Abstract
Many people listen to music for hours every day, often near bedtime. We investigated whether music listening affects sleep, focusing on a rarely explored mechanism: involuntary musical imagery (earworms). In Study 1 (N = 199, mean age = 35.9 years), individuals who frequently listen to music reported persistent nighttime earworms, which were associated with worse sleep quality. In Study 2 (N = 50, mean age = 21.2 years), we randomly assigned each participant to listen to lyrical or instrumental-only versions of popular songs before bed in a laboratory, discovering that instrumental music increased the incidence of nighttime earworms and worsened polysomnography-measured sleep quality. In both studies, earworms were experienced during awakenings, suggesting that the sleeping brain continues to process musical melodies. Study 3 substantiated this possibility by showing a significant increase in frontal slow oscillation activity, a marker of sleep-dependent memory consolidation. Thus, some types of music can disrupt nighttime sleep by inducing long-lasting earworms that are perpetuated by spontaneous memory-reactivation processes.
Keywords: music cognition, stuck-song syndrome, involuntary memory, slow-wave activity, primary auditory cortex, open data, open materials
I came across these jingling rhymes in a newspaper . . . and read them a couple of times. They took instant and entire possession of me. . . . [I] went to bed and rolled, tossed, and jingled right along . . . got up at midnight frantic . . . by sunrise I was out of my mind, and everybody marveled and was distressed at the idiotic burden of my ravings.
—“A Literary Nightmare” (Mark Twain, 1876, p. 167)
Nearly all adolescents and young adults listen to music every day (Krause et al., 2015; North et al., 2004). Because music stimulates brain regions involved in emotion and reward processing (Thaut & Hodges, 2019), it is unsurprising that people regularly use music to improve their mood, typically with the goal of increasing energy and alertness (Chanda & Levitin, 2013). Recent evidence, however, indicates that music is being used by many individuals for the opposite purpose: to try to fall asleep (Trahan et al., 2018).
If music can help one to relax, then it should also help one to fall asleep. That is, after all, the recommendation of the National Institutes of Health (2011) and the National Sleep Foundation (2020). Empirical studies have generally supported these organizations’ recommendation to listen to quiet music during one’s bedtime routine (Feng et al., 2018). For example, one study found that bedtime music was as beneficial to self-reported sleep quality as taking benzodiazepine hypnotic medications (Deshmukh et al., 2009). Although such findings are impressive, systematic reviews of the music-for-sleep literature have indicated an overuse of subjective measures of sleep quality, a reliance on passive control groups, and a moderate to high risk of bias (e.g., only 10% of studies included experimenter blinding; Feng et al., 2018; Jespersen et al., 2015).
Twain’s (1876) anecdote raises the possibility that seemingly benign music might actually worsen sleep quality (see also Sacks, 2010). Songs can become “stuck” in one’s mind, a phenomenon known as involuntary musical imagery or earworms. Earworms are more likely to occur after exposure to music that has a fast tempo with specific pitch contours (Jakubowski et al., 2017), which adolescents and young adults are increasingly exposed to at all hours given the proliferation of smartphones and music streaming (Krause et al., 2015; Sacks, 2010). Earworms are also likely when one is in a low attentional state (Floridou et al., 2017; Williamson et al., 2012), and they may recur over the next 8 hr after one listens to music (Beaman & Williams, 2010; Hyman et al., 2013; Moeck et al., 2018). Considering these characteristics, we hypothesized that evening music listening could heighten risk for earworms that then impact sleep.
In the current investigation, we conducted a survey on music-listening habits and sleep quality (Study 1) as well as a controlled experiment that used the gold standard of sleep measurement and rigorous blinding procedures (Study 2). In both studies, we tested whether earworms have a direct impact on sleep. Interestingly, theorizing on earworms yields contrasting hypotheses on whether a nighttime earworm should hinder or facilitate sleep. According to one view, earworms are pleasant experiences that can distract one from negative thoughts (Beaman & Williams, 2010; Halpern & Bartlett, 2011); if so, then an earworm should help people fall asleep faster by relaxing them or distracting them from other worries (Harvey & Payne, 2002). The alternative view—artfully described by Twain (1876)—is that earworms are intrusive and persistent even when one wants the melody to stop (Hyman et al., 2013; Liikkanen et al., 2015). If so, then the prediction is that earworms will worsen sleep quality. 1
Study 1
Method
Participants
We recruited 209 adults living in the United States from Amazon Mechanical Turk. This convenience sample was powered to detect small- to medium-size correlations, and data collection ended when the targeted sample size was reached. Ten participants were excluded because they did not pass quality-control checks on free responses (e.g., impossible values, bot-like answers). The demographics for the remaining 199 participants are shown in Table S1 in the Supplemental Material available online. The university’s institutional review board approved this study, and all participants provided written informed consent and received monetary compensation.
Statement of Relevance.
Health organizations recommend listening to quiet music before bedtime, but these recommendations largely arise from studies that used self-report measures. Using the gold standard in sleep measurement, we experimentally tested whether bedtime music listening affects sleep quality. Instrumental music actually worsened sleep quality when it caused participants to experience a song stuck in their mind (an earworm). In addition, in a survey study, we found that individuals who frequently listen to music experienced persistent earworms and a decline in sleep quality. These data challenge the wisdom of using music as a hypnotic and identify that the sleeping brain continues to process music for several hours, even after the music stops.
Procedure
Participants completed a series of questionnaires on demographics, sleep quality, music listening, and earworms. Sleep measures were the Pittsburgh Sleep Quality Index (PSQI), the Stanford Sleepiness Scale (SSS), and the Ford Insomnia Response to Stress Test (FIRST). The PSQI assesses global sleep quality via seven component scores (i.e., subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction; Buysse et al., 1989). The SSS assesses daytime sleepiness and alertness (Hoddes et al., 1973). The FIRST assesses vulnerability to stress-related sleep disturbances (Drake et al., 2004). For each sleep measure, higher scores indicate worse sleep outcomes.
To assess music-listening frequency, we asked participants to complete the items on active listening/engagement from the Goldsmiths Musical Sophistication Index (Gold-MSI; Müllensiefen et al., 2014). Example items include “I listen attentively to music for __ per day” and “I spend a lot of my free time doing music-related activities.” To complement the Gold-MSI, we included questions on which times of day participants listened to music, whether they perceived that listening to music at bedtime helped or hurt sleep quality, and what type (or types) of music they listened to during the day and near bedtime. Musical preferences are represented as a word cloud in Figure S1 in the Supplemental Material. Most people preferred classical, rock, or pop music near bedtime.
To assess the general frequency of earworms and individuals’ experiences with earworms, we asked participants to complete the Involuntary Musical Imagery Scale (Floridou et al., 2015). Our interest was in whether earworms were perceived as pleasant or irritating (negative valence factor), particularly when experienced at night. To assess the frequency of sleep-related earworms, we asked participants to indicate on a 7-point scale (ranging from never to every day) how frequently they experienced earworms when they (a) were trying to fall asleep, (b) woke in the middle of the night, and (c) woke in the morning.
