Abstract
Background
Peer influences figure prominently in young adult binge drinking. Women have trended to show a level of alcohol use on par with men during the last decades. It would be of interest to investigate the neural processes of social cognition that may underlie binge drinking and the potential sex differences.
Methods
Here, we examined the data of the Human Connectome Project where we identified a total of 175 binge drinkers (125 men) and 285 non-binge drinkers (97 men) performing a social cognition task during brain imaging. We analyzed the imaging data with published routines and evaluated the results at a corrected threshold.
Results
Both male and female binge relative to non-binge drinkers showed higher perceived friendship. Binge relative to non-binge drinkers demonstrated diminished activations in the anterior medial orbitofrontal cortex (amOFC) during perception of social vs. random interaction, with a more prominent effect size in women. Further, whole-brain regression identified activity of the right posterior insula (rPI) in negative correlation with perceived friendship score in non-binge drinking women. Post-hoc analyses showed significant correlation of rPI activity with perceived friendship, amOFC activity, and a summary measure of alcohol use severity identified by principal component analysis, across all subjects. Mediation and path analysis demonstrated a significant model: amOFC activity → rPI activity → perceived friendship → severity of alcohol use.
Conclusions
These findings support peer influences on binge drinking and suggest neural correlates that may relate altered social cognitive processing to alcohol misuse in young adults.
Keywords: alcohol use disorder, alcohol addiction, peer pressure, positive alcohol effect, fMRI
1. Introduction
1.1. Perceived friendship and binge drinking
Binge drinking is frequently observed in young people and often associated with severe negative, potentially life-long consequences (Garcia et al., 2020; Powers et al., 2016; Stolle et al., 2009; Woo et al., 2017; Zullig et al., 2001). Binge drinkers demonstrate higher drinking motives and report more comorbid drug use (Yoo et al., 2020). Associated with both externalizing and internalizing personality traits (Adan et al., 2017), binge drinking in young adults is also significantly influenced by social experience with peers (Clapp and Shillington, 2001; Luk et al., 2019b).
Extensive evidence suggests that alcohol use behavior, including binge drinking, of adolescents and young adults is heavily influenced by peer's alcohol consumption (Angosta et al., 2019; Carpenter et al., 2019; Edwards et al., 2019; Htet et al., 2020; Luk et al., 2019a; Moran et al., 2019; Morris et al., 2020; White et al., 2019; Willis et al., 2019). Social identification and expectations, norm perceptions, as well as conformity drinking motives were significantly associated with higher levels of alcohol consumption in college students (Davis et al., 2019; Laghi et al., 2019; Tarrant et al., 2019). In a longitudinal study of over 1,800 adolescents, having more substance-using peers elevated the risk of early-onset heavy drinking and developing alcohol use disorders (AUD) in early adulthood (Yuen et al., 2020). Another longitudinal study reported that perceived peer alcohol use and individuals' heavy drinking frequencies changed concurrently over time from ages 18 to 29 (Kennedy et al., 2020). This influence was particularly prominent for individuals higher in the need to belong (Hamilton and Dehart, 2019). Peers and genetic risk factors may have independent influences on individuals' alcohol use (Smith et al., 2019). The influences of peers may carry on through development, with escalating peer substance use during adolescence predicting high substance use at age 25 (Kennedy et al., 2019). In fact, peer influences extend to other health-risking behavior beyond drug and alcohol use (Graupensperger et al., 2019). Research of peer influences has suggested venues in bettering interventional strategies to reduce early alcohol misuse and other risky behaviors (Lau-Barraco et al., 2019; Neighbors et al., 2019; Reboussin et al., 2019; Tabernero et al., 2019).
1.2. Sex differences in the influences of perceived friendship on binge drinking
Men and women are known to demonstrate differences in the clinical characteristics (Beck et al., 1995; Greenfield et al., 2010; Hensing and Spak, 2009; Schulte et al., 2009) as well as the risk factors and consequences of alcohol misuse (Nolen-Hoeksema, 2004). Numerous studies have aimed to characterize the biological and neural bases of these sex differences. For instance, women relative to men are more vulnerable to the influences of negative affect and stress in the pathophysiology of alcohol misuse (Walitzer and Dearing, 2006). As men and women engage distinct neural circuits in affective processing (Shirao et al., 2005; Stevens and Hamann, 2012), they likely demonstrate differences in peer influences on binge drinking. A previous study reported that adolescent girls were susceptible to peer influences on alcohol use to a greater extent and at an earlier age than boys (Boyd et al., 2018). Relative to boys, girls were more sensitive to peers’ pressure as a risk factor for adolescent binge drinking (Dir et al., 2017). In contrast, another work found that the social reinforcement from peers was greater for men than for women in contributing to alcohol misuse (Borsari and Carey, 2006). Other studies reported that sex did not modulate the influences of perceived peer norms on alcohol use (Graupensperger et al., 2020; Weybright et al., 2019). Demographic and clinical heterogeneity of the participants as well as how peer influences were assessed may have contributed to the discrepancy in findings, and more studies are clearly needed to address this issue.
1.3. Imaging the neural correlates of binge drinking
Investigators have combined brain imaging and behavioral tasks to understand the neural bases of binge drinking, with a focus on working memory and association learning (Campanella et al., 2013; Schweinsburg et al., 2010; Squeglia et al., 2012; Squeglia et al., 2011), impulse control and reward-related decision making (Ahmadi et al., 2013; Ames et al., 2014; Cservenka et al., 2015; Herman et al., 2019; Jones et al., 2016; Worbe et al., 2014; Xiao et al., 2013), cue reactivity (Petit et al., 2014; Petit et al., 2012; Petit et al., 2013; Watson et al., 2016), as well as stress response (Blaine et al., 2019; Hagan et al., 2019). In contrast, few studies have investigated the neural correlates of social emotions or perceived social interaction in relation to binge drinking. A recent study of brain responses to the perspective of self vs. other experiencing pain showed that whereas non-binge drinkers engaged areas supporting self to other distinction, binge drinkers do not, suggesting a role of altered empathy in the development of AUD (Rae et al., 2020). Some meta-analyses focused on deficits in social cognition in individuals with AUD, who demonstrated impairment in facial emotion recognition and theory of mind that could leave one vulnerable to social cues (Bora and Zorlu, 2017; Onuoha et al., 2016). This suggests a need to examine the neural correlates of social perception and social emotional processing in binge drinkers. In particular, it remains to be investigated whether men and women engage distinct neural processes interrelating social cognition and binge drinking.
1.4. The present study
We aimed to address these issues using the data set of the Human Connectome Project (HCP). The HCP comprised imaging data collected of a social cognition task from young male and female adults. The social cognition task required individuals to identify “socially” vs. randomly interacting objects. In a recent study we showed responses to perception of social interaction in a wide swath of cortical and subcortical regions critical for social emotional processing and decision making in the social cognition task (Li et al., 2020a). Earlier studies suggested that friendship clique significantly predicted individual body image concern and eating behavior (Hutchinson and Rapee, 2007; Paxton et al., 1999) as well as smoking and alcohol use (Huang et al., 2014). Friendship networks and perceived popularity represented important correlates of adolescent alcohol use (Fujimoto and Valente, 2015). Thus, we posited that perceived friendship, as evaluated by the NIH Emotion Toolbox in the HCP (see Methods), reflect an important aspect of peer influences that contribute to alcohol misuse. We hypothesized that binge as compared to non-binge drinkers demonstrated higher perceived friendship and differences in regional activation to social perception. To this end we performed group (bingers vs. non-bingers) by sex analyses of variance on clinical, behavioral and imaging data and linear regression on imaging data to identify the correlates of perceived friendship. We examined how these neural processes differed between men and women and performed mediation and path analyses to examine the inter-relationship between the neural correlates, perceived friendship, and the severity of alcohol use.
