Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection now has a global resonance and represents a major threat for several patient populations. Observations from initial case series suggested that cancer patients in general might have an unfavorable outcome following coronavirus disease 2019 (COVID-19), due to their underlying conditions and cytotoxic treatments. More recently, data regarding the incidence and clinical evolution of COVID-19 in lymphomas have been reported with the aim to identify those more frequently associated with severe complications and death. Patients with lymphoma appear particularly vulnerable to SARS-CoV-2 infection, only partly because of the detrimental effects of the anti-neoplastic regimens (chemotherapy, pathway inhibitors, monoclonal antibodies) on the immune system. Here, we systematically reviewed the current literature on COVID-19 in adult patients with lymphoma, with particular emphasis on disease course and prognostic factors. We also highlighted the potential differences in COVID-19 clinical picture according to lymphoma subtype, delivered treatment for the hematological disease and its relationship on how these patients have been managed thus far.
Keywords: Lymphoma, SARS-CoV-2 infection, Hematological malignancies, COVID-19, Rituximab, Bendamustine
Core Tip: Recently, the scientific literature has been widely occupied by reports on severe acute respiratory syndrome coronavirus 2 infection. However, patients with cancer have been under-represented, and patients with lymphoma have rarely been described. The real impact of this tremendous pandemic on the life expectancy of patients with different subtypes of lymphoma is still unknown, especially in relation to chemo-, chemo-immunotherapy and/or biologic treatments. Furthermore, the relationship between lymphoma patients’ characteristics and the infection behavior is undescribed. With this review we pointed out what literature clarifies in the prognosis and management of patients with lymphoma during the coronavirus disease 2019 pandemic.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic is a worldwide medical emergency impacting virtually all aspects of medical care. The clinical spectrum of individuals who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is remarkably heterogeneous, ranging from mild flu-like symptoms to life-threatening respiratory failure[1]. Mortality due to the infection is largely dependent on patients age, and the infection fatality ratio is lowest among 5–9-year-old children, with a log-linear increase by age among individuals older than 30 years. Estimated age-specific infection fatality ratios range from 0.001% in those aged 5–9-years-old to 8.29% in those aged 80+. Population age structures, heterogeneous inclusion criteria in terms of comorbidities and burdens in nursing explain some of the heterogeneity between countries in infection fatality ratio[2]. The leading cause of mortality is the acute respiratory distress syndrome. Indeed, after infecting the pneumocytes, SARS-CoV-2 triggers intracellular signaling pathways that promote the release of several proinflammatory mediators, leading to the recruitment of neutrophil and monocyte-macrophages[3-5].
Subgroups of patients with COVID-19 have been identified to be at increased risk of morbidity and mortality, including patients of older age, male sex (vs female) and those with comorbidities, such as hypertension, chronic lung disease, diabetes, immunodeficiency and cancer[6]. In particular, cancer patients often follow a more severe and rapid disease course, with requirement of high-level intensive care and an increased risk of COVID-19-related death[7-10]. In the first published report from the COVID-19 and Cancer Consortium, mortality among 928 analyzed adult patients with any malignancy was 13%, with 23% mortality for any admission to the hospital and 38% mortality for admission to the intensive care unit (ICU)[11]. Among 800 patients with cancer included in the United Kingdom Coronavirus Cancer Monitoring Project, reported mortality in the overall cohort was 28%[12]. A multicenter study in China of 205 patients with cancer reported mortality of 20%[13]. In the latter series, 22 patients with hematologic malignancies (HM) were included and had a mortality rate of 41%. In cancer series, hematologic patients account for 20%-25% of the total including a variable distribution of pathologies. Heterogeneous series addressing SARS-CoV-2 infection in patients with HM have been published, reflecting mortality rates ranging from 30% to 40%; however, these reports offer limited information on the characteristics of the various hematological diseases and their relationship with anticancer treatments. Patients with HM are immunocompromised, which makes them highly susceptible to severe infections. On the other hand, some authors have suggested that some patients with HM might be “protected” from severe COVID-19 morbidity due to an attenuated inflammatory response. In this review, we synthesized the current literature to illustrate the demographic, immunological and clinical features of COVID-19 infection in the specific setting of patients affected by lymphoma, a heterogeneous group of cancers arising from B or T lymphocytes and often associated with various degrees of immune dysfunction.
Lymphoma patients are at high risk of infections: patients with HM (and lymphomas) tend to carry more comorbidities than age and sex matched population, have more frequent contacts with medical systems and are often treated with immunosuppressive medications potentially blunting the antiviral immune responses. Hematologic malignancies affect the production and function of blood cells in fighting off infections[14]. Affected patients often have multiple immune dysfunctions of the innate and adaptive immune system including low immunoglobulin G serum levels (i.e. chronic lymphocytic leukemia or other B cell neoplasms) or functionally impaired granulocytes (i.e. myeloid neoplasms)[15,16]. Crippled cellular and humoral immunity places these patients at risk of a diverse array of infections including COVID-19[17].
Lymphomas are a heterogeneous group of cancers broadly divided into two main histological subtypes: Hodgkin lymphoma (HL) and non-HL (NHL). HL tends to spread in a fairly orderly way from one group of lymph nodes to the next group and it affects young adults aged 20–40 years more frequently, while NHL can spread to extra nodal organs, bone marrow and spleen. The World Health Organization has recognized several forms of NHL, with diffuse large B-cell lymphoma being the most common subtype in adults[18].
Chemotherapy treatment combined with rituximab (widely available immunotherapy against B-lymphocytes) is the current standard upfront treatment for most histologies[19]. Together with lymphodepleting therapies, several intrinsic factors contribute to the typical immunosuppressive status of patients with lymphoma. Among them hypogammaglobulinemia, neutropenia and lymphopenia (both B- and T-cell related) are frequently observed features at disease presentation[20,21]. Furthermore, lymphomas are more likely to develop in patients with underlying immunosuppressive conditions, such as the human immunodeficiency virus infection, rheumatological chronic disorders, autoimmune disease or inherited congenital immune-deficiency states[22]. Lymphoma therapy has historically been based on chemotherapy variably associated with immunotherapy and radiotherapy. Moreover, in recent years, the approval of new molecules with different mechanisms of action (monoclonal antibody, small molecules, biologic agents, cell therapy) has allowed us to expand the therapeutic arsenal available for the treatment of these diseases. Among chemotherapy regimens, bendamustine is a strong inducer of T-cell immune deficiency[23]. Anti-CD20 monoclonal antibodies, such as rituximab or obinutuzumab, induce rapid depletion of more than 95% of CD20-positive mature B-cells, impairing cellular and humoral response towards new pathogens[24-26].
LITERATURE REVIEW
A review of the literature reporting on SARS-CoV-2 infection in lymphoma patients was conducted. In particular, we focused on the relationship with lymphoma characteristics and the clinical course of COVID-19 infection. An electronic search was performed to identify all studies reporting on the management of lymphoma patients during the SARS-CoV-2 pandemic. The PubMed/MEDLINE database was searched on February 6th, 2021. The search strategy was “SARS-CoV-2” OR “COVID-19” AND “lymphoma.” Potential case duplicates were ruled out by analysis of demographic characteristics of the included patients and institution of origin of the reports.
