Abstract
Objective:
To test the null hypothesis that there are no significant differences in pharyngeal airway volumes among adult patients with different vertical skeletal patterns and a clinically normal sagittal skeletal pattern using cone-beam computed tomography (CBCT).
Material and Methods:
The study sample consisted of 100 adult patients (45 men and 55 women; mean age = 24.0 ± 5.3 years) with a normal sagittal skeletal pattern divided into three groups according to the vertical skeletal patterns: high angle (32 patients: 15 women and 17 men), low angle (34 patients: 14 women and 20 men), and normal angle (34 patients: 16 women and 18 men) groups. Nasopharyngeal, oropharyngeal, and total airway volumes of patients in all vertical groups were calculated. Group differences were analyzed using one-way analysis of variance and post hoc Tukey tests.
Results:
Nasopharyngeal airway volume in the high-angle group (mean = 6067.9 ± 1693.9 mm3) was significantly lower than that of the low- and normal-angle groups (P < .01). Oropharyngeal airway volume was highest in the low-angle group (mean = 15,957.6 ± 6817.2 mm3) and significantly decreased in the control (mean = 11,826.1 ± 4831.9 mm3; P = .008) and high angle (mean = 10,869.1 ± 4084.1 mm3; P = .001) groups. Total airway volume was highest in the low-angle group (mean = 24,261.6 ± 8470.1 mm3) and lowest in the high-angle group (mean = 16,937.0 ± 5027.4 mm3; P < .001).
Conclusion:
The null hypothesis was rejected. Significant differences were found in pharyngeal airway volumes among different skeletal vertical patterns.
Keywords: Pharyngeal airway, Vertical skeletal pattern, Cone-beam computed tomography
INTRODUCTION
Vertical malocclusions might develop during pubertal growth due to several etiologic factors, including growth of the maxilla and mandible, dentoalveolar development, eruption of the teeth, and function of the tongue.1 According to Schudy2 and Isaacson et al.,3 backward mandibular rotation and bite opening occurs when vertical growth of condyles is less than that of the facial sutures and alveolar processes.
The relationship between craniofacial morphology and respiratory function has been the focus of investigation since the 19th century.4 A number of studies5–17 have been published on the relationships between the pharyngeal airway and the facial pattern. However, most of the previous studies5–7,11,14,15 were performed on lateral cephalometric films. Because lateral films represent three-dimensional (3D) structures in two dimensions that do not allow the evaluation of those structures' volumes, previous studies were limited.18 In addition, lateral cephalometric films have severe limitations, such as distortion, low reproducibility due to difficulties in landmark identification, differences in magnification, and the superimposition of bilateral craniofacial structures.19
The advantages of using computed tomography (CT) have been previously emphasized; however, the high dose of radiation generated by CT devices is an important limiting factor for routine use in clinics. Cone-beam CT (CBCT) has become a well-accepted diagnostic imaging technique, especially because of its lower radiation doses and faster image acquisition times compared with CT.20
Although a number of studies9,10,12–14,16,17 were performed to investigate the relationship between sagittal skeletal patterns and pharyngeal airway, the relationship between vertical growth patterns and pharyngeal airway was limited.7,8,10,11,15 Within the limitations of those studies,7,10,11,15 which were performed using lateral cephalometric films, an association between pharyngeal airway dimensions and vertical skeletal pattern was demonstrated. To the best of our knowledge, only one study8 has evaluated pharyngeal airway volume in patients with different vertical skeletal patterns, and that unique study had a small sample size of about 21 patients in each vertical group. In addition, sagittal skeletal pattern was not standardized in all vertical groups and included patients with skeletal Class I, II, and III malocclusions and no indication of how many patients had each type. Many authors9,12–14,16,17 have found that pharyngeal airway dimensions and/or volumes are affected by sagittal skeletal patterns. Thus, it seems that the findings reported previously might be affected by sagittal skeletal pattern distribution of the patients in each vertical group.
