Abstract
Variceal bleeding is a serious complication of cirrhosis and portal hypertension. Despite the improvement in management of acute variceal bleed (AVB), it still carries significant mortality. Portal pressure is the main driver of variceal bleeding and also a main predictor of decompensation. Reduction in portal pressure has been the mainstay of management of variceal bleeding. Transjugular intrahepatic porto-systemic stent shunt (TIPSS) is a very effective modality in reducing the portal hypertension and thereby, controlling portal hypertensive bleeding. However, its use in refractory bleeding (rescue/salvage TIPSS) is still associated with high mortality. “Early” use of TIPSS as a “pre-emptive strategy” in patients with AVB at high risk of failure of treatment has shown to be superior to standard treatment in several studies. While patients with Child C cirrhosis (up to 13 points) clearly benefit from early-TIPSS strategy, it’s role in less severe liver disease (Child B) and more severe disease (Child C > 13 points) remains less clear. Moreover, standard of care has improved in the last decade leading to improved 1-year survival in high-risk patients with AVB as compared to earlier “early” TIPSS studies. Lastly in the real world, only a minority of patients with AVB fulfil the stringent criteria for early TIPSS. Therefore, there is unmet need to explore role of early TIPSS in management of AVB in well-designed prospective studies. In this review, we have appraised the role of early TIPSS, patient selection and discussed future directions in the management of patients with AVB.
Keywords: Transjugular intrahepatic portosystemic stent-shunt, Early transjugular intrahepatic portosystemic stent-shunt, Salvage transjugular intrahepatic portosystemic stent-shunt, Portal hypertension, Acute variceal bleed, Hepatic encephalopathy
Core Tip: Outcome of high-risk patients following episode of acute variceal bleeding (AVB) is poor and insertion of transjugular intrahepatic portosystemic stent-shunt (TIPSS) within 72 h of index endoscopy (early or pre-emptive TIPSS) is associated with remarkable outcomes in a selection of patients (Child C up to 13 points). However, it’s efficacy in Child B patients is debatable and criteria for high-risk patients needs to be refined. Moreover, management of variceal bleeding has improved in last decade and provision of early TIPSS (within 72 h) is challenging in most healthcare facilities. In this paper we have discussed the role of early TIPSS, patient selection and future directions in management of AVB.
INTRODUCTION
Acute variceal bleeding (AVB) is a severe complication of portal hypertension and occurs at a rate of around 10%-15% per year in patients with cirrhosis. The risk of variceal bleeding depends on the severity of liver disease, size of varices, and presence of red wale marks[1]. Six-week mortality following an episode of AVB (the endpoint identified as the key outcome in variceal bleeding) is reported to be between 15% and 25%[2-4]. Early mortality was reported to be 50% in the early eighties[5]. The presence of clinically significant portal hypertension is the main factor determining the risk of development of varices and other liver-related decompensations. Transjugular intrahepatic portosystemic stent-shunt (TIPSS) was initially used for management of refractory variceal bleeding only (salvage or rescue TIPSS), followed by prevention of rebleeding or as secondary prophylaxis. There has been recent interest in early or pre-emptive TIPSS (e-TIPSS) in selected patients at high risk of treatment failure and mortality. There remains considerable controversy in the utility of early TIPSS, and we aim to provide a summary of the current evidence and discuss unresolved issues and future directions.
IDENTIFYING PATIENTS AT RISK OF A POOR OUTCOME FOLLOWING AVB
Although the prognosis of AVB has significantly improved over the last decades due to better management of haemorrhage and its associated complications, mortality is still as high as 15%-20%[2].
Patients presenting with AVB do not benefit equally from standard treatment as not all patients have the same risk profile of treatment failure, re-bleeding and mortality. The risk of rebleeding (and subsequently death) is greatest in the first 48-72 h after the initial episode and over 50% of rebleeding episodes occur within the first 10 d[6-8]. Therefore, it is important to identify those patients who are at high risk of treatment failure and death in whom a more aggressive approach, like implantation of early or pre-emptive TIPSS (within 72 h of index bleeding) can be utilised.
Measurement of the hepatic venous pressure gradient (HVPG) is the gold standard method for evaluating portal hypertension[9]. Portal hypertension is defined as an increase of HVPG > 5 mmHg; and HVPG ≥ 10 mmHg is defined as clinically significant portal hypertension as above this threshold, varices usually appear and risk of developing overt clinical decompensation (variceal bleeding, ascites and hepatic encephalopathy) increases[9,10]. If varices remain untreated, rebleeding and death occur in approximately 60% and 30% of patients respectively, one to two years after the index bleeding[1].
HVPG measured within 24 h of the bleeding episode is shown to be a prognostic indicator for outcome following AVB. HVPG > 20 mmHg has been associated with up to 5-fold increased risk of failure to control bleeding and one-year mortality[11,12]. Decrease in portal pressure of ≥ 20% from the baseline or to HVPG ≤ 12 mmHg is associated with significant reduction in risk of decompensation and with improved survival[13].
Recent data also show that decreasing HVPG by > 10% from baseline, or to absolute values < 10 mmHg, reduces the risk of development of varices and AVB regardless of the presence of varices[14]. Therefore, lowering HVPG has been one of the treatment strategies in management of AVB.
Portal hypertension correlates strongly with severity of liver disease measured by Child-Pugh score[13]. The severity of liver disease remains the main determinant of prognosis in patients with AVB[15,16]; There is a strong relationship between the presence of HVPG > 20 mmHg and Child–Pugh class[11,17]. Therefore, Child-Pugh Class C is associated with poor outcome following AVB. Moreover, presence of ascites and bacterial infections are also associated with poor outcome[18].
Severity of bleeding (active bleeding on endoscopy and haematocrit level) as well as presence of portal vein thrombosis are also among the significant predictors of early treatment failure following AVB[19].
Recalibrated MELD score (−5.312 + 0.207 × MELD) has been developed to predict early mortality after an episode of AVB. MELD score of 19 or higher is associated with a higher mortality risk of 20%[2]. The utility of recalibrated MELD in predicting outcome has recently been validated in 2 observational studies[20,21]. Similarly, Child-Pugh Class C is associated with higher mortality risk than in Child–Pugh class A and B cirrhosis, regardless of the presence of active bleeding[21].
In a recently published study acute-on-chronic liver failure (ACLF) at baseline is also found to be an independent risk factor for rebleeding and mortality in patients presenting with AVB. Presence of ACLF almost doubled the risk of rebleeding[22].
SALVAGE TIPSS
In the 1980s, the prognosis in patients with refractory or uncontrolled variceal bleeding was poor with mortality of over 90% in Child-Pugh B and C patients[23]. Though rescue surgical treatments (oesophageal transection or surgical porto-systemic shunting) were effective in decreasing portal hypertension, these procedures were associated with high mortality, ranging from 50% to 90% in this situation[24,25]. Moreover, subsequent liver transplantation may become technically more difficult to perform following porto-systemic shunt surgery[25].
The concept of percutaneous transjugular porto-systemic shunt in context of oesophageal variceal bleeding in humans was first introduced by Colapinto et al[26] in 1982 (in which intrahepatic portosystemic shunt was created by dilating the track with an angioplasty balloon). First (prospective) study evaluating the role of salvage TIPSS in patients with variceal haemorrhage refractory to (then) standard medical and endoscopic treatment was published in 1994[27]. In that study though salvage TIPSS (with bare stent) was associated with immediate control of bleeding in all 20 patients, 40-d mortality was very high at 60% mainly due to liver failure and sepsis[27].
