Abstract
Progesterone, the ovarian steroid hormone, regulates a plentitude of biological processes in tissues ranging from the brain to bones. Recognizing the role of progesterone and its receptors in physiological processes and maladies can prevent and treat various diseases. Apart from its physiological functions, its role in developing diseases, especially breast cancer, is a recent topic of deliberation. There exists conflicting experimental and epidemiological evidence linking progesterone to breast cancer. This review tries to describe the physiological functions of progesterone and its receptors, genomic and non-genomic signaling, splice variants, and a different aspect of progesterone signaling. Furthermore, we seek to address or attempt to discuss the following pertinent questions on steroid hormone signaling; How does progesterone influence breast cancer progression? How does it change the molecular pathways in breast cancer with different receptor statuses, the specific role of each isoform, and how does the ER/and PR ratio affect progesterone signaling?
Keywords: Progesterone, progesterone receptor, physiological functions, cancer
Physiology of progesterone and progesterone receptor
Progesterone (P4) is an endogenous steroid hormone with 21 carbon atoms originating from cholesterol and regulates normal female reproductive functions. Endocrine glands primarily synthesize the hormone progesterone, which belongs to the group of steroid hormones called progestogens [1]. Table 1 summarizes the primary physiological functions of progesterone.
Table 1.
Overview of functions of progesterone in different tissues and systems
| Target system/tissue | Function |
|---|---|
| Endometrium | ● The transition of endometrium from proliferative to secretory phase (Taraborrelli S 2015) |
| ● Promotes ovulation (Taraborrelli S 2015) | |
| Pregnancy | ● Essential for implantation and maintenance of early pregnancy |
| ● Progesterone suppresses myometrial contractility during pregnancy (Conneely OM et al. 2002) | |
| Mammary gland | ● Promotes lobular-alveolar development in preparation for milk secretion (Taraborrelli S 2015) |
| Brain | ● Progesterone controls neurobehavioral expression associated with sexual responsiveness (González-Orozco JC, Camacho-Arroyo I 2019) |
| ● It is related to neuroprotection, neuromodulation, myelination, neurogenesis, neuronal plasticity, and mood (Genazzani AR et al. 2000) | |
| ● Progesterone can act as a neuroprotective agent to treat traumatic brain injury (Wei J, Xiao GM 2013) | |
| Bone | ● Prevent bone loss (Balasch J 2003) |
| Cardio vascular system | ● Exert protective effect on the cardiovascular system, induces vasodilation, and decreases blood pressure (Pang Y et al. 2015) |
| Metabolism | ● Progesterone induces hyperinsulinemia and stimulates deposition of body fat |
| ● It also influences ketone body production (Kalkhoff RK 1982) | |
| Immune system | ● Progesterone inhibits inflammatory innate immune response |
| ● It also alters the distribution and activity of T cells (Hall OJ, Klein SL 2017) |
Ovarian follicles serve as the source of progesterone on target tissues. The effects of progesterone are mediated by the progesterone receptor (PR), a member of the nuclear receptor superfamily of transcription factors that regulate gene expression upon hormonal stimulation. Progesterone receptors were first cloned in 1986 using the same strategies, simultaneously in the laboratories of Pierre Chambon, Edwin Milgrom, and Bert O’Malley; also, it is the first receptor to be identified having genuine isoforms [11]. The receptor exists in the cytoplasm as a multiprotein chaperone complex and functions in a ligand-dependent and independent manner [12]. In the ligand-dependent pathway, when progesterone binds to the inactive PR, it causes a conformational change and releases the chaperone. It then dimerizes and binds to progesterone response elements (PRE) of the target gene and recruit specific transcription factors and coactivators to regulate the transcription of the target gene. The ligand-independent pathway functions in a cell type and promoter-specific manner through cytoplasmic and membrane-generated signals [12]. The structure of the progesterone receptor consists of a highly conserved central DNA binding domain (DBD), a C terminal hormone-binding domain (HBD) which is moderately conserved, and a poorly conserved N terminal region [12] (Figure 1). In addition to these regions, progesterone receptor contains activation function (AF) elements and inhibitory function (IF) elements, which along with co regulators control PR’s transcriptional activity. And PR gene consists of 8 coding exons separated by seven noncoding introns [12,13].
Figure 1.
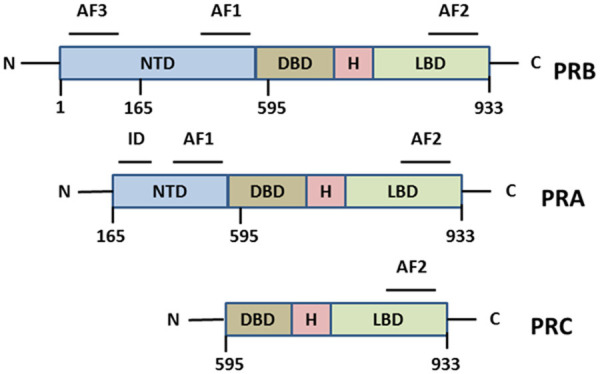
Structure of different isoforms of progesterone receptors. The PR consist of four different domains, NTD represents the N-terminal transactivation domain, DBD represents the DNA binding domain, a hinge region, and LBD represent the Ligand-binding domain. AF1, AF2, and AF3 are activation function domains.
Isoforms and splice variants of progesterone receptor
Two significant isoforms of PR, PRA and PRB, mediates most of the physiological functions of progesterone in humans. These two isoforms are transcribed from a single gene and from a defined promoter site within the gene [14]. PRA and PRB are identical in structure except for a - region of 164 amino acids (that forms the AF3 in PRB) missing in the amino-terminal of PRA. To simply put, PRA is a truncated form of PRB.
Though being isoforms, PRA and PRB regulate different target genes in response to progesterone. PR knock-out mice (both isoforms ablated mice), have shown pleiotropic reproductive abnormalities. To study the reproductive functions of each isoform, Conneely et al. (2001) selectively knocked out the PRA gene. They identified that PRB mediates a subset of reproductive functions of progesterone in PRA knock-out mice models [15]. But the response of the thymus or mammary gland to progesterone was not affected by this ablation. Further, they concluded that PRA and PRB act as two functionally different transcription factors in mediating reproductive functions rather than depend on tissue-specific PRA/PRB ratio.
PRA and PRB are present in the endometrium epithelium during the proliferative phase of the menstrual cycle and increases accordingly with estrogen. At the time of the secretory phase of the menstrual cycle, PRB remains constant, and the level of PRA decreases, suggesting a role for PRB in glandular secretion. Interestingly, over the course of the menstrual cycle, PRA predominates in the stromal cells indicating its role in establishing pregnancy [16]. Knockdown experiments in mice have also shown that PRA is sufficient for the normal uterine functions, including the functioning of ovaries, and necessary for puberty, implantation, and pregnancy. At the same time, PRB alone leads to hyperplasia and inflammation of the endometrial epithelium [16]. Several studies have demonstrated that overexpression of PRA can result in uterine enlargement and endometrial hyperplasia [15,16]. “Progesterone block hypothesis” is a widely accepted hypothesis which states that pregnancy is maintained by progesterone and prevents premature labor. Progesterone withdrawal is a crucial trigger for parturition. Methylation in the promoter region of PRA increases its expression in human pregnancy and accomplishes progesterone withdrawal associated with parturition [16-18]. But expression of pro-inflammatory genes by progesterone depends on PRA: PRB ratio. During pregnancy in myometrial cells, when this ratio favors PRB, it mediates the anti-inflammatory effects of progesterone, and when the ratio favors PRA it promotes pro-inflammatory effects [16,17]. In other words, in pregnancy, trans repressive actions of PRA inhibit the ability of progesterone to exert anti-inflammatory activity via PRB.
In 1990 Wei et al. [19] described a third isoform of progesterone, termed progesterone receptor C in the T47d cell line, which is abundantly synthesized in PR positive cells. PRC is a 45-50KDa protein and results from translation initiation at methionine start codon Met-595. It can form a homodimer or heterodimer with other isoforms [14,19]. AF1, AF3, and an entire DNA binding domain are missing in PRC, so it cannot interact with PRE. But it possesses a nuclear localization signal and two dimerization domains, so PRC can interact with nuclear co-factors and thus influence PRA and PRB [14]. However, Samalecos et al. [20] later proved that PRC does not originate from AUG595 and is not a naturally occurring PR isoform [20,21].
Condon et al. made a striking observation that PRC is overexpressed in the myometrium during labor, and this increase is associated with increased expression of PRB [22]. When PRC: PRB ratio favors PRC, it leads to withdrawal of progesterone by sequestering progesterone from PRB and making it unable to bind to DNA in the laboring myometrium. This altered receptor ratio is contributed by activation of NF-KB pathway in both laboring human fundus and pregnant mouse uterus, resulting in inhibition of PR transactivation and changes in uterine quiescence, leading to labor. In addition to PRA, PRB, and PRC, other isoforms of PRmRNA, PRS, and PRT, have been described from the human testis cDNA library [23]. PRS consists of a novel sequence before exon four termed ‘exon S’ and exon 4-8 of PR gene. The protein encoded by this mRNA lacks the DNA binding domain, but the progesterone binding domain makes it capable of binding to the hormone and mediates nongenomic signaling [23]. The cDNA of isoform T consists of an independent exon, ‘exon T’ before exon four, and exons 4-8 of the PR gene. Like PRS isoform PRT could mediate non-genomic signaling and recruitment of co-factors.
Indeed, PRS and PRT were similar in structure, ‘exonT’ is located in the 5’ region of ‘exonS’, and ‘exonS’ and ‘exonT’ are located between exon 3 and 4 in the human PR gene. Neither S nor T contains a translation initiation site, so the translation is likely to start at the first methionine in exon 4-8 [23].
A study by Marshburn PB et al. [24] revealed the existence of splice variants of PR mRNA lacking exon 4 (del-4 PR), exon 6 (del-6 PR), exons 4 and 6 (del-4&6 PR), and part of exon 4 (del-p4 PR) or part of exon 6 (del-p6 PR) with wild type progesterone receptor in the human endometrium throughout menstrual cycle development. Studies have not followed up on these variants, and their effects on infertility, cancer, and other diseases are unknown. Another splice variant, isolated from endometrial carcinoma cells, contains an insertion of a 232 bp sequence between exon 4 and 5 [25]. They termed it as i45PR mRNA, derived from two independent exons, i45a and i45b. The protein from this mRNA in endometrial cancer cells confers a role for this variant in normal/pathophysiology of the endometrium [25].
