Abstract
Seasonal flu is caused by influenza infection, a virus that spreads easily in human population with periodical epidemic outbreaks. The high mutational rate of influenza viruses leads to the emergence of strains resistant to the current treatments. Due to that, scientific research is focusing on the development of new anti-influenza agents as alternative or complementary treatments. Olive tree (Olea europaea L.) has been a source of ancestral remedies due to its antimicrobial activity. Thus, the aim of this study was to test the anti-influenza activity of a standardized olive leaf extract rich in elenolic acid (EA), Isenolic®, compared with oseltamivir. Isenolic® extract was characterized by High Performance Liquid Chromatography (HPLC)-Mass Spectrometry and its content in EA was determined by HPLC. Cytotoxicity, viral neuraminidase inhibitor activity and cell viability protection against influenza infection of Isenolic® were tested in vitro using sialic acid overexpressing Madin-Darby Canine Kidney cells. Isenolic® formulations showed a 4% and 8% dry basis. Oseltamivir and Isenolic® extracts showed anti-influenza activity. The 8% Isenolic® formulation showed a dose-dependent neuraminidase inhibitor activity higher than the 4% formulation, and preserved cell viability under viral infection. Thus, Isenolic® become a promising natural alternative to existing influenza treatments.
Keywords: Olive leaf extract, elenolic acid, influenza virus, MDCK-SIAT1 cells, antiviral activity, oseltamivir, neuraminidase inhibition activity
Introduction
Seasonal influenza is caused by a burden of airborne respiratory viruses that occurs every year between the meteorological fall and spring of both hemispheres. The virus is highly contagious and produces mild to severe symptoms or even casualties, especially in sensitive populations (very young, individuals with chronic comorbidities, immunocompromised individuals, pregnant women, and frail older adults). The estimations of the 2017 season of influenza lower respiratory tract infections attributed 1.45 × 105 casualties worldwide with higher rates among elderly population (16.4 deaths per 1 × 105 in adults over 70 years). 1
Influenza viruses are enveloped RNA virus members of the Orthomyxoviridae family. There are 4 types of seasonal influenza viruses, types A, B, C and D classified into subtypes according to the surface proteins hemagglutinin (HA) and neuraminidase (NA) combinations. Influenza A and B viruses circulate and cause seasonal epidemics of disease.2,3
There are two classes of antiviral drugs for treatment and prophylaxis of influenza: the M2 ion-channel blockers amantadine and rimantadine acting only against type A influenza viruses; and the neuraminidase enzyme inhibitors (NAI) oseltamivir, zanamivir, acting against type A and type B influenza viruses. The adamantanes (amantadine and rimantadine) are not recommended for prophylaxis or treatment due to the widespread natural resistance to those drugs among human seasonal A (H1N1) and A (H3N2) influenza viruses. 4 By contrast, NAI are strongly advised in clinical practice to start antiviral treatment as soon as possible, 5 but their use are relate to some undesired side-effects, such as nausea, vomiting, diarrhoea and headache.
Despite its effectiveness, there is a major concern about the emergence of new influenza strains resistant to NAI.2,6,7 Before 2007, NAI resistance was sporadic. However, by the late 2007, the oseltamivir-resistant seasonal influenza A (H1N1) viruses began to be more frequent in untreated individuals in Europe and the US and worldwide by 2008.8,9 The World Health Organization (WHO) established the criteria to define the levels of antiviral-resistance in influenza viruses based on the fold-change of their inhibitory concentration value (IC50) compared to reference IC50 of the sensitive strains. For influenza A/B viruses the following cut-off values were set as normal (<10/5-fold increase), reduced inhibition (10–100/5–50-fold increase) and highly reduced inhibition (>100/50-fold increase) respectively. 10 There are dozens of NAI-resistant A (H1N1) and A (H3N2) mutant strains that show highly reduced inhibition for oseltamivir and peramivir. 11 Thus, the search of alternatives to NAIs would provide additional tools to control resistant influenza strains.
Medicinal plants have been used traditionally to treat viral infections. Olive (Olea europaea L.) has been used traditionally as a medicinal plant with different indications in various preparations derived from its leaves, fruits, seeds, wood, bark and oil.16,17 It has been part of the traditional human and veterinary pharmacopeia in Mediterranean countries.18,19 Several studies have reported an antiviral effect of olive against important pathogens such as viral haemorrhagic septicaemia virus, rhinovirus, Herpes, Polio, leukaemia or influenza.16,17
The pharmacological properties of O. europaea have been attributed to its phenolic content. Oleuropein, the main phenolic compound present at olive, is formed by esterification of hydroxytyrosol and a glycosylated monoterpene, the elenolic acid (EA).16,20 It is hypothesised that the real antimicrobial effect of oleuropein would be due to its components hydroxytyrosol 23 and EA, although there are still no studies carried out with EA obtained from natural sources. Early research with calcium elenolate salts revealed its antiviral activity, including influenza and parainfluenza, in vitro and in animal models. 24 Soret MG and Renis HE showed how calcium elenolate treatment reduced influenza virus infection and accelerated its eradication in hamsters.24,26
This research is aimed to study the antiviral effect of natural aqueous extracts from olive leaf rich in EA instead of its salt on a well-known influenza strain.
