Abstract
Mammalian taste bud cells express receptors for numerous peptides implicated elsewhere in the body in the regulation of metabolism, nutrient assimilation, and satiety. The perturbation of several peptide signaling pathways in the gustatory periphery results in changes in behavioral and/or physiological responsiveness to subsets of taste stimuli. We previously showed that Peptide YY (PYY) – which is expressed in both saliva and in subsets of taste cells – can affect behavioral taste responsiveness and reduce food intake and body weight. Here, we investigated the contributions of taste bud-localized receptors for PYY and the related Neuropeptide Y (NPY) on behavioral taste responsiveness. Y1R, but not Y2R, null mice show reduced responsiveness to sweet, bitter, and salty taste stimuli in brief-access taste tests; similar results were seen when wildtype mice were exposed to Y receptor antagonists in the taste stimuli. Finally, mice in which the gene encoding the NPY propeptide was deleted also showed reduced taste responsiveness to sweet and bitter taste stimuli. Collectively, these results suggest that Y1R signaling, likely through its interactions with NPY, can modulate peripheral taste responsiveness in mice.
Keywords: neuropeptide Y, gustation, taste buds, obesity, receptor
INTRODUCTION
Two discoveries in chemosensory research in recent years have realigned our thinking about how taste perception is linked to mechanisms of appetite and satiety. The first was the observation that many cells in the gut express the same molecular machinery required for nutrient detection as that found in taste cells. These gut “taste” receptors detect ingested nutrients and mediate the secretion of peptide hormones implicated in metabolism, nutrient assimilation, and satiety (see Calvo and Egan, 2015; Depoortere, 2014 for a review). The second was the recognition that many of these same peptides, together with their cognate receptors, are expressed in taste receptor cells of the peripheral gustatory system (see Cai et al., 2014b; Dotson et al., 2013 for a review). Together, these two sets of observations suggested a potential regulatory link between the gustatory system and post-ingestive signaling related to nutrient assimilation and metabolism. Indeed, the idea that peripheral taste functions may be modulated by factors associated with metabolic state is supported by a number of studies (e.g., Cai et al., 2014a; Chen et al., 2010; Duca et al., 2014; Ikeda et al., 2013; Maliphol et al., 2013; Sekine et al., 2012; Zhang et al., 2013; Zhou et al., 2009). Even so, the mechanisms by which diet and metabolic state may impact peripheral taste function remains unclear.
In addition to the prospect of hormonal modulation of taste responsiveness by circulating GI peptides, evidence also exists suggesting that cells in the peripheral gustatory system (e.g., taste bud cells, afferent nerve fibers) can be modulated by autocrine and/or paracrine mechanisms (e.g., Herness and Zhao, 2009; Kinnamon and Finger, 2019; Roper and Chaudhari, 2017; Shen et al., 2005; Yang et al., 2020; Yee et al., 2001). Receptors for peptides including GLP-1, glucagon, cholecystokinin, and vasoactive intestinal peptide, as well as the peptides themselves, are expressed in taste receptor cells or the afferent nerves that innervate the taste bud (Dotson et al., 2013; Elson et al., 2010; Herness et al., 2002; Martin et al., 2010; Shin et al., 2008). The anatomical proximity of both agonists and receptor suggest that there is likely paracrine/autocrine signaling in the peripheral gustatory system (see Dotson et al., 2013). Furthermore, many of these peptides and/or peptide receptors have been implicated in the regulation of taste cell physiology or taste behaviors (Brindisi et al., 2019; Cai et al., 2013; De Jonghe et al., 2005; Elson et al., 2010; Hajnal et al., 2005; Hajnal et al., 2007; Herness et al., 2002; Kolodiy et al., 1993; Martin et al., 2009; Martin et al., 2010; Martin et al., 2012; Shin et al., 2008; Swartz et al., 2010; Zhao et al., 2005). For example, we previously reported that peptide tyrosine tyrosine (PYY) is both expressed in taste cells and is transported as an endocrine hormone from circulation to the saliva of both humans and mice (Acosta et al., 2011; La Sala et al., 2013). Augmentation of salivary PYY3–36 through genetic or pharmacological approaches affects behavioral taste responsiveness and reduces food intake and body weight in diet-induced obese mice (Acosta et al., 2011; Hurtado et al., 2013; La Sala et al., 2013).
NPY family peptides and their receptors have been strongly implicated in the regulation of energy homeostasis (Michel et al., 1998; Nguyen et al., 2011; Zhang et al., 2011). The NPY family consists of three 36-amino acid peptides: NPY, PYY, and pancreatic polypeptide (PP), all of which influence energy balance via their unique interactions with G-protein-coupled Y receptors (Y1, Y2, Y4, and Y5; Michel et al., 1998). Subsets of taste cells express all major Y receptors (Hurtado et al., 2012; Zhao et al., 2005).
Both PYY and NPY are expressed in TRCs (Zhao et al., 2005). NPY expression is restricted to a subset of taste cells, and application of the peptide to dissociated taste cells enhances an inward-rectifying K+ conductance (Zhao et al., 2005). Since both NPY and PYY are agonists of the Y1 receptor (Yulyaningsih et al., 2011), it is interesting to speculate whether Y1 receptor-mediated signaling in TRCs may be mediating the loss of behavioral responsiveness observed in animals that have had PYY-mediated signaling disrupted. Here, we detail experiments designed to test the hypothesis that the disruption of Y1 receptor-mediated signaling modulates behavioral responsiveness towards prototypical gustatory stimuli.
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Florida. All procedures in the study were carried out in accordance with the principles of the National Research Council’s guide for the care and use of laboratory animals. The mice were housed at 22–24°C in a twelve-hour dark-light cycle with ad libitum access to water and food unless specified otherwise.
Behavioral assays
Mice
All mice used in behavioral experiments were 8–10 weeks old. The experimental groups (n=8–15) included (Npy1r –/–) (Howell et al., 2003) and (Npy2r –/–) (Sainsbury et al., 2002) mice and 129S-Npytm1Rpa/J mice (Jackson Laboratory, Bar Harbor, MA, USA). C57BL/6J (Jackson Laboratory, Bar Harbor, MA, USA) mice (n=10) served as controls for all experimental groups except for the 129S-Npytm1Rpa/J mice where the 129S1/SvImJ strain (n=8–15) were used as controls. The control strains were recommended by Jackson labs for each line. To contend with cohort effects, novel control groups were tested contemporaneously with all experimental groups. Mice were housed individually in standard cages with bedding. Mice were habituated to their environment for at least seven days before testing began.
Taste Stimuli
Tastants were prepared with purified water (Elix 10; Millipore, Billerica, MA, USA) and reagent grade chemicals. Tastants were presented to the mice at room temperature. Presented tastants include sucrose, NaCl, denatonium benzoate, and citric acid. Testing stimuli consisted of a “no stimulus” water control and 5 or 6 concentrations of each tastant: two different sucrose concentration ranges, a lower range was used for the NPY receptor knockouts and controls (25, 50, 100, 200, and 400 mM; Fisher Scientific, Atlanta, GA, USA) and a high range for the 129S-Npytm1Rpa/J mice and controls (62.5, 125, 250, 500, and 1000 mM); NaCl (30, 100, 300, 600, and 1000 mM; MilliporeSigma, Burlington, MA, USA); denatonium benzoate (DB; 0.05, 0.1, 0.5, and 1, 5 mM; Sigma-Aldrich); and citric acid (CA; 0.3, 1, 3, 10, 30, and 100 mM; Sigma-Aldrich).
The Y1R antagonist BIBO 3304 trifluoroacetate (1000 nM; Tocris Bioscience, Minneapolis, MN, USA) and the Y2R antagonist BIIE 0246 (1000 nM; (Tocris Bioscience, Minneapolis, MN, USA) were also included in some experiments.
Brief-Access Taste Testing
Brief-access taste testing was administered within a Davis Rig Gustometer (Davis MS-160; DiLog Instruments, Tallahassee, FL, USA; Smith, 2001) as previously described (e.g., Crosson et al., 2019; Dotson et al., 2005; Dotson and Spector, 2004, 2005, 2007; Elson et al., 2010; La Sala et al., 2013; Shin et al., 2008). Two testing protocols were used – one for preferred tastants and one for aversive tastants. Testing consisted of 25 min sessions during which mice were presented access to the sipper tubes for 5 sec with 7.5 sec inter-presentation intervals. The animals were first trained to lick a stationary tube of water for 30 min in the Davis rig after being placed on ∼23.5 h restricted water access schedule. Animals then received 2 days of testing with the sucrose stimulus arrays and purified water while maintained on the water-restriction schedule. This was done to familiarize the animals with the testing procedure and the stimulus array. The mice were subsequently tested for three consecutive days. During testing, mice were restricted to 1 g of food and 2 ml of water for 23.5 hours prior to testing.
For aversive stimuli, animals were trained to lick a stationary tube of water for 30 min in the Davis rig after being placed on ∼23.5 h restricted water access schedule. Animals then received 2 days of testing with a ‘water-only’ stimulus array while maintained on the water-restriction schedule. This was done to familiarize the animals with the testing procedure. The mice were subsequently tested for three consecutive days, under a ∼23.5 h restricted water access schedule, with one of the aversive stimulus arrays detailed above and purified water. Following presentation of an aversive tastant, mice were presented with a 1 sec H2O rinse to minimize crossing over effects. If mice dropped below 85% of their starting body weight, they received 1 ml of water after the testing session was completed.