2. Materials and Methods
2.1. Dataset and demographics
For the current study, we have obtained permission from the HCP (Van Essen et al., 2012) to use both the Open and Restricted Access data. As in our previous work (Li et al., 2020a; Li et al., 2020b, c), we employed the 1200 Subjects Release (S1200) data, including behavioral and 3T MR imaging data of 1206 healthy young adult participants (1113 with structural MR scans) collected from 2012 to 2015. Binge drinking was defined as having ≥ 4/5 drinks for women/men on a single day (Wechsler et al., 1994). The binge drinking group comprised 175 adults who reported binge drinking at least once a week for the last 12 months (125 men, 71.4%) (Gowin et al., 2020). A total of 311 adults reported no binge drinking in the prior year. However, 26 of the 311 met criteria for life-time alcohol abuse or dependence and were excluded, leaving 285 adults (97 men, 34.0%) in the non-binge drinking group. Thus, the data of a total of 460 adults (222 men; 22-36 with mean ± SD = 27.7 ± 3.6 years; 238 women, 22-36 or 29.8 ± 3.6 years) were included in this study, with more men than women in the binge drinking group (x2 = 60.72, p < 0.001, chi-square test). Analysis of variance (ANOVA) showed a significant group (F(1,459) = 10.15, p = 0.002) and sex (F(1,459) = 14.43, p < 0.001) main as well as group × sex interaction (F(1,459) = 9.25, p = 0.002) effect in age. Thus, age was included as a covariate in all subsequent analyses. All subjects were physically healthy with no severe neurodevelopmental, neuropsychiatric or neurological disorders. All aspects of the study, including subject recruitment, experimental procedures, and informed consent, including consent to share de-identified data, were conducted according to a protocol in accordance with the Declaration of Helsinki and approved by the Washington University Institutional Review Board (IRB #201204036; title: “Mapping the Human Connectome: Structure, Function and Heritability”).
2.1. Behavioral and clinical measures
The HCP data comprised 13 inter-related drinking metrics to assess the severity of alcohol use. We performed a principal component analysis on the 13 measures and, as expected, identified one principal component (PC1) with an eigenvalue > 1 and accounting for 65.43% of the variance. Note that six of the 13 measures were reversed score so a higher PC1 weight indicated greater severity of drinking. Supplementary Table S1 shows the mean ± SD of the 13 drinking measures and PC1 as well as the statistics of group x sex ANOVA. All participants were evaluated with the NIH-Toolbox Emotion Measures – 18+ (i.e., > 18 years old) battery – which consists of 4 sub-domains: negative affect, psychological well-being, stress and self-efficacy, and social relationships. Perceived friendship, characterized by self-reported perceptions of the availability of friends or companions with whom to interact or affiliate, is within the social relationships sub-domain. There are 8 items in Friendship subscale (Supplementary Methods) each scored from 1 (never feel the friendship) to 5 (always feel the friendship), so the score sums from 8 to 40, with a higher score indicating higher perceived friendship. All analyses of NIH Toolbox Emotion measures are to be conducted with T-scores, which are standard scores in which a T-score of 50 represents the mean of the US general population (based on the 2010 Census) and 10 T-score units represents one standard deviation. The Friendship T score ranged from 16.5 to 67.1 for the current sample.
We performed a group by sex ANOVA of the friendship score with age as a covariate.
2.2. Imaging protocol and social cognition task for fMRI
Detailed in previous studies (Li et al., 2020a; Li et al., 2020b, c), imaging protocols are described in the Supplementary Methods.
Participants completed two runs of a social cognition task each with 5 blocks (~3 m and 27 s each run). The task engaged robust activation of brain regions associated with social cognition (Castelli et al., 2000; Wheatley et al., 2007). The first run contained 2 social and 3 random blocks in a fixed order: social – random – random – social – random; the second run contained 3 social and 2 random blocks in a fixed order: social – social – random – social – random. Developed by (Castelli et al., 2000) and (Wheatley et al., 2007), video clips [20 s, shortened from the original 40-s versions (Barch et al., 2013)] of geometric objects (triangles, squares, circles) “interacted” in some way to simulate causal actions in social blocks, and moved randomly in random blocks. At the end of each video clip, subjects were allowed 3 s to indicate whether the objects interacted socially (as if the objects were taken into account each other’s feelings and thoughts), not sure, or no interaction (i.e. movement appearing random).
We performed a group by sex ANOVA of the difference in accuracy rate (ARSOC-RAN) and reaction time (RTSOC-RAN) between the social and random interaction blocks, with age as a covariate. We also performed a linear regression of ARSOC-RAN and RTSOC-RAN each against the friendship score for all subjects and for male binger, male non-binger, female binger and female non-binger group separately, all with age as a covariate.
2.3. Imaging data modeling and statistics
We followed published routines (Wang et al., 2020; Zhang et al., 2019; Zhornitsky, S. et al., 2019) in data preprocessing, as also described in Supplementary Methods.
We modeled the BOLD signals to identify regional responses. Briefly, a statistical analytical block design was constructed for each individual subject, using a general linear model (GLM) by convolving the canonical hemodynamic response function (HRF) with a boxcar function in SPM. Realignment parameters in all six dimensions were entered in the model as covariates.
We constructed for each individual subject the statistical contrast “social vs. random.” In group analyses, we conducted a full factorial two-way (men vs. women × binger vs. non-binger) ANOVA of the contrast with age as a covariate. We also performed a voxel-wise regression against the perceived friendship scores for all subjects and for each of the four groups of subjects separately with age as a covariate. We evaluated the results at voxel p < 0.001, uncorrected, in combination with cluster p < 0.05, corrected for family-wise error (FWE) of multiple comparisons, on the basis of Gaussian random field theory, as implemented in SPM. We identified brain regions using the Data Processing & Analysis of Brain Imaging toolbox (DPABI) (Yan et al., 2016) and an atlas (Duvernoy, 2009), if the peak was not identified by the DPABI.
Functional regions of interest (ROIs) were defined based on clusters obtained from whole-brain analysis. In ROI analysis, we used MarsBar (http://marsbar.sourceforge.net/) to derive for each individual subject the activity (β’s averaged across voxels) for the ROIs.
2.4. Mediation and path analyses
We performed mediation analyses following published routines (MacKinnon et al., 2007; Wager et al., 2008), as detailed in the Supplementary Methods and our previous work (Hu et al., 2018; Ide et al., 2017; Le et al., 2020; Le et al., 2019; Wang et al., 2020; Zhornitsky, Simon. et al., 2019), to evaluate the relationships between neural markers, perceived friendship and a summary measure of alcohol use severity across all subjects (see Results).
Following up on mediation analysis and the findings of a significant correlation between amOFC and rPI activity (see Results), we performed path analyses (Supplementary Methods) to examine the inter-relationship between amOFC and rPI activity, perceived friendship and alcohol use severity.
3. Results
3.1. Clinical and behavioral measures
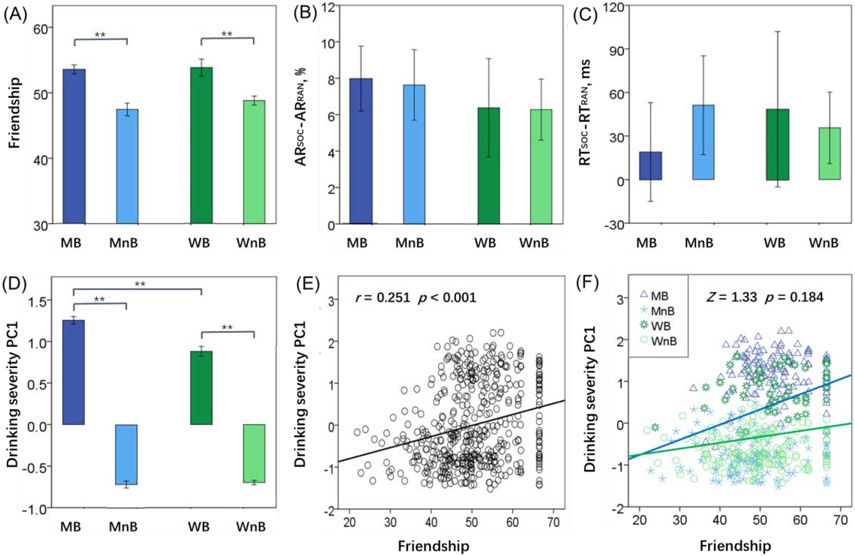
Perceived friendship score showed a significant group main (F(1,459) = 32.66, p < 0.001) but not a sex main (F(1,459) = 0.91, p = 0.341) or group by sex interaction (F(1,459)= 0.421, p = 0.517) effect (Figure 1A). Perceived friendship score was higher in both male and female binge as compared to non-binge drinkers. Supplementary Table S2 shows the results of ANOVA on other measures from the NIH Emotional Toolbox.