Saner KJ et al. [26] described another non-nuclear PR, PR-M (consisting of 314 amino acids), located in the mitochondria. A truncated version of nuclear PR, PR-M gene originates in the distal 3rd intron of the PR gene. It consists of a translation start site that encodes 16 novel N-terminus amino acids followed by a sequence identical to exons 4-8 of nuclear PR. These 16 amino acids have sequence similarity to the N terminal sequence of the outer mitochondrial membrane and are hydrophobic, suggesting a transmembrane domain function for PR-M. It possesses the hinge region and LBD of nPR but lacks the N-terminal domain, the DBD, and a complete NLS. The primary role of this receptor, localized in the outer mitochondrial membrane, is regulation of cellular respiration within the mitochondria [27]. Hitherto any physiological function for other splice variants of progesterone receptor is not known.
The reports of action of progesterone on cells devoid of nuclear PR have led to the discovery of non-genomic participants of progesterone signaling, i.e., the membrane progesterone receptor (mPR), and progesterone receptor membrane components (PGRMCs). mPR belongs to the family of Progestin and AdipoQ Receptors (PAQR), and exist as five isoforms - mPRα, mPRβ, mPRγ, mPRδ, and mPRε [28]. Zhu et al., in 2003 [28,29], first described the presence of mPRα on the membrane of the fish oocyte. Later, this receptor and two other isoforms, namely mPRβ and mPRγ, were identified in vertebrates, including humans [28,30]. It has characteristics of G protein-coupled receptors and possesses seven transmembrane domains. The isoforms, δ, and ε were identified in the Yeast recombination protein expression system [31].
PGRMC is a member of an omnipresent protein family, Membrane-Associated Progesterone Receptor (MAPR), and contains a cytochrome b5 domain [32] (Figure 2). PGRMC1 and PGRMC2 are two members of this group involved in various physiological and pathological processes like breast cancer, ovarian cancer, estrous cycle etc. Together with the serpinel mRNA binding protein I (SERBP1), PGRMC1 controls the anti-apoptotic effect of progesterone in granulosa cells [33], and, its expression may be related to ovarian tumor invasion and metastasis [34].
Figure 2.
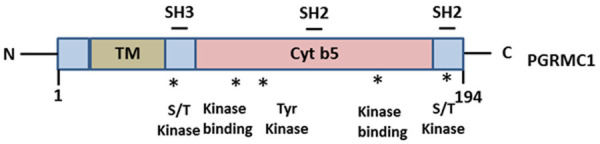
Structure of PGRMC1. PGRMC1 consist of an N terminal TM domain, a cytochrome b5 domain, SH2, and SH3 binding domains, and sites for kinase binding (indicated by asterisks).
Physiological functions of PR isoforms and progesterone signaling
Progesterone and neuroprotection
Besides ovaries and placenta, progesterone is synthesized in both sexes by adrenal glands and within the brain by oligodendrocytes macroglial cells like astrocytes, Schwann cells, and peripheral and central nervous system [35,36]. Remarkably, progesterone level increases up to 10 fold during fetal growth to support the development of neurons. Progesterone could affect the behavioral and cognitive spheres in lactating mice, promising a therapeutic substitute for hostile psychiatric behavior, anxiety, and depression [37]. In a study conducted in 122 healthy premenstrual, reproductive-age women, a low level of progesterone-induced a high intensity of premenstrual mood symptoms such as aggressive behavior and fatigue [38]. Further, higher progesterone levels throughout the post-partum period in lactating rats are related to less aggressive behavior [39] -these all studies suggesting the potential therapeutic use of progesterone in mood disorders.
The action of progesterone and its metabolites in the brain are pleiotropic, and it includes effects on myelination, cognition, glial cell functions, inflammation, neurogenesis, and neural progenitor cell proliferation [40]. Several pre-clinical and clinical studies clearly showed the neuroprotective properties of progesterone. Allopregnanolone, a progesterone metabolite, acts on glial cells by endorsing myelin production and decelerating the evolution of Alzheimer’s disease [36]. Progesterone also has a neuroprotective effect on neurodegenerative diseases,brain trauma, stroke, anoxic brain injury, and spinal cord injury [41]. Traumatic brain injury (TBI) is a multifactorial process, causes sudden damages to the brain caused by an external force. Notably, after traumatic brain injury, progesterone treatment helps to correct and maintain neuronal homeostasis [42,43]. Administration of progesterone in post-injury experimental models of head injury confers protection against TBI-induced cerebral edema and secondary neuronal death [44]. Progesterone may inhibit inflammatory cytokines IL-β and TNF-α in the frontal cortex of the traumatic brain injury (TBI), thus prevent cerebral edema by stabilizing the blood-brain barrier and inhibiting water, ions, and inflammatory molecules from crossing the blood-brain barrier. The sex steroid hormones, especially progesterone, activate MAPK, ERK, and Akt signaling pathways which are known to be associated with neuroprotection [45]. The progesterone receptors localized near the plasma membrane could interact with signal transduction kinases and activate the MAPK pathway [46,47]. Further, progesterone also up-regulates the expression of brain-derived neurotrophic factor (BDNF), a neurotrophin present in CNS, and reduces mitochondrial dysfunction, and all these mechanisms are linked to neuroprotection [45]. Progesterone receptors are localized at different brain parts, mainly the hypothalamus, hippocampus, and cortex. Other than nuclear PR, membrane receptor PGRMC1 also mediates the effects of progesterone in the brain by activating Jak/STAT, Src, and protein kinase G pathways. mPR also identified in several parts of the brain and acts through MAPK and G-protein pathways [48]. These two membrane receptors mediate the neuroprotective effects of progesterone after TBI.
Progesterone and male reproduction
Though considered a female hormone, progesterone also modulates male reproduction. It is now understood that progesterone regulates spermiogenesis, acrosome reaction, and testosterone biosynthesis in Leydig cells [49]. Higher levels of progesterone were identified in testicular tissues. Steroid acute regulatory gene (StAR) necessary for testosterone synthesis is also stimulated by progesterone in the rat testis [50]. Leydig cells in aged rats produce an increased amount of progesterone. Further studies in humans pointed out that testosterone production in testicular cells decreased in older men, and testosterone precursors progesterone and 17a-hydroxyprogesterone increased in the testicular tissue and the spermatic vein, thus having a deleterious effect on the testicular tissue [49].
Further, progesterone promotes sperm acrosome reaction in capacitated spermatozoa, where progesterone has been produced by cumulus cells of oocyte and is present in the follicular fluid [51]. The presence of intracellular and membrane-bound progesterone receptor was identified in human adult testis by Shah C. A. et al. [50]. They recognized PRA, PRB, and a 55kDa band, from the testis and spermatogenic cell lysate. Progesterone receptor isoform S was also identified from the human testicular cDNA library [24].
Progesterone and immune function
Several studies have established the anti-inflammatory role of progesterone in the brain and other tissues such as the intestine [52-56]. Progesterone is found to reduce lipid peroxidation, cellular apoptosis, oxidative stress, and the release of inflammatory cytokines [45,57]. Downregulation of inflammation is a well-established function of progesterone, and it allows maternal immune tolerance of fetal allograft [59]. Progesterone also induces the expression of HLA-G (Human leukocyte antigen), a non-classical MHC-1 molecule expressed on Extravillous trophoblasts (EVTs), responsible for maintaining immune tolerance during pregnancy [58]. Moreover, the presence of progesterone receptors on a wide variety of immune cells suggests a role for progesterone in immune modulation. Progesterone generally inhibits innate immune responses. Studies show that progesterone can suppress the activation of macrophages and dendritic cells also.
When progesterone binds to its receptor, it obstructs the NF-kB pathway and inhibits downstream activation of the NF-κB pathway, including cyclooxygenase-2, to decrease inflammation. It also affects the production of pro-inflammatory cytokines like TNF-α, IFN-γ, and IL-12 and stimulates the anti-inflammatory cytokine IL-10. In vitro treatment of T-cells with progesterone, biased naive T cells from Th1 to Th2 type response and induced synthesis of IL-10, IL-13, and IL-27. In vivo studies showed an increased production of TNF-α in the pregnant endometrium in the presence of progesterone. Lymphocytes in pregnant individuals show higher sensitivity compared to non-pregnant individuals. In B cells, progesterone induces the production of protective asymmetric antibodies. Ex vivo experiments have demonstrated that progesterone causes the differentiation of blood monocytes into dendritic cells, explaining the presence of monocyte-derived dendritic cells in human deciduas. These dendritic cells then modulate the immune response during pregnancy, regulating TH1/TH2 bias through IL12 secretion. This extraordinary interaction between the endocrine and the immune system leads to a dominance of TH2 immune response and successful pregnancy [58].
Progesterone and oxidative stress
Reactive oxygen species are produced due to normal cellular metabolism and initiate various signaling pathways in response to changes in intra and extracellular conditions [59]. Oxidative stress engenders cell survival, proliferation, and metastasis of multiple cancers, but uncontrolled stress leads to cell death. So harnessing oxidative stress as a treatment strategy for various cancers is a topic of current interest [60]. Progesterone induces nitric oxide (NO) production, a regulator of vascular homeostasis, in primary kidney arterial endothelial cells. It functions in these cells by oxidative stress signal/HIFα/eNOS/NO pathway. Here, progesterone inhibits the antioxidant system, increasing oxidative stress and expression of Hypoxia-inducible factors (HIF) and H2O2 level thus leads to NO production [61]. Several studies broached the effect of progesterone on lipid peroxidation and oxidative stress. In repeated mild traumatic brain injuries, progesterone treatment could attenuate neuroinflammation and oxidative stress [62]. Likewise, Lipid peroxidation was reduced consequent to progesterone treatment in a dose-dependent manner in various in vitro free radicle generating systems [63]. When cerebral edema models are treated with progesterone, it increases inhibitory neurotransmitter GABAα and reduces oxidative stress and lipid peroxidation. In pregnancy, the same effects are observed in the brain homogenates and mitochondria, suggesting a role for progesterone in this effect [45]. A recent study reported that long-term progesterone treatment could rescue ovariectomized mice from impaired learning and memory [64] by increasing the antioxidant enzyme SOD activity and reducing Malondialdehyde (MDA), a marker of lipid peroxidation. Further studies identified that in the brain, progesterone is converted to 5α-di-hydro progesterone and further allopregnanolone. Allopregnanolone alleviates lipid peroxidation and ROS production, thus averting peroxide-induced apoptosis and NF-kB activation due to its ability to restore the intracellular redox state [65-67]. In conclusion, progesterone exerts a protective role by reducing oxidative stress and enhancing endogenous free radical scavenging systems. Recent studies in our laboratory have identified role of oxidative stress in progesterone-treated breast cancer cell line MCF-7. Progesterone treatment induces the release of reactive oxygen species in MCF-7 cells in a time- and concentration dependent manner and thus regulates the anti-proliferative activity in breast cancer cell lines [68]. The Enhanced ROS production altered the expression of antioxidant enzymes SOD1 and SOD2 in progesterone treated MCF-7 cells. Further detailed molecular and proteomics studies identified a role for calcium signaling in progesterone-induced growth inhibition by regulating essential proteins VDAC1 and SERCA3, involved in calcium signaling and transport [69].