PREVALENCE OF CANCER AND HM AMONG SARS-COV-2 INFECTED PEOPLE
Human infections with SARS-CoV-2 were first reported in late 2019. At the end of February 2021, the global cumulative numbers were 110.7 million cases and over 2.4 million deaths since the start of the pandemic[27]. The prevalence of cancer in patients with COVID-19 is uncertain. Studies from China reported that 1% to 2% of COVID-19 patients had cancer, and a study from the United States reported that 6% of hospitalized patients with COVID-19 had cancer. In Lombardy, Italy, they observed that 8% of the patients admitted to the ICU for COVID-19 had cancer. In a meta-analysis, the prevalence of cancer was 2% among COVID- 19 patients[28].
Reports about the prevalence of HM among COVID-19 patients are very limited. In a study from Turkey[29], 0.39% of the laboratory-confirmed COVID-19 patients had underlying blood cancer. Patients with HM were reported to be at increased risk for developing COVID-19 as compared to general population, after adjusting for age, gender, race and known COVID-19 risk factors. It has been reported that patients with cancer with different tumor types have differing susceptibility to SARS-CoV-2 infection and COVID-19 phenotypes[30]. Individualized risk tables have been generated for patients with cancer, considering age, sex and tumor subtype, reporting an increased susceptibility to SARS-CoV-2 in patients with HM.
CLINICAL MANAGEMENT AND FATALITY RATES OF PATIENTS WITH COVID-19 AND HM (INCLUDING LYMPHOMAS)
Among papers investigating the characteristics of COVID-19 infection in cancer patients, only some stratified the population by type of malignancy (reported in Table 1). He et al[31] conducted a cohort study at two centers in Wuhan, China, involving 128 hospitalized subjects with HM, 13 (10%) of whom developed COVID-19. There were no significant differences in baseline covariates between subjects with HM developing COVID-19 or not. Case rates for COVID-19 were similar between the two groups, but hospitalized subjects with HM were reported to suffer from more severe disease and higher case fatality rate (CFR). In a study conducted by Mehta et al[32] the CFR in COVID-19 patients with HM was 37%. A study from Spain[33] reported a CFR of 32% among 34 hospitalized COVID-19 patients with HM. Authors concluded that the status of underlying malignancy at the time of COVID-19 correlated with mortality, with disease activity that was directly associated with worse outcomes. Aries et al[34] reported a CFR as high as 40% in a small cohort including 35 patients with HM. In a study conducted by Yang et al[13] among 52 COVID-19 patients with solid tumors or HM, the rate of severe/critical disease was 36.5% and CFR of severe/critical patients was 57.8%. Wood et al[35] described 250 cases of patients with HM and COVID-19 that were enrolled into the ASH Research Collaborative COVID-19 Registry. Consistent with previous reports, patients with HM had poor outcomes, with an overall mortality rate of 28%, which increased to 42% for those patients requiring hospital-level care.
Table 1.
Characteristics of included studies
| Ref. | Location | Type of malignancy included | Duration of study | Total No. of pts with HM included | Matched COVID-19 control | No. of lymphoma pts | No. of NHL pts | No. of HL pts | Mortality rate attributed to COVID-19 (Global) | Mortality rate attributed to COVID-19 (Lymphoma) | Mortality rate attributed to COVID-19 (NHL) | Mortality rate attributed to COVID-19 (HL) |
| Cancer studies including lymphoma pts | ||||||||||||
| Rüthrich et al[36], 2020 | Europe | All | 5 mo | 435 | 2636 | 76 | 71 | 5 | 96/435 (22%) | 20/76 (26%) | NR | NR |
| Lee et al[12], 2020 | UK | All | 1 mo | 1044 | 282878 | 79 | NR | NR | 319/1044 (31%) | 25/79 (31%) | NR | NR |
| Tian et al[50], 2020 | China | All | 9 wk | 232 | 519 | 6 | 6 | 0 | 46/232 (20%) | 2/6 (33%) | 2/6 (33%) | NR |
| HM studies including lymphoma pts | ||||||||||||
| Aries et al[34], 2020 | UK | HM | 2 mo | 35 | No | 8 | 8 | 0 | 14/35 (40%) | NR | NR | / |
| Biernat et al[51], 2020 | Poland | HM | 1 mo | 10 | No | 3 | 3 | 0 | 7/10 (70%) | NR | NR | / |
| Booth et al[52], 2020 | UK | HM | 2 mo | 66 | No | 15 | 15 | 0 | 34/66 (52%) | 6/15 (40%) | 6/15 (40%) | / |
| Cattaneo et al[42], 2021 | Italy | HM | 1 mo | 102 | 101 | 42 | 40 | 2 | 40/102 (39%) | 17/42 (40%) | 16/40 (40%) | 1/2 (50%) |
| Fox et al[53], 2020 | UK | HM | 1 mo | 55 | No | 17 | 17 | 0 | 19/55 (35%) | 7/17 (41%) | 7/17 (41%) | / |
| Garcìa-Suàrez et al[54], 2020 | Spain | HM | 8 wk | 697 | No | 220 | 187 | 33 | 230/697 (33%) | 68/220 (31%) | 59/187 (32%) | 9/33 (27%) |
| Infante et al[55], 2020 | Spain | HM | 1 mo | 41 | No | 15 | 14 | 1 | 15/41 (37%) | NR | NR | NR |
| Lattenist et al[56], 2021 | Belgium | HM | 2 mo | 12 | No | 2 | 2 | 0 | 6/12 (50%) | 2/2 (100%) | 2/2 (100%) | / |
| Malard et al[57], 2020 | France | HM | 1 mo | 25 | No | 7 | 7 | 0 | 10/25 (40%) | 0/7 (0%) | 0/7 (0%) | / |
| Martín-Moro et al[33], 2020 | Spain | HM | 5 wk | 34 | No | 6 | 5 | 1 | 11/34 (32%) | 0/6 (0%) | 0/5 (0%) | 0/1 (0%) |
| Mehta et al[32], 2020 | USA | HM | 3 wk | 54 | No | 20 | 15 | 5 | 20/54 (37%) | 8/20 (40%) | 5/15 (33%) | 3/5 (60%) |
| Passamonti et al[37], 2020 | Italy | HM | 12 wk | 536 | No | 170 | 153 | 17 | 198/536 (37%) | 65/170 (38%) | 62/153 (40%) | 3/17 (18%) |
| Sanchez-Pina et al[58], 2020 | Spain | HM | 1 mo | 39 | 53 | 12 | NR | NR | 14/39 (36%) | 2/12 (14%) | NR | NR |
| van Doesum et al[59], 2020 | Europe | HM | 9 wk | 59 | No | 17 | 15 | 2 | NR | NR | NR | NR |
| Yigenoglu et al[29], 2021 | Turkey | HM | 15 wk | 740 | 188897 | 250 | 223 | 27 | 103/740 (14%) | 28/250 (11%) | 24/223 (11%) | 4/27 (14%) |
| Wood et al[35], 2020 | Worldwide | HM | 3 mo | 250 | No | 79 | 68 | 11 | 70/250 (28%) | 20/79 (25%) | 16/68 (24%) | 4/11 (36%) |
| Lymphoma studies | ||||||||||||
| Regalado-Artamendi et al[40], 2021 | Spain | Lymphoma | 12 wk | 177 | No | 177 | 158 | 9 | 61/177 (29%) | 61/177 (29%) | NR | NR |
| Lamure et al[39], 2020 | France | Lymphoma | 8 wk | 89 | No | 89 | 84 | 5 | 30/85 (34%) | 30/85 (35%) | 29/84 (34%) | 1/5 (20%) |
| Laurenge et al[60], 2021 | France | PCNSL | 2 mo | 13 | No | 13 | 13 | / | 3/13 (23%) | 3/13 (23%) | 3/13 (23%) | |
COVID-19: Coronavirus disease 2019; HM: Hematologic malignancy; HL: Hodgkin lymphoma; NHL: Non-Hodgkin lymphoma; NR: Not reported; PCNSL: Primary central nervous system lymphoma; pts: Patients; UK: United Kingdom; USA: United States of America.