Statistical analysis
Statistical analyses were performed in SPSS (Version 26). All tests were two-tailed, and α was set to .05. We conducted partial correlations and analyses of covariance (ANCOVAs) to investigate the relationships among sleep, music, and earworm frequency and timing. To complement the continuous-data analyses, we additionally classified each participants as either (a) infrequently or never experiencing earworms (two to three times per month to never) or (b) frequently experiencing earworms (every day to once per week). Of those who frequently experienced earworms, we further distinguished whether they experienced frequent sleep-related earworms (every day to once per week) or daytime-only earworms, and 95% confidence intervals (CIs) were estimated using 1,000-sample bootstrapping.
Results
Almost all participants (87%) reported believing that music improves sleep (or at least does not disrupt sleep; see Table S1). But contrary to this general perception, greater music listening/engagement was associated with worse sleep and sleepiness (see Fig. S2 in the Supplemental Material) on the FIRST, rp(195) = .36, p < .001; PSQI, rp(182) = .14, p = .06; and SSS, rp(195) = .15, p = .04. To identify the mechanism by which music listening can be disruptive to sleep, we first examined whether participants commonly experienced earworms (expected) and whether those earworms occurred at night (unknown).
Approximately 77% of participants frequently experienced earworms (infrequent or no earworms: 23.12%; 95% CI = [17.3%, 29.4%]). Interestingly, many participants reported that earworms frequently occurred at sleep-related time points (33.17%; 95% CI = [26.9%, 39.6%]; see Table S2 in the Supplemental Material). The daytime-only earworms group (43.72%; 95% CI = [36.7%, 50.0%]), infrequent or no earworms group, and sleep-related-earworm group were similar on demographic measures, except that women (85.1%) were more likely to experience earworms than men (68.8%; see Table S1). Therefore, subsequent analyses controlled for gender.
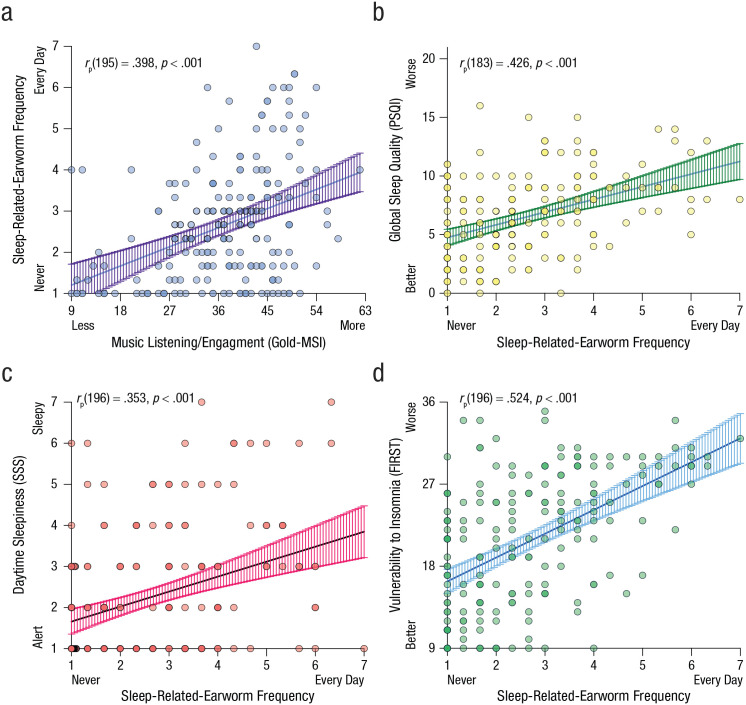
Music-listening habits (assessed via the Gold-MSI) increased the probability of sleep-related earworms, rp(195) = .40, p < .001 (see Fig. 1a), more so than daytime-only earworms (see Table S1). When individuals experienced a sleep-related earworm, it was perceived as more irritating than daytime earworms (assessed via the Involuntary Musical Imagery Scale), F(2, 173) = 5.20, MSE = 62.46, p = .01, η p 2 = .06 (see Table S1). Earworms were irritating seemingly because they were interfering with sleep: We observed medium- to large-size associations between sleep-related-earworm frequency and worse sleep quality (Fig. 1b), worse daytime sleepiness (Fig. 1c), and worse insomnia responses (Fig. 1d). Most striking were the PSQI scores for global sleep quality, which worsened by 54% in the sleep-related-earworm group, F(2, 182) = 9.89, MSE = 11.80, p < .001, η p 2 = .10 (Table S1). The odds of being a PSQI-classified “poor sleeper” (> 5 global score; Buysse et al., 1989) were elevated in the sleep-related-earworm group (77.97%) relative to the daytime-earworm group (52.38%), odds ratio (OR) = 3.28, 95% CI = [1.54, 6.98], χ2(1, N = 143) = 9.54, p = .002, and infrequent/no-earworms group (39.53%), OR = 6.21, 95% CI = [2.48, 15.58], χ2(1, N = 102) = 15.15, p < .001.
Fig. 1.
Scatterplots showing partial correlations in Study 1 between sleep-related-earworm frequency and (a) music listening/engagement, (b) global sleep quality, (c) daytime sleepiness, and (d) vulnerability to insomnia. Overlapping data points are plotted darker. Solid lines show best-fitting regressions, and error bands represent 95% confidence intervals. Partial correlations controlled for gender, and the reduced sample size in (b) reflects missing data. Music listening/engagement was measured with Goldsmiths Musical Sophistication Index (Gold-MSI), global sleep quality was measured with the Pittsburgh Sleep Quality Index (PSQI), daytime sleepiness was measured with the Stanford Sleepiness Scale (SSS), and vulnerability to insomnia was measured with the Ford Insomnia Response to Stress Test (FIRST).
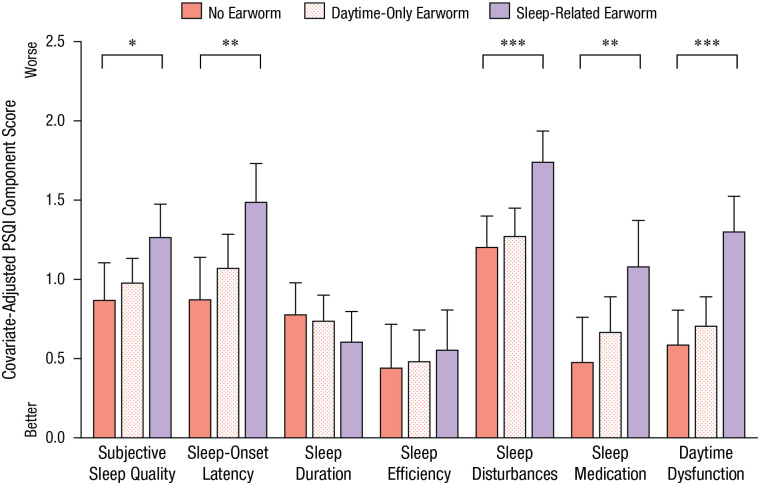
Figure 2 illustrates that earworms were associated with selective aspects of sleep quality. There was a significant interaction of PSQI component and earworm group, F(12, 1092) = 2.92, MSE = 0.54, p = .001, η p 2 = .03. People who had sleep-related earworms had similar sleep durations, bedtimes, and wake times as people who experienced earworms infrequently or predominantly during the daytime (see Table S3 in the Supplemental Material); however, sleep-related earworms (and not daytime-only earworms) were associated with considerably worse scores on other PSQI components, particularly the sleep-disturbances component, F(1, 99) = 16.09, MSE = 0.481, p < .001, η p 2 = .14, and daytime-dysfunction component, F(1, 99) = 22.02, MSE = 0.575, p < .001, η p 2 = .18 (Fig. 2).
