Abstract
O6-Methylguanine (m6G) is formed by the action of alkylating agents such as N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) on DNA. m6G is a highly mutagenic and carcinogenic lesion, and it presents a block to synthesis by DNA polymerases. Here, we provide genetic and biochemical evidence for the involvement of yeast and human DNA polymerase η (Polη) in the replicative bypass of m6G lesions in DNA. The formation of MNNG-induced mutations is almost abolished in the rad30Δ pol32Δ double mutant of yeast, which lacks the RAD30 gene that encodes Polη and the Pol32 subunit of DNA polymerase δ (Polδ). Although Polδ can function in the mutagenic bypass of m6G lesions, our biochemical studies indicate that Polη is much more efficient in replicating through m6G than Polδ. Both Polη and Polδ insert a C or a T residue opposite from m6G; Polη, however, is more accurate, as it inserts a C about twice as frequently as Polδ. Alkylating agents are used in the treatment of malignant tumors, including lymphomas, brain tumors, melanomas, and gastrointestinal carcinomas, and the clinical effectiveness of these agents derives at least in part from their ability to form m6G in DNA. Inactivation of Polη could afford a useful strategy for enhancing the effectiveness of these agents in cancer chemotherapy.
O6-Methylguanine (m6G) is formed in DNA by treatment with alkylating agents such as N-methyl-N′-nitro-N-nitrosoguanidine (MNNG). m6G is highly mutagenic, and the mutagenic and carcinogenic potency of alkylating agents closely parallels their ability to form m6G in DNA (31). In yeast as well as higher eukaryotes, m6G specifically induces G · C to A · T transition mutations (30, 35).
Alkylation at the O6 position of guanine has a profound effect on base pairing properties. Melting studies of DNA duplexes containing m6G have shown that the m6G · T base pair is energetically less stable than the m6G · C base pair (10), and nuclear magnetic resonance studies have indicated that the m6G · T base pair is less hydrogen bonded than the m6G · C base pair (33, 34). Nevertheless, DNA polymerases incorporate T opposite m6G more often than C, because the m6G · T mispair retains the Watson-Crick geometry more closely than the m6G · C base pair. At neutral pH, C is inserted opposite m6G via a wobble configuration, but the phosphodiester links both 3′ and 5′ to the C are distorted in this base pair (18, 19, 24, 40, 41, 46).
m6G is a block to synthesis by prokaryotic and eukaryotic DNA polymerases. Extensive steady-state kinetic analyses have indicated that the Escherichia coli Klenow fragment is inhibited by m6G both at the step of insertion of a nucleotide opposite the lesion and at the step of extension from the m6G · C or m6G · T base pair (7). Sequenase (T7 DNA polymerase) is partially inhibited by m6G at both these steps (42). Eukaryotic DNA polymerase α, required for lagging strand DNA synthesis, is strongly blocked one base before m6G, indicating an inhibition of nucleotide insertion opposite the lesion (42). m6G also blocks DNA polymerase β, which is involved in base excision repair (38). Thus, although the m6G · T base pair is more Watson-Crick-like in geometry than the m6G · C pair, DNA polymerases are quite inefficient in incorporating even a T opposite m6G.
The Saccharomyces cerevisiae RAD30 gene functions in error-free replication of UV-damaged DNA, and RAD30-encoded polymerase η (Polη) replicates past a cis-syn thymine-thymine (T-T) dimer by inserting two adenines across from the two thymines of the dimer (16, 44). In humans, a defect in the yeast RAD30 counterpart causes the variant form of xeroderma pigmentosum (XP-V) (15, 27), and because of a deficit in error-free replication of UV-damaged DNA, XP-V cells are hypermutable with UV light. As a consequence, XP-V individuals suffer from a high incidence of sunlight-induced skin cancers.
Steady-state kinetic studies with yeast and human Polη have shown that this enzyme replicates through the T-T dimer with the same efficiency and fidelity as through the equivalent undamaged Ts (17, 44). Both yeast and human Polη are low fidelity enzymes, misincorporating nucleotides with a frequency of 10−2 to 10−3 (17, 45). We have previously suggested that Polη has a flexible active site which renders the enzyme more tolerant of DNA distortions, enabling it to synthesize DNA past a T-T dimer (17, 44, 45).
Here, we show that yeast and human Polη replicate through the m6G lesion by inserting a C or a T residue opposite the lesion. Although our genetic studies in yeast indicate a role for both Polδ and Polη in the replicative bypass of m6G lesions in DNA, our biochemical studies provide evidence that Polη is much more efficient at it than Polδ. We discuss the possibility that inactivation of Polη could be useful for enhancing the effectiveness of alkylating agents in cancer chemotherapy.
MATERIALS AND METHODS
DNA substrates.
The m6G-containing 75-nucleotide (nt) template oligomer was synthesized by Midland Certified Reagent Co. (Midland, Tex.). DNA substrates S-1, S-2, S-3G, S-3A, S-3T, and S-3C were generated by annealing the 75-nt template, 5′-AGCTACCATGCCTGCCTCAAGAATTCGTAAm6GATGCCTACACTGGAGTACCGGAGCATCGTCGTGACTGGGAAAAC-3′, which contained an m6G at the underlined position 45 nt from the 3′ end, to the 32-, 44-, and four different 45-nt 5′ 32P-labeled oligomer primers: N4456 (5′-GTTTTCCCAGTCACGACGATGCTCCGGTACTC-3′), N4309 (5′-GTTTTCCCAGTCACGACGATGCTCCGGTACTCCAGTGTAGGCAT-3′), or oligonucleotides that contain N4309 with one additional G, A, T, or C residue at its 3′ end, respectively. In the control undamaged DNA substrates, the 75-nt template with the undamaged G residue at position 45 was used. The sequences of DNA substrates containing 18-nt template oligonucleotides annealed to 12-nt primer DNA are shown in the figures. The sequence of the 18-nt oligonucleotides, used as markers, was 5′-AGAGGAAAGTAGXGAAGG, which contained a C (C marker), an A (A marker), a T (T marker), or a G (G marker) residue at the underlined X position.