In Rüthrich et al[36] retrospective analysis of LEOSS study a total of 435 cancer patients with SARS-CoV-2 were included. The majority of patients were hospitalized (98%). Lymphoma and leukemia were documented for 76 (17.5%) and 48 (11%) patients, respectively. The commonest HM was NHL (16.5%). In solid tumors and HM, mortality appeared somewhat comparable, but HM were overrepresented compared to a non-COVID-19 cancer cohort from the United Kingdom, reporting a prevalence of 9.5%[30].
In the study by Passamonti et al[37], 536 HM patients were described. A high frequency of severe infections was reported: dyspnea occurred in 51% of patients and fever in 75% of patients. This was also evidenced by the high proportion (18%) of patients admitted to the ICU and the high number of deaths (198, 37%). Mortality of patients with HM and COVID-19 was nearly four times higher than that of the general population with COVID-19.
Similar conclusions have been reached by the Turkish study conducted by Yigenoglu et al[29] where COVID-19 patients with HM (n = 740) and an age, sex and comorbidity-matched cohort of COVID-19 patients without cancer (n = 740) were enrolled. NHL (30.1%), myelodysplastic syndrome (19.7%) and myeloproliferative neoplasm (15.7%) were the most common HM. The rates of severe and critical disease, hospital and ICU admission and mechanical ventilation support were significantly higher in patients with HM compared with patients without cancer. The length of hospital stay and ICU stay was similar between groups. The CFR was 13.8% in patients with HM and 6.8% in the control group. The lower CFR in this study compared with the other studies may be attributed to a high number of myeloproliferative neoplasm patients who were thought to be less immunocompromised compared with leukemia, multiple myeloma or lymphoma patients. Interestingly, they described higher use of antiviral drugs such as lopinavir/ritonavir in patients with HM.
Finally, recipients of autologous and allogeneic stem cell transplantation (HSCT) who develop COVID-19 have also been reported to have poor survival rates. The Center for International Blood and Marrow Transplant Research reported 318 HSCT recipients diagnosed with COVID-19. Disease severity was mild in 155 (49%) of 318 patients, while severe disease requiring mechanical ventilation occurred in 45 (14%), i.e. 28 (15%) of 184 allogeneic HSCT recipients and 17 (13%) of 134 autologous HSCT recipients. At 30 d after COVID-19 diagnosis, overall survival was 68% (95% confidence interval: 58%–77%) for recipients of allogeneic HSCT and 67% (55-78) for recipients of autologous HSCT[38]. Age 50 years or older, male sex and development of COVID-19 within 12 mo of transplantation were associated with a higher risk of mortality among allogeneic HSCT recipients.
When cancer patients are compared with control groups it appeared evident that the cancer itself constituted an independent prognostic factor in the case of COVID-19 infection. Studies investigating clinical factors associated with worse outcome in HM are summarized in Table 2.
Table 2.
Prognostic factors associated with survival in lymphoma series
| Ref. | Details on study cohort | Univariate analysis for predictors of death | Multivariate analysis for predictors of death |
| Regalado-Artamendi et al[40], 2021 | Lymphoma patients | Age ≥ 70 yr | Age ≥ 70 yr |
| Comorbidities | Comorbidities | ||
| CURB65 ≥ 3 | CURB ≥ 2 | ||
| Low platelet count | Active disease | ||
| Low hemoglobin level | |||
| High D-dimer | |||
| C-reactive protein >10 mg/dL | |||
| LDH > 300 U/L | |||
| Active disease1 (reference to CR) | |||
| DLBCL histology (reference to FL) | |||
| High-risk lymphoma2 (reference to low risk) | |||
| Lamure et al[39], 2020 | Hospitalized lymphoma patients | Age ≥ 70 yr | Age ≥ 70 yr |
| Hypertension | Active disease | ||
| Previous cancer | |||
| Bendamustine treatment | |||
| Active disease |
Partial response or progression.
High risk according to prognostic index at diagnosis. CR: Complete response; CURB65: Confusion, urea concentration, respiratory rate, blood pressure and age > 65; DLBCL: Diffuse large B cell lymphoma; FL: Follicular lymphoma; LDH: Lactate dehydrogenase.
LYMPHOMA SERIES AND CASE REPORTS, CLINICAL FEATURES AND FATALITY RATES
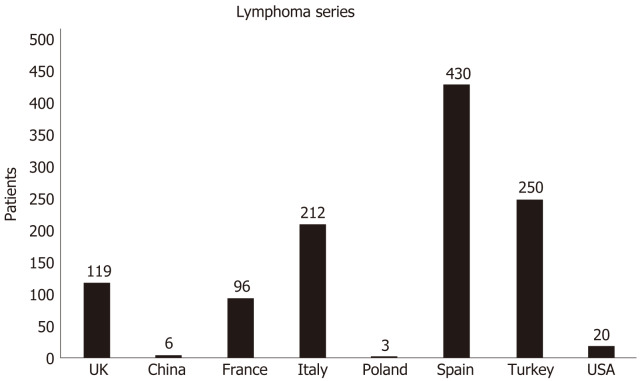
Lymphoma patients represented a small proportion of the entire cancer series, also reflecting the relative prevalence of this disease compared to solid tumors. Figure 1 resumed the number of lymphoma patients described all over the world in the largest HM studies. However, subset data from and disease-specific cohorts are emerging. Two recently published series focused specifically on patients with lymphoma. The first report was from France where Lamure et al[39] described clinical characteristics and outcomes of 89 adult patients with lymphoma hospitalized for COVID-19 in 12 hospitals during the first pandemic wave. Overall, reported 1 mo overall survival was 71%. The most common symptoms at presentation were dyspnea (65%), cough (60%), fever (48%) and diarrhea (24%). The median duration of symptoms before admission was 6 d. Lymphopenia was observed in 66% of patients. During hospitalization, 25 patients (28%) were admitted to the ICU. This CFR was documented despite a significant fraction of patients had received the best available cures against SARS-CoV-2: chloroquine and hydroxychloroquine (11 patients) or antiviral drugs combinations (10 patients). Six patients had received treatment for cytokine shock (tocilizumab, anakinra and eculizumab for two patients each). Seventeen patients (19%) developed a documented coinfection and three an (3%) acute pulmonary embolism.
Figure 1.
Number of lymphoma patients described all over the world in largest hematologic malignancy studies. UK: United Kingdom; USA: United States of America.
The second series from Regalado-Artamendi et al[40] collected 177 cases affected by COVID-19 in Spain. The median incubation time was again 5 d, with fever and cough as the most frequent symptoms at presentation; the presence of dyspnea at presentation was related to CFR. More than 85% of patients required hospital admission, with 9% admitted to the ICU and an overall mortality rate of 34.5%.
Numerous case reports of patients affected by lymphoma and COVID-19 have been reported and summarized in Table 3. These cases have been published over the last 12 mo, witnessing the widespread interest of the scientific community and the difficulties encountered in the management of these patients. Several lymphoma histotypes are described, with disparate outcomes.
Table 3.
