Abstract
The transcription factor ets-2 was phosphorylated at residue threonine 72 in a colony-stimulating factor 1 (CSF-1)- and mitogen-activated protein kinase-independent manner in macrophages isolated from motheaten-viable (me-v) mice. The CSF-1 and ets-2 target genes coding for Bcl-x, urokinase plasminogen activator, and scavenger receptor were also expressed at high levels independent of CSF-1 addition to me-v cells. Akt (protein kinase B) was constitutively active in me-v macrophages, and an Akt immunoprecipitate catalyzed phosphorylation of ets-2 at threonine 72. The p54 isoform of c-jun N-terminal kinase–stress-activated kinase (JNK- SAPK) coimmunoprecipitated with Akt from me-v macrophages, and treatment of me-v cells with the specific phosphatidylinositol 3-kinase inhibitor LY294002 decreased cell survival, Akt and JNK kinase activities, ets-2 phosphorylation, and Bcl-x mRNA expression. Therefore, ets-2 is a target for phosphatidylinositol 3-kinase–Akt–JNK action, and the JNK p54 isoform is an ets-2 kinase in macrophages. Constitutive ets-2 activity may contribute to the pathology of me-v mice by increasing expression of genes like the Bcl-x gene that promote macrophage survival.
Macrophage colony-stimulating factor 1 (CSF-1) and its cognate receptor tyrosine kinase c-fms control the proliferation, differentiation, and survival of cells of the mononuclear phagocyte cell lineage by activating multiple signaling pathways (reviewed in reference 35). One effect of these signaling events in macrophages is the stable, persistent expression of specific genes. For example the urokinase plasminogen activator (uPA) gene (41), the scavenger receptor A (SR) gene (48), and the Bcl-x gene (38) are all targets of CSF-1 action. The ETS family member ets-2 regulates these three CSF-1 target genes (38, 48, 49).
ets-2 is activated by ras-dependent phosphorylation of threonine residue 72, and CSF-1–c-fms signaling leads to persistent phosphorylation of this site (16, 49). Activation of the ras–raf–MEK-1–Erk protein kinase cascade by CSF-1 leads to rapid and persistent phosphorylation of ets-2 in fibroblasts engineered to express exogenous c-fms as well as in macrophage cell lines or primary bone marrow-derived macrophages (BMMs). The MEK inhibitor PD98059 abrogates ets-2 phosphorylation in response to CSF-1–c-fms signaling in the fibroblast model system (16).
One unanswered question is the precise role of ets-2 in CSF-1–c-fms signaling. Does activation of ets-2 by CSF-1 contribute to mitogenic growth, differentiation, or cell survival? Expression of a dominant-negative ets-2 protein in macrophages in transgenic mice resulted in accelerated apoptosis following CSF-1 deprivation (24). In the macrophage cell line BAC1.2F5, overexpression of ets-2 promoted survival of cells in the absence of CSF-1 (38). These observations indicate that ets-2 may be involved in CSF-1-dependent survival of macrophages.
The mouse motheaten (me) and motheaten-viable (me-v) mutants are the result of point mutations that affect splicing of transcripts encoded by the src-homology 2 tyrosine phosphatase 1 gene (SHP-1, also termed hematopoietic cell phosphatase) and lead to expression of proteins with greatly diminished tyrosine phosphatase activity (39, 44). These mutant mice accumulate massive numbers of macrophages and neutrophils in the peripheral tissues, including skin, spleen and lung, and subsequently succumb to an interstitial pneumonia (reviewed in reference 7). The me-v mutant mice also develop an inflammatory disease resembling rheumatoid arthritis (30). SHP-1 apparently plays a central role in cell signaling events that regulate macrophage-dependent inflammatory responses.
SHP-1 may be a negative regulator of CSF-1 signaling (10). SHP-1 is phosphorylated on tyrosine following CSF-1 stimulation of macrophages, but does not directly bind to ligand-activated c-fms (50). CSF-1 treatment of primary macrophages obtained from me mice is reported to result in c-fms hyperphosphorylation, increased phosphorylation of signaling molecules known to be downstream of c-fms, and an increased rate of macrophage proliferation (10). However, another group reported that CSF-1 mitogenic signaling is unaffected in macrophages obtained from me or me-v mice, but that granulocyte-macrophage (GM)-CSF mitogenic signaling is hyperactivated in such macrophages (23). SHP-1 forms a complex with Janus kinase family members, including JAK2 (22), and also forms a complex with two members of a family of receptors involved in negative regulation in the immune system, PIR-B (p91A) and SHPS-1 (BIT) (6, 42, 46). Integrin-mediated adhesion is reported to be altered in me-v macrophages, and this change in cell adhesion correlates with a two- to fivefold increase in phosphatidylinositol 3-kinase (PI 3-kinase) activity in me-v cells compared to wild-type cells (33).
Interestingly, it has been reported that CSF-1 does not activate MEK-1 and Erks in primary macrophages obtained from mice homozygous for the me-v mutation, implying a positive role for SHP-1 in CSF-1 activation of MEK-1 and Erks (31). This observation suggests that studying the me-v mouse model might reveal MEK/Erk-independent pathways leading to ets-2 phosphorylation and activation in macrophages. To test this hypothesis, the phosphorylation of ets-2 in primary macrophages derived from me-v mice was analyzed.
The studies reported here provide evidence for constitutive phosphorylation of ets-2 by the PI 3-kinase/Akt pathway in me-v macrophages. These studies indicate that the p54 isoform of JNK (SAPK) can be found in a complex with Akt in macrophages and that p54 JNK likely is an ets-2 kinase active in me-v macrophages. Phosphorylation of ets-2 correlated with expression of previously identified CSF-1 target genes, including the antiapoptotic Bcl-x gene (38). In transient transfection assays, the promoter for the mouse Bcl-x gene was superactivated over 90-fold by the combination of ets-2 and a membrane-targeted form of Akt. Phosphorylation of ets-2 and activation of target gene expression were found to correlate with increased me-v macrophage survival. In me-v macrophages, both ets-2 phosphorylation and CSF-1-independent cell survival depend largely on a constitutive PI 3-kinase/Akt pathway. These results indicate that constitutive ets-2 activity may contribute to the pathology of me-v mice by regulating expression of genes that promote cell survival in macrophages.