Figure 1.
Clinical and behavioral measures of men binger (MB), men non-binger (MnB), women binger (WB), and women non-binger (WnB): (A) Friendship score; (B) Difference in accuracy rate between social and random blocks or ARSOC-ARRAN; (C) Difference in reaction time between social and random blocks or RTSOC-RTRAN; and (D) Severity of alcohol use as quantified by the weight of the first principal component (PC1) of PCA of all 13 drinking measures. Correlation of drinking PC1 with perceived friendship scores (E) across all subjects and (F) for men and women separately. Note that residuals are plotted here with age accounted for in the regressions. Histograms show mean ± SE. **p ≤ 0.001.
We computed individual accuracy rate (AR) and reaction time (RT) during identification of social vs. random interaction in the social cognition task. The results of ANOVA showed no significant group main (F(1,459) = 0.33, p = 0.564) or sex main (F(1,459) = 0.33, p = 0.566) or group by sex interaction (F(1,459) = 0.09, p = 0.760) effect in RTSOC-RTRAN. ARSOC-ARRAN also showed no significant group main (F(1,459) = 0.10, p = 0.753) or sex main (F(1,459)= 0.82, p = 0.366) or group by sex interaction (F(1,459) = 0.02, p = 0.890) effect (Figure 1B, 1C).
The severity of alcohol use, as indexed by the PC1, showed a significant group main (F(1,459) = 1584.49, p < 0.001), sex main (F(1,459) = 17.42, p < 0.001) and group by sex interaction (F(1,459) = 17.85, p < 0.001) effect (Figure 1D). Bingers showed higher PC1 than non-bingers and men showed higher PC1 then women, with a more significant binger vs. non-binger group difference in men than in women. Further, PC1 was correlated with perceived friendship across all subjects (r=0.251, p=0.000) as well as men (r=0.312, p=0.000) and women (r=0.192, p=0.003) separately. A slope test did not show a significant difference in slope of the regressions between men and women (Z=1.33 p=0.184).
In linear correlations with age as a covariate, friendship score was not correlated with RTSOC-RTRAN (r = −0.045, p = 0.332) or ARSOC-ARRAN (r = −0.005, p = 0.918) across all subjects or for any of the four individual groups (all p’s > 0.221). Likewise, PC1 was not correlated with RTSOC-RTRAN (r = −0.038, p = 0.413) or ARSOC-ARRAN (r = −0.009, p = 0.847) across all subjects or for any of the four individual groups (all p’s > 0.098).
3.2. Brain activations in the social cognition task
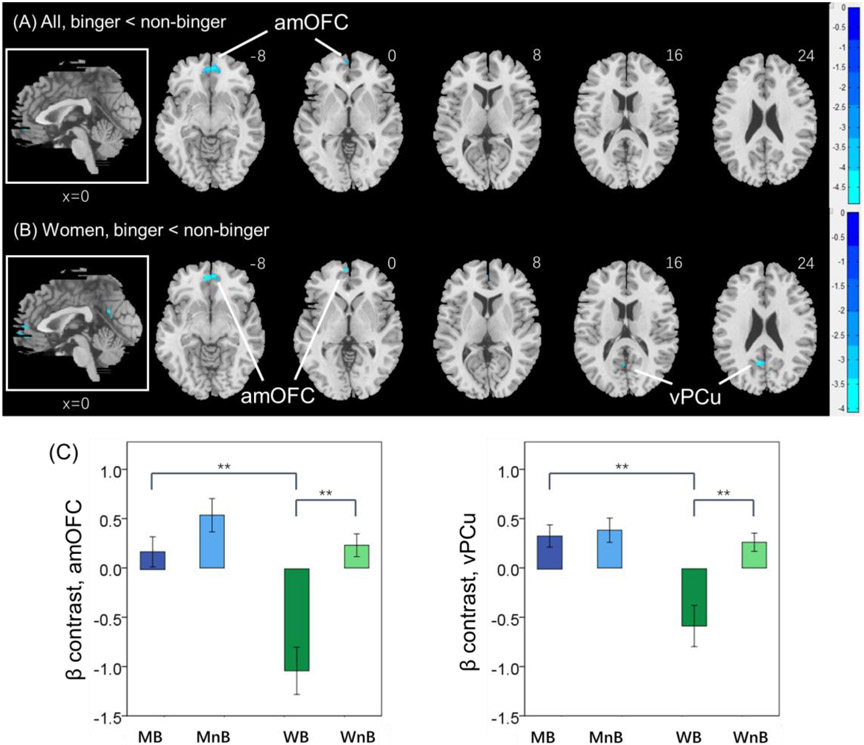
In the social cognition task, the anterior medial orbitofrontal cortex (amOFC; x = 2, y = 50, z = −10; peak voxel Z value = 4.84, volume = 768 mm3) showed higher response to “social – random” in non-binger vs. binger in whole-brain analysis. No clusters showed a significant group by sex interaction effect. Simple effects analyses showed that this group difference was carried mostly by women, who showed higher response to amOFC (0, 50, −8; Z = 4.11, 888 mm3) as well as the left ventral precuneus (vPCu; −8, −58, 24; Z = 4.03, 576 mm3) in non-bingers vs. bingers. No clusters showed significant differences in male binger vs. non-binger in whole-brain analyses. The contrast maps are shown in Figure 2A and 2B. Figure 2C shows the mean ± SE of the β contrasts for the amOFC and vPCu clusters identified from women for each of the four individual groups. We followed up with an ANOVA of the β contrasts with age as a covariate. The results showed that, for both clusters, non-binger relative to binger and men relative to women showed higher activation during perception of social vs. random interaction and, compared to men, women showed significantly lower activity in the binger than non-binger group (Supplementary Table S3).
Figure 2.
Regional brain activations to the contrast “social – random” in the social perception task. (A) binger vs. non-binger; (B) women binger vs. non-binger. Voxel p < 0.001 in combination with a cluster p < 0.05 FWE corrected. Color bars show voxel t values; cool: non-binger > binger. Clusters are overlaid on a T1 structural image in neurological orientation: right = right. The inset shows a mid-sagittal section to highlight the clusters. (C) The β contrast of “social – random” (mean ± SE) of the amOFC and vPCu cluster identified in women shown separately for the four groups. ** p < 0.001.
For each individual cluster we also performed a linear regression of the β contrast against the friendship score for the group from which the ROIs were identified. None of the correlations were significant (all p’s > 0.089).
