DRIVEN BY public health incentives including promoting interoperability (formerly meaningful use), electronic health records (EHRs) have gained widespread traction. Within anesthesiology, perioperative EHRs have gone beyond simply providing enhanced clinical documentation for billing purposes. They now leverage big data to catalyze an array of opportunities for healthcare improvement and innovation, including clinical registry–based research and quality improvement1; advanced statistical modeling2; and more recently, predictive analytics using machine learning and waveform processing.3
Despite new opportunities afforded by large clinical databases, challenges to deriving actionable knowledge from heterogenous EHR data are abundant. After large-scale adoption of EHRs, standards for data quality largely have been driven by administrative needs. Individual EHR data elements are commonly nonstandardized, and compatibility issues in documentation practices across providers, institutions, and EHR vendors pose significant barriers to effective information transmission and retrieval. Another challenge lies in authenticating the sheer volume of data generated; within the average electronic anesthetic record, thousands of physiologic observations are continuously generated from more than 40 parameters.4 This challenge is of particular relevance to cardiothoracic and vascular anesthesia, for which increased demands for high-acuity clinical care allow limited opportunities to curate and correct automatically captured EHR data. This in turn results in anesthetic records that are replete with redundant, conflicting, missing, or inaccurate EHR data (eg, multiple arterial blood pressure sources; electrocardiogram interference from electrocautery; intermittently disconnected/clamped arterial, central venous, and pulmonary arterial catheter monitors), often rendering raw data uninterpretable for secondary use. Finally, even with steps taken to handle the “4 Vs of big data” (volume, velocity, variety, veracity), EHR data often are unusable to clinical providers who may feel overwhelmed by the sheer size and complexity of the available information and commonly lack proper information technology tools to access the data. This lack of user-friendly and convenient access to structured data for clinical providers is frequently the rate-limiting step for clinical research and quality improvement.
Making Sense of Big Data Within Perioperative EHRs
To improve stewardship of big data, considerations first must be given to the multiple levels of large-scale perioperative EHR data available, each serving unique functions. In its most granular form, perioperative EHR data are available at the individual case level, providing clinicians with personalized feedback on cases performed5 and supplying local quality improvement programs with objective data for individual provider performance review and peer-to-peer comparisons. These data also can be used for quality improvement initiatives, such as development of real-time clinical decision support4 and opportunities for obtaining Maintenance of Certification in Anesthesiology Part 4 credits. Conversely, in aggregate forms, perioperative EHR data are available at institutional and multicenter levels and can be used for studying low-incidence outcomes (eg, mortality6) that otherwise would be challenging to analyze within smaller datasets and more common outcomes (eg, postoperative atrial fibrillation7) or processes of care (eg, lung protective ventilation8) for which anesthesiology practice benchmarking may be performed to enable comparisons across providers and institutions.9 Managing data at this level requires substantial coordination of efforts and cooperation of participants, both at the institutional and individual levels.
In addition to a growing need for stewardship of big data, a need for systematic processes to implement new knowledge into clinical practice deserves equal attention. Changes to best practice driven by research findings, leading to new hypotheses that can in turn be tested through new analyses, form an important quality improvement feedback loop for improving care. When these complementary processes are paired together, research and quality improvement comprise a conceptual model commonly described as a learning health system (Fig 1).10
Fig 1.