Materials and methods
Antiviral agents
The branded olive leaf extract Isenolic® standardized in 4 and 8% of EA by HPLC was obtained by a proprietary process by Pharmactive Biotech Products S.L. The samples were stored in darkness and a dry place prior to analysis.
Oseltamivir phosphate was purchased from Sigma-Aldrich (Sigma-Aldrich, St Louis, MO, USA) and stock solution was prepared in Milli-Q water purified by a Milli-Q Millipore system (Bedford, MA, USA). Acetonitrile of HPLC grade was purchased from Scharlab (Barcelona, Spain).
Isenolic® characterization
Identification of EA derivates in Isenolic® was accomplished by High Performance Liquide Chromatography-associated Mass Spectrometry (HPLC-MS) analysis, using a HPLC-MS QStar device equipped with a Q-TOF mass analyser and electrospray ionization system (AB Sciex; Alcobendas, Spain). Separation and ESI operation were performed according to the methodology previously described. 27 Chromatograms were registered at 240, 280 and 330 nm and mass spectra from m/z 100 to 2500 uma at 100, 200 and 250 V. 27
Quantification of EA content in both formulations of Isenolic® was carried out by HPLC analysis at 242 nm considering oleuropein (Sigma-Aldrich; St Louis, MO, USA) as external standard in a 1220 Infinity series system equipped with autosampler and UV detector (Agilent Technologies; Santa Clara, CA, USA). Briefly, a Luna PFP(2) column (5 µm, 100 Å, 250 × 4.6 mm) from Phenomenex was used as stationary phase. As mobile phase, a gradient of acetic acid at 2.5% in water as solvent A and acetonitrile as solvent B was applied to ensure the separation as follows: from 0 to 20% of B in 50 min, from 20 to 100% of B in 5 min, maintained 100% of B for 5 min and finally form 100 to 0% of B in 10 min. The total run time was 70 min at a flow rate of 0.6 ml/min. Samples of Isenolic® extracts were diluted in water:acetonitrile 50:50 and the HPLC injection volume was 20 µl. An external calibration curve of oleuropein was assessed by triplicate and the linearity of the system was proven (r > 0.99). EA content in the samples was calculated by interpolation form the calibration curve.
Virus and cells
Human Influenza A virus (FR-8 A/Brisbane/10/2007 A (H3N2)) was obtained from International Reagent Resource. Sialic acid overexpressing Madin-Darby Canine Kidney (MDCK-SIAT1) cells were purchased from Sigma Aldrich (St Louis, MO, USA). Cells were cultured in Dulbecco's Modified Eagle's Medium, DMEM, supplemented with 2 mM glutamine (MERK), 9% glucose (Sigma Aldrich), 10% of foetal bovine serum (FBS) from Lonza (BioWhittaker) and 1% gentamicin (G-418, Thermo Fischer Scientific; Waltham, MA, USA).
Cytotoxicity
Stock solutions of both Isenolic® formulations were prepared at 12 mg/ml in water plus 0.01% chloroform. Cytotoxicity evaluation of Isenolic® was performed in MDCK-SIAT1 canine kidney cells using the extract vehicle, water + 0.01% chloroform (Sigma Aldrich; St Louis, MO, USA), and 20% DMSO (Sigma Aldrich; St Louis, MO, USA) as experimental controls. Triplicate cultures of 1 × 104 cells were grown in 96-well microtiter plates for 24 h. Then, cells were incubated with both Isenolic® formulations (4% and 8% EA dry basis) at concentrations from 6 to 1200 µg/ml in a 24 h period of exposure. The remaining cell metabolic activity was determined by 3-(4, 5-dimethylthiazol-2ol) 2, 5 diphenyl tetrazolium bromide, MTT, reduction assay. After removing Isenolic® containing supernatants, cells were washed twice with Optimem (GIBCO) and 0.15 mg/ml MTT (Sigma Aldrich; St Louis, MO, USA) was added for an incubation period of 4 h at 37 °C. Finally, 200 µl of DMSO was added to dissolve MTT crystals and absorbance was read at 570/655 nm in a microplate reader. The 50% cytotoxic concentration (CC50) was considered as the concentration at which cell viability was reduced by 50%. CC50 and other interpolation values were determined by non-linear regression analysis of three independent essays.
