Abstract
Inflammatory bowel diseases (IBDs), including Crohn’s disease and ulcerative colitis, are chronic, progressive, immune-mediated diseases of adults and children that have no cure. IBD can cause significant morbidity and lead to complications such as strictures, fistulas, infections, and cancer. In children, IBD can also result in growth impairment and pubertal delays. IBD is highly heterogenous, with severity ranging from mild to severe and symptoms ranging from mild to debilitating. Delay in IBD diagnosis, especially in Crohn’s disease, is common and associated with adverse outcomes. Early diagnosis and prompt institution of treatment are the cornerstones for improving outcomes and maximizing health. Early diagnosis requires a low threshold of suspicion and red flags to guide early specialist referral at the primary provider level. Although the armamentarium of IBD medications is growing, many patients will not respond to treatment, and the selection of first-line therapy is critical. Risk stratification of disease severity, based on clinical, demographic, and serologic markers, can help guide selection of first-line therapy. Clinical decision support tools, genomics, and other biomarkers of response to therapy and risk of adverse events are the future of personalized medicine. After starting appropriate therapy, it is important to confirm remission using objective end points (treat to target) with continued control of inflammation with adjustment of therapy using surrogate biomarkers (tight control). Lastly, IBD therapy extends far beyond medications, and other aspects of the overall health and wellbeing of the patient are critical. These include preventive health, nutrition, and psychobehavioral support addressing patients’ concerns around complementary therapy and medication adherence, prevention of disability, and ensuring open communication.
Keywords: Inflammatory Bowel Disease, Crohn’s Disease, Ulcerative Colitis, Early Diagnosis, Early Therapy, Personalized Therapy, Adult, Pediatric
Inflammatory bowel diseases (IBDs), including the main subtypes Crohn’s disease (CD) and ulcerative colitis (UC), are chronic, progressive, immune-mediated diseases of the intestinal tract that have no cure.1,2 IBDs are associated with significant morbidity, disability, and risk of complications.3,4 Although they can occur at any age, IBDs are most common among adolescents and young adults. In developed counties, IBD incidence, although stable at approximately 1%, is higher than mortality, leading to compounding prevalence and rising burden of IBDs.5 In developing countries, IBD incidence and prevalence are also on the rise, especially in the last few decades.6 Direct and indirect costs of IBD are substantial. Within the United States, direct health care costs among IBD patients are 3 times as high compared as those among non-IBD patients.7,8
Early management of IBD, including both diagnosis and treatment, are critical to improve quality of life and prevent complications. This review summarizes the existing literature on, and proposes a framework for how to best manage, CD and UC early in the disease course in adult and pediatric patients.
Early Diagnosis and Treatment: Gateways for Improved Prognosis in Inflammatory Bowel Diseases
Inflammatory Bowel Diseases Are Progressive Diseases
The natural history of both CD and UC can vary from mild disease with few symptoms to complicated disease with strictures and fistulas. In a French population-based study of incident CD, the cumulative probability of perianal CD varied between 11% and 19% at 1–10 years after diagnosis.9 In a study of 983 patients with CD in Asia, stricturing or penetrating CD occurred in 41% and perianal disease in 25% of patients.10 At the other end of the spectrum, incidental terminal ileitis can be diagnosed in 1.6% of individuals undergoing nondiagnostic colonoscopy, with an uncertain but likely low rate of progression to overt CD.11 With respect to UC, most patients have mild to moderate severity and 10%–15% of patients can experience a severe course.12 In a population-based cohort study, proctosigmoid location of colitis occurred in 73% of patients; of these, disease extension occurred in 23% of patients at 7 years of follow-up and it was a marker of worse prognosis.13 The risk of surgery for CD and UC can be up to 46.6% and 15.6% 10 years after diagnosis, respectively, and has decreased significantly during the last 6 decades.14
The symptoms associated with IBD can be waxing and waning, but the underlying systemic inflammation can lead to progressive, cumulative, and often irreversible intestinal damage and risk of complications if not treated adequately.15 Complications associated with ongoing inflammation in CD include strictures, obstructions, fistulas, abscesses, and surgery,3,16 and those associated with UC include loss of colonic and anorectal function, surgery, and colorectal cancer.2,4,17 Other complications include anemia, nutritional deficiencies, loss of bone density, and progressive loss of quality of life. In children, persistent inflammation is associated with growth impairment, risking permanent loss of height.18 Similar to other chronic diseases, such as rheumatoid arthritis, the concept of cumulative damage is now acknowledged in IBD and can be measured using validated tools, such as the Lemann Index.19
Limited Correlations Among Inflammation, Symptoms, and Complications
The concordance between intestinal inflammation and symptoms can be limited, especially in CD.20,21 In a population-based cohort, more than 20% of CD patients were found to have strictures and penetrating disease at the time of diagnosis, suggesting that clinically silent inflammation may precede formal diagnosis.22 The STRIDE (Selecting Therapeutic Targets in Inflammatory Bowel Disease) recommendations, updated recently, are meant to target endoscopic healing, minimize disability, and restore quality of life and adequate growth in children, in addition to control of symptoms.23 Therefore, rather than a gradual “step-up” approach, in which treatment adjustment is based on the severity of symptoms, the recommended treat-to-target approach aims to achieve endoscopic healing and control of inflammation and involves early, aggressive treatment in appropriate patients.23
Delay in Diagnosis Leads to Adverse Outcomes
Delay in diagnosis is more common in CD than UC (median delay, 7.6 months; interquartile range [IQR], 3.1–15.0 months and 3.3 months; IQR, 1.9–7.3 months, respectively; P < .001).24 Data from the Swiss IBD cohort demonstrate that CD diagnosis can be delayed in pediatric and adult patients by 3 months (IQR, 1–9 months) and 6 months (IQR, 1–24 months), respectively. Adults were more likely to present with strictures (P < .001) and require bowel surgery (P < .001). Furthermore, the duration of diagnostic delay has been associated with complications such as strictures and internal fistulae.25 In a study, of adult CD patients in France, delay in diagnosis longer than 13 months was associated with higher risk of a major surgery (P = .05).26
Evidence of Disease Activity Before Diagnosis
There is a significant increase in gastrointestinal symptoms before IBD diagnosis (9.6% and 10.4% 5 years before CD and UC diagnosis, respectively, compared with 5.8% of controls).27 In addition, patients who were diagnosed with irritable bowel syndrome or depression were less likely to receive timely specialist referral (irritable bowel syndrome: hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.60–0.99, depression: HR, 0.77, 95% CI, 0.60–0.98), suggesting that delays in IBD diagnosis may be related to focusing on comorbid or inaccurate diagnoses.27 In a population-based study using data from the Danish registry, Vadstrup et al28 found a significant increase in costs associated with health care services, prescription medicine, home care services, and labor productivity loss in the 10 years before IBD diagnosis.
Growth Impairment and Pubertal Delays in Children
IBD and ongoing inflammation can lead to impairment of growth and pubertal development to children. Impaired linear growth may be the only presenting symptom of IBD in pediatric patients.29,30 Growth impairment is multifactorial in nature, representing the summation of nutritional deficiencies, altered eating habits, inflammatory cytokines, and corticosteroid use. Marked growth impairment at diagnosis, defined as a height z-score < −2.5, is a poor prognostic sign.31 Furthermore, IBD patients exhibit “catch up” growth beyond the typically expected age for growth plate closure (median additional years to reach adult height: male patients with CD, 1.9 years and male patients with UC, 2.5 years; female patients with CD: 2.6 years and female patients with UC, 2.8 years), suggesting that achieving remission even after reaching adolescence may allow for maximal growth potential.18
Early Management Can Alter Disease Course and Prevent Complications
Data have consistently demonstrated that long-term IBD complications are mitigated by early treatment and remission. In the long-term follow-up data from the CALM trial, patients with early CD who achieved deep remission, compared to those who did not, had a significantly lower risk of new fistulas, abscesses, hospitalization, or surgery for CD (adjusted HR, 0.19; 95% CI, 0.07–0.31) during a median 3 years’ follow-up.32 Other CD studies have similarly demonstrated that early treatment is independently associated with improved long-term outcomes.33 Data on the impact of early therapy in UC are more limited, but, as UC is also a progressive disease with risk of colorectal cancer, surgery, and loss of colonic function, it is reasonable to treat early to mitigate these risks.
How to Diagnose Inflammatory Bowel Disease Early
IBD is heterogenous with wide variation in presentation. Disease activity at presentation can range from mild to severe and, as discussed, symptoms may be mild to none. These can make the IBD diagnosis challenging, especially at a primary care level. Danese et al34 have proposed a Red Flags Index consisting of a 21-item questionnaire to help providers triage and identify patients with concerning symptoms in a timely manner and refer to specialists appropriately. A useful adjunct to symptom-based criteria is fecal calprotectin (FC), a stool marker of inflammation; in a large meta-analysis, FC ≤ 40 μg/g carried a ≤1% probability of IBD, effectively excluding the diagnosis of IBD.35 Similarly, in the Red Flags Index validation study, combining the index with FC (Red Flags Index ≥8 and/or FC >250 μg/g) increased the positive predictive value from 4% to 21% and negative predictive value from 97% to 100%.36 Diagnostic criteria for IBD involve a composite of clinical symptoms, endoscopic, biomarker, and cross-sectional imaging features, which are beyond the scope of this review. We refer readers to the excellent guidelines laid out by the American College of Gastroenterology, European Crohn’s and Colitis Organization, and British Society of Gastroenterology.37–40
Risk Stratification and Prognostication at Diagnosis
Appropriately risk-stratifying prognosis in patients recently diagnosed with IBD is essential to inform selection of the most appropriate treatment and monitoring strategies. CD and UC phenotype and disease activity at initial presentation can vary widely from limited and mild to extensive and complicated. Certain baseline clinical features are associated with a more aggressive disease course with higher risk of progression and complications. In higher-risk patients, early control of inflammation with immune-modifying therapies is critical. However, immunosuppressive therapies are associated with safety concerns in the short- and long-term, and entail significant health care costs. Therefore, a consideration of risks, benefits, and alternatives is key to develop an individualized therapeutic plan for the informed patient.