DNA polymerase reactions.
Yeast and human Polη were purified as described previously (16, 17). Standard DNA polymerase reactions (10 μl) contained 40 mM Tris-HCl (pH 7.5), 5 mM MgCl2, 1 mM dithiothreitol, bovine serum albumin (100 μg/ml), 10% glycerol, 100 μM deoxynucleoside triphosphate (dNTP), and 20 nM 5′ 32P-labeled oligonucleotide primer annealed to an oligonucleotide template. Reactions were initiated by adding a DNA polymerase enzyme, yeast Polδ (10 nM), yeast Polη (2.5 nM), or human Polη (2.5 or 5 nM). For the identification of the nucleotide incorporated opposite m6G, we used an 18-nt template primed with a 12-nt oligomer. Polη and Polδ bind poorly to this short DNA substrate; therefore, higher amounts of Polδ (40nM) and Polη (10 nM) were used in these experiments. After incubation for 5 min at 30°C, reactions were terminated by the addition of 40 μl of loading buffer containing 20 mM EDTA, 95% formamide, 0.3% bromphenol blue, and 0.3% cyanol blue. The reaction products were resolved on 10 or 20% polyacrylamide gels containing 8 M urea and were dried before autoradiography at −70°C with intensifying screens. Gel band intensities were quantified by PhosphorImager and the ImageQuant software (Molecular Dynamics).
Steady-state kinetic analyses.
Analysis of kinetic parameters for deoxynucleotide incorporation opposite m6G or primer extension from this lesion was done as described before (3, 12, 29). Briefly, yeast Polη was incubated with increasing concentrations of a single deoxynucleotide (0 to 2,400 μM) for 1 min under standard reaction conditions. Gel band intensities of the substrates and products were quantified by PhosphorImager and the ImageQuant software. The percentage of primer extended was plotted as a function of dNTP concentration, and the data were fitted by nonlinear regression using SigmaPlot 5.0 to the Michaelis-Menten equation describing a hyperbola, v = (Vmax × [dNTP]/(Km + [dNTP]). Apparent Km and Vmax steady-state parameters were obtained from the fit and were used to calculate the frequency of deoxynucleotide incorporation (finc) and extension (f0ext) using the following equation: finc or ext = (Vmax/Km)incorrect pair/(Vmax/Km)correct pair.
MNNG sensitivity and mutagenesis in yeast.
All the yeast strains used for these experiments were derived from EMY74.7. For determining MNNG-induced forward mutations at the CAN1S locus, cells were grown overnight in yeast extract-peptone-dextrose (YPD) medium, sonicated to disperse clumps, washed, and resuspended in 0.1 M sodium acetate buffer, pH 5.0. Appropriate volumes of stock MNNG solution (made as 1 mg/ml in 0.1 M sodium acetate buffer, pH 5.0, and stored at −20°C) were added to 1-ml suspensions of cells adjusted to 1.5 × 108 cells per ml. Samples were incubated in the presence of MNNG with vigorous shaking for 20 min at 30°C. The reaction was terminated by the addition of 1 ml of 10% sodium thiosulfate. Appropriate dilutions of cells were plated on YPD for viability determinations and on synthetic complete medium lacking arginine but containing canavanine for determining the frequency of can1r mutations. Plates were incubated at 30°C and counted after 3 and 4 to 5 days for viability and mutagenesis determinations, respectively.
RESULTS
Genetic evidence for the involvement of DNA polymerases η and δ in the mutagenic bypass of m6G lesions in yeast.
To identify the DNA polymerase(s) involved in replication past m6G in eukaryotes, we examined, in S. cerevisiae, the frequency of MNNG-induced CAN1s to can1r forward mutations in deletion mutants of the POL32 gene that encodes one of the subunits of the replicative DNA polymerase Polδ (11) and of RAD30 that encodes Polη (16). Yeast Polδ is comprised of three subunits of 125, 58, and 55 kDa. The 125-kDa catalytic subunit and the 58-kDa subunits are essential for viability, but the 55-kDa subunit, which is encoded by the POL32 gene, is not essential (11).
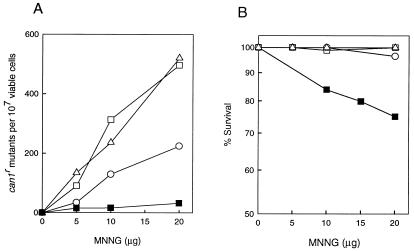
As shown in Fig. 1A, the frequency of MNNG-induced can1r mutations was reduced in the pol32Δ mutant compared with the wild type but was not affected in the rad30Δ mutant. MNNG-induced can1r mutagenesis was, however, almost abolished in the pol32Δ rad30Δ double mutant. These results indicate a role for Polδ and Polη in error-prone replication of m6G in DNA, and they further suggest that Polδ performs this task in a more error-prone manner than Polη. Consistent with the absence of m6G-induced mutagenesis in the rad30Δ pol32Δ strain, this strain exhibits enhanced sensitivity even at the low MNNG concentrations used in these experiments (Fig. 1B).
FIG. 1.