Fig. 2.
Mean score in Study 1 for each of the seven components of the Pittsburgh Sleep Quality Index (PSQI), separately for the no-earworm, daytime-only-earworm, and sleep-related-earworm groups. Analyses were adjusted for gender. Error bars represent +1 SE. Asterisks indicate significant differences between groups (*p < .05, **p < .01, ***p < .001).
To investigate whether the various sleep measures contributed unique variance to explaining sleep-related-earworm frequency, we conducted a hierarchical linear regression analysis. The correlational matrix of sleep measures and the regression analysis are shown in Table S4 and Table S5, respectively, in the Supplemental Material. After controlling for gender (Step 1), we entered SSS, FIRST, and the seven PSQI component scores (Step 2). The sleep measures in Step 2 collectively explained 38.4% of the variance in sleep-related-earworm frequency, ∆R2 = .384, F(9, 175) = 12.18, p < .001. FIRST scores (β = 0.30, p < .001), PSQI sleep-disturbances scores (β = 0.16, p = .032), and PSQI daytime-dysfunction scores (β = 0.19, p = .018) were uniquely associated with sleep-related-earworm frequency (Table S5).
Finally, we investigated whether the relationship between music listening/engagement and sleep measures was mediated by sleep-related-earworm frequency. As shown in Table S6 in the Supplemental Material, after controlling for gender (Step 1), we entered SSS, FIRST, and the seven PSQI component scores as predictors of Gold-MSI music listening/engagement (Step 2). The sleep measures were significantly associated with music listening/engagement, ∆R2 = .146, F(9, 175) = 3.316, p < .001. Next, we repeated this regression analysis but controlled for sleep-related-earworm frequency. Sleep-related-earworm frequency was significantly associated with music listening/engagement, ∆R2 = .167, F(1, 182) = 36.561, p < .001, and controlling for earworm frequency reduced the association between music listening and sleep quality to nonsignificant levels, ∆R2 = .047, F(9, 173) = 1.154, p = .328 (Table S6). Thus, earworm frequency mediated the relationship between music listening/engagement and sleep quality.
Discussion
Earworms are not limited to the day but can occur after awakening from sleep. Frequently listening to music was related to increased earworms at night, with sleep-related earworms mediating the association between music listening and sleep quality. Therefore, a possible mechanism is that involuntary musical imagery burdens sleep quality, even when individuals adopt a normal bedtime, even when they allow for a reasonable time-in-bed duration, and even when they believe that music helps sleep.
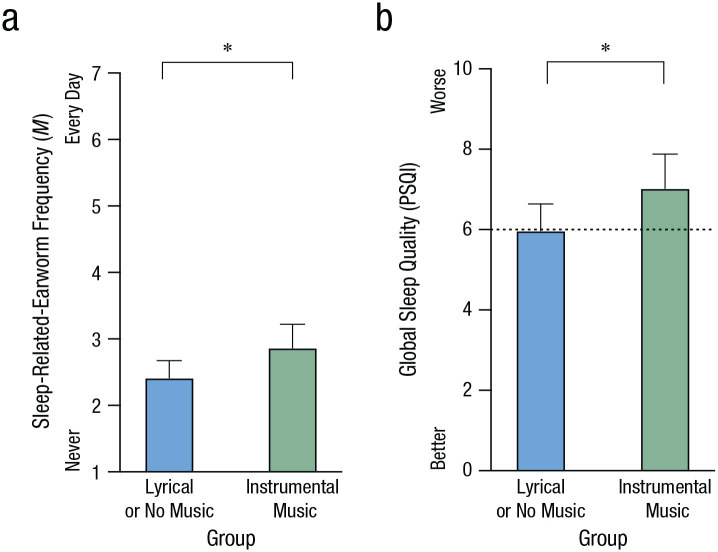
In Study 2, we sought to gain control over earworm induction by playing familiar popular music before bedtime, and we measured subsequent sleep by polysomnography. We used three popular lyrical songs, with half of participants receiving delyricized versions of the identical songs (instrumental condition). The intuitive view is that instrumental-only music should lead to better sleep quality and fewer sleep-related earworms. However, an alternative view emerged from an exploratory analysis of participants’ bedtime music preferences in Study 1: Instrumental music near bedtime—but not lyrical music—was associated with worse sleep quality and more sleep-related earworms (see Fig. 3).
Fig. 3.
Music preferences during bedtime routine in Study 1 in relation to (a) sleep-related earworms and (b) global sleep quality. Participants were separated into two groups: 75 who reported preferring instrumental music near bedtime (e.g., classical, ambient, lo-fi) and 117 comparison participants who preferred either lyrical music or no music near bedtime. In (b), the dashed line indicates the cutoff for poor sleep quality as defined by the Pittsburgh Sleep Quality Index (PSQI). Analyses were adjusted for gender. Error bars represent the upper bounds of 95% confidence intervals. Asterisks indicate significant differences between groups (p < .05).
Study 2
Method
Participants
We recruited 50 young adults (age: M = 21.16 years, SD = 2.77; 70% female; 60% Caucasian) to complete overnight polysomnography recording in a sound-, light-, and temperature-controlled laboratory. The sample size was powered to detect large effect sizes, and data collection continued until the study budget was exhausted. Eligibility criteria included being at least 18 years of age, having no history of psychiatric or neurological disorders, having no diagnosis of sleep disorders (e.g., insomnia, narcolepsy, sleep apnea), and not taking medications that affect sleep. Two participants were excluded for not completing all procedures (final N = 48). The university’s institutional review board approved this study, and participants provided written informed consent.
Polysomnography
We used the Comet XL Plus system (Grass Technologies, West Warwick, RI) to record overnight sleep. We collected electroencephalography (EEG) data at a rate of 200 samples per second from Fp1, Fp2, F3, F4, F7, F8, Fz, C3, C4, P3, P4, Pz, T3, T4, T5, T6, O1, and O2 sites (grounded at Fpz and Cz sites and referenced to contralateral mastoids). We additionally included left and right electrooculography, mentalis electromyography, and breathing measures to screen for sleep apnea (nasal pressure, chest and abdomen movements, and fingertip pulse oximetry). A certified polysomnography technician who was blind to participants’ conditions identified the sleep stages for each 30-s epoch according to the American Academy of Sleep Medicine guidelines.