Several (retrospective) studies were published afterwards, evaluating the role of salvage (rescue) TIPSS (using uncovered stents) in setting of refractory variceal bleeding[28-30]. Salvage TIPSS was effective in controlling the variceal bleeding but early mortality rate remained high in these patients, approaching 48% at 45-d. Majority of the patients died due to multi-organ failure and sepsis. Child-Pugh (CP > 11), APACHE II and MELD scores (> 20) were associated with increased mortality[29,30]. These studies were uncontrolled, mainly involved uncovered stents and sclerotherapy was the choice of endoscopic treatment.
Standard treatment of AVB has improved considerably in the recent decade and covered TIPSS has lower risk of stent dysfunction as compared to bare metal stents[31,32]. In subsequently reported retrospective studies of salvage TIPSS using both covered and uncovered stents, the use of covered stent did not culminate in survival advantage at both 6 wk and 1-year[33,34]. However, use of bare metal stent was associated with increased rate of re-bleeding due to stent dysfunction and salvage TIPSS appeared to be futile in patients with Child-Pugh score of > 13[34].
A recently published Chinese retrospective study of 58 patients, in which 55 patients had covered stents, showed better 6-weeks and 1-year transplant free survivals (87.7% and 81.8%, respectively) following salvage TIPSS[35]. Treatment failure at 6 wk was associated with bare stents and white cell count. It is important to note that 62% patients had Child B disease and over 60% had hepatitis B related disease. Only 30% of patients had Child C disease. Median MELD score was 10 and mean Child score was 8.7, indicating that majority of patients had less severe disease (but with high portal pressure)[35]. Moreover, 82% of patients had variceal embolization[35], an effective tool to prevent re-bleeding[36,37].
EARLY TIPSS
Randomised control trials in e-TIPSS
It is important to clarify the concept of e-TIPSS. e-TIPSS strategy refers to a pre-emptive placement of TIPSS in those at a high-risk of treatment failure before treatment failure or re-bleeding occurs. In this setting, TIPSS is usually placed within 24–72 h of successful therapeutic endoscopy (with patients already on pharmacological therapy with vasoactive drugs and antibiotics). The rationale of this strategy is that reducing portal pressure early on, will prevent rebleeding, the associated liver failure and development of multi-organ failure with a lot worse outcome. This is in contrast to salvage TIPSS, where TIPSS is placed in patients with refractory variceal bleeding, not controlled with standard treatment; and this group of patients has very high mortality (as described above).
As stated earlier, reduction in portal hypertension is one of the mainstays of management of AVB. Utilising this evidence, Jalan et al[38] introduced the concept of preventive insertion of TIPSS (pre-emptive or early TIPSS placement, within 72 h) to lower portal pressure in cirrhotic patients with AVB in 1990s. They published a randomised control trial (RCT) in 1997 including 58 patients and compared endoscopic band ligation (EBL) with e-TIPSS (with bare-metal stent) randomised within 24 h after controlling of first episode of AVB. Mean time to TIPSS in that study was 2.2 d. e-TIPSS placement was superior to EBL in preventing rebleeding and was cost-effective in this setting. However, no survival difference was seen, although ITU requirement was significantly less with TIPSS. The Child-Pugh score of 9 was similar in the two groups, although there were some Child’s A patients included[38]. Patient selection was not as strict as for subsequent studies. This could explain the lack of difference of survival.
Since then, the role of e-TIPSS in the management of acute variceal bleeding in patients with cirrhosis has been evaluated in several studies. The safety and efficacy of e-TIPSS in high-risk cirrhotic patients has been evaluated in a few RCTs (Table 1).
Table 1.
Early transjugular intrahepatic portosystemic stent-shunt in acute variceal bleeding: Key studies
|
Ref.
|
Main inclusion criteria
|
Primary and secondary outcomes
|
Results
|
Comments
|
| Randomised controlled trials | ||||
| Monescillo et al[12], 2004 (Italy) | HVPG > 20 mmHg within 24 h of admission. | (1) Primary: Sensitivity and specificity of HVPG cut-off value (20 mmHg) in predicting transplant-free survival (TFS), and assessment of TFS as well as short- and long-term survival; and (2) Secondary: Transfusional needs, ICU stay, complications during the first week of treatment, and causes of death. | 6-wk mortality = 17% in e-TIPSS vs 38% in control (P ≤ 0.05). 1-yr mortality = 31% in e-TIPSS vs 65% in control (P ≤ 0.05). Treatment failure = 12% in e-TIPSS vs 50% in control (P = 0.001). | 46% of study population had Child C and 40% had Child B cirrhosis. mean Child score = 9.2. SOC does not reflect current management and only bare metal stents were used. |
| García-Pagán et al[39], 2010 (Europe) | Child- B with active bleeding or Child C < 14 points. | (1) Composite Primary: Failure to control bleeding and failure to prevent clinically significant VB within 1 yr; and (2) Secondary: Mortality at 6 wk and at 1 yr, failure to control acute bleeding, early rebleeding, rate of rebleeding between 6 wk and 1 yr, other complications of PHTN, number of days in ICU, days spent in the hospital, use of alternative treatments. | 6-wk survival = 97% in e-TIPSS vs 67% in control (NNT = 3.3). 1-yr survival = 86% in e-TIPSS vs 61% in control (P < 0.001). 1-yr re-bleeding = 3% in e-TIPSS vs 50% in control (P < 0.001, NNT = 2.1). | mean Child score = 9.4. mean MELD score = 16.2. About 50% of study participants had Child C cirrhosis. Majority had ALD. NSBB (propranolol or nadolol) was administered with EBL in 25 patients. |
| Lv et al[44], 2019 (China) | Child B and C < 14 points, regardless of active bleeding. | (1) Primary: Transplant-free survival; and (2) Secondary: Failure to control bleeding or rebleeding, new or worsening ascites, overt HE, and other complications of portal hypertension and adverse events. | 6-wk TFS = 99% in e-TIPSS vs 84% in SOC (P = 0.02). 1-yr TFS = 86% in e-TIPSS vs 73% in SOC (P = 0.046; NNT = 8). 1-yr re-bleeding/uncontrolled bleeding = 11% in e-TIPSS vs 34% in SOC (P < 0.0001). | mean Child Score = 8.0. mean MELD score = 13.8. More than 55% patients had Child-Pugh B without active bleeding. 75% of patients had Hepatitis B and had Child B cirrhosis. No significant difference in the incidence of HE was observed between two groups. |