MNNG-induced mutations at the CAN1 locus in rad30Δ and pol32Δ yeast strains. can1r mutation frequency (A) and viability (B) in MNNG-treated yeast strains are shown. Cells grown overnight in YPD medium were treated with MNNG at the concentrations indicated for a 20-min period. Appropriate dilutions were spread onto synthetic complete medium lacking arginine and containing canavanine for the determination of CAN1s to can1r mutation frequencies and onto YPD plates for viability determinations. Each curve represents the average of two or more experiments. ▵, EMY74.7 (wild type) (RAD30 POL32); ○, YPO-69 (pol32Δ); □, YR30.2 (rad30Δ); ■, YR30.97 (rad30Δ pol32Δ).
Efficient m6G bypass by yeast Polη.
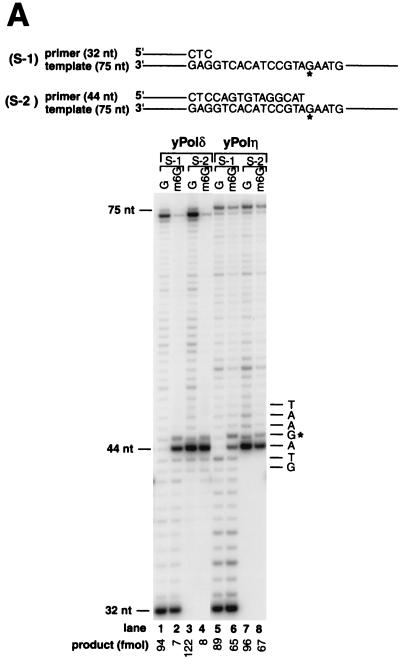
To examine the ability of yeast Polδ and Polη to replicate across m6G in DNA, we performed running-start and standing-start experiments using a 75-nt template DNA substrate containing a single m6G lesion 45 nt from the 3′ end and primed with a 5′ 32P-labeled 32- or 44-nt oligomer, respectively. Under conditions where approximately 50% of the primers were extended by both DNA polymerases (Fig. 2A), yeast Polδ replicated through only ∼7% of the m6G lesions (Fig. 2A, lanes 2 and 4) compared to synthesis on template containing an undamaged G residue (Fig. 2A, lanes 1 and 3). In contrast, compared to replication on undamaged DNA (Fig. 2A, lanes 5 and 7), yeast Polη replicated through m6G ∼10 times more efficiently (∼70%) than yeast Polδ (Fig. 2A, lanes 6 and 8). Furthermore, yeast Polδ exhibits a strong stall site right before the lesion, indicating an inhibition of insertion across from m6G, and another weaker stall site opposite the lesion, indicating some inhibition of extension 3′ to the modified base (Fig. 2A, lane 2). These two stall sites are also observed with yeast Polη, but they are much weaker (Fig. 2A, lane 6). These results indicate that whereas the m6G lesion presents a strong block for yeast Polδ, yeast Polη bypasses this lesion quite readily.
FIG. 2.
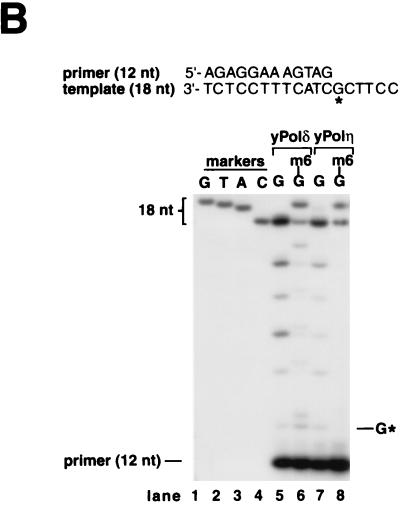
Translesion DNA synthesis by yeast Polη and yeast Polδ on templates containing m6G. (A) Running-start and standing-start DNA synthesis past m6G by yeast Polη and yeast Polδ. Sequences adjacent to the primer:template junction are shown for 75-nt template and 32-nt (S-1 substrate, lanes 1, 2, 5, and 6) or 44-nt (S-2 substrate, lanes 3, 4, 7, and 8) primer. The primers were 32P-labeled at their 5′ end. The position of undamaged G (lanes 1, 3, 5, and 7) or m6G (lanes 2, 4, 6, and 8) on the template is indicated by G*. Yeast Polδ (10 nM) (lanes 1 to 4) or yeast Polη (2.5 nM) (lanes 5 to 8) was incubated with the DNA substrate (20 nM) for 5 min at 30°C. Reaction products were resolved on a 10% denaturing polyacrylamide gel and were visualized by autoradiography. The amount of synthesis past the undamaged G or m6G is indicated. (B) Nucleotides incorporated opposite m6G by yeast Polη and yeast Polδ. Standing-start reactions were carried out on a G (lanes 5 and 7)- or m6G (lanes 6 and 8)-containing 18-nt template primed with a 5′ 32P-labeled 12-nt oligomer. The position corresponding to the G or m6G residue in the template is indicated by G*. Reactions were carried out as described for panel A above, except that the following DNA polymerase concentrations were used: yeast Polδ, 40 nM (lanes 5 and 6); and yeast Polη, 10 nM (lanes 7 and 8). Reaction mixtures were resolved on 20% denaturing polyacrylamide gel, and electrophoretic mobilities of the 18-nt reaction products (lanes 5 to 8) were compared with those of 18-nt synthetic oligomers containing a G (lane 1), a T (lane 2), an A (lane 3), or a C (lane 4) residue at position 13.