Case reports and case series of coronavirus disease 2019 infection in lymphoma patients
| Ref. | No. of patients described | Sex | Age | Details on lymphoma diagnosis | Details on lymphoma treatment | Outcome of COVID-19 infection | Global outcome |
| Li et al[61], 2020 | 1 | M | 26 yr | PMLBCL | R-DA-EPOCH | Recovered | Alive |
| Tepasse et al[62], 2020 | 2 | M | 65 yr | DLBCL with CNS relapse | R-DeVIC | Not recovered | Dead |
| M | 66 yr | MCL in CR | Rituximab maintenance | Not recovered | Dead | ||
| O'Kelly et al[63], 2020 | 1 | cHL second relapse | Pembrolizumab | Recovered | Alive | ||
| Baang et al[64], 2021 | 1 | M | 60 yr | Relapsed/Refractory MCL | R-CHOP | Recovered | Alive |
| Moore et al[65], 2020 | 1 | F | 63 yr | NHL | Obinotuzumab maintenance | Recovered | Alive |
| Alsuliman et al[66], 2020 | 2 | M | 71 yr | MCL relapsed | Ibrutinib | Recovered | Alive |
| M | NR | MCL relapsed | Ibrutinib | Recovered | Alive | ||
| Hoffmann et al[67], 2021 | 3 | F | 68 yr | DLBCL, FL | R-CHOP | Recovered | Alive |
| M | 60 yr | DLBCL, FL | R-ICE | Not recovered | Dead | ||
| M | 75 yr | DLBCL | R-CHOP | Not recovered | Dead | ||
| Yonal-hindilerden et al[68], 2021 | 1 | F | 55 yr | Relapsed/Refractory cHL | Brentuximab | Not recovered | Dead |
| Fujii et al[69], 2021 | 1 | M | 43 yr | cHL | A + AVD | Recovered | Alive |
| Kamel, 2021 | 1 | M | 58 yr | ALCL | None | Not recovered | Dead |
| Santana et al[70], 2021 | 1 | F | 47 yr | FL | Rituximab maintenance | Recovered | Alive |
| Velier et al[71], 2021 | 1 | F | 61 yr | WM | None | Recovered | Dead |
| Pelcovits et al[72], 2021 | 1 | M | 43 yr | High Grade B Cell Lymphoma, NOS | R-CODOX-M/IVAC | Recovered | Alive |
| Otsuka et al[73], 2020 | 1 | M | 56 yr | MCL | R-hyper CVAD/MA | Not recovered | Dead |
A + AVD: Brentuximab vedotin, dacarbazine, doxorubicin, vinblastine; ALCL: Anaplastic large-cell lymphoma; cHL: Classic Hodgkin lymphoma; CNS: Cerebral nervous system; COVID-19: Coronavirus disease 2019; CR: Complete remission; DLBCL: Diffuse large B-cell lymphoma; F: Female; FL: Follicular lymphoma; M: Male; MCL: Mantle cell lymphoma; NHL: Non-Hodgkin lymphoma; NOS: Not otherwise specified; PMLBCL: Primary mediastinal large B-cell lymphoma; R-CHOP: Rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; R-CODOX-M/IVAC: Rituximab, cyclophosphamide, vincristine, doxorubicin and methotrexate alternating with ifosfamide, etoposide and cytarabine; R-DA-EPOCH: Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin and rituximab; R-DeVIC: Rituximab, dexamethasone, etoposide, ifosfamide carboplatin; R-hyper CVAD/MA: Rituximab/cyclophosphamide/vincristine sulfate/doxorubicin and hydrochloride/dexamethasone/methotrexate/cytarabine; R-ICE: Rituximab, ifosfamide, carboplatin and etoposide; WM: Waldenstrom macroglobulinemia.
PROGNOSTIC FACTORS ASSOCIATED WITH SURVIVAL IN PATIENTS WITH LYMPHOMA
As previously mentioned, in most of the cancer series including HM, male sex, active disease and advanced age were associated with higher CFR attributed to COVID-19[30,36,41,42]. Passamonti et al[37] observed that overall survival in patients affected by HM and COVID-19 was independently predicted by age, type of malignancy, disease status and the severity of COVID-19. NHL (with no mention of histological subtype), acute myeloid leukemia and plasma cell neoplasms, together with progressive disease status, were independently predictive of poor outcomes. Among patients with NHLs, 4 (31%) of 13 patients on rituximab maintenance, 27 (47%) of 57 on active treatment with rituximab–chemotherapy and 8 (44%) of 18 on chemotherapy alone died. No association between overall survival and time since HM diagnosis or last treatment was described. In Lamure et al[39] series from France, which specifically focused on hospital admitted lymphoma patients with a median follow-up of 33 d from admission, 30 d overall survival was 71%, (95% confidence interval: 62%-81%). Factors independently associated with death were advanced age (> 70 years) and relapsed/refractory lymphoma. Interestingly, treatment with bendamustine (n = 9) was associated with a higher risk of death. No significant difference in the rate of death was described for patients with different lymphoma histology.
In the Regalado-Artamendi et al[40] series from Spain, also specifically addressing lymphoma patients, the overall mortality rate was 34.5%. Age > 70 years, heart disease, chronic kidney disease and confusion, urea concentration, respiratory rate, blood pressure and age > 65 score ≥ 2 were statistically significant mortality predictors, resembling previous reports in cancer patients. Among the variables related to lymphoma, the presence of active disease was a strong predictor of death. However, active treatment, the number of previous lines or type of treatment did not modify mortality risk. Quite surprisingly but confirming previous reports, the use of monoclonal antibodies (i.e. rituximab) was not associated with impaired survival for lymphoma patients. The detrimental effect of therapy based on bendamustine was not independently confirmed in this study.
A subanalysis in regard to lymphoma histology observed that aggressive tumors (i.e. diffuse large B-cell lymphoma) were associated with significantly worse overall survival compared with indolent forms (i.e. follicular lymphoma; 50% vs 80%, P = 0.0028). However, the study was not able to demonstrate clear differences between the various lymphoma histologies and therapeutic schemes; these variables were grouped into categories that could have limited the statistical power of this subanalysis. Finally, the persistence of SARS-CoV-2-positive PCR after week 6 was significantly associated with mortality. In the previously cited series describing the outcome of transplanted patients, the subgroup of patients with lymphoma (among other HM) was associated with a higher risk of death compared with plasma cell disorder or myeloma in autologous HSCT recipients[38].
REPORTS OF SPONTANEOUS REMISSIONS IN PATIENTS WITH LYMPHOMAS
Few cases along the literature indicate that some patients may benefit of lymphoma remission when infected by COVID-19. In one case, a dramatic transient reduction in plasmatic Epstein–Barr virus (EBV)-DNA viral copies during COVID-19 pneumonia and resolution of lymphoma relapse were reported[43]. In another report, a 61-year-old man with EBV-positive classical HL with progressive lymphadenopathy and weight loss was admitted with breathlessness and wheezing and was diagnosed with PCR-positive SARS-CoV-2 pneumonia. No corticosteroid or immunochemotherapy was administered. Four months later, palpable lymphadenopathy had reduced, and an interim positron emission tomography–computed tomography scan revealed widespread resolution of the lymphadenopathy. The EBV viral PCR had also fallen[44]. The authors hypothesized that the SARS-CoV-2 infection triggered an antitumor immune response, as it has been described with other infections in the context of high-grade NHL. It is noteworthy that in both cases EBV reactivation was present.
