Abstract
Current European guidelines on chronic coronary syndromes recommend the use of low-dose aspirin (or clopidogrel if intolerance or contraindication occurs) throughout life. However, as the risk of recurrent vascular events is high, particularly in some patients (i.e. diffuse multivessel coronary artery disease, diabetes, recurrent myocardial infarction, peripheral artery disease, or chronic kidney disease,…), these guidelines also consider that in those patients at moderate or high risk of ischemic events, but without a high bleeding risk, dual antithrombotic therapy should be considered. According to these guidelines, treatment options for dual antithrombotic therapy in combination with aspirin may include clopidogrel 75 mg/daily, prasugrel 10 mg/daily, ticagrelor 60 mg bid or rivaroxaban 2.5 mg bid. Remarkably, despite the results of the clinical trials that sustain these recommendations clearly diverge, guidelines do not differentiate between them. However, although all these drugs have demonstrated a significant reduction in major cardiovascular events in patients with stable atherosclerotic disease, only the addition of rivaroxaban has been associated with a reduction in cardiovascular and overall mortality in the secondary analysis. This may be related to the fact that the activation of platelets and factor X plays a key role in the development of atherothrombosis, and, consequently, both targets should be considered for the appropriate management of these patients.
Keywords: Atherosclerosis, COMPASS, chronic coronary syndrome, ischemic heart disease, MACE, rivaroxaban
1. INTRODUCTION
Although there are some differences between higher-income and lower-income countries rates, atherosclerotic cardiovascular disease remains the leading cause of mortality worldwide, and it is associated with a high economic burden [1, 2]. Thus, it has been estimated that in Europe, cardiovascular disease is one of the main contributors to potential years of life lost [3]. Remarkably, the health burden of atherosclerotic cardiovascular disease will increase in the following years due to the ageing of the population and worse, unhealthy lifestyle of individuals (sedentary lifestyle, obesity, smoking) [4].
Although the atherosclerotic cardiovascular disease may have long and stable periods, instability can occur at any time during the evolution due to plaque rupture or erosion.As a result, despite the patient may be asymptomatic (silentperiod), the disease is dynamic and progressive, and efforts should be performed in order to optimize treatment to decrease cardiovascular morbidity and mortality [5].
Cardiovascular risk factor control is mandatory in patients with the atherosclerotic disease to decrease the risk of new events [5]. Unfortunately, a high proportion of patients with chronic ischemic disease do not achieve cardiovascular risk factors goals [6]. In a substudy of the COMPASS trial, blood pressure, smoking status, cholesterol level, presence of diabetes, body mass index, and level of physical activity were analyzed. Less than 3% of patients had all risk factors controlled, about 50% of patients had 0-2 uncontrolled risk factors and nearly 19% of patients had more than four risk factors uncontrolled. Of note, rates of ischemic events and cardiovascular death increased with the number of cardiovascular risk factors uncontrolled [7]. However, the impact of antithrombotic therapy was independent of the number of risk factors, indicating that both the approaches have an independent impact on the prognosis of patients and should be optimized.
Antithrombotic therapy is essential to reduce ischemic events among patients with atherosclerotic vascular disease [5]. Current guidelines about chronic coronary syndromes recommend the use of aspirin 75-100 mg/daily (or clopidogrel 75 mg/daily) in patients with atherosclerotic vascular disease [5]. However, these guidelines recognize that despite antithrombotic monotherapy, some patients have an unacceptably high risk of new ischemic events. In this context, these guidelines state that the addition of a second antithrombotic drug to aspirin for long-term secondary prevention should be considered in patients at a high risk of ischemic events and without high bleeding risk and may be considered in those patients with at least a moderately increased risk of ischemic events and without high bleeding risk [5]. Unfortunately, despite there are important disparities in the results of the different clinical trials that have analyzed the impact of the addition of a second antithrombotic drug on the reduction of ischemic events and mortality in patients with atherosclerotic vascular disease (Table 1) [8-16], guidelines do not clearly differentiate between the available strategies [5].
Table 1.
Treatment options for dual antithrombotic therapy in combination with aspirin recommended by european guidelines about chronic coronary syndromes.
| Drug (dose) | Indication | MACE | Mortality |
|---|---|---|---|
| Clopidogrel (75 g od) |
Post-MI in patients who have tolerated DAPT for 1 year | 4.3% vs 5.9%, HR 0.71; 95% CI 0.59-0.85 |
Overall death: 2.0% vs 1.5%; P=0.05 Cardiac mortality; 0.9% vs 1.0%; P=0.98 Vascular mortality: 0.1% vs 0.1%; P=0.98 |
| Prasugrel (10 mg od*) |
Post-PCI for MI in patients who have tolerated DAPT for 1 year | 3.7% vs 8.8%; HR 0.407; 95% CI 0.281-0.589 |
Overall death: 1.9% vs 2.0%; P=0.85 Cardiac death: 0.9% vs 1.2%; P=0.517 |
| Rivaroxaban (2.5 mg bid) | Post-MI >1 year or multivessel coronary artery disease | 4.1% vs 5.4% HR 0.76; 95% CI 0.66-0.86 |
Overall death: 3.4% vs 4.1%; P=0.01 CV death: 1.7% vs 2.2%; P=0.02 |
| Ticagrelor (60 mg bid) |
Post-MI in patients who have tolerated DAPT for 1 year | 7.77% vs 9.04% HR 0.84; 95% CI 0.74-0.95 |
Overall death: 4.69% vs 5.16%; P=0.14 CV death: 2.86% vs 3.39%; P=0.07 |
Abbreviations: MI: myocardial infarction; DAPT: dual antiplatelet therapy; HR: Hazard Ratio; CI: confidence. interval; CV: cardiovascular.
Note: * 5 mg o.d.; if body weight <60 kg or age >75 years.
Adapted from references #5,8-16.
2. RIVAROXABAN IN THE MANAGEMENT OF PATIENTS WITH STABLE ATHEROSCLEROTIC VASCULAR DISEASE THE COMPASS TRIAL
The COMPASS trial included 27,395 patients with stable atherosclerotic vascular disease, either coronary artery disease or peripheral artery disease. The patients received rivaroxaban (2.5 mg twice daily) plus aspirin (100 mg once daily), rivaroxaban (5 mg twice daily), or aspirin (100 mg once daily). The primary outcome was a composite of cardiovascular death, stroke, or myocardial infarction (MACE). The study was prematurely stopped after 23 months of follow-up, due to the benefits of the rivaroxaban plus aspirin strategy over aspirin. This is important, as the efficacy Kaplan-Meier curves progressively separated over time, particularly after one year of treatment, indicating that the benefit of rivaroxaban plus aspirin increases with treatment time [13].
The combination of rivaroxaban plus aspirin significantly reduced the risk of MACE by 24% compared with aspirin (4.1% vs 5.4%; Hazard Ratio [HR] 0.76; 95% confidence interval [CI] 0.66-0.86), and the risk of stroke by 42% (0.9% vs 1.6%; HR 0.58; 95% CI 0.44-0.76), and showed a lower risk of cardiovascular hospitalization by 8% (14.2% vs 15.3%; HR 0.92; 95% CI 0.86-1.00). More importantly, the risk of cardiovascular mortality and death from any cause reduced by 22% and 18%, respectively (1.7% vs 2.2%; HR 0.78; 95% CI 0.64-0.96 and 3.4% vs 4.1%; HR 0.82; 95% CI 0.71-0.96, respectively) [13].
With regard to the risk of hemorrhage, major bleeding was more common with the approach of rivaroxaban plus aspirin than with aspirin (3.1% vs 1.9%; HR 1.70; 95% CI 1.40-2.05), but not nonfatal symptomatic intracranial hemorrhage (0.2% vs 0.2%; HR 1.10; 95% CI 0.59-2.04) or fatal bleeding (0.2% vs 0.1%; HR 1.49; 95% CI 0.67-3.33) [13].
Although when analyzing the results of the COMPASS trial in relative terms, it seemed that the beneficial effects of the combination of rivaroxaban plus aspirin could be in some way offset by the risk of bleeding, the fact is that when considering the absolute numbers, the benefit-risk ratio undoubtedly favors the new therapeutic approach (i.e. rates of death from any cause 3.4% vs 4.1% vs rates of fatal bleeding 0.2% vs 0.1%). In fact, the net clinical benefit outcome of cardiovascular death, stroke, myocardial infarction, fatal bleeding, or symptomatic bleeding into critical organ was better with the combination of rivaroxaban plus aspirin than with aspirin alone (4.7% vs 5.9%; HR 0.80; 95% CI 0.70-0.91) [13].