Questionnaires
Participants maintained a sleep diary for 7 days. They also completed the PSQI, the FIRST, Raven’s Progressive Matrices (Raven, 1938), and measures of demographics, perceived stress, daytime sleepiness, vocabulary, and morningness-eveningness (Horne & Ostberg, 1976).
Music conditions
Each participant was randomly assigned to listen to either the lyrical or the instrumental-only versions of three popular songs. The songs were “Don’t Stop Believin’” by Journey, “Call Me Maybe” by Carly Rae Jepsen, and “Shake It Off” by Taylor Swift. These songs are known to trigger earworms and were selected to ensure high familiarity across the young adult participants (Jakubowski et al., 2017; OnePoll survey, cited in Macdonald, 2016). The songs were played at a quiet volume (42 dB) while the participant sat at a desk with the lights dimmed to simulate a bedtime routine.
Randomization and experimenter blinding
Prior to study enrollment, the principal investigator used blocked randomization with sets of two, four, and six to determine condition assignments. Using these random assignments, we created an individual music file for each participant, labeled with the participant’s arbitrary ID number so that staff remained blind to conditions when they clicked the file and when the media player opened. The computers were hardwired to the bedroom speakers (preventing music in the technician room), and the bedrooms were soundproofed, thereby further ensuring blinding fidelity.
Procedure
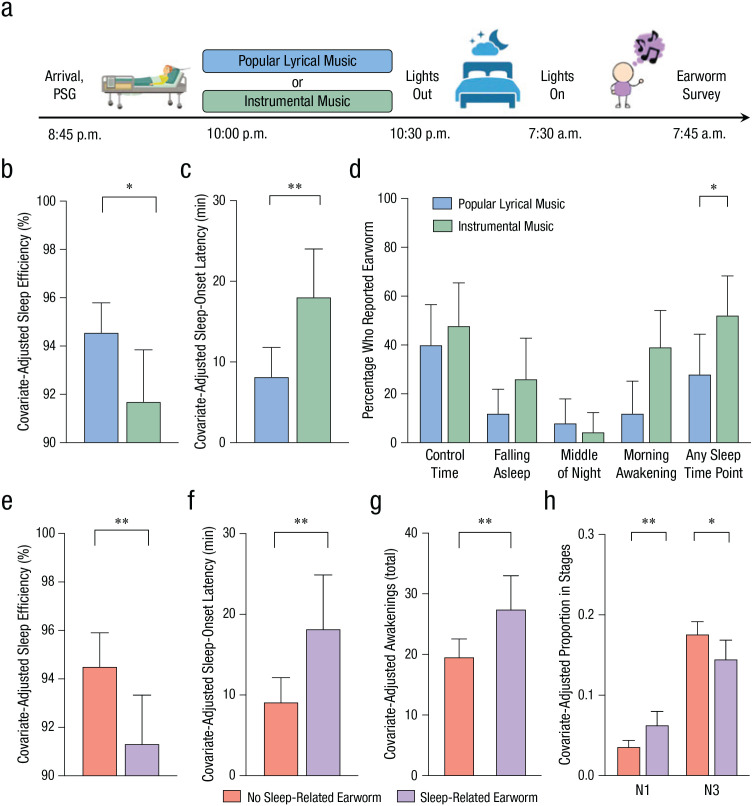
The procedure is illustrated in Figure 4a. At 8:45 p.m., participants arrived at the sleep laboratory. The laboratory was controlled for lighting (45 lux) and temperature (68 °F [20 °C]) to create a quiet, dim bedroom environment. Participants sat at the desk in their bedroom, had their blood pressure measured, and completed questionnaires while the research assistant applied electrodes. Following electrode application, participants’ blood pressure was measured again, and they completed a premusic visual analog scale asking how relaxed, nervous, energetic, sleepy, and stressed they felt.
Fig. 4.
Procedure and results of Study 2. Participants arrived at a light- and sound-controlled laboratory (a), and each participant was randomly assigned to listen to either popular lyrical music or instrumental-only versions at a quiet volume. Polysomnography (PSG) was used to measure sleep quality, and participants completed an earworm survey after waking. The top row of graphs shows (b) prebed sleep efficiency, (c) sleep-onset latency, and (d) the percentage of participants who reported earworms at separate time points. Results are shown separately for each music condition, and asterisks indicate significant differences between conditions (*p < .05, **p < .01). The bottom row of graphs shows (e) sleep efficiency, (f) sleep-onset latency, (g) the number of awakenings, and (h) sleep quality at N1 (light sleep) and N3 (deep sleep). Results are shown separately for participants who reported earworms and those who did not. Asterisks indicate significant differences between earworm conditions (*p < .05, **p < .01). Gender, insomnia stress vulnerability, and morningness-eveningness preferences were included as covariates in these analyses. Error bars represent upper bounds of 95% confidence intervals.
At 10:00 p.m., participants were told that they were going to be given a downtime period. Participants were instructed that there would be music playing and that they should not use their phones or other distractions. Then, the researchers left the room, closed the door, and played the music file. Afterward, the researchers again measured the participants’ blood pressure and administered the postmusic visual analog scale. Following biocalibration, lights-out time was at approximately 10:30 p.m.
The next morning, participants woke up at approximately 7:30 a.m. Participants were given 10 min to use the bathroom and get dressed. Then participants responded whether they had a sound, song, or melody stuck in their head at that moment (control time point) or during a sleep-related time point (while trying to fall asleep, while they woke up during the night, while they woke up that morning). Participants were then dismissed, although they returned one to two nights later to complete an unrelated experiment (Gao et al., 2020).
Statistical analyses
We used t tests to assess condition differences in demographics (to identify covariates, if necessary) and whether blood pressure (mean arterial pressure) and visual-analog-scale ratings changed after participants listened to music. We conducted ANCOVAs on the polysomnography measures to determine whether the music condition and earworms impacted sleep quality.
Results
Participants in the lyrical- and instrumental-music conditions were similar on musical experience, age, race/ethnicity, recent sleep, trait-level stress, crystallized intelligence, and fluid intelligence (see Table S7 in the Supplemental Material). There were some condition differences in gender, morningness-eveningness questionnaire scores, and FIRST scores (see Table S7), which were covaried in the following analyses.