3.3. Neural correlates of perceived friendship in the social cognition task
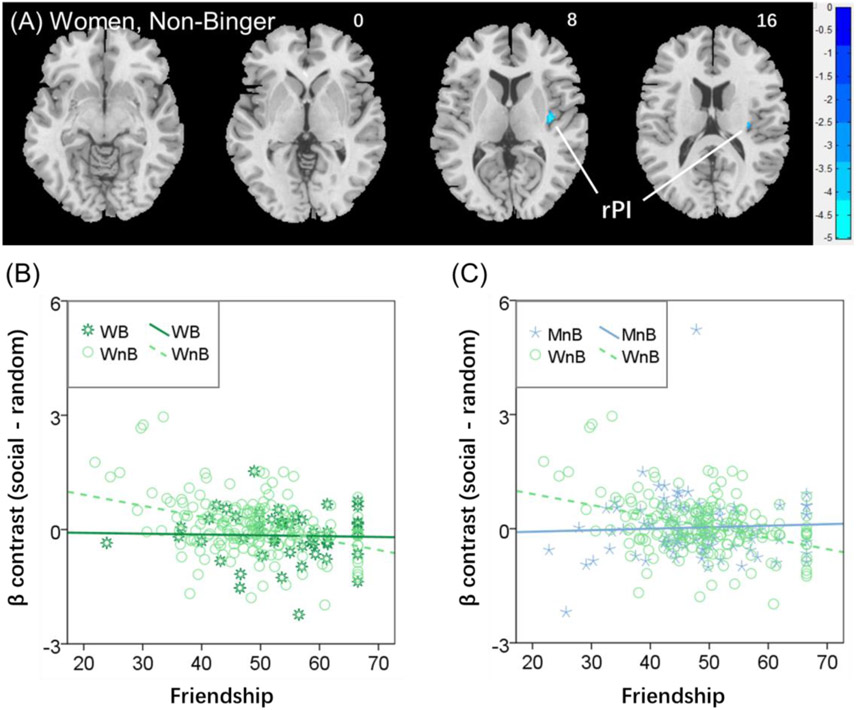
We performed whole-brain linear regression of the contrast “social – random” against perceived friendship score for the entire sample and for each of the four individual groups separately. At voxel p < 0.001, uncorrected in combination with a cluster p < 0.05 FWE corrected, only non-binger women yielded a significant cluster in the right posterior insula (rPI; 36, −12, 8, Z = 4.85, 472 mm3, Figure 3A). We extracted the β estimate of the rPI for all individual subjects and confirmed with slope tests that the correlation of the β contrast with perceived friendship score was significantly different between binger and non-binger women (z = 2.00, p = 0.0455; Figure 3B) and between non-binger women and non-binger men (z = −3.33, p = 0.0009; Figure 3C). We summarize the statistics of the correlation between the β estimate of the rPI and perceived friendship score for each group in Supplementary Table S4. Across all subjects, the β estimate of the rPI and perceived friendship score was significantly correlated (r= −0.192, p < 0.001).
Figure 3.
(A) Linear regression of the contrast “social – random” against perceived friendship score identified a cluster in the right posterior insula (rPI) in negative correlation for women non-binger (WnB). Voxel p < 0.001, uncorrected in combination with a cluster p < 0.05 FWE corrected. Color bars show voxel t values; cool: negative correlation. Clusters are overlaid on a T1 structural image in neurological orientation: right=right. We performed slope tests to examine the difference in the regression between (B) women binger (WB, dark green) and WnB (light green) and between (C) WnB and men non-binger (MnB, light blue). Both showed a significant difference in slope. Note that residuals are plotted here with age accounted for in the regressions.
Preclinical studies have suggested the amOFC and posterior insula as the hedonic “hotspots” of the brain (Castro and Berridge, 2017). Thus, we performed a linear regression of amOFC and rPI activities during perception of social interaction across all subjects, with age as a covariate. The results showed that across all subjects, amOFC and rPI activities (β estimates) were significantly correlated (r = 0.236, p < 0.001).
3.4. The inter-relationship of rPI activity, perceived friendship, and severity of alcohol use
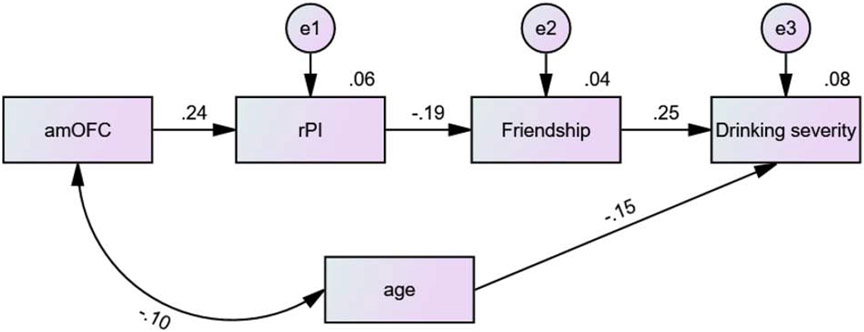
As described in the Methods, a principal component analysis of the 13 drinking measures of the HCP identified a single factor (PC1) indexing alcohol use severity. Across all subjects, individual weight of the PC1 was positively correlated with the friendship score (r = 0.251, p < 0.001) and negatively with the effect size of the rPI (r = −0.095, p = 0.041) with age as covariate. Thus, we performed a mediation analysis to examine the inter-relationship between PC1, friendship score, and the effect size of the rPI during identification of social interaction, with age as a covariate. We considered all six possible models and only one model (rPI β estimate → perceived friendship → severity of alcohol use) showed a significant (and complete) mediation. The six models and the statistics are shown in Supplementary Table S5.
Further, because amOFC and rPI activities were correlated and previous studies have suggested amOFC regulation of rPI activity (see Discussion), we performed a path analysis to examine whether the model amOFC β → rPI β → perceived friendship → severity of alcohol use demonstrated a significant statistical fit. The results showed that, whether age alone or both age and sex were included as covariates, the model demonstrated a significant fit as well as better fit as compared to other models. Figure 4 shows the best model (fit indices: RMSEA = 0.031 [90% CI: 0.000 0.077], χ2/df = 1.43, SRMR = 0.031, and CFI = 0.976). The other models and fit statistics are shown in Supplementary Figure S1 and Supplementary Table S6.
Figure 4.
Path model to show the inter-relationship of amOFC and rPI activity, perceived friendship and severity of drinking. The other models and fit statistics are shown in Supplementary Figure S1 and Supplementary Table S6.
4. Discussion
Young adult binge as compared to non-binge drinkers demonstrated significantly higher perceived friendship as evaluated by the NIH Emotion Toolbox. This difference was observed for both men and women. Binge relative non-binge drinkers showed diminished responses in the amOFC and vPCu during perception of social vs. random interactions, with a more significant effect in women. The mOFC and vPCu are both involved in processing social interactions and rewards; diminished regional activities may suggest dysfunctional social emotional processing in binge drinkers. Further, the rPI showed a significant negative correlation in regional responses to social perception with perceived friendship and severity of alcohol use. The PI supports processing of somatosensory and interoceptive information. Mediation and path analyses characterized the inter-relationship between amOFC and rPI activity, perceived friendship and the severity of alcohol use. We discuss the main findings in the below.
4.1. Binge drinking and regional responses to social vs. random interactions
Despite no differences in behavioral performance, bingers as compared with non-bingers showed lower activation to the perception of social vs. random interactions in the amOFC and vPCu, in the social cognition task. A number of studies have implicated the mOFC in social emotional processing. Lesions of the OFC compromised the ability to empathize and interact socially (Rudebeck and Rich, 2018). Young healthy adults showed activations in the mOFC watching video clips of social vs. non-social interactions (Mainieri et al., 2013). The amOFC is involved in mediating social touch (hand-holding)-induced analgesia in women (López-Solà et al., 2019). The mOFC mediated the effects of social support on the reduction of thalamus and amygdala responses to negative emotions (Mulej Bratec et al., 2020). The mOFC responded to motivated behavior directed to benefit not only oneself but also in-group and out-group others (Bortolini et al., 2017). Notably, in a previous meta-analysis of imaging studies of emotional processing, we demonstrated that the amOFC was particularly involved in processing positive emotions (Yang et al., 2020). Thus, whereas binge drinking individuals reported higher perceived friendship, they paradoxically demonstrated less activation of the amOFC and perhaps positive emotions during identification of socially vs. randomly interacting stimuli, as compared to non-binge drinking individuals. The mOFC was also implicated in reward-based decision-making (Chau et al., 2018). Diminished activation of the mOFC may broadly suggest dysfunctional social reward processing in binge drinkers.
Densely inter-connected with the mOFC (Cavada et al., 2000), the precuneus (PCu) has also been implicated in social judgment and valuation. The PCu and mOFC were activated concurrently when people made judgments that required understanding whether to act out of empathy and forgiveness (Farrow et al., 2001). In a behavioral task to quantify the motivation for seeking social rewards, the mOFC and PCu were engaged when participants chose social vs. object reward (Dubey et al., 2020). Further, the vPCu appeared to support the extent of behavioral engagement in a cognitive task (Zhang and Li, 2010; Zhang and Li, 2012). Binge as compared to non-binge drinkers, particularly in women, would seem to be less engaged and rewarded in the process of identifying social interaction.