Progesterone and cardiovascular system
Progesterone exhibits a beneficial effect on the cardiovascular system. It lowers blood pressure, hinders coronary hyperactivity, and has vasodilatory and natriuretic effects [8]. Human aorta vascular smooth muscle cells express both PRA and PRB, and expression of PRA is more in women than males. Most of the rapid actions of progesterone in the cardiovascular system are attributed to membrane progesterone receptors. Progesterone increases rapid nitric oxide production in both human and animal vascular endothelial cell models by regulating the synthesis of endothelial NO synthase (eNOS) and activating membrane progesterone receptor alpha [8]. NO is the primary regulator of blood vessel dilation and acts by relaxing the surrounding vascular smooth muscle cells. mPRβ and mPRγ is also reported in human endothelial and smooth muscle vascular cells. Mitochondrial PR in the myocardial cells regulates oxidative cellular respiration and beta-oxidation in a ligand-dependent mechanism that assigns the increased myocardial energy production during pregnancy [70].
Progesterone and bone metabolism
In females, estradiol, and progesterone-function together to maintain bone balance. Estradiol prevents bone resorption, while progesterone increases bone formation or bone turnover, a slow process mediated by progesterone receptors A and B expressed on human osteoblasts [71,72]. Several studies have suggested the importance of oral micronized progesterone, similar to ovarian progesterone, in preventing and treating bone loss in pre-or peri-menopausal women with regular, estrogen-sufficient menstrual cycles who are also experiencing ovulatory disturbances [73]. However, the role of PR signaling in bone remains controversial [74,75].
Metabolic effects of progesterone
Progesterone affects carbohydrate metabolism by inducing hyperinsulinemia via a direct action on pancreatic islets while promoting glycogen storage in the liver. Interestingly, progesterone has a negative impact on the effect of insulin on glucose metabolism in adipose tissues and skeletal muscle. In lipid metabolism, it has an anabolic effect and catabolic effect on protein metabolism. All, these actions of progesterone appears to be the body’s adaptation for normal pregnancy [9]. Two randomized studies in postmenopausal women concluded that progesterone does not affect lipid profile [76,77], making it favorable for HRT as it doesn’t modify blood lipids induced by estrogen, as done by other progestogens [78,79]. It has also been shown that rat adipocytes express PR, and inhibiting lipolysis [80]. A recent study on blood glucose level showed that progesterone induces the transcription of gluconeogenic genes and increases blood glucose level through induction of hepatic PGRMC1 under insulin-resistant conditions [81,82].
Furthermore, progesterone activates Glycogen phosphorylase, a key enzyme in glycogen metabolism, leading to a rise in blood sugar levels [83]. In pregnancy,several studies point out a direct relationship between higher progesterone levels and gestational diabetics characterized by glucose intolerance, a consequence of inadequate insulin supply [84-86]. Pregnant rats treated with progesterone were more prone to insulin resistance than placebo control [87] due to reduced expression of Glut4 in skeletal muscles, leading to decreased glucose uptake [83]. PR knock-out female mice demonstrated greater glucose tolerance and lower fasting blood sugar due to increased βcell mass and βcell proliferation [88].
Progesterone signaling in multiple diseases
Covid-19
Covid-19 belongs to the ß coronavirus cluster, a zoonotic coronavirus disease, behind the SARS and the Middle East respiratory syndrome [89]. Despite limited knowledge on its source, current data suggests that SARS-CoV-2 was an amalgamation of unknown origin of coronavirus and bat coronavirus [89]. Although in the beginning, much attention was laid down on the elderly or those with morbidities as being at high risk for contracting/dying of COVID-19, epidemiological studies have revealed that males are more susceptible than females. In Italy and Spain, the majority of COVID-19 deaths were male than in females. Apart from this, figures from China also indicated a gender gap in mortality rate. Data from other countries, including the USA, South Korea, Germany, and United Kingdom also confirmed the similar pattern of death rate [89]. One of the possible reasons could be the expression and distribution of Angiotensin-converting enzyme-2 (ACE 2), a responsible receptor for SARS-CoV-2 encoded by the ACE 2 gene [90], implying a positive correlation between the ACE 2 receptor and coronavirus infection [91]. Taken this into account, many studies have quantified the expression of ACE 2 receptors in human cells based on gender ethnicity.
Single-cell RNA-sequencing (RNA-seq) analysis revealed that Asian males are more vulnerable to the virus than females since they had higher expression of ACE 2 receptors compared to females [92]. In addition, a study conducted in the Chinese population indicated that the expression level of ACE-2 was excessively high in Asian males than females [92]. Another reason could be the presence of steroid hormones like 17β-estradiol (E2) and progesterone (P4) and their strong immunomodulatory action at peak concentrations [93]. Among them, progesterone (P4) is an important immunomodulatory and anti-inflammatory hormone produced at high concentrations during pregnancy by the placenta. Pinna G et al. [37] from the University of Illinois in Chicago outlines some of the evidence in Trends in Endocrinology & Metabolism, pointing out that female reproductive hormones possibly play a role in sex preference trends of the coronavirus disease 2019 (COVID-19). Progesterone, the well-known steroid hormone functioning in reproduction, also regulates essential immunomodulatory functions such as redesigning the competence of immune cells and bringing strong anti-inflammatory actions [94]. By activating the progesterone-induced blocking factor, progesterone can suppress cellular cytotoxicity, regulate T cell receptor signaling, and may also suppress degranulation [58]. Most immune cells, including epithelial cells, macrophages, dendritic cells, lymphocytes, mast cells, eosinophils, express progesterone receptors [95]. In humans and rodents, progesterone prevents the synthesis of pro-inflammatory cytokines IL-1β and interleukin 12 by macrophages and dendritic cells. Progesterone also influences the skewing of CD4+ T-helper cell responses from Th1-type to Th2-type and thus the production of anti-inflammatory cytokines IL-4 and IL-10 [95-97]. Progesterone treatment of cord blood cells increases the percentage of FOXP3+ Treg cells (thus promoting immune tolerance), and decreases pro-inflammatory Th17 cells. Progesterone-depleted adult female mice confer protection from lethal influenza A virus pneumonia when administered with progesterone at concentrations sufficient to mimic the luteal phase [98]. Progesterone treatment in mice ensued an earlier recovery without effects on viral load by decreasing the lung inflammation, improving pulmonary functions, and repairing and promoting cell proliferation. Although the immune reactions produced by influenza A virus infection and SARS-CoV-2 are different, Hall OJ et al. 2016 provides essential insight into progesterone’s immunomodulatory and healing effects. Butterworth et al., in 1967 itself, and other multiple studies, subsequently confirmed that women produce higher levels of circulating immunoglobulins IgG and IgM than men [99].
E2 and P4 also boost immune tolerance by expanding regulatory T cells (Treg) [96,97] (Figure 3). Considering these immunomodulatory actions of progesterone, a recent study described progesterone treatment as a safe and effective procedure in hospitalized men with COVID-associated hypoxia [100]. Clinical trials are also testing the effectiveness of progesterone or estradiol treatment in COVID-19 patients [93].
Figure 3.
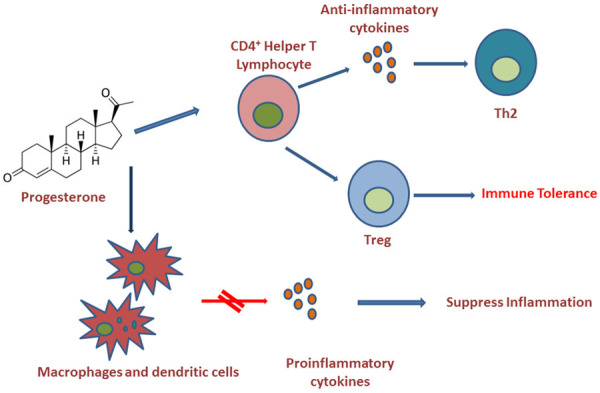
Anti-inflammatory and immunomodulatory actions of progesterone. Progesterone inhibits synthesis of pro-inflammatory cytokines by macrophages and dendritic cells. Progesterone also helps to produce anti-inflammatory cytokines by CD4+ T-helper cells and switch to Th2 type anti-inflammatory response. Moreover, both estradiol and progesterone stimulate the expansion of T-regulatory cells, thus supporting immune tolerance.
Breast cancer
As a partner to estrogen, progesterone is the most crucified female hormone related to cancer. A role for progesterone in the development and progression of breast and gynecological cancers is gaining more attention, as various research groups reported contradictory results. Studies in cell lines showed biphasic effects of progestins. In 1985 Horwitz and Freidenberg demonstrated the inhibitory action of progestin in T47D cell line independent of estrogen induction [101]. In T47D cell line, cyclin-dependent kinase inhibitor p27Kip1 (p27) gene mediates the inhibitory effects of progesterone by transcriptional upregulation [102]. Alkhalaf M et al. (2002) investigated the molecular mechanisms underlying progesterone’s growth inhibition of breast cancer cell lines [103]. They showed that MCF-7 cell lines undergo differentiation, not apoptosis, upon treatment with progesterone, and phosphorylation of Akt is the crucial mediator of this process. Groshong SD et al. (1997), using T47DYB cells, demonstrated progesterone’s capability to stimulate or inhibit cell growth depending on whether treatment is transient or continuous [104]. G1 phase cell cycle arrest after progesterone treatment is accompanied by cellular changes that permit other, possibly tissue-specific, factors to influence the final proliferative or differentiative state.
Later Lange CA et al. (1999) hypothesized about the contradictory actions of progesterone, which acts as a priming factor for secondary agents like cytokines and growth factors [105]. Other transient or intermittent doses of progesterone are growth stimulatory, while continuous or sustained high-dose progesterone is growth inhibitory. This model of progesterone action implied the importance of timing of progesterone treatment in clinical practice, i.e., continuous or periodic administration. It also suggested the difference in physiological consequence of endogenous cyclical progesterone of the menstrual cycle and continuous progesterone during pregnancy. Anti-apoptotic effects of progesterone add controversy to the topic. Hissom JR and Moore MR first reported proliferative effects of progesterone in T47D cells [106]. They identified that promegestone alone, at physiological progestin concentration, significantly stimulates growth. Progesterone also protects breast cancer cells against serum depletion and radiation-induced apoptosis in PR-positive breast cancer cell lines [107].