Viral infection
Influenza infectivity was evaluated in MDCK-SIAT1 cells using Tris-EDTA buffer and 20% DMSO as experimental controls. Triplicate cultures of 1 × 104 cells were grown in the aforementioned medium for 24 h. Dilutions of the virus in Tris-EDTA buffer at multiplicity of infection (MOI): 0.01, 0.1, 1 and 2 were then added to the cells. After 48 h of exposure, viral particles were removed with double Optimem washing and cell viability was measured by the MTT tetrazolium reduction assay following the protocol described previously. The experiments were repeated at least three times in triplicate assays.
Antiviral activity
Antiviral activity evaluation of Isenolic® was performed in MDCK-SIAT1 cells infected with Influenza A (H3N2) using the commercial kit Neuraminidase Assay Kit MAK121 (Sigma-Aldrich; St Louis, MO, USA) and comparing to Oseltamivir as positive experimental control. Cultures of 1 × 104 cells per well were grown in 96-well plates and pre-treated with Isenolic® 4% and 8% formulations at concentrations from 6 to 150 µg/ml and 0.8 µg/ml oseltamivir for 1 h. Next, the extract is removed by double medium refreshment before exposing cells to the virus at MOI:1 for 1 h. After 48 h of infection, viral particles were removed with DMEM supplemented with 2 µg/ml TPK-Trypsin, and neuraminidase activity was determined as an indicator of viral production. Briefly, neuraminidase activity test is an enzymatic assay in which sialic acid released by neuraminidase results in a colorimetric/fluorometric product, directly proportional to neuraminidase activity in the sample. Aliquots of 20 μl of supernatant samples were mixed with 80 μl of the Master-Mix reaction provided in the commercial kit in 96 μl well plates and incubated at 37°C. The absorbance of the samples and standards was measured at 30 min λex = 530 / λem = 585 nm in fluorometric assay. The IC50 was considered as the concentration at which neuraminidase activity was reduced by 50%. The IC50 parameter and other interpolation values were determined by non-linear regression analysis of at least three independent assays. Selectivity index (SI) was calculated as the ratio of CC50/IC50 as an indicator of specific antiviral action of 4% and 8% Isenolic® formulations. The experiments were repeated at least three times in triplicate assays.
The effect of Isenolic® as antiviral agent was measured as cell viability. Cytopathic effect of Influenza A (H3N2) virus was assayed in MDCK-SIAT1 cells pre-treated or not with 8% Isenolic®. 1 × 104 cells per well were grown in 96-well plates and pre-treated or not with 8% Isenolic® at 100 µg/ml or 0.8 µg/ml oseltamivir for 1 h as positive experimental control. Isenolic® excess was removed by washing cells twice with fresh medium. Then, the cells were exposed to a cytotoxic concentration of the virus at MOI: 2 for 1 h. After 48 h of infection, viral particles were removed and cell viability was evaluated by the MTT tetrazolium reduction assay. The experiments were repeated at least three times in triplicate assays.
Data analysis
Data are expressed as mean ± standard deviation (SD) in bar graphs and as mean ± standard error of the mean (SEM) in tables. Student's t-test was performed to evaluate the statistical difference between CC50 and IC50 values, other paired results and observations for groups of different treatments referred to non-treatment control groups. Differences between groups were considered statistically significant for p values lower than 0.05. Data calculation and statistical analysis were performed using GraphPad Prism software.
Results
Isenolic® characterization
HPLC-MS analysis allowed the identification EA isomers and various derivatives in Isenolic® extracts (Table 1). Characteristic molecular ions of EA (m/z 241.1) were registered at retention times of 62.7 and 66.7 min, indicating the presence of two isomers. These two isomers of EA were as well identified by HPLC and their total concentration was quantified in both Isenolic® formulations, obtaining values of 4.6% and 8.1% dry basis (Figure 1). The content of EA derivates was analysed by using several standard substances (dimethyl elenolic oleoside and EA glucoside 2). All of them appeared in negligible concentrations (below 0.1%).
Table 1.
Identification of elenolic acid (EA) and derivatives in isenolic® by high performance liquid chromatography-associated Mass Spectrometry (HPLC-MS).
| Compound | TR (min) | λmax (nm) | [M-H]− (m/z) | Fragments (m/z) | 2[M-H]− (m/z) |
|---|---|---|---|---|---|
| Demethyl EA glucoside (DMEA-Glu-1) (Oleoside) | 20.6 | 233 | 389.1 | 227.0, 183.0 | 779.2 |
| Demethyl EA glucoside (DMEA-Glu-2) (Secologanoside) | 34.0 | 236 | 389.2 | 345.1, 138.9 | ND |
| Caffeoyl diglucoside+ EA glucoside 1 (EA-Glu 1) | 37.9 | 231/300sh/312 | 489.0 403.0 | 463.0 ([M + Cl]-), 241.1, 139.1 | ND |
| EA glucoside 2 (EA-Glu 2) | 40.6 | 240/342 | 403.0 | 223.1, 179.0, 119.1, 112.9, 100.9 | 807.3 |
| Demethyl EA glucoside (DMEA-Glu-3) | 47.0 | 238 | 389.0 | 139.1 | ND |
| 6-hydroxyloganin+ EA glucoside 3 (EA-Glu 3) | 56.6 | 246 | 405.1 403.1 | 240.9 | 811.0 |
| EA 1 (EA-1) | 62.7 | 239 | 241.1 | 165.1, 139.1, 127.1, 121.1, 111.2, 101.0 | ND |
| EA 2 (EA-2) | 66.7 | 240 | 241.1 | ND | ND |
| EA derivative | 73.7 | 281 | 395.0 | 241.1, 209.1, 181.1, 139.1 | ND |
Retention time (Rt), UV-Vis absorption maximum (λmax) and characteristic molecular and fragments ions. ND: not detected.