People often listen to music to regulate their mood, and consistent with this notion, results showed that mean arterial pressure decreased significantly from premusic to postmusic time points, F(1, 46) = 4.98, MSE = 31.38, p = .031, η p 2 = .098 (see Table S8 in the Supplemental Material). Furthermore, listening to music decreased self-reported stress, F(1, 46) = 15.17, MSE = 61.24, p < .001, η p 2 = .248; decreased nervousness, F(1, 46) = 12.26, MSE = 105.38, p = .001, η p 2 = .210; and increased relaxation, F(1, 46) = 11.27, MSE = 145.09, p = .002, η p 2 = .197 (see Fig. S3 in the Supplemental Material). None of these effects interacted with music condition (all ps > .10; see Table S9 in the Supplemental Material). Therefore, if mood alteration is the causal mechanism by which music influences sleep (rather than earworm induction), then sleep quality should not differ across music conditions.
In contrast to the mood-alteration hypothesis, we observed significantly worse polysomnography outcomes in the instrumental-music condition than in the lyrical-music condition. Prebedtime instrumental music caused poorer sleep efficiency (Fig. 4b), F(1, 42) = 4.63, MSE = 15.89, p = .037, η p 2 = .10, and greater difficulty falling asleep (Fig. 4c), F(1, 42) = 8.16, MSE = 107.65, p = .007, η p 2 = .16, while preserving total sleep time and other aspects of sleep quality (ps > .05; see Table S10 in the Supplemental Material).
To investigate involuntary musical imagery as the mechanism by which instrumental music led to worse sleep, we first examined the frequency of earworms at sleep-related time points and the control time point. Instrumental music (52%) seemed to increase susceptibility to sleep-related earworms relative to lyrical music (28%), OR = 4.30, 95% CI = [0.99, 18.60], Wald χ2(1, N = 48) = 3.80, p = .051 (Fig. 4d). These patterns were selective to sleep-related earworms; there was no condition effect on control-time-only earworms, OR = 1.20, 95% CI = [0.12, 11.88], Wald χ2(1, N = 29) = 0.02, p = .88.
According to the view that earworms are a pleasant experience, nighttime earworms should lead to better sleep outcomes. By contrast, Figure 4 shows that sleep quality was considerably worse in participants who had a sleep-related earworm. Sleep-related earworms were associated with poorer sleep efficiency (Fig. 4e), F(1, 42) = 7.46, MSE = 14.98, p = .009, η p 2 = .15; greater difficulty falling asleep (Fig. 4f), F(1, 42) = 8.45, MSE = 107.04, p = .006, η p 2 = .17; more awakenings (Fig. 4g), F(1, 42) = 8.15, MSE = 83.28, p = .007, η p 2 = .16; and a shift from deeper sleep (N3) toward lighter sleep (N1; Fig. 4h)—N1 component: F(1, 42) = 11.95, MSE = 0.001, p = .001, η p 2 = .22; N3 component: F(1, 42) = 5.84, MSE = 0.002, p = .020, η p 2 = .12. These patterns occurred without altering total sleep time (F < 1, p > .10; see Table S10 for other nonsignificant results). When we repeated this analysis on control-time earworms, all effects were nonsignificant (ps > .05).
Discussion
In Study 2, by randomly assigning each participant to listen to three repetitive and familiar songs in either their original or delyricized instrumental versions, we provided causal evidence for bedtime instrumental music affecting sleep quality via inducing earworms (converging with the findings of Study 1; see Fig. 3). One possibility is that instrumental music induces a lower attentional state, and low attentional states are known to increase earworms (Floridou et al., 2017). Another possibility is that because the lyrical music was familiar, participants may have attempted to generate (“fill in”) the lyrics, and doing so might instigate earworms (Margulis, 2014). Future work can test these views—and determine the generalizability of instrumental music’s effects on sleep—through careful manipulations of ambient noise, classical music, and familiar, repetitive, delyricized songs. We recommend that such investigation be carried out similarly as in Study 2, using in-laboratory polysomnography, experimenter blinding, and control over all elements of the participants’ environment and behaviors.
A remaining unknown is how involuntary musical imagery can persist during sleep. In both Study 1 and Study 2, approximately one quarter of participants woke up from sleep with an earworm. This occurred even though they had not listened to music for hours and even though there were no external cues to trigger the musical melodies. Why would earworms occur at such time points? One hypothesis is that musical melodies, just like procedural and episodic memories, are spontaneously replayed during sleep to promote consolidation into cortical networks (Rasch & Born, 2013). Therefore, for Study 3, we conducted quantitative-EEG analyses of the data acquired in Study 2 to investigate whether earworms were associated with the same sleep-physiology hallmarks of memory consolidation. We tested three quantitative-EEG measures that are often observed in memory-consolidation studies: N3 slow oscillation activity, theta activity, and spindle density (Antony et al., 2012; Gao et al., 2020; Rasch & Born, 2013).
Study 3
Method
Preprocessing, spectral analysis, and spindle detection
Using the data from Study 2, we applied Wamsley et al.’s (2012) algorithm to automatically detect sleep spindles and BrainVision Analyzer 2.0 (Brain Products, Gilching, Germany) to conduct spectral power analysis. Trained research personnel visually inspected all records to exclude waking epochs and epochs containing movement or electrode artifacts. Afterward, we filtered EEG data with high- and low-pass cutoffs of 0.3 Hz and 35 Hz, respectively. Then, we separated each epoch into 4-s segments and applied a symmetric Hanning window with 50% overlap. Next, we conducted a fast Fourier transformation to generate spectral power (μV2) for each channel for typical bandwidths, focusing on slow oscillation (0.5–1 Hz), theta (4–8 Hz), and spindle/sigma (12–16 Hz) bandwidths. On the basis of the memory-consolidation literature, we focused analyses on EEG activity during N3 that arose from frontal channels (averaging Fp1, Fp2, F3, F4, F7, F8, Fz).
Statistical analyses
We used ANCOVAs to test the differences in EEG activity between music conditions and between participants with (vs. without) sleep-related earworms. For descriptive visualization of spatial patterns for our significant main effects, we plotted spectral power across the scalp, using independent-samples t tests to highlight channel-level differences in condition. We plotted channel markers at several significance levels (.01, .05) to allow for some differentiation of the relative magnitude of between-conditions differences across channels, although it should be noted that single-channel effects do not survive Bonferroni corrections (single-channel Bonferroni correction would require p < .003).