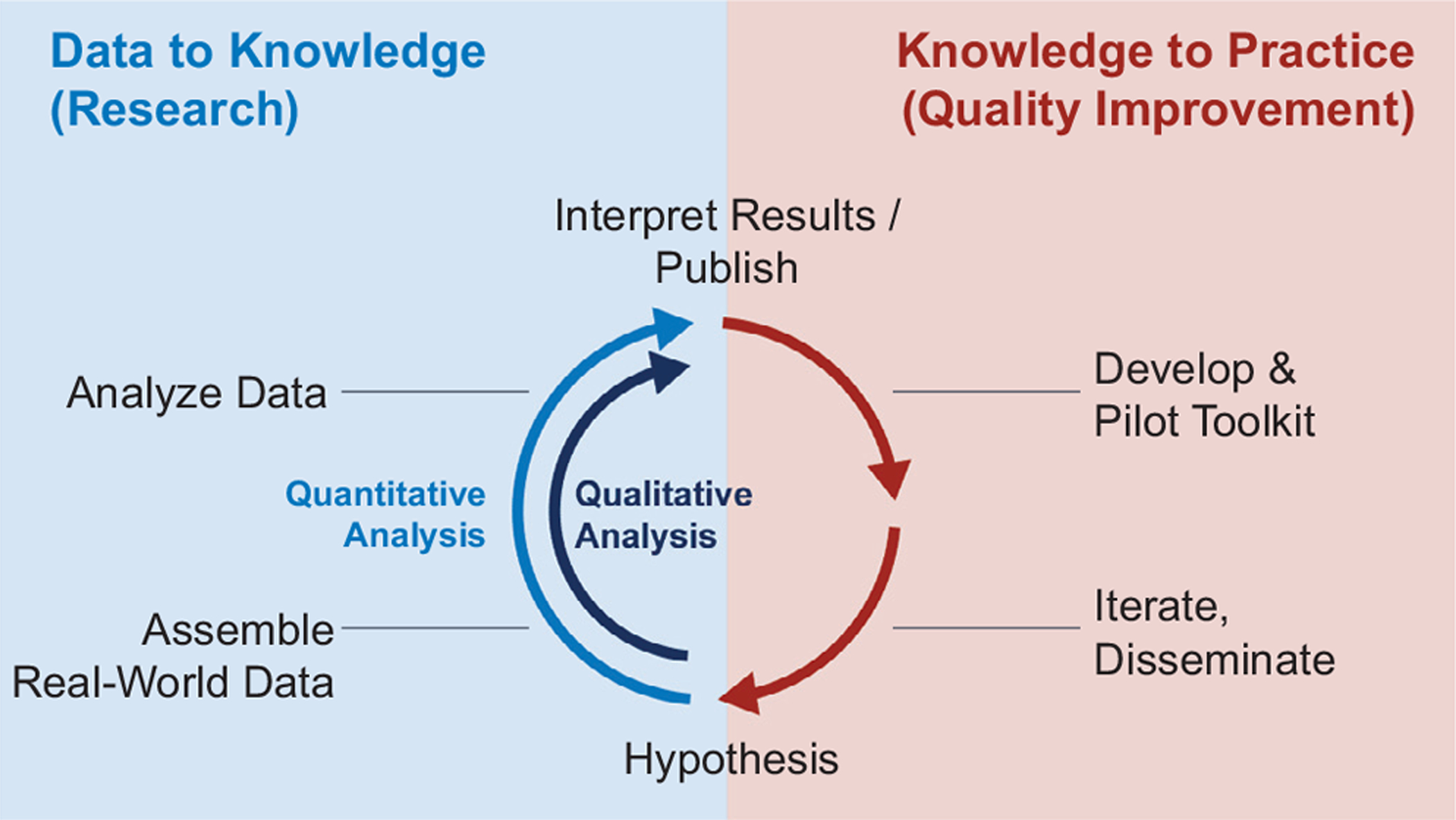
Learning health systems: conceptual framework.
The Multicenter Perioperative Outcomes Group as a Learning Health System
Within anesthesiology, one example of a multicenter learning health system driven by EHR data is the Multicenter Perioperative Outcomes Group (MPOG), a nonprofit consortium of more than 50 academic and community hospitals.11 Through the MPOG infrastructure, perioperative data are aggregated via automated extraction followed by clinician validation, including a preoperative anesthesia history and physical; intraoperative observations (eg, physiologic monitors, ventilator data); interventions (eg, airway documentation, medications, fluids/blood products, cardiopulmonary bypass start/stop); providers; laboratory values; and administrative and billing data.12 Highly structured, deidentified data are transmitted to a central repository on a monthly basis and are made accessible to researchers as regulated by a committee of representatives from all participating institutions and to quality improvement programs as governed by the MPOG Quality Improvement Committee. At selected MPOG institutions, perioperative EHR data are integrated with surgical registry data (eg, the Society of Thoracic Surgeons Adult Cardiac Surgery and General Thoracic Surgery databases and the American College of Surgeons National Surgical Quality Improvement Program), enabling associations to be studied between perioperative care processes available via MPOG and surgical outcomes available via surgical outcomes registries. Through systematic approaches to knowledge generation and dissemination as modeled by MPOG and other anesthesia-based research and quality improvement collaboratives,13,14 learning health systems within anesthesiology offer an evidenced-based framework for collaboration and improving perioperative care.