MATERIALS AND METHODS
Cell culture, Northern analysis, and transfections.
The methods for culturing RAW264 cells and for DNA-mediated transfection have been described previously (16). RNA was isolated and analyzed by Northern blotting as previously described (16). The method for deriving BMMs has been described previously (41). Briefly, bone marrow cells were cultured in RPMI containing 5% heat-denatured fetal calf serum and supplemented with 50 ng of CSF-1 per ml for 5 days. At this point, BMMs were deprived of CSF-1 for 8 to 24 h and then restimulated with CSF-1 (50 ng/ml) for various times as indicated. For experiments with the PI 3-kinase inhibitor LY294002 (AG Scientific, Inc., San Diego, Calif.) cells were treated with 100 μM drug for 15 min prior to stimulation.
The me-v mice were obtained from Jackson Laboratories. The mutation is in the background C57BL/6J, and mice of this strain were used as controls (wild type). All mice were genotyped by a method involving PCR amplification of the region containing the me-v point mutation (39) directly from genomic DNA. The amplified DNA was hybridized to labeled 15-mers representing the wild-type sequence and the me-v lesion. DNA from wild-type or homozygous mutant mice hybridized to either one of these probes, respectively, while DNA from mice heterozygous for the mutation hybridized to both.
Immunohistochemistry.
BMMs were cultured in Lab-Tek two-well chamber slides (NUNC). Cells were deprived of CSF-1 for 24 h and stimulated for 8 h with 50 ng of CSF-1 per ml. Cells were washed in Tris-buffered saline (TBS) (50 mM Tris [pH 7.5], 150 mM NaCl), fixed for 30 min in 4% paraformaldehyde, washed three times with TBS, and permeabilized for 5 min with TBS containing 1% Triton X-100. Endogenous peroxide activity was blocked by incubation with 0.1% hydrogen peroxide and 10% methanol in TBS. Cells were washed and treated with 2% normal goat serum in TBS. The affinity-purified phosphothreonine 72-specific anti-ets-2 antibody (16) was used at a 1:75 dilution for 2 h at room temperature and followed by three washes with TBS containing 0.2% NP-40. Antirabbit immunoglobulin G conjugated with biotin (Boehringer Mannheim) was incubated for 1 h at a 1:200 dilution and washed as described above. A 1:2,000 dilution of streptavidin-horseradish peroxidase (Boehringer Mannheim) was used for 1 h and washed in TBS. Antibody binding was detected with the metal-enhanced 3,3′-diaminobenzidine substrate kit (Pierce Biochemicals).
Immune kinase assays and Western analysis.
Antibodies for phosphotyrosine residues, Erks, Akt, and JNK were purchased commercially (Upstate Biotechnology or Santa Cruz Biotechnology). Akt assays were also confirmed with an antibody kindly provided by Phil Tsichlis. The procedures for Erk immune kinase assays have been described previously (16). The procedures for Akt kinase assays have been described previously (21). The substrates employed for Erk, Akt, and JNK assays were a recombinant ets-2 protein corresponding to amino acids 60 to 167 (16), histone H2B (Sigma), and a glutathione S-transferase–jun fusion protein representing amino acids 1 to 79 of c-jun (25). Phosphorylated products produced in immune kinase assays were quantitated with a Molecular Dynamics PhosphorImager. Western analysis was performed as previously described (16) with a Lumi-Imager for quantification (Boehringer Mannheim).
Cell apoptosis and viability assays.
Three assays were used to determine cell apoptosis and cell viability. The first was a flow cytometry assay that measured fragmented DNA indicative of apoptotic cells (reviewed in reference 11). Briefly, cells were fixed in 70% ethanol, fragmented DNA was extracted with phosphate-buffered saline containing 1 mg of RNase per ml, 10 mM sodium citrate, and 0.1% Triton X-100 (30 min at 37°C), and total DNA was stained with propidium iodide (50 μg/ml) for 15 min. Samples were analyzed by flow cytometry (Becton-Dickinson FACSCalibur). The peak of DNA appearing just before the G1-specific peak (the sub-G0 peak, or hypodiploid peak) was quantitated by using ModFit LT; version 2.0 software (Verity Software House, Topsham, Maine). The ratio of this sub-G0 DNA peak to the total DNA recovered is equivalent to the fraction of apoptotic cells. The second assay depended on the striking change in nuclear morphology that occurs in macrophages undergoing apoptosis. Cells were fixed with 3.7% paraformaldehyde in phosphate-buffered saline and stained with 10 μM bis-benzamide (Sigma) for 5 min at room temperature. Nuclear morphology was examined by fluorescent microscopy with an Eclipse E800 microscope (Nikon). Images were captured with MicroMax camera (Princeton Instruments, Inc.). Two hundred to 300 cells were counted and scored as viable or apoptotic based on nuclear morphology, and each experiment was performed in duplicate. The third assay employed was a trypan blue dye exclusion assay to measure cell viability. The results from all three assays were identical.
RESULTS
Phosphorylation of ets-2 and expression of ets-2 target genes in me-v macrophages are independent of exogenous CSF-1 and Erk activity.