4.2. Perceived friendship and the posterior insula
Both male and female bingers perceived more friendship than non-bingers, in accordance with earlier findings (Boman et al., 2013; Tinajero et al., 2019). In examining the neural correlates of individual variation, we observed activities of the rPI in negative correlation with perceived friendship in non-binging women. The insula is heavily involved in socio-emotional processing, as demonstrated in behavioral paradigms that engage emotional experience, empathy, and social cognition (Boucher et al., 2015; Burleson and Quigley, 2019; Cheng et al., 2020; Dal Monte et al., 2013; Fan et al., 2011; Pugnaghi et al., 2011; Uddin et al., 2017; Wang et al., 2019). The PI has also been implicated in the pathophysiology of mental disorders involving social emotional dysfunction (Francis et al., 2019; Turel et al., 2018).
Importantly, although rPI activity in relation to perceived friendship did not reveal in whole-brain regression analysis, the rPI activity was significantly correlated with a summary measure of the severity of alcohol use, as shown by the PCA of drinking metrics, across all subjects. This allowed us to perform a mediation analysis and establish a directional relationship between rPI activity, perceived friendship and drinking severity. That is, diminished rPI activity contributed to higher perceived friendship which, in turn, led to greater severity of alcohol use.
4.3. Potential roles of amOFC and rPi in perceived friendship and alcohol use
Both preclinical and human studies support the interaction of mOFC and PI in social emotional and reward processing. In rats, neurons both in the OFC and the insula tracked sucrose intensity and those in the OFC responded to movement decision for consummation in the discrimination task (Fonseca et al., 2018). In rodents, opioid/orexin stimulations of the mOFC and PI enhanced the hedonic impact of sucrose taste and elevated Fos expression throughout the reward circuits (Castro and Berridge, 2017). In humans, when affective vocalizations were heard, the amOFC supported emotional control through functional connectivity with the PI (Koch et al., 2020), consistent with concerted roles of the OFC and PI in social emotional processing (Britton et al., 2006).
More broadly, interactions of the OFC and PI have been implicated in physiological responses central to social emotions and a number of psychopathologies involving altered social and emotional processing. For instance, higher functional connectivity between the insula and OFC were associated with interoceptive accuracy in a heartbeat counting task (Chong et al., 2017). Reduced OFC response and higher OFC-PI connectivity during loss in a gambling task was associated with depression in adolescents (Jin et al., 2017). In morphometric studies thicker PI and OFC were each associated with disease duration and pain severity in women with irritable bowel syndrome (Piché et al., 2013).
Thus, the findings of a significant correlation between amOFC and rPI activities during identification of social interaction was consistent with this literature. Further, we showed in path analysis the directional relationship between amOFC and rPI activity during social cognition, perceived friendship, and the severity of alcohol use. As described earlier, the amOFC and rPI appeared to play a distinct role in processing positive emotions (Harrison et al., 2017; Myers-Schulz and Koenigs, 2012; Yang et al., 2020) and social emotions (Burleson and Quigley, 2019; Cheng et al., 2020; Wang et al., 2019). These findings suggest that a less than positive emotional state during identification of social interaction may represent a marker of inter-subject variation in perceived friendship and heavier alcohol use.
4.4. Limitations of the study and conclusions
A few limitations need to be considered for the study. Although we identified regional activations that may reflect differences in social emotional processing between binge and non-binge drinkers, these activities did not appear to relate to perceived friendship in linear regressions. Thus, we were not able to investigate whether these processes mediate the influences of perceived friendship on alcohol misuse. It is probable that, with geometric objects simulating social interaction, the social cognition task of the HCP does not capture social emotions as experienced during human interactions. More studies clearly are needed to investigate this issue further. Secondly, we discussed the psychological relevance of amOFC and rPI co-activation. However, these propositions need to be tested directly, perhaps with behavioral paradigms involving not only genuine human interaction but also allowing a contrast between positive and negative interaction. Finally, the sample comprised more bingers in men and more non-bingers in women and, although we have examined sex differences specifically for the findings revealed in men or women alone, it remains unclear how this asymmetry in group representation may have influenced the results.
In conclusion, we replicated previous findings that perceived friendship is strongly associated with binge drinking and the severity of alcohol use in young adults. We showed that this is true for both men and women. For bingeing relative to non-bingeing individuals (women in particular), perception of social interaction in a simulated social task engaged less activity of the amOFC, a region central to processing social and positive emotions. The amOFC may influence rPI activity and, in turn, perceived friendship and alcohol use severity. Future studies with a more realistic paradigm of human interaction are needed to confirm this finding and to explore how perceive friendship and other peer influences contribute to alcohol misuse in young adults.
Supplementary Material
Highlights.
Binge relative to non-binge drinkers showed higher perceived friendship.
Binge drinkers showed diminished mOFC activations during social perception.
MOFC and rPI responses influence drinking severity via perceived friendship.
More research can examine other dimensions of peer influences on drinking.
Funding Support and Acknowledgement
The current study is supported by NIH grants AA021449, MH113134, DA023248, DA045189, AG067024 (C-SRL) and a scholarship from the China Scholarship Council for GL to visit Yale University. Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adan A, Forero DA, Navarro JF, 2017. Personality Traits Related to Binge Drinking: A Systematic Review. Front Psychiatry 8, 134–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi A, Pearlson GD, Meda SA, Dager A, Potenza MN, Rosen R, Austad CS, Raskin SA, Fallahi CR, Tennen H, Wood RM, Stevens MC, 2013. Influence of alcohol use on neural response to Go/No-Go task in college drinkers. Neuropsychopharmacology 38(11), 2197–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames SL, Wong SW, Bechara A, Cappelli C, Dust M, Grenard JL, Stacy AW, 2014. Neural correlates of a Go/NoGo task with alcohol stimuli in light and heavy young drinkers. Behav Brain Res 274, 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angosta J, Steers M-LN, Steers K, Lembo Riggs J, Neighbors C, 2019. Who cares if college and drinking are synonymous? Identification with typical students moderates the relationship between college life alcohol salience and drinking outcomes. Addictive behaviors 98, 106046–106046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, Glasser MF, Curtiss S, Dixit S, Feldt C, Nolan D, Bryant E, Hartley T, Footer O, Bjork JM, Poldrack R, Smith S, Johansen-Berg H, Snyder AZ, Van Essen DC, Consortium, W.U.-M.H., 2013. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage 80, 169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KH, Thombs DL, Mahoney CA, Fingar KM, 1995. Social Context and Sensation Seeking: Gender Differences in College Student Drinking Motivations. International Journal of the Addictions 30(9), 1101–1115. [DOI] [PubMed] [Google Scholar]
- Blaine SK, Nautiyal N, Hart R, Guarnaccia JB, Sinha R, 2019. Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addict Biol 24(5), 1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman J, Stogner J, Miller B, 2013. Binge Drinking, Marijuana Use, and Friendships: The Relationship Between Similar and Dissimilar Usage and Friendship Quality. Journal of psychoactive drugs 45, 218–226. [DOI] [PubMed] [Google Scholar]
- Bora E, Zorlu N, 2017. Social cognition in alcohol use disorder: a meta-analysis. Addiction 112(1), 40–48. [DOI] [PubMed] [Google Scholar]