To identify the deoxynucleotides inserted opposite m6G, we assayed yeast Polη and yeast Polδ on an 18-nt template containing a G or an m6G residue at position 13 from the 3′ end and primed with a standing-start 12-nt primer (Fig. 2B). The relative electrophoretic mobilities of the products of the DNA synthesis reaction were compared to 18-nt oligomer markers containing a C, an A, a T, or a G residue at position 13, on a 20% polyacrylamide gel (Fig. 2B, lanes 1 to 4). As expected, DNA synthesis on undamaged templates by yeast Polδ or yeast Polη resulted in the incorporation of the correct C residue across from G at position 13 (Fig. 2B, lanes 5 and 7). On the damaged template, yeast Polδ replicated through the lesion by inserting a C (∼30%) or a T (∼70%) across from m6G (Fig. 2B, lane 6), while yeast Polη replicated through this lesion by inserting a C (∼60%) or a T (∼40%) (Fig. 2B, lane 8). Thus, while both DNA polymerases replicate through m6G in an error-free as well as an error-prone manner, yeast Polη inserts the correct residue C about twice as frequently as yeast Polδ.
Steady-state kinetic analyses of base insertion and extension during m6G bypass by yeast Polη.
To characterize further the bypass of m6G lesion by yeast Polη, we measured the kinetic parameters of base insertion and extension during translesion DNA synthesis. The kinetics of insertion of a single deoxynucleotide opposite an m6G and the kinetics of addition of the next correct nucleotide to various 3′-primer termini situated across from m6G were determined as a function of deoxynucleotide concentration under steady-state conditions (3, 12, 29). From the kinetics of deoxynucleotide incorporation, the steady-state apparent Km and Vmax values for each deoxynucleotide were obtained from the curve fitted to the Michaelis-Menten equation. The frequency of nucleotide misincorporation, finc, and the frequency of mismatch extension, foext, were calculated as the ratio of the efficiency (Vmax/Km) of incorrect nucleotide incorporated or extended from, to the efficiency (Vmax/Km) of correct nucleotide incorporated or extended from, respectively (Tables 1 and 2).
TABLE 1.
Kinetics of incorporation of nucleotides opposite m6G by yeast Polη
| DNA substrate | dNTP added | Km (μM) | Vmax (%/min) | Vmax/Km | finc |
|---|---|---|---|---|---|
| Insertion opposite G | |||||
| 5′----CAT | dCTP | 0.21 ± 0.08 | 9.2 ± 1.4 | 43.8 | 1.0 |
| -----GTAGAA-- | dTTP | 8.6 ± 1.2 | 3.9 ± 0.09 | 0.45 | 1 × 10−2 |
| Insertion opposite m6G | |||||
| 5′----CAT | dGTP | 59 ± 11 | 7.5 ± 0.3 | 0.13 | 3.0 × 10−3 |
| -----GTAGAA-- | dATP | 17 ± 3.7 | 4.5 ± 0.45 | 0.26 | 5.9 × 10−3 |
| | | dTTP | 3.4 ± 0.56 | 10.2 ± 0.3 | 3.0 | 6.8 × 10−2 |
| m6 | dCTP | 5.1 ± 1.7 | 9.6 ± 0.59 | 1.9 | 4.3 × 10−2 |
TABLE 2.
Kinetics of extension from nucleotides opposite m6G by yeast Polη
| DNA substratea | Km (μM) | Vmax (%/min) | Vmax/Km | foext |
|---|---|---|---|---|
| Extension from C or T opposite G | ||||
| 5′----CATC | 0.42 ± 0.02 | 5.3 ± 0.11 | 12.6 | 1.0 |
| -----GTAGAA-- | ||||
| 5′----CATT | 24.7 ± 4 | 1.58 ± 0.37 | 0.063 | 5 × 10−3 |
| -----GTAGAA-- | ||||
| Extension from G, A, T, or C opposite m6G | ||||
| 5′----CATG | 7.6 ± 2.1 | 1.69 ± 0.13 | 0.22 | 1.7 × 10−2 |
| -----GTAGAA-- | ||||
| | | ||||
| m6 | ||||
| 5′----CATA | 13.4 ± 1.2 | 1.24 ± 0.08 | 0.093 | 7.4 × 10−3 |
| -----GTAGAA-- | ||||
| | | ||||
| m6 | ||||
| 5′----CATT | 11.47 ± 0.25 | 3.6 ± 0.1 | 2.45 | 1.9 × 10−1 |
| -----GTAGAA-- | ||||
| | | ||||
| m6 | ||||
| 5′----CATC | 0.81 ± 0.12 | 4.8 ± 0.13 | 5.93 | 4.7 × 10−1 |
| -----GTAGAA-- | ||||
| | | ||||
| m6 |
dTTP was added to each reaction mixture.
As indicated by the Vmax/Km values, yeast Polη incorporates C opposite the m6G lesion about 20-fold less efficiently than C opposite G (Table 1), and extension from the C · m6G base pair is about twofold less efficient than extension from the C · G base pair (Table 2). Yeast Polη incorporates T opposite m6G about sevenfold better than T opposite G (Table 1), and it extends the T · m6G base pair about 40-fold more efficiently than the T · G mispair (Table 2). The order and the ratio of deoxynucleotide insertion opposite m6G by Polη were T:C:A:G and ∼23:15:2:1 (Table 1), and the order and the frequency of extension from different 3′-terminal deoxynucleotides paired with the m6G template residue were C:T:G:A and ∼64:26:2:1 (Table 2). Thus, opposite m6G, yeast Polη inserts the incorrect T slightly better than the correct C, but it is more efficient at extending from C opposite m6G than from T opposite this lesion. From these analyses, we estimate that yeast Polη would bypass m6G by inserting a C or a T residue and then extending from the resulting base pair with an efficiency of 2.0 × 10−2 and 1.3 × 10−2, respectively, relative to the efficiency of insertion of a C opposite an undamaged G residue and extension from this base pair (Tables 1 and 2). These kinetic results are in accord with the level of incorporation of C (60%) and T (40%) during replication through an m6G lesion by yeast Polη (Fig. 2B).
m6G bypass by human Polη.