It is important to differentiate between disease activity and severity. Although the former refers to the burden of inflammation at any given point in time, the latter takes into account the disease phenotype and course, and is helpful in determining prognosis and predicting complications (Figure 1). For example, a patient with low disease activity (little to no symptoms and low inflammatory markers) may actually have high severity (due to disease history or behavior) increasing the risk for disease progression, and should be managed more proactively and aggressively. We will review predictors of IBD severity and activity.
Figure 1.

Inflammatory bowel disease severity vs disease activity variables. ASCA, anti-Saccharomyces cerevisiae antibodies; ANCA, anti-neutrophil cytoplasmic antibodies; GMCSF, granulocyte-macrophage colony-stimulating factor; TI, terminal ileum.
Age of Inflammatory Bowel Disease Diagnosis
Numerous studies suggest that younger age at diagnosis is tied to adverse outcomes in both CD and UC.41 In CD, being diagnosed before the age of 40 years is associated with an increased risk (odds ratio [OR], 2.1; 95% CI, 1.3–3.6) of disabling disease 5 years after diagnosis, including higher rates of surgery, hospitalization, steroid dependence, and disease recurrence.42,43 In UC, younger age at diagnosis is linked to more frequent relapses, colectomy, and colorectal cancer.44,45 There are conflicting data on the disease course in the very-early-onset IBD population of patients diagnosed before age 6 years, with studies reporting both similar and worse outcomes in very-early-onset IBD compared to the general pediatric population.46 Similar to the youngest patients with IBD, elderly-onset IBD (diagnosed at 60–65 years of age) has conflicting evidence on prognosis, with 1 study reporting fewer hospitalizations and less frequent requirement of immunosuppression in patients diagnosed after age 60 years,47 and a meta-analysis suggested that, although elderly IBD presents less commonly with complications, there are similar or higher rates of surgery compared to a nonelderly population.48 It is being increasingly recognized that frailty, rather than chronologic age, may be the driver of adverse outcomes in this group.49,50
Other Demographic Variables
There are significant differences in IBD phenotype and outcomes based on race and ethnicity, likely due to a multitude of factors, both social and biologic. Emergency department use, hospitalization, complicated disease course, and IBD-related disability are more common in minority and lower socioeconomic status groups.51,52 In a study of 770 patients with IBD, South Asian immigrants living in the United Kingdom were more likely to have extensive UC and colonic CD compared with their native counterparts,53 which might be related, in part, to limited access to care.54 Genes implicated in IBD risk also differ in non-White compared with White patients with IBD.55 Being aware of the higher vulnerability to adverse IBD-related outcomes in these vulnerable groups should prompt careful monitoring to limit adverse outcomes.
There are increasing data on sex-based differences in IBD phenotype and outcomes, which may signal differences in pathogenic pathways and progression. Of note, extraintestinal manifestations (EIMs) consistently tend to be more common in female patients (CD: OR, 2.3; 95% CI, 1.9–2.8 and UC: OR, 1.5; 95% CI, 1.1–2.3, in a large Dutch Bio-Bank).56 Similarly, in a retrospective pediatric study of nearly 1000 patients with CD, girls were more likely to have EIMs and less likely to have growth impairment compared with boys,57 the latter is possibly related to lower insulin-like growth factor-1 level in boys.58
Disease Phenotype and Clinical Characteristics
The International Organization for the Study of Inflammatory Bowel Disease and the American Gastroenterological Association (AGA) provide a framework for categorization of CD and UC severity into mild-, moderate-, and high-risk groups based on phenotype and other characteristics.59,60
In CD, the presence of large or deep mucosal lesions on endoscopy or magnetic resonance imaging (MRI), history of a fistula, abscess, or intestinal resection are predictors of worse outcomes.59,60 In a prospective population-based inception cohort of 213 patients with CD, penetrating behavior was associated with a higher risk of progression to perianal disease (HR, 5.65; 95% CI, 2.65–12.03) and was a risk factor for resection (HR, 3.92; 95% CI, 1.86–8.67) and hospitalization (HR, 1.01; 95% CI, 1.00–1.01).16 Ileal and upper gastrointestinal disease location and extensive disease are also markers of severe disease.61 Cigarette smoking is associated with complications and need for therapy escalation.61
In CD, the threshold of suspicion should be low for high-quality imaging of the pelvis, such as MRI, to rule our perianal CD. In a retrospective study of 136 pediatric CD patients, presence of anal fissures and skin tags, non-White race, and elevated C-reactive protein (CRP) were risk factors for perianal CD.62 In another study of 274 patients in China with recently diagnosed CD, all of whom underwent MRI pelvis, asymptomatic perianal fistulas were diagnosed in 17.5% of patients and colonic location of CD was a risk factor for asymptomatic perianal fistulas.63
In UC, corresponding predictors of aggressive disease advised by the International Organization for the Study of Inflammatory Bowel Disease include active colonic ulcers and prior use of biologics.59 Extensive colitis, deep ulcers, need for corticosteroids, hospitalization, Clostridium difficile, and cytomegalovirus infection also indicate higher colectomy risk.64 Of note, disease extension from limited disease to extensive or pancolitis is associated with worse prognosis.45,65
In addition, co-occurrence of other immune-mediated inflammatory diseases can occur, most commonly with psoriasis and asthma, but also, in more rare instances, with other gastrointestinal diseases (eg, celiac, nonalcoholic fatty liver disease,66 and eosinophilic esophagitis).67,68 Presence of a concomitant immune-mediated inflammatory disease is associated with worse outcomes. In a systematic review of 93 studies, the risk of extensive or pancolitis and IBD-related surgery was higher in IBD patients with immune-mediated inflammatory diseases (risk ratio, 1.38; 95% Cl, 1.25–1.52; P < .01; I2 = 86% and risk ratio, 1.17; 95% Cl, 1.01–1.36; P = .03; I2 = 85%, respectively).69
Serologic Biomarkers
Of routinely available serologic biomarkers, CRP, an interleukin-6–dependent acute-phase reactant, is correlated with inflammatory burden, albeit nonspecifically, and is used for IBD risk stratification, with higher CRP (≥5 mg/dL) consistent with moderate to severe disease.59,70 In addition, commercially available IBD serologic markers can help with prognostication. These include perinuclear antineutrophil antibody and several antimicrobial antibodies, notably anti-Saccharomyces cerevisiae antibody, antibody to Escherichia coli outer-membrane porin C, and antibody to flagellin (CBir1). In the Pediatric RISK Stratification study, a large prospective study of CD, patients positive for 2 or more serologic markers (anti-Saccharomyces cerevisiae antibody, outer-membrane porin C, and/or CBir1) progressed to a penetrating or stricturing complication more quickly than those with only 1 serologic marker, and those receiving anti-TNF in this cohort had a reduced likelihood of progression to penetrating complications, indicating a window to change disease complication.41 Another autoantibody of interest is that to granulocyte-macrophage colony-stimulating factor, of which high expression has been associated with stricturing and penetrating behavior in adult and pediatric CD.71,72
Fecal Calprotectin
Stool markers of inflammation, of which FC is the most widely used, are more specific for bowel inflammation compared to serum markers.73 FC is limited by patients’ reticence to collect stool and lack of specificity for IBD, but it remains an important tool for stratifying newly diagnosed patients with IBD for risk of progression and response to first therapy. Practically, it is important to discuss the collection with patients, high-lighting that the sample can be from any time, only a small amount of stool is necessary for the analysis and, given instability at room temperature leading to falsely low values, it should be remitted to the laboratory immediately or placed in the refrigerator,74 not the freezer, for the most accurate results. If the result is unexpectedly high, it is appropriate to repeat based on clinical judgment, as there can be variability among measurements within the same individual even on the same day.75 Lastly, there is variability between assays and it is important to use assay-specific cutoff values until there is standardization.76
FC has been shown to accurately discriminate between endoscopic disease severity in both UC73 and CD.77 Kennedy et al78 demonstrated that an elevated FC (>250 μg/g) at index visit for CD was associated with increased rates of disease progression. More recently, the same group reported that achieving an FC <250 μg/g within 12 months of diagnosis was associated with a reduction in the risk of disease progression.79
Genetic Risk Predictors
There are more than 200 identified risk loci for IBD.55,80 IBD susceptibility mutations in nucleotide-binding oligomerization domain 2 (NOD2), which is involved in the host–microbe immune response, were the first identified and confer the greatest risk for IBD.81,82 In a large genotype–phenotype study of CD, NOD2 was noted to be strongly tied to ileal disease location and younger age at diagnosis and, when accounting for ileal disease location, an association with stricturing disease no longer remained.83
Polygenic risk scores (a summation of risk alleles weighted by effect size derived from genome-wide association studies) have been studied, but the methods used to create the scores vary widely. Within the RISK cohort, neither a polygenic risk score nor NOD2 were associated with stricturing or fistulizing behavior.41 As more expansive, genome-wide polygenic risk scores improve and evolve with the help of more sophisticated techniques, they continue to warrant further exploration as a prognostic test.84
Risk Prediction Tools
Risk prediction models are being incorporated consistently across diseases toward risk stratification and shared decision-making at the individual level. For CD patients, Siegel et al85 developed and validated a web-based prediction tool (PROSPECT) to individualize risk of complications using clinical, serologic, and genetic markers. Variables in the prediction tool included the presence of small bowel disease (HR, 2.12; 95% CI, 1.05–4.29), left colonic disease (HR, 0.73; 95% CI, 0.49–1.09), perianal disease (HR, 4.12; 95% CI, 1.01–16.88), anti-Saccharomyces cerevisiae antibody (HR, 1.35; 95% CI, 1.16–1.58), anti-CBir1 (HR, 1.29; 95% CI, 1.07–1.55), anti-neutrophil cytoplasmic antibody (HR, 0.77; 95% CI, 0.62–0.95), and the NOD2 frameshift mutation/SNP13 (HR, 2.13; 95% CI, 1.33–3.40), with a predictive accuracy of 73% and 75% in adults and children, respectively. This is expected to be available for clinical use in early 2021.