The results of the COMPASS trial are in line with the pathophysiology of atherothrombosis. Thus, when the rupture or erosion of an atherosclerotic plaque occurs, not only platelets are activated, but also facto X that is converted into factor Xa after tissue factor is locally released [17-19]. As a result, targeting only platelets by mono or dual antiplatelet therapy seems insufficient to reduce the risk of recurrence of atherosclerotic events, as previous studies have demonstrated [8-12, 14-16]. By contrast, the COMPASS trial has shown that the dual pathway inhibition with the combination of rivaroxaban and aspirin has synergic effects with a good safety profile [20-22].
In addition, preclinical data have confirmed the beneficial effects of Factor Xa inhibition on atherothrombosis, particularly with rivaroxaban. Thus, it has been reported that the inhibition of factor Xa has anti-endothelial senescence and anti-atherosclerotic effects, has protective, repairing and fibrinolytic effects on vascular endothelium, attenuates neointima formation after vascular injury and improves neovascularization. In addition, Factor Xa inhibition reduces inflammation and platelet-dependent thrombin generation [23-31].
However, this beneficial effect of Factor Xa inhibition on atherosclerotic protection should not be extended to all Factor Xa inhibitors. Remarkably, the APPRAISE-2 study showed that the addition of apixaban (5 mg bid) to antiplatelet therapy did not translate into a reduction of recurrent ischemic events in high-risk patients after an acute coronary syndrome, but increased the major bleeding risk [32]. However, this could be related to the dose used in this study, in contrast to the dose of rivaroxaban in the COMPASS trial. On the other hand, it has been reported that rivaroxaban and apixaban differ in their capacity to inhibit factor Xa. Thus, the association rates for binding to either free factor Xa or factor Xa are incorporated into the prothrombinase complex and also dissociation rates are markedly faster with rivaroxaban than with apixaban, leading to greater effects of rivaroxaban on global tests of coagulation [33].
3. EFFECTS OF THE RIVAROXABAN PLUS ASPIRIN APPROACH IN PARTICULAR SUBGROUPS OF PATIENTS WITH STABLE ATHEROSCLEROTIC VASCULAR DISEASE
3.1. Patients with Coronary Artery Disease
Approximately 91% of patients (n=24,824) included in the COMPASS trial had stable coronary artery disease. In the COMPAS trial, coronary artery disease was defined as myocardial infarction within the past 20 years, or multivessel coronary disease with symptoms or with a history of stable/unstable angina, or multivessel percutaneous coronary intervention, or multivessel coronary artery bypass graft surgery. In case of age less than 65 years, patients also required to have atherosclerosis ≥2 vascular beds or ≥2 additional risk factors [13].
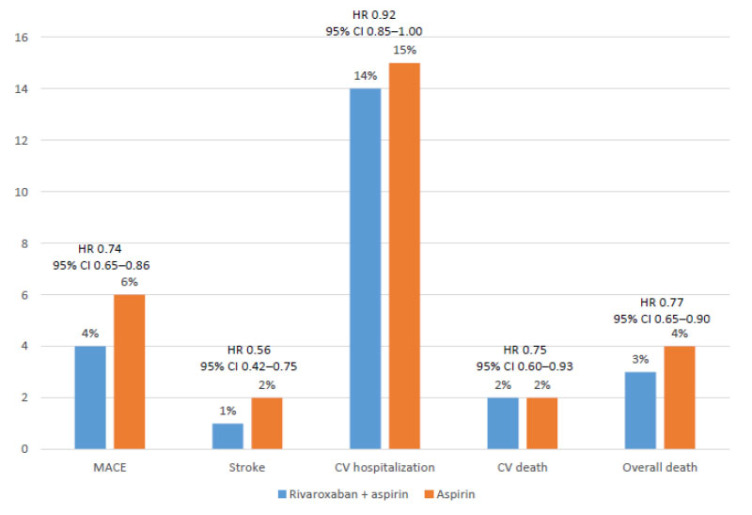
In a specific analysis of the COMPASS trial of those patients with coronary artery disease, 69% had a history of previous myocardial infarction and 62% multi-vessel disease. Data about the efficacy and safety or the combination of rivaroxaban plus aspirin compared with aspirin in this group of patients were consistent with those of the overall study (Figs. 1 and 2) [34].
Fig. (1).
Efficacy outcomes among patients with stable coronary artery disease. Data from the COMPASS study [34]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
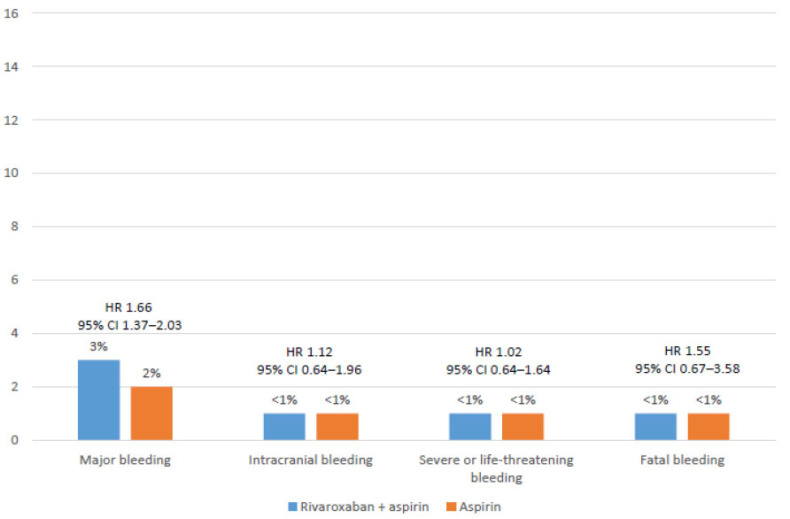
Fig. (2).
Safety outcomes among patients with stable coronary artery disease. Data from the COMPASS study [34]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In summary, after a mean follow-up of nearly 2 years, compared with aspirin alone, the rivaroxaban plus aspirin approach significantly reduced the risk of MACE (HR 0.74; 95% CI 0.65-0.86; P<0.0001), and stroke (HR 0.56; 95% CI 0.42-0.75: P<0.0001), and showed a lower risk of cardiovascular hospitalization (HR 0.92; 95% CI 0.85-1.00; P=0.046), cardiovascular death (HR 0.75; 95% CI 0.60-0.93; P=0.01) and overall death (HR 0.77; 95% CI 0.65-0.90; P=0.0012) (Fig. 1). With regard to the risk of hemorrhage, although major bleeding was more common with the combination (HR 1.66; 95% CI 1.37-2.03; P<0.0001), the risk of fatal bleeding and intracranial hemorrhage similarly occurred in both groups (Fig. 2) [34].
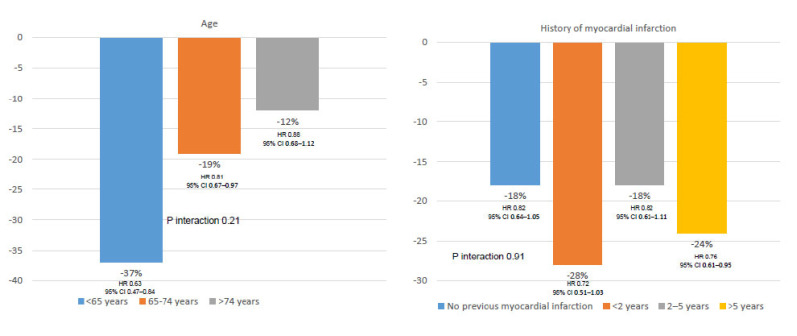
However, as ischemic events were much more common than bleeding outcomes, the net clinical benefit outcome of MACE, fatal bleeding or symptomatic bleeding into a critical organ, markedly favored the combination of rivaroxaban plus aspirin (HR 0.78; 95% CI 0.69-0.90; P=0.0003) [34]. In addition, as the risk of ischemic events was stable during the follow-up, but the risk of major bleeding decreased, particularly after the first year of treatment, the net clinical benefit of rivaroxaban plus aspirin approach increases with time of treatment, compared with aspirin alone (Table 2) [34]. Importantly, the net clinical benefit of rivaroxaban plus aspirin over aspirin was independent of age or the history of myocardial infarction or time of evolution since myocardial infarction (Fig. 3) [34].
Table 2.
Effects of rivaroxaban + aspirin vs aspirin on mace and major bleeding in patients with stable coronary artery disease according to the duration of treatment data from the compass study.
| MACE | Major Bleeding | |||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| <1 year | 0.79 | 0.65-0.96 | 2.32 | 1.75-3.07 |
| 1-2 years | 0.66 | 0.52-0.83 | 1.19 | 0.84-1.68) |
| >2 years | 0.82 | 0.58-1.16 | 1.05 | 0.63-1.75 |
Note:Table performed with data taken from reference #34.
Fig. (3).
Net clinical benefit of rivaroxaban + aspirin vs aspirin in patients with stable coronary artery disease according to age and the history of myocardial infarction. Data from the COMPASS study [34]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
On the other hand, it has been reported that less than one-third of patients undergoing surgical revascularization have at least 1 occluded graft within one year after surgery [35]. A pre-planned analysis of the COMPASS study showed that although both approaches have similar graft failure rates after 1 year of surgery, there was a trend to a reduction of MACE by the combination of rivaroxaban plus aspirin in this subgroup of patients [36].