| Dunne et al[46], 2020 (United Kingdom) | Child B and C, 8-13 points (regardless of active bleeding at the endoscopy). | (1) Primary: 1-yr survival; and (2) Secondary: Survival at 6 wk, early rebleeding (within 6 wk) and late rebleeding (between 6 wk and 1 yr), and the development of HE. | 1-yr survival = 79.3% in e-TIPSS vs 75.9% in SOC (P = 0.79). e-TIPSS group showed a trend to reduced variceal re-bleeding (P = 0.09). | Median Child score = 9.8. Median MELD score = 17. More than 90% of participants had ALD. More than 55% had Child-C disease. 23/29 received TIPSS, 13 within 72 h. 18/29 (62%) in SOC group had carvedilol, 3 had cardio-selective beta- blocker and 2 had rescue- TIPSS for early re-bleeding. Incidence of HE was higher in e-TIPSS group (P < 0.05). |
| Observational studies | ||||
| Garcia-Pagán et al[49], 2013 (Europe) | Child-B with active bleeding or Child-C < 14 points. | (1) Composite primary: Failure to control acute bleeding or to prevent clinically significant variceal rebleeding; and (2) Secondary: mortality, development of other complications related to portal hypertension and the percentage of follow-up days spent in hospital. | 1-yr survival = 86 % in e-TIPSS vs 70% in SOC (P = 0.056); e-TIPSS had lower incidence of failure to control bleeding or rebleeding than patients receiving SOC (3 vs 15, P <0.001). | mean Child score = 10. mean MELD score= 17. No significant difference in incidence of HE. Incidence of development of new or worsening ascites was low in e-TIPSS group (P < 0.01). |
| Rudler et al[52], 2014 (France) | Child-C 10–13 cirrhosis or Child-B with active bleeding | (1) Primary: prevention of rebleeding at 1 yr; and (2) Secondary: 3 and 6-mo survival, liver transplantation, control of bleeding, rate of rebleeding at 6 wk, between 6 wk and 1 yr, and the occurrence of adverse events (HE, acute cardiac failure, sepsis). | 1-yr survival = 71% in e-TIPSS vs 74% in control (P = 0.77). 1-yr free of rebleeding = 97% in e-TIPSS vs 51% in control (P < 0.001). | mean Child score = 11.2. mean MELD score = 21.5. 77% had ALD and 77% had Child-C cirrhosis. Patients with previous history of variceal bleeding or with PVT were also included. |
| Thabut et al[50], 2017 (France) | Child-C (< 14) or Child-B with active bleeding | Survival at 5-d, 6-wk and 1-yr. | 1-yr survival = 85% in e-TIPSS vs 59% in control (P = 0.04). | 67% had ALD. 52% undergoing TIPSS had Child C cirrhosis. 35% were eligible for e-TIPSS. Severity of liver disease was the only parameter that influenced survival. |
| Hernández-Gea et al[51], 2018 (Europe and Canada) | Child-C score (< 14 points) or Child-B plus active bleeding | (1) Primary: Survival at 6 weeks and 1 year; and (2) Secondary: (a) The composite end-point of failure to control acute bleeding (up to day 5), early rebleeding (from day 5 to day 42), and late rebleeding (from day 42); (b) onset or worsening of ascites; and (c) development of HE. | 6-wk survival = 92% in p-TIPSS vs 77% in control. Overall, 1-yr survival = 78% in p-TIPPS vs 62% in control (P = 0.014). 1-yr survival in Child C patients = 78% in e-TIPSS vs 53% in control (P = 0.002). 1-yr survival in Child-B + AB = 77% in p-TIPSS vs 75% in control (P = 0.935). | Median MELD score= 15.5. Median Child Score= 10. More than 75% of patients had ALD. Development of de novo or worsening of previous ascites was significantly less in p-TIPSS group (P < 0.001). No difference in incidence of HE was observed in two groups. |
| Lv et al[45], 2018 (China) | Any grade of cirrhosis (with Child score < 14) and AVB. | (1) Primary: All-cause mortality; and (2) Secondary: Failure to control acute bleeding or rebleeding, new or worsening ascites and development of overt HE. | Overall 6-wk mortality = 3.6% in e-TIPSS vs 10.6 % in SOC (P = 0.002). Overall 1-yr mortality = 14.1% in e-TIPSS vs 17.3% in SOC (P = 0.218). e-TIPSS group had significantly lower mortality in MELD ≥ 19 category. | Patients with Child A cirrhosis were also included. Only small number (< 20%) had Child C cirrhosis. Survival benefit was not seen in Child B patients without active bleeding. Incidence of HE was not significantly different between two groups. |
| Trebicka et al[22], 2020 (Multicentre) | Child-C, Child- B with active bleeding. | (1) Primary: All-cause mortality or liver transplantation at 6 wk and 1 yr; and (2) Secondary: Rebleeding. | 6-wk mortality = 13.6 % in e-TIPSS vs 51% in SOC group of patients with ACLF (P = 0.002). 1-yr mortality = 22.7% in e-TIPSS vs 56.5% in SOC group with ACLF (P = 0.002). | Patients with ACLF had a higher rate of rebleeding compared to patients without ACLF (42-d: P < 0.001; 1-yr: 22.9% vs 17.7%, P = 0.024). |
e-TIPSS: Early transjugular intrahepatic portosystemic stent-shunt; RCT: Randomised controlled trial; HVPG: Hepatic venous pressure gradient; HCC: Hepatocellular carcinoma; PHTN: Portal hypertension; PVT: Portal vein thrombosis; TFS: Transplant-free survival; HIV: Human immunodeficiency virus; ICU: Intensive care unit; NSBB: Non-selective beta-blockers; EBL: Endoscopic band ligation; IGV: Isolated gastric varices; MELD: Model for end-stage liver disease; ALD: Alcohol-related liver disease; NNT: Number needed to treat; HE: Hepatic encephalopathy; SOC: Standard of care; ACLF: Acute on chronic liver failure.
Monescillo et al[12] performed the first study applying high-risk selection criteria by using measurements of HVPG. Fifty two patients with HVPG ≥ 20 mmHg measured with 24 h of bleeding episodes were randomised to either TIPSS group or standard therapy. Their study showed that “early” TIPSS placement was associated with a significantly lower rate of treatment failure (50% vs 12%) and lower 6-wk mortality (38% vs 19%). 46% of study population had Child C disease[12]. However, bare-metal stents were used in TIPSS patients and standard of care (SOC) in the non-TIPSS group did not reflect current practice (sclerotherapy rather than combination of endoscopic band ligation and non-selective beta-blocker therapy). Patients in non-TIPSS arm received only non-selective beta-blockers (NSBBs) to prevent rebleeding and EBL was used in whom NSBBs were not tolerated or were contraindicated.
Measurement of early HVPG for risk stratification and treatment assignment in AVB is not easily applicable in clinical practice nor readily available. Therefore, it is important to identify non-invasive predictors of treatment failure and early mortality in patients with AVB. In this regard, Abraldes et al[11] not only showed a strong relationship between the presence of HVPG > 20 mmHg and Child-Pugh class C but also showed that 6-wk mortality is more strongly determined by the severity of underlying liver disease (assessed by Child- Pugh classification) than by HVPG > 20 mmHg. Therefore, subsequent studies used clinical criteria to define high-risk patients and used only covered stents. A schema of the study design of these trials is illustrated in Figure 1 and Figure 2.
Figure 1.
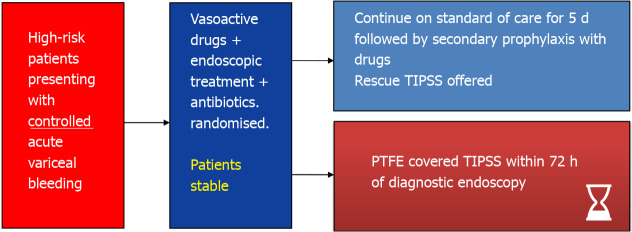
Early transjugular intrahepatic portosystemic stent-shunt – study design. High risk criteria: Child’s C or Child’s B + active bleeding, Child-Pugh score 8-13, Child’s B + C; Maximum threshold: CPS > 13; TIPSS: Transjugular intrahepatic portosystemic stent-shunt; PTFE: Polytetrafluoroethylene.