Clinical risk features (disease severity), as well as degree of intestinal inflammation (disease activity), can be very useful to stratify outcomes of IBD and guide personalized therapy. Newer biomarkers, serologic and genetic, will help improve the precision of IBD therapy.
Early Therapy of Inflammatory Bowel Disease
Risk stratification is a key element of determining the initial selection of IBD therapy. Medication safety profile, patient preference, and payer preference are other important variables to consider. For the purpose of this discussion, we risk-stratify patients into mild, moderate, or severe disease based on guidance laid out by the AGA (Figure 2)60,64 and illustrate therapeutic options for CD and UC in Figures 3 and 4, respectively.
Figure 2.

Stratification of inflammatory bowel disease risk of progression based on disease severity and activity. BMI, body mass index; CDAI, Crohn’s Disease Activity Index; ESR, erythrocyte sedimentation rate; UCEIS, Ulcerative Colitis Endoscopic Index of Severity. *Refer to Figure 1. **From Torres et a1,1 adapted with permission. ***From Rubin et al,38 adapted with permission.
Figure 3.
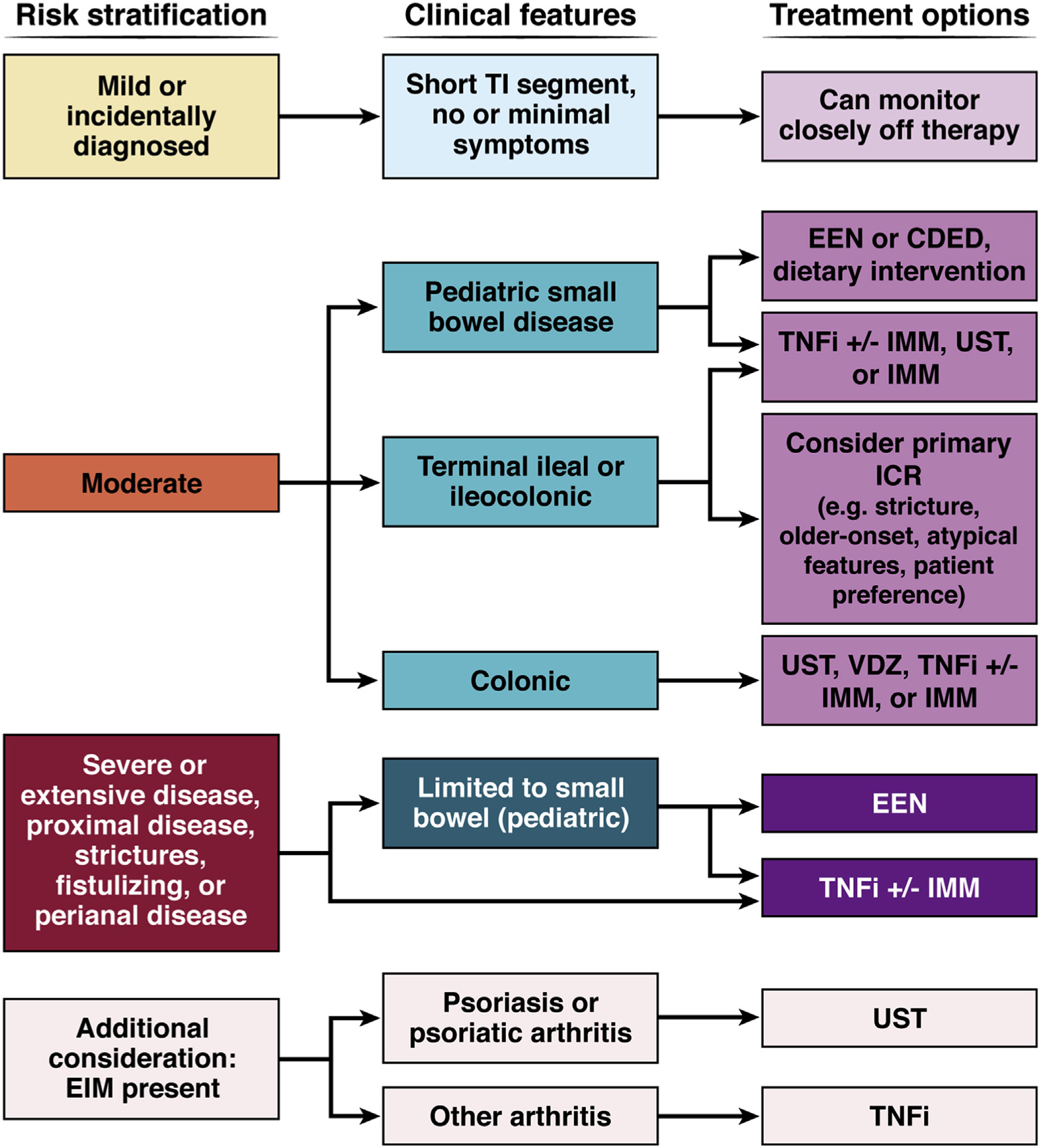
Therapeutic strategies for the management of recently diagnosed CD. IMMs include thiopurines and methotrexate. CDED, Crohn’s disease exclusion diet; TI, terminal ileum.
Figure 4.

Therapeutic strategies for the management of recently-diagnosed ulcerative colitis. IMMs include thiopurines and methotrexate. 5-ASA, 5-amino salicylic acid; ASUC, acute severe ulcerative colitis; IPAA, ileal pouch anal anastomosis.
Early Therapy for Crohn’s Disease
Medical therapy.
There is good evidence-based guidance for the management of moderate to severe CD, but the treatment of mild CD is less clear in the absence of clinical trials devoted to this group of patients. In a small minority of cases that have mild inflammatory CD with mild endoscopic activity, no symptoms and no evidence of stricturing or fistulizing, the risk of progression is likely to be low.86 It may be reasonable to monitor such patients off therapy with close follow-up and frequent symptom reassessment and monitoring of FC and CRP. If patients have mild disease activity, then an 8-week course of budesonide can be tried and patients can be monitored off therapy after stopping steroids. Immunomodulators (IMMs) may be a consideration in moderate disease nonresponsive to budesonide; however, long-term safety concerns limit this approach.60
Due to lack of head-to-head trials, biologic positioning is reliant on indirect data, such as network meta-analyses and real-world retrospective studies. In the former, the surface under the cumulative ranking (SUCRA), a measure of the relative ranking of each treatment, is used for comparisons. SUCRA ranges from 0 to 1, with a higher number indicating higher ranking. For the management of early moderate to severe CD, tumor necrosis factor inhibitors (TNFis) remain the mainstay of treatment. In a network meta-analysis of randomized controlled trial data,87 infliximab (IFX) and adalimumab (ADA) were superior to other therapies for moderate CD (SUCRA 0.93 and 0.75, respectively). Of note, biosimilars are approved as inexpensive alternatives to IFX.88 Ustekinumab (UST) was associated with a lower risk of serious adverse events and infections (SUCRA 0.72 and 0.71, respectively), and may be a consideration among risk-averse patients. In moderate CD, especially when limited to the colon and no high-risk features, vedolizumab (VDZ) and UST are efficacious first-line options with good safety profiles. In addition, although response to VDZ is lower among TNFi exposed, biologic-naïve patients have higher rates of response. Results from ongoing head-to-head trials such as UST vs adalimumab (ClinicalTrials.gov ID: NCT03464136) and risankizumab vs UST (ClinicalTrials.gov ID: NCT04524611) will help clarify the positioning of biologics.
In the case of severe CD, or when particularly high-risk features, such as fistulizing or stricturing disease, or proximal involvement, a TNFi, particularly IFX, remains the first choice. Of note, there is no benefit of concomitant mesalamine with biologic therapy in CD and it should be stopped at the time of escalation to a biologic.89
Combination therapy of a biologic with IMM, best-studied with TNFi, is an important consideration in severe disease, especially penetrating or perianal disease. It is associated with improved outcomes, a decrease in loss of TNFi response, and fewer long-term complications.90,91 The mechanism of action may be due to decrease in immunogenicity and impact on drug level. Similar effects have not been seen with adding IMM to UST of VDZ therapy, possibly due to a lower role of immunogenicity in drug metabolism.92 For patients with perianal CD, a multidisciplinary approach with early referral to a colorectal surgeon is key to improved outcomes.93
Ileocolic resection as a first-line therapy.
Surgery is often viewed as a late-stage therapeutic option for CD, to be positioned after medical therapies, and the rate of surgery for CD has declined over time.94 The LIR!C (Laparoscopic Ileocolic Resection Versus Infliximab Treatment of Distal Ileitis in Crohn’s Disease) trial, a randomized controlled trial of IFX vs laparoscopic ileocolic resection (ICR) in adult patients with limited terminal ileal disease, found no difference in safety or measures of quality of life at 1 year between the 2 groups.95 Furthermore, on long-term follow-up (median 5 years), the ICR group had no subsequent resection, 42% received no treatment at all, and 74% received no biologic treatment.96 More recent cost-effectiveness analyses found that ICR is more cost-effective than IFX.97 ICR should be considered a viable initial treatment modality not only in patients presenting with a complication at diagnosis, but also in those with limited nonpenetrating ileocecal CD in the setting of shared decision-making or when concerns about biologic safety are a significant consideration.
Dietary therapy.