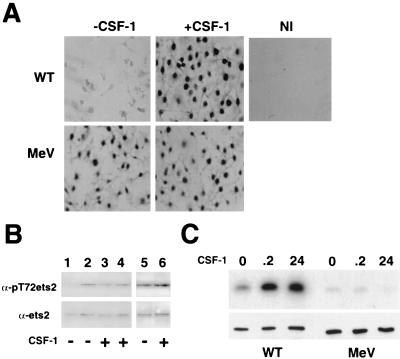
CSF-1 stimulates phosphorylation of ets-2 at threonine residue 72 via the raf/MEK/Erk pathway (16). However, the mitogen-activated protein (MAP) kinases Erk-1 and Erk-2 are reported not to be responsive to CSF-1 stimulation of macrophages isolated from me-v mice (31). To examine whether Erk-independent phosphorylation of ets-2 can occur in macrophages, the phosphorylation of ets-2 at threonine residue 72 was monitored in primary macrophages isolated from mice homozygous for the me-v mutation. For these experiments, a polyclonal antibody that specifically recognizes the phosphothreonine 72-modified version of ets-2 was employed (16). Immunohistochemistry with this antibody demonstrated that low levels of phosphorylated ets-2 were detected in wild-type BMMs deprived of CSF-1, but that nuclear phospho-ets-2 could be readily detected in these cells treated with CSF-1 (Fig. 1A). If nonimmune serum was substituted in the analysis, no histochemical signal was detected (Fig. 1A). Experiments using BMMs derived from me-v mice produced an unexpected result. In these cells, ets-2 was expressed and phosphorylated in a manner independent of addition of exogenous CSF-1 (Fig. 1A).
FIG. 1.
ets-2 is persistently phosphorylated at residue threonine 72 in me-v macrophages. (A) Detection of phosphorylated ets-2 by immunohistochemistry. Cells were grown for 24 h without CSF-1, fixed, and incubated with ets-2 phosphothreonine antibody (−CSF-1) or stimulated for 8 h with 50 ng of CSF-1 per ml prior to fixation and incubation with either the same ets-2 antibody (+CSF-1) or nonimmune serum (NI). The dark nuclear staining indicates reactivity for ets-2 phosphothreonine 72 antibody. WT, wild type. (B) ets-2 phosphorylation detected in nuclear extracts by Western analysis with the same ets-2 antibody as in panel A (top panel) or an antibody that detects ets-2 regardless of phosphorylation status (bottom panel). Extracts were prepared from BMMs derived from wild-type cells (lanes 1 and 3), from BMMs pooled from three me-v mice (lanes 2 and 4), or from spleen macrophages pooled from three me-v mice (lanes 5 and 6). Cells were grown without CSF-1 for 12 h (lanes 1 and 2) or 48 h (lanes 5) or continuously with 50 ng of CSF-1 per ml (lanes 3, 4, and 6). (C) BMMs derived from wild-type or me-v mice, as indicated, were grown without CSF-1 for 16 h and then stimulated with 50 ng of CSF-1 per ml for the times indicated. Erk immune kinase assays were performed by using the ets-2 “pointed” domain recombinant protein substrate. The phosphorylated ets-2 substrate was detected by autoradiography (top panel), while Western blots using an anti-Erk antibody demonstrated that equal amounts of Erks were immunoprecipitated (bottom panel).
In order to confirm these results, extracts prepared from both wild-type and me-v macrophages that had been deprived of CSF-1 for 12 h or grown continuously in the presence of CSF-1 were analyzed by Western blotting with the discriminating anti-phospho-ets-2 antibody or a nondiscriminating ets-2 antibody (Fig. 1B, top and bottom panels, respectively). For wild-type cells, the removal of CSF-1 for 12 h resulted in a slight 1.8-fold decrease in ets-2 steady-state levels and a more significant 8-fold decrease in levels of phosphothreonine 72 ets-2 (Fig. 1B, compare lanes 1 and lane 3). In contrast, phosphorylated ets-2 levels in me-v BMMs were insensitive to withdrawal of CSF-1 for 12 h (Fig. 1B, lanes 2 and 4). In addition, ets-2 was phosphorylated in primary macrophages derived from spleens of me-v mice that had been cultured in the absence of CSF-1 for 48 h (Fig. 1B, lanes 5 and 6). Even after 72 h of CSF-1 withdrawal, the amount of phosphorylated ets-2 detected in me-v BMMs did not change (data not shown). The level of phosphorylated ets-2 in me-v macrophages deprived of CSF-1 was equivalent to the levels observed in CSF-1-treated wild-type cells (Fig. 1B, compare lanes 2 and 3).
Immune kinase assays using Erk-specific antibodies corroborated that Erks were not transiently activated following 12 min of CSF-1 treatment of BMMs derived from me-v mice (Fig. 1C, left panel, wild-type BMMs; right panel, me-v BMMs) (31). Additionally, persistent activation of Erks after 24 h of CSF-1 stimulation (16) was not observed in me-v cells (Fig. 1C).
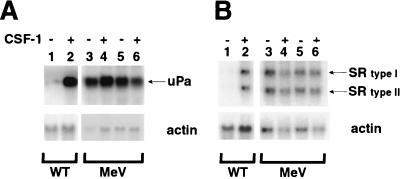
Two well-defined target genes of the CSF-1/ets-2 pathway in macrophages are those coding for uPA (16, 41) and SR (48). As demonstrated in Fig. 2, the levels of expression of uPA and SR mRNAs were approximately eightfold higher in wild-type BMMs grown in the presence of CSF-1 than those in cells deprived of CSF-1 (Fig. 2A and B, lane 2 versus lane 1). In me-v BMMs, uPA and SR mRNAs were expressed at high levels whether or not CSF-1 was present (Fig. 2A and B, lanes 3 and 4). Levels of expression of uPA and SR mRNAs were also found to be CSF-1 independent in primary spleen macrophages cultured from me-v mice (Fig. 2A and B, lanes 5 and 6). The phosphorylation of ets-2 in me-v macrophages correlated with increased expression of target genes.
FIG. 2.
The ets-2 target genes coding for uPa and SR are expressed in a CSF-1-independent fashion in me-v macrophages. Pooled macrophages (three mice) were grown in medium lacking CSF-1 for 24 h (−CSF) or stimulated with 50 ng of CSF-1 per ml for 8 h following cytokine starvation (+CSF). Total RNA was prepared and analyzed by Northern blotting. (A) Expression of uPA mRNA in wild-type (WT) BMMs (lanes 1 and 2), me-v (MeV) BMMs (lane 3 and 4), or me-v spleen macrophages (lanes 5 and 6). The arrow indicates the position of the 2.2-kb mRNA for uPA. (B) Expression of SR mRNA in wild-type BMMs (lanes 1 and 2), me-v BMMs (lane 3 and 4), or me-v spleen macrophages (lanes 5 and 6). The arrows indicate the position of the SR type I or type II mRNA (4 and 3.2 kb, respectively). Blots in both panels were reprobed with a mouse γ-actin probe as a control for sample loading (bottom panels).