In normal human mammary cells, PRA and PRB express in equimolar ratios [108]. But this ratio changes in breast cancer cells and PR isoform expression and ratio significantly affect breast cancer progression. In breast cancer cell lines, PRA regulates more genes than PRB, with a modest overlap in genes controlled by both isoforms. Studies identified PRB as more proliferative while increased expression of PRA responsible for metastasis. In the presence of its ligand, PRB is transcriptionally more active, and in its absence, PRA has more dominant roles [109]. The main downstream effectors of PR signaling are cyclin D1, WNT4, and RANKL, which promote breast carcinogenesis [110]. And overexpression of PRA leads to transcription of specific genes involved in cell proliferation and metastasis. TNFRSF11A, one among those genes, encodes RANK (receptor activator of NF-Κb), the receptor for RANKL. Progesterone has a direct effect on PR-positive cells in normal breast, but in PR negative cells, it acts through RANKL induced paracrine actions, which in turn leads to the proliferation of PR negative mammary epithelial cells [111]. Changes in RANKL signaling has a positive correlation with primary breast cancer development. Studies reported that hypoxia up-regulates RANK/RANKL signaling and increases breast cancer cell migration through PI3K/Akt-HIF-1α pathway [112]. RANK/RANKL also increases the expression of cyclinD1 and its overexpression correlated with poor prognosis in ER+PR+ patients. Surprisingly RANKL is neither expressed nor stimulated by progesterone in most breast cancer cell lines, including MCF-7 and T47D. But in vivo studies confirmed RANKL protein expression and a positive correlation with serum progesterone levels [113].
Moreover, tumors expressing PRA respond more effectively to antiprogestins [113]. Brca1/p53-deficient mice overexpress PRA, and a progesterone antagonist mifepristone (RU 486) prevented mammary tumor formation in those animals [114]. In a postmenopausal model of an obese ovariectomized rat with a chemically induced tumor, the PRA expression increased, enhancing tumor growth compared to its control rat [113]. PRA and PRB ratio in breast cancer is an essential indicator of treatment outcome and prognosis. Increased expression of PRA results in resistance or less response to tamoxifen treatment, and also, mifepristone, an antiprogestin, inhibits cell proliferation in PRA overexpressing tumors [113,115].
Non-genomic signaling of progesterone can also affect the pathophysiology of breast cancer. Studies identified overexpression of mPR and PGRMC1 in breast cancer cell lines [109]. In TNBC cell line progesterone exerts its effects through mPRα and mPRβ and suppresses the growth and metastasis of TNBC cells to the brain through mPRα [116]. Progesterone also reverses the mesenchymal phenotype to epithelial-like phenotype in MDAMB231 cells through mPRα [109]. Moreover, primary breast tumors express PGRMC1, and its expression is related to increased tumor size and lymph node metastasis [117]. In another study, PGRMC1 was shown to increase the chemotherapeutic resistance in breast cancer and attenuate the apoptotic effect of chemotherapeutic drug doxorubicin [118]. PGRMC1 is expressed in both ER+PR+ and TNBC cell lines, and controls the breast tumorigenesis through PI3K/AKT/mTOR and EGFR signaling pathway [119].
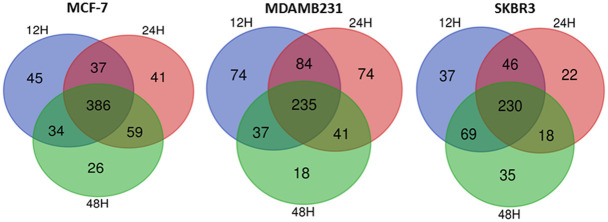
In our laboratory, we have used different breast cancer cell lines having different receptor status. We have used the progesterone which is identical to the natural hormone and not the progestins, to study its effect on breast cancer growth regulation. Progesterone and progestin differ in chemical structure and progestins may mimic some of the action of progesterone but have different effect on progesterone receptor [120]. Using proteomics approach, identified the proteins which is responsive to progesterone in MCF-7, MDAMB231 and SKBR3 cell lines. Figure 4 represents the total proteins expressed at different cell lines in response to progesterone [Figure 4].
Figure 4.
Venn-Euler diagram of commonly expressed proteins in different breast cancer cell lines. Breast cancer cell lines MCF-7, MDAMB-231, and SKBR3 were treated with progesterone for 48 hr. Protein samples were collected by cell lysis and further analyzed by LC-MS-MS analysis and total proteins are represented as Venn-Euler diagram.
The proteins were classified using the Panther classification system (Table 2), and important up-regulated and down-regulated proteins are listed in Table 3. Functional studies in MCF7 (ER+PR+HER2+) (69) and MDAMB231 (ER-PR-HER2-) [unpublished data] indicate that progesterone has growth inhibitory functions in breast cancer cells, irrespective of their receptor status, suggesting a cross-talk or the involvement of other receptors in mediating progesterone signaling.
Table 2.
List of major pathways stimulated by progesterone in different breast cancer cell lines
| MCF-7 | MDAMB231 | SKBR3 | |
|---|---|---|---|
| Molecular Function | ● Binding | ● Binding | ● Binding |
| ● Catalytic activity | ● Catalytic activity | ● Catalytic activity | |
| ● Structural molecule activity | ● Structural molecule activity | ● Structural molecule activity | |
| Biological process | ● Cellular process | ● Cellular process | ● cellular process |
| ● Biological regulation | ● Metabolic process | ● Metabolic process | |
| ● Metabolic process | ● Response to stimulus | ● Response to stimulus | |
| ● Response to stimulus | ● Biological regulation | ● Biological regulation | |
| ● Signaling | |||
| ● Localization | |||
| Pathways | ● Apoptosis signaling | ● Apoptosis signaling | ● Apoptosis signaling |
| ● Cytoskeletal regulation by Rho GTPase | ● Cytoskeletal regulation by Rho GTPase | ● Cytoskeletal regulation by Rho GTPase | |
| ● FGF signaling pathway | ● FGF signaling pathway | ● FGF signaling pathway | |
| ● EGF receptor signaling pathway | ● EGF receptor signaling pathway | ● EGF receptor signaling pathway | |
| ● Inflammation pathway | ● Inflammation pathway | ● Inflammation pathway | |
| ● Glycolysis | ● Glycolysis | ● Glycolysis | |
| ● TCA cycle | ● TCA cycle | ● TCA cycle | |
| Protein class | ● translational protein | ● Chaperons | ● Chaperons |
| ● metabolite interconversion enzyme | ● translational protein | ● translational protein | |
| ● Cytoskeletal proteins | ● metabolite interconversion enzyme | ● metabolite interconversion enzyme | |
| ● Chaperone | ● Cytoskeletal proteins | ● Cytoskeletal proteins | |
| ● Calcium-binding protein | ● Calcium-binding protein | ● Calcium-binding protein | |
| ● Protein modifying enzymes | ● Protein modifying enzymes | ● Protein modifying enzymes | |
| ●Protein binding activity modulator | ● Protein binding activity modulator |
Table 3.
List of major proteins up-regulated and downregulated by progesterone from proteomics analysis, in different breast cancer cell lines
| Down-regulated Proteins | Up-regulated Proteins | |
|---|---|---|
| MCF7 | Protein S100 A11 | peroxiredoxin 6 |
| apoptosis-inducing factor 1 | protein disulfide isomerase A4 | |
| 14 3 3 protein zeta delta | superoxide dismutase | |
| ras-related protein Rab | exportin 2 isoform 2 | |
| calumenin | metallothionein 2 | |
| protein SET isoform 4 | ||
| nucleophosmin isoform 1 | ||
| SKBR3 | Ezrin | Calreticulin |
| chloride intracellular channel protein 1 | Peroxiredoxin | |
| Calnexin | Protein SET isoform 4 | |
| Pyruvate kinase | phosphoglycerate kinase 1 | |
| 14 3 3 protein epsilon | citrate synthase mitochondrial precursor | |
| 14 3 3 protein sigma | endoplasmic reticulum resident protein 29 isoform 1 | |
| heat shock 70 kDa protein | protein disulfide isomerase | |
| Profilin1 | malate dehydrogenase mitochondrial | |
| MDAMB231 | Histone H1 | 14 3 3 protein sigma Homo sapiens |
| calumenin isoform e | 14 3 3 protein eta Homo sapiens | |
| protein disulfide isomerase A4 | protein S100 A6 Homo sapiens | |
| ERO1 like protein alpha | annexin A2 |
So far, another research group has also reported the receptor independence of progesterone in breast cancer [121,122]. They have described the ability of progesterone to suppress the invasion and migration of breast cancer cells irrespective of their receptor status. Serum- and glucocorticoid-regulated kinase gene (SGK1) and N-Myc downstream-regulated gene 1 (NDRG1) were up-regulated in response to progesterone treatment, thus leading to the inactivation of a set of genes related to invasion and migration. MicroRNAs miR-29a and miR-101-1 targeting the 3’-UTR of SGK1 is also down-regulated upon progesterone treatment which confers the upregulation of SGK1 and NDRG1. However, further studies are required in this arena to use progesterone as a treatment option i.e., pre-operative progesterone treatment for better survival of breast cancer patients.
Progesterone signaling in ovarian, endometrial and colorectal cancer
Estradiol induces proliferation in ovarian cancer cell lines, while progesterone exhibits both stimulatory and inhibitory effects depending on its concentration [123]. ER and PR expression in ovarian cancer cells are also related to better survival and longevity [124]. In ovarian high-grade serous carcinoma (HGSOC), progesterone, through its receptor, produces necroptosis of p53-deficient fallopian tube epithelium cells [125] and suggested progesterone as a chemopreventive drug for HGSOC. Progesterone treatment also reduced the cell proliferation in endometrioid ovarian carcinoma [126]. Type1 endometrial cancer cells, which are well-differentiated, express ER and PR. The expression of these receptors determines the clinical response rate and overall survival of patients. Progesterone treatment induces apoptosis and inhibits metastasis in endometrial cancer cells by acting through cyclin D1, FOXO1; p21; and p27, and MMP1 (matrix metalloproteinase-1), MMP-2, MMP-7, and MMP-9 [119]. Elsewhere, when estradiol plus progestin was used for a short period, the occurrence rate of colorectal cancer drastically reduced [127]. Furthermore, postmenopausal hormone replacement therapy (HRT) has been shown to reduce the risk of colorectal carcinoma. Sasso CV et al. reported that progesterone plus estradiol treatment in colorectal tumor cells induces apoptosis through activation of estrogen receptor β [128]. Folic acid inhibits colorectal cancer cell growth and migration through progesterone receptor activation [129].