The aim of the present study was to evaluate the pharyngeal airway volumes of patients presenting with normal sagittal skeletal pattern and different vertical skeletal patterns (high- and low-angle patterns) and to compare the findings with a well-matched control group (normal angle) using 3D analysis on CBCT. In addition, some angular and linear measurements were performed to exclude the effects of sagittal skeletal pattern on pharyngeal airway volumes.
MATERIAL AND METHODS
The present retrospective study was approved by the local ethical committee in Erciyes University. All patients signed an informed consent form allowing use of their data for scientific purposes. CBCT scans used in the present study were part of diagnostic records collected to assess impacted teeth and/or orthodontic treatment needs, and no patient was contacted and no CBCTs were taken for the purpose of the present study.
Study sample calculation was performed based on a formula previously described by Pandis.21 A sample size of at least 27 patients in each group would be necessary to detect a difference of 2500 mm3 (±2500 mm3) pharyngeal pathway volume with a test power of 95% (p = .05 significance level). To increase the power of the study, more patients were included in each vertical group. A total of 100 adult patients, aged 18 to 30 years, were randomly selected from the archive of Erciyes University. The patients' data (anamnesis, plaster models, photographs, clinic diagnosis forms) were examined; exclusion criteria were chronic mouth breathing, permanent snoring, tonsillectomy, adenoidectomy, previous orthodontic treatment and orthognathic surgery, cleft lip and/or palate and/or related syndromes, history of trauma, and body mass index higher than 28. Patients with enlarged adenoids and/or tonsils were also excluded from the study. All selected patients had dental (based on Angle classification by using plaster models) and skeletal Class I (1° < ANB < 5°)22 relationships.
The selected patients were divided into three groups based on vertical growth pattern using SN-MP angle (high angle = >38°; low angle = <26°; and control group or normal angle = 26–38°).11,22 The sample included 32 patients in the high-angle group (15 women and 17 men; mean age = 23.9 ± 4.8 years), 34 patients in the low-angle group (14 women and 20 men; mean age = 24.3 ± 6.6 years), and 34 patients in the normal-angle group (16 women and 18 men; mean age = 23.9 ± 4.4 years). All patients in the high-, low-, and normal-angle groups had a skeletal Class I relationship (ANB = 3.2 ± 1.5°, 2.9 ± 1.5°, and 3.3 ± 1.4°, respectively), and the SN-MP angle was 42.2 ± 3.3°, 23.8 ± 2.5°, and 32.5 ± 3.8°, respectively.
Craniofacial features of the patients were examined by four angular (SNA, SNB, ANB, and SN-MP) and two linear (Co-A and Co-Gn) measurements performed on lateral cephalometric films (Figure 1); bizygomatic width (the distance between zygomatic right and zygomatic left) was measured on frontal radiographs (Figure 2) obtained from CBCT using Simplant Pro software, version 13 (Materialise Dental, Leuven, Belgium).
Figure 1.
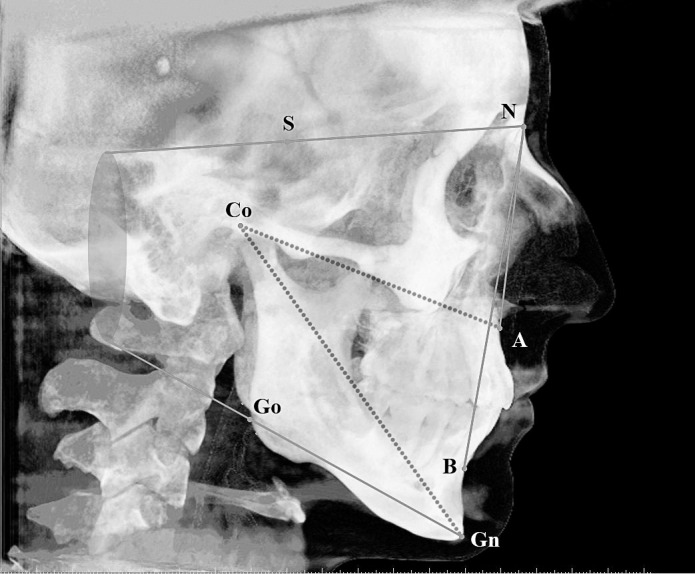
Cephalometric measurements used in the present study: SNA (°), SNB (°), ANB (°), SN-MP (°), Co-A (mm), and Co-Gn (mm).