Statistical Analysis
For the normally avoided stimuli, the average number of licks per trial for each concentration was divided by that animal’s average licks per trial to water yielding a tastant/water lick ratio (Glendinning et al., 2002). To control for the low rate of water licking when animals are tested with normally preferred stimuli, a “tastant minus licks to water” difference score was also derived by taking the mean number of licks to water and subtracting it from the mean number of licks at each concentration (Jiang et al., 2008; Spector et al., 1996; Treesukosol et al., 2009). All scores were analyzed with analyses of variance (ANOVAs). The main variable of interest was genotype. If a main effect of genotype was not observed, genotype X concentration interaction were explored. If a significant interaction was observed, post hoc t tests were conducted to determine which concentrations differed between the experimental groups. The conventional statistic p ≤ 0.05 was applied as the statistical rejection criterion. Effect sizes were estimated using eta squared (η2) or Cohen’s d. Only mice that had at least one trial at every concentration were included in the analysis of a given stimulus. One Y1R KO was excluded from the analysis of denatonium responsiveness. One Y2R KO, one Y2R control, and one mouse tested with the Y1 receptor antagonist were excluded from the analysis of sucrose responsiveness in the deprived condition. Lastly, two NPY KOs were excluded from the analysis of sucrose responsiveness in the food and water restricted condition.
Curves were fitted to the mean data for each genotype using a logistic function of the form:
where x log10 concentration, c log10 concentration at the inflection point, b slope, a the asymptotic lick ratio, and d minimum asymptote of lick ratio. These logistic functions help to quantify the differences in stimulus sensitivity between the groups.
RESULTS
Previously, we found that both Y1R and Y2R proteins are expressed in taste receptor cells (TRCs; La Sala et al., 2013). Thus, we next asked if deletion of either receptor would impact behavioral responsiveness to prototypical taste stimuli. When tested in the water-deprived condition, the response of the Y1R germline KOs did not differ from controls (Figure 1A). However, the Y1R KOs showed decreased responsiveness to sucrose as assessed by the tastant minus licks to water difference score [F(4,72) = 5.04, p = 0.001, interaction, η2 = 0.07] (Figure 1B) when tested in the food and water restricted condition. Post hoc t-tests revealed that the Y1R KOs showed significantly decreased responsiveness to sucrose at the highest concentration [t(18) = 3.19, p = 0.005, Cohen’s d = 1.43].
Figure 1. Y1R KO mice show reduced behavioral taste responsiveness to sweet, bitter and salty-tasting stimuli.
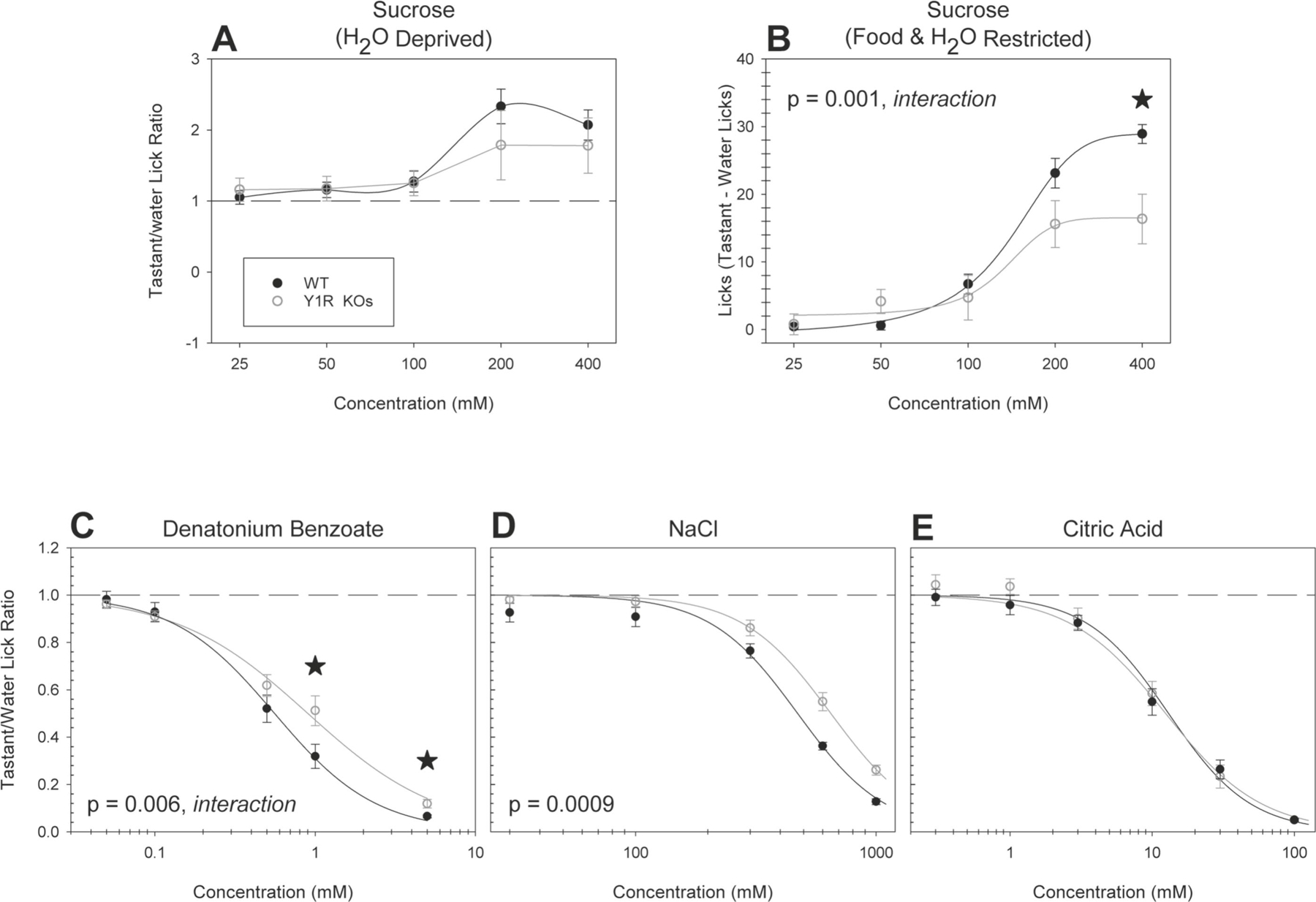
Brief-access taste testing of Y1R KOs (n = 10; open circles) and WT mice (n = 10; solid circles) in response to (A) sucrose (after water deprivation; p = 0.63), (B) sucrose (after food and water restriction; p = 0.005, interaction), (C) denatonium benzoate (p = 0.006, interaction), (D) NaCl (p = 0.0009), and (E) citric acid (p = 0.50). For mice sampling sucrose after food and water restriction, a “tastant minus licks to water” difference score was derived by taking the mean number of licks to water and subtracting it from the mean number of licks at each concentration. For all other conditions and stimuli, the average number of licks per trial for each concentration was divided by that animal’s average licks per trial to water yielding a tastant/water lick ratio. Data are presented ± SEM. When a significant interaction was observed, stars indicate where that post hoc t-tests determined that a given concentration differed between the experimental groups.
When tested with normally avoided stimuli, Y1R KOs displayed a slight decrease in responsiveness to both the bitter-tasting stimulus denatonium benzoate [F(1,17) = 3.93, p = 0.006, interaction, η2 = 0.01] (Figure 1C) and to sodium chloride [F(1,18) = 15.93, p = 0.0009, η2 = 0.47] (Figure 1D). Post hoc t-tests revealed that the animals showed decreased responsiveness to denatonium benzoate at the two highest concentrations [1mM - t(17) = 2.35, p = 0.03, Cohen’s d = 1.08; 5mM - t(17) = 2.62, p = 0.02, Cohen’s d = 1.20]. No significant differences were seen between Y1R KOs and control mice (Figure 1E) when sampling citric acid. By contrast, behavioral responses of Y2R KO mice did not differ from controls for any stimuli (Figure 2A–F). The average number of test trials taken by all experimental groups is detailed in Table 1. Y1R KOs took fewer trials, relative to controls, when sampling all tested stimuli (Table 1).
Figure 2. Y2R KO mice do not differ from WT mice in behavioral responsiveness to prototypical taste stimuli.