Results
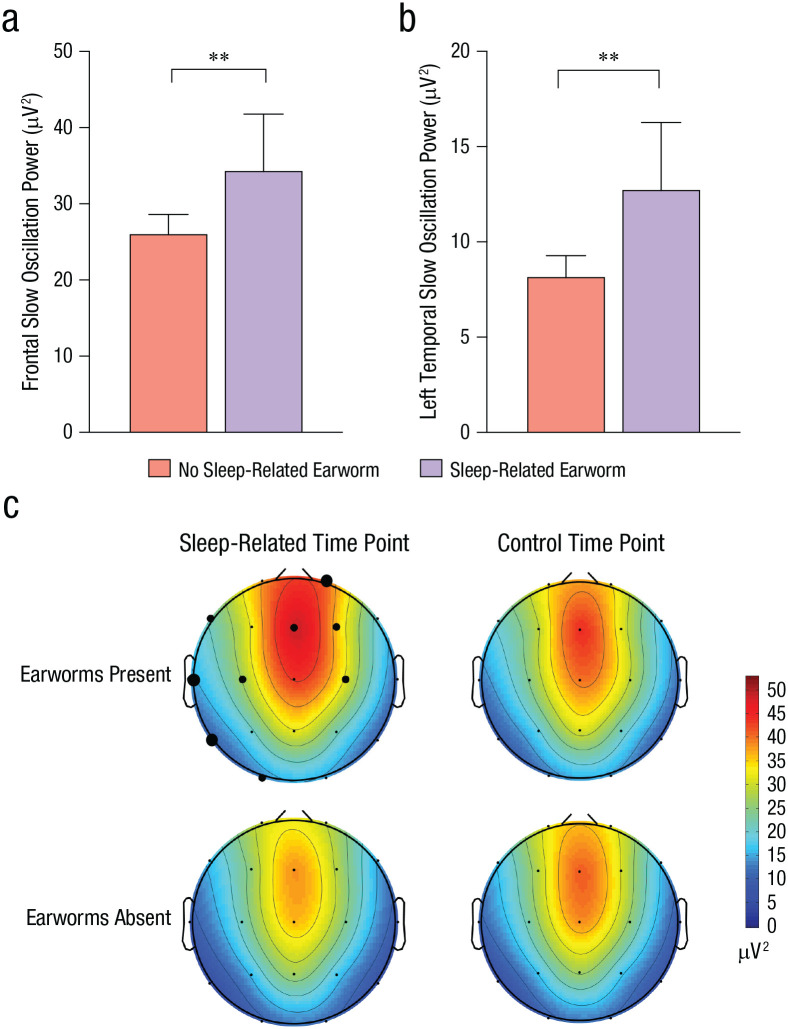
Slow oscillations, theta power, and spindle density were similar across the lyrical- and instrumental-music conditions (ps > .05; see Table S11 in the Supplemental Material). However, Figure 5a shows that sleep-related earworms were associated with significantly greater frontal slow oscillations, a classic signature of sleep-dependent memory consolidation, F(1, 46) = 6.69, MSE = 117.551, p = .013, η p 2 = .127 (theta power and spindle density were nonsignificant; ps > .05; Table S11). Interestingly, greater slow oscillation activity extended to left temporal sites (Figs. 5b and 5c), F(1, 42) = 10.39, MSE = 212.901, p = .002, η p 2 = .198, the same brain region that increases in activation when experiencing earworms while awake (Kraemer et al., 2005). We examined the robustness of these effects by including the same covariates as in Study 2 (gender, morningness-eveningness questionnaire, FIRST) as well as covarying the proportion of time in N3. Sleep-related earworms remained associated with significantly greater slow oscillation activity in frontal channels, F(1, 41) = 7.05, MSE = 123.78, p = .011, η p 2 = .147, and left temporal channels (T3, T5), F(1, 37) = 10.09, MSE = 20.75, p = .003, η p 2 = .214.
Fig. 5.
Results of Study 3. The graphs show slow oscillation activity in averaged (a) frontal channels and (b) left temporal (T3, T5) channels for participants who reported and did not report sleep-related earworms. Error bars represent upper bounds of 95% confidence intervals. Asterisks indicate significant differences between earworm groups (p < .01). The heat maps (c) show oscillation power in frontal and left temporal regions at sleep-related and control time points, separately for each earworm group. The size of the black dots indicates the level of significance (small: p > .05, medium: p < .05, large: p < .01).
These EEG effects were selective to sleep-related earworms; earworms at the control time point showed no associations with slow oscillation activity (Fig. 5c), F(1, 46) = 0.217, MSE = 134.02, p = .643, η p 2 < .01; theta activity, F(1, 46) = 1.05, MSE = 2.82, p = .312, η p 2 = .02; or spindle density, F(1, 46) = 0.139, MSE = 0.30, p = .711, η p 2 < .01 (Table S11). Preexperimental differences in homeostatic sleep pressure did not explain the slow oscillation patterns; there were no significant associations with previous-week sleep duration, r(45) = −.02, p = .89; proportion of night spent in N3, r(46) = .06, p = .68; PSQI sleep quality, r(45) = .19, p = .21; daytime sleepiness, r(45) = .04, p = .78; or FIRST scores, r(45) = −.01, p = .94.
Discussion
Study 3 implicated memory-consolidation processes as the proximal mechanism of sleep-related earworms. Frontal slow oscillations are a hallmark of memory consolidation, and alternative explanations such as homeostatic pressure were not supported by correlational analyses with recent sleep. Interestingly, earworm-related slow oscillation activity extended beyond frontal sites to include left temporal channels, which correspond to the auditory cortices. Elegant neuroimaging work previously connected earworm experiences to increased activation of the primary auditory cortex (Kraemer et al., 2005). Thus, even when one falls asleep, the brain continues to process musical information, spontaneously reactivating melodies during sleep.
General Discussion
With the advent of smartphones and the proliferation of music streaming services, never before have people listened to music so frequently (International Federation of the Phonographic Industry, 2019). And songs are now designed to be “catchy” rather than simply to be aesthetically pleasing; the rare type of jingle that caused Mark Twain’s earworm has become commonplace. The convergence of increasingly catchy music with increasing exposure to music across the day and night is a recipe for a society-wide growth in involuntary musical imagery (Sacks, 2010). The current findings, which emanate from both laboratory and survey designs, provide cohesive evidence that frequent listening to familiar music increases nighttime earworms and that nighttime earworms worsen sleep quality. In this section, we will detail the mechanistic and translational implications of these findings.
Descriptive work on earworms indicates that they are most likely to occur for fast-tempo and repetitive music and in individuals who listen to music frequently (Floridou et al., 2017; Jakubowski et al., 2017; Margulis, 2014; Williamson et al., 2012). Our findings reinforce these principles, but with a twist: Music habits affect the timing of earworms such that earworms are more likely to occur at night (presumably because higher attentional states during daytime hours keep earworms suppressed; e.g., Floridou et al., 2017). Given existing theories, it was not a foregone conclusion that nighttime earworms would detrimentally affect sleep (Dickson & Schubert, 2019). According to the view that earworms are nonproblematic or even pleasant (Beaman & Williams, 2010; Halpern & Bartlett, 2011), one would expect an earworm to help people relax, distract people from their worries, or otherwise block presleep cognitive intrusions (Harvey & Payne, 2002). Contrary to such notions, results from both the experimental and survey studies showed that when earworms occurred at night, they were perceived as more irritating than when they occurred during the day (Hyman et al., 2013; Liikkanen et al., 2015), understandably, because the earworms were disrupting sleep.