Exclusive enteral nutrition (EEN) is the use of polymeric or elemental formula typically for 8–12 weeks to induce remission in CD. European Pediatric Consensus guidelines recommend EEN as the first-line therapy to induce remission in pediatric patients with luminal CD.31 This is based on numerous randomized controlled trials demonstrating that EEN is as effective as corticosteroids. Meta-analyses of these randomized controlled trials have reported clinical remission rates of 73%–95%.98,99 Relapse after this period of induction is common, typically leading to maintenance management with IMM or biologics.100 To increase adherence, a combination of partial enteral nutrition and the CD exclusion diet may be more tolerable and as effective in inducing remission in mild to moderate pediatric CD.101 Studies on EEN in adults are limited and have shown mixed results but warrant consideration in select cases.102
The modification of whole foods in diet as a therapeutic strategy in IBD is an emerging area of interest, such as the Trial of Specific Carbohydrate and Mediterranean Diets to Induce Remission of CD (DINE-CD).103 Dietary studies are highly heterogenous due to the relevance of macro- and micronutrients in diet, as well as food additives, processing, and packaging.104 Currently, there is no recommendation for whole foods–based management of IBD, but future studies will delineate this further.
Early Therapy of Ulcerative Colitis
In UC, mild disease can be managed with oral and/or topical mesalamine therapy, generally with adequate control of disease.38 For moderate UC, VDZ and UST are effective options,105,106 and may be better first-choice options than TNFi, given safety profile. In the VARSITY trial, the only head-to-head trial of 2 biologics (n = 769), VDZ was superior to ADA in achieving clinical remission (31.3% vs 22.5%; P = .006) and endoscopic improvement (39.7% vs 27.7%; P < .001) in moderate to severe UC.105 In case VDZ or UST may not be feasible due to payer preference, TNFi, particularly IFX, is an effective option with good safety profile.107 Thiopurines may be considered in moderate UC after weighing risks against benefits.64 In patients with severe UC requiring hospitalization, IFX is the preferred biologic for induction and maintenance of remission, with or without IMM. Combination therapy in the SUCCESS trial was associated with improved clinical, but not endoscopic, outcomes compared to IFX monotherapy.108 When using IFX as a monotherapy in moderate to severe UC, given the potential for the colon to act as a “sink” for drugs, we advocate for checking early drug concentrations to ensure proper dosing and detecting immunogenicity early along with other pharmacokinetic and safety-related variables.109 There is no clear benefit of concomitant mesalamine with biologic therapy in UC and it can be stopped in patients escalating to biologics.110 Some patients may present with acute severe UC where TNFi, cyclosporine, or subtotal colectomy followed by ileal pouch anal anastomosis can be a good initial strategy; discussion of management of acute severe UC is beyond the scope of this article.
Taking Extraintestinal Manifestation Into Consideration
EIMs are common in IBD, estimated to affect 30%–40% of patients.111,112 In a Swiss cohort study, symptoms of EIMs before IBD in one-quarter of the patients113; collaboration across specialties, including but not limited to rheumatologists, dermatologists, ophthalmologists, is vital not only to prompt referral of patients with EIM suspicious for IBD to allow for expedient initiation of therapy for both conditions, but also to allow for consideration of both in the selection of therapy, dose, and chronicity of treatment. For example, VDZ, which is gut-selective, is associated with an increased risk of de novo or worsening EIM.114,115 In patients with significant symptoms of EIM, one should weigh the potential benefit of VDZ against another therapy, such as TNFi, that might more broadly target gut and systemic inflammation.116
Predictors of Response to Inflammatory Bowel Disease Therapy
The number of therapeutic agents in the IBD armamentarium are numbered, and each agent has limited efficacy. In addition, the first biologic, regardless of choice, has the highest probability of success. For all of these reasons, it is important to be thoughtful in selecting the initial therapy. Clinical decision support tools (CDSTs) and prediction models are being developed to help with this.
Smoking has been associated with lower response to TNFi, and among responders, shorter response duration.117 Using ACT1 and ACT2 trial data, Vande Casteele et al118 developed a CDST incorporating IFX clearance, stool frequency, rectal bleeding scores, white blood cell count, and body weight to predict endoscopic healing in UC at different time points, with accuracy in the range of 80% or higher. External validation models, however, had lower predictive value, with an area under of the curve of 0.67 (95% CI, 0.61–0.74). Similar CDSTs have been developed to predict response to CD and UC with VDZ.119,120 Such tools require clinical variables that are widely available, have no associated costs, and are feasible to incorporate in discussions with the patient. Ultimately, biomarkers that are specific to underlying mechanisms of action may be most accurate in helping select the right therapy for the patient. Although these CDSTs and predictors show promise, none have been prospectively validated and are not yet ready for full incorporation into routine clinical care.
Predictors of Adverse Events With Inflammatory Bowel Disease Therapy
Genetic heterogeneity pertaining to drug metabolism impacts adverse events and can be leveraged to guide therapy. Thiopurine methyltransferase (TPMT), the first identified variant impacting IBD therapy, is involved in thiopurine metabolism with decreased activity resulting in severe leukopenia. Thiopurines should be avoided or the dose lowered based on genotype and the associated TPMT activity.121 Nudix hydrolase (NUDT15) mutations have been implicated in leukopenia risk with thiopurine in European and Asian populations.122,123 A combination of TPMT and NUDT15 mutations accounts for nearly 50% of cases with severe thiopurine-induced leukopenia.123 Testing for TPMT genotype or enzyme level and NUDT15 genotype before initiating thiopurine therapy is recommended. In addition, polymorphism in the HLA class II region (rs2647087) is associated with pancreatitis risk in thiopurine-treated individuals, 9% risk in heterozygotes and 17% risk in homozygotes.124
Beyond IMMs, there is promise in using genetic variants to further aid in identification of adverse events with biologic therapies. Most notably, in a genome-wide association study of 1240 biologic-naïve, primarily European patients, the Personalising Anti-TNF Therapy in Crohn’s disease (PANTS) Consortium found that the allele HLA-DQA1*05 significantly increased the risk of anti-drug antibody development to TNFi drugs (HR, 1.90; 95% CI, 1.60–2.25).125 Additional analyses have revealed that differences in haplotypes are associated with differences in immunogenicity to IFX vs ADA.126 More recently, enrichment in a polymorphism in TNF-α (rs1799964) was found in patients with paradoxical psoriasis with TNFi compared to those without in a small cohort of 53 patients with IBD.127 Further study to validate these findings is needed, but genetic variants may have an expanded role in future selection of early therapies.
Treat-to-Target Strategy
Choice of initial therapy should be accompanied by a plan for a treat-to-target strategy with decisions made about how response to therapy will be assessed, how therapy will be optimized, and long-term monitoring strategies to be undertaken after achieving target goals to support continued tight control.
Selecting the Target
The STRIDE program, initiated by the International Organization for the Study of Inflammatory Bowel Disease, published a consensus statement on treat-to-target strategies with the primary therapeutic goal being clinical and endoscopic remission.128 This was recently updated to include normalization of biomarkers as a short-term target following the landmark CALM trial.23 CALM, a randomized controlled trial of 244 patients with recently diagnosed moderate to severe CD treated with TNFi early in their disease course, showed a treat-to-target strategy of escalating TNFi and thiopurine therapy based on clinical symptoms and biomarker normalization resulted in more patients achieving the primary end point (mucosal healing [Crohn’s Disease Endoscopic Index of Severity <4] with absence of deep ulcers) at week 48 compared to using clinical symptoms alone (adjusted risk difference, 16.1%; 95% CI, 3.9–28.3; P = .010).129 The long-term extension of CALM has further demonstrated that attainment of target goals of either endoscopic or deep remission by week 48 led to reduction in progression of disease during a median 3 years’ follow-up.32
Given the issue of nonadherence with collecting a stool sample to measure FC, there is increasing interest in identifying serum-based biomarkers as therapeutics targets. A serologic panel of 13 protein markers, the Endoscopic Healing Index (Monitr, Prometheus Biosciences, San Diego, CA), validated in CD to distinguish endoscopic activity, is comparable to FC and likely superior to CRP alone.130 Another modality of interest is point-of-care intestinal ultrasound, which allows for noninvasive and inexpensive evaluation of variables correlated with disease activity (bowel wall thickness, Doppler signal, and wall layer stratification) and has been used to measure initial response to therapy,131 as well as ongoing assessment of disease activity in both CD and UC.132,133 MRI is a reasonable alternative monitoring modality, with 2 small studies reporting the ability of MRI to accurately discriminate mucosal healing based on cut points of Ma-RIA and Nancy scores.134,135
We recommend identifying an appropriate target, depending on disease characteristics, availability of resources and patient preference, and treating to achieve normalization of the target, followed by periodic reassessment.
Achieving the Target: Therapy Optimization and Drug Monitoring
Whatever the choice of initial therapy, it is important to optimize it to achieve maximal benefit to the patient as well as to fully exploit its efficacy in the setting of expanding, but still limited, therapeutic choices. This optimization entails dose and interval adjustments or the addition of a medication to use in combination and is often based on therapeutic drug monitoring (TDM). This can be as simple as dose escalation of mesalamine (defined as increasing dose by 2.4 g/day), which, even in clinically quiescent patients, led to reduction of calprotectin levels to <100 μg/g and was tied to longer time to relapse.136
TDM is typically used to optimize both thiopurines and biologics (Table 1). With thiopurines, monitoring metabolites has long been accepted.137 AGA guidelines currently recommend the use of TDM to achieve target drug concentrations and assess for presence of high-level neutralizing anti-drug antibodies at time of treatment failure for TNFi therapies.121 However, controversy remains over the utility of proactive TDM with TNFi agents.138 Proactive TDM for biologics may also offer a cost benefit compared to empirical adjustments of therapy.139 A further nuance to TDM with biologics is the choice of target concentration. Both the AGA121 and the BRIDGe (Building Research in IBD Globally) Group have suggested target concentrations based on the currently available literature; BRIDGe offers a web-based tool to assist in choosing target drug concentrations based on patient characteristics (Table 1).140,141 If these targets are not achieved, escalation of dose, shortening of interval, and/or adding an immunomodulator, in the case of TNFi, are recommended, with subsequent reassessment of drug concentration until targets are reached.