Figure 2.
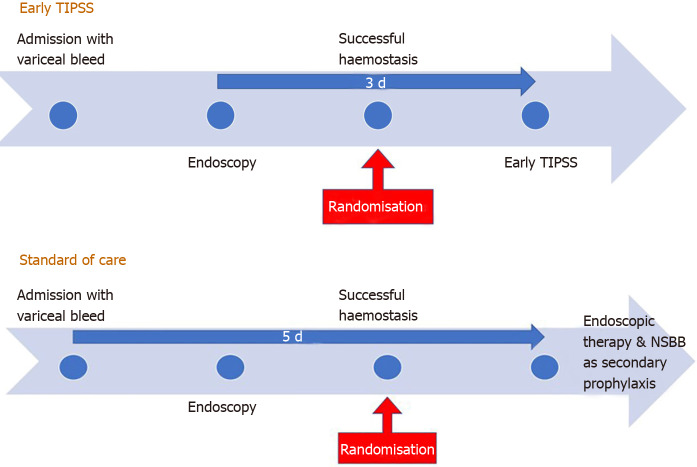
Design of early transjugular intrahepatic portosystemic stent-shunt and standard of care. TIPSS: Transjugular intrahepatic portosystemic stent-shunt.
In García-Pagán et al[39] landmark RCT published in 2010, patients with Child-Pugh C < 14 or Child-Pugh B with active bleeding at index endoscopy were considered high-risk patients. While there is clear justification of including patients with Child C disease in this category[11], the selection of Child B patients (with active bleeding on endoscopy) was not very clear. The composite primary end point in their study was of failure to control acute bleeding or to prevent clinically significant variceal rebleeding within 1 year. Their trial of 63 patients showed that early covered TIPSS (placed within 72 h of index bleeding) not only reduced re-bleeding at 1 year (3% vs 50%, P < 0.001) but also improved 6-wk [97% vs 67%; absolute risk reduction = 30%; number needed to treat (NNT) = 3.3]; and 1-year survival rates (86 vs 61%, P < 0.001; NNT = 4.0) in high-risk patients with cirrhosis when compared to standard of care (NSBB plus EBL). It is important to note that rates of treatment failure and death were higher in Child C patients than in those with Child-B disease, and mortality rates in Child B category did not appear to be significantly different statistically between SOC and TIPSS arms (2/16 vs 1/16). However, the trial was not powered enough to conduct appropriate subgroup analyses[39]. Patients with prognostic factors unlikely to benefit from TIPSS placement were excluded from this trial and the subsequent studies (Table 1).
In the light of emerging evidence, subsequent guidelines incorporated the use of pre-emptive or e- TIPSS, as a treatment option in patients with AVB at high risk of treatment failure[40-43] (Table 2).
Table 2.
Summary of current Guidelines regarding early transjugular intrahepatic portosystemic stent-shunt
|
Ref.
|
Guidelines
|
e-TIPSS recommendations
|
| [40] | Baveno VI Consensus Workshop (2015) | An early TIPSS (p-TIPSS) with PTFE-covered stents within 72 h (ideally < 24 h) must be considered in patients bleeding from EV, GOV1 and GOV2 at high risk of treatment failure [e.g., Child-Pugh class C < 14 points or Child-Pugh class B with active bleeding) after initial pharmacological and endoscopic therapy (1b; A)]. Criteria for high-risk patients should be refined. |
| [41] | American Association for the Study of Liver Diseases (2017) | In patients at high risk of failure or rebleeding (CTP class C cirrhosis or CTP class B with active bleeding on endoscopy) who have no contraindications for TIPSS, an “early” (pre-emptive) TIPSS within 72 h from EGD/EVL may benefit selected patients. |
| [42] | The European Association for the Study of the Liver (2018) | Early pre-emptive covered TIPSS (placed within 24–72 h) can be suggested in selected high-risk patients, such as those with Child class C with score < 14 (I; 2). However, the criteria for high-risk patients, particularly Child B with active bleeding, remains debatable and needs further study. Up to 10%–15% of patients have persistent bleeding or early rebleeding despite treatment with vasoactive drugs plus variceal ligation, and prophylactic antibiotics. TIPSS should be used as the rescue therapy of choice in such cases (I; 1). |
| [43] | British Society of Gastroenterology (2020) | In patients who have Child’s C disease (C10-13) or MELD ≥ 19, and bleeding from oesophageal varices or GOV1 and GOV2 gastric varices and are hemodynamically stable, early or pre-emptive TIPSS can be considered within 72 h of a variceal bleed where local resources allow (weak recommendation, moderate quality of evidence). However, large multi-centre randomised controlled trials are necessary to determine whether patients with Child’s B disease and active bleeding or with MELD 12-18 benefit from early pre-emptive TIPSS. |
PTFE: Polytetrafluoroethylene; p-TIPSS: Pre-emptive transjugular intrahepatic portosystemic shunt; EV: Oesophageal varices, GOV: Gastro-oesophageal varices; CTP: Child-Turcotte-Pugh; EGD: Oesophago-gastro-duodenoscopy; EVL: Endoscopic variceal ligation; MELD: Model for end-stage liver disease.
An RCT from China included 132 cirrhotic patients who were randomly assigned (2:1) to receive pre-emptive TIPSS or standard of care (NSBB + EBL)[44]. This RCT showed better 1-year transplantation-free survival (primary outcome) in e-TIPSS group than in the control group; with greatest benefit for those with a MELD score between 12 and 19 (P = 0.04, NNT = 8)[44]. However, all patients with Child B and C (< 14 points) cirrhosis were included regardless of active bleeding at the index endoscopy. Secondly, the patient demographics were significantly different from other studies and most patients were Child-Pugh B without active bleeding (57%). Over 75% of patients had Hepatitis B related cirrhosis. Only 43% of patients were high risk according to the previously described criteria[39], and considered to benefit from e-TIPSS intervention[45]. Therefore, absolute risk difference of 13% for 1-year (transplant-free) survival in e-TIPSS group appeared to be lower than in the previous RCTs (34% and 25%)[39]. There was no significant difference in incidence of hepatic encephalopathy between the two groups.
A recently published RCT from the UK included 58 patients with a Child-Pugh score of 8-13, without previous treatment for portal hypertension related bleeding, regardless of active bleeding on endoscopy[46]. Patients were randomised to receive e-TIPSS or standard of care (carvedilol + EBL). There was no difference in 1-year survival rate (primary outcome) between the SOC and e-TIPSS groups (75.9% vs 79.3% respectively, P = 0.79). More than 90% of participants had alcohol related liver disease and majority (over 80%) were actively consuming alcohol at inclusion reflecting real Western world population. Over 55% had Child-C disease with median MELD score of 17, comparable with Garcia-Pagan study[39]. In the e-TIPSS group, 23/29 patients (79%) actually underwent TIPSS and only 13 within 72 h, but all within 5 days. There was no difference in worsening or new ascites, with more encephalopathy (46.1% vs 20.7%, P < 0.05) and a trend towards lower variceal rebleeding in the e-TIPSS group (P = 0.09). Notably, previous RCT[39] and recent individual data metanalysis[47] did not show significant difference in development of hepatic encephalopathy between the two groups. Though the study was not powered enough to reach valid conclusions, it demonstrated better survival in the SOC arm than the previous European RCT[39] (76% vs 61%), although SOC survival rate is comparable to the Chinese RCT[44].