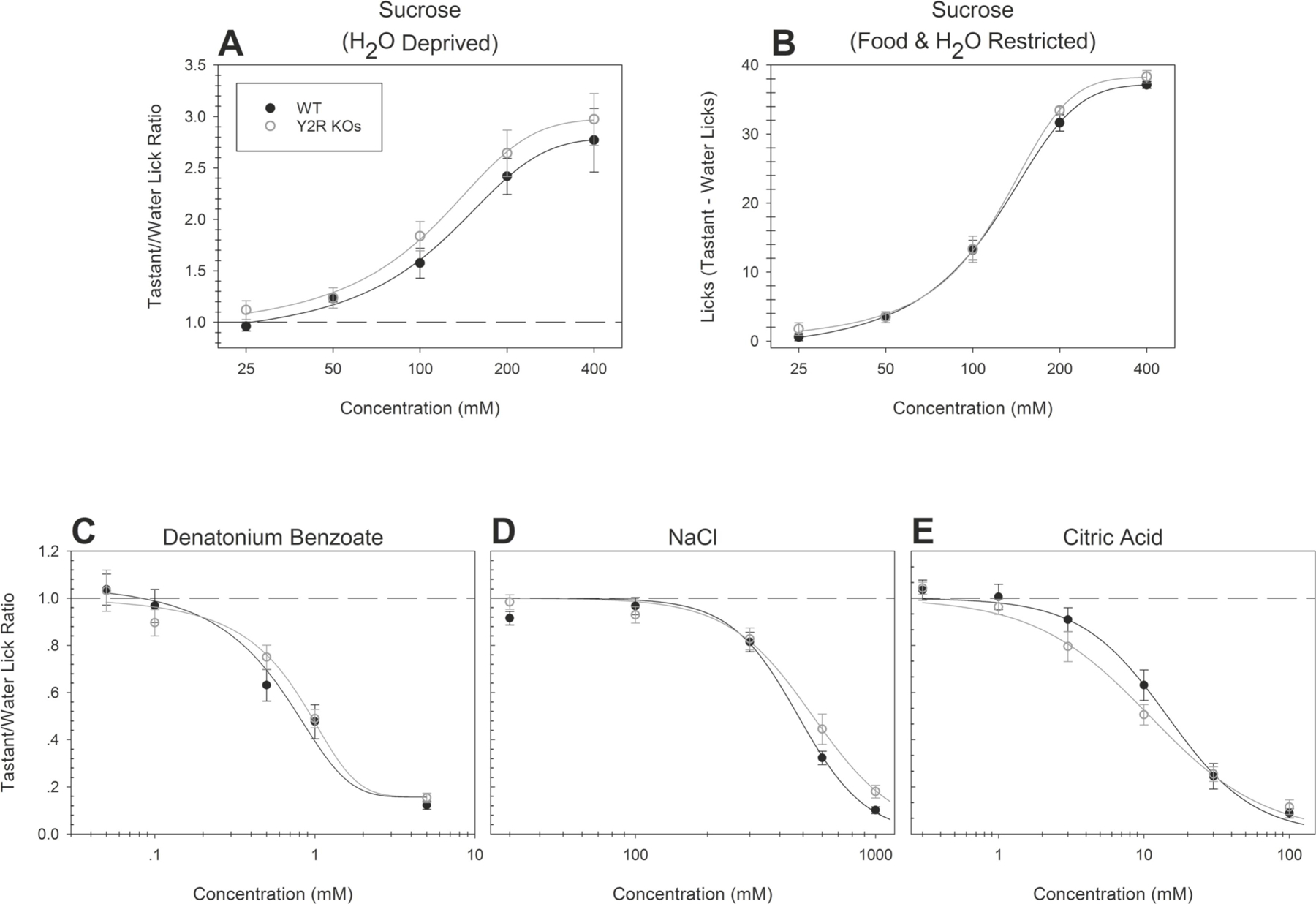
Brief-access taste testing of Y2R KOs (n = 9; open circles) and WT mice (n = 10; solid circles) in response to (A) sucrose (after water deprivation; p = 0.34), (B) sucrose (after food and water restriction; p = 0.31), (C) denatonium benzoate (p = 0.87), (D) NaCl (p = 0.17), and (E) citric acid (p = 0.37). For mice sampling sucrose after food and water restriction, a “tastant minus licks to water” difference score was derived by taking the mean number of licks to water and subtracting it from the mean number of licks at each concentration. For all other conditions and stimuli, the average number of licks per trial for each concentration was divided by that animal’s average licks per trial to water yielding a tastant/water lick ratio. Data are presented ± SEM.
Table 1:
Average Number of Trials Taken During Testing
| Sucrose (WD) | Sucrose (F&W) | Den | NaCl | Citric Acid | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | EG ± SD | CG ± SD | P value | EG ± SD | CG ± SD | P value | EG ± SD | CG ± SD | P value | EG ± SD | CG ± SD | P value | EG ± SD | CG ± SD | P value |
| Y1R KO | 28.5 ± 6.1 | 63.8 ± 16.0 | 0.000004 | 35.2 ± 25.6 | 76.9 ± 25.5 | 0.002 | 54.9 ± 9.1 | 70.6 ± 10.1 | 0.002 | 85.6 ± 9.7 | 98.9 ± 8.4 | 0.005 | 56.6 ± 8.3 | 72 ± 12.1 | 0.004 |
| Y2R KO | 78.4 ± 25.6 | 80 ±14.9 | 0.87 | 140.8± 38.6 | 139.1± 22.7 | 0.91 | 53.1 ± 12.5 | 62.8 ± 9.3 | 0.07 | 80.2 ± 14.1 | 82.4 ± 8.1 | 0.68 | 57.2±16.6 | 60.± 9.9 | 0.66 |
| Y1R A | 71.8 ± 10.6 | 81.2 ± 18.4 | 0.18 | 101.± 29.2 | 107.2 ±31.3 | 0.66 | 53.6 ± 8.7 | 74.5 ± 13.8 | 0.0007 | 71.2 ± 6.8 | 67.6 ± 14 | 0.47 | 51.5 ± 5.8 | 51.6 ±10.1 | 0.98 |
| Y2R A | 69.7± 23.2 | 81.2 ± 18.4 | 0.26 | 79.2 ± 30.3 | 107.2 ±31.3 | 0.07 | |||||||||
| NPY KO | 40.9 ± 13.1 | 41.6 ± 10.4 | 0.88 | 21.5 ± 13 | 29 ± 16 | 0.19 | 32.1 ± 6.9 | 35.4 ± 6.3 | 0.34 | 79.5 ± 15.5 | 68.1 ± 10.4 | 0.11 | 44.4 ± 14.5 | 48.3 ±12.7 | 0.58 |
To control for the possibility that the Y1R deletion is impacting behavioral taste responses through actions in extraoral tissues, we next assessed the effects of Y1R and Y2R receptor antagonists in the taste solutions. When testing the animals’ responsiveness to the antagonist when presented without the presence of a taste stimulus, animals did not respond to either antagonist differently than they responded to water [p = 0.54; Figure 3]. Mice receiving the Y1R antagonist BIBO 3304 trifluoroacetate in taste solutions showed decreased behavioral responsiveness to sucrose when tested in the water-deprived condition [F(1,17) = 12.42, p = 0.003, η2 = 0.42] (Figure 4A). When tested in the food and water restricted condition, the response of these animals did not differ from vehicle-treated controls (Figure 4B).
Figure 3. The response of mice to the NPY receptor antagonists does not differ from their response to water.
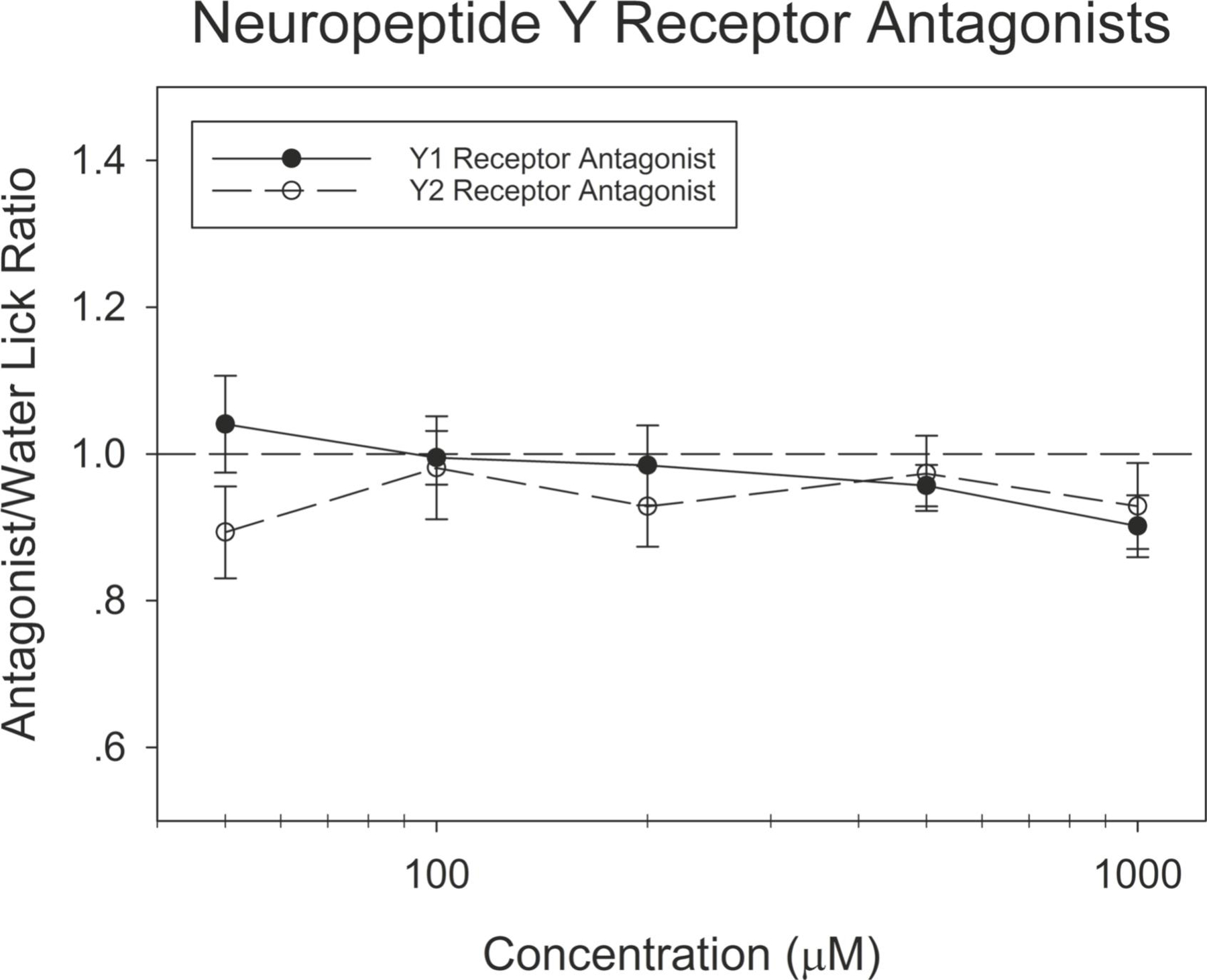
Brief-access taste testing of WT mice receiving the antagonist BIBO 3304 trifluoroacetate (n = 10; solid circles) or the antagonist BIIE 0246 (n = 10; open circles). The response of mice to either NPY receptor antagonist did not differ from their response to water. The average number of licks per trial for each concentration was divided by that animal’s average licks per trial to water yielding a tastant/water lick ratio. Data are presented ± SEM.