A 61-year-old patient affected from follicular lymphoma also noted a shrinkage of a para-aortic lymph nodal lesion compared to baseline during SARS-CoV-2 infection[45]. Finally, complete spontaneous remission of diffuse large B-cell lymphoma of the maxillary sinus after concurrent SARS-CoV-2 infection was reported, with the patient’s facial swelling resolving during the hospitalization[46].
Since these reports represent anecdotal observations, further data are needed to address or confirm the relationship between the virus and lymphoma subtypes as well its behavior in parallel to anti-neoplastic response.
CONCLUSION
In our opinion, our search for lymphoma patients among other cancer in the recent COVID-19 literature may deliver some important messages for the scientific community. The analysis we performed reveals that there is an increased risk of COVID-19 related serious events (ICU admission, mechanical ventilation support or death) in patients with lymphomas as compared to COVID-19 patients without cancer and confirms the high vulnerability of such patients in the current pandemic. Overall, among the HM series, lymphoma represented the commonest malignancy. In lymphoma patients COVID-19 presentation symptoms occurred a median of 5 to 6 d before hospitalization, being represented by fever, cough and dyspnea. The mortality rate, taking into account the different characteristics of the populations studied, and different lymphoma subtypes was relatively high, attesting at approximately 30% after 1-2 mo of follow-up, at least in hospitalized patients.
In a meta-analysis of hematologic malignancies and COVID-19 that incorporated data from more than 3000 patients, pooled risk of death for lymphomas was 32%[28].
Active disease at COVID-19 infection presentation or lymphoma status as progressive disease appeared to be among the strongest predictors of early death. Among histotypes, no definitive conclusions can be drawn, while the use of bendamustine (but not anti-CD20 antibodies) has been associated with increased risk of death in at least one study. Published results indicate that the start of treatment should not be delayed given that active treatment has not been associated to increased risk of mortality. Instead, achieving disease remission could lead to better outcomes. Currently, little is known about specific phenotypic and/or functional T cell changes associated with symptomatic and asymptomatic SARS-CoV-2 infection, as in patients treated with immune checkpoint inhibitors. In cancer patients[47,48], treatment with immune checkpoint inhibitors did not increase risk of adverse events compared to standard chemotherapy and did not seem to increase COVID-19 susceptibility. However, no data are reported on patients with lymphoma.
With several vaccines available, it would be extremely important to protect frail categories as soon as possible. The humoral response of patients with lymphoma to COVID-19 vaccines has been investigated by several groups[49]. Altogether, these data suggest that the humoral response in lymphoma patients is impaired as compared to other HM, especially after treatment with anti-CD20 containing therapies. Different vaccination strategies are therefore warranted for lymphoma patients. Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of patients with COVID-19 infection.
Footnotes
Conflict-of-interest statement: The authors do not have any conflict of interest. No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review started: April 21, 2021
First decision: July 7, 2021
Article in press: August 9, 2021
Specialty type: Hematology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dou AX, Yoshida N, Zhu F S-Editor: Wang JL L-Editor: Filipodia P-Editor: Zhang YL
Contributor Information
Valentina Bonuomo, Section of Haematology, Department of Medicine, University of Verona, Verona 37134, Italy.
Isacco Ferrarini, Section of Haematology, Department of Medicine, University of Verona, Verona 37134, Italy.
Michele Dell'Eva, Section of Haematology, Department of Medicine, University of Verona, Verona 37134, Italy.
Eugenio Sbisà, Section of Haematology, Department of Medicine, University of Verona, Verona 37134, Italy.
Mauro Krampera, Section of Haematology, Department of Medicine, University of Verona, Verona 37134, Italy.
Carlo Visco, Section of Haematology, Department of Medicine, University of Verona, Verona 37134, Italy. carlo.visco@univr.it.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med . 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Driscoll M, Ribeiro Dos Santos G, Wang L, Cummings DAT, Azman AS, Paireau J, Fontanet A, Cauchemez S, Salje H. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature . 2021;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet . 2020;395:1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, Rajagopal S, Pai AR, Kutty S. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front Immunol . 2020;11:1648. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet . 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salzberger B, Buder F, Lampl B, Ehrenstein B, Hitzenbichler F, Holzmann T, Schmidt B, Hanses F. Epidemiology of SARS-CoV-2. Infection . 2021;49:233–239. doi: 10.1007/s15010-020-01531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, Xiong Y, Xiong H, Wang C, Chen C, Xiong F, Zhang Y, Peng Y, Ge S, Zhen B, Yu T, Wang L, Wang H, Liu Y, Chen Y, Mei J, Gao X, Li Z, Gan L, He C, Shi Y, Qi Y, Yang J, Tenen DG, Chai L, Mucci LA, Santillana M, Cai H. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov . 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol . 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 Transmission in Patients With Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol . 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, Cruz C. Do patients with cancer have a poorer prognosis of COVID-19? Ann Oncol . 2020;31:1088–1089. doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, Shete S, Hsu CY, Desai A, de Lima Lopes G Jr, Grivas P, Painter CA, Peters S, Thompson MA, Bakouny Z, Batist G, Bekaii-Saab T, Bilen MA, Bouganim N, Larroya MB, Castellano D, Del Prete SA, Doroshow DB, Egan PC, Elkrief A, Farmakiotis D, Flora D, Galsky MD, Glover MJ, Griffiths EA, Gulati AP, Gupta S, Hafez N, Halfdanarson TR, Hawley JE, Hsu E, Kasi A, Khaki AR, Lemmon CA, Lewis C, Logan B, Masters T, McKay RR, Mesa RA, Morgans AK, Mulcahy MF, Panagiotou OA, Peddi P, Pennell NA, Reynolds K, Rosen LR, Rosovsky R, Salazar M, Schmidt A, Shah SA, Shaya JA, Steinharter J, Stockerl-Goldstein KE, Subbiah S, Vinh DC, Wehbe FH, Weissmann LB, Wu JT, Wulff-Burchfield E, Xie Z, Yeh A, Yu PP, Zhou AY, Zubiri L, Mishra S, Lyman GH, Rini BI, Warner JL COVID-19 and Cancer Consortium. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet . 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, Chackathayil J, Cheng VW, Curley HM, Fittall MW, Freeman-Mills L, Gennatas S, Goel A, Hartley S, Hughes DJ, Kerr D, Lee AJ, Lee RJ, McGrath SE, Middleton CP, Murugaesu N, Newsom-Davis T, Okines AF, Olsson-Brown AC, Palles C, Pan Y, Pettengell R, Powles T, Protheroe EA, Purshouse K, Sharma-Oates A, Sivakumar S, Smith AJ, Starkey T, Turnbull CD, Várnai C, Yousaf N UK Coronavirus Monitoring Project Team, Kerr R, Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet . 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, Lu H, Liu J, Yang J, Dong Y, Pan D, Shu C, Li J, Wei J, Huang Y, Peng L, Wu M, Zhang R, Wu B, Li Y, Cai L, Li G, Zhang T, Wu G. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol . 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Society of Hematology. Blood Cancers. Available from: https://www.hematology.org/education/patients/blood-cancers .