3.2. Patients With Peripheral Artery Disease
In the COMPASS trial, a total of 7,470 patients had peripheral artery disease. Peripheral artery disease was defined as having a history of peripheral artery disease of the lower extremities (previous revascularization, amputation or intermittent claudication), of the carotid arteries (previous revascularization or asymptomatic ≥50% carotid artery stenosis), or patients with coronary artery disease and an ankle-brachial index <0.90 [37].
Among patients with peripheral artery disease, compared with aspirin alone, the rivaroxaban plus aspirin approach reduced the risk of MACE by 28% (HR 0.72; 95% CI 0.57-0.90; P=0.0047), and the risk of stroke by 46% (HR 0.54; 95% CI 0.33-0.87), with a trend towards a lower risk of cardiovascular and overall mortality. Remarkably, with regard to the prespecified limb outcomes, the combination of rivaroxaban plus aspirin reduced the risk of acute limb ischemia (HR 0.56; 95% CI 0.32-0.99; P=0.042), major adverse limb event (MALE) (HR 0.54; 95% CI 0.35-0.84; P=0.0054), all vascular amputations (HR 0.40;95% CI 0.20-0.79; P=0.0069), major amputation (HR 0.30; 95% CI 0.11-0.80; P=0.011), and major adverse limb event plus major amputation (HR 0.54; 95% CI 0.35-0.82; P=0.0037) [37].
Although major bleeding was more common with the combined therapy, mainly of a gastrointestinal origin, fatal bleeding and non-fatal symptomatic intracranial hemorrhage similarly occurred in both groups (<1%) [37]. As a result, the net benefit outcome of MACE, and critical organ or fatal bleeding was found to be reduced with the combination of rivaroxaban plus aspirin by 25% (HR 0.75; 95% CI 0.60-0.94; P=0.011), and the net benefit outcome of MACE or major adverse limb events, major amputation, or fatal or critical organ bleeding reduced by 28% (HR 0.72; 95% CI 0.59-0.87; P=0.0008) [37].
Another substudy of the COMPASS trial showed that among patients with lower extremity peripheral artery disease, the development of MALE was associated with a poor prognosis, with a marked risk of subsequent hospitalization for vascular amputations, death, and MACE after one year of the index event. Of note, in these patients, the combination of rivaroxaban plus aspirin was of particular benefit [38].
3.3. Patients with Heart Failure
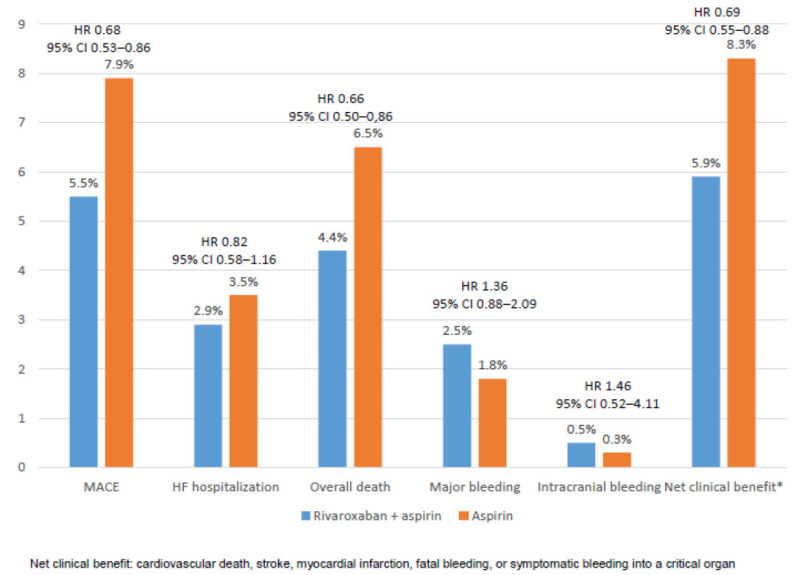
Patients with stable atherosclerotic vascular disease and heart failure have a higher risk of MACE [39]. In the COMPASS trial, patients with NYHA functional class III or IV heart failure or left ventricular ejection fraction < 30% were excluded from the study. However, in the COMPASS trial, a total of 5,902 subjects (22%) had a history of heart failure, 12% with a left ventricular ejection fraction <40%. The effects of the combination of rivaroxaban plus aspirin compared with aspirin alone on MACE was independent of the history of heart failure and left ventricular ejection fraction, but larger absolute risk reduction was obtained in those patients with heart failure. Similarly, the risk of major bleeding was independent of the presence of heart failure. Among patients with heart failure, the rivaroxaban plus aspirin approach reduced the risk of MACE by 32%, heart failure hospitalization by 18%, overall death by 34%, with a significant increase of major bleeding, but not intracranial bleeding (Fig. 4) [40].
Fig. (4).
Efficacy and safety outcomes among patients with heart failure. Data from the COMPASS study [40]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The COMMANDER HF showed in patients with coronary artery disease, low ejection fraction, and recent heart failure exacerbation, that although low dose rivaroxaban reduced thrombotic outcomes, it did not reduce MACE [41]. When analyzing the results of the COMMANDER HF, ATLAS ACS-2, and COMPASS trials, data suggest that the combination or rivaroxaban 2.5 mg bid plus aspirin is beneficial in patients with a stable atherosclerotic vascular disease with mild to moderate heart failure, but not in those patients with a recent decompensated heart failure or advanced heart failure with reduced ejection fraction [13, 40-42]. In fact, the clinical practice update on heart failure 2019 indicates that for patients with coronary artery disease, the addition of rivaroxaban 2.5 mg bid to aspirin reduces the risk of vascular events in patients without heart failure and with mild heart failure, but not in patients with advanced heart failure, as in these patients, myocardial dysfunction and congestion rather than vascular events determine the prognosis [43].
3.4. Patients with Diabetes
Diabetes is common among patients with the chronic coronary syndrome, and the concomitance of both markedly worsens cardiovascular prognosis [44]. In a prespecified analysis of the COMPASS trial, the effects of rivaroxaban plus aspirin (100 mg daily) versus aspirin alone, according to the presence of diabetes, were compared. In the COMPASS trial, approximately 38% of patients had diabetes at baseline. The reduction of MACE with the combination of rivaroxaban plus aspirin (vs aspirin) similarly occurred regardless of the presence of diabetes (diabetic patients: HR 0.74; 95% CI 0.61-0.90, P=0.002; nondiabetic patients HR 0.77; 95% CI 0.64-0.93; P=0.005; Pinteraction=0.77). In addition, the risk of major bleeding was not influenced by the history of diabetes. However, due to the higher baseline risk of patients with diabetes, the absolute benefits seemed greater in this population [45].
3.5. Patients with Renal Insufficiency
Chronic kidney disease increases the risk of both thromboembolic and bleeding events [46, 47]. In addition, it has been reported that full anticoagulation, with or without antiplatelet therapy, increases the risk of hemorrhage in patients with renal insufficiency [48, 49].
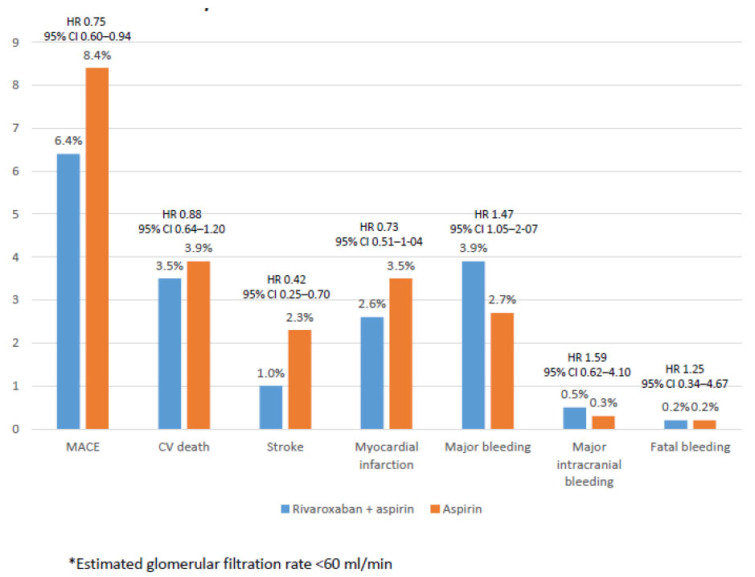
In the COMPASS trial, 6,276 patients (22.9%) had a glomerular filtration rate <60 ml/min. As expected, both MACE and major bleeding were more frequent in patients with renal dysfunction. Despite that, the relative efficacy and safety of the rivaroxaban plus aspirin approach compared with aspirin alone was independent of renal function. Among patients with renal insufficiency, the combination of rivaroxaban and aspirin (vs aspirin alone) reduced the risk of MACE by 25% and the risk of stroke by 58%, and tended to reduce the risk of myocardial infarction by 27%, and cardiovascular death by 12%. Although major bleeding was more common with the combined therapy, rates of major intracranial bleeding and fatal bleeding were low and similarly occurred between both groups (Fig. 5) [50].