Figure 4. A Y1R antagonist reduces behavioral taste responsiveness to sweet, bitter and salty-tasting stimuli.
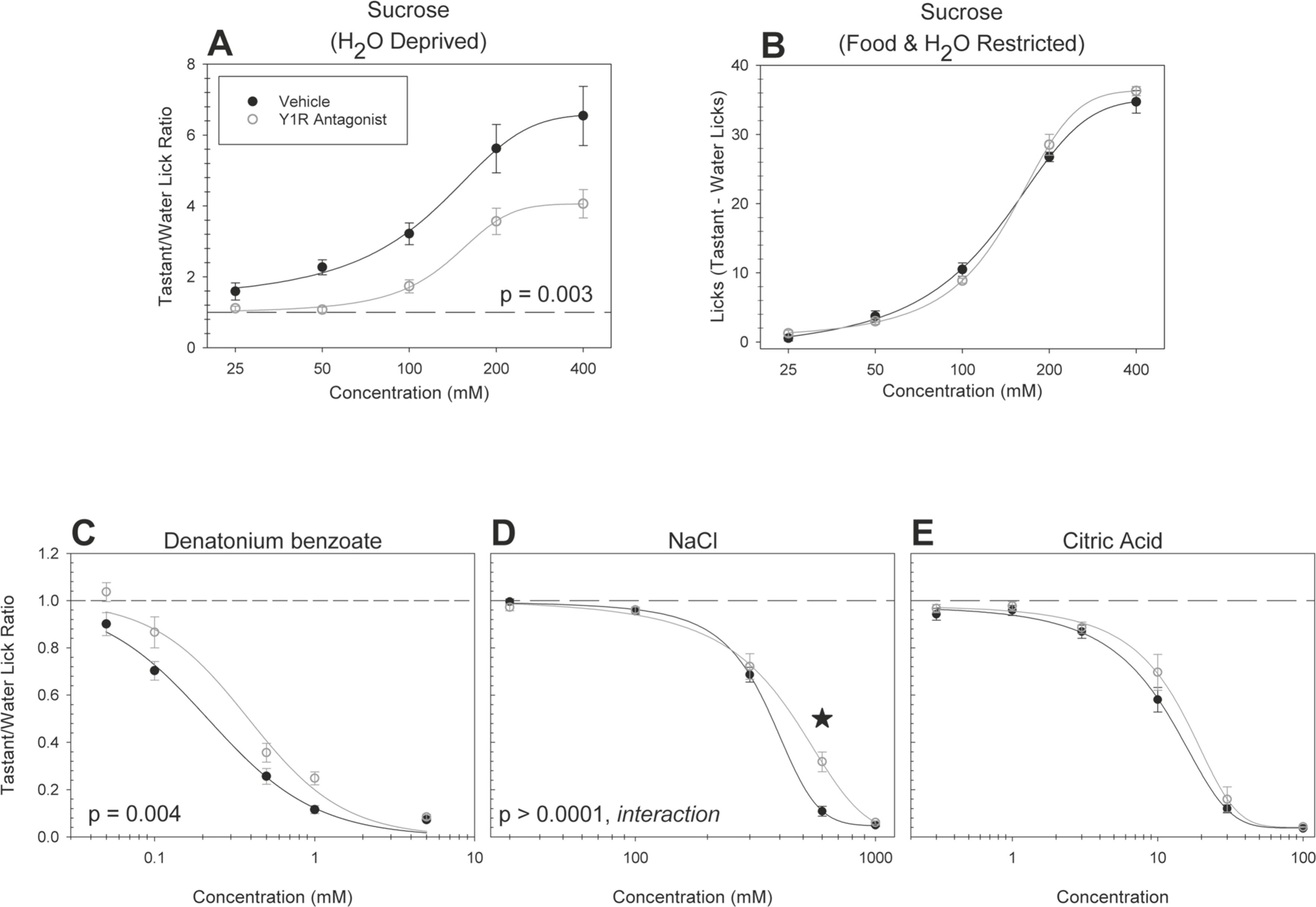
Brief-access taste testing of WT mice receiving the antagonist BIBO 3304 trifluoroacetate (n = 10; open circles) or vehicle (n = 10; solid circles) in the taste stimulus solution: (A) sucrose (after water deprivation; p = 0.003), (B) sucrose (after food and water restriction; p = 0.56), (C) denatonium benzoate (p = 0.004), (D) NaCl (p = 0.00001, interaction), and (E) citric acid (p = 0.80). For mice sampling sucrose after food and water restriction, a “tastant minus licks to water” difference score was derived by taking the mean number of licks to water and subtracting it from the mean number of licks at each concentration. For all other conditions and stimuli, the average number of licks per trial for each concentration was divided by that animal’s average licks per trial to water yielding a tastant/water lick ratio. Data are presented ± SEM. When a significant interaction was observed, stars indicate where that post hoc t tests determined that a given concentration differed between the experimental groups.
When sampling normally avoided stimuli adulterated with the Y1R antagonist, mice displayed decreased responsiveness to denatonium benzoate [F(1,18) = 11.15, p = 0.004, η2 = 0.38] (Figure 4C). These animals took few trials, relative to vehicle treated controls, when sampling denatonium (Table 1). Antagonist-treated mice also displayed decreased responsiveness to sodium chloride [F(4,72) = 8.88, p < 0.0001, interaction, η2 = 0.42] (Figure 4D). Post hoc t-tests revealed that the animals showed decreased responsiveness to sodium chloride at the 600mM concentration [t(18) = 4.51, p = 0.0003, Cohen’s d = 2.02]. There was no significant difference in responsiveness to citric acid (Figure 4E). Consistent with the results observed with the Y2R KO mice, the Y2R antagonist BIIE 0246 did not affect responses to sucrose (Figure 5A and B; aversive stimuli not tested). These data demonstrate that adulterating stimuli with any receptor antagonist does not, obligatorily, impact upon behavioral responsiveness as assessed in the Davis rig.
Figure 5. A Y2R antagonist does not impact behavioral responsiveness to sucrose.