Better survival in SOC group could be explained by improved initial management of AVB (vasoactive drugs, antibiotics and endoscopic band ligation), with better access to intensive care. Furthermore, carvedilol was used to a greater extent. The improved SOC could be major factor in the lack of difference in survival between the two groups. In the SOC arm, 18/29 patients received carvedilol (at a median dose of 6.25 mg a day). Carvedilol with its additional alpha-1 antagonism profile, seems to have greater effect on reducing HVPG than other traditional NSBB (propranolol and nadolol) and may have a beneficial effect in SOC group but this needs further validation. This study has led to much debate in relation to patient selection[48].
Observational studies
The benefits of e-TIPSS have been shown by several (but not all) observational studies. Most of these studies used similar clinical high-risk and exclusion criteria as the study by Garcia-Pagan[49] (Table 1). It is important to note that in a French national audit and in large multicentre study, only a minority of patients eligible for e-TIPSS (according to defined criteria) actually received e-TIPSS (6.7% and 9.8% respectively)[50,51]. Survival benefit of e-TIPSS was only seen in those with Childs-Pugh C disease in a large multicentre study including 671 patients[51]. These large observational multicentre studies underscore the lack of adherence of physicians to concept of e-TIPSS and difficulty in arranging e-TIPSS (within limited timeframe) in a real-life practice. Most physicians did not believe in the role of e-TIPSS. Two European studies did not find a statistically significant increase in survival in e-TIPSS group[49,52]. One of these studies included patients with Child-Pugh score up to 15 points i-e, patients with significantly advanced liver disease[52].
A recent observational study from China included 1425 patients with cirrhosis and variceal bleed[45]. Most of the patients had cirrhosis due to viral hepatitis and e-TIPSS was also offered to Child-Pugh A patients and Child-Pugh B patients without active bleeding. Survival benefit was observed in patients fulling the high-risk criteria used in Garcia Pagan RCT[39] and with MELD score ≥ 19 but not in patients with Child-Pugh A or Child-Pugh B without active bleeding.
In a recently published retrospective study, e-TIPSS has also shown improved 6-weeks and 1-year survival (P < 0.05) in patients with ACLF[22]. 671 patients were eligible for e-TIPSS and only 66 received e-TIPSS. 22 out of 66 e-TIPSS patients had ACLF. However, the findings need to be interpreted with caution due to the small sample size and require validation in larger prospective studies.
Systemic reviews and meta-analyses
A few metanalyses of studies looking at the role of early TIPSS in patients with AVB have been published in recent years. A well-designed meta-analysis of two earlier RCTs[39] and two observational studies[49,52] comparing e-TIPSS with standard of care showed that e-TIPSS is associated with reduced overall mortality (odds ratio = 0.38, 95%CI: 0.17-0.83, P = 0.02)[53] (Table 3). It is important to note that sensitivity analysis looking separately at Child B patients with active bleeding and those with Child C (< 14 score) showed that survival benefit was only observed in Child C (< 14 score) patients but not so in Child B patients. There was also significant reduction in rebleeding with e-TIPSS without significant difference in incidence of hepatic encephalopathy. Moderate heterogenicity was observed among the studies and the recent RCTs by Lv et al[44] and Dunne et al[46] were not included in this metanalysis. The authors concluded that further study was required to identify factors associated with poor outcome after e-TIPSS.
Table 3.
Early transjugular intrahepatic portosystemic stent-shunt in acute variceal bleeding: Key meta-analyses
|
Ref.
|
Design
|
Results
|
Comments
|
| Deltenre et al[43] | 4 studies (2 RCTs[12,39] and 2 Observational[49,52] ) included. | e-TIPSS was associated with fewer deaths [odds ratio (OR) = 0.38, P = 0.02], and with lower rates of bleeding (OR = 0.08, 95%CI: 0.04–0.17, P < 0.001) within 1 year when compared to SOC, without increase in incidence of encephalopathy (OR = 0.84, 95% CI: 0.50–1.42, P = 0.5). | There was moderate heterogeneity between studies. No significant difference in mortality was observed between Child–Pugh B and C patients. This could be explained by inclusion of sicker patients (C-P score < 14) in Rudler et al[52] study. |
| Nicoară-Farcău et al[47] | Individual data meta-analysis from 7 studies (3 RCTs[12,39,44] and 4 observational studies[45,49,51,52]), comprising 1327 patients (310 received e-TIPSS, 1017 received SOC (drugs + endoscopic treatment). | Overall, e- TIPSS significantly increased 1- year survival compared with SOC [hazard ratio (HR) 0.443; P < 0.001]. e-TIPSS significantly reduced the risk of failure to control bleeding/preventing variceal rebleeding (HR = 0.338; P < 0.001) and ascites without increasing risk of HE, compared with SOC. | Only individual data of those patients fulfilling the high-risk criteria (Child-Pugh B with active bleeding and Child-Pugh C < 14 points) from included studies were included. On multivariate analysis patients with Child-Pugh score > 7 points had a significantly worse survival than those with Child-Pugh score ≤ 7. Both prospective and observational studies were included and latest UK RCT[46] and the multicentre French audit[50] were not included. |
| Tripathi et al[54] | 3 RCTs[39,44,46] comparing e-TIPSS (with covered stent) with SOC, comprising 152 patients. | e-TIPSS significantly reduced incidence of re-bleeding (RR = 0.20; P ≤ 0.001). Improvement in overall survival at 1 and 2 yr was not statistically significant between two groups (RR = 0.62; P = 0.16 and RR = 0.62; P = 0.19 respectively). | There was no significant difference in incidence of HE. RCTs are underpowered to reach firm conclusion about the survival benefit of e-TIPSS. |
e-TIPSS: Early transjugular intrahepatic portosystemic stent-shunt; RCT: Randomised controlled trial; SOC: Standard of care; HE: Hepatic encephalopathy.
A recently published individual patient data meta-analysis assessed the efficacy of e-TIPSS in high-risk patients[47]. They included 7 studies: 3 randomized controlled trials[12,39,44] and 4 observational studies[45,49,51,52] comprising 1327 patients. As discussed previously, one of the RCTs[44] and one of the observational studies[45] included patients in all Child-Pugh categories, therefore only individual data of those patients fulfilling the current high-risk criteria (Child-Pugh B with active bleeding and Child- Pugh C up to 13 points) were included in this individual meta-analysis. This meta-analysis showed overall survival benefit of e-TIPSS over standard of care. Six-week and 1-year survival were significantly higher in the e-TIPSS group than in the SOC group (93% vs 76.8% and 79% vs 62%, respectively P < 0.001). Moreover, benefit of e-TIPSS was observed in both CP- B patients with active bleeding (P = 0.008;) and in CP-C patients (P < 0.001). Number of patients needed to treat to save one life was 4.23 (95%CI: 3.57–6.94). Multivariate analysis showed that patients with a CP score > 7 points had a significantly worse survival than those with CP score of 7 points or less. e-TIPSS significantly reduced the risk of failure to control bleeding/preventing variceal rebleeding (P < 0.001) in all patients. Moreover, risk of developing new or worsening ascites was significantly reduced by the e-TIPSS in the overall population (P < 0.001). This meta-analysis showed no significant differences in the risk of developing hepatic encephalopathy in the overall population (P = 0.553). However, a limitation of this meta-analysis is the inclusion of both prospective and observational studies, and the authors concluded that further prospective studies are necessary. The latest UK RCT[46] and the multicentre French audit[50] were not included in this meta-analysis, thus somewhat limiting its utility.