Fig. (5).
Efficacy outcomes among patients with renal insufficiency*. Data from the COMPASS study [50]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.6. Patients with Stroke
Patients with atherosclerotic vascular disease have an increased risk of stroke [51, 52]. In primary prevention, treatment with aspirin is associated with a 12% reduction of MACE and in secondary prevention, with a 19% risk reduction [53].
In the COMPASS trial, the combination of rivaroxaban plus aspirin was associated with a significant reduction of stroke compared with aspirin (HR 0.58; 95% CI 0.44-0.76; P<0.0001). Similarly, ischemic/uncertain strokes (HR 0.51; 95% CI 0.38-0.68 P<0.0001) and fatal and disabling stroke (modified Rankin Scale, 3-6) (HR 0.58; 95% CI 0.37-0.89; P=0.01) were also decreased by the combination of rivaroxaban plus aspirin in comparison with aspirin alone. Of note, the relative efficacy of the rivaroxaban plus aspirin approach was consistent in those patients with prior stroke [54]. A recent exploratory analysis has also shown that compared with aspirin, the combination of rivaroxaban plus aspirin is associated with significant reductions in cardioembolic strokes and embolic strokes of undetermined source [55].
4. DISCUSSION
The European guidelines of chronic coronary syndromes recognized that in patients with moderate (as at least one of the following: multivessel/diffuse coronary artery disease, diabetes, recurrent myocardial infarction, peripheral artery disease, heart failure, or chronic kidney disease) or high risk (diffuse multivessel coronary artery disease with at least one of the following: diabetes, recurrent myocardial infarction, peripheral artery disease, or chronic kidney disease) of ischemic events and those who do not have a high bleeding risk (history of intracerebral hemorrhage or ischemic stroke, history of other intracranial pathology, recent gastrointestinal bleeding or anemia due to possible gastrointestinal blood loss, other gastrointestinal pathology associated with increased bleeding risk, liver failure, bleeding diathesis or coagulopathy, extreme old age or frailty, or severe renal failure), dual antithrombotic therapy should be considered [5]. Different alternatives are proposed for dual antithrombotic therapy (i.e. clopidogrel 75 mg/daily, prasugrel 10 mg/daily, ticagrelor 60 mg bid and rivaroxaban 2.5 mg bid), but no differences are seen in the recommendations between these drugs [5]. However, the results of the clinical trials that support these recommendations have important differences. For example, in the PEGASUS-TIMI 54 trial, although the addition of ticagrelor 60 mg to aspirin significantly reduced the risk of MACE, no significant effect was seen on ischemic stroke, major adverse limb events or overall mortality [10, 12, 14, 16]. In fact, in contrast to clopidogrel, ticagrelor or prasugrel, the addition of rivaroxaban 2.5 mg bid to aspirin has demonstrated to have a positive impact not only on MACE, but also on mortality as a secondary end-point [5, 8-16]. This is in line with the pathophysiology of atherothr-ombosis, in which the activation of both platelets, and factor X is implied and consequently, the management of patients with atherosclerotic vascular disease should include both targets [20]. In fact, the last European guidelines on diabetes indicate that in high-risk patients, the combination of low-dose rivaroxaban and aspirin may be beneficial for coronary artery disease [56]. Therefore, double antiplatelet therapy is more beneficial in the acute setting but to a lesser extent in chronic conditions, in contrast to rivaroxaban 2.5 mg bid.
In addition, as an atherosclerotic vascular disease can affect different vascular beds, the focus of treatment should not be limited to the reduction of MACE, but also MALE. Among patients with peripheral artery disease, in the CHARISMA trial, the addition of clopidogrel to aspirin (vs aspirin alone) did not translate into a reduction of MACE or mortality [57]. In the TRA 2P-TIMI 50 trial, compared with aspirin, the addition of vorapaxar to aspirin significantly reduced the risk of hospitalization for acute limb ischemia, but not the risk of MACE [58]. In the PEGASUS-TIMI 54, compared with aspirin, the addition of ticagrelor 90 mg to aspirin only translated into a significant reduction of MALE, but not MACE, ischemic stroke or cardiovascular death, whereas the addition of ticagrelor 60 mg to aspirin significantly reduced the risk of MACE, and cardiovascular death, but not MALE or ischemic stroke [12]. By contrast, the COMPASS trial demonstrated that the rivaroxaban plus aspirin approach provided comprehensive protection with reductions in the risk of MACE, MALE, and cardiovascular mortality and overall mortality [37]. The last European guidelines on diabetes indicate that rivaroxaban 2.5 mg b.i.d. plus aspirin 100 mg o.d. should be considered in patients with diabetes and symptomatic lower extremity artery disease [56]. In addition, the global vascular guidelines on the management of chronic limb-threatening ischemia also state that low-dose aspirin and rivaroxaban, 2.5 mg twice daily, should be considered to reduce adverse cardiovascular events and lower extremity ischemic events in patients with chronic limb-threatening ischemia [59].
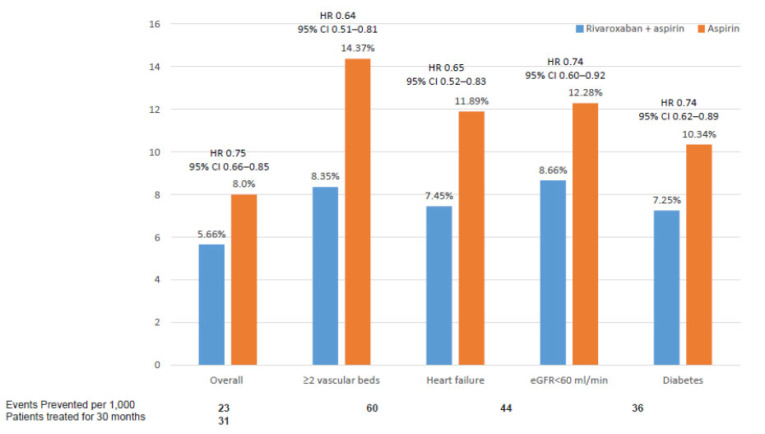
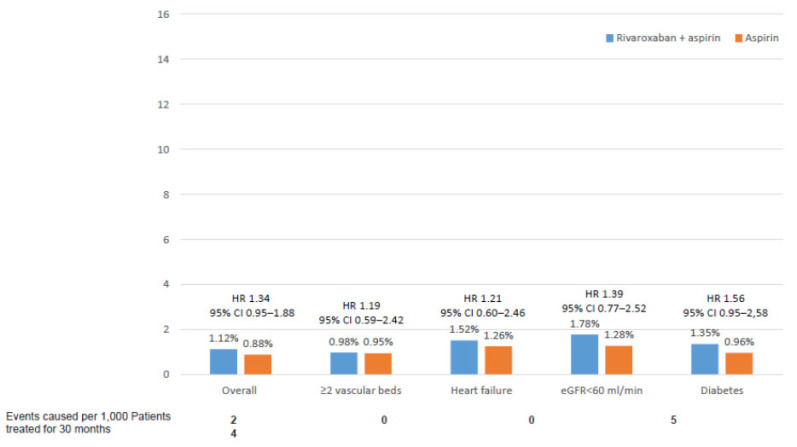
Although the results of the COMPASS trial were consistent across different subgroups of patients, it is important to ascertain which patients may benefit more from a COMPASS strategy. In this context, a recent substudy of the COMPASS trial has identified those patients with the highest net clinical benefit with the rivaroxaban plus aspirin approach [60]. This study showed that those patients with ≥2 vascular beds affected, heart failure, diabetes or renal insufficiency had the best net clinical benefit that included MACE, acute limb ischemia, and vascular amputation. In addition, in these patients, the risk of fatal or symptomatic into a critical organ bleeding was low (Fig. 6 and 7) [60]. On the other hand, different lifetime models have been developed in order to establish the benefits and bleeding risk for each individual patient, to facilitate treatment decisions in clinical practice [61]. In Table (3), patients who may benefit more from a COMPASS strategy are summarized [60].
Fig. (6).
Net Clinical Benefit (MACE, acute limb ischemia, and vascular amputation) according to different clinical conditions. Data from the COMPASS study [60]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (7).
Fatal or symptomatic into a critical organ bleeding according to different clinical conditions. Data from the COMPASS study [60]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 3.