Figure 2.
Measurement used on the frontal radiographs (bizygomatic width) obtained from CBCT.
All images were taken in a standard supine position (scanning time = 14–18 seconds; collimation height = 13 cm; exposure time = 3.6 seconds; and voxel size = 0.3 mm3) using the same device (NewTom 5G, QR, Verona, Italy). Patients were asked to bite in maximum intercuspation but not to swallow and not to move their head or tongue while the CBCT was being taken.
The 3D images were transformed to DICOM (Digital Imaging and Communications in Medicine) and handled by Simplant Pro software. Each 3D-rendered image was then reoriented using the Frankfort horizontal plane as the horizontal reference plane. The sagittal reference plane was constructed from the nasion and mid-orbital point and perpendicular to the horizontal reference plane. The axial plane was constructed from the nasion and perpendicular to the horizontal and sagittal planes. The borderlines of the pharyngeal airway consisted of the anterior border, that is, the vertical plane through the posterior nasal spine (PNS) perpendicular to the sagittal plane; the posterior border, the posterior wall of the pharynx; and the inferior border, a plane tangent to the most caudal medial projection of the third cervical vertebra perpendicular to the sagittal plane.8 The plane perpendicular to the sagittal plane through the posterior nasal spine and lower medial border of the first cervical vertebra divided the pharyngeal airway into two segments, the upper (nasopharyngeal airway) and lower (oropharyngeal airway) compartments (Figure 3). The previously described 3D volumetric measurements were blindly and randomly analyzed with the Simplant software by an experienced maxillofacial radiologist who had no knowledge of the patient's vertical pattern.
Figure 3.
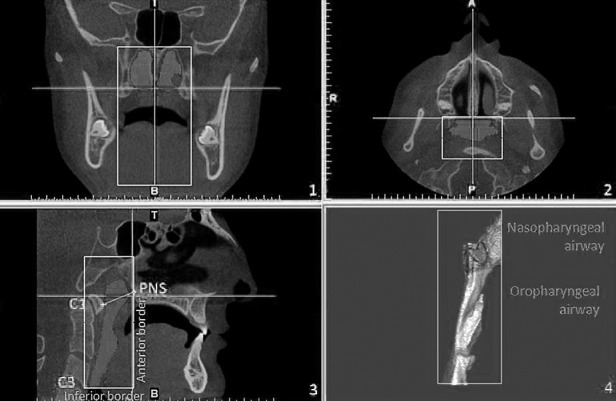
Three-dimensional measurement of pharyngeal airway volumes.
Statistical Analysis
To determine the random error associated with digitizing and measurements, 30 radiographs (10 radiographs from each group) were randomly selected. All procedures, such as landmark identification, tracing, and 3D measurements, were repeated 2 weeks after the first examination by the same investigators (3D volumetric measurements by Dr Sekerci; craniofacial measurements by Dr Buyuk) without knowledge the first measurements. Pearson correlation coefficients were performed to assess the reliability of the measurements. In addition, the differences between the two readings for both volumetric and cephalometric measurements were tested to estimate the systematic error by means of a paired t-test.
Because the normality test of Kolmogorov Smirnov showed normal distribution of the data, the comparisons between the groups were analyzed with parametric tests. Distribution of gender and chronological age in each vertical group was tested by means of χ2 and one-way analysis of variance (ANOVA) tests, respectively. A Student t-test was performed to examine gender differences for airway volumes. One-way ANOVA was performed to test potential differences among groups and post hoc Tukey honestly significant difference for individual differences. All statistical analyses were performed using the SPSS software package program (SPSS for Windows 98, version 11.0, SPSS Inc, Chicago, Ill) at P < .05.