A recent meta-analysis of 152 patients in three prospective RCTs, including the latest UK RCT[39,44,46], concluded that e-TIPSS is more effective in preventing variceal rebleeding than standard of care (EBL and medical management) without increase in adverse events[54]. e-TIPSS with covered stents significantly reduced incidence of bleeding (RR = 0.20, 95%CI: 0.09–0.42, P < 0.001). This was associated an improvement in overall survival, but it did not quite reach statistical significance, at 1 and 2 years (RR = 0.62, 95%CI: 0.33–1.19 and RR = 0.62, P = 0.16 95%CI: 0.31–1.26, respectively). Incidence of hepatic encephalopathy was similar across the studies[54].
FUTURE DIRECTIONS
Patients with advanced liver disease i-e with Child-Pugh C score (up to 13 points) and MELD score ≥ 19 benefit from the e-TIPSS intervention in the described studies. However, benefit of this intervention in patients with less severe disease i-e with Child-Pugh B or MELD < 19 is not very robust and there is further need to define the high-risk category.
Though patients with child score > 13 points are considered too sick for early TIPSS intervention with high mortality, it is not very clear from the literature if there is a maximal threshold of severity of liver disease beyond which there is no benefit from e-TIPSS intervention. Indeed certain patients with ACLF may benefit from e-TIPSS following AVB. This concept needs further revalidation in a multi-centre trial collecting large numbers of patients.
Outcomes after an episode of variceal bleed have improved in the last decade with improved 1-year survival in patients receiving standard care[44,46] as compared to the earlier landmark RCT[39], causing reluctancy to adopt e-TIPSS approach among the practicing physicians. Moreover, providing e-TIPSS (within 72 h of admission) is challenging in most healthcare systems, even in centres providing 24/7 TIPSS service, and is a significant barrier to adoption of e-TIPSS. Indeed, recruitment in trials[39,46] was very slow and careful reading of the manuscripts suggests that the included patients may not be truly representative of the entire population of patients with severe cirrhosis and variceal bleeding. With such stringent inclusion criteria, the applicability of this therapeutic approach is questionable in a larger cohort of cirrhotic patients.
Even if TIPSS was performed outside the 72 h window, so called “late e-TIPSS”, it may not have a significant impact on the outcomes given the time frame for acute bleeding is 5 d as defined by the Baveno consensus[40]. Indeed, benefits of e-TIPSS placement following oesophageal variceal bleeding have been observed for up to 28 d after index endoscopy[55,56]. Therefore, a more pragmatic approach to the time window for e-TIPSS is an important consideration when designing future trials.
CONCLUSION
The role of e-TIPSS in acute variceal bleeding requires further prospective study with adequately powered trials. Studies should focus on careful patient selection, investigate optimal timing of TIPSS, and explore quality of life and health economics.
Footnotes
Conflict-of-interest statement: Khan F has no conflict of interest. Tripathi D receives Speaker fees and consultancy for Gore Medical, and Research grant award for early TIPSS trial (NIHR, UK).
Provenance and peer review: Invited article; Externally peer reviewed.
Corresponding Author's Membership in Professional Societies: British Society of Gastroenterology, No. 61414; European Association for the Study of the Liver, No. 12367; British Association for the study of the Liver; Royal College of Physicians and Surgeons of Glasgow; General Medical Council, No. 4146063.
Peer-review started: April 19, 2021
First decision: May 12, 2021
Article in press: October 29, 2021
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Debnath P S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
Contributor Information
Faisal Khan, Gastroenterology Unit, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TH, United Kingdom.
Dhiraj Tripathi, Liver Unit, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TH, United Kingdom; NIHR Birmingham Biomedical Research Centre, University Hospitals Birmingham, NHS Foundation Trust and University of Birmingham, Birmingham B15 2TH, United Kingdom; Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham B15 2TH, United Kingdom. d.tripathi@bham.ac.uk.
References
- 1.D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999;19:475–505. doi: 10.1055/s-2007-1007133. [DOI] [PubMed] [Google Scholar]
- 2.Reverter E, Tandon P, Augustin S, Turon F, Casu S, Bastiampillai R, Keough A, Llop E, González A, Seijo S, Berzigotti A, Ma M, Genescà J, Bosch J, García-Pagán JC, Abraldes JG. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146:412–19.e3. doi: 10.1053/j.gastro.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Amitrano L, Guardascione MA, Manguso F, Bennato R, Bove A, DeNucci C, Lombardi G, Martino R, Menchise A, Orsini L, Picascia S, Riccio E. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol. 2012;107:1872–1878. doi: 10.1038/ajg.2012.313. [DOI] [PubMed] [Google Scholar]
- 4.Fortune BE, Garcia-Tsao G, Ciarleglio M, Deng Y, Fallon MB, Sigal S, Chalasani NP, Lim JK, Reuben A, Vargas HE, Abrams G, Lewis MD, Hassanein T, Trotter JF, Sanyal AJ, Beavers KL, Ganger D, Thuluvath PJ, Grace ND, Groszmann RJ Vapreotide Study Group. Child-Turcotte-Pugh Class is Best at Stratifying Risk in Variceal Hemorrhage: Analysis of a US Multicenter Prospective Study. J Clin Gastroenterol. 2017;51:446–453. doi: 10.1097/MCG.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham DY, Smith JL. The course of patients after variceal hemorrhage. Gastroenterology. 1981;80:800–809. [PubMed] [Google Scholar]
- 6.de Franchis R, Primignani M. Why do varices bleed? Gastroenterol Clin North Am. 1992;21:85–101. [PubMed] [Google Scholar]
- 7.Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, Berger E, Blum U, Gabelmann A, Hauenstein K. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165–171. doi: 10.1056/NEJM199401203300303. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico G, Pagliaro L, Bosch J. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332–354. doi: 10.1002/hep.1840220145. [DOI] [PubMed] [Google Scholar]
- 9.Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573–582. doi: 10.1038/nrgastro.2009.149. [DOI] [PubMed] [Google Scholar]