Table 1.
Target Thiopurine Metabolite Levels and Biologic Trough Concentrations
| Drug | Target | BRIDGe141 | AGA Guideline121 |
|---|---|---|---|
|
| |||
| Thiopurine monotherapy | Clinical remission | — | 6-TGN 230–450 pmol/8 × 108 |
| Infliximab (and biosimilars) | Clinical remission Endoscopic healing |
wk 14 and beyond: ≥3 μg/mLa ≥7 μg/mL |
≥5 μg/mL |
| Adalimumabb | Clinical remission Endoscopic healing |
wk 4 and beyond: ≥5 μg/mLa ≥7 μg/mL |
≥7.5 μg/mL |
| Certolizumab | Clinical remission | wk 6: ≥32 μg/mL Remission: ≥15 μg/mL |
≥20 μg/mL |
| Golimumab | Clinical remission | wk 6: ≥2.5 μg/mL Remission: ≥1 μg/mL |
No recommendation |
| VDZc and USTd | No recommendation | No recommendation | |
BRIDGe, Building Research in IBD Globally.
If active, do not abandon therapy unless >10 μg/mL.
Adalimumab, on achieving a steady state in maintenance, does not need to be a trough concentration.
Suggested targets for VDZ163: wk 6 ≥25 μg/mL; maintenance ≥15 μg/mL.
Suggested targets for UST164: wk 8: 3.7–8.7+ μg/mL; maintenance ≥1.3 μg/mL.
Maintaining the Target: Tight Control
Once achieving target goals, patients should continue to be monitored serially every 3–6 months with clinical visit and noninvasive inflammatory markers (FC and CRP). Prospective studies show that FC can increase and predict relapse 3–4 months before clinical symptoms in both UC and CD, making it a viable biomarker for monitoring, even in the quiescent state.142 In the post-hoc analysis of the TAILORIX (Drug Concentration Versus Symptom-Driven Dose Adaptation of IFX in Patients With Active CD) study, FC was highly predictive of mucosal healing in their cohort after week 2, and, in the setting of a persistently abnormal FC, they recommended assessing a drug concentration at the same time to determine a possible pharmacokinetic cause of failure to achieve endoscopic and histologic remission.143 Future studies will determine the role of the Endoscopic Healing Index as a biomarker to monitor tight control.
360-Degree Inflammatory Bowel Disease Care
Beyond prognostication and medical and surgical management, outcomes in IBD are improved when there is a 360-degree approach to care of the patient (Figure 5), interweaving preventative care, nutrition, psychobehavioral management, and socioeconomic considerations, as well as fostering open communication with the patient and family. An interdisciplinary care team, often described as a “medical home,” should include services to address all of these facets of care and, when implemented early in the disease course, has been shown to improve patient outcomes.144,145 There is potential, with the rise of telemedicine and remote monitoring tools, to expand comprehensive services for patients with IBD to locations formerly with issues of access.146,147
Figure 5.

360° care of the patient with IBD. Please see Supplementary Figure 1 for screening tool to identify patients who may need 360-degree care services. CBC, complete blood count; CMP, comprehensive metabolic panel; CRP, C-reactive protein; CTE, computed tomography enterography; DEXA, dual energy X-ray absorptiometry; EBV, Epstein-Barr virus; ESR, erythrocyte sedimentation rate; IgG, immunoglobulin G; MMA, methylmalonic acid; MRE, magnetic resonance enterography; PPD, purified protein derivative; PPSV23, pneumococcal polysaccharide vaccine 23; sAb, surface antibody; sAg, surface antigen; SB, small bowel; SBFT, small bowel follow through; SBUS, small bowel ultrasound.
Preventative Care
There are well-accepted guidelines for preventative care in IBD,148,149 but it has also been shown that preventative care is often overlooked.150,151 It is important for the gastroenterologist to engage in preventative care for their patients with IBD, whether it be by informing the primary physician of services needed, informing the patient and empowering them to seek the preventative services, or offering those services themselves in clinic, for example, vaccinations. Early in the disease course, preventative care, in particular vaccinations, should be highlighted as strategies that are important in reducing the risk of disease and treatment-related complications. Supplementary Figures 1–7, which include detailed sections on preventative care, are comprehensive checklists to promote 360-degree care starting from the very first visit.
Nutrition
Nutritional services should be offered to patients with IBD to address nutritional deficiencies, aid in catch-up growth in pediatrics, provide support for enteral nutrition as needed, and explore any specific patient concerns about diet. There is burgeoning patient interest in the use of specialized diets (eg, specific carbohydrate diet and anti-inflammatory diet) as an adjunct to medical therapy.152 It is useful to be open to discussion and provide referral to a well-informed nutritionist to provide accurate information and support.
Psychobehavioral Support
There is a well-described increase in anxiety and depression in IBD patients, and patients with psychological comorbidities are more likely to be hospitalized (OR, 4.13; 95% CI, 1.25–13.61).153 When a biopsychosocial integrated care model that included cognitive behavior therapy was instituted for IBD patients, there were improvements in disease activity and quality of life, with significant reductions in the use of opioids (P = .037), hospitalizations (48% to 30%), and inpatient care costs (P = .005).154 High resilience, or an ability to recover from adversity, has been shown to be associated with lower disease activity, improved quality of life, and fewer operations in CD.155 Screening for psychological comorbidities and low resilience early in the disease course with referral for psychobehavioral support could lead to improvements in outcomes among patients with IBD.
Complementary Therapies
Complementary treatments are also commonly pursued by patients with IBD156 and, just as with diet and nutrition, it is important to engage with your patient in open discussion about these therapies to foster a therapeutic relationship.157
Medication Management
Medications in IBD need to be taken long-term and require a high level of adherence to be effective and to avoid relapse and/or development of anti-drug antibodies. Patient understanding and “buy-in” to therapy is critical. A clinical pharmacist, if available, can facilitate a discussion with patients regarding these medications to promote understanding.158 Furthermore, there are available resources to off-set the financial burden from medications.7 Numerous advocacy groups (eg, Crohn’s and Colitis Foundation; AGA; and North American Society for Pediatric Gastroenterology, Hepatology & Nutrition) have useful resources for education and support, directing patients to these trusted sources can be invaluable.
Providing Support for Disability
IBD takes a toll on school and work life. Absenteeism and presenteeism are common in patients with active IBD.159,160 In pediatrics, discussion about the need for accommodations at school should be routinely asked and a plan provided as needed. For adults, physicians should devise a plan with the patient how to best address their concerns and needs for support based on their specific situation and preferences.
Communication
Lastly, open communication between the patient and provider is key to success.161 Effective shared decision-making is important in fostering satisfaction with treatment decisions, reducing decisional conflict and regret,162 and improving adherence.
Conclusions
In summary, early diagnosis and early management of IBDs are critical to minimizing complications and ensuring positive outcomes. Selection of appropriate first-line therapy should be based on risk assessment, disease activity, and clinical characteristics of the patient. Ongoing head-to-head trials will provide further clarity as to the relative positioning of available therapies. Comprehensive care of the IBD patients involves a thoughtful, individualized, and well-rounded assessment and treatment plan, taking into consideration feedback from the patient. Personalized IBD care is fast-evolving, and sophisticated prediction models incorporating multi-omic platforms are likely to be the future of IBD therapeutics.
Supplementary Material
Acknowledgments
The authors thank Jill Gregory, certified medical illustrator, Icahn School of Medicine at Mount Sinai, for the illustrations.
Funding
Manasi Agrawal receives research support from the Dickler Family Fund, New York Community Trust, and the Helmsley Charitable Trust Fund for SECURE-IBD. Elizabeth A. Spencer receives research support from an National Institutes of Health T32 (5T32GM082773-13), NY Crohn’s Foundation, and the Goldsmith Family Fund. Ryan C. Ungaro is supported by an National Institutes of Health K23 Career Development Award (K23KD111995-01A1).
Abbreviations used in this paper:
- ADA
adalimumab
- AGA
American Gastroenterological Association
- CD
Crohn’s disease
- CDST
clinical decision support tool
- CI
confidence interval
- CRP
C-reactive protein
- EEN
exclusive enteral nutrition
- EIM
extraintestinal manifestation
- FC
fecal calprotectin
- HR
hazard ratio
- IBD
inflammatory bowel disease
- ICR
ileocolic resection
- IFX
infliximab
- IMM
immunomodulator
- IQR
interquartile range
- MRI
magnetic resonance imaging
- NUDT15
nudix hydrolase
- OR
odds ratio
- SUCRA
surface under the cumulative ranking
- TDM
therapeutic drug monitoring
- TPMT
thiopurine methyltransferase
- TNFi
tumor necrosis factor inhibitor
- UC
ulcerative colitis
- UST
ustekinumab
- VDZ
vedolizumab
Footnotes
Conflicts of interest
These authors disclose the following: Jean-Frederic Colombel has received research grants from AbbVie, Janssen Pharmaceuticals and Takeda; has received payment for lectures from AbbVie, Amgen, Allergan, Bristol-Myers Squibb Company, Ferring Pharmaceuticals, Shire, Takeda and Tillots; has received consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb Company, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Gilead, Iterative Scopes, Ipsen, Immunic, lmtbio, Inotrem, Janssen Pharmaceuticals, Landos, LimmaTech Biologics AG, Medimmune, Merck, Novartis, O Mass, Otsuka, Pfizer, Shire, Takeda, Tigenix, Viela bio; and hold stock options in Intestinal Biotech Development. Ryan C. Ungaro has served as a consultant and/or advisory board member for Bristol Myers Squibb, Eli Lilly, Janssen, Pfizer, and Takeda. He has received research support from AbbVie, Boehringer Ingelheim, and Pfizer. The remaining authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dxdoi.org/10.1053/j.gastro.2021.04.063.
