021;10:e64806. doi: 10.7554/eLife.64806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 40.Massie A, Schallier A, Kim SW, Fernando R, Kobayashi S, Beck H, De Bundel D, Vermoesen K, Bannai S, Smolders I, Conrad M, Plesnila N, Sato H, Michotte Y. Dopaminergic neurons of system x(c)--deficient mice are highly protected against 6-hydroxydopamine-induced toxicity. FASEB J. 2011;25:1359–1369. doi: 10.1096/fj.10-177212. [DOI] [PubMed] [Google Scholar]
- 41.Conrad M, Angeli JP, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2016;15:348–366. doi: 10.1038/nrd.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J, Wang Z, Jiang C, Ying M, Koehler RC, Stockwell BR, Wang J. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2:e90777. doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borgese N, Aggujaro D, Carrera P, Pietrini G, Bassetti M. A role for N-myristoylation in protein targeting: NADH-cytochrome b5 reductase requires myristic acid for association with outer mitochondrial but not ER membranes. J Cell Biol. 1996;135:1501–1513. doi: 10.1083/jcb.135.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stockwell BR. A powerful cell-protection system prevents cell death by ferroptosis. Nature. 2019;575:597–598. doi: 10.1038/d41586-019-03145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X, Wang H, Cao L, Tang D. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34:5617–5625. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 50.Mello SS, Attardi LD. Deciphering p53 signaling in tumor suppression. Curr Opin Cell Biol. 2018;51:65–72. doi: 10.1016/j.ceb.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Gu W. The complexity of p53-mediated metabolic regulation in tumor suppression. Semin Cancer Biol. 2021 doi: 10.1016/j.semcancer.2021.03.010. S1044-579X(21)00060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci U S A. 2016;113:e6806–e6812. doi: 10.1073/pnas.1607152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu M, Sun Y, Xu F, Liang Y, Liu H, Yi Y. Annexin A2 silencing inhibits proliferation and epithelial-to-mesenchymal transition through p53-dependent pathway in NSCLCs. J Cancer. 2019;10:1077–1085. doi: 10.7150/jca.29440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rademaker G, Costanza B, Bellier J, Herfs M, Peiffer R, Agirman F, Maloujahmoum N, Habraken Y, Delvenne P, Bellahcène A, Castronovo V, Peulen O. Human colon cancer cells highly express myoferlin to maintain a fit mitochondrial network and escape p53-driven apoptosis. Oncogenesis. 2019;8:21. doi: 10.1038/s41389-019-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flöter J, Kaymak I, Schulze A. Regulation of metabolic activity by p53. Metabolites. 2017;7:21. doi: 10.3390/metabo7020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sang M, Luo R, Bai Y, Dou J, Zhang Z, Liu F, Feng F, Xu J, Liu W. Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer. Theranostics. 2019;9:6209–6223. doi: 10.7150/thno.36283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang CC, Zhu LF, Xu XH, Ning TY, Ye JH, Liu LK. Membrane type 1 matrix metalloproteinase induces an epithelial to mesenchymal transition and cancer stem cell-like properties in SCC9 cells. BMC Cancer. 2013;13:171. doi: 10.1186/1471-2407-13-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suh Y, Yoon CH, Kim RK, Lim EJ, Oh YS, Hwang SG, An S, Yoon G, Gye MC, Yi JM, Kim MJ, Lee SJ. Claudin-1 induces epithelial-mesenchymal transition through activation of the c-Abl-ERK signaling pathway in human liver cells. Oncogene. 2013;32:4873–4882. doi: 10.1038/onc.2012.505. [DOI] [PubMed] [Google Scholar]
- 61.Szarvas T, vom Dorp F, Ergün S, Rübben H. Matrix metalloproteinases and their clinical relevance in urinary bladder cancer. Nat Rev Urol. 2011;8:241–254. doi: 10.1038/nrurol.2011.44. [DOI] [PubMed] [Google Scholar]