Although it is known that earworms can occur during the day and logical that they should occur at night before one falls asleep, it struck us as remarkable that approximately one quarter of participants woke from sleep with an earworm. These participants had not listened to music for 8 hr and were not exposed to external cues that would have triggered involuntary musical imagery. The implication is that musical processing continues during sleep (spontaneously). This notion converges well with the literature demonstrating that procedural and episodic memories are spontaneously replayed during sleep (Rasch & Born, 2013) and was substantiated by evidence that earworms were associated with an increase in frontal slow oscillation activity (a marker of sleep-dependent memory consolidation). Sleep-related earworms were even associated with increased left temporal activity during sleep, which has previously been implicated as a neural underpinning of earworms experienced while awake (Kraemer et al., 2005).
The current work has translational implications for using bedtime music to resolve sleep difficulties, an approach reported by 62% of individuals in one large survey (Trahan et al., 2018). Music clearly improves one’s mood, which may reinforce listening behaviors and lead one to expect that sleep difficulties can be treated by bedtime music. In spite of substantial public and clinical interest in using music to assist with sleep, there has been minimal experimental investigation of whether music listening affects objective sleep outcomes (Cordi et al., 2019; Jespersen et al., 2019). The current work demonstrated that the type of music, the duration of music, and the timing of music listening can all influence sleep outcomes at night. Just because music listening is enjoyable does not mean that more music is always better for health outcomes.
If one experiences earworms at bedtime, there are two empirically validated strategies for eliminating or reducing their occurrence. First, frequent music listening is the most consistent risk factor for acquiring earworms (Williamson et al., 2012), meaning that changing how, when, and where one listens to music should affect earworm frequency (see also stimulus control for insomnia for deconditioning of music behaviors from the bed context; Bootzin et al., 2016). Second, earworms are most likely to occur in low attentional states and can be prevented by engaging in a moderately demanding cognitive activity (Floridou et al., 2017). Although we would discourage some cognitive activities in bed (e.g., video games), other mildly cognitively demanding activities would be beneficial, such as writing down one’s worries (Harvey & Payne, 2002) or a to-do list (Scullin et al., 2018).
In conclusion, there are few behaviors as prevalent in young adults as listening to music, and many regularly listen to music as part of their bedtime routine. Listening to music feels relaxing, but familiar and repetitive music can trigger involuntary musical imagery that worsens sleep quality and daytime functioning. Evidently, musical processing in the brain does not cease when the music stops or even when one falls asleep. Such effects were anticipated by Mark Twain in his “Literary Nightmare” story nearly 150 years ago and call for a reevaluation of recommendations to use music as part of one’s bedtime routine.
Supplemental Material
Supplemental material, sj-docx-1-pss-10.1177_0956797621989724 for Bedtime Music, Involuntary Musical Imagery, and Sleep by Michael K. Scullin, Chenlu Gao and Paul Fillmore in Psychological Science
Acknowledgments
We thank Natalya Pruett, Hannah Ballard, Nikita Chapagain, Mary High, Taylor Luster, Stacy Nguyen, Kiersten Scott, Taylor Terlizzese, Ali Villagran, and Daniel Zeter for assistance with data collection and data entry.
We recorded our predictions prior to data collection, although we did not preregister them on a public repository. Members of our laboratory were split among those who predicted that earworms would benefit sleep (n = 2), have no impact on sleep (n = 3), or negatively affect sleep (n = 2).
Footnotes
ORCID iD: Michael K. Scullin  https://orcid.org/0000-0002-7578-7587
https://orcid.org/0000-0002-7578-7587
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797621989724
Transparency
Action Editor: M. Natasha Rajah
Editor: Patricia J. Bauer
Author Contributions
M. K. Scullin developed the study concepts and designed the study. C. Gao collected the data. All the authors performed statistical analyses and interpreted the data. M. K. Scullin and C. Gao wrote the manuscript. P. Fillmore provided critical revisions. All the authors approved the final manuscript for submission.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work was supported by a National Science Foundation (NSF) CAREER award (1943323) to M. K. Scullin and by the NSF Division of Research and Learning (1920730). The funding sources had no role in study design, data collection, data analysis and interpretation, writing of the manuscript, and decision to submit the manuscript.
Open Practices: All data and materials have been made publicly available via OSF and can be accessed at https://osf.io/7tfqz. The design and analysis plans for the studies were not preregistered (but see Note 1). This article has received the badges for Open Data and Open Materials. More information about the Open Practices badges can be found at http://www.psychologicalscience.org/publications/badges.


References
- Antony J. W., Gobel E. W., O’Hare J. K., Reber P. J., Paller K. A. (2012). Cued memory reactivation during sleep influences skill learning. Nature Neuroscience, 15(8), 1114–1116. 10.1038/nn.3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman C. P., Williams T. I. (2010). Earworms (stuck song syndrome): Towards a natural history of intrusive thoughts. British Journal of Psychology, 101(Pt. 4), 637–653. 10.1348/000712609X479636 [DOI] [PubMed] [Google Scholar]
- Bootzin R. R., Smith L. J., Franzen P. L., Shapiro S. L. (2016). Stimulus control therapy. In Sateia M. J., Buysse D. (Eds.), Insomnia: Diagnosis and treatment (pp. 268–276). CRC Press. [Google Scholar]
- Buysse D. J., Reynolds C. F., III, Monk T. H., Berman S. R., Kupfer D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Chanda M. L., Levitin D. J. (2013). The neurochemistry of music. Trends in Cognitive Sciences, 17(4), 179–193. 10.1016/j.tics.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Cordi M. J., Ackermann S., Rasch B. (2019). Effects of relaxing music on healthy sleep. Scientific Reports, 9(1), Article 9079. 10.1038/s41598-019-45608-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh A. D., Sarvaiya A. A., Seethalakshmi R., Nayak A. S. (2009). Effect of Indian classical music on quality of sleep in depressed patients: A randomized controlled trial. Nordic Journal of Music Therapy, 18(1), 70–78. 10.1080/08098130802697269 [DOI] [Google Scholar]
- Dickson G. T., Schubert E. (2019). How does music aid sleep? Literature review. Sleep Medicine, 63, 142–150. 10.1016/j.sleep.2019.05.016 [DOI] [PubMed] [Google Scholar]