- Borsari B, Carey KB, 2006. How the quality of peer relationships influences college alcohol use. Drug Alcohol Rev 25(4), 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolini T, Bado P, Hoefle S, Engel A, Zahn R, de Oliveira Souza R, Dreher J-C, Moll J, 2017. Neural bases of ingroup altruistic motivation in soccer fans. Sci Rep 7(1), 16122–16122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher O, Rouleau I, Lassonde M, Lepore F, Bouthillier A, Nguyen DK, 2015. Social information processing following resection of the insular cortex. Neuropsychologia 71, 1–10. [DOI] [PubMed] [Google Scholar]
- Boyd SJ, Sceeles EM, Tapert SF, Brown SA, Nagel BJ, 2018. Reciprocal relations between positive alcohol expectancies and peer use on adolescent drinking: An accelerated autoregressive cross-lagged model using the NCANDA sample. Psychol Addict Behav 32(5), 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I, 2006. Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage 31(1), 397–409. [DOI] [PubMed] [Google Scholar]
- Burleson MH, Quigley KS, 2019. Social interoception and social allostasis through touch: Legacy of the Somatovisceral Afference Model of Emotion. Social Neuroscience, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella S, Peigneux P, Petit G, Lallemand F, Saeremans M, Noël X, Metens T, Nouali M, De Tiège X, De Witte P, Ward R, Verbanck P, 2013. Increased cortical activity in binge drinkers during working memory task: a preliminary assessment through a functional magnetic resonance imaging study. PLoS One 8(4), e62260–e62260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RW, Treloar Padovano H, Emery NN, Miranda R Jr., 2019. Rate of alcohol consumption in the daily life of adolescents and emerging adults. Psychopharmacology (Berl) 236(11), 3111–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C, 2000. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage 12(3), 314–325. [DOI] [PubMed] [Google Scholar]
- Castro DC, Berridge KC, 2017. Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc Natl Acad Sci U S A 114(43), E9125–E9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Compañy T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suárez F, 2000. The Anatomical Connections of the Macaque Monkey Orbitofrontal Cortex. A Review. Cerebral Cortex0 10(3), 220–242. [DOI] [PubMed] [Google Scholar]
- Chau BKH, Jarvis H, Law C-K, Chong TTJ, 2018. Dopamine and reward: a view from the prefrontal cortex. Behavioural Pharmacology 29(7). [DOI] [PubMed] [Google Scholar]
- Cheng TW, Vijayakumar N, Flournoy JC, Op de Macks Z, Peake SJ, Flannery JE, Mobasser A, Alberti SL, Fisher PA, Pfeifer JH, 2020. Feeling left out or just surprised? Neural correlates of social exclusion and overinclusion in adolescence. Cognitive, Affective, & Behavioral Neuroscience 20(2), 340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JSX, Ng GJP, Lee SC, Zhou J, 2017. Salience network connectivity in the insula is associated with individual differences in interoceptive accuracy. Brain Structure and Function 222(4), 1635–1644. [DOI] [PubMed] [Google Scholar]
- Clapp JD, Shillington AM, 2001. ENVIRONMENTAL PREDICTORS OF HEAVY EPISODIC DRINKING. The American Journal of Drug and Alcohol Abuse 27(2), 301–313. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Jones SA, Nagel BJ, 2015. Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Dev Cogn Neurosci 16, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Krueger F, Solomon JM, Schintu S, Knutson KM, Strenziok M, Pardini M, Leopold A, Raymont V, Grafman J, 2013. A voxel-based lesion study on facial emotion recognition after penetrating brain injury. Soc Cogn Affect Neurosci 8(6), 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JP, Pedersen ER, Tucker JS, Dunbar MS, Seelam R, Shih R, D’Amico EJ, 2019. Long-term Associations Between Substance Use-Related Media Exposure, Descriptive Norms, and Alcohol Use from Adolescence to Young Adulthood. Journal of Youth and Adolescence 48(7), 1311–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dir AL, Bell RL, Adams ZW, Hulvershorn LA, 2017. Gender Differences in Risk Factors for Adolescent Binge Drinking and Implications for Intervention and Prevention. Front Psychiatry 8, 289–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey I, Georgescu AL, Hommelsen M, Vogeley K, Ropar D, d.C. Hamilton AF, 2020. Distinct neural correlates of social and object reward seeking motivation. European Journal of Neuroscience 52(9), 4214–4229. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, 2009. The Human Brain. Second Edition. Springer-Verlag, Wien/New York. [Google Scholar]
- Edwards KA, Witkiewitz K, Vowles KE, 2019. Demographic differences in perceived social norms of drug and alcohol use among Hispanic/Latinx and non-Hispanic White college students. Addictive Behaviors 98, 106060. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greek M, Northoff G, 2011. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience & Biobehavioral Reviews 35(3), 903–911. [DOI] [PubMed] [Google Scholar]
- Farrow T, Zheng Y, Wilkinson I, Spence S, Deakin J, Tarrier N, Griffiths P, Woodruff P, 2001. Investigating the functional anatomy of empathy and forgiveness. NeuroReport 12, 2433–2438. [DOI] [PubMed] [Google Scholar]
- Fonseca E, de Lafuente V, Simon SA, Gutierrez R, 2018. Sucrose intensity coding and decision-making in rat gustatory cortices. Elite 7, e41152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SM, Camchong J, Brickman L, Goelkel-Garcia L, Mueller BA, Tseng A, Lim KO, Jacob S, 2019. Hypoconnectivity of insular resting-state networks in adolescents with Autism Spectrum Disorder. Psychiatry Research: Neuroimaging 283, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Valente TW, 2015. Multiplex congruity: Friendship networks and perceived popularity as correlates of adolescent alcohol use. Social Science & Medicine 125, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Rouchy E, Galéra C, Tzourio C, Michel G, 2020. The relation between ADHD symptoms, perceived stress and binge drinking in college students. Psychiatry Research 284, 112689. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Manza P, Ramchandani VA, Volkow ND, 2020. Neuropsychosocial markers of binge drinking in young adults. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupensperger S, Benson AJ, Bray BC, Evans MB, 2019. Social cohesion and peer acceptance predict student-athletes' attitudes toward health-risk behaviors: A within- and between-group investigation. J Sci Med Sport 22(12), 1280–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupensperger S, Turrisi R, Jones D, Evans MB, 2020. Dynamic characteristics of groups and individuals that amplify adherence to perceived drinking norms in college club sport teams: A longitudinal multilevel investigation. Psychol Addict Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Pettinati HM, O'Malley S, Randall PK, Randall CL, 2010. Gender differences in alcohol treatment: an analysis of outcome from the COMBINE study. Alcohol Clin Exp Res 34(10), 1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MJ, Modecki K, Moctezuma Tan L, Luecken L, Wolchik S, Sandler I, 2019. Binge drinking in adolescence predicts an atypical cortisol stress response in young adulthood. Psychoneuroendocrinology 100, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton HR, Dehart T, 2019. Needs and Norms: Testing the Effects of Negative Interpersonal Interactions, the Need to Belong, and Perceived Norms on Alcohol Consumption. Journal of Studies on Alcohol and Drugs 80(3), 340–348. [PubMed] [Google Scholar]
- Harrison BJ, Fullana MA, Via E, Soriano-Mas C, Vervliet B, Martínez-Zalacaín I, Pujol J, Davey CG, Kircher T, Straube B, Cardoner N, 2017. Human ventromedial prefrontal cortex and the positive affective processing of safety signals. Neuroimage 152, 12–18. [DOI] [PubMed] [Google Scholar]
- Hensing G, Spak F, 2009. Introduction: Gendering Socio Cultural Alcohol and Drug Research. Alcohol and Alcoholism 44(6), 602–606. [DOI] [PubMed] [Google Scholar]
- Herman AM, Critchley HD, Duka T, 2019. Binge drinking is associated with attenuated frontal and parietal activation during successful response inhibition in fearful context. European Journal of Neuroscience 50(3), 2297–2310. [DOI] [PubMed] [Google Scholar]