RESULTS
The coefficients of reliability of the craniofacial and pharyngeal airway volume measurements were above 0.991 and 0.993, respectively. The results of the paired t-test showed that there were no significant differences between the first and second examinations for measurements, confirming that they were free of systematic error (P > .05).
Demographic values (gender distribution and chronological ages) of the vertical groups are shown in Table 1. All vertical groups were matched on chronological age and gender distribution as tested by one-way ANOVA and Pearson χ2, respectively (P > .05).
Table 1.
Descriptive Statistics for the Groups
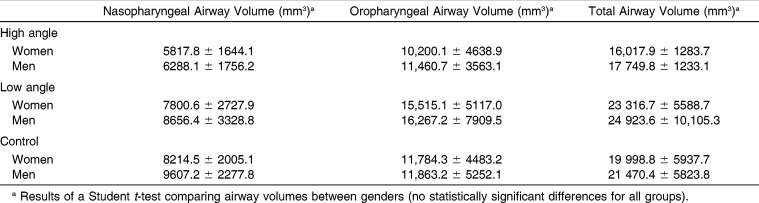
Comparisons of nasopharyngeal, oropharyngeal, and total airway volumes between genders are shown in Table 2. No statistically significant differences were found between genders, and thus, the subjects were pooled for further statistical comparisons.
Table 2.
Descriptive Statistics and Comparisons of Airway Volumes Between Genders
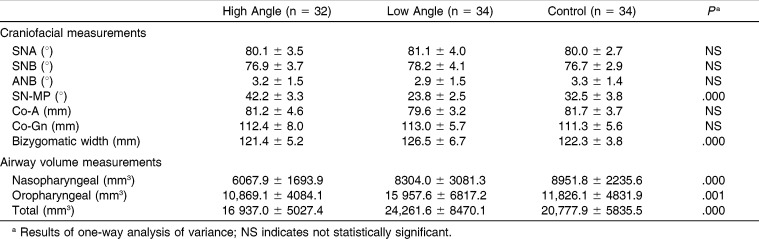
According to the results of ANOVA, statistically significant differences were found for SN-MP angle (P < .001), bizygomatic width (P < .001), nasopharyngeal (P < .001), oropharyngeal (P < .01), and total airway volumes (P < .001) among the vertical groups. There were no statistically significant differences for SNA, SNB, ANB, Co-A, and Co-Gn measurements (P > .05) (Table 3). The bizygomatic width was highest in low-angle group (mean = 126.5 ± 6.7 mm) compared with the normal-angle group (mean = 122.3 ± 3.8 mm; P < .01) and the high-angle group (mean = 121.4 ± 5.2 mm; P < .001). No statistically significant difference was found between the high- and normal-angle groups (P > .05).
Table 3.
Descriptive Statistics and Comparisons of Craniofacial and Airway Volume Measurements Among Groups
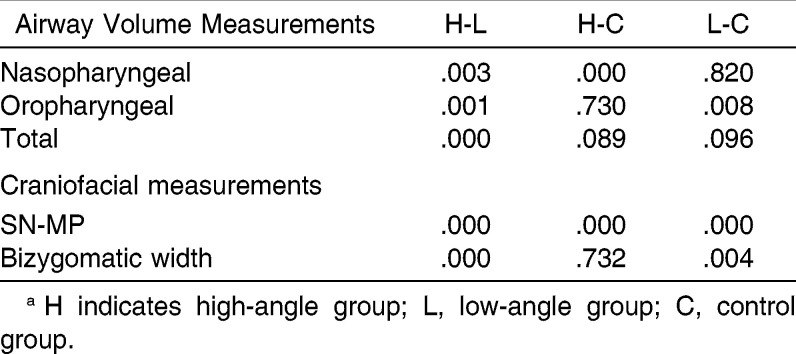
Nasopharyngeal airway volume in the high-angle group (6067.9 ± 1693.9 mm3) was significantly lower than that of the low-angle group (8304.0 ± 3081.3 mm3; P = .003) and the normal-angle group (8951.8 ± 2235.6 mm3; P < 0.001). Oropharyngeal airway volume was highest in the low-angle group (15,957.6 ± 6817.2 mm3) and significantly decreased in the control group (11,826.1 ± 4831.9 mm3; P = 0.008) and the high-angle group (10,869.1 ± 4084.1 mm3; P = 0.001). Total airway volume was highest in the low-angle group (24,261.6±8470.1 mm3) and lowest in the high-angle group (16,937.0 ± 5027.4 mm3) (P <0.001) (Tables 3 and 4).