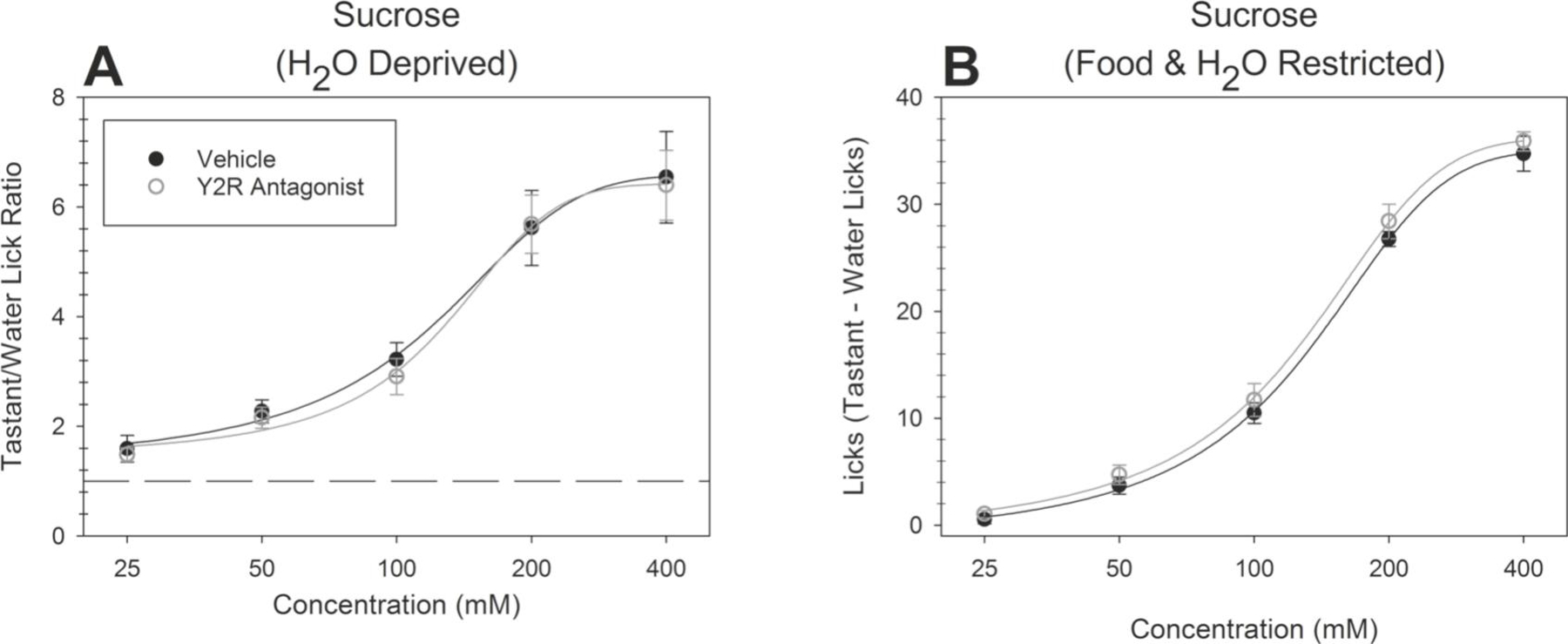
Brief-access taste testing of WT mice receiving the antagonist BIIE 0246 (n = 10; open circles) or vehicle (n = 10; solid circles) in the taste stimulus solution: (A) sucrose (after water deprivation; p = 0.81), (B) sucrose (after food and water restriction; p = 0.23). When mice sampled sucrose in the water restricted condition, the average number of licks per trial for each concentration was divided by that animal’s average licks per trial to water yielding a tastant/water lick ratio. For mice sampling sucrose after food and water restriction, a “tastant minus licks to water” difference score was derived by taking the mean number of licks to water and subtracting it from the mean number of licks at each concentration. Data are presented ± SEM.
Previously, we found that the hormone PYY is expressed in TRCs and that deletion of the gene encoding for the protein has impacts on taste-related behavioral responsiveness (Acosta et al., 2011; La Sala et al., 2013). NPY, a member of the NPY family of peptides along with PYY and pancreatic polypeptide, is also expressed in TRCs (Zhao et al., 2005). Thus, we next asked if deletion of the gene encoding NPY would impact behavioral responsiveness to prototypical taste stimuli. NPY germline KOs showed decreased responsiveness to sucrose [F(1,26) = 10.87, p = 0.003, η2 = 0.30] (Figure 6B) when tested in the food and water restricted condition. When tested in the water-deprived condition, the response of the NPY KOs did not differ from controls (Figure 6A).
Figure 6. Npy null mice show reduced behavioral taste responsiveness to sweet and bitter, but not salty or sour, tasting stimuli.
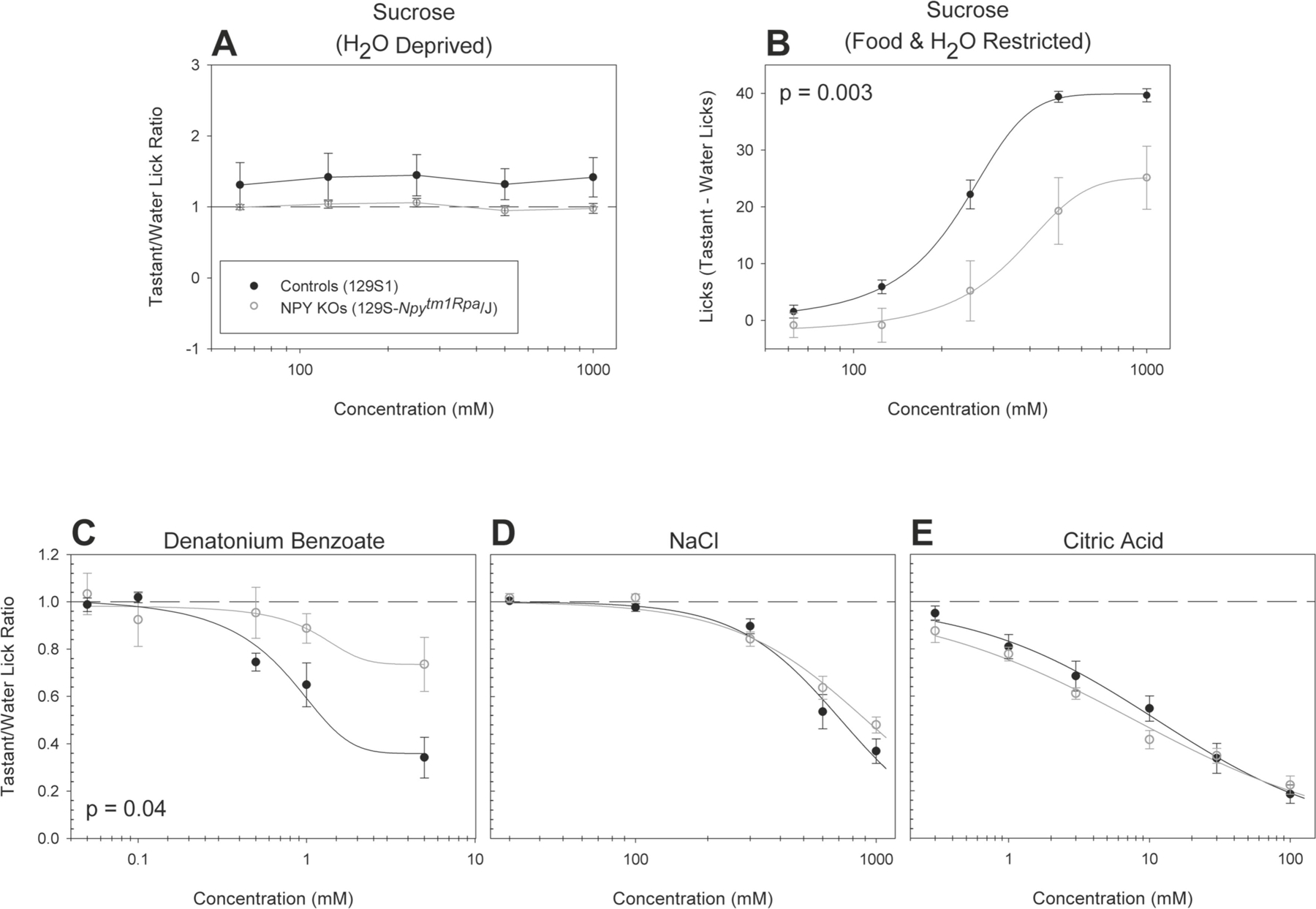
Brief-access taste testing of 129S-Npytm1Rpa/J (n = 15 for sucrose, n = 8 for all other stimuli; open circles) and WT mice (n = 15 for sucrose, n = 8 for all other stimuli; solid circles) in response to (A) sucrose (after water deprivation; p = 0.18), (B) sucrose (after food and water restriction; p = 0.003), (C) denatonium benzoate (p = 0.009, interaction), (D) NaCl (p = 0.18), and (E) citric acid (p = 0.26). For mice sampling sucrose after food and water restriction, a “tastant minus licks to water” difference score was derived by taking the mean number of licks to water and subtracting it from the mean number of licks at each concentration. For all other conditions and stimuli, the average number of licks per trial for each concentration was divided by that animal’s average licks per trial to water yielding a tastant/water lick ratio. Data are presented ± SEM. When a significant interaction was observed, stars indicate where that post hoc t tests determined that a given concentration differed between the experimental groups.
NPY KOs also showed decreased responsiveness to the bitter-tasting stimulus denatonium benzoate [F(1,14) = 4.94, p = 0.04, η2 = 0.26] (Figure 6C). No significant differences were seen between NPY KOs and control mice (Figure 6D and E) when sampling two other aversive stimuli, sodium chloride or citric acid.
DISCUSSION
We assessed the contribution of Y receptor signaling on taste behavioral responsiveness using germline Y receptor KOs, as well as with highly specific Y receptor antagonists. The latter approach helped to control for the possibility that the deletion of a Y receptor gene would indirectly impact taste behaviors through effects in extraoral tissues. Moreover, the approach also helped to control for possible developmental compensatory changes in hormonal signaling present in our germline KO mice. These highly specific Y receptor antagonists work with IC50s in the low nM range in vitro (Dumont et al., 2000; Wieland et al., 1998). We conducted experiments flanking this range to select concentrations that do not appear to have a taste to mice.
Both Y1R germline KOs, as well as Y1R antagonist treated WT mice showed decreased responsiveness to sweet-, bitter-, and salty-tasting stimuli. By contrast, neither the Y2R KO mice nor mice receiving the Y2R antagonist differed from controls in their responsiveness for any of the tested stimuli. Interestingly, mice presented with the Y1R antagonist only showed decreased sucrose responsiveness in the water-deprived condition, whereas Y1R KOs display the deficit only in the food and water restricted condition. The source of this unexplained variation is unknown. While it is certainly possible that these differences result from random variation (e.g., technical issues), we speculate that this difference reflects the disruption of normal Y1R-mediated signaling in extraoral tissues in the KOs. For example, it is well known that Y1R is highly concentrated in the nucleus accumbens, a region involved in reward, motivation, and the regulation of palatable feeding (e.g., Hsieh et al., 2013; Kask et al., 1998; Skibicka et al., 2012; van den Heuvel et al., 2015; Wieland et al., 1998; Zheng et al., 2010). These results suggest that the interplay between motivation, sensory processing, and the central circuits that encode perceived food value can subtly impact upon the behavior of an animal. The relationship between these factors is likely complex and will require additional research to disentangle.