Patients that may benefit more from a compass approach.
| • Coronary Artery Disease And/Or Peripheral Artery Disease AnFd: |
|---|
| • ≥2 vascular beds. |
| • Heart failure. |
| • Low estimated glomerular filtration rate. |
| • Diabetes mellitus. |
| • High risk REACH. |
| • High risk CART |
Note: REACH (REduction of Atherothrombosis for Continued Health); CART (Classification and Regression Tree).
High risk REACH patients: those with a history of vascular disease with 2 or more vascular beds affected, a history of heart failure, or renal insufficiency defined as an estimated glomerular filtration rate <60 ml/min.
High risk CART patients: those with either a history of vascular disease with 2 or more affected vascular beds, a history of heart failure or diabetes.
Table performed with data taken from reference #60.
The representativeness of the COMPASS trial has been analyzed in different “real-life” studies. Thus, after applying the inclusion and exclusion criteria to those patients included in the REACH registry, more than a half of the patients met the COMPASS criteria. The main reasons for exclusion included high-bleeding risk (52%), anticoagulant use (45%), a requirement for dual antiplatelet therapy within 1 year of an acute coronary syndrome or percutaneous coronary intervention (26%), history of recent ischemic stroke (12%), and severe renal failure (2%) [62]. In Spain, CICCOR was a single-center prospective cohort study that included a total of 1,268 outpatients with stable ischemic heart disease. This study showed that mortality rates in this population remained high, and greater than in the general population [63]. In the CICCOR registry, 46% of patients had at least one exclusion criteria for the COMPASS study and 18% did not meet with all the inclusion criteria. As a result, approximately 35% of the patients from the CICCOR registry could have been included in the COMPASS trial. The main reasons for exclusion were the need for dual antiplatelet therapy within the first year of an acute coronary syndrome or percutaneous coronary intervention (70%), and high risk of bleeding (33%). Of note, among those patients from the CICCOR registry that could have been included in the COMPASS trial, the rates of MACE and cardiovascular mortality were significantly higher than those of the aspirin alone arm of the COMPASS trial, suggesting than in real-life patients, outcomes are higher and may benefit more from a rivaroxaban plus aspirin approach [64]. Taking into account that one of the main reasons for exclusion is the first year after an acute coronary syndrome or coronary percutaneous intervention, this implies, that after this first year, a high number of patients with atherosclerotic vascular disease could benefit from a COMPASS strategy, particularly in those patients at high cardiovascular risk. Therefore, the implementation of the rivaroxaban plus aspirin approach in suitable patients is mandatory.
Finally, due to the good results on MACE and mortality of the combination of rivaroxaban plus aspirin, this could be a cost-effective approach among patients with atherosclerotic vascular disease, particularly those at the highest risk [65, 66].
CONCLUSION
Current European guidelines recognize that in those patients with a moderate or high risk of ischemic events, but without a high bleeding risk, dual antithrombotic therapy should be considered, as in these patients the risk or recurrent vascular events is unacceptably high despite treatment with aspirin (or clopidogrel) in monotherapy. Considering that in atherothrombosis, the activation of platelets and factor X plays a key role, both targets should be considered for the appropriate management of these patients. In fact, no reduction of mortality has been observed when dual antiplatelet therapy has been used among patients with atherosclerotic vascular disease. By contrast, the COMPASS trial demonstrated that compared with aspirin alone, the rivaroxaban plus aspirin approach did not only reduce MACE, but also MALE and cardiovascular and overall mortality. Therefore, the COMPASS strategy should be preferentially be used in patients with stable atherosclerotic vascular disease, particularly those at moderate or high risk of recurrent vascular events, such as those with ≥2 vascular beds affected, heart failure, renal insufficiency, diabetes or high-risk REACH/CART.
ACKNOWLEDGEMENTS
Writing and editorial assistance was provided by Content Ed Net (Madrid, Spain) with funding from Bayer Hispania.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was financially supported by Bayer Hispania.
CONFLICT OF INTEREST
FAY has received personal fees for educational activities or participation in boards from Daiichi Sankyo, Bayer, Böhringer Ingelheim and Bristol Myers Squibb.
VB has received honoraria consultancies and/or lecture fees from Bayer, Boehringer Ingelheim, BMS/Pfizer and Daiichi Sankyo.
MBA has received honoraria consultancies and/or lecture fees from Amgen, Bayer, Boehringer Ingelheim, BMS/Pfizer, MSD and Rovi.
JMA has received honoraria consultancies and/or lecture fees from Bayer, Boehringer Ingelheim, BMS/Pfizer and Daiichi Sankyo.
JGM has no relationships that could be construed as a conflict of interest.
JAAD has received honoraria consultancies and/or lecture fees from: Bayer, Sanofi, Astra-Zeneca, Boehringer Ingelheim.
MRO has received honoraria consultancies and/or lecture fees from Bayer, Boehringer Ingelheim, BMS/Pfizer and Daiichi Sankyo.
RVG has no relationships that could be construed as a conflict of interest.
AVM has received honoraria consultancies and/or lecture fees from Bayer, Pfizer, Daiichi Sankyo and Boehringer Ingelheim.
REFERENCES
- 1.Barquera S., Pedroza-Tobías A., Medina C., Hernández-Barrera L., Bibbins-Domingo K., Lozano R., Moran A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015;46(5):328–338. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Shen X., DiMario S., Philip K. Gender disparities in health resource utilization in patients with atherosclerotic cardiovascular disease: A retrospective cross-sectional study. Adv. Ther. 2019;36(12):3424–3434. doi: 10.1007/s12325-019-01107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmis A., Gale C.P., Flather M., Maniadakis N., Vardas P. Cardiovascular disease statistics from the European atlas: inequalities between high- and middle-income member countries of the ESC. Eur. Heart J. Qual. Care Clin. Outcomes. 2018;4(1):1–3. doi: 10.1093/ehjqcco/qcx045. [DOI] [PubMed] [Google Scholar]
- 4.Odden M.C., Coxson P.G., Moran A., Lightwood J.M., Goldman L., Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the United States. Am. J. Med. 2011;124(9):827–33.e5. doi: 10.1016/j.amjmed.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., Prescott E., Storey R.F., Deaton C., Cuisset T., Agewall S., Dickstein K., Edvardsen T., Escaned J., Gersh B.J., Svitil P., Gilard M., Hasdai D., Hatala R., Mahfoud F., Masip J., Muneretto C., Valgimigli M., Achenbach S., Bax J.J. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 6.Kotseva K., De Backer G., De Bacquer D., Rydén L., Hoes A., Grobbee D., Maggioni A., Marques-Vidal P., Jennings C., Abreu A., Aguiar C., Badariene J., Bruthans J., Castro Conde A., Cifkova R., Crowley J., Davletov K., Deckers J., De Smedt D., De Sutter J., Dilic M., Dolzhenko M., Dzerve V., Erglis A., Fras Z., Gaita D., Gotcheva N., Heuschmann P., Hasan-Ali H., Jankowski P., Lalic N., Lehto S., Lovic D., Mancas S., Mellbin L., Milicic D., Mirrakhimov E., Oganov R., Pogosova N., Reiner Z., Stöerk S., Tokgözoğlu L., Tsioufis C., Vulic D., Wood D. EUROASPIRE Investigators*. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: Results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur. J. Prev. Cardiol. 2019;26(8):824–835. doi: 10.1177/2047487318825350. [DOI] [PubMed] [Google Scholar]