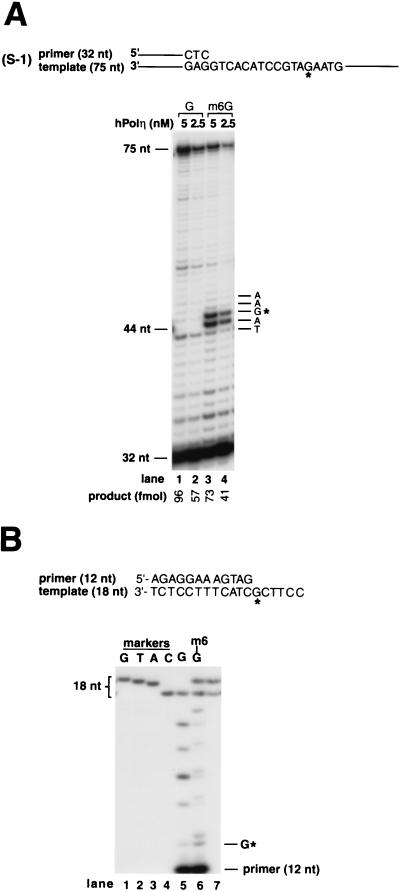
We also examined the ability of human Polη (hPolη) to bypass the m6G lesion. As shown in Fig. 3A, hPolη replicated through m6G ∼70% as efficiently as through undamaged DNA. hPolη exhibits two stall sites, one right before m6G and the other opposite the lesion, indicating that there is some inhibition of deoxynucleotide insertion opposite m6G as well as inhibition of extension from the base opposite the lesion. hPolη bypasses m6G by inserting a C or a T opposite this lesion about equally frequently (Fig. 3B, lane 6).
FIG. 3.
Translesion DNA synthesis activity of hPolη on template containing m6G. (A) Running-start DNA synthesis past m6G by hPolη. The position of undamaged G or the corresponding m6G is indicated by G*. Five nanomolar (lanes 1 and 3) or 2.5 nM (lanes 2 and 4) hPolη was incubated with DNA substrate (20 nM) for 5 min at 30°C under standard reaction conditions. Undamaged DNA template, lanes 1 and 2; m6G template, lanes 3 and 4. (B) Deoxynucleotide incorporation opposite m6G by hPolη. The position of the m6G or the undamaged G residue in template DNA is indicated by G*. hPolη (10 nM) was incubated with 20 nM undamaged (lane 5) or m6G-containing (lane 6) DNA substrate under standard reaction conditions. Electrophoretic mobilities of 18-nt reaction products (lanes 5 and 6) were compared with those of 18-nt synthetic oligomer markers containing a G, T, A, or C residue at position 13 (lanes 1 to 4, respectively). Two of these 18-nt marker oligomers, containing a C (lower band) or T (upper band) at position 13, were mixed and run in lane 7.
DISCUSSION
Our genetic studies in yeast indicate that Polδ and Polη provide alternate pathways for the mutagenic bypass of m6G. Since the frequency of MNNG-induced can1r mutations is reduced in the pol32Δ mutant but not in the rad30Δ mutant, these studies further suggest that Polη bypasses the m6G lesion in a more error-free manner than Polδ. Also, our biochemical studies indicate that Polη replicates through m6G more accurately than Polδ, as it inserts a C opposite this lesion about twice as frequently as Polδ. Although Polδ replicates through the m6G lesion quite inefficiently in vitro, association with RFC and PCNA may enhance this reaction in vivo. Because of its required role in DNA replication, Polδ will be the first polymerase to arrive at the m6G lesion; however, at times Polδ may stall at the lesion site, necessitating the participation of Polη in this process. Rad6-Rad18-dependent protein ubiquitination (1) may play a crucial role in the replacement of Polδ by Polη.
Yeast and human Polη replicate through a cis-syn T-T dimer with the same efficiency and accuracy as undamaged DNA (17, 44). m6G is, however, somewhat inhibitory to Polη, and as indicated from steady-state kinetic studies, replication through this lesion is about 50-fold less efficient than the replication of undamaged G (Tables 1 and 2). These observations may be reflective of the more frequent formation of a T-T dimer than an m6G lesion in DNA, and that could have imposed a more intense selection pressure on Polη for the more efficient and accurate bypass of a T-T dimer than the m6G lesion.
Kinetic studies with the Klenow fragment of E. coli DNA polymerase I have indicated that relative to the insertion of a C opposite G, this enzyme incorporates a C or a T residue opposite m6G poorly, with a frequency of 1.3 × 10−4 and 3.3 × 10−4, respectively (7). Also, relative to the extension from a G · C base pair, the Klenow fragment extends from an m6G · C or an m6G · T base pair with a frequency of 4.3 × 10−3 and 12.9 × 10−3, respectively (7). By contrast, Polη inserts a C or a T residue opposite m6G with a frequency of 4.3 × 10−2 and 6.8 × 10−2, respectively (Table 1), and extends from the m6G · C or the m6G · T base pair with a frequency of 4.7 × 10−1 and 1.9 × 10−1, respectively (Table 2). Thus, Polη is over 100-fold more efficient than the Klenow fragment in inserting a C or T opposite from m6G, and it is also more efficient in extending from the resulting base pair.
In eukaryotes, replicative DNA polymerases Polα (42) and, as shown here, Polδ are inhibited by m6G. Although Polβ can replicate through m6G in DNA, it does so 10,000-fold less efficiently than the replication of undamaged DNA, and Polβ inserts primarily a T residue (∼95%) opposite m6G (38). Further, our genetic studies in yeast have yielded no evidence that might impute a role for Polβ in m6G bypass. Thus, Polβ is unlikely to have a role in m6G bypass.