Figure 1.
Identification of EA isomers in isenolic® 4% (A) and 8% (B) formulations by high performance liquid chromatography (HPLC) at 242 nm. *: EA isomers corresponding peaks.
Cytotoxicity
Cytotoxicity of Isenolic® formulations was assessed by MTT assay. As shown in Figure 2, cell viability remained unaffected at concentrations up to 60 µg/ml for both concentrations of 4% and 8% EA. Cell viability started to be affected at 120 µg/ml, showing the 8% formulation a more pronounced effect. Therefore, cytotoxic concentrations of both formulations presented statistical differences: CC50 values of 4% and 8% formulations were 218.2 and 149.7 µg/ml respectively (P < 0.001). At concentrations higher than 300 µg/ml, cell viability fell to rates comparable to that found at of 20% DMSO for both formulations (Figure 2 and Table 2). Cytotoxicity of oseltamivir was previously tested in MDCK-SIAT1 cells and cell viability was over 90% up to 150 μg/ml concentration (data not shown), in accordance with other authors studies. 28
Figure 2.
Influence of isenolic® concentrations on viability of MDCK-SIAT1 cells for 4% (A) and 8% (B) formulations compared to dimethyl sulfoxide (DMSO) and non-linear regression representation of dose-response curves for isenolic® formulations (C). Data are represented as mean ± standard deviation of at least three independent assays. Statistical differences were evaluated by Student's t-test comparing concentrations with non-treated (NT) cells. *** P < 0.001 vs NT.
Table 2.
Cytotoxic concentration (CC50), inhibitory concentration (IC50) and selectivity index (SI) of isenolic® formulations.
| Isenolic® formulation | CC50 (µg/ml) a | IC50 (µg/ml) a | S.I. |
|---|---|---|---|
| 4% EA | 218.2 ± 8.9 | 171.4 ± 13.3 | 1.3 |
| 8% EA | 149.7 ± 2.1*** | 65.5 ± 4.8* | 2.3 |
CC50 and IC50 values are expressed as mean ± SEM of at least two independent assays and were interpolated from non-linear regression analysis. Statistical differences of CC50 and IC50 values comparing 4% and 8% formulations were evaluate by Student's t-test *P < 0.05 vs 4% EA; ***P < 0.001 vs 4% EA.
Viral infection
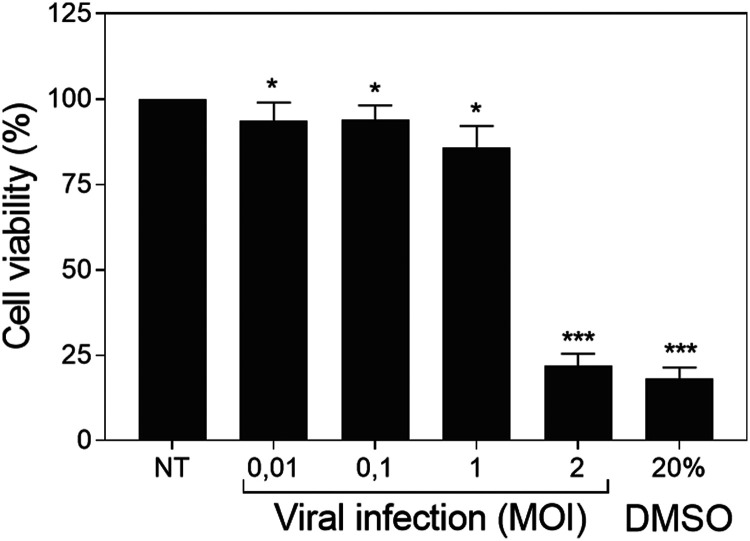
Cell viability was affected at large extent at MOI 2, whereas lower viral dosages did not shrink substantially the metabolic activity of the target cells that sustained cell viability rates above 80%. At MOI 0.01, 0.1 and 1 the MTT test showed cell viability rates of 93.7, 93.9 and 85.9% respectively compared to the untreated cells. However, at MOI 2 cell viability was reduced to 21.9%, similar to the experimental rate found with 20% DMSO (Figure 3).
Figure 3.