- 62.Huang CH, Yang WH, Chang SY, Tai SK, Tzeng CH, Kao JY, Wu KJ, Yang MH. Regulation of membrane-type 4 matrix metalloproteinase by SLUG contributes to hypoxia-mediated metastasis. Neoplasia. 2009;11:1371–1382. doi: 10.1593/neo.91326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu J, Qin L, He T, Qin J, Hong J, Wong J, Liao L, Xu J. The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell Res. 2011;21:275–289. doi: 10.1038/cr.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mao Y, Zhang N, Xu J, Ding Z, Zong R, Liu Z. Significance of heterogeneous Twist2 expression in human breast cancers. PLoS One. 2012;7:e48178. doi: 10.1371/journal.pone.0048178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang CH, Xu GL, Jia WD, Li JS, Ma JL, Ren WH, Ge YS, Yu JH, Liu WB, Wang W. Activation of STAT3 signal pathway correlates with twist and E-cadherin expression in hepatocellular carcinoma and their clinical significance. J Surg Res. 2012;174:120–129. doi: 10.1016/j.jss.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 66.Sánchez-Tilló E, Lázaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, Engel P, Postigo A. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29:3490–3500. doi: 10.1038/onc.2010.102. [DOI] [PubMed] [Google Scholar]
- 67.Lee JY, Park MK, Park JH, Lee HJ, Shin DH, Kang Y, Lee CH, Kong G. Loss of the polycomb protein Mel-18 enhances the epithelial-mesenchymal transition by ZEB1 and ZEB2 expression through the downregulation of miR-205 in breast cancer. Oncogene. 2014;33:1325–1335. doi: 10.1038/onc.2013.53. [DOI] [PubMed] [Google Scholar]
- 68.Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, Chen ZN, Jiang X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang HC, Lin YL, Hsu CC, Chao YJ, Hou YC, Chiu TJ, Huang PH, Tang MJ, Chen LT, Shan YS. Pancreatic stellate cells activated by mutant KRAS-mediated PAI-1 upregulation foster pancreatic cancer progression via IL-8. Theranostics. 2019;9:7168–7183. doi: 10.7150/thno.36830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lleonart ME, Abad E, Graifer D, Lyakhovich A. Reactive oxygen species-mediated autophagy defines the fate of cancer stem cells. Antioxid Redox Signal. 2018;28:1066–1079. doi: 10.1089/ars.2017.7223. [DOI] [PubMed] [Google Scholar]
- 71.Chen JJ, Galluzzi L. Fighting resilient cancers with iron. Trends Cell Biol. 2018;28:77–78. doi: 10.1016/j.tcb.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 72.Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, Viswanathan SR, Chattopadhyay S, Tamayo P, Yang WS, Rees MG, Chen S, Boskovic ZV, Javaid S, Huang C, Wu X, Tseng YY, Roider EM, Gao D, Cleary JM, Wolpin BM, Mesirov JP, Haber DA, Engelman JA, Boehm JS, Kotz JD, Hon CS, Chen Y, Hahn WC, Levesque MP, Doench JG, Berens ME, Shamji AF, Clemons PA, Stockwell BR, Schreiber SL. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo W, Zhang S, Chen Y, Zhang D, Yuan L, Cong H, Liu S. An important role of the hepcidin-ferroportin signaling in affecting tumor growth and metastasis. Acta Biochim Biophys Sin (Shanghai) 2015;47:703–715. doi: 10.1093/abbs/gmv063. [DOI] [PubMed] [Google Scholar]
- 74.Brown CW, Amante JJ, Goel HL, Mercurio AM. The α6β4 integrin promotes resistance to ferroptosis. J Cell Biol. 2017;216:4287–4297. doi: 10.1083/jcb.201701136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen P, Li X, Zhang R, Liu S, Xiang Y, Zhang M, Chen X, Pan T, Yan L, Feng J, Duan T, Wang D, Chen B, Jin T, Wang W, Chen L, Huang X, Zhang W, Sun Y, Li G, Kong L, Chen X, Li Y, Yang Z, Zhang Q, Zhuo L, Sui X, Xie T. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics. 2020;10:5107–5119. doi: 10.7150/thno.44705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, Xia H, Zhou J, Li G, Li J, Li W, Wei S, Vatan L, Zhang H, Szeliga W, Gu W, Liu R, Lawrence TS, Lamb C, Tanno Y, Cieslik M, Stone E, Georgiou G, Chan TA, Chinnaiyan A, Zou W. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim DH, Kim WD, Kim SK, Moon DH, Lee SJ. TGF-β1-mediated repression of SLC7A11 drives vulnerability to GPX4 inhibition in hepatocellular carcinoma cells. Cell Death Dis. 2020;11:406. doi: 10.1038/s41419-020-2618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu T, Ma Y, Yuan Q, Hu H, Hu X, Qian Z, Rolle JK, Gu Y, Li S. Enhanced ferroptosis by oxygen-boosted phototherapy based on a 2-in-1 nanoplatform of ferrous hemoglobin for tumor synergistic therapy. ACS Nano. 2020;14:3414–3425. doi: 10.1021/acsnano.9b09426. [DOI] [PubMed] [Google Scholar]