Table 4.
Multiple Comparisons of Airway Volume Measurements Among Groups by Means of the Tukey Honestly Significant Difference Testa
DISCUSSION
According to Aras et al.,23 cephalometric techniques to evaluate pharyngeal airway are insufficient compared with CT. Because of the high doses of radiation with CT, however, the use of CBCT was suggested for the assessment of pharyngeal airway volume. The coefficients of reliability of the pharyngeal airway volume measurements in the present study were above 0.993, agreeing with the findings of previous studies12,13,16,25,26 using CBCT.
Several two-dimensional and 3D studies8,9,12–14,16,17,24–26 have been performed to evaluate the relationship between airway and sagittal skeletal patterns. It was observed that patients with skeletal Class I and III had significantly larger pharyngeal airway volumes than patients with Class II.13,14,16 Although the ANB angle might be affected by the anteroposterior position of the nasion relative to the maxilla and mandible,11,13,14,16 it was the most commonly used cephalometric measurement to determine sagittal relationship of the jaws.8–13,15,17,26 Thus, we decided to use the ANB angle to define the sagittal relationship of the jaws and exclude its effect on pharyngeal airway volume.
In the present study, men had larger nasopharyngeal, oropharyngeal, and total airway volumes than women. However, this difference was not statistically significant (P > .05). No significant gender difference was found in previous studies,8,13,17 confirming this finding. However, Chiang et al.27 found a significant gender-related difference for the airway volume. Gender and chronological age distribution and/or the use of different anatomic landmarks to define the airway might be possible factors affecting this difference. In our study, the groups were well matched regarding chronological age and gender distribution as tested by one-way ANOVA and χ2 tests (P > .05).
Anteroposterior relationships of the maxilla and mandible in the present study were within normal limits, and no statistically significant differences were observed for SNA, SNB, ANB, Co-A, and Co-Gn among the high-, low-, and normal-angle groups, eliminating the possible effects of the sagittal skeletal pattern.8,9,12–14 On the other hand, significant differences were found for SN-MP angle and bizygomatic width among the groups. The difference observed for SN-MP was expected as the subjects were divided into three groups according to this criterion. Recently, size of the face measured as bizygomatic width on frontal radiographs was found to be significantly correlated with inferior, superior, and total airway volumes in a study by Grauer et al.8 In the present study, subjects in the low-angle group had the largest bizygomatic width (P < .01), and this factor may be a possible explanations as to why patients in the low-angle group had larger pharyngeal airway volumes than patietnts in the high- and normal-angle groups. Although no statistically significant difference was present for the SNB angle among the groups, it was lowest in the high-angle group. Another explanation for the airway differences among the groups might be the retruded mandible in the high-angle group. According to Kim et al.,9 preadolescent children with retruded mandibles had decreased total pharyngeal airway volumes.
After conducting a bibliographic search in PubMed's Medline using the keywords CBCT, pharyngeal airway, vertical skeletal pattern, 3D analysis, and airway, we found only one study to date (one by Grauer et al.8) that presented pharyngeal airway volume comparisons among vertical growth patterns using CBCT. The authors8 stated that there were no significant differences in the inferior, superior, and total airway volumes among the long, short, and normal groups of nongrowing patients. However, we found significant differences for all pharyngeal airway volume measurements among the vertical groups. The technique to define airway volumes and chronological age distributions were similar in both studies. One of the differences, however, was that the previous study included fewer patients in groups than we did; in addition, the previous study used different parameters (facial index values) to define the vertical groups. However, the most important difference, in our opinion, might be the sagittal relationship of the patients. As stated by the authors,8 many patients with long faces were also classified as skeletal Class II or Class III, whereas those with short faces tended to be classified as skeletal Class I. It was understood that a sagittal standardization was not performed to eliminate the effects of sagittal relationship of the jaws. In light of the findings of previous studies,9,12–14,16,17 significant differences for pharyngeal airway volumes were present among sagittal skeletal patterns.