- 15.Safdar A, Armstrong D. Infections in patients with hematologic neoplasms and hematopoietic stem cell transplantation: neutropenia, humoral, and splenic defects. Clin Infect Dis . 2011;53:798–806. doi: 10.1093/cid/cir492. [DOI] [PubMed] [Google Scholar]
- 16.Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull . 2008;87:49–62. doi: 10.1093/bmb/ldn034. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Berger NA, Xu R. When hematologic malignancies meet COVID-19 in the United States: Infections, death and disparities. Blood Rev . 2021;47:100775. doi: 10.1016/j.blre.2020.100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, Walewski J, André M, Johnson PW, Pfreundschuh M, Ladetto M ESMO Guidelines Committee. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol . 2015;26 Suppl 5:v116–v125. doi: 10.1093/annonc/mdv304. [DOI] [PubMed] [Google Scholar]
- 19.Pfreundschuh M, Kuhnt E, Trümper L, Osterborg A, Trneny M, Shepherd L, Gill DS, Walewski J, Pettengell R, Jaeger U, Zinzani PL, Shpilberg O, Kvaloy S, de Nully Brown P, Stahel R, Milpied N, López-Guillermo A, Poeschel V, Grass S, Loeffler M, Murawski N MabThera International Trial (MInT) Group. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol . 2011;12:1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 20.Averbuch D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C, Gyssens IC, Kern WV, Klyasova G, Marchetti O, Engelhard D, Akova M ECIL4, a joint venture of EBMT, EORTC, ICHS, ESGICH/ESCMID and ELN. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica . 2013;98:1826–1835. doi: 10.3324/haematol.2013.091025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maschmeyer G, De Greef J, Mellinghoff SC, Nosari A, Thiebaut-Bertrand A, Bergeron A, Franquet T, Blijlevens NMA, Maertens JA European Conference on Infections in Leukemia (ECIL) Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL) Leukemia . 2019;33:844–862. doi: 10.1038/s41375-019-0388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Visco C, Barcellini W, Maura F, Neri A, Cortelezzi A, Rodeghiero F. Autoimmune cytopenias in chronic lymphocytic leukemia. Am J Hematol . 2014;89:1055–1062. doi: 10.1002/ajh.23785. [DOI] [PubMed] [Google Scholar]
- 23.Gafter-Gvili A, Polliack A. Bendamustine associated immune suppression and infections during therapy of hematological malignancies. Leuk Lymphoma . 2016;57:512–519. doi: 10.3109/10428194.2015.1110748. [DOI] [PubMed] [Google Scholar]
- 24.Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol . 2010;47:187–198. doi: 10.1053/j.seminhematol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Hua Q, Zhu Y, Liu H. Severe and fatal adverse events risk associated with rituximab addition to B-cell non-Hodgkin's lymphoma (B-NHL) chemotherapy: a meta-analysis. J Chemother . 2015;27:365–370. doi: 10.1179/1973947815Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 26.Tudesq JJ, Cartron G, Rivière S, Morquin D, Iordache L, Mahr A, Pourcher V, Klouche K, Cerutti D, Le Quellec A, Guilpain P. Clinical and microbiological characteristics of the infections in patients treated with rituximab for autoimmune and/or malignant hematological disorders. Autoimmun Rev . 2018;17:115–124. doi: 10.1016/j.autrev.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. COVID-19 Weekly Epidemiological Update 22, 2020 December. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/weekly_epidemiological_update_22.pdf .
- 28.Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, Martín-Moro F, Razanamahery J, Riches JC, Zwicker J, Patell R, Vekemans MC, Scarfò L, Chatzikonstantinou T, Yildiz H, Lattenist R, Mantzaris I, Wood WA, Hicks LK. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood . 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yigenoglu TN, Ata N, Altuntas F, Bascı S, Dal MS, Korkmaz S, Namdaroglu S, Basturk A, Hacıbekiroglu T, Dogu MH, Berber İ, Dal K, Erkurt MA, Turgut B, Ulgu MM, Celik O, Imrat E, Birinci S. The outcome of COVID-19 in patients with hematological malignancy. J Med Virol . 2021;93:1099–1104. doi: 10.1002/jmv.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee LYW, Cazier JB, Starkey T, Briggs SEW, Arnold R, Bisht V, Booth S, Campton NA, Cheng VWT, Collins G, Curley HM, Earwaker P, Fittall MW, Gennatas S, Goel A, Hartley S, Hughes DJ, Kerr D, Lee AJX, Lee RJ, Lee SM, Mckenzie H, Middleton CP, Murugaesu N, Newsom-Davis T, Olsson-Brown AC, Palles C, Powles T, Protheroe EA, Purshouse K, Sharma-Oates A, Sivakumar S, Smith AJ, Topping O, Turnbull CD, Várnai C, Briggs ADM, Middleton G, Kerr R UK Coronavirus Cancer Monitoring Project Team. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol . 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He W, Chen L, Yuan G, Fang Y, Chen W, Wu D, Liang B, Lu X, Ma Y, Li L, Wang H, Chen Z, Li Q, Gale RP. COVID-19 in persons with haematological cancers. Leukemia . 2020;34:1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, Pradhan K, Thota R, Reissman S, Sparano JA, Gartrell BA, Smith RV, Ohri N, Garg M, Racine AD, Kalnicki S, Perez-Soler R, Halmos B, Verma A. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov . 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín-Moro F, Marquet J, Piris M, Michael BM, Sáez AJ, Corona M, Jiménez C, Astibia B, García I, Rodríguez E, García-Hoz C, Fortún-Abete J, Herrera P, López-Jiménez J. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol . 2020;190:e16–e20. doi: 10.1111/bjh.16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aries JA, Davies JK, Auer RL, Hallam SL, Montoto S, Smith M, Sevillano B, Foggo V, Wrench B, Zegocki K, Agrawal S, Le Dieu R, Truelove E, Erblich T, Araf S, Okosun J, Oakervee H, Cavenagh JD, Gribben JG, Riches JC. Clinical outcome of coronavirus disease 2019 in haemato-oncology patients. Br J Haematol . 2020;190:e64–e67. doi: 10.1111/bjh.16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood WA, Neuberg DS, Thompson JC, Tallman MS, Sekeres MA, Sehn LH, Anderson KC, Goldberg AD, Pennell NA, Niemeyer CM, Tucker E, Hewitt K, Plovnick RM, Hicks LK. Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH Research Collaborative Data Hub. Blood Adv . 2020;4:5966–5975. doi: 10.1182/bloodadvances.2020003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rüthrich MM, Giessen-Jung C, Borgmann S, Classen AY, Dolff S, Grüner B, Hanses F, Isberner N, Köhler P, Lanznaster J, Merle U, Nadalin S, Piepel C, Schneider J, Schons M, Strauss R, Tometten L, Vehreschild JJ, von Lilienfeld-Toal M, Beutel G, Wille K LEOSS Study Group. COVID-19 in cancer patients: clinical characteristics and outcome-an analysis of the LEOSS registry. Ann Hematol . 