Influenza infectivity evaluation. Influence of viral multiplicity of infection (MOI) on MDCK-SIAT1 cells viability for influenza and dimethyl sulfoxide (DMSO). Data are represented as mean ± standard deviation of four independent assays. Statistical differences were evaluated by Student's t-test comparing concentrations with non-treated nor infected (NT) cells. * P < 0.05 vs NT; *** P < 0.001 vs NT.
Antiviral activity
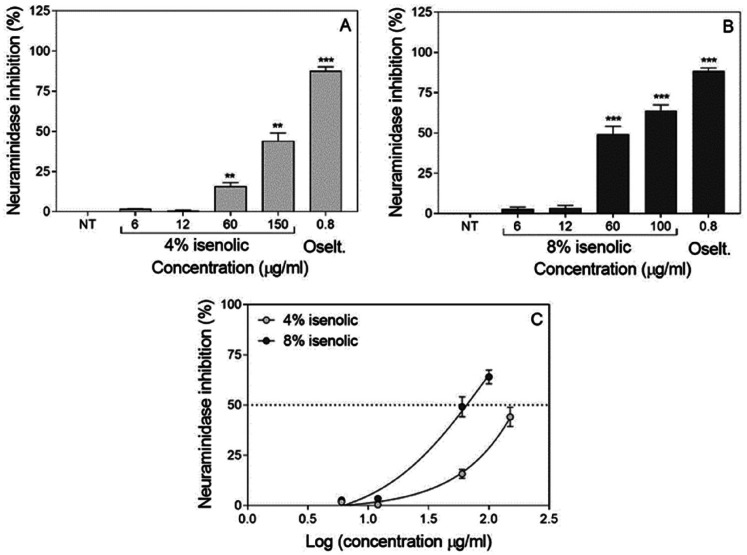
The effects of pre-treatments with 8% and 4% Isenolic® formulations were evaluated indirectly by the assessment of the neuraminidase activity. Both Isenolic® formulations at concentrations higher than 12 µg/ml reduced neuraminidase activity as did the use of 0.8 µg/ml oseltamivir (that showed an 88.0% inhibition rate), finding an increase at inhibition rate at higher concentration of EA at the formulation (Figure 4). The maximum inhibitory effect found for Isenolic® was 64.0% for the 8% EA formulation added at 100 µg/ml; whereas at the same concentration, the inhibition was only 28.6% with Isenolic® at 4% EA (P = 0.0014). 4% EA formulation reached its maximum 44.1% inhibition when was added at 150 µg/ml (Table 2). The Inhibitory concentration (IC50) values of Isenolic® were significantly different at 4% and 8% EA: 65.5 µg/ml for the 8% formulation and 171.4 µg/ml for the 4% formulation (P < 0.01). Selectivity index (SI) values were 2.3 and 1.3 for the 8% and 4% formulations, respectively (Table 2).
Figure 4.
Influence of concentration of Isenolic® on viral neuraminidase inhibitory effect after influenza infection of MDCK-SIAT1 cells for 4% (A) and 8% (B) formulations compared to oseltamivir (Oselt.) and non-linear regression representation of dose-response curves for isenolic® formulations (C). Data are represented as mean ± standard deviation of at least three independent assays. Statistical differences were evaluated by Student's t-test comparing concentrations with non-treated (NT) cells. ** P < 0.01 vs NT; *** P < 0.001 vs NT.
Cell viability
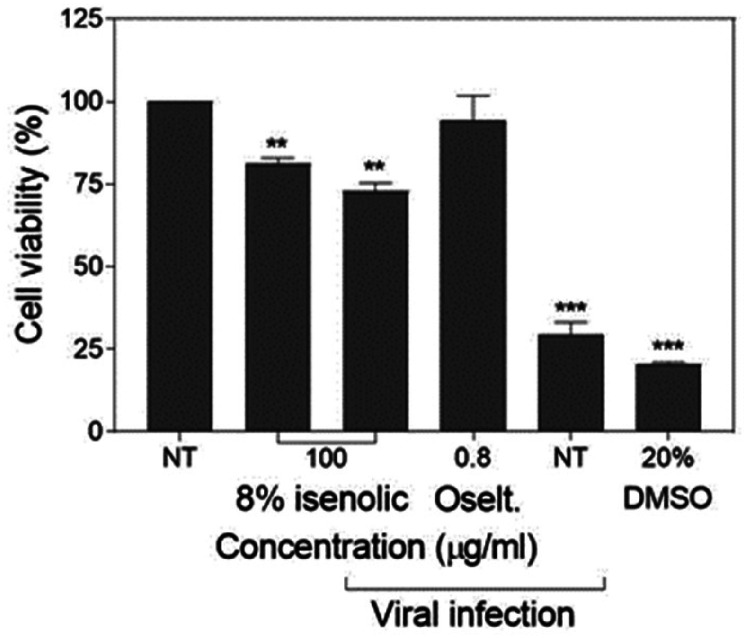
The preventive effect of Isenolic® at 8% EA on cell viability under infection by influenza virus was determined by MTT assay. In not pre-treated cells the influenza infection at high cytopathic concentration (MOI 2) lowered the viability down to 29.6%, close to the deleterious effect of the addition of 20% DMSO (20.7%). Nevertheless, the pre-treated cells with the 8% EA formula added at 100 µg/ml preserved 72.9% cell viability in the presence of the virus (P < 0.01), being a significative lower viability compared to non-infected pre-treated cells (81.1%) (P < 0.01). Only the use of the pharmacological drug 0.8 µg/ml oseltamivir gave better preservation data (the metabolic activity of the cells was maintained at 94.3% comparing to the controls) (Figure 5).