Within the limitations of the present study, pharyngeal airway volumes were lowest in the high-angle group. Considering this finding, orthodontists should define the best treatment that should have positive effects on the pharyngeal airway.
CONCLUSIONS
The null hypothesis was rejected. Significant differences were found for pharyngeal airway volumes among different vertical skeletal patterns using CBCT.
Nasopharyngeal, oropharyngeal, and total airway volumes were lowest in the high-angle group; thus, the appropriate treatment of malocclusion in those patients should also have positive effects on pharyngeal airway.
Oropharyngeal and total airway volumes were the highest in the low angle group.
REFERENCES
- 1.Nielsen IL. Vertical malocclusions: etiology, development, diagnosis and some aspects of treatment. Angle Orthod. 1991;61:247–260. doi: 10.1043/0003-3219(1991)061<0247:VMEDDA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Schudy FF. The rotation of the mandible resulting from growth: its implications in orthodontic treatment. Angle Orthod. 1965;35:36–50. doi: 10.1043/0003-3219(1965)035<0036:TROTMR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Isaacson JR, Isaacson RJ, Speidel TM, Worms FW. Extreme variation in vertical facial growth and associated variation in skeletal and dental relations. Angle Orthod. 1971;41:219–229. doi: 10.1043/0003-3219(1971)041<0219:EVIVFG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Meyer C. On adenoid vegatation in the naso-pharyngeal cavity: their pathology, diagnosis and treatment. Med Chir Trans. 1872;53:191–215. doi: 10.1177/095952877005300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNamara JA. Influence of respiratory pattern on craniofacial growth. Angle Orthod. 1981;51:269–300. doi: 10.1043/0003-3219(1981)051<0269:IORPOC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Kerr WJ. The nasopharynx, face height, and overbite. Angle Orthod. 1985;55:31–36. doi: 10.1043/0003-3219(1985)055<0031:TNFHAO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Joseph AA, Elbaum J, Cisneros GJ, Eisig SB. A cephalometric comparative study of the soft tissue airway dimensions in persons with hyperdivergent and normodivergent facial patterns. J Oral Maxillofac Surg. 1998;56:135–139. doi: 10.1016/s0278-2391(98)90850-3. [DOI] [PubMed] [Google Scholar]
- 8.Grauer D, Cevidanes LS, Styner MA, Ackerman JL, Proffit WR. Pharyngeal airway volume and shape from cone-beam computed tomography: relationship to facial morphology. Am J Orthod Dentofacial Orthop. 2009;136:805–814. doi: 10.1016/j.ajodo.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YJ, Hong JS, Hwang YI, Park YH. Three-dimensional analysis of pharyngeal airway in preadolescent children with different anteroposterior skeletal patterns. Am J Orthod Dentofacial Orthop. 2010;137:e301–e311. doi: 10.1016/j.ajodo.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Zhong Z, Tang Z, Gao X, Zeng XL. A comparison study of upper airway among different skeletal craniofacial patterns in nonsnoring Chinese children. Angle Orthod. 2010;80:267–274. doi: 10.2319/030809-130.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ucar FI, Uysal T. Orofacial airway dimensions in subjects with Class I malocclusion and different growth patterns. Angle Orthod. 2011;81:460–468. doi: 10.2319/091910-545.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El H, Palomo JM. An airway study of different maxillary and mandibular sagittal positions. Eur J Orthod. 2013;35:262–270. doi: 10.1093/ejo/cjr114. [DOI] [PubMed] [Google Scholar]