- 10.Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Gao H, Makuch R Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–2261. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 11.Abraldes JG, Villanueva C, Bañares R, Aracil C, Catalina MV, Garci A-Pagán JC, Bosch J Spanish Cooperative Group for Portal Hypertension and Variceal Bleeding. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol. 2008;48:229–236. doi: 10.1016/j.jhep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, Sierra A, Guevara C, Jiménez E, Marrero JM, Buceta E, Sánchez J, Castellot A, Peñate M, Cruz A, Peña E. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793–801. doi: 10.1002/hep.20386. [DOI] [PubMed] [Google Scholar]
- 13.Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902–908. doi: 10.1053/jhep.2003.50133. [DOI] [PubMed] [Google Scholar]
- 14.Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas RM, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–1608. doi: 10.1016/S0140-6736(18)31875-0. [DOI] [PubMed] [Google Scholar]
- 15.Kamath PS, Mookerjee RP. Individualized care for portal hypertension: Not quite yet. J Hepatol. 2015;63:543–545. doi: 10.1016/j.jhep.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 17.Magaz M, Baiges A, Hernández-Gea V. Precision medicine in variceal bleeding: Are we there yet? J Hepatol. 2020;72:774–784. doi: 10.1016/j.jhep.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Tandon P, Abraldes JG, Keough A, Bastiampillai R, Jayakumar S, Carbonneau M, Wong E, Kao D, Bain VG, Ma M. Risk of Bacterial Infection in Patients With Cirrhosis and Acute Variceal Hemorrhage, Based on Child-Pugh Class, and Effects of Antibiotics. Clin Gastroenterol Hepatol. 2015;13:1189–96.e2. doi: 10.1016/j.cgh.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 19.D'Amico G, De Franchis R Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 20.Rudler M, Bureau C, Carbonell N, Mathurin P, Saliba F, Mallat A, Massard J, Golmard JL, Bernard-Chabert B, Dib N, Thabut D French Club for the Study of Portal Hypertension (CFEHTP) Recalibrated MELD and hepatic encephalopathy are prognostic factors in cirrhotic patients with acute variceal bleeding. Liver Int. 2018;38:469–476. doi: 10.1111/liv.13632. [DOI] [PubMed] [Google Scholar]
- 21.Conejo I, Guardascione MA, Tandon P, Cachero A, Castellote J, Abraldes JG, Amitrano L, Genescà J, Augustin S. Multicenter External Validation of Risk Stratification Criteria for Patients With Variceal Bleeding. Clin Gastroenterol Hepatol. 2018;16:132–139.e8. doi: 10.1016/j.cgh.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 22.Trebicka J, Gu W, Ibáñez-Samaniego L, Hernández-Gea V, Pitarch C, Garcia E, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Silva-Junior G, Martinez J, Genescà J, Bureau C, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud L, Ferreira CN, Barcelo R, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Weiss E, Catalina MV, Erasmus HP, Uschner FE, Schulz M, Brol MJ, Praktiknjo M, Chang J, Krag A, Nevens F, Calleja JL, Robic MA, Conejo I, Albillos A, Rudler M, Alvarado E, Guardascione MA, Tantau M, Bosch J, Torres F, Pavesi M, Garcia-Pagán JC, Jansen C, Bañares R International Variceal Bleeding Observational Study Group and Baveno Cooperation. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol. 2020;73:1082–1091. doi: 10.1016/j.jhep.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Bornman PC, Terblanche J, Kahn D, Jonker MA, Kirsch RE. Limitations of multiple injection sclerotherapy sessions for acute variceal bleeding. S Afr Med J. 1986;70:34–36. [PubMed] [Google Scholar]
- 24.Durtschi MB, Carrico CJ, Johansen KH. Esophageal transection fails to salvage high-risk cirrhotic patients with variceal bleeding. Am J Surg. 1985;150:18–23. doi: 10.1016/0002-9610(85)90004-2. [DOI] [PubMed] [Google Scholar]
- 25.Rikkers LF, Jin G, Burnett DA, Buchi KN, Cormier RA. Shunt surgery versus endoscopic sclerotherapy for variceal hemorrhage: late results of a randomized trial. Am J Surg. 1993;165:27–32; discussion 32. doi: 10.1016/s0002-9610(05)80400-3. [DOI] [PubMed] [Google Scholar]
- 26.Colapinto RF, Stronell RD, Birch SJ, Langer B, Blendis LM, Greig PD, Gilas T. Creation of an intrahepatic portosystemic shunt with a Grüntzig balloon catheter. Can Med Assoc J. 1982;126:267–268. [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick PA, Dick R, Panagou EB, Chin JK, Greenslade L, McIntyre N, Burroughs AK. Emergency transjugular intrahepatic portasystemic stent shunting as salvage treatment for uncontrolled variceal bleeding. Br J Surg. 1994;81:1324–1327. doi: 10.1002/bjs.1800810922. [DOI] [PubMed] [Google Scholar]
- 28.Allaire M, Walter A, Sutter O, Nahon P, Ganne-Carrié N, Amathieu R, Nault JC. TIPS for management of portal-hypertension-related complications in patients with cirrhosis. Clin Res Hepatol Gastroenterol. 2020;44:249–263. doi: 10.1016/j.clinre.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Rubin RA, Haskal ZJ, O'Brien CB, Cope C, Brass CA. Transjugular intrahepatic portosystemic shunting: decreased survival for patients with high APACHE II scores. Am J Gastroenterol. 1995;90:556–563. [PubMed] [Google Scholar]
- 30.Tzeng WS, Wu RH, Lin CY, Chen JJ, Sheu MJ, Koay LB, Lee C. Prediction of mortality after emergent transjugular intrahepatic portosystemic shunt placement: use of APACHE II, Child-Pugh and MELD scores in Asian patients with refractory variceal hemorrhage. Korean J Radiol. 2009;10:481–489. doi: 10.3348/kjr.2009.10.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripathi D, Redhead D. Transjugular intrahepatic portosystemic stent-shunt: technical factors and new developments. Eur J Gastroenterol Hepatol. 2006;18:1127–1133. doi: 10.1097/01.meg.0000236871.78280.a7. [DOI] [PubMed] [Google Scholar]
- 32.Patidar KR, Sydnor M, Sanyal AJ. Transjugular intrahepatic portosystemic shunt. Clin Liver Dis. 2014;18:853–876. doi: 10.1016/j.cld.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casadaban LC, Parvinian A, Zivin SP, Lakhoo J, Minocha J, Knuttinen MG, Ray CE Jr, Bui JT, Gaba RC. MELD score for prediction of survival after emergent TIPS for acute variceal hemorrhage: derivation and validation in a 101-patient cohort. Ann Hepatol. 2015;14:380–388. [PubMed] [Google Scholar]
- 34.Maimone S, Saffioti F, Filomia R, Alibrandi A, Isgrò G, Calvaruso V, Xirouchakis E, Guerrini GP, Burroughs AK, Tsochatzis E, Patch D. Predictors of Re-bleeding and Mortality Among Patients with Refractory Variceal Bleeding Undergoing Salvage Transjugular Intrahepatic Portosystemic Shunt (TIPS) Dig Dis Sci. 2019;64:1335–1345. doi: 10.1007/s10620-018-5412-x. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y, Wang X, Xi X, Li X, Luo X, Yang L. Emergency Transjugular Intrahepatic Portosystemic Shunt: an Effective and Safe Treatment for Uncontrolled Variceal Bleeding. J Gastrointest Surg. 2019;23:2193–2200. doi: 10.1007/s11605-019-04146-8. [DOI] [PubMed] [Google Scholar]