The disruption of Y1R-mediated signaling affected behavioral responsiveness in a taste-salient brief-access assay where post-ingestive influences on behavior are minimized (e.g., Nelson et al., 2003). The use of such an assay increases our confidence that the behavior we observed was being influenced by group differences in gustatory signals emanating from the periphery. However, since we know that Y receptor-mediated signaling can also influence appetite and motivation, it is possible that the observed differences in licking in these germline KO mice may be due to changes in the relative appetitiveness of the stimuli resulting from changes in the central nervous system.
Ingestive behavior can be subdivided into discrete segments. Appetitive behaviors are those that bring the animal towards a meal. Consummatory behaviors are those that are elicited after chemical stimuli comprising the meal bind with receptors in the oral cavity (see Craig, 1917; Sherrington, 1906). The Davis rig allows for some segregation of these behavioral components by providing a measure of trial initiation, which can be thought of as appetitive behavior, as well as by measuring unconditioned lick responsiveness, which can be thought of as consummatory behavior (e.g., Li et al., 2014; Loney and Meyer, 2018; Mathes et al., 2012; Mathes and Spector, 2011; Myers et al., 2020; Schier et al., 2019; Schier and Spector, 2016; Smith, 2001; Treesukosol et al., 2013). Not only did the Y1R KOs demonstrate a decrement in consummatory behavior, these animals also displayed a reduction in appetitive behavior indicated by the fact that the animal took few trials when sampling all of the tested stimuli. Collectively, our data are consistent with the known influences of CNS-mediated NPY related signaling on appetitive behavior (e.g., Ammar et al., 2005; Ammar et al., 2000; Sederholm et al., 2002; Seeley et al., 1995; Treesukosol et al., 2013). Interestingly, these same reports also detail a lack of impact of this signaling on consummatory behavior, suggesting that these types of behaviors are neurally dissociable and mediated by different brain circuits. For example, intracerebroventricular administration of NPY increases intake of a sucrose when presented in a one-bottle test, which involves both appetitive and consummatory behavior, but not when the sucrose is infused intraorally, a measure which focuses more on the consummatory behavior (e.g., Ammar et al., 2005; Ammar et al., 2000; Sederholm et al., 2002; Seeley et al., 1995). Indeed, the relative lack of impact that the Y1 receptor antagonist had on appetitive behavior and robust impact that it had on consummatory behavior is consistent with the notion that modulation of consummatory behavior is primarily controlled by orosensory signals emanating from the periphery (e.g., Ferrario et al., 2016; Fu et al., 2021; Rolls, 2005; Schier and Spector, 2019). It should be noted that some reports suggest that the influence of central NPY signaling on appetitive and consummatory behavior is more complex and varied (e.g., Baird et al., 2006; Torregrossa et al., 2006). Indeed, recent data suggest that these varied influence on appetitive and consummatory behaviors may be mediated via different NPY receptors (e.g., Y1R vs Y5R; see Keen-Rhinehart et al., 2013; Schneider et al., 2013 for a review). More research is needed to fully flesh out which centrally expressed receptors and signaling systems, in conjunction with orosensory signals, are influencing consummatory behavior.
Similar to Y1R KOs, NPY germline KOs showed decreased responsiveness to sucrose and denatonium benzoate. It is interesting to speculate that the changes in taste-related behavioral responsiveness seen upon disruption of Y1R-mediated signaling is due to the disruption of NPY-mediated signaling in taste buds. The slight difference in the behavior of these groups could be explained by the loss of NPY-mediated signaling in extra-oral tissues of the NPY germline KOs.
Numerous peptides implicated elsewhere in nutrient metabolism are expressed in TRCs, while their cognate receptors are expressed in taste cells or found in fibers of afferent taste nerves (see Dotson et al., 2013; Shigemura and Ninomiya, 2016 for a review). However, the function of these signaling systems in peripheral taste tissues remains poorly understood. It has long been hypothesized that output from the taste periphery may be modulated in the context of an animal’s metabolic state and nutritional needs (e.g., Cabanac, 1971; Cabanac and Fantino, 1977; Geerling and Loewy, 2008; Han et al., 2017; Kral, 2006; Lee et al., 2019; Richter, 1936; Scherr and King, 1982; Stellar, 1993; Stellar and Epstein, 1991). One purported mechanism for this phenomenon is that circulating gastrointestinal peptides modulate the functioning of the peripheral gustatory system. For example, it has been postulated that alterations in the levels of circulating gastrointestinal peptides mediate the changes in taste perception observed after gastric bypass surgery (e.g., Ahmed et al., 2018; Bueter et al., 2011; Cummings and Shannon, 2003; Le Roux et al., 2011; Münzberg et al., 2015).
The functional significance of peptides expressed as paracrine factors in TRCs is still poorly understood. The anatomical proximity of agonists and receptors suggest that these peptides may play a role in TRC functioning. Paracrine signaling by neuropeptides in TRCs may influence behavioral responsiveness to tastants by affecting cell-to-cell communication within the taste bud, thereby influencing the total output from taste buds to the CNS (see Herness and Zhao, 2009 for a review). We previously showed that the Y1 receptor is expressed in TRCs of the circumvallate papillae (CV) in mice (Hurtado et al., 2012; La Sala et al., 2013). Results from these studies do not provide clarity on whether the behavioral changes resulting from disruption of NPY and/or Y1R signaling reflect changes in endocrine or paracrine signaling. Herness and colleagues also showed expression of the Y1R, along with the NPY peptide, in TRCs of the rat CV (Herness and Zhao, 2009; Zhao et al., 2005). Herness found that the NPY peptide was expressed primarily in a subset of T2r-expressing, bitter tastant-responsive TRCs, while Y1R was found primarily in type 1 taste receptor, T1r2-expressing, sweet tastant-responsive TRCs. These results indicate that cells that express NPY are distinct from those that express Y1R, suggesting that NPY expressing cells may be influencing the response properties of taste buds by paracrine modulation of neighboring TRCs. However, predicting complex systemic responses from isolated cellular data can be risky. For example, the expression patterns of key molecular components that mediate taste transduction between rats and mice (e.g., Ma et al., 2007). These species also differ in their taste-related behavioral responsiveness (e.g., Kolodiy et al., 1993; Sclafani et al., 2010). More importantly, the experiments suggesting that Y1R activation leads to TRC hyperpolarization was done using CV taste buds. The response properties of TRCs from different papillae often differ substantially (e.g., Dana and McCaughey, 2015; Kim et al., 2003; Shingai and Beidler, 1985) and may contribute differentially to the functional aspects of taste (e.g., sensory discriminative vs. affective functioning; Spector, 2003 for a review; Spector and Glendinning, 2009). Consequently, the influence of a given molecular manipulation on taste-related behavior, which results from the processing of sensory input by the entirety of the gustatory system, may not be easily predicted by observing the output of individual TRCs. Lastly, the TRCs assessed by Zhao, Herness and colleagues were not tested in the presence of taste stimuli (Zhao et al., 2005). Indeed, it is most likely that NPY does not operate in isolation but coordinately other peptides and with small molecule neurotransmitters in neuromodulatory roles (Herness and Zhao, 2009).
Highlights.
Mouse vallate taste buds express all four Y receptor subtypes (Y1R, Y2R, Y4R, Y5R).
Disruption of Y1R signaling reduces sweet, bitter and salty tastant responsiveness.
Npy null mice show reduced behavioral responsiveness to sweet and bitter stimuli.