- 7.Vanassche T., Verhamme P., Anand S.S., Shestakovska O., Fox K.A., Bhatt D.L., Avezum A., Alings M., Aboyans V., Maggioni A.P., Widimsky P., Berkowitz S.D., Yusuf S., Connolly S.J., Eikelboom J.W., Bosch J. Risk factors and clinical outcomes in chronic coronary and peripheral artery disease: An analysis of the randomized, double-blind COMPASS trial. Eur. J. Prev. Cardiol. 2020;27(3):296–307. doi: 10.1177/2047487319882154. [DOI] [PubMed] [Google Scholar]
- 8.Mauri L., Kereiakes D.J., Yeh R.W., Driscoll-Shempp P., Cutlip D.E., Steg P.G., Normand S.L., Braunwald E., Wiviott S.D., Cohen D.J., Holmes D.R., Jr, Krucoff M.W., Hermiller J., Dauerman H.L., Simon D.I., Kandzari D.E., Garratt K.N., Lee D.P., Pow T.K., Ver Lee P., Rinaldi M.J., Massaro J.M. DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 2014;371(23):2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh R.W., Kereiakes D.J., Steg P.G., Windecker S., Rinaldi M.J., Gershlick A.H., Cutlip D.E., Cohen D.J., Tanguay J.F., Jacobs A., Wiviott S.D., Massaro J.M., Iancu A.C., Mauri L. DAPT Study Investigators. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J. Am. Coll. Cardiol. 2015;65(20):2211–2221. doi: 10.1016/j.jacc.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt D.L., Bonaca M.P., Bansilal S., Angiolillo D.J., Cohen M., Storey R.F., Im K., Murphy S.A., Held P., Braunwald E., Sabatine M.S., Steg P.G. Reduction in ischemic events with ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS-TIMI 54. J. Am. Coll. Cardiol. 2016;67(23):2732–2740. doi: 10.1016/j.jacc.2016.03.529. [DOI] [PubMed] [Google Scholar]
- 11.Bansilal S., Bonaca M.P., Cornel J.H., Storey R.F., Bhatt D.L., Steg P.G., Im K., Murphy S.A., Angiolillo D.J., Kiss R.G., Parkhomenko A.N., Lopez-Sendon J., Isaza D., Goudev A., Kontny F., Held P., Jensen E.C., Braunwald E., Sabatine M.S., Oude Ophuis A.J. Ticagrelor for secondary prevention of atherothrombotic events in patients with multivessel coronary disease. J. Am. Coll. Cardiol. 2018;71(5):489–496. doi: 10.1016/j.jacc.2017.11.050. [DOI] [PubMed] [Google Scholar]
- 12.Bonaca M.P., Bhatt D.L., Storey R.F., Steg P.G., Cohen M., Kuder J., Goodrich E., Nicolau J.C., Parkhomenko A., López-Sendón J., Dellborg M., Dalby A., Špinar J., Aylward P., Corbalán R., Abola M.T.B., Jensen E.C., Held P., Braunwald E., Sabatine M.S. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J. Am. Coll. Cardiol. 2016;67(23):2719–2728. doi: 10.1016/j.jacc.2016.03.524. [DOI] [PubMed] [Google Scholar]
- 13.Eikelboom J.W., Connolly S.J., Bosch J., Dagenais G.R., Hart R.G., Shestakovska O., Diaz R., Alings M., Lonn E.M., Anand S.S., Widimsky P., Hori M., Avezum A., Piegas L.S., Branch K.R.H., Probstfield J., Bhatt D.L., Zhu J., Liang Y., Maggioni A.P., Lopez-Jaramillo P., O’Donnell M., Kakkar A.K., Fox K.A.A., Parkhomenko A.N., Ertl G., Störk S., Keltai M., Ryden L., Pogosova N., Dans A.L., Lanas F., Commerford P.J., Torp-Pedersen C., Guzik T.J., Verhamme P.B., Vinereanu D., Kim J.H., Tonkin A.M., Lewis B.S., Felix C., Yusoff K., Steg P.G., Metsarinne K.P., Cook Bruns N., Misselwitz F., Chen E., Leong D., Yusuf S. COMPASS Investigators. Rivaroxaban with or without aspirin in stable cardiovascular disease. N. Engl. J. Med. 2017;377(14):1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 14.Bonaca M.P., Bhatt D.L., Cohen M., Steg P.G., Storey R.F., Jensen E.C., Magnani G., Bansilal S., Fish M.P., Im K., Bengtsson O., Oude Ophuis T., Budaj A., Theroux P., Ruda M., Hamm C., Goto S., Spinar J., Nicolau J.C., Kiss R.G., Murphy S.A., Wiviott S.D., Held P., Braunwald E., Sabatine M.S. PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015;372(19):1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 15.Garratt K.N., Weaver W.D., Jenkins R.G., Pow T.K., Mauri L., Kereiakes D.J., Winters K.J., Christen T., Allocco D.J., Lee D.P. Prasugrel plus aspirin beyond 12 months is associated with improved outcomes after TAXUS Liberté paclitaxel-eluting coronary stent placement. Circulation. 2015;131(1):62–73. doi: 10.1161/CIRCULATIONAHA.114.013570. [DOI] [PubMed] [Google Scholar]
- 16.Bonaca M.P., Bhatt D.L., Steg P.G., Storey R.F., Cohen M., Im K., Oude Ophuis T., Budaj A., Goto S., López-Sendón J., Diaz R., Dalby A., Van de Werf F., Ardissino D., Montalescot G., Aylward P., Magnani G., Jensen E.C., Held P., Braunwald E., Sabatine M.S. Ischaemic risk and efficacy of ticagrelor in relation to time from P2Y12 inhibitor withdrawal in patients with prior myocardial infarction: insights from PEGASUS-TIMI 54. Eur. Heart J. 2016;37(14):1133–1142. doi: 10.1093/eurheartj/ehv531. [DOI] [PubMed] [Google Scholar]
- 17.Bauersachs R., Zannad F. Rivaroxaban: A new treatment paradigm in the setting of vascular protection? Thromb. Haemost. 2018;118(S 01):S12–S22.. doi: 10.1055/s-0038-1636530. [DOI] [PubMed] [Google Scholar]
- 18.Frangogiannis N.G. Pathophysiology of myocardial infarction. Compr. Physiol. 2015;5(4):1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 19.Vogel B., Claessen B.E., Arnold S.V., Chan D., Cohen D.J., Giannitsis E., Gibson C.M., Goto S., Katus H.A., Kerneis M., Kimura T., Kunadian V., Pinto D.S., Shiomi H., Spertus J.A., Steg P.G., Mehran R. ST-segment elevation myocardial infarction. Nat. Rev. Dis. Primers. 2019;5(1):39. doi: 10.1038/s41572-019-0090-3. [DOI] [PubMed] [Google Scholar]
- 20.Coppens M., Weitz J.I., Eikelboom J.W.A. Synergy of dual pathway inhibition in chronic cardiovascular disease. Circ. Res. 2019;124(3):416–425. doi: 10.1161/CIRCRESAHA.118.313141. [DOI] [PubMed] [Google Scholar]
- 21.Barrios V., Almendro-Delia M., Facila L., Garcia-Moll X., Mazón P., Camafort M., Cepeda J.M., Mediavilla Garcia J.D., Pose Reino A., Suarez Fernandez C. Rivaroxaban: searching the integral vascular protection. Expert Rev. Clin. Pharmacol. 2018;11(7):719–728. doi: 10.1080/17512433.2018.1495559. [DOI] [PubMed] [Google Scholar]
- 22.Ramacciotti E., Weitz J.I. Rivaroxaban plus aspirin for cardiovascular protection: Rationale for the vascular dose and dual pathway inhibition. Thromb. Res. 2019;184:44–49. doi: 10.1016/j.thromres.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 23.Maeda M., Tsuboi T., Hayashi T. An Inhibitor of Activated Blood Coagulation Factor X Shows Anti-Endothelial Senescence and Anti-Atherosclerotic Effects. J. Vasc. Res. 2019;56(4):181–190. doi: 10.1159/000499975. [DOI] [PubMed] [Google Scholar]
- 24.Posthuma J.J., Posma J.J.N., van Oerle R., Leenders P., van Gorp R.H., Jaminon A.M.G., Mackman N., Heitmeier S., Schurgers L.J., Ten Cate H., Spronk H.M.H. Targeting coagulation factor Xa promotes regression of advanced atherosclerosis in apolipoprotein-e deficient mice. Sci. Rep. 2019;9(1):3909. doi: 10.1038/s41598-019-40602-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rattazzi M., Faggin E., Bertacco E., Nardin C., Pagliani L., Plebani M., Cinetto F., Guidolin D., Puato M., Pauletto P. Warfarin, but not rivaroxaban, promotes the calcification of the aortic valve in ApoE-/- mice. Cardiovasc. Ther. 2018;36(4):e12438. doi: 10.1111/1755-5922.12438. [DOI] [PubMed] [Google Scholar]