- 79.Elliott MR, Ravichandran KS. The dynamics of apoptotic cell clearance. Dev Cell. 2016;38:147–160. doi: 10.1016/j.devcel.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo X, Gong HB, Gao HY, Wu YP, Sun WY, Li ZQ, Wang G, Liu B, Liang L, Kurihara H, Duan WJ, Li YF, He RR. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ. 2021;28:1971–1989. doi: 10.1038/s41418-020-00719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rothe T, Gruber F, Uderhardt S, Ipseiz N, Rossner S, Oskolkova O, Bluml S, Leitinger N, Bicker W, Bochkov VN, Yamamoto M, Steinkasserer A, Schett G, Zinser E, Kronke G. 12/15-Lipoxygenase-mediated enzymatic lipid oxidation regulates DC maturation and function. J Clin Invest. 2015;125:1944–1954. doi: 10.1172/JCI78490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, Johnson JJ, Zhang LM, Klein-Seetharaman J, Celis E, Kagan VE, Gabrilovich DI. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol. 2014;192:2920–2931. doi: 10.4049/jimmunol.1302801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ubellacker JM, Tasdogan A, Ramesh V, Shen B, Mitchell EC, Martin-Sandoval MS, Gu Z, McCormick ML, Durham AB, Spitz DR, Zhao Z, Mathews TP, Morrison SJ. Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 2020;585:113–118. doi: 10.1038/s41586-020-2623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, Vedunova MV, Fimognari C, Bachert C, Coppieters F, Lefever S, Skirtach AG, Krysko O, Krysko DV. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8:e001369. doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 87.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, Sahai E, Reis e Sousa C. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Reis e Sousa C. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–1037. e14. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kurtova AV, Xiao J, Mo Q, Pazhanisamy S, Krasnow R, Lerner SP, Chen F, Roh TT, Lay E, Ho PL, Chan KS. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517:209–213. doi: 10.1038/nature14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu S, Min J, Wang F. Ferroptosis: an emerging player in immune cells (News & Views) Science Bulletin. 2021 doi: 10.1016/j.scib.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 92.Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212:555–568. doi: 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Drijvers JM, Gillis JE, Muijlwijk T, Nguyen TH, Gaudiano EF, Harris IS, LaFleur MW, Ringel AE, Yao CH, Kurmi K, Juneja VR, Trombley JD, Haigis MC, Sharpe AH. Pharmacologic screening identifies metabolic vulnerabilities of CD8(+) T cells. Cancer Immunol Res. 2021;9:184–199. doi: 10.1158/2326-6066.CIR-20-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muri J, Thut H, Bornkamm GW, Kopf M. B1 and marginal zone B cells but not follicular B2 cells require Gpx4 to prevent lipid peroxidation and ferroptosis. Cell Rep. 2019;29:2731–2744. e4. doi: 10.1016/j.celrep.2019.10.070. [DOI] [PubMed] [Google Scholar]
- 95.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wan C, Sun Y, Tian Y, Lu L, Dai X, Meng J, Huang J, He Q, Wu B, Zhang Z, Jiang K, Hu D, Wu G, Lovell JF, Jin H, Yang K. Irradiated tumor cell-derived microparticles mediate tumor eradication via cell killing and immune reprogramming. Sci Adv. 2020;6:eaay9789. doi: 10.1126/sciadv.aay9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, Brown LM, Uchida K, O’Connor OA, Stockwell BR. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem Biol. 2019;26:623–633. e9. doi: 10.1016/j.chembiol.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23:270–278. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ, Stockwell BR. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS, Bos PH, Reznik E, Ye LF, Tyurina YY, Lin AJ, Shchepinov MS, Chan AY, Peguero-Pereira E, Fomich MA, Daniels JD, Bekish AV, Shmanai VV, Kagan VE, Mahal LK, Woerpel KA, Stockwell BR. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018;14:507–515. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Woo JH, Shimoni Y, Yang WS, Subramaniam P, Iyer A, Nicoletti P, Rodriguez Martinez M, Lopez G, Mattioli M, Realubit R, Karan C, Stockwell BR, Bansal M, Califano A. Elucidating compound mechanism of action by network perturbation analysis. Cell. 2015;162:441–451. doi: 10.1016/j.cell.2015.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eaton JK, Ruberto RA, Kramm A, Viswanathan VS, Schreiber SL. Diacylfuroxans are masked nitrile oxides that inhibit GPX4 covalently. J Am Chem Soc. 2019;141:20407–20415. doi: 10.1021/jacs.9b10769. [DOI] [PubMed] [Google Scholar]