References
- 1.Torres J, Mehandru S, Colombel JF, et al. Crohn’s disease. Lancet 2017;389:1741–1755. [DOI] [PubMed] [Google Scholar]
- 2.Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, et al. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–297. [DOI] [PubMed] [Google Scholar]
- 4.Torres J, Billioud V, Sachar DB, et al. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis 2012;18:1356–1363. [DOI] [PubMed] [Google Scholar]
- 5.Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 2019;156:1345–1353.e4. [DOI] [PubMed] [Google Scholar]
- 6.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 7.Park KT, Ehrlich OG, Allen JI, et al. The cost of inflammatory bowel disease: an initiative from the Crohn’s & Colitis Foundation. Inflamm Bowel Dis 2020;26:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Gennep S, Evers SW, Rietdijk ST, et al. High disease burden drives indirect costs in employed inflammatory bowel disease patients: the WORK-IBD study. Inflamm Bowel Dis 2021;27:352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brochard C, Rabilloud ML, Hamonic S, et al. Natural history of perianal Crohn’s disease: long-term follow-up of a population-based cohort [published online ahead of print December 24, 2020]. Clin Gastroenterol Hepatol 10.1016/j.cgh.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Ng SC, Leung WK, Shi HY, et al. Epidemiology of inflammatory bowel disease from 1981 to 2014: results from a territory-wide population-based registry in Hong Kong. Inflamm Bowel Dis 2016;22:1954–1960. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal M, Bento-Miranda M, Walsh S, et al. Prevalence and progression of incidental terminal ileitis on non-diagnostic colonoscopy: a systematic review and meta-analysis [published online ahead of print February 14, 2021]. J Crohns Colitis 10.1093/ecco-jcc/jjab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fumery M, Singh S, Dulai PS, et al. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol 2018; 16:343–356.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burisch J, Ungaro R, Vind I, et al. Proximal disease extension in patients with limited ulcerative colitis: a Danish population-based inception cohort. J Crohns Colitis 2017;11:1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013; 145:996–1006. [DOI] [PubMed] [Google Scholar]
- 15.Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol 2015; 13:1042–1050.e2. [DOI] [PubMed] [Google Scholar]
- 16.Zhao M, Lo BZS, Vester-Andersen MK, et al. A 10-year follow-up study of the natural history of perianal crohn’s disease in a danish population-based inception cohort. Inflamm Bowel Dis 2019;25:1227–1236. [DOI] [PubMed] [Google Scholar]
- 17.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004;126:451–459. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Liu C, King E, et al. Continued statural growth in older adolescents and young adults with Crohn’s disease and ulcerative colitis beyond the time of expected growth plate closure. Inflamm Bowel Dis 2020; 26:1880–1889. [DOI] [PubMed] [Google Scholar]
- 19.Pariente B, Torres J, Burisch J, et al. SA1895 Validation of the Lémman index in Crohn’s disease. Gastroenterology 2020;158:S–469. [Google Scholar]
- 20.Cellier C, Sahmoud T, Froguel E, et al. ; Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut 1994;35:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis 2011;17:1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s Disease in a population-based cohort. Gastroenterology 2010;139:1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570–1583. [DOI] [PubMed] [Google Scholar]
- 24.Walker GJ, Lin S, Chanchlani N, et al. Quality improvement project identifies factors associated with delay in IBD diagnosis. Aliment Pharmacol Ther 2020;52:471–480. [DOI] [PubMed] [Google Scholar]
- 25.Schoepfer A, Santos J, Fournier N, et al. Systematic analysis of the impact of diagnostic delay on bowel damage in paediatric versus adult onset Crohn’s disease. J Crohns Colitis 2019;13:1334–1342. [DOI] [PubMed] [Google Scholar]
- 26.Nahon S, Lahmek P, Paupard T, et al. Diagnostic delay is associated with a greater risk of early surgery in a French cohort of Crohn’s disease patients. Dig Dis Sci 2016; 61:3278–3284. [DOI] [PubMed] [Google Scholar]
- 27.Blackwell J, Saxena S, Jayasooriya N, et al. Prevalence and duration of gastrointestinal symptoms before diagnosis of Inflammatory Bowel Disease and predictors of timely specialist review: a population-based study [published online ahead of print July 15, 2020]. J Crohns Colitis 10.1093/ecco-jcc/jjaa146. [DOI] [PubMed] [Google Scholar]
- 28.Vadstrup K, Alulis S, Borsi A, et al. Cost burden of crohn’s disease and ulcerative colitis in the 10-year period before diagnosis–A Danish register-based study from 2003–2015. Inflamm Bowel Dis 2020. August 20; 26(9):1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motil KJ, Grand RJ, Davis-Kraft L, et al. Growth failure in children with inflammatory bowel disease: a prospective study. Gastroenterology 1993;105:681–691. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths AM, Nguyen P, Smith C, et al. Growth and clinical course of children with Crohn’s disease. Gut 1993;34:939–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis 2014;8:1179–1207. [DOI] [PubMed] [Google Scholar]
- 32.Ungaro RC, Yzet C, Bossuyt P, et al. Deep remission at 1 year prevents progression of early Crohn’s disease. Gastroenterology 2020;159:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodríguez-Lago I, Hoyo JD, Pérez-Girbés A, et al. Early treatment with anti-tumor necrosis factor agents improves long-term effectiveness in symptomatic stricturing Crohn’s disease. United European Gastroenterol J 2020;8:1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danese S, Fiorino G, Mary JY, et al. Development of Red Flags Index for early referral of adults with symptoms and signs suggestive of Crohn’s disease: an IOIBD initiative. J Crohns Colitis 2015;9:601–606. [DOI] [PubMed] [Google Scholar]
- 35.Menees SB, Powell C, Kurlander J, et al. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol 2015;110:444–454. [DOI] [PubMed] [Google Scholar]
- 36.Fiorino G, Bonovas S, Gilardi D, et al. Validation of the Red Flags Index for early diagnosis of Crohn’s disease: a prospective observational IG-IBD study among general practitioners [published online ahead of print September 28, 2020]. J Crohns Colitis 10.1093/ecco-jcc/jjaa111. [DOI] [PubMed] [Google Scholar]
- 37.Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 38.Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 39.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68(Supple 3):s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohn’s Colitis 2018;13:144–164. [DOI] [PubMed] [Google Scholar]
- 41.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 2017;389:1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol 2009;44:431–440. [DOI] [PubMed] [Google Scholar]
- 43.Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn’s disease. Gastroenterology 2006;130:650–656. [DOI] [PubMed] [Google Scholar]
- 44.Roth LS, Chande N, Ponich T, et al. Predictors of disease severity in ulcerative colitis patients from Southwestern Ontario. World J Gastroenterol 2010;16:232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Etchevers MJ, Aceituno M, García-Bosch O, et al. Risk factors and characteristics of extent progression in ulcerative colitis. Inflamm Bowel Dis 2009;15:1320–1325. [DOI] [PubMed] [Google Scholar]
- 46.Ouahed J, Spencer E, Kotlarz D, et al. Very early onset inflammatory bowel disease: a clinical approach with a focus on the role of genetics and underlying immune deficiencies. Inflammatory Bowel Diseases 2019; 26:820–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heresbach D, Alexandre JL, Bretagne JF, et al. Crohn’s disease in the over-60 age group: a population based study. Eur J Gastroenterol Hepatol 2004;16:657–664. [DOI] [PubMed] [Google Scholar]
- 48.Ananthakrishnan AN, Shi HY, Tang W, et al. Systematic Review and meta-analysis: phenotype and clinical outcomes of older-onset inflammatory bowel disease. J Crohns Colitis 2016;10:1224–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kochar B, Cai W, Cagan A, et al. Pretreatment frailty is independently associated with increased risk of infections after immunosuppression in patients with inflammatory bowel diseases. Gastroenterology 2020; 158:2104–2111.e2. [DOI] [PubMed] [Google Scholar]
- 50.Faye AS, Wen T, Soroush A, et al. Increasing prevalence of frailty and its association with readmission and mortality among hospitalized patients with IBD [published online ahead of print January 1, 2020]. Dig Dis Sci 10.1007/s10620-020-06746-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galoosian A, Rezapour M, Liu B, et al. Race/ethnicity-specific disparities in in-hospital mortality and hospital charges among inflammatory bowel disease-related hospitalizations in the United States. J Clin Gastroenterol 2020;54:e63–e72. [DOI] [PubMed] [Google Scholar]
- 52.Agrawal M, Cohen-Mekelburg S, Kayal M, et al. Disability in inflammatory bowel disease patients is associated with race, ethnicity and socio-economic factors. Aliment Pharmacol Ther 2019;49:564–571. [DOI] [PubMed] [Google Scholar]
- 53.Walker DG, Williams HR, Kane SP, et al. Differences in inflammatory bowel disease phenotype between South Asians and Northern Europeans living in North West London, UK. Am J Gastroenterol 2011;106:1281–1289. [DOI] [PubMed] [Google Scholar]
- 54.Agrawal M, Burisch J, Colombel JF, et al. Viewpoint: inflammatory bowel diseases among immigrants from low- to high-incidence countries: opportunities and considerations. J Crohns Colitis 2020;14:267–273. [DOI] [PubMed] [Google Scholar]
- 55.Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 2015;47:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Severs M, Spekhorst LM, Mangen MJ, et al. Sex-related differences in patients with inflammatory bowel disease: results of 2 prospective cohort studies. Inflamm Bowel Dis 2018;24:1298–1306. [DOI] [PubMed] [Google Scholar]
- 57.Gupta N, Bostrom AG, Kirschner BS, et al. Gender differences in presentation and course of disease in pediatric patients with Crohn disease. Pediatrics 2007; 120:e1418–e1425. [DOI] [PubMed] [Google Scholar]
- 58.Gupta N, Lustig RH, Kohn MA, et al. Sex differences in statural growth impairment in Crohn’s disease: role of IGF-1. Inflamm Bowel Dis 2011;17:2318–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siegel CA, Whitman CB, Spiegel BMR, et al. Development of an index to define overall disease severity in IBD. Gut 2018;67:244–254. [DOI] [PubMed] [Google Scholar]
- 60.Sandborn W, Binion D, Persley K, et al. AGA Institute Guidelines for the Identification, Assessment and Initial Medical Treatment in Crohn’s Disease: Clinical Decision Support Tool. Available at: https://s3.amazonaws.com/agaassets/pdf/guidelines/IBDCarePathway.pdf. Accessed May 16, 2021.