ER and PR cross-talk
The importance of PR in selecting endocrine therapy in both the adjuvant and metastatic settings has not been proven yet. The only factor predictive of tamoxifen benefit in a meta-analysis of adjuvant tamoxifen therapy was ER status [13,130]. Likewise, in a meta-analysis comparing adjuvant aromatase inhibitors (AIs) to tamoxifen, the expression of PR did not confirm any selective advantage of AI therapy [13,131]. PR loss occurs more commonly in metastatic primary breast tumors than HER2 and ESR1 loss [132]. Studies reported that considerable cross-talk happens between PR and ER signaling pathways, whereby the activation of one has a substantial influence on the other. Notably, when its ligand activates PR in the presence of estrogen, it cross-talks with ER in breast cancer cells. It redirects ER chromatin binding, suggesting PR’s direct role in regulating ER action [133]. The treatment of breast cancer cells in vivo and in vitro with progesterone can reprogram ER binding to thousands of new cis-regulatory elements and change the gene expression profiles causing cell cycle arrest. In short, progesterone redirects ER-mediated transcription by the appropriation of the ER complex to prevent breast tumor growth; the positive patient outcome is the translational phase of this newly identified transcriptional machinery [133]. R5020, a synthetic progestogen, inhibited estradiol-induced proliferation in ex vivo cultured primary breast cancer samples from patient tumors [134].
Moreover, progesterone suppressed estradiol-mediated breast tumor growth in mouse xenograft, and, when in combination with tamoxifen, tumor growth was reduced more effectively than tamoxifen alone [134]. By identifying the tumors responsive to progesterone-induced PR reprogramming of ER, we could exploit PR’s therapeutic importance. A trial of a single injection depot progesterone before surgery for breast cancers in 976 patients confirmed a significant improvement in survival upshots in patients with the higher-risk node-positive disease [135].
Progesterone and timing of breast cancer surgery
The effect of the menstrual cycle phase on primary breast cancer surgery has been a subject of controversy over the last 30 years. Ratajczak HV et al. (1988) first proposed an effect of the estrous stage at the time of surgical resection of an estrogen receptor-bearing mammary adenocarcinoma in a mouse model [136]. In the following year, Hrushesky WJM et al. (1989) addressed the same hypothesis in humans with a retrospective study of 44 premenopausal women. They underwent resection of primary breast cancer, and in women operated during the perimenstrual period, disease recurrence and metastasis were more frequent and more rapid [137]. Later several studies found contradictory results. Studies by Badwe RA et al. and Veronesi U et al., supported the hypothesis, whereas Goldhirsch A et al., and Nathan B et al., found no effect on the survival of patients. Sainsbury R et al. observed that surgery on the follicular phase survives longer than the luteal stage [138-142]. A multicentre prospective study done in 2008 reported 3-year overall and disease-free survival results. It concluded that surgery timing with the menstrual cycle phase had no significant impact on 3-year survival [143]. Love RR et al. (2015) conducted a phase III randomized clinical trial to test the hypothesis, and in their study, any advantage of adjuvant luteal phase oophorectomy was not shown [144]. In a randomized trial conducted by Badwe RA et al., participated 1000 women with operable breast cancer were either treated with surgery alone or along with a single intramuscular hydroxyprogesterone 500 mg injection between 5 and 14 days before surgery [135]. After sixty-five months of follow-up studies, the overall survival rate in nod positive patients who received progesterone treatment was significant compared to surgery alone. Transcriptome sequence analysis of primary breast tumor samples collected from patients before and after hydroxyprogesterone treatment suggested that cellular stress is altered by progesterone exposure, thus conferring the beneficial effect of progesterone on cancer cells by alleviating the impact of surgical stress [145]. So far, this disputed hypothesis remains a puzzle, and many scientific groups are trying to shed light on the molecular mechanisms behind this hypothesis.
Summary
In the past years, the field of progesterone research has achieved remarkable progress. For years PR has been considered as a marker for functional activity of ER. But recent studies placed progesterone into the spotlight and redefined the PR’s role in cancers and other physiological functions. Herein, we have discussed the pivotal physiological functions of progesterone and its different isoforms. Initially, there were only two receptors known for progesterone-PRA, and PRB. But at present, four more isoforms, non-genomic receptors and many other splice variants, apart from these classical receptors (PRA and PRB), were discovered, but their specific functions are unclear. Based on recent studies, we should focus on progesterone as essential in reproductive fields and a vital tool for managing many clinical illnesses, including cerebral edema, Alzheimer’s disease, diabetic neuropathy, osteoporosis, and cancer. Progesterone is considered a neurosteroid, and its therapeutic potential for brain damages could represent a potential treatment option. Moreover, we should extensively discuss its ability to reduce oxidative stress through enhancing the free radicle scavenging system, anti-inflammatory potential, beneficial effect on the cardiovascular system, bone balance, and the metabolic process.
Furthermore, different research groups have studied the involvement of progesterone signaling in emerging diseases like COVID-19. A latest study points out that progesterone therapy improves COVID-19 outcomes in men [100]. Clinical trials regarding the therapeutic potential of progesterone in COVID-19 patients also suggest a weapon against the pandemic.
Beyond its physiological functions, progesterone was always considered a culprit for breast cancer tumorigenesis and progression. But contradictory research work claiming progesterone as a warrior fighting against breast cancer progression, migration, and invasion partly proved it as innocuous. This field achieved a different aspect with the hypothesis of Hurshesky et al. regarding the timing of breast cancer surgery and menstrual cycle. According to his study, surgery at the luteal phase increased the survival rate of patients, and concurrent molecular evidence was obtained in our studies using progesterone and breast cancer cell lines. Moreover, another mechanism of action for progesterone in breast cancer is emerging from our studies and other research groups, i.e., progesterone executes its functions in breast cancer cells regardless of its receptor status. More studies on these results will lead to the possible treatment intervention of progesterone in breast cancer. Finally, further studies are necessary to recognize the molecular mechanisms of progesterone and its receptors in the pathophysiology of diseases and physiological processes to take therapeutic advantage of this underrated steroid hormone.
Acknowledgements
This study was funded by the Department of Biotechnology, Government of India. Juberiya M Azeez and Susmi TR appreciates the support and grants from the Department of Biotechnology, Government of India. Viji Remadevi and Vini Ravindran appreciates the support and grant provided by Indian Council for Medical Research (ICMR), Government of India. The views and opinions expressed in this article are those of the authors.
Disclosure of conflict of interest
None.
References
- 1.Bohra A, Bhateja S. Carcinogenesis and sex hormones: a review. Endocrinol Metab Synd. 2015;4:1–14. [Google Scholar]
- 2.Taraborrelli S. Physiology, production and action of progesterone. Acta Obstet Gynecol Scand. 2015;94:8–16. doi: 10.1111/aogs.12771. [DOI] [PubMed] [Google Scholar]
- 3.Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O’Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–355. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- 4.González-Orozco JC, Camacho-Arroyo I. Progesterone actions during central nervous system development. Front Neurosci. 2019;13:503. doi: 10.3389/fnins.2019.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genazzani A, Stomati M, Morittu A, Bernardi F, Monteleone P, Casarosa E, Gallo R, Salvestroni C, Luisi M. Progesterone, progestagens and the central nervous system. Hum Reprod. 2000;15:14–27. doi: 10.1093/humrep/15.suppl_1.14. [DOI] [PubMed] [Google Scholar]
- 6.Wei J, Xiao GM. The neuroprotective effects of progesterone on traumatic brain injury: current status and future prospects. Acta Pharmacol Sin. 2013;34:1485–1490. doi: 10.1038/aps.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balasch J. Sex steroids and bone: current perspectives. Hum Reprod Update. 2003;9:207–222. doi: 10.1093/humupd/dmg017. [DOI] [PubMed] [Google Scholar]
- 8.Pang Y, Dong J, Thomas P. Progesterone increases nitric oxide synthesis in human vascular endothelial cells through activation of membrane progesterone receptor-α. Am J Physiol Endocrinol Metab. 2015;308:E899–E911. doi: 10.1152/ajpendo.00527.2014. [DOI] [PubMed] [Google Scholar]
- 9.Kalkhoff RK. Metabolic effects of progesterone. Am J Obstet Gynecol. 1982;142:735–738. doi: 10.1016/s0002-9378(16)32480-2. [DOI] [PubMed] [Google Scholar]
- 10.Hall OJ, Klein SL. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol. 2017;10:1097–1107. doi: 10.1038/mi.2017.35. [DOI] [PubMed] [Google Scholar]
- 11.Laudet V, Gronemeyer H. Academic Press; 2002. PR. In Factsbook, The Nuclear Receptor FactsBook. [Google Scholar]