Polη differs from other eukaryotic DNA polymerases in its ability to replicate through the cis-syn T-T dimer and 8-oxoguanine (8-oxoG) lesions efficiently and accurately (13, 17, 44). We show here that Polη bypasses the m6G lesion with a reasonable efficiency, and it is more adept at inserting the correct nucleotide C opposite m6G than Polδ. All of these lesions distort the DNA helix. Although the two T's in the T-T dimer can base pair with A's, a dimer is still a block to most DNA polymerases, presumably because of the intolerance of their active site for the DNA distortion caused by the dimer (2, 14, 21, 22, 43). 8-oxoG in the syn conformation mimics T and has the correct geometry to form a stable base pair with A via two hydrogen bonds, whereas 8-oxoG in the anticonformation forms a normal Watson-Crick base pair with C that involves the same three hydrogen bonds as in the G · C base pair (23, 25, 28, 32). In the 8-oxoG · C base pair, however, the template strand is distorted significantly in the vicinity of the lesion (23, 25, 28, 32). Eukaryotic replicative DNA polymerases α, δ, and ɛ bypass 8-oxoG by incorporating an A rather than a C opposite the lesion (13, 36), presumably because their active site is unable to adapt to the distortion conferred by the 8-oxoG · C base pair. Polη, on the other hand, bypasses 8-oxoG by predominantly inserting a C opposite the lesion (13). Polη is also more efficient at inserting a C opposite the m6G lesion than Polδ, even though the phosphodiester backbone is distorted in the m6G · C base pair (18, 19, 24, 40, 41, 46). The m6G · C base pair, however, is more hydrogen bonded than the m6G · T base pair (33, 34). The ability of Polη to replicate through the T-T dimer, the 8-oxoG lesion, and the m6G lesion could derive from an active site which is indifferent to DNA distortion caused by these lesions but which can utilize the ability of these modified bases to form base pairs.
The involvement of Polη in m6G bypass suggests that inactivation of this enzyme could be useful for increasing the effectiveness of alkylating agents in cancer treatment. Chloroethylating agents, in combination with methylating agents such as procarbazine and temozolomide, are presently used to treat malignant tumors, particularly lymphomas, brain neoplasms, malignant melanomas, multiple myeloma, and gastrointestinal carcinomas (6). The clinical effectiveness of these agents is attributed, in part, to their forming O6-alkylguanine adducts in DNA (26). Intrinsic and acquired resistance to alkylating agents, however, limits the efficacy of these drugs, and O6-methylguanine DNA methyltransferase (MGMT), which transfers the methyl group from m6G to its active site cysteine residue, contributes to this resistance (4, 39). High levels of MGMT prevent the cytotoxic effect by removing O6-alkylguanine DNA adducts, and inactivation of MGMT by a potent inhibitor, O6-benzylguanine, sensitizes cells to killing by temozolomide (6). Consistent with the involvement of Polη in m6G bypass, XP-V cells exhibit enhanced sensitivity to alkylating agents that form O6-alkylguanine in DNA (37). Thus, in cells where MGMT has been specifically inactivated by O6-benzylguanine, additional inactivation of Polη by a specific inhibitor may confer enhanced sensitivity to alkylating agents, arising from a defect in both the removal of m6G and its bypass during replication. Hence, simultaneous inactivation of MGMT and Polη may prove to be an effective strategy for enhancing the sensitivity of tumor cells to alkylating agents and for augmenting the effectiveness of these drugs in chemotherapy.
In humans, DNA mismatch repair (MMR) potentiates the cytotoxicity of O6-alkylguanine, and cells acquire resistance to these agents by inactivating MMR (5, 20). As Polη inserts a C or a T residue opposite m6G, and since the human Msh2-Msh6 protein complex binds the m6G · T and m6G · C base pairs equally well, removal of either of these nucleotides (C or T) from the newly synthesized DNA strand by the MMR system might lead to a reiterative process of excision and synthesis, resulting in cell death (8). This could account for the role of MMR in enhancing the cytotoxicity of alkylating agents. In MMR-deficient cells, even the inactivation of MGMT fails to sensitize cells to temozolomide (9), and thus, in the absence of MMR, even high levels of O6-alkylguanine adducts are not cytotoxic. In cells inactivated for Polη, however, sensitivity to alkylating agents may be maintained even in the absence of functional MMR.
ACKNOWLEDGMENTS
We thank P. M. Burgers for yeast Polδ.
This work was supported by NIH grants GM19261 and CA80882.