- 61.Torres J, Caprioli F, Katsanos KH, et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J Crohns Colitis 2016;10:1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singer AAM, Bloom DA, Adler J. Factors associated with development of perianal fistulas in pediatric patients with Crohn’s disease. Clin Gastroenterol Hepatol 2021; 19:1071–1073. [DOI] [PubMed] [Google Scholar]
- 63.Zhi M, Zhang M, Jiang X, et al. Su1916–Absence of perianal symptoms does not mean no perianal fistula in Crohn’s disease: base on MRI examination. Gastroenterology 2019;156:S659. [Google Scholar]
- 64.Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA Clinical Practice Guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology 2020;158:1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu Y, Chen B, Li Y, et al. Risk factors and long-term outcome of disease extent progression in Asian patients with ulcerative colitis: a retrospective cohort study. BMC Gastroenterol 2019;19:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bessissow T, Le NH, Rollet K, et al. Incidence and predictors of nonalcoholic fatty liver disease by serum biomarkers in patients with inflammatory bowel disease. Inflamm Bowel Dis 2016;22:1937–1944. [DOI] [PubMed] [Google Scholar]
- 67.Limketkai BN, Shah SC, Hirano I, et al. Epidemiology and implications of concurrent diagnosis of eosinophilic oesophagitis and IBD based on a prospective population-based analysis. Gut 2019;68:2152–2160. [DOI] [PubMed] [Google Scholar]
- 68.Burisch J, Jess T, Egeberg A. Incidence of immune-mediated inflammatory diseases among patients with inflammatory bowel diseases in Denmark. Clin Gastroenterol Hepatol 2019;17:2704–2712.e3. [DOI] [PubMed] [Google Scholar]
- 69.Attauabi M, Zhao M, Bendtsen F, et al. Systematic review with meta-analysis: the impact of co-occurring immune-mediated inflammatory diseases on the disease course of inflammatory bowel diseases [published online ahead of print July 6, 2020]. Inflamm Bowel Dis 10.1093/ibd/izaa167. [DOI] [PubMed] [Google Scholar]
- 70.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gathungu G, Kim MO, Ferguson JP, et al. Granulocyte-macrophage colony-stimulating factor autoantibodies: a marker of aggressive Crohn’s disease. Inflamm Bowel Dis 2013;19:1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han X, Uchida K, Jurickova I, et al. Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn’s disease. Gastroenterology 2009;136:1261–1271; e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis 2013;19:332–341. [DOI] [PubMed] [Google Scholar]
- 74.Haisma SM, van Rheenen PF, Wagenmakers L, et al. Calprotectin instability may lead to undertreatment in children with IBD. Arch Dis Child 2020;105:996–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lasson A, Stotzer P-O, Öhman L, et al. The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns 2014;9:26–32. [DOI] [PubMed] [Google Scholar]
- 76.Pansart C, Roblin X, Paul S. Preanalytical heterogeneity in fecal calprotectin measurement needs to be considered for tight control. Clin Gastroenterol Hepatol 2020;18:524–525. [DOI] [PubMed] [Google Scholar]
- 77.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 2010;105:162–169. [DOI] [PubMed] [Google Scholar]
- 78.Kennedy NA, Jones GR, Plevris N, et al. Association between level of fecal calprotectin and progression of Crohn’s disease. Clin Gastroenterol Hepatol 2019; 17:2269–2276.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plevris N, Fulforth J, Lyons M, et al. Normalization of fecal calprotectin within 12 months of diagnosis is associated with reduced risk of disease progression in patients with Crohn’s disease [published online ahead of print August 13, 2020]. Clin Gastroenterol Hepatol 10.1016/j.cgh.2020.08.022. [DOI] [PubMed] [Google Scholar]
- 80.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001;411:603–606. [DOI] [PubMed] [Google Scholar]
- 82.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001;411:599–603. [DOI] [PubMed] [Google Scholar]
- 83.Cleynen I, Boucher G, Jostins L, et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet 2016; 387:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet 2018;50:1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siegel CA, Horton H, Siegel LS, et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment Pharmacol Ther 2016;43:262–271. [DOI] [PubMed] [Google Scholar]
- 86.Agrawal M, Bento-Miranda M, Walsh S, et al. ; Su1976. The prevalence of incidental terminal ileitis in persons undergoing non-diagnostic colonoscopy: a meta-analysis. Gastroenterology 2020;158:S–729. [Google Scholar]
- 87.Singh S, Fumery M, Sandborn WJ, et al. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment Pharmacol Ther 2018;48:394–409. [DOI] [PubMed] [Google Scholar]
- 88.RENFLEXIS® (infliximab-abda) [package insert]. Merck, 2020. [Google Scholar]
- 89.Ungaro RC, Limketkai BN, Jensen CB, et al. Stopping mesalamine therapy in patients with Crohn’s disease starting biologic therapy does not increase risk of adverse outcomes. Clin Gastroenterol Hepatol 2020; 18:1152–1160.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Targownik LE, Benchimol EI, Bernstein CN, et al. Combined biologic and immunomodulatory therapy is superior to monotherapy for decreasing the risk of inflammatory bowel disease-related complications. J Crohns Colitis 2020;14:1354–1363. [DOI] [PubMed] [Google Scholar]
- 91.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 92.Hu A, Kotze PG, Tan W, et al. 279. Combination therapy does not improve clinical and endoscopic remission rates with vedolizumab or ustekinumab in Crohn’s disease and ulcerative colitis. Gastroenterology 2020; 158:S–53. [Google Scholar]
- 93.Panés J, Rimola J. Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat Rev Gastroenterol Hepatol 2017;14:652–653. [DOI] [PubMed] [Google Scholar]
- 94.Olivera P, Spinelli A, Gower-Rousseau C, et al. Surgical rates in the era of biological therapy: up, down or un-changed? Curr Opin Gastroenterol 2017;33:246–253. [DOI] [PubMed] [Google Scholar]
- 95.Ponsioen CY, de Groof EJ, Eshuis EJ, et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: a randomised controlled, open-label, multicentre trial. Lancet Gastroenterol Hepatol 2017;2:785–792. [DOI] [PubMed] [Google Scholar]
- 96.Stevens TW, Haasnoot ML, D’Haens GR, et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: retrospective long-term follow-up of the LIR!C trial. Lancet Gastroenterol Hepatol 2020; 5:900–907. [DOI] [PubMed] [Google Scholar]
- 97.de Groof EJ, Stevens TW, Eshuis EJ, et al. Cost-effectiveness of laparoscopic ileocaecal resection versus infliximab treatment of terminal ileitis in Crohn’s disease: the LIR!C Trial. Gut 2019;68:1774–1780. [DOI] [PubMed] [Google Scholar]
- 98.Dziechciarz P, Horvath A, Shamir R, et al. Meta-analysis: enteral nutrition in active Crohn’s disease in children. Aliment Pharmacol Ther 2007;26:795–806. [DOI] [PubMed] [Google Scholar]
- 99.Heuschkel RB, Menache CC, Megerian JT, et al. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr 2000;31:8–15. [DOI] [PubMed] [Google Scholar]
- 100.Frivolt K, Schwerd T, Werkstetter KJ, et al. Repeated exclusive enteral nutrition in the treatment of paediatric Crohn’s disease: predictors of efficacy and outcome. Aliment Pharmacol Ther 2014;39:1398–1407. [DOI] [PubMed] [Google Scholar]
- 101.Levine A, Wine E, Assa A, et al. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology 2019;157:440–450.e8. [DOI] [PubMed] [Google Scholar]
- 102.Wall C, Day A, Gearry R. Use of exclusive enteral nutrition in adults with Crohn’s disease: a review. World J Gastroenterol 2013;19 43:7652–7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trial of Specific Carbohydrate and Mediterranean diets to induce remission of Crohn’s Disease (DINE-CD). Available at: https://clinicaltrials.gov/ct2/show/NCT03058679. Accessed May 16, 2021.