- 104.Eaton JK, Furst L, Ruberto RA, Moosmayer D, Hilpmann A, Ryan MJ, Zimmermann K, Cai LL, Niehues M, Badock V, Kramm A, Chen S, Hillig RC, Clemons PA, Gradl S, Montagnon C, Lazarski KE, Christian S, Bajrami B, Neuhaus R, Eheim AL, Viswanathan VS, Schreiber SL. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat Chem Biol. 2020;16:497–506. doi: 10.1038/s41589-020-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ooko E, Saeed MEM, Kadioglu O, Sarvi S, Colak M, Elmasaoudi K, Janah R, Greten HJ, Efferth T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine. 2015;22:1045–1054. doi: 10.1016/j.phymed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 106.Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2:517–532. doi: 10.18632/oncoscience.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trang Thi M, Hamai A, Hienzsch A, Caneque T, Mueller S, Wicinski J, Cabaud O, Leroy C, David A, Acevedo V, Ryo A, Ginestier C, Birnbaum D, Charafe-Jauffret E, Codogno P, Mehrpour M, Rodriguez R. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Na Chem. 2017;9:1025–1033. doi: 10.1038/nchem.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mai TT, Hamai A, Hienzsch A, Caneque T, Muller S, Wicinski J, Cabaud O, Leroy C, David A, Acevedo V, Ryo A, Ginestier C, Birnbaum D, Charafe-Jauffret E, Codogno P, Mehrpour M, Rodriguez R. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat Chem. 2017;9:1025–1033. doi: 10.1038/nchem.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, Mourao A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O’Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 110.Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, Cheng Q, Zhang P, Dai W, Chen J, Yang F, Yang HT, Linkermann A, Gu W, Min J, Wang F. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Angeli JPF, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Foerster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O’Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hilvo M, Denkert C, Lehtinen L, Mueller B, Brockmoeller S, Seppanen-Laakso T, Budczies J, Bucher E, Yetukuri L, Castillo S, Berg E, Nygren H, Sysi-Aho M, Griffin JL, Fiehn O, Loibl S, Richter-Ehrenstein C, Radke C, Hyotylainen T, Kallioniemi O, Iljin K, Oresic M. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011;71:3236–3245. doi: 10.1158/0008-5472.CAN-10-3894. [DOI] [PubMed] [Google Scholar]
- 114.Do Van B, Gouel F, Jonneaux A, Timmerman K, Gele P, Petrault M, Bastide M, Laloux C, Moreau C, Bordet R, Devos D, Devedjian JC. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol Dis. 2016;94:169–178. doi: 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 115.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, Cheng Q, Zhang P, Dai W, Chen J, Yang F, Yang HT, Linkermann A, Gu W, Min J, Wang F. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xie Y, Song X, Sun X, Huang J, Zhong M, Lotze MT, Zeh HJ Rd, Kang R, Tang D. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem Biophys Res Commun. 2016;473:775–780. doi: 10.1016/j.bbrc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 117.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966–4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayir H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, IngoId I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trumbach D, Mao G, Qu F, Bayir H, Fullekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang CC, Hsiao LD, Tseng HC, Kuo CM, Yang CM. Pristimerin inhibits MMP-9 expression and cell migration through attenuating NOX/ROS-Dependent NF-kappaB activation in rat brain astrocytes challenged with LPS. J Inflamm Res. 2020;13:325–341. doi: 10.2147/JIR.S252659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sehm T, Rauh M, Wiendieck K, Buchfelder M, Eyüpoglu IY, Savaskan NE. Temozolomide toxicity operates in a xCT/SLC7a11 dependent manner and is fostered by ferroptosis. Oncotarget. 2016;7:74630–74647. doi: 10.18632/oncotarget.11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davies TG, Wixted WE, Coyle JE, Griffiths-Jones C, Hearn K, McMenamin R, Norton D, Rich SJ, Richardson C, Saxty G, Willems HM, Woolford AJ, Cottom JE, Kou JP, Yonchuk JG, Feldser HG, Sanchez Y, Foley JP, Bolognese BJ, Logan G, Podolin PL, Yan H, Callahan JF, Heightman TD, Kerns JK. Monoacidic inhibitors of the Kelch-like ECH-associated protein 1: nuclear factor erythroid 2-related factor 2 (KEAP1:NRF2) protein-protein interaction with high cell potency identified by fragment-based discovery. J Med Chem. 2016;59:3991–4006. doi: 10.1021/acs.jmedchem.6b00228. [DOI] [PubMed] [Google Scholar]