- Htet H, Saw YM, Saw TN, Htun NMM, Lay Mon K, Cho SM, Thike T, Khine AT, Kariya T, Yamamoto E, Hamajima N, 2020. Prevalence of alcohol consumption and its risk factors among university students: A cross-sectional study across six universities in Myanmar. PLoS One 15(2), e0229329–e0229329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Ide JS, Chao HH, Castagna B, Fischer KA, Zhang S, Li C-SR, 2018. Structural and functional cerebral bases of diminished inhibitory control during healthy aging. Hum Brain Mapp 39(12), 5085–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GC, Unger JB, Soto D, Fujimoto K, Pentz MA, Jordan-Marsh M, Valente TW, 2014. Peer Influences: The Impact of Online and Offline Friendship Networks on Adolescent Smoking and Alcohol Use. Journal of Adolescent Health 54(5), 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson DM, Rapee RM, 2007. Do friends share similar body image and eating problems? The role of social networks and peer influences in early adolescence. Behaviour Research and Therapy 45(7), 1557–1577. [DOI] [PubMed] [Google Scholar]
- Ide JS, Zhornitsky S, Hu S, Zhang S, Krystal JH, Li C-SR, 2017. Sex differences in the interacting roles of impulsivity and positive alcohol expectancy in problem drinking: A structural brain imaging study. Neuroimage Clin 14, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Narayanan A, Perlman G, Luking K, DeLorenzo C, Hajcak G, Klein DN, Kotov R, Mohanty A, 2017. Orbitofrontal cortex activity and connectivity predict future depression symptoms in adolescence. Biological psychiatry. Cognitive neuroscience and neuroimaging 2(7), 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Cservenka A, Nagel BJ, 2016. Binge drinking impacts dorsal striatal response during decision making in adolescents. Neuroimage 129, 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy TM, Howard AL, Mitchell JT, Hoza B, Arnold LE, Hechtman LT, Swanson JM, Stehli A, Molina BSG, 2019. Adult substance use as a function of growth in peer use across adolescence and young adulthood in the context of ADHD: Findings from the MTA. Addictive behaviors 99, 106106–106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy TM, Walther CAP, Pedersen SL, McKone KMP, Gnagy EM, Pelham WE Jr, Molina BSG, 2020. Beers with Peers: Childhood ADHD and Risk for Correlated Change in Perceived Peer and Personal Alcohol Use Across Young Adulthood. Alcoholism: Clinical and Experimental Research 44(11), 2350–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Galli A, Volman I, Kaldewaij R, Toni I, Roelofs K, 2020. Neural Control of Emotional Actions in Response to Affective Vocalizations. Journal of Cognitive Neuroscience 32, 1–12. [DOI] [PubMed] [Google Scholar]
- Laghi F, Bianchi D, Pompili S, Lonigro A, Baiocco R, 2019. Heavy episodic drinking in late adolescents: The role of theory of mind and conformity drinking motives. Addictive Behaviors 96, 18–25. [DOI] [PubMed] [Google Scholar]
- Lau-Barraco C, Linden-Carmichael AN, Stamates AL, Preonas PD, Braitman AL, 2019. The Influence of a Brief Alcohol Intervention on Alcohol Use Trajectories in Nonstudent Emerging Adult Drinkers. Substance use & misuse 54(12), 2025–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TM, Chao H, Levy I, Li C-SR, 2020. Age-Related Changes in the Neural Processes of Reward-Directed Action and Inhibition of Action. Front Psychol 11, 1121–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TM, Zhornitsky S, Wang W, Ide J, Zhang S, Li C-SR, 2019. Posterior Cingulate Cortical Response to Active Avoidance Mediates the Relationship between Punishment Sensitivity and Problem Drinking. J Neurosci 39(32), 6354–6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Chen Y, Wang W, Dhingra I, Zhornitsky S, Tang X, Li C-SR, 2020a. Sex Differences in Neural Responses to the Perception of Social Interactions. Front Hum Neurosci 14, 565132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang S, Le TM, Tang X, Li C-SR, 2020b. Neural responses to negative facial emotions: Sex differences in the correlates of individual anger and fear traits. Neuroimage 221, 117171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang S, Le TM, Tang X, Li C-SR, 2020c. Neural Responses to Reward in a Gambling Task: Sex Differences and Individual Variation in Reward-Driven Impulsivity. Cereb Cortex Commun 1(1), tgaa025–tgaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Solà M, Geuter S, Koban L, Coan JA, Wager TD, 2019. Brain mechanisms of social touch-induced analgesia in females. PAIN 160(9). [DOI] [PubMed] [Google Scholar]
- Luk JW, Haynie DL, Vaca FE, Li K, Hingson R, Simons-Morton BG, 2019a. Close Friends' Drinking and Personal Income as Mediators of Extreme Drinking: A Prospective Investigation. Journal of studies on alcohol and drugs 80(6), 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk JW, Haynie DL, Vaca FE, Li K, Hingson R, Simons-Morton BG, 2019b. Close Friends’ Drinking and Personal Income as Mediators of Extreme Drinking: A Prospective Investigation. Journal of Studies on Alcohol and Drugs 80(6), 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS, 2007. Mediation analysis. Annu Rev Psychol 58, 593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainieri AG, Heim S, Straube B, Binkofski F, Kircher T, 2013. Differential role of the Mentalizing and the Mirror Neuron system in the imitation of communicative gestures. Neuroimage 81,294–305. [DOI] [PubMed] [Google Scholar]
- Moran MB, Villanti AC, Johnson A, Rath J, 2019. Patterns of Alcohol, Tobacco, and Substance Use Among Young Adult Peer Crowds. Am J Prev Med 56(6), e185–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H, Larsen J, Catterall E, Moss AC, Dombrowski SU, 2020. Peer pressure and alcohol consumption in adults living in the UK: a systematic qualitative review. BMC Public Health 20(1), 1014–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulej Bratec S, Bertram T, Starke G, Brandi F, Xie X, Sorg C, 2020. Your presence soothes me: a neural process model of aversive emotion regulation via social buffering. Soc Cogn Affect Neurosci 15(5), 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M, 2012. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Molecular psychiatry 17(2), 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors C, Krieger H, Rodriguez LM, Rinker DV, Lembo JM, 2019. Social identity and drinking: Dissecting social networks and implications for novel interventions. J Prev Interv Community 47(3), 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, 2004. Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev 24(8), 981–1010. [DOI] [PubMed] [Google Scholar]
- Onuoha RC, Quintana DS, Lyvers M, Guastella AJ, 2016. A Meta-analysis of Theory of Mind in Alcohol Use Disorders. Alcohol and Alcoholism 51(4), 410–415. [DOI] [PubMed] [Google Scholar]
- Paxton SJ, Schutz HK, Wertheim EH, Muir SL, 1999. Friendship clique and peer influences on body image concerns, dietary restraint, extreme weight-loss behaviors, and binge eating in adolescent girls. American Psychological Association, US, pp. 255–266. [DOI] [PubMed] [Google Scholar]
- Petit G, Kornreich C, Dan B, Verbanck P, Campanella S, 2014. Electrophysiological correlates of alcohol- and non-alcohol-related stimuli processing in binge drinkers: A follow-up study. Journal of Psychopharmacology 28(11), 1041–1052. [DOI] [PubMed] [Google Scholar]
- Petit G, Kornreich C, Maurage P, Noël X, Letesson C, Verbanck P, Campanella S, 2012. Early attentional modulation by alcohol-related cues in young binge drinkers: An event-related potentials study. Clinical Neurophysiology 123(5), 925–936. [DOI] [PubMed] [Google Scholar]
- Petit G, Kornreich C, Verbanck P, Campanella S, 2013. Gender differences in reactivity to alcohol cues in binge drinkers: A preliminary assessment of event-related potentials. Psychiatry Research 209(3), 494–503. [DOI] [PubMed] [Google Scholar]
- Piché M, Chen J-I, Roy M, Poitras P, Bouin M, Rainville P, 2013. Thicker Posterior Insula Is Associated With Disease Duration in Women With Irritable Bowel Syndrome (IBS) Whereas Thicker Orbitofrontal Cortex Predicts Reduced Pain Inhibition in Both IBS Patients and Controls. The Journal of Pain 14(10), 1217–1226. [DOI] [PubMed] [Google Scholar]
- Powers J, Duffy L, Burns L, Loxton D, 2016. Binge drinking and subsequent depressive symptoms in young women in Australia. Drug and Alcohol Dependence 161, 86–94. [DOI] [PubMed] [Google Scholar]
- Pugnaghi M, Meletti S, Castana L, Francione S, Nobili L, Mai R, Tassi L, 2011. Features of somatosensory manifestations induced by intracranial electrical stimulations of the human insula. Clinical Neurophysiology 122(10), 2049–2058. [DOI] [PubMed] [Google Scholar]