- 12.Scarpin KM, Graham JD, Mote PA, Clarke CL. Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl Recept Signal Nucl Recept Signal. 2009;7:e009. doi: 10.1621/nrs.07009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim E, Palmieri C, Tilley WD. Renewed interest in the progesterone receptor in breast cancer. Br J Cancer. 2016;115:909–911. doi: 10.1038/bjc.2016.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cork DM, Lennard TW, Tyson-Capper AJ. Alternative splicing and the progesterone receptor in breast cancer. Breast Cancer Res. 2008;10:207. doi: 10.1186/bcr2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;179:97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 16.Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21:155–173. doi: 10.1093/humupd/dmu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97:E719–730. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci. 2011;18:6–19. doi: 10.1177/1933719110382922. [DOI] [PubMed] [Google Scholar]
- 19.Wei LL, Gonzalez-Aller C, Wood WM, Miller LA, Horwitz KB. 5’-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated “C”-receptor and unique A-receptor messages. Mol Endocrinol. 1990;4:1833–1840. doi: 10.1210/mend-4-12-1833. [DOI] [PubMed] [Google Scholar]
- 20.Samalecos A, Gellersen B. Systematic expression analysis and antibody screening do not support the existence of naturally occurring progesterone receptor (PR)-C, PR-M, or other truncated PR isoforms. Endocrinology. 2008;149:5872–5887. doi: 10.1210/en.2008-0602. [DOI] [PubMed] [Google Scholar]
- 21.Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Hum Reprod Update. 2009;15:119–138. doi: 10.1093/humupd/dmn044. [DOI] [PubMed] [Google Scholar]
- 22.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 23.Hirata S, Shoda T, Kato J, Hoshi K. Novel isoforms of the mRNA for human female sex steroid hormone receptors. J Steroid Biochem Mol Biol. 2002;83:25–30. doi: 10.1016/s0960-0760(02)00255-8. [DOI] [PubMed] [Google Scholar]
- 24.Marshburn PB, Zhang J, Bahrani-Mostafavi Z, Matthews ML, White J, Hurst BS. Variant progesterone receptor mRNAs are co-expressed with the wild-type progesterone receptor mRNA in human endometrium during all phases of the menstrual cycle. Mol Hum Reprod. 2005;11:809–815. doi: 10.1093/molehr/gah244. [DOI] [PubMed] [Google Scholar]
- 25.Yamanaka T, Hirata S, Shoda T, Hoshi K. Progesterone receptor mRNA variant containing novel exon insertions between exon 4 and exon 5 in human uterine endometrium. Endocr J. 2002;49:473–482. doi: 10.1507/endocrj.49.473. [DOI] [PubMed] [Google Scholar]
- 26.Saner KJ, Welter BH, Zhang F, Hansen E, Dupont B, Wei Y, Price TM. Cloning and expression of a novel, truncated, progesterone receptor. Mol Cell Endocrinol. 2003;200:155–163. doi: 10.1016/s0303-7207(02)00380-5. [DOI] [PubMed] [Google Scholar]
- 27.Price TM, Dai Q. The role of a mitochondrial progesterone receptor (PR-M) in progesterone action. Semin Reprod Med. 2015;33:185–194. doi: 10.1055/s-0035-1552583. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valadez-Cosmes P, Vázquez-Martínez ER, Cerbón M, Camacho-Arroyo I. Membrane progesterone receptors in reproduction and cancer. Mol Cell Endocrinol. 2016;434:166–175. doi: 10.1016/j.mce.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 31.Pang Y, Dong J, Thomas P. Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and {epsilon} (mPRδ and mPR{epsilon}) and mPRδ involvement in neurosteroid inhibition of apoptosis. Endocrinology. 2013;154:283–295. doi: 10.1210/en.2012-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Peluso JJ. Multiplicity of progesterone’s actions and receptors in the mammalian ovary. Biol Reprod. 2006;75:2–8. doi: 10.1095/biolreprod.105.049924. [DOI] [PubMed] [Google Scholar]
- 34.Koensgen D, Mustea A, Klaman I, Sun P, Zafrakas M, Lichtenegger W, Denkert C, Dahl E, Sehouli J. Expression analysis and RNA localization of PAI-RBP1 (SERBP1) in epithelial ovarian cancer: association with tumor progression. Gynecol Oncol. 2007;107:266–273. doi: 10.1016/j.ygyno.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Espinoza TR, Wright DW. The role of progesterone in traumatic brain injury. J Head Trauma Rehabil. 2011;26:497–499. doi: 10.1097/HTR.0b013e31823088fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang JM, Irwin RW, Liu L, Chen S, Brinton RD. Regeneration in a degenerating brain: potential of allopregnanolone as a neuroregenerative agent. Curr Alzheimer Res. 2007;4:510–517. doi: 10.2174/156720507783018262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem Res. 2008;33:1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- 38.Ziomkiewicz A, Pawlowski B, Ellison PT, Lipson SF, Thune I, Jasienska G. Higher luteal progesterone is associated with low levels of premenstrual aggressive behavior and fatigue. Biol Psychol. 2012;91:376–382. doi: 10.1016/j.biopsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Kaore SN, Langade DK, Yadav VK, Sharma P, Thawani VR, Sharma R. Novel actions of progesterone: what we know today and what will be the scenario in the future? J Pharm Pharmacol. 2012;64:1040–1062. doi: 10.1111/j.2042-7158.2012.01464.x. [DOI] [PubMed] [Google Scholar]
- 40.Cooke PS, Nanjappa MK, Yang Z, Wang KK. Therapeutic effects of progesterone and its metabolites in traumatic brain injury may involve non-classical signaling mechanisms. Front Neurosci. 2013;7:108. doi: 10.3389/fnins.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh M, Su C. Progesterone and neuroprotection. Horm Behav. 2013;63:284–290. doi: 10.1016/j.yhbeh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson CL, Bath PM. Feasibility of progesterone treatment for ischaemic stroke. J Cereb Blood Flow Metab. 2016;36:487–491. doi: 10.1177/0271678X15616782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siddiqui AN, Siddiqui N, Khan RA, Kalam A, Jabir NR, Kamal MA, Firoz CK, Tabrez S. Neuroprotective role of steroidal sex hormones: an overview. CNS Neurosci Ther. 2016;22:342–350. doi: 10.1111/cns.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson AC, Roche SL, Byrne AM, Ruiz-Lopez AM, Cotter TG. Progesterone receptor signalling in retinal photoreceptor neuroprotection. J Neurochem. 2016;136:63–77. doi: 10.1111/jnc.13388. [DOI] [PubMed] [Google Scholar]
- 45.Stein DG, Wright DW, Kellermann AL. Does progesterone have neuroprotective properties? Ann Emerg Med. 2008;51:164–172. doi: 10.1016/j.annemergmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Meffre D, Labombarda F, Delespierre B, Chastre A, De Nicola AF, Stein DG, Schumacher M, Guennoun R. Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience. 2013;231:111–124. doi: 10.1016/j.neuroscience.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 47.Aboukhabar HA, Shaban SM. Impact of progesterone administration on outcome in patients with severe traumatic brain injury. Res Opin Anesth Intensive Care. 2017;4:84–89. [Google Scholar]
- 48.Guennoun R. Progesterone in the brain: hormone, neurosteroid and neuroprotectant. Int J Mol Sci. 2020;21:5271. doi: 10.3390/ijms21155271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oettel M, Mukhopadhyay AK. Progesterone: the forgotten hormone in men? Aging Male. 2004;7:236–257. doi: 10.1080/13685530400004199. [DOI] [PubMed] [Google Scholar]
- 50.Shah C, Modi D, Sachdeva G, Gadkar S, Puri C. Coexistence of intracellular and membrane-bound progesterone receptors in human testis. J Clin Endocrinol Metab. 2005;90:474–483. doi: 10.1210/jc.2004-0793. [DOI] [PubMed] [Google Scholar]
- 51.Bronson RA, Peresleni T, Golightly M. Progesterone promotes the acrosome reaction in capacitated human spermatozoa as judged by flow cytometry and CD46 staining. Mol Hum Reprod. 1999;5:507–512. doi: 10.1093/molehr/5.6.507. [DOI] [PubMed] [Google Scholar]
- 52.Lei B, Mace B, Dawson HN, Warner DS, Laskowitz DT, James ML. Anti-inflammatory effects of progesterone in lipopolysaccharide-stimulated BV-2 microglia. PLoS One. 2014;9:e103969. doi: 10.1371/journal.pone.0103969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp Neurol. 2005;193:522–530. doi: 10.1016/j.expneurol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Pan DS, Liu WG, Yang XF, Cao F. Inhibitory effect of progesterone on inflammatory factors after experimental traumatic brain injury. Biomed Environ Sci. 2007;20:432–438. [PubMed] [Google Scholar]
- 55.Sarkaki AR, Khaksari Haddad M, Soltani Z, Shahrokhi N, Mahmoodi M. Time- and dose-dependent neuroprotective effects of sex steroid hormones on inflammatory cytokines after a traumatic brain injury. J Neurotrauma. 2013;30:47–54. doi: 10.1089/neu.2010.1686. [DOI] [PubMed] [Google Scholar]
- 56.Chen G, Shi J, Ding Y, Yin H, Hang C. Progesterone prevents traumatic brain injury-induced intestinal nuclear factor kappa B activation and proinflammatory cytokines expression in male rats. Mediators Inflamm. 2007;2007:93431. doi: 10.1155/2007/93431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein DG. Progesterone in the treatment of acute traumatic brain injury: a clinical perspective and update. Neuroscience. 2011;191:101–106. doi: 10.1016/j.neuroscience.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 58.Shah NM, Lai PF, Imami N, Johnson MR. Progesterone-Related Immune Modulation of Pregnancy and Labor. Front Endocrinol (Lausanne) 2019;10:198. doi: 10.3389/fendo.2019.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Postovit L, Widmann C, Huang P, Gibson SB. Harnessing oxidative stress as an innovative target for cancer therapy. Oxid Med Cell Longev. 2018;2018:6135739. doi: 10.1155/2018/6135739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan XH, Fan YY, Yang CR, Gao XR, Zhang LL, Hu Y, Wang YQ, Jun H. Progesterone amplifies oxidative stress signal and promotes NO production via H2O2 in mouse kidney arterial endothelial cells. J Steroid Biochem Mol Biol. 2016;155:104–111. doi: 10.1016/j.jsbmb.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 62.Webster KM, Wright DK, Sun M, Semple BD, Ozturk E, Stein DG, O’Brien TJ, Shultz SR. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury. J Neuroinflammation. 2015;12:238. doi: 10.1186/s12974-015-0457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hernández-Rabaza V, López-Pedrajas R, Almansa I. Progesterone, lipoic acid, and sulforaphane as promising antioxidants for retinal diseases: a review. Antioxidants (Basel) 2019;8:53. doi: 10.3390/antiox8030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He L, Yang H, Zhai LD, Shao H, Li YS. A preliminary study on progesterone antioxidation in promoting learning and memory of young ovariectomized mice. Arch Med Sci. 2011;7:397–404. doi: 10.5114/aoms.2011.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chainy GBN, Sahoo DK. Hormones and oxidative stress: an overview. Free Radic Res. 2020;54:1–26. doi: 10.1080/10715762.2019.1702656. [DOI] [PubMed] [Google Scholar]