- 123.Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z, Cai K, Zhao Y, Luo Z. HCAR1/MCT1 Regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. 2020;33:108487. doi: 10.1016/j.celrep.2020.108487. [DOI] [PubMed] [Google Scholar]
- 124.Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, Tarangelo A, Ward CC, Cho K, Patti GJ, Nomura DK, Olzmann JA, Dixon SJ. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem Biol. 2019;26:420–432. e9. doi: 10.1016/j.chembiol.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Muller C, Zandkarimi F, Merl-Pham J, Bao X, Anastasov N, Kossl J, Brandner S, Daniels JD, Schmitt-Kopplin P, Hauck SM, Stockwell BR, Hadian K, Schick JA. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Song X, Xie Y, Kang R, Hou W, Sun X, Epperly MW, Greenberger JS, Tang D. FANCD2 protects against bone marrow injury from ferroptosis. Biochem Biophys Res Commun. 2016;480:443–449. doi: 10.1016/j.bbrc.2016.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kagan VE, Mao GW, Qu F, Angeli JPF, Doll S, St Croix C, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar H, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayir H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, Zhong M, Yuan H, Zhang L, Billiar TR, Lotze MT, Zeh HJ 3rd, Kang R, Kroemer G, Tang D. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20:1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 130.Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J, Zhang Z, Wang X. Membrane Damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol Cell. 2021;81:355–369. e10. doi: 10.1016/j.molcel.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Z, Yao Z, Wang L, Ding H, Shao J, Chen A, Zhang F, Zheng S. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14:2083–2103. doi: 10.1080/15548627.2018.1503146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, Ding H, Tan S, Chen A, Zhang F, Zheng S. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2020;16:1482–1505. doi: 10.1080/15548627.2019.1687985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rouault TA. Biogenesis of iron-sulfur clusters in mammalian cells: new insights and relevance to human disease. Dis Model Mech. 2012;5:155–164. doi: 10.1242/dmm.009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, Baer CE, Dixon SJ, Mercurio AM. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell. 2019;51:575–586. e4. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chiang SK, Chen SE, Chang LC. A dual role of heme oxygenase-1 in cancer cells. Int J Mol Sci. 2018;20:39. doi: 10.3390/ijms20010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Knutson MD. Non-transferrin-bound iron transporters. Free Radic Biol Med. 2019;133:101–111. doi: 10.1016/j.freeradbiomed.2018.10.413. [DOI] [PubMed] [Google Scholar]
- 137.Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P, Wang J, Wu Q, Fang X, Duan L, Wang S, Wang K, An P, Shao T, Chung RT, Zheng S, Min J, Wang F. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726–739. doi: 10.1182/blood.2019002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Feng H, Schorpp K, Jin J, Yozwiak CE, Hoffstrom BG, Decker AM, Rajbhandari P, Stokes ME, Bender HG, Csuka JM, Upadhyayula PS, Canoll P, Uchida K, Soni RK, Hadian K, Stockwell BR. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020;30:3411–3423. e7. doi: 10.1016/j.celrep.2020.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478:838–844. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 140.Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X, Wang H, Cao L, Tang D. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34:5617–5625. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K, Possemato R. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017;551:639–643. doi: 10.1038/nature24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, Tyagi S, Ma L, Westbrook TF, Steinberg GR, Nakada D, Stockwell BR, Gan B. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22:225–234. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee JY, Nam M, Son HY, Hyun K, Jang SY, Kim JW, Kim MW, Jung Y, Jang E, Yoon SJ, Kim J, Kim J, Seo J, Min JK, Oh KJ, Han BS, Kim WK, Bae KH, Song J, Kim J, Huh YM, Hwang GS, Lee EW, Lee SC. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci U S A. 2020;117:32433–32442. doi: 10.1073/pnas.2006828117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gervois P, Torra IP, Fruchart JC, Staels B. Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin Chem Lab Med. 2000;38:3–11. doi: 10.1515/CCLM.2000.002. [DOI] [PubMed] [Google Scholar]