- Drake C., Richardson G., Roehrs T., Scofield H., Roth T. (2004). Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep, 27(2), 285–291. 10.1093/sleep/27.2.285 [DOI] [PubMed] [Google Scholar]
- Feng F., Zhang Y., Hou J., Cai J., Jiang Q., Li X., Zhao Q., Li B.-A. (2018). Can music improve sleep quality in adults with primary insomnia? A systematic review and network meta-analysis. International Journal of Nursing Studies, 77, 189–196. 10.1016/j.ijnurstu.2017.10.011 [DOI] [PubMed] [Google Scholar]
- Floridou G. A., Williamson V. J., Stewart L. (2017). A novel indirect method for capturing involuntary musical imagery under varying cognitive load. Quarterly Journal of Experimental Psychology, 70(11), 2189–2199. 10.1080/17470218.2016.1227860 [DOI] [PubMed] [Google Scholar]
- Floridou G. A., Williamson V. J., Stewart L., Müllensiefen D. (2015). The Involuntary Musical Imagery Scale (IMIS). Psychomusicology: Music, Mind, and Brain, 25(1), 28–36. 10.1037/pmu0000067 [DOI] [Google Scholar]
- Gao C., Fillmore P., Scullin M. K. (2020). Classical music, educational learning, and slow wave sleep: A targeted memory reactivation experiment. Neurobiology of Learning and Memory, 171, Article 107206. 10.1016/j.nlm.2020.107206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern A. R., Bartlett J. C. (2011). The persistence of musical memories: A descriptive study of earworms. Music Perception, 28(4), 425–432. 10.1525/mp.2011.28.4.425 [DOI] [Google Scholar]
- Harvey A. G., Payne S. (2002). The management of unwanted pre-sleep thoughts in insomnia: Distraction with imagery versus general distraction. Behaviour Research and Therapy, 40(3), 267–277. 10.1016/s0005-7967(01)00012-2 [DOI] [PubMed] [Google Scholar]
- Hoddes E., Zarcone V., Smythe H., Phillips R., Dement W. C. (1973). Quantification of sleepiness: A new approach. Psychophysiology, 10(4), 431–436. [DOI] [PubMed] [Google Scholar]
- Horne J. A., Ostberg O. (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology, 4(2), 97–110. [PubMed] [Google Scholar]
- Hyman I. E., Jr., Burland N. K., Duskin H. M., Cook M. C., Roy C. M., McGrath J. C., Roundhill R. F. (2013). Going Gaga: Investigating, creating, and manipulating the song stuck in my head. Applied Cognitive Psychology, 27(2), 204–215. 10.1002/acp.2897 [DOI] [Google Scholar]
- International Federation of the Phonographic Industry. (2019). Music listening 2019. https://www.ifpi.org/wp-content/uploads/2020/07/Music-Listening-2019-1.pdf
- Jakubowski K., Finkel S., Stewart L., Müllensiefen D. (2017). Dissecting an earworm: Melodic features and song popularity predict involuntary musical imagery. Psychology of Aesthetics, Creativity, and the Arts, 11(2), 122–135. 10.1037/aca0000090 [DOI] [Google Scholar]
- Jespersen K. V., Koenig J., Jennum P., Vuust P. (2015). Music for insomnia in adults. Cochrane Database of Systematic Reviews, 8, Article CD010459. 10.1002/14651858.CD010459.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen K. V., Otto M., Kringelbach M., Van Someren E., Vuust P. (2019). A randomized controlled trial of bedtime music for insomnia disorder. Journal of Sleep Research, 28(4), Article e12817. 10.1111/jsr.12817 [DOI] [PubMed] [Google Scholar]
- Kraemer D. J., Macrae C. N., Green A. E., Kelley W. M. (2005). Sound of silence activates auditory cortex. Nature, 434(7030), Article 158. 10.1038/434158a [DOI] [PubMed] [Google Scholar]
- Krause A. E., North A. C., Hewitt L. Y. (2015). Music-listening in everyday life: Devices and choice. Psychology of Music, 43(2), 155–170. 10.1177/0305735613496860 [DOI] [Google Scholar]
- Liikkanen L. A., Jakubowski K., Toivanen J. M. (2015). Catching earworms on Twitter: Using big data to study involuntary musical imagery. Music Perception, 33(2), 199–216. 10.1525/mp.2015.33.2.199 [DOI] [Google Scholar]
- Macdonald J. (2016, August 5). Britain’s top 50 earworms revealed - and you won’t be able to get them out of your head. Mirror. https://www.mirror.co.uk/news/weird-news/britains-top-50-earworms-revealed-8568664
- Margulis E. H. (2014). On repeat: How music plays the mind. Oxford University Press. [Google Scholar]
- Moeck E. K., Hyman I. E., Jr., Takarangi M. K. T. (2018). Understanding the overlap between positive and negative involuntary cognitions using instrumental earworms. Psychomusicology: Music, Mind, and Brain, 28(3), 164–177. 10.1037/pmu0000217 [DOI] [Google Scholar]
- Müllensiefen D., Gingras B., Musil J., Stewart L. (2014). The musicality of non-musicians: An index for assessing musical sophistication in the general population. PLOS ONE, 9(2), Article e89642. 10.1371/journal.pone.0089642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. (2011). Your guide to healthy sleep. https://www.nhlbi.nih.gov/files/docs/public/sleep/healthy_sleep.pdf
- National Sleep Foundation. (2020). Music and sleep: Music can help you sleep better. https://www.sleepfoundation.org/bedroom-environment/music-and-sleep
- North A. C., Hargreaves D. J., Hargreaves J. J. (2004). Uses of music in everyday life. Music Perception, 22(1), 41–77. 10.1525/mp.2004.22.1.41 [DOI] [Google Scholar]
- Rasch B., Born J. (2013). About sleep’s role in memory. Physiological Reviews, 93(2), 681–766. 10.1152/physrev.00032.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J. C. (1938). Raven’s Progressive Matrices. Western Psychological Services. [Google Scholar]
- Sacks O. (2010). Musicophilia: Tales of music and the brain. Vintage Canada. [Google Scholar]
- Scullin M. K., Krueger M. L., Ballard H. K., Pruett N., Bliwise D. L. (2018). The effects of bedtime writing on difficulty falling asleep: A polysomnographic study comparing to-do lists and completed activity lists. Journal of Experimental Psychology: General, 147(1), 139–146. 10.1037/xge0000374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaut M. H., Hodges D. A. (Eds.). (2019). The Oxford handbook of music and the brain. Oxford University Press. [Google Scholar]
- Trahan T., Durrant S. J., Müllensiefen D., Williamson V. J. (2018). The music that helps people sleep and the reasons they believe it works: A mixed methods analysis of online survey reports. PLOS ONE, 13(11), Article e0206531. 10.1371/journal.pone.0206531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twain M. (1876, February). A literary nightmare. The Atlantic Monthly, 37(2), 167–169. [Google Scholar]
- Wamsley E. J., Tucker M. A., Shinn A. K., Ono K. E., McKinley S. K., Ely A. V., Goff D. C., Stickgold R., Manoach D. S. (2012). Reduced sleep spindles and spindle coherence in schizophrenia: Mechanisms of impaired memory consolidation? Biological Psychiatry, 71(2), 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson V. J., Jilka S. R., Fry J., Finkel S., Müllensiefen D., Stewart L. (2012). How do “earworms” start? Classifying the everyday circumstances of involuntary musical imagery. Psychology of Music, 40(3), 259–284. 10.1177/0305735611418553 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pss-10.1177_0956797621989724 for Bedtime Music, Involuntary Musical Imagery, and Sleep by Michael K. Scullin, Chenlu Gao and Paul Fillmore in Psychological Science