We conclude NPY-dependent Y1R signaling modulates murine peripheral taste responses.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health [grant numbers: P30-DC010763, R01-DK62302 to S.Z., and R01-DC012819 to C.D.D and S.D.M.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acosta A, Hurtado MD, Gorbatyuk O, La Sala M, Duncan D, Aslanidi G, Campbell-Thompson M, Zhang L, Herzog H, Voutetakis A, Baum BJ, Zolotukhin S, 2011. Salivary PYY: A Putative Bypass to Satiety. PLoS One 6, e26137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed K, Penney N, Darzi A, Purkayastha S, 2018. Taste Changes after Bariatric Surgery: a Systematic Review. Obesity surgery 28, 3321–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammar AA, Nergardh R, Fredholm BB, Brodin U, Sodersten P, 2005. Intake inhibition by NPY and CCK-8: A challenge of the notion of NPY as an “Orexigen”. Behav Brain Res 161, 82–87. [DOI] [PubMed] [Google Scholar]
- Ammar AA, Sederholm F, Saito TR, Scheurink AJ, Johnson AE, Sodersten P, 2000. NPY-leptin: opposing effects on appetitive and consummatory ingestive behavior and sexual behavior. Am J Physiol Regul Integr Comp Physiol 278, R1627–1633. [DOI] [PubMed] [Google Scholar]
- Baird JP, Gray NE, Fischer SG, 2006. Effects of neuropeptide Y on feeding microstructure: dissociation of appetitive and consummatory actions. Behav Neurosci 120, 937–951. [DOI] [PubMed] [Google Scholar]
- Brindisi MC, Brondel L, Meillon S, Barthet S, Grall S, Fenech C, Lienard F, Schlich P, Astruc K, Mouillot T, Jacquin-Piques A, Leloup C, Verges B, Penicaud L, 2019. Proof of concept: Effect of GLP-1 agonist on food hedonic responses and taste sensitivity in poor controlled type 2 diabetic patients. Diabetes Metab Syndr 13, 2489–2494. [DOI] [PubMed] [Google Scholar]
- Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, Unwin RJ, Lutz TA, Spector AC, le Roux CW, 2011. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav 104, 709–721. [DOI] [PubMed] [Google Scholar]
- Cabanac M, 1971. Physiological Role of Pleasure. Science 173, 1103–+. [DOI] [PubMed] [Google Scholar]
- Cabanac M, Fantino M, 1977. Origin of olfacto-gustatory alliesthesia: intestinal sensitivity to carbohydrate concentration? Physiol Behav 18, 1039–1045. [DOI] [PubMed] [Google Scholar]
- Cai H, Cong WN, Daimon CM, Wang R, Tschop MH, Sevigny J, Martin B, Maudsley S, 2013. Altered lipid and salt taste responsivity in ghrelin and GOAT null mice. PLoS One 8, e76553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Daimon CM, Cong WN, Wang R, Chirdon P, de Cabo R, Sevigny J, Maudsley S, Martin B, 2014a. Longitudinal analysis of calorie restriction on rat taste bud morphology and expression of sweet taste modulators. J Gerontol A Biol Sci Med Sci 69, 532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Maudsley S, Martin B, 2014b. What is the role of metabolic hormones in taste buds of the tongue. Frontiers of hormone research 42, 134–146. [DOI] [PubMed] [Google Scholar]
- Calvo SS, Egan JM, 2015. The endocrinology of taste receptors. Nature reviews. Endocrinology 11, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Yan J, Suo Y, Li J, Wang Q, Lv B, 2010. Nutritional status alters saccharin intake and sweet receptor mRNA expression in rat taste buds. Brain Res 325, 53–62. [DOI] [PubMed] [Google Scholar]
- Craig W, 1917. Appetites and Aversions as Constituents of Instincts. Proc Natl Acad Sci U S A 3, 685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson SM, Marques A, Dib P, Dotson CD, Munger SD, Zolotukhin S, 2019. Taste Receptor Cells in Mice Express Receptors for the Hormone Adiponectin. Chem Senses 44, 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Shannon MH, 2003. Ghrelin and gastric bypass: is there a hormonal contribution to surgical weight loss? J Clin Endocrinol Metab 88, 2999–3002. [DOI] [PubMed] [Google Scholar]
- Dana RM, McCaughey SA, 2015. Gustatory responses of the mouse chorda tympani nerve vary based on region of tongue stimulation. Chem Senses 40, 335–344. [DOI] [PubMed] [Google Scholar]
- De Jonghe BC, Hajnal A, Covasa M, 2005. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am J Physiol Regul Integr Comp Physiol 288, R292–300. [DOI] [PubMed] [Google Scholar]
- Depoortere I, 2014. Taste receptors of the gut: emerging roles in health and disease. Gut 63, 179–190. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Geraedts MC, Munger SD, 2013. Peptide regulators of peripheral taste function. Seminars in cell & developmental biology 24, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson CD, Roper SD, Spector AC, 2005. PLCbeta2-independent behavioral avoidance of prototypical bitter-tasting ligands. Chem Senses 30, 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson CD, Spector AC, 2004. The relative affective potency of glycine, L-serine and sucrose as assessed by a brief-access taste test in inbred strains of mice. Chem Senses 29, 489–498. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Spector AC, 2005. Drinking spout orifice size affects licking behavior in inbred mice. Physiol Behav 85, 655–661. [DOI] [PubMed] [Google Scholar]
- Dotson CD, Spector AC, 2007. Behavioral discrimination between sucrose and other natural sweeteners in mice: implications for the neural coding of T1R ligands. J Neurosci 27, 11242–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca FA, Swartz TD, Covasa M, 2014. Effect of diet on preference and intake of sucrose in obese prone and resistant rats. PLoS One 9, e111232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont Y, Cadieux A, Doods H, Pheng LH, Abounader R, Hamel E, Jacques D, Regoli D, Quirion R, 2000. BIIE0246, a potent and highly selective non-peptide neuropeptide Y Y(2) receptor antagonist. Br J Pharmacol 129, 1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson AE, Dotson CD, Egan JM, Munger SD, 2010. Glucagon signaling modulates sweet taste responsiveness. FASEB J 24, 3960–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Labouebe G, Liu S, Nieh EH, Routh VH, Xu S, O’Connor EC, 2016. Homeostasis Meets Motivation in the Battle to Control Food Intake. J Neurosci 36, 11469–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu O, Minokoshi Y, Nakajima KI, 2021. Recent Advances in Neural Circuits for Taste Perception in Hunger. Front Neural Circuits 15, 609824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD, 2008. Central regulation of sodium appetite. Experimental physiology 93, 177–209. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC, 2002. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses 27, 461–474. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Covasa M, Bello NT, 2005. Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol 289, R1675–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, De Jonghe BC, Covasa M, 2007. Dopamine D2 receptors contribute to increased avidity for sucrose in obese rats lacking CCK-1 receptors. Neuroscience 148, 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Keast RSJ, Roura E, 2017. Salivary leptin and TAS1R2/TAS1R3 polymorphisms are related to sweet taste sensitivity and carbohydrate intake from a buffet meal in healthy young adults. Br J Nutr 118, 763–770. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, 2009. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol Behav 97, 581–591. [DOI] [PubMed] [Google Scholar]
- Herness S, Zhao FL, Lu SG, Kaya N, Shen T, 2002. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J Neurosci 22, 10018–10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell OW, Scharfman HE, Herzog H, Sundstrom LE, Beck-Sickinger A, Gray WP, 2003. Neuropeptide Y is neuroproliferative for post-natal hippocampal precursor cells. J Neurochem 86, 646–659. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Chen PN, Yu CH, Liao JM, Kuo DY, 2013. The neuropeptide Y Y1 receptor knockdown modulates activator protein 1-involved feeding behavior in amphetamine-treated rats. Molecular brain 6, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado MD, Acosta A, Riveros PP, Baum BJ, Ukhanov K, Brown AR, Dotson CD, Herzog H, Zolotukhin S, 2012. Distribution of y-receptors in murine lingual epithelia. PLoS One 7, e46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado MD, Sergeyev VG, Acosta A, Spegele M, La Sala M, Waler NJ, Chiriboga-Hurtado J, Currlin SW, Herzog H, Dotson CD, Gorbatyuk OS, Zolotukhin S, 2013. Salivary peptide tyrosine-tyrosine 3–36 modulates ingestive behavior without inducing taste aversion. J Neurosci 33, 18368–18380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Sekine H, Takao K, Ikeda M, 2013. Expression and localization of taste receptor genes in the vallate papillae of rats: effect of zinc deficiency. Acta Otolaryngol 133, 957–964. [DOI] [PubMed] [Google Scholar]
- Jiang E, Blonde G, Garcea M, Spector AC, 2008. Greater superficial petrosal nerve transection in rats does not change unconditioned licking responses to putatively sweet taste stimuli. Chem Senses 33, 709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask A, Rägo L, Harro J, 1998. Evidence for involvement of neuropeptide Y receptors in the regulation of food intake: studies with Y1-selective antagonist BIBP3226. Br J Pharmacol 124, 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Ondek K, Schneider JE, 2013. Neuroendocrine regulation of appetitive ingestive behavior. Front Neurosci 7, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A, 2003. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun 312, 500–506. [DOI] [PubMed] [Google Scholar]
- Kinnamon SC, Finger TE, 2019. Recent advances in taste transduction and signaling. F1000Res 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodiy N, Brosvic GM, Pak D, Loeffler S, 1993. Taste preference behavior in Long-Evans rats and Egyptian spiny mice. Bulletin of the Psychonomic Society 31, 307–310. [Google Scholar]
- Kral TV, 2006. Effects on hunger and satiety, perceived portion size and pleasantness of taste of varying the portion size of foods: a brief review of selected studies. Appetite 46, 103–105. [DOI] [PubMed] [Google Scholar]
- La Sala MS, Hurtado MD, Brown AR, Bohorquez DV, Liddle RA, Herzog H, Zolotukhin S, Dotson CD, 2013. Modulation of taste responsiveness by the satiation hormone peptide YY. FASEB J [DOI] [PMC free article] [PubMed]