- 145.Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao H, Kang R, Wang X, Tang D, Dai E. Lipid storage and lipophagy regulates ferroptosis. Biochem Biophys Res Commun. 2019;508:997–1003. doi: 10.1016/j.bbrc.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 146.Gan W, Dai X, Dai X, Xie J, Yin S, Zhu J, Wang C, Liu Y, Guo J, Wang M, Liu J, Hu J, Quinton RJ, Ganem NJ, Liu P, Asara JM, Pandolfi PP, Yang Y, He Z, Gao G, Wei W. LATS suppresses mTORC1 activity to directly coordinate Hippo and mTORC1 pathways in growth control. Nat Cell Biol. 2020;22:246–256. doi: 10.1038/s41556-020-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gu Y, Albuquerque CP, Braas D, Zhang W, Villa GR, Bi J, Ikegami S, Masui K, Gini B, Yang H, Gahman TC, Shiau AK, Cloughesy TF, Christofk HR, Zhou H, Guan KL, Mischel PS. mTORC2 regulates amino acid metabolism in cancer by phosphorylation of the cystine-glutamate antiporter xCT. Mol Cell. 2017;67:128–138. e7. doi: 10.1016/j.molcel.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu T, Jiang L, Tavana O, Gu W. The Deubiquitylase OTUB1 Mediates ferroptosis via stabilization of SLC7A11. Cancer Res. 2019;79:1913–1924. doi: 10.1158/0008-5472.CAN-18-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hasegawa M, Takahashi H, Rajabi H, Alam M, Suzuki Y, Yin L, Tagde A, Maeda T, Hiraki M, Sukhatme VP, Kufe D. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget. 2016;7:11756–11769. doi: 10.18632/oncotarget.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact. 1998;111-112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 151.Kang YP, Mockabee-Macias A, Jiang C, Falzone A, Prieto-Farigua N, Stone E, Harris IS, DeNicola GM. Non-canonical glutamate-cysteine ligase activity protects against ferroptosis. Cell Metabolism. 2021;33:174–189. e7. doi: 10.1016/j.cmet.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martinez AM, Mirkovic J, Stanisz ZA, Patwari FS, Yang WS. NSC-34 motor neuron-like cells are sensitized to ferroptosis upon differentiation. FEBS Open Bio. 2019;9:582–593. doi: 10.1002/2211-5463.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Molecular Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shin D, Lee J, You JH, Kim D, Roh JL. Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol. 2020;30:101418. doi: 10.1016/j.redox.2019.101418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J, Xie Y, Liu J, Klionsky DJ, Kroemer G, Lotze MT, Zeh HJ, Kang R, Tang D. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system Xc(-) activity. Curr Biol. 2018;28:2388–2399. e5. doi: 10.1016/j.cub.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Du J, Zhou Y, Li Y, Xia J, Chen Y, Chen S, Wang X, Sun W, Wang T, Ren X, Wang X, An Y, Lu K, Hu W, Huang S, Li J, Tong X, Wang Y. Identification of Frataxin as a regulator of ferroptosis. Redox Biol. 2020;32:101483. doi: 10.1016/j.redox.2020.101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biology. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162–168. doi: 10.1016/j.freeradbiomed.2018.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang WH, Ding CC, Sun T, Rupprecht G, Lin CC, Hsu D, Chi JT. The hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep. 2019;28:2501–2508. e4. doi: 10.1016/j.celrep.2019.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang WH, Huang Z, Wu J, Ding CC, Murphy SK, Chi JT. A TAZ-ANGPTL4-NOX2 axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancer. Mol Cancer Res. 2020;18:79–90. doi: 10.1158/1541-7786.MCR-19-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Elgendy SM, Alyammahi SK, Alhamad DW, Abdin SM, Omar HA. Ferroptosis: an emerging approach for targeting cancer stem cells and drug resistance. Crit Rev Oncol Hematol. 2020;155:103095. doi: 10.1016/j.critrevonc.2020.103095. [DOI] [PubMed] [Google Scholar]
- 164.Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