Figure 5.
MDCK-SIAT1 cell viability preservation of isenolic® 8% formulation and oseltamivir (oselt.) after influenza infection. Data are represented as mean ± standard deviation of three independent assays. Statistical differences were evaluated by Student's t-test comparing concentrations with non-treated (NT) nor infected cells. ** P < 0.01 vs NT nor infected; *** P < 0.001 vs NT nor infected.
Discussion
The assessment of the anti-influenza effects of Isenolic® formulations was carried out by the study of the inhibition of virus-induced cytopathic effects according to the guidance to test the anti-infective potential of natural products by Cos et al. 29 Results showed that cytotoxicity of 4% formulation was lower than that of 8% formulation, but the 8% EA formulation was more efficient in reducing neuraminidase activity. Taking all together, the selectivity index (SI) was calculated. SI gives an idea of how effective and safe a drug would be during in vivo treatment for a given viral infection. Since SI value was approximately two-fold higher for the 8% EA formulation than for the 4% EA, it is implied that the anti-influenza A activity of the 8% formulation was safer. Although the 8% EA formulation gave an IC50 value below 100 µg/ml and below the threshold suggested by Cos et al. 29 to define an extract with a relevant and selective activity against the pathogen, its selectivity index was low due to its cytotoxicity against the used cells. Nevertheless, due to its IC50 and its higher selectivity index, compared to the 4% formulation, we decided to use the 8% EA extract for further research on the cell viability preservation.
Interestingly, the pre-treatment of the target cells with the Isenolic® 8% EA formulation preserved most of the metabolic activity of the cells after the influenza A infection. It confirmed the potential of 8% Isenolic® as agent to preserve the target cells from the viral cytopathic effect.
It is known that olive leaves extracts can contain antimicrobial metabolites: oleuropein, hydroxytyrosol and EA. 30 Antiviral effects have been described in hydroxytyrosol and oleuropein. Both inhibit HIV-1 infection 31 by blocking the viral entry, integration and replication into the host cells. Additionally, the influenza activity has been inhibited by hydroxytyrosol. The susceptible subtypes comprise A(H1N1), A(H3N2), A(H5N1), and A(H9N2) strains. But our results described herein does not reflect the typical actions found in hydroxytyrosol. The pre-treatment of the target cells with hydroxytyrosol did not prevent the infection and, at least, the A(H9N2) neuraminidase is not inhibited by the compound. The proposed mechanism of inhibition in hydroxytyrosol would be related to abnormalities provoked by the substance in the surface of the virion envelope. 23
The anti-influenza effect of Isenolic® can be attributed to its EA content, because the extract showed an EA dose-dependent inhibition. The role of several elenolic derivates against the influenza viruses is known since decades, especially in works done with the calcium salt of EA. Accordingly to our results, there is a patent was filed in 1973 claiming the use of elenolic derivates as anti-influenza drug. 34 The patent described an in vivo animal model in which calcium elenolate administrated intranasally reduced virus infection and accelerated its eradication in the lungs. 26 Earlier studies showed antiviral effects of EA salts against several enveloped and non-enveloped viruses at a concentration of at least 0.5 mg/ml of elenolate. 25 However, the antiviral mechanism of action of EA remains unknown. Previously, it was stipulated that EA salts interfere with the virus life cycle and, in the case of retroviruses, with the inhibition of reverse transcriptase and protease. 25 Recently, some studies indicated neuraminidase inhibition as a possible mechanism of action of EA derivatives. Myxoviruses neuraminidase resulted inhibited at dosage between 24–120 µg/ml of pure salt. 35 Additionally, prior incubation of sensitive cells with calcium elenolate and amino acids such as histidine, glycine, cysteine, or lysine resulted in losses of the salt virucidal activity. 25 Originally this finding suggested EA derivates interacted directly with specific virion proteins, but not HA or NA, and blocks the infection cycle at some stage. Nevertheless, the effect of certain amino acid derivates 36 and the efficacy of a blend composed by natural substances and amino acids as neuraminidase inhibitors have been reported, 37 suggesting the need for studies more focused on the activity of EA alone.