2021;100:383–393. doi: 10.1007/s00277-020-04328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, Angelucci E, Krampera M, Cairoli R, Della Porta MG, Fracchiolla N, Ladetto M, Gambacorti Passerini C, Salvini M, Marchetti M, Lemoli R, Molteni A, Busca A, Cuneo A, Romano A, Giuliani N, Galimberti S, Corso A, Morotti A, Falini B, Billio A, Gherlinzoni F, Visani G, Tisi MC, Tafuri A, Tosi P, Lanza F, Massaia M, Turrini M, Ferrara F, Gurrieri C, Vallisa D, Martelli M, Derenzini E, Guarini A, Conconi A, Cuccaro A, Cudillo L, Russo D, Ciambelli F, Scattolin AM, Luppi M, Selleri C, Ortu La Barbera E, Ferrandina C, Di Renzo N, Olivieri A, Bocchia M, Gentile M, Marchesi F, Musto P, Federici AB, Candoni A, Venditti A, Fava C, Pinto A, Galieni P, Rigacci L, Armiento D, Pane F, Oberti M, Zappasodi P, Visco C, Franchi M, Grossi PA, Bertù L, Corrao G, Pagano L, Corradini P ITA-HEMA-COV Investigators. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol . 2020;7:e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, Dandoy C, Gauthier J, Gowda L, Perales MA, Seropian S, Shaw BE, Tuschl EE, Zeidan AM, Riches ML, Shah GL. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol . 2021;8:e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamure S, Duléry R, Di Blasi R, Chauchet A, Laureana C, Deau-Fischer B, Drenou B, Soussain C, Rossi C, Noël N, Choquet S, Bologna S, Joly B, Kohn M, Malak S, Fouquet G, Daguindau E, Bernard S, Thiéblemont C, Cartron G, Lacombe K, Besson C. Determinants of outcome in Covid-19 hospitalized patients with lymphoma: A retrospective multicentric cohort study. EClinicalMedicine . 2020;27:100549. doi: 10.1016/j.eclinm.2020.100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regalado-Artamendi I, Jiménez-Ubieto A, Hernández-Rivas JÁ, Navarro B, Núñez L, Alaez C, Córdoba R, Peñalver FJ, Cannata J, Estival P, Quiroz-Cervantes K, Riaza Grau R, Velasco A, Martos R, Domingo-González A, Benito-Parra L, Gómez-Sanz E, López-Jiménez J, Matilla A, Herraez MR, Penalva MJ, García-Suárez J, Díez-Martín JL, Bastos-Oreiro M. Risk Factors and Mortality of COVID-19 in Patients With Lymphoma: A Multicenter Study. Hemasphere . 2021;5:e538. doi: 10.1097/HS9.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang J, Jin G, Liu T, Wen J, Li G, Chen L, Wang W, Wang Y, Liao W, Song J, Ding Z, Chen XP, Zhang B. Clinical characteristics and risk factors for mortality in cancer patients with COVID-19. Front Med . 2021;15:264–274. doi: 10.1007/s11684-021-0845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cattaneo C, Daffini R, Pagani C, Salvetti M, Mancini V, Borlenghi E, D'Adda M, Oberti M, Paini A, De Ciuceis C, Barbullushi K, Cancelli V, Belotti A, Re A, Motta M, Peli A, Bianchetti N, Anastasia A, Dalceggio D, Roccaro AM, Tucci A, Cairoli R, Muiesan ML, Rossi G. Clinical characteristics and risk factors for mortality in hematologic patients affected by COVID-19. Cancer . 2020;126:5069–5076. doi: 10.1002/cncr.33160. [DOI] [PubMed] [Google Scholar]
- 43.Pasin F, Mascalchi Calveri M, Calabrese A, Pizzarelli G, Bongiovanni I, Andreoli M, Cattaneo C, Rignanese G. Oncolytic effect of SARS-CoV2 in a patient with NK lymphoma. Acta Biomed . 2020;91:ahead of print. doi: 10.23750/abm.v91i3.10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Challenor S, Tucker D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br J Haematol . 2021;192:415. doi: 10.1111/bjh.17116. [DOI] [PubMed] [Google Scholar]
- 45.Sollini M, Gelardi F, Carlo-Stella C, Chiti A. Complete remission of follicular lymphoma after SARS-CoV-2 infection: from the "flare phenomenon" to the "abscopal effect". Eur J Nucl Med Mol Imaging . 2021;48:2652–2654. doi: 10.1007/s00259-021-05275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckner TW, Dunphy C, Fedoriw YD, van Deventer HW, Foster MC, Richards KL, Park SI. Complete spontaneous remission of diffuse large B-cell lymphoma of the maxillary sinus after concurrent infections. Clin Lymphoma Myeloma Leuk . 2012;12:455–458. doi: 10.1016/j.clml.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Mandala M, Lorigan P, De Luca M, Bianchetti A, Merelli B, Bettini AC, Bonomi L, Nahm S, Vitale MG, Negrini G, Di Croce A, Ascierto PA, Rulli E, Tondini CA. SARS-CoV-2 infection and adverse events in patients with cancer receiving immune checkpoint inhibitors: an observational prospective study. J Immunother Cancer . 2021;9 doi: 10.1136/jitc-2020-001694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klebanov N, Pahalyants V, Murphy WS, Theodosakis N, Zubiri L, Klevens RM, Kwatra SG, Lilly E, Reynolds KL, Semenov YR. Risk of COVID-19 in Patients with Cancer Receiving Immune Checkpoint Inhibitors. Oncologist . 2021;26:e898–e901. doi: 10.1002/onco.13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell . 2021 doi: 10.1016/j.ccell.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, Cai Y, Lu Z, Wang J, Wang Y, Liu S, Cheng B, Zhang M, Wang L, Niu S, Yao Z, Deng X, Zhou F, Wei W, Li Q, Chen X, Chen W, Yang Q, Wu S, Fan J, Shu B, Hu Z, Wang S, Yang XP, Liu W, Miao X, Wang Z. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol . 2020;21:893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biernat MM, Kolasińska A, Kwiatkowski J, Urbaniak-Kujda D, Biernat P, Janocha-Litwin J, Szymczyk-Nużka M, Bursy D, Kalicińska E, Simon K, Mazur G, Wróbel T. Early Administration of Convalescent Plasma Improves Survival in Patients with Hematological Malignancies and COVID-19. Viruses . 2021;13 doi: 10.3390/v13030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Booth S, Willan J, Wong H, Khan D, Farnell R, Hunter A, Eyre T, Katz H, Dungarwalla M, Chen L, Browning J, Polzella P, Gray N, Neelakantan P, Dhillon EK, Dutton D, Sternberg A, Prideaux S, Collins GP, Peniket A. Regional outcomes of severe acute respiratory syndrome coronavirus 2 infection in hospitalised patients with haematological malignancy. Eur J Haematol . 2020;105:476–483. doi: 10.1111/ejh.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox TA, Troy-Barnes E, Kirkwood AA, Chan WY, Day JW, Chavda SJ, Kumar EA, David K, Tomkins O, Sanchez E, Scully M, Khwaja A, Lambert J, Singer M, Roddie C, Morris EC, Yong KL, Thomson KJ, Ardeshna KM. Clinical outcomes and risk factors for severe COVID-19 in patients with haematological disorders receiving chemo- or immunotherapy. Br J Haematol . 2020;191:194–206. doi: 10.1111/bjh.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.García-Suárez J, de la Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V, Hernández-Rivas JÁ, Gil-Manso R, Kwon M, Sánchez-Godoy P, Martínez-Barranco P, Colás-Lahuerta B, Herrera P, Benito-Parra L, Alegre A, Velasco A, Matilla A, Aláez-Usón MC, Martos-Martínez R, Martínez-Chamorro C, Susana-Quiroz K, Del Campo JF, de la Fuente A, Herráez R, Pascual A, Gómez E, Pérez-Oteyza J, Ruiz E, Alonso A, González-Medina J, Martín-Buitrago LN, Canales M, González-Gascón I, Vicente-Ayuso MC, Valenciano S, Roa MG, Monteliu PE, López-Jiménez J, Escobar CE, Ortiz-Martín J, Diez-Martin JL, Martinez-Lopez J Asociación Madrileña de Hematología y Hemoterapia (AMHH) Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol . 2020;13:133. doi: 10.1186/s13045-020-00970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Infante MS, González-Gascón Y Marín I, Muñoz-Novas C, Churruca J, Foncillas MÁ, Landete E, Marín K, Ryan P, Hernández-Rivas JÁ. COVID-19 in patients with hematological malignancies: A retrospective case series. Int J Lab Hematol . 2020;42:e256–e259. doi: 10.1111/ijlh.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lattenist R, Yildiz H, De Greef J, Bailly S, Yombi JC. COVID-19 in Adult Patients with Hematological Disease: Analysis of Clinical Characteristics and Outcomes. Indian J Hematol Blood Transfus . 2020:1–5. doi: 10.1007/s12288-020-01318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malard F, Genthon A, Brissot E, van de Wyngaert Z, Marjanovic Z, Ikhlef S, Banet A, Lapusan S, Sestilli S, Corre E, Paviglianiti A, Adaeva R, M 'Hammedi-Bouzina F, Labopin M, Legrand O, Dulery R, Mohty M. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant . 2020;55:2180–2184. doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez-Pina JM, Rodríguez Rodriguez M, Castro Quismondo N, Gil Manso R, Colmenares R, Gil Alos D, Paciello ML, Zafra D, Garcia-Sanchez C, Villegas C, Cuellar C, Carreño-Tarragona G, Zamanillo I, Poza M, Iñiguez R, Gutierrez X, Alonso R, Rodríguez A, Folgueira MD, Delgado R, Ferrari JM, Lizasoain M, Aguado JM, Ayala R, Martinez-Lopez J, Calbacho M. Clinical course and risk factors for mortality from COVID-19 in patients with haematological malignancies. Eur J Haematol . 2020;105:597–607. doi: 10.1111/ejh.13493. [DOI] [PubMed] [Google Scholar]
- 59.van Doesum J, Chinea A, Pagliaro M, Pasquini MC, van Meerten T, Bakker M, Ammatuna E. Clinical characteristics and outcome of SARS-CoV-2-infected patients with haematological diseases: a retrospective case study in four hospitals in Italy, Spain and the Netherlands. Leukemia . 2020;34:2536–2538. doi: 10.1038/s41375-020-0960-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laurenge A, Ursu R, Houillier C, Abdi B, Tebano G, Quemeneur C, Choquet S, Di Blasi R, Lozano F, Morales A, Durán-Peña A, Sirven-Villaros L, Mathon B, Mokhtari K, Bielle F, Martin-Duverneuil N, Delattre JY, Marcelin AG, Pourcher V, Alentorn A, Idbaih A, Carpentier AF, Leblond V, Hoang-Xuan K, Touat M. SARS-CoV-2 infection in patients with primary central nervous system lymphoma. J Neurol . 2021 doi: 10.1007/s00415-020-10311-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Q, Zhu F, Xiao Y, Liu T, Liu X, Wu G, Zhang L. A Primary Mediastinal Large B-Cell Lymphoma Patient With COVID-19 Infection After Intensive Immunochemotherapy: A Case Report. Front Oncol . 2020;10:924. doi: 10.3389/fonc.2020.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tepasse PR, Hafezi W, Lutz M, Kühn J, Wilms C, Wiewrodt R, Sackarnd J, Keller M, Schmidt HH, Vollenberg R. Persisting SARS-CoV-2 viraemia after rituximab therapy: two cases with fatal outcome and a review of the literature. Br J Haematol . 2020;190:185–188. doi: 10.1111/bjh.16896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Kelly B, McGettrick P, Angelov D, Fay M, McGinty T, Cotter AG, Sheehan G, Lambert JS. Outcome of a patient with refractory Hodgkin lymphoma on pembrolizumab, infected with SARS-CoV-2. Br J Haematol . 2020;190:e1–e3. doi: 10.1111/bjh.16798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baang JH, Smith C, Mirabelli C, Valesano AL, Manthei DM, Bachman MA, Wobus CE, Adams M, Washer L, Martin ET, Lauring AS. Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 Replication in an Immunocompromised Patient. J Infect Dis . 2021;223:23–27. doi: 10.1093/infdis/jiaa666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore JL, Ganapathiraju PV, Kurtz CP, Wainscoat B. A 63-Year-Old Woman with a History of Non-Hodgkin Lymphoma with Persistent SARS-CoV-2 Infection Who Was Seronegative and Treated with Convalescent Plasma. Am J Case Rep . 2020;21:e927812. doi: 10.12659/AJCR.927812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alsuliman T, Faict S, Malard F, Genthon A, Brissot E, Van de Wyngaert Z, Ikhlef S, Banet A, Lapusan S, Sestili S, Corre E, M'hammedi-Bouzina F, Schaeffer L, Legrand O, Dulery R, Mohty M, Marjanovic Z. Does Ibrutinib impact outcomes of viral infection by SARS-CoV-2 in mantle cell lymphoma patients? Curr Res Transl Med . 2021;69:103273. doi: 10.1016/j.retram.2020.103273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoffmann MS, Ganguly S. Delayed COVID-19 Respiratory Failure in Patients with Lymphoma on Rituximab-based Chemoimmunotherapy. Clin Lymphoma Myeloma Leuk . 2021;21:e548–e550. doi: 10.1016/j.clml.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yonal-Hindilerden I, Hindilerden F, Mastanzade M, Tiryaki TO, Tasan-Yenigun S, Bilen Y, Aksoz S, Cagatay AA, Nalcaci M. Case Report: Severe COVID-19 Pneumonia in a Patient With Relapsed/Refractory Hodgkin's Lymphoma. Front Oncol . 2021;11:601709. doi: 10.3389/fonc.2021.601709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fujii H, Tsuji T, Sugitani M, Matsumoto Y, Yuba T, Tanaka S, Suga Y, Matsuyama A, Goda S, Omura A, Shiotsu S, Takumi C, Ono S, Hiraoka N. Prolonged persistence of SARS-CoV-2 infection during A+AVD therapy for classical Hodgkin's lymphoma: A case report. Curr Probl Cancer . 2021:100739. doi: 10.1016/j.currproblcancer.2021.100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santana ANC, Melo FX, Xavier FD, Amado VM. Migratory pulmonary infiltrates in a patient with COVID-19 and lymphoma. J Bras Pneumol . 2021;47:e20200528. doi: 10.36416/1806-3756/e20200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Velier M, Priet S, Appay R, Atieh T, Lepidi H, Kaplanski G, Jarrot PA, Koubi M, Costello R, Dignat-George F, de Lamballerie X, Tichadou A, Arcani R, Couderc AL, Touati J, Varoquaux A, Berda-Haddad Y, Venton G. Severe and Irreversible Pancytopenia Associated With SARS-CoV-2 Bone Marrow Infection in a Patient With Waldenstrom Macroglobulinemia. Clin Lymphoma Myeloma Leuk . 2021;21:e503–e505. doi: 10.1016/j.clml.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pelcovits A, Pandita A, Farmakiotis D, Egan P. Lymphocyte-depleting chemotherapy for aggressive hematologic malignancies in two patients with positive SARS-CoV-2 PCR. Leuk Res . 2021;100:106473. doi: 10.1016/j.leukres.2020.106473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Otsuka Y, Kobayashi T. Case Report: A Patient with COVID-19 under Myelosuppression Induced by Chemotherapy. Am J Trop Med Hyg . 2020;103:1983–1985. doi: 10.4269/ajtmh.20-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]