An Akt immunoprecipitate catalyzes ets-2 phosphorylation in vitro, and the PI 3-kinase inhibitor LY294002 diminishes levels of phosphorylated ets-2 in vivo.
The results presented above indicated that Erk-independent phosphorylation and activation of ets-2 occurred in me-v macrophages. One potential candidate for the Erk-independent pathway is the PI 3-kinase/Akt pathway. The PI 3-kinase/Akt pathway can be activated in a ras-dependent or ras-independent fashion (17, 34) and has been implicated in growth, differentiation, and survival pathways in many cell types, including myeloid cells (8, 32). Additionally, a recent report demonstrated that membrane-associated PI 3-kinase levels were two- to fivefold higher in me-v macrophages than in wild-type cells (33), a result that we have reproduced in our laboratory (data not shown).
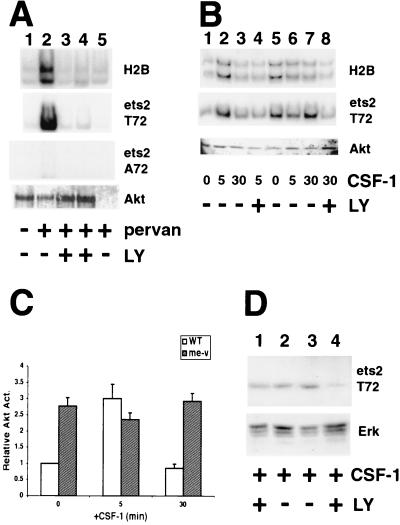
As a first step, Akt immunoprecipitates prepared from the macrophage cell line RAW264 were assayed for ets-2 kinase activity (Fig. 3A). For these experiments, either the characterized Akt substrate histone H2B or a recombinant ets-2 polypeptide corresponding to the “pointed” domain (amino acids 67 to 170) (16) was used as a substrate. In immune kinase assays, Akt immunoprecipitates were able to phosphorylate either histone H2B or the ets-2 substrate following treatment of cells with the cell-permeable tyrosine phosphatase inhibitor pervanadate (3) (Fig. 3A, lane 2 versus lane 1). Phosphorylation of both histone H2B and ets-2 substrates was inhibited by inclusion of the specific PI 3-kinase inhibitor LY294002 in addition to pervanadate (47) (Fig. 3A, lanes 3 and 4, 50 and 100 μM, respectively).
FIG. 3.
An Akt immunoprecipitate catalyzes phosphorylation of ets-2 at position threonine 72, and LY294002 inhibits ets-2 phosphorylation in me-v macrophages. (A) Akt immune kinase assays performed with RAW264 cells using histone H2B, ets-2 T72, and ets-2 A72 as substrates (panels as indicated). Akt was isolated from 107 cells grown in normal medium (lane 1), treated for 5 min with 300 μM pervanadate (pervan) (lane 2), or treated for 5 min with both 300 μM pervanadate and 50 or 100 μM PI 3-kinase inhibitor LY294002 (LY) (lanes 3 and 4, respectively). The sample in lane 5 is a control in which Akt antibody was not included. The bottom panel is a Western blot performed with the Akt antibody to demonstrate that Akt was present in all samples. (B) Akt immune kinase assays performed on 107 wild-type (lanes 1 to 4) or me-v primary macrophages (lanes 5 to 8) with histone H2B or ets-2 substrates (as labeled). Cells were grown without CSF-1 for 24 h and then stimulated with 50 ng of CSF-1 per ml for the times indicated. LY294002 (100 μM) was included 30 min prior to addition of CSF-1 (lanes 4 and 8). The bottom panel is a Western blot of the immunoprecipitated material probed with anti-Akt antibody. (C) The average of four independent Akt immune kinase assays performed on wild-type (WT [open bars]) or me-v (shaded bars) macrophages with the ets-2 substrate (including the experiment shown in 3B). The results are presented relative to wild-type samples grown in the absence of CSF-1. The error bars indicate the standard deviation. (D) Western analysis of wild-type (lanes 1 and 2) or me-v (lanes 3 and 4) macrophages by using the anti-ets-2 pT72-specific antibody (top panel). Cells were grown in the presence of 50 ng of CSF-1 per ml and treated with 100 μM LY294002 for 16 h (lanes 1 and 4). The blot shown was reprobed with an anti-Erk antibody as a sample loading control (bottom panel).
To test whether an Akt immunoprecipitate catalyzed phosphorylation of the ets-2 substrate at residue threonine 72, the wild-type substrate was compared to a substrate that had alanine substituted for threonine at position 72 (16). While the threonine 72 ets-2 protein was a substrate in the Akt immune kinase assay, the alanine 72 substrate was not (Fig. 3A, [ets-2 A72 panel]), indicating that the Akt-associated ets kinase activity phosphorylated the same residue of ets-2 as the raf/Erk pathway (16).
To assess whether the Akt pathway could be responsible for the constitutive phosphorylation of ets-2 observed in macrophages isolated from me-v mice, Akt kinase activities were measured in wild-type and me-v macrophages (Fig. 3B). Both histone H2B and ets-2 substrates were used for this analysis, and identical results were obtained for both substrates (Fig. 3B, top panel versus bottom panel). Akt activity increased about threefold following restimulation of wild-type macrophages deprived of CSF-1 in the representative experiment presented (Fig. 3B, lane 2 versus lane 1) and then returned to basal levels following 30 min of CSF-1 treatment (Fig. 3B, lane 3). Pretreatment of cells with LY294002 resulted in a threefold inhibition of CSF-1-dependent Akt activity (Fig. 3B, lane 4). In comparison, Akt activity in me-v cells was already 2.8-fold greater than the wild-type cell basal activity in the absence of exogenous CSF-1 (Fig. 3B, lanes 5). Akt activity was not significantly increased by treatment of cells with CSF-1 for 5 or 30 min (Fig. 3B, lanes 6 and 7, respectively). Pretreatment of cells with LY294002 resulted in a threefold inhibition of CSF-1-independent Akt activity with either of the histone H2B or ets-2 substrates (Fig. 3B, lane 8).