- 26.Álvarez E., Paradela-Dobarro B., Raposeiras-Roubín S., González-Juanatey J.R. Protective, repairing and fibrinolytic effects of rivaroxaban on vascular endothelium. Br. J. Clin. Pharmacol. 2018;84(2):280–291. doi: 10.1111/bcp.13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara T., Fukuda D., Tanaka K., Higashikuni Y., Hirata Y., Yagi S., Soeki T., Shimabukuro M., Sata M. Inhibition of activated factor X by rivaroxaban attenuates neointima formation after wire-mediated vascular injury. Eur. J. Pharmacol. 2018;820:222–228. doi: 10.1016/j.ejphar.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 28.Borst O., Münzer P., Alnaggar N., Geue S., Tegtmeyer R., Rath D., Droppa M., Seizer P., Heitmeier S., Heemskerk J.W.M., Jennings L.K., Storey R.F., Angiolillo D.J., Rocca B., Spronk H., Ten Cate H., Gawaz M., Geisler T. Inhibitory mechanisms of very low-dose rivaroxaban in non-ST-elevation myocardial infarction. Blood Adv. 2018;2(6):715–730. doi: 10.1182/bloodadvances.2017013573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara T., Fukuda D., Tanaka K., Higashikuni Y., Hirata Y., Nishimoto S., Yagi S., Yamada H., Soeki T., Wakatsuki T., Shimabukuro M., Sata M. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Atherosclerosis. 2015;242(2):639–646. doi: 10.1016/j.atherosclerosis.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Wu T.C., Chan J.S., Lee C.Y., Leu H.B., Huang P.H., Chen J.S., Lin S.J., Chen J.W. Rivaroxaban, a factor Xa inhibitor, improves neovascularization in the ischemic hindlimb of streptozotocin-induced diabetic mice. Cardiovasc. Diabetol. 2015;14:81. doi: 10.1186/s12933-015-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanada F., Muratsu J., Otsu R., Shimizu H., Koibuchi N., Uchida K., Taniyama Y., Yoshimura S., Rakugi H., Morishita R. Local production of activated factor X in atherosclerotic plaque induced vascular smooth muscle cell senescence. Sci. Rep. 2017;7(1):17172. doi: 10.1038/s41598-017-17508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander J.H., Lopes R.D., James S., Kilaru R., He Y., Mohan P., Bhatt D.L., Goodman S., Verheugt F.W., Flather M., Huber K., Liaw D., Husted S.E., Lopez-Sendon J., De Caterina R., Jansky P., Darius H., Vinereanu D., Cornel J.H., Cools F., Atar D., Leiva-Pons J.L., Keltai M., Ogawa H., Pais P., Parkhomenko A., Ruzyllo W., Diaz R., White H., Ruda M., Geraldes M., Lawrence J., Harrington R.A., Wallentin L. APPRAISE-2 Investigators. Apixaban with antiplatelet therapy after acute coronary syndrome. N. Engl. J. Med. 2011;365(8):699–708. doi: 10.1056/NEJMoa1105819. [DOI] [PubMed] [Google Scholar]
- 33.Kim P.Y., Yeh C.H., Dale B.J., Leslie B.A., Stafford A.R., Fredenburgh J.C., Hirsh J., Weitz J.I. Mechanistic basis for the differential effects of rivaroxaban and apixaban on global tests of coagulation. TH Open. 2018;2(2):e190–e201. doi: 10.1055/s-0038-1649507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connolly S.J., Eikelboom J.W., Bosch J., Dagenais G., Dyal L., Lanas F., Metsarinne K., O’Donnell M., Dans A.L., Ha J.W., Parkhomenko A.N., Avezum A.A., Lonn E., Lisheng L., Torp-Pedersen C., Widimsky P., Maggioni A.P., Felix C., Keltai K., Hori M., Yusoff K., Guzik T.J., Bhatt D.L., Branch K.R.H., Cook Bruns N., Berkowitz S.D., Anand S.S., Varigos J.D., Fox K.A.A., Yusuf S. COMPASS investigators. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):205–218. doi: 10.1016/S0140-6736(17)32458-3. [DOI] [PubMed] [Google Scholar]
- 35.Shroyer A.L., Grover F.L., Hattler B., Collins J.F., McDonald G.O., Kozora E., Lucke J.C., Baltz J.H., Novitzky D. Veterans Affairs Randomized On/Off Bypass (ROOBY) Study Group. On-pump versus off-pump coronary-artery bypass surgery. N. Engl. J. Med. 2009;361(19):1827–1837. doi: 10.1056/NEJMoa0902905. [DOI] [PubMed] [Google Scholar]
- 36.Lamy A., Eikelboom J., Sheth T., Connolly S., Bosch J., Fox K.A.A., Zhu J., Lonn E., Dagenais G., Widimsky P., Branch K.R.H., Bhatt D.L., Zheng Z., Straka Z., Dagenais F., Kong Y., Marsden T., Lee S.F., Copland I., Yusuf S. Rivaroxaban, aspirin, or both to prevent early coronary bypass graft occlusion: The COMPASS-CABG Study. J. Am. Coll. Cardiol. 2019;73(2):121–130. doi: 10.1016/j.jacc.2018.10.048. [DOI] [PubMed] [Google Scholar]
- 37.Anand S.S., Bosch J., Eikelboom J.W., Connolly S.J., Diaz R., Widimsky P., Aboyans V., Alings M., Kakkar A.K., Keltai K., Maggioni A.P., Lewis B.S., Störk S., Zhu J., Lopez-Jaramillo P., O’Donnell M., Commerford P.J., Vinereanu D., Pogosova N., Ryden L., Fox K.A.A., Bhatt D.L., Misselwitz F., Varigos J.D., Vanassche T., Avezum A.A., Chen E., Branch K., Leong D.P., Bangdiwala S.I., Hart R.G., Yusuf S. COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):219–229. doi: 10.1016/S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 38.Anand S.S., Caron F., Eikelboom J.W., Bosch J., Dyal L., Aboyans V., Abola M.T., Branch K.R.H., Keltai K., Bhatt D.L., Verhamme P., Fox K.A.A., Cook-Bruns N., Lanius V., Connolly S.J., Yusuf S. Major adverse limb events and mortality in patients with peripheral artery disease: The COMPASS Trial. J. Am. Coll. Cardiol. 2018;71(20):2306–2315. doi: 10.1016/j.jacc.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Shah K.S., Xu H., Matsouaka R.A., Bhatt D.L., Heidenreich P.A., Hernandez A.F., Devore A.D., Yancy C.W., Fonarow G.C. Heart failure with preserved, borderline, and reduced ejection fraction: 5-Year outcomes. J. Am. Coll. Cardiol. 2017;70(20):2476–2486. doi: 10.1016/j.jacc.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 40.Branch K.R., Probstfield J.L., Eikelboom J.W., Bosch J., Maggioni A.P., Cheng R.K., Bhatt D.L., Avezum A., Fox K.A.A., Connolly S.J., Shestakovska O., Yusuf S. Rivaroxaban with or without aspirin in patients with heart failure and chronic coronary or peripheral artery disease. Circulation. 2019;140(7):529–537. doi: 10.1161/CIRCULATIONAHA.119.039609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zannad F., Anker S.D., Byra W.M., Cleland J.G.F., Fu M., Gheorghiade M., Lam C.S.P., Mehra M.R., Neaton J.D., Nessel C.C., Spiro T.E., van Veldhuisen D.J., Greenberg B. COMMANDER HF Investigators. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N. Engl. J. Med. 2018;379(14):1332–1342. doi: 10.1056/NEJMoa1808848. [DOI] [PubMed] [Google Scholar]
- 42.Mega J.L., Braunwald E., Wiviott S.D., Bassand J.P., Bhatt D.L., Bode C., Burton P., Cohen M., Cook-Bruns N., Fox K.A., Goto S., Murphy S.A., Plotnikov A.N., Schneider D., Sun X., Verheugt F.W., Gibson C.M. ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012;366(1):9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 43.Seferovic P.M., Ponikowski P., Anker S.D., Bauersachs J., Chioncel O., Cleland J.G.F., de Boer R.A., Drexel H., Ben Gal T., Hill L., Jaarsma T., Jankowska E.A., Anker M.S., Lainscak M., Lewis B.S., McDonagh T., Metra M., Milicic D., Mullens W., Piepoli M.F., Rosano G., Ruschitzka F., Volterrani M., Voors A.A., Filippatos G., Coats A.J.S. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019;21(10):1169–1186. doi: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- 44.Naito R., Miyauchi K. Coronary artery disease and type 2 diabetes mellitus. Int. Heart J. 2017;58(4):475–480. doi: 10.1536/ihj.17-191. [DOI] [PubMed] [Google Scholar]
- 45.Bhatt D.L., Eikelboom J.W., Connolly S.J., Steg P.G., Anand S.S., Verma S., Branch K.R.H., Probstfield J., Bosch J., Shestakovska O., Szarek M., Maggioni A.P., Widimský P., Avezum A., Diaz R., Lewis B.S., Berkowitz S.D., Fox K.A.A., Ryden L., Yusuf S. COMPASS Steering Committee and Investigators. Role of combination antiplatelet and anticoagulation therapy in diabetes mellitus and cardiovascular disease: Insights from the COMPASS Trial. Circulation. 2020;141(23):1841–1854. doi: 10.1161/CIRCULATIONAHA.120.046448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Go A.S., Fang M.C., Udaltsova N., Chang Y., Pomernacki N.K., Borowsky L., Singer D.E. ATRIA Study Investigators. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119(10):1363–1369. doi: 10.1161/CIRCULATIONAHA.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jun M., James M.T., Manns B.J., Quinn R.R., Ravani P., Tonelli M., Perkovic V., Winkelmayer W.C., Ma Z., Hemmelgarn B.R. Alberta Kidney Disease Network. The association between kidney function and major bleeding in older adults with atrial fibrillation starting warfarin treatment: population based observational study. BMJ. 2015;350:h246. doi: 10.1136/bmj.h246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hohnloser S.H., Hijazi Z., Thomas L., Alexander J.H., Amerena J., Hanna M., Keltai M., Lanas F., Lopes R.D., Lopez-Sendon J., Granger C.B., Wallentin L. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur. Heart J. 2012;33(22):2821–2830. doi: 10.1093/eurheartj/ehs274. [DOI] [PubMed] [Google Scholar]