- 104.Sasson AN, Ananthakrishnan AN, Raman M. Diet in treatment of inflammatory bowel diseases. Clin Gastroenterol Hepatol 2021;19:425–435 e3. [DOI] [PubMed] [Google Scholar]
- 105.Sands BE, Peyrin-Biroulet L, Loftus EV Jr, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019;381:1215–1226. [DOI] [PubMed] [Google Scholar]
- 106.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 107.Singh S, Murad MH, Fumery M, et al. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol 2020; 18:2179–2191.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392–400.e3. [DOI] [PubMed] [Google Scholar]
- 109.Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 2015;149:350–355.e2. [DOI] [PubMed] [Google Scholar]
- 110.Ungaro RC, Limketkai BN, Jensen CB, et al. Stopping 5-aminosalicylates in patients with ulcerative colitis starting biologic therapy does not increase the risk of adverse clinical outcomes: analysis of two nationwide population-based cohorts. Gut 2019;68:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol 2011;106:110–119. [DOI] [PubMed] [Google Scholar]
- 112.Bernstein CN, Blanchard JF, Rawsthorne P, et al. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol 2001;96:1116–1122. [DOI] [PubMed] [Google Scholar]
- 113.Vavricka SR, Rogler G, Gantenbein C, et al. Chronological order of appearance of extraintestinal manifestations relative to the time of IBD diagnosis in the swiss inflammatory bowel disease cohort. Inflamm Bowel Dis 2015;21:1794–1800. [DOI] [PubMed] [Google Scholar]
- 114.Dubinsky MC, Cross RK, Sandborn WJ, et al. Extraintestinal manifestations in vedolizumab and anti-TNF-treated patients with inflammatory bowel disease. Inflamm Bowel Dis 2018;24:1876–1882. [DOI] [PubMed] [Google Scholar]
- 115.Diaz LI, Keihanian T, Schwartz I, et al. Vedolizumab-induced de novo extraintestinal manifestations. Gastroenterol Hepatol 2020;16:75. [PMC free article] [PubMed] [Google Scholar]
- 116.Chateau T, Bonovas S, Le Berre C, et al. Vedolizumab treatment in extra-intestinal manifestations in inflammatory bowel disease: a systematic review. J Crohns Colitis 2019;13:1569–1577. [DOI] [PubMed] [Google Scholar]
- 117.Parsi MA, Achkar JP, Richardson S, et al. Predictors of response to infliximab in patients with Crohn’s disease. Gastroenterology 2002;123:707–713. [DOI] [PubMed] [Google Scholar]
- 118.Vande Casteele N, Jairath V, Jeyarajah J, et al. Development and validation of a clinical decision support tool that incorporates pharmacokinetic data to predict endoscopic healing in patients treated with infliximab. Clin Gastroenterol Hepatol 2021;19:1209–1217.e2. [DOI] [PubMed] [Google Scholar]
- 119.Dulai PS, Boland BS, Singh S, et al. Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with Crohn’s disease. Gastroenterology 2018;155:687–695.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dulai PS, Singh S, Vande Casteele N, et al. Development and validation of clinical scoring tool to predict outcomes of treatment with vedolizumab in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020; 18:2952–2961.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017;153:827–834. [DOI] [PubMed] [Google Scholar]
- 122.Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet 2016;48:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schaeffeler E, Jaeger SU, Klumpp V, et al. Impact of NUDT15 genetics on severe thiopurine-related hematotoxicity in patients with European ancestry. Genet Med 2019;21:2145–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heap GA, Weedon MN, Bewshea CM, et al. HLA-DQA1-HLA-DRB1 variants confer susceptibility to pancreatitis induced by thiopurine immunosuppressants. Nat Genet 2014;46:1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sazonovs A, Kennedy NA, Moutsianas L, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology 2020;158:189–199. [DOI] [PubMed] [Google Scholar]
- 126.Powell Doherty RD, Liao H, Satsangi JJ, et al. Extended analysis identifies drug-specific association of 2 distinct HLA class II haplotypes for development of immunogenicity to adalimumab and infliximab. Gastroenterology 2020;159:784–787. [DOI] [PubMed] [Google Scholar]
- 127.Bucalo A, Rega F, Zangrilli A, et al. Paradoxical psoriasis induced by anti-TNFα treatment: evaluation of disease-specific clinical and genetic markers. Int J Mol Sci 2020:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 129.Colombel J-F, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2017;390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 130.D’Haens G, Kelly O, Battat R, et al. Development and validation of a test to monitor endoscopic activity in patients with Crohn’s disease based on serum levels of proteins. Gastroenterology 2020;158:515–526.e10. [DOI] [PubMed] [Google Scholar]
- 131.Zorzi F, Ghosh S, Chiaramonte C, et al. Response assessed by ultrasonography as target of biological treatment for Crohn’s disease. Clin Gastroenterol Hepatol 2020;18:2030–2037. [DOI] [PubMed] [Google Scholar]
- 132.Maaser C, Petersen F, Helwig U, et al. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut 2020;69:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kucharzik T, Wittig BM, Helwig U, et al. Use of intestinal ultrasound to monitor Crohn’s disease activity. Clin Gastroenterol Hepatol 2017;15:535–542.e2. [DOI] [PubMed] [Google Scholar]
- 134.Ordas I, Rimola J, Rodriguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology 2014;146:374–382. e1. [DOI] [PubMed] [Google Scholar]
- 135.Laurent V, Naude S, Vuitton L, et al. Accuracy of diffusion-weighted magnetic resonance colonography in assessing mucosal healing and the treatment response in patients with ulcerative colitis. J Crohns Colitis 2017; 11:716–723. [DOI] [PubMed] [Google Scholar]
- 136.Osterman MT, Aberra FN, Cross R, et al. Mesalamine dose escalation reduces fecal calprotectin in patients with quiescent ulcerative colitis. Clin Gastroenterol Hepatol 2014;12:1887–1893.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dubinsky MC, Lamothe S, Yang HY, et al. Pharmaco-genomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000;118:705–713. [DOI] [PubMed] [Google Scholar]
- 138.Agrawal M, Dubinsky MC, Colombel JF. Therapeutic drug monitoring of anti-tumour necrosis factor agents in inflammatory bowel diseases: the jury is still out. J Crohns Colitis 2020;14:1035–1036. [DOI] [PubMed] [Google Scholar]
- 139.Guidi L, Pugliese D, Tonucci TP, et al. Therapeutic drug monitoring is more cost-effective than a clinically based approach in the management of loss of response to infliximab in inflammatory bowel disease: an observational multicentre study. J Crohns Colitis 2018;12:1079–1088. [DOI] [PubMed] [Google Scholar]
- 140.Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2019;17:1655–1668.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.BRIDGe Group. Which biologic drug is your patient on?. Available at: https://www.bridgeibd.com/biologic-therapy-optimizer. Accessed May 16, 2021.
- 142.Mao R, Xiao YL, Gao X, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis 2012; 18:1894–1899. [DOI] [PubMed] [Google Scholar]
- 143.Dreesen E, Baert F, Laharie D, et al. Monitoring a combination of calprotectin and infliximab identifies patients with mucosal healing of Crohn’s disease. Clin Gastroenterol Hepatol 2020;18:637–646.e11. [DOI] [PubMed] [Google Scholar]
- 144.Regueiro M, Click B, Anderson A, et al. Reduced un-planned care and disease activity and increased quality of life after patient enrollment in an inflammatory bowel disease medical home. Clin Gastroenterol Hepatol 2018; 16:1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ungaro R, Ho M, Stanley S, et al. Sa1761. An interdisciplinary care program for recently diagnosed inflammatory bowel disease patients is associated with increased clinical remission rates and lower steroid use. Gastroenterology 2020;158:S–413. [Google Scholar]
- 146.Habashi P, Bouchard S, Nguyen GC. Transforming access to specialist care for inflammatory bowel disease: the PACE Telemedicine Program. J Can Assoc Gastroenterol 2019;2:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet 2017; 390:959–968. [DOI] [PubMed] [Google Scholar]
- 148.Rufo PA, Denson LA, Sylvester FA, et al. Health super-vision in the management of children and adolescents with IBD: NASPGHAN recommendations. J Pediatr Gastroenterol Nutr 2012;55:93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG Clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol 2017;112:241–258. [DOI] [PubMed] [Google Scholar]
- 150.Selby L, Kane S, Wilson J, et al. Receipt of preventive health services by IBD patients is significantly lower than by primary care patients. Inflamm Bowel Dis 2008; 14:253–258. [DOI] [PubMed] [Google Scholar]
- 151.Lester R, Lu Y, Tung J. Survey of immunization practices in patients with inflammatory bowel disease among pediatric gastroenterologists. J Pediatr Gastroenterol Nutr 2015;61:47–51. [DOI] [PubMed] [Google Scholar]
- 152.Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol 2019;17:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mikocka-Walus A, Knowles SR, Keefer L, et al. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm Bowel Dis 2016;22:752–762. [DOI] [PubMed] [Google Scholar]
- 154.Mikocka-Walus AA, Turnbull D, Holtmann G, et al. An integrated model of care for inflammatory bowel disease sufferers in Australia: development and the effects of its implementation. Inflamm Bowel Dis 2012; 18:1573–1581. [DOI] [PubMed] [Google Scholar]
- 155.Sehgal P, Ungaro RC, Foltz C, et al. High levels of psychological resilience associated with less disease activity, better quality of life, and fewer surgeries in inflammatory bowel disease [published online ahead of print July 22, 2020]. Inflamm Bowel Dis 10.1093/ibd/izaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Langhorst J, Wulfert H, Lauche R, et al. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J Crohns Colitis 2015;9:86–106. [DOI] [PubMed] [Google Scholar]
- 157.Nguyen GC, Croitoru K, Silverberg MS, et al. Use of complementary and alternative medicine for inflammatory bowel disease is associated with worse adherence to conventional therapy: the COMPLIANT Study. Inflamm Bowel Dis 2016;22:1412–1417. [DOI] [PubMed] [Google Scholar]
- 158.Tiao DK, Chan W, Jeganathan J, et al. Inflammatory bowel disease pharmacist adherence counseling improves medication adherence in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2017;23:1257–1261. [DOI] [PubMed] [Google Scholar]
- 159.Eloi C, Foulon G, Bridoux-Henno L, et al. Inflammatory bowel diseases and school absenteeism. J Pediatr Gastroenterol Nutr 2019;68:541–546. [DOI] [PubMed] [Google Scholar]
- 160.Manceur AM, Ding Z, Muser E, et al. Burden of Crohn’s disease in the United States: long-term healthcare and work-loss related costs. J Med Econ 2020;23:1092–1101. [DOI] [PubMed] [Google Scholar]
- 161.Rubin DT, Dubinsky MC, Martino S, et al. Communication between physicians and patients with ulcerative colitis: reflections and insights from a qualitative study of in-office patient-physician visits. Inflamm Bowel Dis 2017;23:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014:Cd001431. [DOI] [PubMed] [Google Scholar]
- 163.Adedokun OJ, Xu Z, Marano C, et al. Ustekinumab pharmacokinetics and exposure response in a phase 3 randomized trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020;18:2244–2255.e9. [DOI] [PubMed] [Google Scholar]
- 164.Dreesen E, Verstockt B, Bian S, et al. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2018;16:1937–1946.e8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