Four independent Akt immune kinase experiments with the ets-2 substrate demonstrated that the results were reproducible, and the differences between wild-type and me-v cells were significant (Fig. 3C). The analysis suggested that an Akt-associated kinase can phosphorylate ets-2 in a CSF-1-dependent, transient manner in wild-type cells. However, in me-v cells, the Akt-associated ets-2 kinase activity was constitutive.
To establish a direct link between PI 3-kinase–Akt activation and ets-2 activation, the phosphorylation of ets-2 following LY294002 treatment of macrophages was examined by Western analysis (Fig. 3D, top panel). This analysis revealed that 100 μM LY294002 caused an approximately 1.5-fold reduction in phosphorylated ets-2 in wild-type cells grown in the presence of CSF-1 (Fig. 3D, lane 1 versus lane 2). The inability of LY294002 to inhibit ets-2 phosphorylation in wild-type cells likely reflected that Erks are fully active and capable of phosphorylating ets-2 (16) (Fig. 1C). However, treatment of me-v cells with 100 μM LY294002 for 16 h diminished the level of phosphorylated ets-2 about sixfold compared to that in untreated cells (Fig. 3D, lane 4 versus lane 3). The PI 3-kinase inhibitor wortmannin also decreased ets-2 phosphorylation in me-v cells (data not shown). ets-2 phosphorylation was dependent on the PI 3-kinase/Akt pathway in me-v BMMs.
JNK p54 isoform coimmunoprecipitates with Akt in me-v macrophages and has ets-2 kinase activity.
The threonine 72 ets-2 phosphorylation site is a proline-directed site (PLLTP) that is closely related to the optimal Erk phosphorylation site [PL(S/T)P] (2). However, this site is distinct from the consensus Akt substrate site [RXRXX(S/T) (hydrophobic residue)] (1). This implied that ets-2 was probably not directly phosphorylated by Akt, but rather by a kinase that coimmunoprecipitated with Akt. The sequence of the ets-2 site suggested that another MAP kinase family member might be a candidate for the Akt-associated kinase. JNKs were selected as the most likely candidates, because there is evidence linking JNK kinase activation to the PI 3-kinase pathway in several cell types (4, 28, 43).
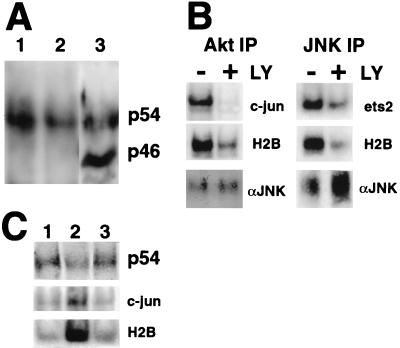
To determine if JNKs were in a complex with Akt in me-v macrophages, an anti-Akt antibody was used to immunoprecipitate Akt and associated proteins, and the complex was analyzed by Western blotting with an anti-JNK antibody that recognizes all three known JNK family members and their isoforms (see Materials and Methods). The immunoprecipitates were compared to a whole-cell extract prepared from me-v BMMs (Fig. 4A). The analysis demonstrated that both the p54 and p46 major isoforms of JNK were expressed in BMMs and that the p46 isoform was about twofold more abundant than the p54 isoform (Fig. 4A, lane 3). When an Akt immunoprecipitate was analyzed, the p54 JNK isoform was found to be coimmunoprecipitated with Akt, but the p46 isoform was not detected (Fig. 4A, lanes 1 and 2). The association between Akt and JNK was independent of CSF-1 treatment of the cells.
FIG. 4.
p54 JNK coimmunoprecipitates with Akt and is an ets-2 kinase in me-v macrophages. (A) Akt immunoprecipitates (lanes 1 and 2) were analyzed by Western blotting with the anti-JNK antibody. Cells were grown in the absence of CSF-1 for 24 h (lanes 1) or continuously in the presence of 50 ng of CSF-1 per ml (lanes 2). Whole-cell extracts were also prepared and analyzed on a lane on the same gel (lane 3). The positions of the p54 and p46 JNK isoforms are indicated. (B) Akt (left panels) or JNK (right panels) immune kinase assays performed on me-v macrophages using N-terminal c-jun, ets-2 “pointed,” and histone H2B substrates, as indicated. Cells were grown continually in the presence of 50 ng of CSF-1 per ml and treated with 100 μM LY294002 (LY) for 16 h as indicated. The bottom panel is a Western blot with an anti-JNK antibody to demonstrate that equivalent amounts of JNK were present in untreated and LY294002-treated samples. IP, immunoprecipitate. (C) Wild-type BMMs were deprived of CSF-1 for 24 h (lane 1) and then restimulated with CSF-1 for 5 or 30 min (lanes 2 and 3, respectively). Cell extracts were prepared and incubated with anti-Akt antibody. Half of the Akt immunoprecipitate was analyzed by Western blotting with a JNK antibody (top panel, p54). The other half was assayed for kinase activity by using both the c-jun (middle panel) and histone H2B substrates (lower panel).
Immune kinase assays were performed with both anti-Akt and anti-JNK antibodies for immunoprecipitation (Fig. 4B). This analysis demonstrated that the Akt immunoprecipitate contained c-jun N-terminal kinase activity and that this activity was inhibited three- to fourfold by treatment of cells with 100 μM LY294002 (Fig. 4B, top left panel). The JNK immunoprecipitate had histone H2B kinase activity that was inhibited 2.5-fold by LY294002 treatment, indicating that Akt could be coprecipitated with JNK (Fig. 4B, right middle panel). In addition, the JNK immunoprecipitate contained ets-2 kinase activity that was inhibited twofold by LY294002 treatment. These results implied that the p54 isoform of JNK is an ets-2 kinase found in a complex with Akt in macrophages.