- Rae CL, Gierski F, Smith KW, Nikolaou K, Davies A, Critchley HD, Naassila M, Duka T, 2020. Differential brain responses for perception of pain during empathic response in binge drinkers compared to non-binge drinkers. Neuroimage Clin 27, 102322–102322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboussin BA, Furr-Holden DM, Green KM, Ialongo NS, Rabinowitz JA, Matson PA, Maher B, Nelson V, Milam AJ, 2019. Social Influences on Drinking Trajectories From Adolescence to Young Adulthood in an Urban Minority Sample. Journal of studies on alcohol and drugs 80(2), 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Rich EL, 2018. Orbitofrontal cortex. Current Biology 28(18), R1083–R1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte MT, Ramo D, Brown SA, 2009. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev 29(6), 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF, 2010. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol 44(1), 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirao N, Okamoto Y, Okada G, Ueda K, Yamawaki S, 2005. Gender differences in brain activity toward unpleasant linguisticstimuli concerning interpersonal relationships: an fMRI study. European Archives of Psychiatry and Clinical Neuroscience 255(5), 327–333. [DOI] [PubMed] [Google Scholar]
- Smith RL, Salvatore JE, Aliev F, Neale Z, Barr P, Spit for Science Working, G., Dick DM, 2019. Genes, Roommates, and Residence Halls: A Multidimensional Study of the Role of Peer Drinking on College Students' Alcohol Use. Alcohol Clin Exp Res 43(6), 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF, 2012. Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. Journal of studies on alcohol and drugs 73(5), 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF, 2011. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res 35(10), 1831–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Hamann S, 2012. Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia 50(7), 1578–1593. [DOI] [PubMed] [Google Scholar]
- Stolle M, Sack P-M, Thomasius R, 2009. Binge drinking in childhood and adolescence: epidemiology, consequences, and interventions. Dtsch Arztebl Int 106(19), 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero C, Luque B, Cuadrado E, 2019. A Multilevel Study of Alcohol Consumption in Young Adults: Self-Efficacy, Peers' Motivations and Protective Strategies. Int J Environ Res Public Health 16(16), 2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant M, Smith J, Ball S, Winlove C, Gul S, Charles N, 2019. Alcohol consumption among university students in the night-time economy in the UK: A three-wave longitudinal study. Drug and Alcohol Dependence 204, 107522. [DOI] [PubMed] [Google Scholar]
- Tinajero C, Cadaveira F, Rodríguez MS, Páramo MF, 2019. Perceived Social Support from Significant Others among Binge Drinking and Polyconsuming Spanish University Students. Int J Environ Res Public Health 16(22), 4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turel O, He Q, Brevers D, Bechara A, 2018. Delay discounting mediates the association between posterior insular cortex volume and social media addiction symptoms. Cognitive, Affective, & Behavioral Neuroscience 18(4), 694–704. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O, 2017. Structure and Function of the Human Insula. J Clin Neurophysiol 34(4), 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, Della Penna S, Feinberg D, Glasser MF, Harel N, Heath AC, Larson-Prior L, Marcus D, Michalareas G, Moeller S, Oostenveld R, Petersen SE, Prior F, Schlaggar BL, Smith SM, Snyder AZ, Xu J, Yacoub E, 2012. The Human Connectome Project: A data acquisition perspective. Neuroimage 62(4), 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN, 2008. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59(6), 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walitzer KS, Dearing RL, 2006. Gender differences in alcohol and substance use relapse. Clin Psychol Rev 26(2), 128–148. [DOI] [PubMed] [Google Scholar]
- Wang, Zhornitsky S, Li CSP, Le TM, Joormann J, Li C-SR, 2019. Social anxiety, posterior insula activation, and autonomic response during self-initiated action in a Cyberball game. J Affect Disord 255, 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhornitsky S, Le TM, Zhang S, Li C-SR, 2020. Heart Rate Variability, Cue- Evoked Ventromedial Prefrontal Cortical Response, and Problem Alcohol Use in Adult Drinkers. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 5(6), 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TD, Newton-Mora M, Pirkle J, 2016. Event-related potential correlates of processing alcohol-related pictures in young adult binge drinkers. The American Journal of Drug and Alcohol Abuse 42(1), 77–87. [DOI] [PubMed] [Google Scholar]
- Wechsler, Davenport, Dowdall, Moeykens, Castillo, 1994. Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. Jama the Journal of the American Medical Association. [PubMed] [Google Scholar]
- Weybright EH, Beckmeyer JJ, Caldwell LL, Wegner L, Smith EA, 2019. With a little help from my friends? A longitudinal look at the role of peers versus friends on adolescent alcohol use. J Adolesc 73, 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley T, Milleville SC, Martin A, 2007. Understanding Animate Agents: Distinct Roles for the Social Network and Mirror System. Psychological Science 18(6), 469–474. [DOI] [PubMed] [Google Scholar]
- White HR, Kilmer JR, Fossos-Wong N, Hayes K, Sokolovsky AW, Jackson KM, 2019. Simultaneous Alcohol and Marijuana Use Among College Students: Patterns, Correlates, Norms, and Consequences. Alcohol Clin Exp Res 43(7), 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis E, Adams R, Keene J, 2019. If Everyone Is Doing It, It Must Be Safe: College Students’ Development of Attitudes toward Poly-Substance Use. Substance Use & Misuse 54(11), 1886–1893. [DOI] [PubMed] [Google Scholar]
- Woo B, Wang K, Tran T, 2017. Racial and ethnic differences in associations between psychological distress and the presence of binge drinking: Results from the California health interview survey. Addictive Behaviors 65, 1–6. [DOI] [PubMed] [Google Scholar]
- Worbe Y, Irvine M, Lange I, Kundu P, Howell NA, Harrison NA, Bullmore ET, Robbins TW, Voon V, 2014. Neuronal correlates of risk-seeking attitudes to anticipated losses in binge drinkers. Biol Psychiatry 76(9), 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Gong Q, Huang X, Li X, Xue G, Wong S, Lu Z-L, Palmer P, Wei Y, Jia Y, Johnson CA, 2013. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychology of Addictive Behaviors 27(2), 443–454. [DOI] [PubMed] [Google Scholar]
- Yan C-G, Wang X-D, Zuo X-N, Zang Y-F, 2016. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 14(3), 339–351. [DOI] [PubMed] [Google Scholar]
- Yang M, Tsai S-J, Li C-SR, 2020. Concurrent amygdalar and ventromedial prefrontal cortical responses during emotion processing: a meta-analysis of the effects of valence of emotion and passive exposure versus active regulation. Brain Structure and Function 225(1), 345–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HH, Cha SW, Lee SY, 2020. Patterns of Alcohol consumption and Drinking Motives Among Korean Medical Students. Med Sci Monit 26, e921613–e921613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen WS, Chan G, Bruno R, Clare P, Mattick R, Aiken A, Boland V, McBride N, McCambridge J, Slade T, Kypri K, orwood J, Hutchinson D, Najman J, De Torres C, Peacock A, 2020. Adolescent Alcohol Use Trajectories: Risk Factors and Adult Outcomes. Pediatrics 146(4), e20200440. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li C-SR, 2010. A neural measure of behavioral engagement: task-residual low-frequency blood oxygenation level-dependent activity in the precuneus. Neuroimage 49(2), 1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C-SR, 2012. Task-related, low-frequency task-residual, and resting state activity in the default mode network brain regions. Front Psychol 3, 172–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhornitsky S, Le TM, Li CR, 2019. Hypothalamic Responses to Cocaine and Food Cues in Individuals with Cocaine Dependence. Int J Neuropsychopharmacol 22(12), 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhornitsky S, Zhang S, Ide JS, Chao HH, Wang W, Le TM, Leeman RF, Bi J, Krystal JH, Li C-SR, 2019. Alcohol Expectancy and Cerebral Responses to Cue-Elicited Craving in Adult Nondependent Drinkers. Biological psychiatry. Cognitive neuroscience and neuroimaging 4(5), 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhornitsky S, Zhang S, Ide JS, Chao HH, Wang W, Le TM, Leeman RF, Bi J, Krystal JH, Li CR, 2019. Alcohol Expectancy and Cerebral Responses to Cue-Elicited Craving in Adult Nondependent Drinkers. Biol Psychiatry Cogn Neurosci Neuroimaging 4(5), 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullig KJ, Valois RF, Huebner ES, Oeltmann JE, Drane JW, 2001. Relationship between perceived life satisfaction and adolescents’ substance abuse. Journal of Adolescent Health 29(4), 279–288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.