REFERENCES
- 1.Bailly V, Lauder S, Prakash S, Prakash L. Yeast DNA repair proteins Rad6 and Rad18 form a heterodimer that has ubiquitin conjugating, DNA binding, and ATP hydrolytic activities. J Biol Chem. 1997;272:23360–23365. doi: 10.1074/jbc.272.37.23360. [DOI] [PubMed] [Google Scholar]
- 2.Ciarrocchi G, Pedrini A M. Determination of pyrimidine dimer unwinding angle by measurement of DNA electrophoretic mobility. J Mol Biol. 1982;155:177–183. doi: 10.1016/0022-2836(82)90445-4. [DOI] [PubMed] [Google Scholar]
- 3.Creighton S, Bloom L B, Goodman M F. Gel fidelity assay measuring nucleotide misinsertion, exonucleolytic proofreading, and lesion bypass efficiencies. Methods Enzymol. 1995;262:232–256. doi: 10.1016/0076-6879(95)62021-4. [DOI] [PubMed] [Google Scholar]
- 4.Day R S, III, Ziolkowski C H J, Scudiero D A, Meyer S A, Mattern M R. Human tumor cell strains defective in the repair of alkylation damage. Carcinogenesis. 1980;1:21–32. doi: 10.1093/carcin/1.1.21. [DOI] [PubMed] [Google Scholar]
- 5.de Wind N, Dekker M, Berns A, Radman M, te Riele H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell. 1995;82:321–330. doi: 10.1016/0092-8674(95)90319-4. [DOI] [PubMed] [Google Scholar]
- 6.Dolan M E, Pegg A E. O6-benzylguanine and its role in chemotherapy. Clin Cancer Res. 1997;3:837–847. [PubMed] [Google Scholar]
- 7.Dosanjh M K, Galeros G, Goodman M F, Singer B. Kinetics of extension of O6-methylguanine paired with cytosine or thymine in defined oligonucleotide sequences. Biochemistry. 1991;30:11595–11599. doi: 10.1021/bi00113a015. [DOI] [PubMed] [Google Scholar]
- 8.Duckett D R, Drummond J T, Murchie A I H, Reardon J T, Sancar A, Lilley D M J, Modrich P. Human MutSα recognizes damaged DNA base pairs containing O6-methylguanine, O4-methylthymine, or the cisplatin-d(GpG)adduct. Proc Natl Acad Sci USA. 1996;93:6443–6447. doi: 10.1073/pnas.93.13.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink D, Aebi S, Howell S B. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4:1–6. [PubMed] [Google Scholar]
- 10.Gaffney B L, Joens R A. Thermodynamic comparison of the base pairs formed by the carcinogenic lesion O6-methylguanine with reference both to Watson-Crick pairs and to mismatched pairs. Biochemistry. 1989;28:5881–5889. doi: 10.1021/bi00440a026. [DOI] [PubMed] [Google Scholar]
- 11.Gerik K J, Li X, Pautz A, Burgers P M J. Characterization of the two small subunits of Saccharomyces cerevisiae DNA polymerase δ. J Biol Chem. 1998;273:19747–19755. doi: 10.1074/jbc.273.31.19747. [DOI] [PubMed] [Google Scholar]
- 12.Goodman M F, Creighton S, Bloom L B, Petruska J. Biochemical basis of DNA replication fidelity. Crit Rev Biochem Mol Biol. 1993;28:83–126. doi: 10.3109/10409239309086792. [DOI] [PubMed] [Google Scholar]
- 13.Haracska L, Yu S-L, Johnson R E, Prakash L, Prakash S. Efficient and accurate replication in the presence of 7,8-dihydro-8-oxoguanine by DNA polymerase η. Nat Genet. 2000;25:458–461. doi: 10.1038/78169. [DOI] [PubMed] [Google Scholar]
- 14.Husain I, Griffith J, Sancar A. Thymine dimers bend DNA. Proc Natl Acad Sci USA. 1988;85:2558–2562. doi: 10.1073/pnas.85.8.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson R E, Kondratick C M, Prakash S, Prakash L. hRAD30 mutations in the variant form of xeroderm pigmentosum. Science. 1999;285:263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- 16.Johnson R E, Prakash S, Prakash L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Polη. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 17.Johnson R E, Washington M T, Prakash S, Prakash L. Fidelity of human DNA polymerase η. J Biol Chem. 2000;275:7447–7450. doi: 10.1074/jbc.275.11.7447. [DOI] [PubMed] [Google Scholar]
- 18.Kalnik M W, Li B F L, Swann P F, Patel D J. O6-ethylguanine carcinogenic lesions in DNA: an NMR study of O6etG · C pairing in dodecanucleotide duplexes. Biochemistry. 1989;28:6182–6192. doi: 10.1021/bi00441a009. [DOI] [PubMed] [Google Scholar]
- 19.Kalnik M W, Li B F, Swann P F, Patel D J. O6-ethylguanine carcinogenic lesions in DNA: an NMR study of O6etG · T pairing in dodecanucleotide duplexes. Biochemistry. 1989;28:6170–6181. doi: 10.1021/bi00441a008. [DOI] [PubMed] [Google Scholar]
- 20.Kat A, Thilly W G, Fang W-H, Longley M J, Li G-M, Modrich P. An alkylation-tolerant, mutator human cell line is deficient in strand-specific mismatch repair. Proc Natl Acad Sci USA. 1993;90:6424–6428. doi: 10.1073/pnas.90.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemmink J, Boelens R, Koning T, van der Marel G A, van Boom J H, Kaptein R. 1H NMR study of the exchangeable protons of the duplex d(GCGTTGCG).d(CGCAACGC) containing a thymine photodimer. Nucleic Acids Res. 1987;15:4645–4653. doi: 10.1093/nar/15.11.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J-K, Patel D, Choi B-S. Contrasting structural impacts induced by cis-syn cyclobutane dimer and (6-4) adduct in DNA duplex decamers: implication in mutagenesis and repair activity. Photochem Photobiol. 1995;62:44–50. doi: 10.1111/j.1751-1097.1995.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 23.Kouchakdjian M, Bodepudi V, Shibutani S, Eisenberg M, Johnson F, Grollman A P, Patel D J. NMR structural studies of the ionizing radiation adduct 7-hydro-8-oxodeoxyguanosine (-8-oxo-7H-dG) opposite deoxyadenosine in a DNA duplex. 8-oxo-7H-dG(syn).dA(anti) alignment at lesion site. Biochemistry. 1991;30:1403–1412. doi: 10.1021/bi00219a034. [DOI] [PubMed] [Google Scholar]