Extracts prepared from wild-type macrophages were examined in order to establish if Akt and p54 JNK were in a complex in normal as well as me-v cells (Fig. 4C). The analysis revealed that Akt and p54 JNK were in a complex in a CSF-1-independent fashion (Fig. 4C, top panel, lane 1 versus lanes 2 and 3). Immune kinase assays of the Akt immunoprecipitate indicated that JNK activity, measured with c-jun substrate, was rapidly activated three- to fourfold within 5 min of CSF-1 stimulation of cells (Fig. 4C, lanes 2). The Akt-associated JNK activity returned to basal levels within 30 min (Fig. 4C, middle panel, lanes 3). The activation of JNK in the Akt immunoprecipitate in wild-type cells paralleled histone H2B kinase activity (Fig. 3B, lanes 1 to 3, and bottom panel, 4C, lanes 1 to 3), as well as ets-2 kinase activity (Fig. 3B, lanes 1 to 3).
CSF-1-independent survival of me-v cells depends on PI 3-kinase signaling.
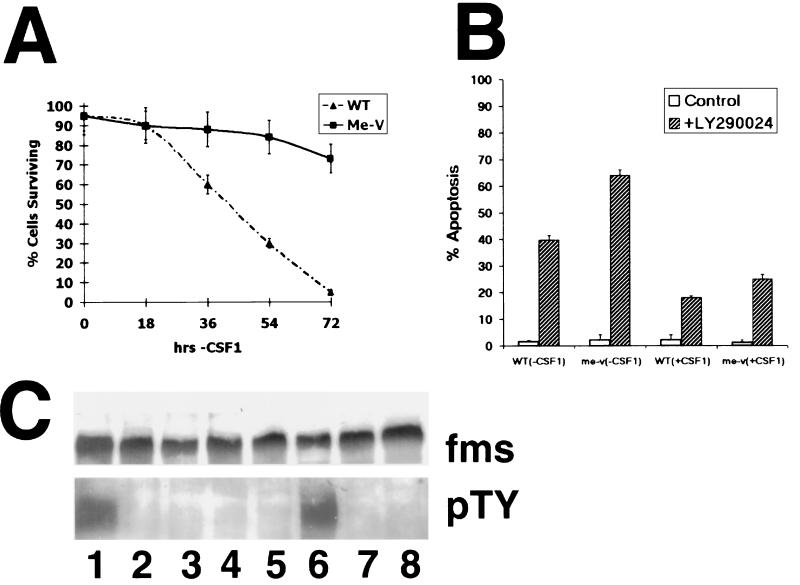
The well-established role of the PI 3-kinase/Akt pathways in preventing cell apoptosis (12, 13, 27) suggested that me-v macrophages might be resistant to cell death induced by withdrawal of exogenous CSF-1 when compared to normal cells. To test this prediction, the survival of me-v macrophages in response to CSF-1 withdrawal was studied (Fig. 5A). Following 72 h of CSF-1 withdrawal, less than 10% of wild-type BMMs survived, while 70 to 80% of me-v cells were viable (Fig. 5A). The results shown were obtained with the nuclear morphology assay to determine the extent of cell apoptosis and were independently confirmed by using the sub-G0 flow cytometry and cell viability assays (see Materials and Methods; data not shown).
FIG. 5.
Increased survival of me-v macrophages after CSF-1 withdrawal dependent on the PI 3-kinase pathway. (A) BMMs derived from normal and me-v mice were cultured in media lacking CSF-1 for the indicated times (2 × 106 cells plated per time point), fixed, and stained with 10 μM bis-benzimide. Fluorescent microscopy was used to distinguish the nuclear morphology of viable cells and apoptotic cells (see Materials and Methods). Results of the average of three experiments are shown, presented as the percentage of cells surviving. Error bars indicate the standard deviation. WT, wild type. (B) Fraction of apoptotic cells (2 × 106 cells plated) following treatment with 100 μM LY294002 for 24 h (hatched bars) in the presence or absence of 50 ng of CSF-1 per ml as indicated. Apoptosis was quantified by using the flow cytometry sub-G0 assay (see Materials and Methods). The average result of three experiments is represented. Error bars indicate the standard deviation of measurements. (C) me-v macrophages were grown continuously in the presence of CSF-1 (lane 1) or in media lacking CSF-1 for 8, 12, 16, 24 (lanes 2 to 5), 48, or 72 h (lanes 7 and 8). Cells starved of CSF-1 for 24 h had CSF-1 added to the medium for 10 min before harvest (lane 6). c-fms was immunoprecipitated with a specific antibody. The immunoprecipitate was divided in half and analyzed by Western blotting with either the c-fms (fms) antibody (top panel) or antiphosphotyrosine (pTY) antibody (bottom panel).
To assess whether me-v macrophage survival depended on PI 3-kinase signaling, cells were treated with the drug LY294002, and cell apoptosis was determined (Fig. 5B). These experiments demonstrated that cell apoptosis was triggered in both wild-type and me-v cells grown in the presence of CSF-1 following 24 h of LY294002 treatment, with approximately 18 and 25% of macrophages, respectively, found to undergo apoptosis, compared to less than 2% of cells that were not treated with the drug (Fig. 5B). The effect of LY294002 on me-v and wild-type macrophages grown in the absence of CSF-1 was even more dramatic, with 64 and 40% of cells apoptotic after 24 h, respectively, in contrast to cells not treated with the PI 3-kinase inhibitor (Fig. 5B). The results shown were obtained by using the flow cytometry sub-G0 assay and were independently confirmed by using nuclear morphology as an index for apoptosis (data not shown). The results suggested that both wild-type and me-v cells were dependent on PI 3-kinase signaling to maintain cell survival.
The expression and activation of the CSF-1 receptor, c-fms, were studied in me-v cells grown in the absence of CSF-1 (Fig. 5C). The experiment indicated that c-fms was expressed; however, tyrosine-phosphorylated c-fms was not detected in cells following 8 to 72 h of growth in the absence of CSF-1 (Fig. 5C, lanes 2 to 5, 7, and 8). Readdition of CSF-1 to cells grown in the absence of CSF-1 for 24 h resulted in detection of phosphorylated tyrosine residues within 10 min (Fig. 5C, lane 6).