- 66.Schumacher M, Akwa Y, Guennoun R, Robert F, Labombarda F, Desarnaud F, Robel P, De Nicola AF, Baulieu EE. Steroid synthesis and metabolism in the nervous system: trophic and protective effects. J Neurocytol. 2000;29:307–326. doi: 10.1023/a:1007152904926. [DOI] [PubMed] [Google Scholar]
- 67.Zampieri S, Mellon SH, Butters TD, Nevyjel M, Covey DF, Bembi B, Dardis A. Oxidative stress in NPC1 deficient cells: protective effect of allopregnanolone. J Cell Mol Med. 2009;13:3786–3796. doi: 10.1111/j.1582-4934.2008.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azeez JM, Sithul H, Hariharan I, Sreekumar S, Prabhakar J, Sreeja S, Pillai MR. Progesterone regulates the proliferation of breast cancer cells - in vitro evidence. Drug Des Devel Ther. 2015;9:5987–5999. doi: 10.2147/DDDT.S89390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Azeez JM, Vini R, Remadevi V, Surendran A, Jaleel A, Santhosh Kumar TR, Sreeja S. VDAC1 and SERCA3 mediate progesterone-triggered Ca2(+) signaling in breast cancer cells. J Proteome Res. 2018;17:698–709. doi: 10.1021/acs.jproteome.7b00754. [DOI] [PubMed] [Google Scholar]
- 70.Dai Q, Likes CE 3rd, Luz AL, Mao L, Yeh JS, Wei Z, Kuchibhatla M, Ilkayeva OR, Koves TR, Price TM. A mitochondrial progesterone receptor increases cardiac beta-oxidation and remodeling. J Endocr Soc. 2019;3:446–467. doi: 10.1210/js.2018-00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prior JC. Progesterone as a bone-trophic hormone. Endocr Rev. 1990;11:386–398. doi: 10.1210/edrv-11-2-386. [DOI] [PubMed] [Google Scholar]
- 72.Mac Namara P, Loughrey HC. Progesterone receptor A and B isoform expression in human osteoblasts. Calcif Tissue Int. 1998;63:39–46. doi: 10.1007/s002239900487. [DOI] [PubMed] [Google Scholar]
- 73.Prior JC. Progesterone for the prevention and treatment of osteoporosis in women. Climacteric. 2018;21:366–374. doi: 10.1080/13697137.2018.1467400. [DOI] [PubMed] [Google Scholar]
- 74.Rickard DJ, Iwaniec UT, Evans G, Hefferan TE, Hunter JC, Waters KM, Lydon JP, O’Malley BW, Khosla S, Spelsberg TC, Turner RT. Bone growth and turnover in progesterone receptor knockout mice. Endocrinology. 2008;149:2383–2390. doi: 10.1210/en.2007-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yao W, Dai W, Shahnazari M, Pham A, Chen Z, Chen H, Guan M, Lane NE. Inhibition of the progesterone nuclear receptor during the bone linear growth phase increases peak bone mass in female mice. PLoS One. 2010;5:e11410. doi: 10.1371/journal.pone.0011410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Honisett SY, Pang B, Stojanovska L, Sudhir K, Komesaroff PA. Progesterone does not influence vascular function in postmenopausal women. J Hypertens. 2003;21:1145–1149. doi: 10.1097/00004872-200306000-00014. [DOI] [PubMed] [Google Scholar]
- 77.Prior JC, Elliott TG, Norman E, Stajic V, Hitchcock CL. Progesterone therapy, endothelial function and cardiovascular risk factors: a 3-month randomized, placebo-controlled trial in healthy early postmenopausal women. PLoS One. 2014;9:e84698. doi: 10.1371/journal.pone.0084698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith GI, Reeds DN, Okunade AL, Patterson BW, Mittendorfer B. Systemic delivery of estradiol, but not testosterone or progesterone, alters very low density lipoprotein-triglyceride kinetics in postmenopausal women. J Clin Endocrinol Metab. 2014;99:E1306–1310. doi: 10.1210/jc.2013-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang Y, Tian W. The effects of progesterones on blood lipids in hormone replacement therapy. Lipids Health Dis. 2017;16:219. doi: 10.1186/s12944-017-0612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boonyaratanakornkit V, Pateetin P. The role of ovarian sex steroids in metabolic homeostasis, obesity, and postmenopausal breast cancer: molecular mechanisms and therapeutic implications. Biomed Res Int. 2015;2015:140196. doi: 10.1155/2015/140196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Masuyama H, Hiramatsu Y. Potential role of estradiol and progesterone in insulin resistance through constitutive androstane receptor. J Mol Endocrinol. 2011;47:229–239. doi: 10.1530/JME-11-0046. [DOI] [PubMed] [Google Scholar]
- 82.Lee SR, Choi WY, Heo JH, Huh J, Kim G, Lee KP, Kwun HJ, Shin HJ, Baek IJ, Hong EJ. Progesterone increases blood glucose via hepatic progesterone receptor membrane component 1 under limited or impaired action of insulin. Sci Rep. 2020;10:16316. doi: 10.1038/s41598-020-73330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol Endocrinol Metab. 2002;282:E1139–1146. doi: 10.1152/ajpendo.00184.2001. [DOI] [PubMed] [Google Scholar]
- 84.Rebarber A, Istwan NB, Russo-Stieglitz K, Cleary-Goldman J, Rhea DJ, Stanziano GJ, Saltzman DH. Increased incidence of gestational diabetes in women receiving prophylactic 17alpha-hydroxyprogesterone caproate for prevention of recurrent preterm delivery. Diabetes Care. 2007;30:2277–2280. doi: 10.2337/dc07-0564. [DOI] [PubMed] [Google Scholar]
- 85.Carr DB, Gabbe S. Gestational diabetes: detection, management, and implications. Clinical Diabetes. 1998;16:4–12. [Google Scholar]
- 86.Li M, Song Y, Rawal S, Hinkle SN, Zhu Y, Tekola-Ayele F, Ferrara A, Tsai MY, Zhang C. Plasma prolactin and progesterone levels and the risk of gestational diabetes: a prospective and longitudinal study in a multiracial cohort. Front Endocrinol (Lausanne) 2020;11:83. doi: 10.3389/fendo.2020.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.González C, Alonso A, Alvarez N, Díaz F, Martínez M, Fernández S, Patterson AM. Role of 17beta-estradiol and/or progesterone on insulin sensitivity in the rat: implications during pregnancy. J Endocrinol. 2000;166:283–291. doi: 10.1677/joe.0.1660283. [DOI] [PubMed] [Google Scholar]
- 88.Picard F, Wanatabe M, Schoonjans K, Lydon J, O’Malley BW, Auwerx J. Progesterone receptor knockout mice have an improved glucose homeostasis secondary to beta -cell proliferation. Proc Natl Acad Sci U S A. 2002;99:15644–15648. doi: 10.1073/pnas.202612199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bwire GM. Coronavirus: why men are more vulnerable to COVID-19 than women? SN Compr Clin Med. 2020:1–3. doi: 10.1007/s42399-020-00341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X, Wen F, Huang X, Ning G, Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li W, Sui J, Huang IC, Kuhn JH, Radoshitzky SR, Marasco WA, Choe H, Farzan M. The S proteins of human coronavirus NL63 and severe acute respiratory syndrome coronavirus bind overlapping regions of ACE2. Virology. 2007;367:367–374. doi: 10.1016/j.virol.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mauvais-Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161:bqaa127. doi: 10.1210/endocr/bqaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paul SM, Pinna G, Guidotti A. Allopregnanolone: from molecular pathophysiology to therapeutics. A historical perspective. Neurobiol Stress. 2020;12:100215. doi: 10.1016/j.ynstr.2020.100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 96.Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, Livi C, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- 97.Szekeres-Bartho J, Wegmann TG. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J Reprod Immunol. 1996;31:81–95. doi: 10.1016/0165-0378(96)00964-3. [DOI] [PubMed] [Google Scholar]
- 98.Hall OJ, Limjunyawong N, Vermillion MS, Robinson DP, Wohlgemuth N, Pekosz A, Mitzner W, Klein SL. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog. 2016;12:e1005840. doi: 10.1371/journal.ppat.1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Butterworth M, McClellan B, Allansmith M. Influence of sex in immunoglobulin levels. Nature. 1967;214:1224–1225. doi: 10.1038/2141224a0. [DOI] [PubMed] [Google Scholar]
- 100.Ghandehari S, Matusov Y, Pepkowitz S, Stein D, Kaderi T, Narayanan D, Hwang J, Chang S, Goodman R, Ghandehari H, Mirocha J, Bresee C, Tapson V, Lewis M. Progesterone in addition to standard of care vs standard of care alone in the treatment of men hospitalized with moderate to severe COVID-19: a randomized, controlled pilot trial. Chest. 2021;160:74–84. doi: 10.1016/j.chest.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horwitz KB, Freidenberg GR. Growth inhibition and increase of insulin receptors in antiestrogen-resistant T47DCO human breast cancer cells by progestins: implications for endocrine therapies. Cancer Res. 1985;45:167–173. [PubMed] [Google Scholar]
- 102.Gizard F, Robillard R, Gervois P, Faucompré A, Révillion F, Peyrat JP, Hum WD, Staels B. Progesterone inhibits human breast cancer cell growth through transcriptional upregulation of the cyclin-dependent kinase inhibitor p27Kip1 gene. FEBS Lett. 2005;579:5535–5541. doi: 10.1016/j.febslet.2005.08.084. [DOI] [PubMed] [Google Scholar]
- 103.Alkhalaf M, El-Mowafy A, Karam S. Growth inhibition of MCF-7 human breast cancer cells by progesterone is associated with cell differentiation and phosphorylation of Akt protein. Eur J Cancer Prev. 2002;11:481–488. doi: 10.1097/00008469-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 104.Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1) Mol Endocrinol. 1997;11:1593–1607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- 105.Lange CA, Richer JK, Horwitz KB. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol Endocrinol. 1999;13:829–836. doi: 10.1210/mend.13.6.0290. [DOI] [PubMed] [Google Scholar]
- 106.Hissom JR, Moore MR. Progestin effects on growth in the human breast cancer cell line T-47D--possible therapeutic implications. Biochem Biophys Res Commun. 1987;145:706–711. doi: 10.1016/0006-291x(87)91022-9. [DOI] [PubMed] [Google Scholar]
- 107.Vares G, Ory K, Lectard B, Levalois C, Altmeyer-Morel S, Chevillard S, Lebeau J. Progesterone prevents radiation-induced apoptosis in breast cancer cells. Oncogene. 2004;23:4603–4613. doi: 10.1038/sj.onc.1207601. [DOI] [PubMed] [Google Scholar]