- 24.Leonard G A, Thomson J, Watson W P, Brown T. High-resolution structure of a mutagenic lesion in DNA. Proc Natl Acad Sci USA. 1990;87:9573–9576. doi: 10.1073/pnas.87.24.9573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipscomb L A, Peek M E, Morningstar M L, Verghis S M, Miller E M, Rich A, Essignman J M, Williams L D. X-ray structure of a DNA decamer containing 7,8-dihydro-8-oxoguanine. Proc Natl Acad Sci USA. 1995;92:719–723. doi: 10.1073/pnas.92.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludlum D B. The chloroethylnitrosoureas: sensitivity and resistance to cancer chemotherapy at the molecular level. Cancer Investig. 1997;15:588–598. doi: 10.3109/07357909709047601. [DOI] [PubMed] [Google Scholar]
- 27.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 28.McAuley-Hecht K E, Leonard G A, Gibson N J, Thomson J B, Watson W P, Hunter W N, Brown T. Crystal structure of a DNA duplex containing 8-hydroxydeoxyguanine-adenine base pairs. Biochemistry. 1994;33:10266–10270. doi: 10.1021/bi00200a006. [DOI] [PubMed] [Google Scholar]
- 29.Mendelman L V, Petruska J, Goodman M F. Base mispair extension kinetics. Comparison of DNA polymerase α and reverse transcriptase. J Biol Chem. 1990;265:2338–2346. [PubMed] [Google Scholar]
- 30.Mitra G, Pauly G T, Kumar R, Pei G K, Hughes S H, Moschel R C, Barbacid M. Molecular analysis of O6-substituted guanine-induced mutagenesis of ras oncogenes. Proc Natl Acad Sci USA. 1989;86:8650–8654. doi: 10.1073/pnas.86.22.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newbold R F, Warren W, Medcalf A S C, Amos J. Mutagenicity of carcinogenic methylating agents is associated with a specific DNA modification. Nature. 1980;283:596–599. doi: 10.1038/283596a0. [DOI] [PubMed] [Google Scholar]
- 32.Oda Y, Uesugi S, Ikehara M, Nishimura S, Kawase Y, Ishikawa H, Inoue H, Ohtsuka E. NMR studies of a DNA containing 8-hydroxydeoxyguanosine. Nucleic Acids Res. 1991;19:1407–1412. doi: 10.1093/nar/19.7.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel D J, Shapiro L, Kozlowski S A, Gaffney B L, Jones R A. Structural studies of the O6meG · C interaction in the d(C-G-C-G-A-A-T-T-C-O6meG-C-G) duplex. Biochemistry. 1986;25:1027–1036. doi: 10.1021/bi00353a012. [DOI] [PubMed] [Google Scholar]
- 34.Patel D J, Shapiro L, Kozlowski S A, Gaffney B L, Jones R A. Structural studies of the O6meG · T interaction in the d(C-G-C-G-A-A-T-T-C-O6meG-C-G) duplex. Biochemistry. 1986;26:1036–1042. doi: 10.1021/bi00353a013. [DOI] [PubMed] [Google Scholar]
- 35.Prakash L, Sherman F. Mutagenic specificity: reversion of iso-1-cytochrome c mutants of yeast. J Mol Biol. 1973;79:65–82. doi: 10.1016/0022-2836(73)90270-2. [DOI] [PubMed] [Google Scholar]
- 36.Shibutani S, Takeshita M, Grollman A P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349:431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 37.Simon L, Hazard R M, Maher V M, McCormick J J. Enhanced cell killing and mutagenesis by ethylnitrosourea in xeroderma pigmentosum cells. Carcinogenesis (London) 1981;2:567–570. doi: 10.1093/carcin/2.6.567. [DOI] [PubMed] [Google Scholar]
- 38.Singh J, Su L, Snow E T. Replication across O6-methylguanine by human DNA polymerase β in vitro. J Biol Chem. 1996;271:28391–28398. doi: 10.1074/jbc.271.45.28391. [DOI] [PubMed] [Google Scholar]
- 39.Sklar R, Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981;289:417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- 40.Spratt T E, Levy D E. Structure of the hydrogen bonding complex of O6-methylguanine with cytosine and thymine during DNA replication. Nucleic Acids Res. 1997;25:3354–3361. doi: 10.1093/nar/25.16.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan H-B, Swann P F, Chance E M. Kinetic analysis of the coding properties of O6-methylguanine in DNA: the crucial role of the conformation of the phosphodiester bond. Biochemistry. 1994;33:5335–5346. doi: 10.1021/bi00183a042. [DOI] [PubMed] [Google Scholar]
- 42.Voigt J M, Topal M D. O6-methylguanine-induced replication blocks. Carcinogenesis (Oxford) 1995;16:1775–1782. doi: 10.1093/carcin/16.8.1775. [DOI] [PubMed] [Google Scholar]
- 43.Wang C-I, Taylor J-S. Site-specific effect of thymine dimer formation on dAn.dTn tract bending and its biological implications. Proc Natl Acad Sci USA. 1991;88:9072–9076. doi: 10.1073/pnas.88.20.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Washington M T, Johnson R E, Prakash S, Prakash L. Accuracy of thymine-thymine dimer bypass by Saccharomyces cerevisiae DNA polymerase η. Proc Natl Acad Sci USA. 2000;97:3094–3099. doi: 10.1073/pnas.050491997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Washington M T, Johnson R E, Prakash S, Prakash L. Fidelity and processivity of Saccharomyces cerevisiae DNA polymerase η. J Biol Chem. 1999;274:36835–36838. doi: 10.1074/jbc.274.52.36835. [DOI] [PubMed] [Google Scholar]
- 46.Williams L D, Shaw B R. Protonated base pairs explain the ambiguous pairing properties of O6-methylguanine. Proc Natl Acad Sci USA. 1987;84:1779–1783. doi: 10.1073/pnas.84.7.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]