The Bcl-x gene is a target of Akt and ets-2 signaling.
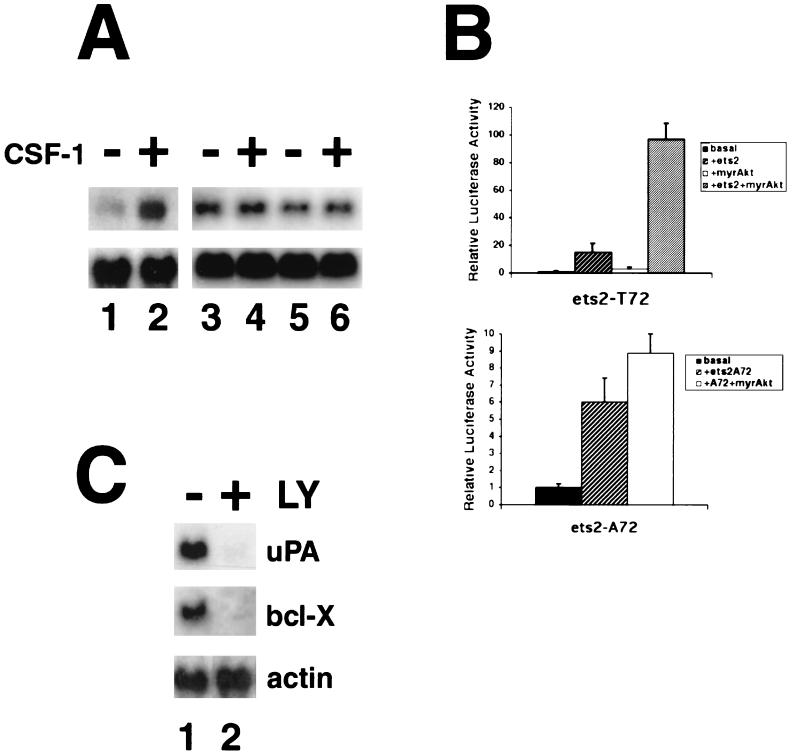
How might the phosphorylation of ets-2 by the PI 3-kinase/Akt pathway contribute to the survival of me-v cells? One hypothesis is that PI 3-kinase/Akt pathway-dependent phosphorylation of ets-2 leads to activation of genes that prevent apoptosis in me-v macrophages. The Bcl-x gene was selected as a potential target gene for Akt and ets-2 action in me-v cells, because this antiapoptotic gene was reported to be an ets-2 target in the macrophage cell line BAC-1.2F5 (38). Northern analysis of RNA isolated from me-v macrophages revealed that Bcl-x RNA was expressed independently of exogenous CSF-1 (Fig. 6A, lanes 3 to 6), unlike wild-type cells, in which Bcl-x expression was dependent on CSF-1 (Fig. 6A, lanes 1 and 2).
FIG. 6.
The mouse Bcl-x gene is a target of Akt–ets-2 action. (A) Total RNA was isolated from wild-type or me-v BMMs (two independent RNA samples each prepared from cells pooled from two mice). Cells were cultured in medium lacking CSF-1 for 24 h (lanes 1, 3, and 5) or restimulated with CSF-1 for 16 h (lanes 2, 4, and 6). RNA was analyzed by Northern blotting with a probe specific for mouse Bcl-x cDNA. A major 2.6-kb Bcl-x mRNA species was detected. Blots were reprobed with a mouse γ-actin probe as a sample loading control (bottom panel). (B) Transient transfections were performed in RAW264 cells. In each experiment, 4 μg of the Bcl-x reporter pGL2-0.6R was transfected in the presence or absence of 1 μg of the ets-2 expression vector pGCN-ets-2 (either wild-type T72 or mutated A72 forms, as indicated), 0.2 μg of a cytomegalovirus expression vector for the myristylated form of Akt (myr-Akt), or the combination of both ets-2 and myr-Akt as indicated. The average result of four experiments is shown. Error bars indicate the standard deviation of the measurements. (C) RNA was prepared from me-v BMMs that were untreated (lane 1) or treated with 100 μM LY294002 for 16 h (lane 2) and analyzed by Northern blotting. Blots were hybridized with probes specific for uPA or Bcl-x, as indicated and then reprobed with γ-actin (a loading control).
To examine whether the Bcl-x gene was directly regulated by ets-2 and Akt, a mouse Bcl-x proximal promoter reporter was studied in transient cotransfection experiments with the combination of a dominant-active form of Akt and ets-2 (Fig. 6B). The Bcl-x sequences studied comprised the region located approximately 600 bp upstream of the transcription start site fused to a firefly luciferase reporter gene (pGLK2-0.6R) (19). A myristylated, membrane-targeted form of Akt was used for the analysis (5). This constitutively active form of Akt was able to activate the Bcl-x reporter around 3-fold (Fig. 6B), while ets-2 alone stimulated the Bcl-x reporter 15-fold in this set of experiments. The combination of membrane-targeted Akt and ets-2 was able to superactivate the Bcl-x reporter, with an average induction of 97-fold observed (Fig. 6B [ets2-T72 panel]). An ets-2 protein with the phosphoacceptor site at threonine 72 mutated to alanine 72 was not able to superactivate the Bcl-x reporter in combination with myristylated Akt in the transient assay (Fig. 6B [ets2-A72 panel]).
The effect of LY294002 treatment on Ets-2 target gene expression was analyzed in me-v macrophages by Northern analysis (Fig. 6C). The experiments demonstrated that 16 h after LY294002 treatment, the levels of expression of uPA and Bcl-x mRNA were decreased approximately eightfold in me-v macrophages (Fig. 6C). Thus, ets-2 phosphorylation and target gene expression correlated with PI 3-kinase-dependent cell survival in me-v cells.