- 49.Fox K.A., Piccini J.P., Wojdyla D., Becker R.C., Halperin J.L., Nessel C.C., Paolini J.F., Hankey G.J., Mahaffey K.W., Patel M.R., Singer D.E., Califf R.M. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur. Heart J. 2011;32(19):2387–2394. doi: 10.1093/eurheartj/ehr342. [DOI] [PubMed] [Google Scholar]
- 50.Fox K.A.A., Eikelboom J.W., Shestakovska O., Connolly S.J., Metsarinne K.P., Yusuf S. Rivaroxaban plus aspirin in patients with vascular disease and renal dysfunction: From the COMPASS Trial. J. Am. Coll. Cardiol. 2019;73(18):2243–2250. doi: 10.1016/j.jacc.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 51.Bhatt D.L., Eagle K.A., Ohman E.M., Hirsch A.T., Goto S., Mahoney E.M., Wilson P.W., Alberts M.J., D’Agostino R., Liau C.S., Mas J.L., Röther J., Smith S.C., Jr, Salette G., Contant C.F., Massaro J.M., Steg P.G. REACH Registry Investigators. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304(12):1350–1357. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 52.Ducrocq G., Amarenco P., Labreuche J., Alberts M.J., Mas J.L., Ohman E.M., Goto S., Lavallée P., Bhatt D.L., Steg P.G. A history of stroke/transient ischemic attack indicates high risks of cardiovascular event and hemorrhagic stroke in patients with coronary artery disease. Circulation. 2013;127(6):730–738. doi: 10.1161/CIRCULATIONAHA.112.141572. [DOI] [PubMed] [Google Scholar]
- 53.Baigent C., Blackwell L., Collins R., Emberson J., Godwin J., Peto R., Buring J., Hennekens C., Kearney P., Meade T., Patrono C., Roncaglioni M.C., Zanchetti A. Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma M., Hart R.G., Connolly S.J., Bosch J., Shestakovska O., Ng K.K.H., Catanese L., Keltai K., Aboyans V., Alings M., Ha J.W., Varigos J., Tonkin A., O’Donnell M., Bhatt D.L., Fox K., Maggioni A., Berkowitz S.D., Bruns N.C., Yusuf S., Eikelboom J.W. Stroke outcomes in the COMPASS Trial. Circulation. 2019;139(9):1134–1145. doi: 10.1161/CIRCULATIONAHA.118.035864. [DOI] [PubMed] [Google Scholar]
- 55.Perera K.S., Ng K.K.H., Nayar S., Catanese L., Dyal L., Sharma M., Connolly S.J., Yusuf S., Bosch J., Eikelboom J.W., Hart R.G. Association between low- dose Rivaroxaban with or without aspirin and ischemic stroke subtypes: A secondary analysis of the COMPASS Trial. JAMA Neurol. 2020;77(1):42–48. doi: 10.1001/jamaneurol.2019.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., Federici M., Filippatos G., Grobbee D.E., Hansen T.B., Huikuri H.V., Johansson I., Jüni P., Lettino M., Marx N., Mellbin L.G., Östgren C.J., Rocca B., Roffi M., Sattar N., Seferović P.M., Sousa-Uva M., Valensi P., Wheeler D.C. ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 57.Bhatt D.L., Flather M.D., Hacke W., Berger P.B., Black H.R., Boden W.E., Cacoub P., Cohen E.A., Creager M.A., Easton J.D., Hamm C.W., Hankey G.J., Johnston S.C., Mak K.H., Mas J.L., Montalescot G., Pearson T.A., Steg P.G., Steinhubl S.R., Weber M.A., Fabry-Ribaudo L., Hu T., Topol E.J., Fox K.A. CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J. Am. Coll. Cardiol. 2007;49(19):1982–1988. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 58.Bonaca M.P., Scirica B.M., Creager M.A., Olin J., Bounameaux H., Dellborg M., Lamp J.M., Murphy S.A., Braunwald E., Morrow D.A. Vorapaxar in patients with peripheral artery disease: results from TRA2{degrees}P-TIMI 50. Circulation. 2013;127(14):1522-1529, 1529e1-6. doi: 10.1161/CIRCULATIONAHA.112.000679. [DOI] [PubMed] [Google Scholar]
- 59.Conte M.S., Bradbury A.W., Kolh P., White J.V., Dick F., Fitridge R., Mills J.L., Ricco J.B., Suresh K.R., Murad M.H., Aboyans V., Aksoy M., Alexandrescu V.A., Armstrong D., Azuma N., Belch J., Bergoeing M., Bjorck M., Chakfé N., Cheng S., Dawson J., Debus E.S., Dueck A., Duval S., Eckstein H.H., Ferraresi R., Gambhir R., Gargiulo M., Geraghty P., Goode S., Gray B., Guo W., Gupta P.C., Hinchliffe R., Jetty P., Komori K., Lavery L., Liang W., Lookstein R., Menard M., Misra S., Miyata T., Moneta G., Munoa Prado J.A., Munoz A., Paolini J.E., Patel M., Pomposelli F., Powell R., Robless P., Rogers L., Schanzer A., Schneider P., Taylor S., De Ceniga M.V., Veller M., Vermassen F., Wang J., Wang S. GVG Writing Group for the Joint Guidelines of the Society for Vascular Surgery (SVS), European Society for Vascular Surgery (ESVS), and World Federation of Vascular Societies (WFVS) Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur. J. Vasc. Endovasc. Surg. 2019;58(1S):S1-S109, 109.e33. doi: 10.1016/j.ejvs.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anand S.S., Eikelboom J.W., Dyal L., Bosch J., Neumann C., Widimsky P., Avezum A.A., Probstfield J., Cook Bruns N., Fox K.A.A., Bhatt D.L., Connolly S.J., Yusuf S. COMPASS Trial Investigators. Rivaroxaban plus aspirin versus aspirin in relation to vascular risk in the COMPASS Trial. J. Am. Coll. Cardiol. 2019;73(25):3271–3280. doi: 10.1016/j.jacc.2019.02.079. [DOI] [PubMed] [Google Scholar]
- 61.de Vries T.I., Eikelboom J.W., Bosch J., Westerink J., Dorresteijn J.A.N., Alings M., Dyal L., Berkowitz S.D., van der Graaf Y., Fox K.A.A., Visseren F.L.J. Estimating individual lifetime benefit and bleeding risk of adding rivaroxaban to aspirin for patients with stable cardiovascular disease: results from the COMPASS trial. Eur. Heart J. 2019;40(46):3771–3778a. doi: 10.1093/eurheartj/ehz404. [DOI] [PubMed] [Google Scholar]
- 62.Darmon A., Bhatt D.L., Elbez Y., Aboyans V., Anand S., Bosch J., Branch K.R., Connolly S.J., Dyal L., Eikelboom J.W., Fox K.A.A., Keltai K., Probstfield J., Yusuf S., Abtan J., Sorbets E., Eagle K.A., Ducrocq G., Steg P.G. External applicability of the COMPASS trial: an analysis of the reduction of atherothrombosis for continued health (REACH) registry. Eur. Heart J. 2018;39(9):750–757a. doi: 10.1093/eurheartj/ehx658. [DOI] [PubMed] [Google Scholar]
- 63.Sánchez Fernández J.J., Ruiz Ortiz M., Ogayar Luque C., Cantón Gálvez J.M., Romo Peñas E., Mesa Rubio D., Delgado Ortega M., Castillo Domínguez J.C., Anguita Sánchez M., López Aguilera J., Carrasco Ávalos F., Pan Álvarez-Ossorio M. Long-term survival in a spanish population with stable ischemic heart disease. The CICCOR registry. Rev. Esp. Cardiol. (Engl. Ed.) 2019;72(10):827–834. doi: 10.1016/j.rec.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 64.M. Ruiz Ortiz M, Fernández J.J.S., Ogáyar Luque C., et al. Potential applicability of low-doses of rivaroxaban in patients with stable ischemic heart disease in real-life: substudy of the CICCOR registry. Rev. Esp. Cardiol. 2019;72(Supl 1):737. A6017-202. [Google Scholar]
- 65.Tsilimigras D. I., Moris D., Karaolanis G., Kakkos S. K., Filis K., Sigala F. Rivaroxaban versus clopidogrel for peripheral artery disease: A clinico-economic approach of the COMPASS Trial. Curr Pharm Des. 2018;24(38):4516–4517. doi: 10.2174/1381612825666190101100832. [DOI] [PubMed] [Google Scholar]
- 66.Ademi Z., Zomer E., Tonkin A., Liew D. Cost-effectiveness of rivaroxaban and aspirin compared to aspirin alone in patients with stable cardiovascular disease: An Australian perspective. Int. J. Cardiol. 2018;270:54–59. doi: 10.1016/j.ijcard.2018.06.091. [DOI] [PubMed] [Google Scholar]