- 108.Lamb CA, Fabris VT, Jacobsen B, Molinolo AA, Lanari C. Biological and clinical impact of imbalanced progesterone receptor isoform ratios in breast cancer. Endocr Relat Cancer. 2018 doi: 10.1530/ERC-18-0179. ERC-18-0179. [DOI] [PubMed] [Google Scholar]
- 109.Pedroza DA, Subramani R, Lakshmanaswamy R. Classical and non-classical progesterone signaling in breast cancers. Cancers (Basel) 2020;12:2440. doi: 10.3390/cancers12092440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brisken C. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nat Rev Cancer. 2013;13:385–396. doi: 10.1038/nrc3518. [DOI] [PubMed] [Google Scholar]
- 111.Kiesel L, Kohl A. Role of the RANK/RANKL pathway in breast cancer. Maturitas. 2016;86:10–16. doi: 10.1016/j.maturitas.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 112.Tang ZN, Zhang F, Tang P, Qi XW, Jiang J. Hypoxia induces RANK and RANKL expression by activating HIF-1α in breast cancer cells. Biochem Biophys Res Commun. 2011;408:411–416. doi: 10.1016/j.bbrc.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 113.Grimm SL, Hartig SM, Edwards DP. Progesterone receptor signaling mechanisms. J Mol Biol. 2016;428:3831–3849. doi: 10.1016/j.jmb.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 114.Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. 2006;314:1467–1470. doi: 10.1126/science.1130471. [DOI] [PubMed] [Google Scholar]
- 115.Hopp TA, Weiss HL, Hilsenbeck SG, Cui Y, Allred DC, Horwitz KB, Fuqua SA. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res. 2004;10:2751–2760. doi: 10.1158/1078-0432.ccr-03-0141. [DOI] [PubMed] [Google Scholar]
- 116.Zhou L, Zhou W, Zhang H, Hu Y, Yu L, Zhang Y, Zhang Y, Wang S, Wang P, Xia W. Progesterone suppresses triple-negative breast cancer growth and metastasis to the brain via membrane progesterone receptor α. Int J Mol Med. 2017;40:755–761. doi: 10.3892/ijmm.2017.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ruan X, Zhang Y, Mueck AO, Willibald M, Seeger H, Fehm T, Brucker S, Neubauer H. Increased expression of progesterone receptor membrane component 1 is associated with aggressive phenotype and poor prognosis in ER-positive and negative breast cancer. Menopause. 2017;24:203–209. doi: 10.1097/GME.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 118.Kabe Y, Nakane T, Koike I, Yamamoto T, Sugiura Y, Harada E, Sugase K, Shimamura T, Ohmura M, Muraoka K, Yamamoto A, Uchida T, Iwata S, Yamaguchi Y, Krayukhina E, Noda M, Handa H, Ishimori K, Uchiyama S, Kobayashi T, Suematsu M. Haem-dependent dimerization of PGRMC1/Sigma-2 receptor facilitates cancer proliferation and chemoresistance. Nat Commun. 2016;7:11030. doi: 10.1038/ncomms11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pedroza DA, Rajamanickam V, Subramani R, Bencomo A, Galvez A, Lakshmanaswamy R. Progesterone receptor membrane component 1 promotes the growth of breast cancers by altering the phosphoproteome and augmenting EGFR/PI3K/AKT signalling. Br J Cancer. 2020;123:1326–1335. doi: 10.1038/s41416-020-0992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Asi N, Mohammed K, Haydour Q, Gionfriddo MR, Vargas OL, Prokop LJ, Faubion SS, Murad MH. Progesterone vs. synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/s13643-016-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Godbole M, Tiwary K, Badwe R, Gupta S, Dutt A. Progesterone suppresses the invasion and migration of breast cancer cells irrespective of their progesterone receptor status - a short report. Cell Oncol (Dordr) 2017;40:411–417. doi: 10.1007/s13402-017-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Godbole M, Togar T, Patel K, Dharavath B, Yadav N, Janjuha S, Gardi N, Tiwary K, Terwadkar P, Desai S, Prasad R, Dhamne H, Karve K, Salunkhe S, Kawle D, Chandrani P, Dutt S, Gupta S, Badwe RA, Dutt A. Up-regulation of the kinase gene SGK1 by progesterone activates the AP-1-NDRG1 axis in both PR-positive and -negative breast cancer cells. J Biol Chem. 2018;293:19263–19276. doi: 10.1074/jbc.RA118.002894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pedernera E, Gómora MJ, Morales-Vásquez F, Pérez-Montiel D, Mendez C. Progesterone reduces cell survival in primary cultures of endometrioid ovarian cancer. J Ovarian Res. 2019;12:15. doi: 10.1186/s13048-019-0486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen S, Dai X, Gao Y, Shen F, Ding J, Chen Q. The positivity of estrogen receptor and progesterone receptor may not be associated with metastasis and recurrence in epithelial ovarian cancer. Sci Rep. 2017;7:16922. doi: 10.1038/s41598-017-17265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu NY, Huang HS, Chao TH, Chou HM, Fang C, Qin CZ, Lin CY, Chu TY, Zhou HH. Progesterone Prevents High-Grade Serous Ovarian Cancer by Inducing Necroptosis of p53-defective fallopian tube epithelial cells. Cell Rep. 2017;18:2557–2565. doi: 10.1016/j.celrep.2017.02.049. [DOI] [PubMed] [Google Scholar]
- 126.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130–162. doi: 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schürmann R, Cronin M, Meyer JU. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:2417–2419. [PubMed] [Google Scholar]
- 128.Sasso CV, Santiano FE, Campo Verde Arboccó F, Zyla LE, Semino SN, Guerrero-Gimenez ME, Pistone Creydt V, López Fontana CM, Carón RW. Estradiol and progesterone regulate proliferation and apoptosis in colon cancer. Endocr Connect. 2019;8:217–229. doi: 10.1530/EC-18-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuo CT, Lee WS. Progesterone receptor activation is required for folic acid-induced anti-proliferation in colorectal cancer cell lines. Cancer Lett. 2016;378:104–110. doi: 10.1016/j.canlet.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 130.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dowsett MFJ, Bradley R, Ingle J, Aihara T, Bliss J, Boccardo F, Coates A, Coombes RC, Cuzick J, Dubsky P, Gnant M, Kaufmann M, Kilburn L, Perrone F, Rea D, Thurlimann B, van de Velde C, Pan H, Peto R, Davies C, Gray R. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 132.Yeung C, Hilton J, Clemons M, Mazzarello S, Hutton B, Haggar F, Addison CL, Kuchuk I, Zhu X, Gelmon K, Arnaout A. Estrogen, progesterone, and HER2/neu receptor discordance between primary and metastatic breast tumours-a review. Cancer Metastasis Rev. 2016;35:427–437. doi: 10.1007/s10555-016-9631-3. [DOI] [PubMed] [Google Scholar]
- 133.Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA, Serandour AA, Birrell SN, Bruna A, Saadi A, Menon S, Hadfield J, Pugh M, Raj GV, Brown GD, D’Santos C, Robinson JL, Silva G, Launchbury R, Perou CM, Stingl J, Caldas C, Tilley WD, Carroll JS. Progesterone receptor modulates ERα action in breast cancer. Nature. 2015;523:313–317. doi: 10.1038/nature14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, Gräf S, Ha G, Haffari G, Bashashati A, Russell R, McKinney S, Langerød A, Green A, Provenzano E, Wishart G, Pinder S, Watson P, Markowetz F, Murphy L, Ellis I, Purushotham A, Børresen-Dale AL, Brenton JD, Tavaré S, Caldas C, Aparicio S. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Badwe R, Hawaldar R, Parmar V, Nadkarni M, Shet T, Desai S, Gupta S, Jalali R, Vanmali V, Dikshit R, Mittra I. Single-injection depot progesterone before surgery and survival in women with operable breast cancer: a randomized controlled trial. J. Clin. Oncol. 2011;29:2845–2851. doi: 10.1200/JCO.2010.33.0738. [DOI] [PubMed] [Google Scholar]
- 136.Ratajczak HV, Sothern RB, Hrushesky WJ. Estrous influence on surgical cure of a mouse breast cancer. J Exp Med. 1988;168:73–83. doi: 10.1084/jem.168.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hrushesky WJ, Bluming AZ, Gruber SA, Sothern RB. Menstrual influence on surgical cure of breast cancer. Lancet. 1989;2:949–952. doi: 10.1016/s0140-6736(89)90956-2. [DOI] [PubMed] [Google Scholar]
- 138.Badwe RA, Richards MA, Fentiman IS, Gregory W, Saad Z, Chaudary MA, Bentley A, Rubens RD. Surgical procedures, menstrual cycle phase, and prognosis in operable breast cancer. Lancet. 1991;338:815–816. doi: 10.1016/0140-6736(91)90695-l. [DOI] [PubMed] [Google Scholar]
- 139.Veronesi U, Luini A, Mariani L, Del Vecchio M, Alvez D, Andreoli C, Giacobone A, Merson M, Pacetti G, Raselli R, et al. Effect of menstrual phase on surgical treatment of breast cancer. Lancet. 1994;343:1545–1547. doi: 10.1016/s0140-6736(94)92942-4. [DOI] [PubMed] [Google Scholar]
- 140.Goldhirsch A, Gelber R, Forbes J, Price K, Castiglione M, Rudenstam C, Lindtner J, Hacking A, Senn H. Timing of surgery in breast cancer. Lancet. 1991;338:691–692. [Google Scholar]
- 141.Nathan B, Bates T, Anbazhagan R, Norman AR. Timing of surgery for breast cancer in relation to the menstrual cycle and survival of premenopausal women. Br J Surg. 1993;80:43. doi: 10.1002/bjs.1800800115. [DOI] [PubMed] [Google Scholar]
- 142.Sainsbury R, Jones M, Parker D, Hall R, Close H. Timing of surgery for breast cancer and menstrual cycle. Lancet. 1991;338:391–392. [PubMed] [Google Scholar]
- 143.Thorpe H, Brown SR, Sainsbury JR, Perren TJ, Hiley V, Dowsett M, Nejim A, Brown JM. Timing of breast cancer surgery in relation to menstrual cycle phase: no effect on 3-year prognosis: the ITS study. Br J Cancer. 2008;98:39–44. doi: 10.1038/sj.bjc.6604120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Love RR, Laudico AV, Van Dinh N, Allred DC, Uy GB, Quang le H, Salvador JD, Siguan SS, Mirasol-Lumague MR, Tung ND, Benjaafar N, Navarro NS Jr, Quy TT, De La Peña AS, Dofitas RB, Bisquera OC Jr, Linh ND, To TV, Young GS, Hade EM, Jarjoura D. Timing of adjuvant surgical oophorectomy in the menstrual cycle and disease-free and overall survival in premenopausal women with operable breast cancer. J Natl Cancer Inst. 2015;107:djv064. doi: 10.1093/jnci/djv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chatterjee S, Chaubal R, Maitra A, Gardi N, Dutt A, Gupta S, Badwe RA, Majumder PP, Pandey P. Pre-operative progesterone benefits operable breast cancer patients by modulating surgical stress. Breast Cancer Res Treat. 2018;170:431–438. doi: 10.1007/s10549-018-4749-3. [DOI] [PubMed] [Google Scholar]