- 36.Bian S, Tian XG, Hu JH, Wang GC, Zhang CQ. Percutaneous transhepatic variceal embolization combined with endoscopic ligation for the prevention of variceal rebleeding. J Dig Dis. 2013;14:388–395. doi: 10.1111/1751-2980.12049. [DOI] [PubMed] [Google Scholar]
- 37.Tian X, Shi Y, Hu J, Wang G, Zhang C. Percutaneous transhepatic variceal embolization with cyanoacrylate vs. transjugular intrahepatic portal systematic shunt for esophageal variceal bleeding. Hepatol Int. 2013;7:636–644. doi: 10.1007/s12072-013-9433-4. [DOI] [PubMed] [Google Scholar]
- 38.Jalan R, Forrest EH, Stanley AJ, Redhead DN, Forbes J, Dillon JF, MacGilchrist AJ, Finlayson ND, Hayes PC. A randomized trial comparing transjugular intrahepatic portosystemic stent-shunt with variceal band ligation in the prevention of rebleeding from esophageal varices. Hepatology. 1997;26:1115–1122. doi: 10.1002/hep.510260505. [DOI] [PubMed] [Google Scholar]
- 39.García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 40.de Franchis R Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 42.European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Tripathi D, Stanley AJ, Hayes PC, Travis S, Armstrong MJ, Tsochatzis EA, Rowe IA, Roslund N, Ireland H, Lomax M, Leithead JA, Mehrzad H, Aspinall RJ, McDonagh J, Patch D. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173–1192. doi: 10.1136/gutjnl-2019-320221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lv Y, Yang Z, Liu L, Li K, He C, Wang Z, Bai W, Guo W, Yu T, Yuan X, Zhang H, Xie H, Yao L, Wang J, Li T, Wang Q, Chen H, Wang E, Xia D, Luo B, Li X, Yuan J, Han N, Zhu Y, Niu J, Cai H, Xia J, Yin Z, Wu K, Fan D, Han G AVB-TIPS Study Group. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:587–598. doi: 10.1016/S2468-1253(19)30090-1. [DOI] [PubMed] [Google Scholar]
- 45.Lv Y, Zuo L, Zhu X, Zhao J, Xue H, Jiang Z, Zhuge Y, Zhang C, Sun J, Ding P, Ren W, Li Y, Zhang K, Zhang W, He C, Zhong J, Peng Q, Ma F, Luo J, Zhang M, Wang G, Sun M, Dong J, Bai W, Guo W, Wang Q, Yuan X, Wang Z, Yu T, Luo B, Li X, Yuan J, Han N, Zhu Y, Niu J, Li K, Yin Z, Nie Y, Fan D, Han G. Identifying optimal candidates for early TIPS among patients with cirrhosis and acute variceal bleeding: a multicentre observational study. Gut. 2019;68:1297–1310. doi: 10.1136/gutjnl-2018-317057. [DOI] [PubMed] [Google Scholar]
- 46.Dunne PDJ, Sinha R, Stanley AJ, Lachlan N, Ireland H, Shams A, Kasthuri R, Forrest EH, Hayes PC. Randomised clinical trial: standard of care versus early-transjugular intrahepatic porto-systemic shunt (TIPSS) in patients with cirrhosis and oesophageal variceal bleeding. Aliment Pharmacol Ther. 2020;52:98–106. doi: 10.1111/apt.15797. [DOI] [PubMed] [Google Scholar]
- 47.Nicoară-Farcău O, Han G, Rudler M, Angrisani D, Monescillo A, Torres F, Casanovas G, Bosch J, Lv Y, Thabut D, Fan D, Hernández-Gea V, García-Pagán JC Preemptive TIPS Individual Data Metanalysis, International Variceal Bleeding Study and Baveno Cooperation Study groups. Effects of Early Placement of Transjugular Portosystemic Shunts in Patients With High-Risk Acute Variceal Bleeding: a Meta-analysis of Individual Patient Data. Gastroenterology. 2021;160:193–205.e10. doi: 10.1053/j.gastro.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 48.García-Pagán JC, Bosch J, Trebicka J, Abraldes JG, Albillos A, Grønbaek H, Giráldez Á, Zipprich A, Bureau C, Hernández-Gea V International Variceal Bleeding Observational Study Group, Baveno Cooperation. Letter: improve survival! Aliment Pharmacol Ther. 2020;52:927–928. doi: 10.1111/apt.15926. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Pagán JC, Di Pascoli M, Caca K, Laleman W, Bureau C, Appenrodt B, Luca A, Zipprich A, Abraldes JG, Nevens F, Vinel JP, Sauerbruch T, Bosch J. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol. 2013;58:45–50. doi: 10.1016/j.jhep.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 50.Thabut D, Pauwels A, Carbonell N, Remy AJ, Nahon P, Causse X, Cervoni JP, Cadranel JF, Archambeaud I, Bramli S, Ehrhard F, Ah-Soune P, Rostain F, Pariente A, Vergniol J, Dupuychaffray JP, Pelletier AL, Skinazi F, Guillygomarc'h A, Vitte RL, Henrion J, Combet S, Rudler M, Bureau C des Hépato-Gastroentérologues des Hôpitaux Généraux (ANGH); Club Francophone pour l'Etude de l'Hypertension Portale (CFETHTP); CHOC Study Group collaborators. Cirrhotic patients with portal hypertension-related bleeding and an indication for early-TIPS: a large multicentre audit with real-life results. J Hepatol. 2017;68:73–81. doi: 10.1016/j.jhep.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Hernández-Gea V, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Ibañez-Samaniego L, Silva-Junior G, Martinez J, Genescà J, Bureau C, Trebicka J, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud LL, Noronha Ferreira C, Barcelo R, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Krag A, Nevens F, Calleja JL, Jansen C, Robic MA, Conejo I, Catalina MV, Albillos A, Rudler M, Alvarado E, Guardascione MA, Tantau M, Bosch J, Torres F, Garcia-Pagán JC International Variceal Bleeding Observational Study Group and Baveno Cooperation. Preemptive-TIPS Improves Outcome in High-Risk Variceal Bleeding: An Observational Study. Hepatology. 2019;69:282–293. doi: 10.1002/hep.30182. [DOI] [PubMed] [Google Scholar]
- 52.Rudler M, Cluzel P, Corvec TL, Benosman H, Rousseau G, Poynard T, Thabut D. Early-TIPSS placement prevents rebleeding in high-risk patients with variceal bleeding, without improving survival. Aliment Pharmacol Ther. 2014;40:1074–1080. doi: 10.1111/apt.12934. [DOI] [PubMed] [Google Scholar]
- 53.Deltenre P, Trépo E, Rudler M, Monescillo A, Fraga M, Denys A, Doerig C, Fournier N, Moreno C, Moradpour D, Bureau C, Thabut D. Early transjugular intrahepatic portosystemic shunt in cirrhotic patients with acute variceal bleeding: a systematic review and meta-analysis of controlled trials. Eur J Gastroenterol Hepatol. 2015;27:e1–e9. doi: 10.1097/MEG.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 54.Tripathi D, O'Neill F, Patch D, Joseph A, Aithal G. P59 Systematic review and meta-analysis of early transjugular intrahepatic portosystemic stent-shunt (TIPSS) in the management of acute variceal bleeding. Gut. 2020;69 Suppl 1:A35–A36. [Google Scholar]
- 55.Dunne P, Sinha R, Tripathi D, Hayes P. ATU-06 Does the timing of TIPSS in patients with acute oesophageal variceal bleeding alter patient outcome? Gut. 2019;68 Suppl 2:A109. [Google Scholar]
- 56.Bucsics T, Schoder M, Mandorfer M, Schwabl P, Riedl F, Bauer D, Trauner M, Peck-Radosavljevic M, Karner J, Karnel F, Reiberger T. SAT199: Effectiveness of "early" TIPS implantation vs "late" TIPS vs standard endoscopic treatment for acute variceal bleeding in patients with liver cirrhosis. J Hepatol. 2018;68:S605–S842. [Google Scholar]