Methods for Enhancing the Use of EHR Data: The MPOG Approach
To handle issues of data interoperability, quality, scope, and access endemic to the EHR, clinicians and developers at MPOG offer 1 approach and present the following key considerations for using EHR data to improve care:
Development of universal EHR data concepts. In order for anesthesia practices (eg, airway management, medication administration) to be understood in a comparable way across institutions, “data mapping” utilities can be used to transform site- and vendor-specific EHR documentation into universal, semantically interoperable EHR data concepts. Data mapping requires an initial effort from clinically trained providers at each participating site, who are fluent in site-specific EHR documentation patterns, to map local EHR data concepts to universal, collaborative-adopted EHR data concepts.
Continuous assessments of data quality. After data mapping and before transfer to a centralized database, data from participating sites are assessed regularly for completeness and accuracy using “data diagnostics” tools. Through data visualizations, deficiencies across data category and time domains can be identified and communicated to clinically trained site representatives in order to improve EHR data documentation and mapping processes. For example, an abrupt decrease in the proportion of cases using endotracheal tubes or neuromuscular blockade reversal detected at a participating site, although potentially representing a fundamental change in practice patterns, also may represent a change in EHR documentation (eg, providers documenting airway management as a custom free-text entry rather than using predefined structured airway documentation) or EHR concept mapping (eg, use of a new neuromuscular blockade reversal agent [sugammadex] requiring new EHR data mapping).
Transformation of data into clinically meaningful measures. In many cases, deficiencies within individual EHR data concepts can be mitigated through computable “phenotypes,” or logical inferences drawn from multiple data sources. For example, a general anesthesia phenotype logically synthesizes data from neuromuscular blocker medication doses, expired volatile anesthetic, and airway interventions to accurately identify cases using general anesthesia. Once developed, phenotypes are reusable across projects and may catalyze research or quality improvement initiatives previously impeded by the time and effort required to curate EHR data into reliable, clinically meaningful measures.
Self-serve and intuitive access to EHR data. In many cases, clinical researchers and quality improvement teams lack the technical resources to rapidly access and analyze EHR data, even with departmental support and institutional review board approval. To overcome this hurdle, MPOG uses a self-serve EHR data browser tool, “DataDirect,” which provides a user-friendly graphical interface with data intuitively organized into pick-list categories (eg, medications, laboratory values, physiologic data). After approval by institutional EHR data stakeholders and the MPOG clinical research committee, providers are able to query and access EHR data in a secure computing environment, displayed in simple, tabular/spreadsheet form.
Methods for Practicing Anesthesiologists to Use Data to Drive Change
As noted by cardiologist and informatician, Homer Warner, health informatics is “10% medicine, 10% technology, and 80% sociology.” In many cases, even the most well-intended, evidence-based plans for implementation of new clinical practices are doomed to failure if a culture of change is not embraced within a particular group of healthcare providers. In high-stakes healthcare settings that demand high reliability such as with cardiothoracic and vascular anesthesia, changes to practice patterns are justifiably met with high scrutiny.
To overcome hurdles of clinical change, implementation science within anesthesiology can focus on creating a community of engaged providers sharing common goals. This can be facilitated by providing easily accessible performance feedback to participating sites in aggregate for comparison to peer sites and to individual providers for comparison with peers within a single institution. To enable reliable, accessible performance feedback, an approach adopted by MPOG has been to engage a national community of anesthesiologists through automated e-mails sent on a monthly basis summarizing care for an individual provider’s patients during the previous month. Summary e-mails describe performance metrics, comparing providers with peers at their institution. With one-click navigation, providers can review anesthetic records for cases with high performance and for cases for which an opportunity to improve may have existed or where evidence-based best clinical practices may not have been followed. Within MPOG, examples of such measures include transfusion management, lung protective ventilation, adherence to a postoperative transfer of care protocol, and avoidance of hyperglycemia/hypoglycemia and hypotension when clinically relevant.15
To facilitate a community of engaged clinical providers, one model adopted within Michigan is the Collaborative Quality Initiative (CQI).16 Funded by Blue Cross Blue Shield of Michigan, CQIs exist for 17 different medical specialties, including anesthesiology, and serve to create an environment for clinicians to share data and develop best practices around areas of care with high cost and high variation. CQI interactions occur (1) in person through site visits, quarterly statewide meetings, and annual retreats at national professional meetings; (2) through bimonthly web meetings to review quality measure development and discuss trends in performance; and (3) online through provider e-mails and a dedicated website with updates and a “BaseCamp” listserv. Through participation in statewide activities, anesthesiologists are able to share ideas in a supportive, nonpunitive environment and encourage sites to adopt best practices through structural changes to clinical workflow and provider education.