By contrast to the previous studies, this research was done with the naturally present EA isomers in the olive leaf extract, instead of calcium elenolate. It avoided the problems derived by the presence of the ion, because Calcium would stabilise the viral neuraminidase activity 38 and, therefore, induce wrong conclusions. Possibly derived from this interaction, we found antiviral effects at concentrations lower than those stipulated in this patent for EA (50 µg/ml-50 mg/ml to EA from the patent vs 4.8 µg/ml-8 µg/ml to EA from Isenolic®).
Conclusions
As far as we know, this is the first time where an olive extract rich in EA showed a reduction of neuraminidase activity and prevents the metabolic inactivation of the host cells in infection experiments. The two formulations of the Isenolic® showed an anti-influenza activity increasing in a dose-dependent relation. Thus, Isenolic® would become a complement or alternative to well established drugs to fight against NAI resistant and sensitive influenza strains.
Acknowledgements
The authors would like to thank Dr Ricardo Madrid and his team from BioAssays S.L. for their participation in the in vitro study and Dr Alberto Espinel and Dr Enrique Cubas-Cano, from Pharmactive Biotech Products S.L., for the critical readout of the manuscript.
Footnotes
Declaration of conflicting interests: Pharmactive Biotech Products, S.L. is the manufacturer of Isenolic® and provided the product samples for the study. As this work has been funded by the pharmaceutical company Pharmactive Biotech Products S.L. authors from this company may have conflict of interest. The HPLC-MS analysis has been performed by the academic researchers from Universidad Autónoma de Madrid.
Funding: This work has been funded by Pharmactive Biotech Products S.L. and by the grants awarded from the Spanish Ministry of Science, Innovation and universities to Aurora Salamanca (PTQ-16-08297) and from Community of Madrid to Daniel González-Hedström (IND2017/BIO7701).
ORCID iD: Daniel González-Hedström https://orcid.org/0000-0001-9425-1115
References
- 1.Troeger CE, Blacker BF, Khalil IA, et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2019; 7: 69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.te Velthuis AJW, Fodor E. Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis. Nat Rev Microbiol 2016; 14: 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krug RM, Aramini JM. Emerging antiviral targets for influenza A virus. Trends Pharmacol Sci 2009; 30: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster RG, Govorkova EA. Continuing challenges in influenza. Ann N Y Acad Sci 2014; 1323: 115–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clinical Infectious Diseases 2019; 68: e1–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi K, Furuta Y, Fukuda Y, et al. In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir Chem Chemother 2003; 14: 235–241. [DOI] [PubMed] [Google Scholar]
- 7.Ginex T, Luque FJ. Searching for effective antiviral small molecules against influenza A virus: a patent review. Expert Opinion on Therapeutic Patents 2020; 31(1): 53–66. [DOI] [PubMed] [Google Scholar]
- 8.Meijer A, Lackenby A, Hungnes O, et al. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007-08 season. Emerg Infect Dis 2009; 15: 552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dharan NJ, Gubareva LV, Meyer JJ, et al. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA 2009; 301: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 10.Practical guidance for national influenza centres establishing or implementing neuraminidase inhibitor susceptibility surveillance ii WHO Library Cataloguing-in-Publication Data Practical guidance for national influenza centres establishing or implementin. 2019.
- 11.Abed Y, Baz M, Boivin G. Impact of neuraminidase mutations conferring influenza resistance to neuraminidase inhibitors in the N1 and N2 genetic backgrounds. Antivir Ther 2006; 11: 971–976. [PubMed] [Google Scholar]
- 12.Jackson D, Barclay W, Zürcher T. Characterization of recombinant influenza B viruses with key neuraminidase inhibitor resistance mutations. J Antimicrob Chemother 2005; 55: 162–169. [DOI] [PubMed] [Google Scholar]
- 13.Baz M, Abed Y, Papenburg J, et al. Emergence of oseltamivir-resistant pandemic H1N1 virus during prophylaxis. N Engl J Med 2009; 361: 2296–2297. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen HT, Sheu TG, Mishin VP, et al. Assessment of pandemic and seasonal influenza A (H1N1) virus susceptibility to neuraminidase inhibitors in three enzyme activity inhibition assays. Antimicrob Agents Chemother 2010; 54: 3671–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubareva LV, Besselaar TG, Daniels RS, et al. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors, 2015–2016. Antiviral Res 2017; 146: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan Y, Panchal S, Vyas N, et al. Olea europaea: a phyto-pharmacological review. Phytochemistry 2007; 1: 114–118. [Google Scholar]