- Le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Löwenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA, 2011. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol 301, R1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Augustine V, Zhao Y, Ebisu H, Ho B, Kong D, Oka Y, 2019. Chemosensory modulation of neural circuits for sodium appetite. Nature 568, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Treesukosol Y, Moghadam A, Smith M, Ofeldt E, Yang D, Li T, Tamashiro K, Choi P, Moran TH, Smith WW, 2014. Behavioral characterization of the hyperphagia synphilin-1 overexpressing mice. PLoS One 9, e91449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney GC, Meyer PJ, 2018. Brief Exposures to the Taste of Ethanol (EtOH) and Quinine Promote Subsequent Acceptance of EtOH in a Paradigm that Minimizes Postingestive Consequences. Alcohol Clin Exp Res 42, 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Yang R, Thomas SM, Kinnamon JC, 2007. Qualitative and quantitative differences between taste buds of the rat and mouse. BMC Neurosci 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliphol AB, Garth DJ, Medler KF, 2013. Diet-induced obesity reduces the responsiveness of the peripheral taste receptor cells. PLoS One 8, e79403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Dotson CD, Shin YK, Ji S, Drucker DJ, Maudsley S, Munger SD, 2009. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann N Y Acad Sci 1170, 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Shin YK, White CM, Ji S, Kim W, Carlson OD, Napora JK, Chadwick W, Chapter M, Waschek JA, Mattson MP, Maudsley S, Egan JM, 2010. Vasoactive intestinal peptide-null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression. Diabetes 59, 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Passilly-Degrace P, Chevrot M, Ancel D, Sparks SM, Drucker DJ, Besnard P, 2012. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. Journal of lipid research 53, 2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathes CM, Bueter M, Smith KR, Lutz TA, le Roux CW, Spector AC, 2012. Roux-en-Y gastric bypass in rats increases sucrose taste-related motivated behavior independent of pharmacological GLP-1-receptor modulation. Am J Physiol Regul Integr Comp Physiol 302, R751–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathes CM, Spector AC, 2011. The selective serotonin reuptake inhibitor paroxetine does not alter consummatory concentration-dependent licking of prototypical taste stimuli by rats. Chem Senses 36, 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T, 1998. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev 50, 143–150. [PubMed] [Google Scholar]
- Münzberg H, Laque A, Yu S, Rezai-Zadeh K, Berthoud HR, 2015. Appetite and body weight regulation after bariatric surgery. Obes Rev 16 Suppl 1, 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KP, Summers MY, Geyer-Roberts E, Schier LA, 2020. The Role of Post-Ingestive Feedback in the Development of an Enhanced Appetite for the Orosensory Properties of Glucose over Fructose in Rats. Nutrients 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T, Munger S, Boughter JJ, 2003. Taste sensitivities to PROP and PTC vary independently in mice. Chem Senses 28, 695–704. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Herzog H, Sainsbury A, 2011. Neuropeptide Y and peptide YY: important regulators of energy metabolism. Curr Opin Endocrinol Diabetes Obes 18, 56–60. [DOI] [PubMed] [Google Scholar]
- Richter CP, 1936. Increased salt appetite in adrenalectomized rats. American Journal of Physiology 115, 155–161. [Google Scholar]
- Rolls ET, 2005. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav 85, 45–56. [DOI] [PubMed] [Google Scholar]
- Roper SD, Chaudhari N, 2017. Taste buds: cells, signals and synapses. Nat Rev Neurosci 18, 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Furtinger S, Jenkins A, Cox HM, Sperk G, Hökfelt T, Herzog H, 2002. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci U S A 99, 8938–8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherr S, King KR, 1982. Sensory and metabolic feedback in the modulation of taste hedonics. Physiol Behav 29, 827–832. [DOI] [PubMed] [Google Scholar]
- Schier LA, Hyde KM, Spector AC, 2019. Conditioned taste aversion versus avoidance: A re-examination of the separate processes hypothesis. PLoS One 14, e0217458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier LA, Spector AC, 2016. Post-oral sugar detection rapidly and chemospecifically modulates taste-guided behavior. Am J Physiol Regul Integr Comp Physiol 311, R742–R755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier LA, Spector AC, 2019. The Functional and Neurobiological Properties of Bad Taste. Physiological reviews 99, 605–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JE, Wise JD, Benton NA, Brozek JM, Keen-Rhinehart E, 2013. When do we eat? Ingestive behavior, survival, and reproductive success. Horm Behav 64, 702–728. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Bahrani M, Zukerman S, Ackroff K, 2010. Stevia and saccharin preferences in rats and mice. Chem Senses 35, 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederholm F, Ammar AA, Sodersten P, 2002. Intake inhibition by NPY: role of appetitive ingestive behavior and aversion. Physiol Behav 75, 567–575. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Payne CJ, Woods SC, 1995. Neuropeptide Y fails to increase intraoral intake in rats. Am J Physiol 268, R423–427. [DOI] [PubMed] [Google Scholar]
- Sekine H, Takao K, Yoshinaga K, Kokubun S, Ikeda M, 2012. Effects of zinc deficiency and supplementation on gene expression of bitter taste receptors (TAS2Rs) on the tongue in rats. Laryngoscope 122, 2411–2417. [DOI] [PubMed] [Google Scholar]
- Shen T, Kaya N, Zhao FL, Lu SG, Cao Y, Herness S, 2005. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells. Neuroscience 130, 229–238. [DOI] [PubMed] [Google Scholar]
- Sherrington CS, 1906. The integrative action of the nervous system. C. Scribner’s sons, New York,. [Google Scholar]
- Shigemura N, Ninomiya Y, 2016. Recent Advances in Molecular Mechanisms of Taste Signaling and Modifying. Int Rev Cell Mol Biol 323, 71–106. [DOI] [PubMed] [Google Scholar]
- Shin Y, Martin B, Golden E, Dotson C, Maudsley S, Kim W, Jang H, Mattson M, Drucker D, Egan J, Munger S, 2008. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem 106, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai T, Beidler LM, 1985. Response characteristics of three taste nerves in mice. Brain Res 335, 245–249. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Shirazi RH, Hansson C, Dickson SL, 2012. Ghrelin interacts with neuropeptide Y Y1 and opioid receptors to increase food reward. Endocrinology 153, 1194–1205. [DOI] [PubMed] [Google Scholar]
- Smith JC, 2001. The history of the Davis Rig. Appetite 36, 93–98. [DOI] [PubMed] [Google Scholar]
- Spector AC, 2003. Psychophysical Evaluation of Taste Function in Nonhuman Mammals, in: Doty RL (Ed.), Handbook of Olfaction and Gustation Marcel Dekker, New York, pp. 861–879. [Google Scholar]
- Spector AC, Glendinning JI, 2009. Linking peripheral taste processes to behavior. Curr Opin Neurobiol 19, 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Redman R, Garcea M, 1996. The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci 110, 1096–1109. [PubMed] [Google Scholar]
- Stellar E, 1993. Salt appetite: its neuroendocrine basis. Acta neurobiologiae experimentalis 53, 475–484. [PubMed] [Google Scholar]
- Stellar E, Epstein AN, 1991. Neuroendocrine factors in salt appetite. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 42, 345–355. [PubMed] [Google Scholar]
- Swartz TD, Hajnal A, Covasa M, 2010. Altered orosensory sensitivity to oils in CCK-1 receptor deficient rats. Physiol Behav 99, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa AM, Davis JD, Smith GP, 2006. Orosensory stimulation is sufficient and postingestive negative feedback is not necessary for neuropeptide Y to increase sucrose intake. Physiol Behav 87, 773–780. [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Bi S, Moran TH, 2013. Overexpression of neuropeptide Y in the dorsomedial hypothalamus increases trial initiation but does not significantly alter concentration-dependent licking to sucrose in a brief-access taste test. Physiol Behav 110–111, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Blonde GD, Spector AC, 2009. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol 296, R855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel JK, Furman K, Gumbs MC, Eggels L, Opland DM, Land BB, Kolk SM, Narayanan NS, Fliers E, Kalsbeek A, DiLeone RJ, la Fleur SE, 2015. Neuropeptide Y activity in the nucleus accumbens modulates feeding behavior and neuronal activity. Biol Psychiatry 77, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN, 1998. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br J Pharmacol 125, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Dzowo YK, Wilson CE, Russell RL, Kidd GJ, Salcedo E, Lasher RS, Kinnamon JC, Finger TE, 2020. Three-dimensional reconstructions of mouse circumvallate taste buds using serial blockface scanning electron microscopy: I. Cell types and the apical region of the taste bud. J Comp Neurol 528, 756–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC, 2001. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol 440, 97–108. [DOI] [PubMed] [Google Scholar]
- Yulyaningsih E, Zhang L, Herzog H, Sainsbury A, 2011. NPY receptors as potential targets for anti-obesity drug development. Br J Pharmacol 163, 1170–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bijker MS, Herzog H, 2011. The neuropeptide Y system: pathophysiological and therapeutic implications in obesity and cancer. Pharmacol Ther 131, 91–113. [DOI] [PubMed] [Google Scholar]
- Zhang XJ, Wang YQ, Long Y, Wang L, Li Y, Gao FB, Tian HM, 2013. Alteration of sweet taste in high-fat diet induced obese rats after 4 weeks treatment with exenatide. Peptides 47, 115–123. [DOI] [PubMed] [Google Scholar]
- Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S, 2005. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci U S A 102, 11100–11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Townsend RL, Shin AC, Patterson LM, Phifer CB, Berthoud HR, 2010. High-fat intake induced by mu-opioid activation of the nucleus accumbens is inhibited by Y1R-blockade and MC3/4R- stimulation. Brain Res 1350, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LH, Liu XM, Feng XH, Han LO, Liu GD, 2009. Expression of alpha-gustducin in the circumvallate papillae of taste buds of diabetic rats. Acta Histochem 111, 145–149. [DOI] [PubMed] [Google Scholar]