Future Directions
Moving forward, it is incumbent upon perioperative clinicians to become increasingly familiar with the opportunities and challenges afforded by large perioperative EHR databases. Not all processes conducive to excellent perioperative care are easily measurable, and not all measurements contained within EHR data are clinically meaningful or actionable. However, to the extent that perioperative EHR data provide new opportunities for generating clinical knowledge, a well-developed feedback loop—involving individual providers, quality improvement champions, and institutional stakeholders—is a critical counterpart for implementation of research findings. Collectively, perioperative clinicians must ensure that limitations inherent to EHR data do not lead to misinterpretation and incentivizing of misguided practices and must leverage opportunities present within EHR data to inform best practices and advance core missions of cardiothoracic and vascular anesthesia.
Acknowledgments
The authors gratefully acknowledge the work of Emily Smith (Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, MI) for her contributions to the graphic design of the figure used in this article.
Conflict of Interest
The study was supported in part by the by the National Heart, Lung, and Blood Institute, Grant K01-HL141701-02, Bethesda, MD.
MR Mathis reports grants from the US National Institute of Health (NHLBI, K01-HL141701-02) during the development of the study.
References
- 1.Deng F, Hickey JV. Anesthesia information management systems: An underutilized tool for Outcomes research. AANA J 2015;83:189–95. [PubMed] [Google Scholar]
- 2.Huen SC, Parikh CR. Predicting acute kidney injury after cardiac surgery: A systematic review. Ann Thorac Surg 2012;93:337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatib F, Jian Z, Buddi S, et al. Machine-learning algorithm to predict hypotension based on high-fidelity arterial pressure waveform analysis. Anesthesiology 2018;129:663–74. [DOI] [PubMed] [Google Scholar]
- 4.Kheterpal S, Shanks A, Tremper KK. Impact of a novel multiparameter decision support system on intraoperative processes of care and postoperative outcomes. Anesthesiology 2018;128:272–82. [DOI] [PubMed] [Google Scholar]
- 5.McCormick PJ, Yeoh C, Vicario-Feliciano RM, et al. Improved compliance with anesthesia quality measures after implementation of automated monthly feedback. J Oncol Pract 2019;15:e583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vassileva CM, Aranki S, Matthew Brennan J, et al. Evaluation of the Society of Thoracic Surgeons online risk calculator for assessment of risk in patients presenting for aortic valve replacement after prior coronary artery bypass graft: An analysis using the STS Adult Cardiac Surgery Database. Ann Thorac Surg 2015;100:2109–16. [DOI] [PubMed] [Google Scholar]
- 7.Cameron MJ, Tran DTT, Abboud J, et al. Prospective external validation of three preoperative risk scores for prediction of new onset atrial fibrillation after cardiac surgery. Anesth Analg 2018;126:33–8. [DOI] [PubMed] [Google Scholar]
- 8.Mathis MR, Duggal NM, Likosky DS, et al. Intraoperative mechanical ventilation and postoperative pulmonary complications after cardiac surgery. Anesthesiology 2019;131:1046–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson ME. Benchmarking anesthesiologists’ performance: Understanding factors that impact productivity. ASA Newsl 2016;80:40–2. [Google Scholar]
- 10.Marsolo K, Margolis PA, Forrest CB, et al. A digital architecture for a network-based learning health system: Integrating chronic care management, quality improvement, and research. EGEMS (Wash DC) 2015;3:1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Multicenter Perioperative Outcomes Group. Available at: http://www.mpog.org. Accessed September 29, 2019.
- 12.Freundlich RE, Ehrenfeld JM. Perioperative information systems: Opportunities to improve delivery of care and clinical outcomes in cardiac and vascular surgery. J Cardiothorac Vasc Anesth 2018; 32:1458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannenberg AA, Warner MA. The registry imperative. Anesthesiology 2009;111:687–9. [DOI] [PubMed] [Google Scholar]
- 14.Whitlock EL, Feigner JR, Chen L-L. Perioperative mortality, 2010 to 2014: A retrospective cohort study using the National Anesthesia Clinical Outcomes Registry. Anesthesiology 2015;123:1312–21. [DOI] [PubMed] [Google Scholar]
- 15.Multicenter Perioperative Outcomes Group. Quality: our measures. Available at: https://mpog.org/quality/our-measures/. Accessed October 9, 2019.
- 16.Share DA, Campbell DA, Birkmeyer N, et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff 2011;30:636–45. [DOI] [PubMed] [Google Scholar]