- 17.Hashmi MA, Khan A, Hanif M, et al. Traditional uses, phytochemistry, and pharmacology of Olea europaea (olive). Evid Based Complement Alternat Med. 2015; 2015: 541591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akerreta S, Calvo MI, Cavero RY. Ethnoveterinary knowledge in navarra (Iberian Peninsula). J Ethnopharmacol 2010; 130: 369–378. [DOI] [PubMed] [Google Scholar]
- 19.Rivera D, Alcaraz F, Verde A, et al. Is there nothing new under the sun? The influence of herbals and pharmacopoeias on ethnobotanical traditions in Albacete (Spain). J Ethnopharmacol 2017; 195: 96–117. [DOI] [PubMed] [Google Scholar]
- 20.Esti Cinquanta, La Notte E. Phenolic compounds in different olive varieties. J Agric Food Chem 1998; 46: 32–35. [DOI] [PubMed] [Google Scholar]
- 21.Nikolaivits E, Termentzi A, Skaltsounis AL, et al. Enzymatic tailoring of oleuropein from olea europaea leaves and product identification by HRMS/MS spectrometry. J Biotechnol 2017; 253: 48–54. [DOI] [PubMed] [Google Scholar]
- 22.Omar SH. Oleuropein in olive and its pharmacological effects. Sci Pharm 2010; 78: 133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada K, Ogawa H, Hara A, et al. Mechanism of the antiviral effect of hydroxytyrosol on influenza virus appears to involve morphological change of the virus. Antiviral Res 2009; 83: 35–44. [DOI] [PubMed] [Google Scholar]
- 24.Soret M. Antiviral activity of calcium elenolate on parainfluenza infection of hamsters. Antimicrob Agents Chemother 1969; 9: 160–166. [DOI] [PubMed] [Google Scholar]
- 25.Renis H. In vitro antiviral activity of calcium elenotate. Antimicrob Agents Chemother 1969; 9: 167–172. [PubMed] [Google Scholar]
- 26.Renis HE. Archives of virology influenza virus infection of hamsters. A model for evaluatinfl antiviral drufs. Arch Virol 1977; 54: 85–93. [DOI] [PubMed] [Google Scholar]
- 27.Prodanov M, Vacas V, Hernández T, et al. Chemical characterisation of Malvar grape seeds (Vitis vinifera L.) by ultrafiltration and RP-HPLC-PAD-MS. J Food Compos Anal 2013; 31: 284–292. [Google Scholar]
- 28.Ghaffari H, Tavakoli A, Moradi A, et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci 2019; 26: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cos P, Vlietinck AJ, Berghe DV, et al. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol 2006; 106: 290–302. [DOI] [PubMed] [Google Scholar]
- 30.Thielmann J, Kohnen S, Hauser C. Antimicrobial activity of Olea europaea Linné extracts and their applicability as natural food preservative agents. Int J Food Microbiol 2017; 251: 48–66. [DOI] [PubMed] [Google Scholar]
- 31.Bedoya LM, Beltrán M, Obregón-Calderón P, et al. Hydroxytyrosol: a new class of microbicide displaying broad anti-HIV-1 activity. AIDS 2016; 30: 2767–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee-Huang S, Huang PL, Zhang D, et al. Discovery of small-molecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol: part I. Integrase inhibition. Biochem Biophys Res Commun 2007; 354: 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao J, Zhang DW, Zhang JZH, et al. Computational study of bindings of olive leaf extract (OLE) to HIV-1 fusion protein gp41. FEBS Lett 2007; 581: 2737–2742. [DOI] [PubMed] [Google Scholar]
- 34.Mary AN, Harold ER, Gerald E. Un derwood. Method for treating influenza viral infections . US3737550A.
- 35.Renis HE. Inactivation of myxoviruses by calcium elenolate. Antimicrob Agents Chemother 1975; 8: 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kati WM, Montgomery D, Maring C, et al. Novel α- and β-amino acid inhibitors of influenza virus neuraminidase. Antimicrob Agents Chemother 2001; 45: 2563–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jariwalla RJ, Roomi MW, Gangapurkar B, et al. Suppression of influenza A virus nuclear antigen production and neuraminidase activity by a nutrient mixture containing ascorbic acid, green tea extract and amino acids. BioFactors 2007; 31: 1–15. [DOI] [PubMed] [Google Scholar]
- 38.Chong AKJ, Pegg MS, von Itzstein M. Influenza virus sialidase: effect of calcium on steady-state kinetic parameters. Biochim Biophys Acta (BBA)/Protein Struct Mol 1991; 1077: 65–71. [DOI] [PubMed] [Google Scholar]
- 39.Smith BJ, Huyton T, Joosten RP, et al. Structure of a calcium-deficient form of influenza virus neuraminidase: implications for substrate binding. Acta Crystallogr Sect D Biol Crystallogr 2006; 62: 947–952. [DOI] [PubMed] [Google Scholar]
- 40.Burmeister WP, Cusack S, Ruigrok RWH. Calcium is needed for the thermostability of influenza B virus neuraminidase. J Gen Virol 1994; 75: 381–388. [DOI] [PubMed] [Google Scholar]