- 13.El H, Palomo JM. Airway volume for different dentofacial skeletal patterns. Am J Orthod Dentofacial Orthop. 2011;139:e511–e521. doi: 10.1016/j.ajodo.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Alves M, Jr, Franzotti ES, Baratieri C, Nunes LK, Nojima LI, Ruellas AC. Evaluation of pharyngeal airway space amongst different skeletal patterns. Int J Oral Maxillofac Surg. 2012;41:814–819. doi: 10.1016/j.ijom.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Oz U, Orhan K, Rubenduz M. Two-dimensional lateral cephalometric evaluation of varying types of Class II subgroups on posterior airway space in postadolescent girls: a pilot study. J Orofac Orthop. 2013;74:18–27. doi: 10.1007/s00056-012-0121-0. [DOI] [PubMed] [Google Scholar]
- 16.Claudino LV, Mattos CT, Ruellas AC, Sant' Anna EF. Pharyngeal airway characterization in adolescents related to facial skeletal pattern: a preliminary study. Am J Orthod Dentofacial Orthop. 2013;143:799–809. doi: 10.1016/j.ajodo.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Zheng ZH, Yamaguchi T, Kurihara A, Li HF, Maki K. Three-dimensional evaluation of upper airway in patients with different anteroposterior skeletal patterns. Orthod Craniofac Res. 2014;17:38–48. doi: 10.1111/ocr.12029. [DOI] [PubMed] [Google Scholar]
- 18.Aboudara C, Nielsen I, Huang JC, Maki K, Miller AJ, Hatcher D. Comparison of airway space with conventional lateral headfilms and 3-dimensional reconstruction from cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2009;135:468–479. doi: 10.1016/j.ajodo.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 19.Lowe AA, Fleetham JA, Adachi S, Ryan CF. Cephalometric and computed tomographic predictors of obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop. 1995;107:589–595. doi: 10.1016/s0889-5406(95)70101-x. [DOI] [PubMed] [Google Scholar]
- 20.Quereshy FA, Savell TA, Palomo JM. Applications of cone beam computed tomography in the practice of oral and maxillofacial surgery. J Oral Maxillofac Surg. 2008;66:791–796. doi: 10.1016/j.joms.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Pandis N. Sample calculations for comparison of 2 means. Am J Orthod Dentofacial Orthop. 2012;141:519–521. doi: 10.1016/j.ajodo.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Celikoglu M, Kazanci F, Miloglu O, Oztek O, Kamak H, Ceylan I. Frequency and characteristics of tooth agenesis among an orthodontic patient population. Med Oral Patol Oral Cir Bucal. 2010;15:e797–e801. doi: 10.4317/medoral.15.e797. [DOI] [PubMed] [Google Scholar]
- 23.Aras I, Olmez S, Dogan S. Comparative evaluation of nasopharyngeal airways of unilateral cleft lip and palate patients using three-dimensional and two-dimensional methods. Cleft Palate Craniofac J. 2012;49:e75–e81. doi: 10.1597/12-004. [DOI] [PubMed] [Google Scholar]
- 24.Celikoglu M, Nur M, Kilkis D, Sezgin OS, Bayram M. Mesiodistal tooth dimension and anterior and overall Bolton ratios evaluated by cone beam computed tomography. Aust Orthod J. 2013;29:153–158. [PubMed] [Google Scholar]
- 25.Nur M, Kayipmaz S, Bayram M, Celikoglu M, Kilkis D, Sezgin OS. Conventional frontal radiographs compared with frontal radiographs obtained from cone beam computed tomography. Angle Orthod. 2012;82:579–584. doi: 10.2319/080311-488.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu Allhaija ES, Al-Khateeb SN. Uvulo-glosso-pharyngeal dimensions in different anteroposterior skeletal patterns. Angle Orthod. 2005;75:1012–1018. doi: 10.1043/0003-3219(2005)75[1012:UDIDAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Chiang CC, Jeffres MN, Miller A, Hatcher DC. Three-dimensional airway evaluation in 387 subjects from one university orthodontic clinic using cone beam computed tomography. Angle Orthod. 2012;82:985–992. doi: 10.2319/122811-801.1. [DOI] [PMC free article] [PubMed] [Google Scholar]