DISCUSSION
The finding that MEK-1 and Erks were not activated by CSF-1 in me-v BMMs (31) suggested that the me-v model system might provide a unique genetic background that would facilitate identification of Erk-independent signaling pathways that activate ets-2 in response to CSF-1. Analysis of macrophages derived from me-v mice unexpectedly demonstrated that phosphorylation of ets-2 was constitutive and independent of CSF-1 in these cells. In addition, the well-characterized ets-2 target genes coding for uPA, SR, and Bcl-x were constitutively expressed.
Erk-independent phosphorylation of ets-2 and activation of target genes in me-v macrophages were linked to the constitutive activation of the PI 3-kinase/Akt pathway and of an Akt-associated ets-2 (threonine 72) kinase activity. Furthermore, the p54 JNK isoform coimmunoprecipitated with Akt in macrophages and immune kinase assays demonstrated that JNK could catalyze phosphorylation of an ets-2 substrate dependent on PI 3-kinase signaling. Thus, in addition to being a substrate for the Raf/Erk pathway (16), ets-2 is a substrate for a novel PI 3-kinase/Akt/p54 JNK signaling pathway. The PI 3-kinase pathway is transiently activated by CSF-1 in wild-type cells, but is constitutively active in me-v macrophages. These results also highlight a difference between JNK p46 and p54 isoforms and suggest that the extended C-terminal domain of the p54 isoform may be involved in the association between Akt and JNK.
We have previously demonstrated that an epitope-tagged version of p54 JNK2 expressed in fibroblasts was incapable of catalyzing the phosphorylation of ets-2 substrate under conditions in which the PI 3-kinase pathway was not activated (16). JNK2 is reported to bind to the N-terminal portion of c-jun 25 times more efficiently than JNK1, and this direct interaction increases phosphorylation of c-jun by JNK2 relative to JNK1 (25). ets-2 was likely a poor substrate for JNK2 in the previously reported experiments, because it does not directly form a complex with JNK2. Taken with the results presented here, these data suggest the hypothesis that JNK substrate specificity is altered by association with the Akt complex. An adapter protein present in the Akt-JNK complex may recruit ets-2 and allow direct phosphorylation by p54 JNK even in the absence of a high-affinity interaction between ets-2 and JNK. Thus, the in vivo substrate range for the 10 characterized JNK isoforms may be dictated by the signaling complex with which they associate, in addition to high-affinity physical interactions with substrates (20). Further characterization of the Akt-JNK complex in macrophages will determine if this model is valid.
Our results imply that SHP-1 is a negative regulator of the PI 3-kinase/Akt/JNK pathway in macrophages. However, the question of what is the actual substrate for SHP-1 that lies upstream of the PI 3-kinase pathway remains open. The alterations in signaling in cells deficient in SHP-1 function are likely pleiotropic, reflecting that SHP-1 regulates multiple signaling pathways. In addition to c-fms (10), the GM-CSF receptor (23), JAK-2 (22), and negative-signaling receptors p91/PIR-B and SHPS-12 (6, 42, 46) have all been reported as substrates for SHP-1 and all can potentially activate the PI 3-kinase/Akt pathway. The outcome of effects on multiple ligand-receptor pairs is likely aberrant signaling, leading not only to PI 3-kinase–Akt activation, but also to abrogation of MEK-1–Erk signaling.
Recent work has revealed that, in some cell types, the PI 3-kinase/Akt pathway can negatively regulate the raf/MEK-1/Erk pathway via phosphorylation of regulatory sites within c-raf (36, 51). Our finding that the PI 3-kinase pathway is constitutively upregulated in me-v macrophages provides a molecular explanation for the lack of CSF-1-dependent MEK-1 and Erk activity in me-v macrophages. More interestingly, these results demonstrate an additional level of cross talk between these two pathways, the phosphorylation and activation of ets-2. At least one nuclear target of raf-Erk signaling remains phosphorylated and active in spite of the potential negative cross talk with the PI 3-kinase/Akt pathway in me-v macrophages.
Why is ets-2 a target for both raf and Akt signaling pathways? The data presented here indicate a link between the PI 3-kinase and Akt signaling pathways that promote me-v macrophage survival and ets-2 activation of antiapoptotic targets like Bcl-x. Perhaps the raf/MEK/Erk and PI 3-kinase/Akt pathways share some common targets to ensure that, under the physiological conditions that dictate negative interactions between the two pathways, programmed cell death is not triggered inappropriately. Work demonstrating that the proapoptotic factor BAD may be a target for both of these signaling pathways supports this idea (15, 37).
ets-2 is one of several transcription factors that have recently been identified as targets of Akt action in mammalian cells, including forkhead transcription factors, the cyclic AMP-responsive factor CREB, and NF-κB (9, 14, 26, 29, 40). Our results indicate that increased cell survival contingent on Akt-JNK activation of ets-2 may in part account for the massive overaccumulation of macrophages and subsequent pathology observed in me-v mice and therefore may have implications for understanding the molecular basis of macrophage-mediated damage in human inflammatory diseases such as rheumatoid arthritis (45).
ACKNOWLEDGMENTS
We acknowledge Lori Nelsen for expert technical assistance; Paul Herman (Ohio State University [OSU]) for critical discussions; Clay Marsh, Anil Jacobs, and Mark Coggeshall (OSU) for advice on Akt immune kinase assays; Gabriel Nunez (University of Michigan) for the gift of the mouse Bcl-x plasmids; Phil Tsichlis (Thomas Jefferson University) for Akt antibody and plasmids; the OSU Comprehensive Cancer Center; and the Keck Genetic Facility.
J.K.H. was supported by a Fellowship from the Lymphoma Research Foundation of America, Inc., and by T-32 Oncology Training grant CA 09338-21. This work was supported by NIH grant RO1-CA-53271 (M.C.O.).
J.L.S. and A.E.S. contributed equally to this work.
REFERENCES
- 1.Alessi D R, Caudwell F B, Andjelkovic M, Hemmings B A, Cohen P. Molecular basis for the substrate specificity of protein kinase B: comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez E, Northwood I C, Gonzalez F A, Latour D A, Seth A, Abate C, Curran T, Davis R J. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991;266:15277–15285. [PubMed] [Google Scholar]
- 3.Andjelkovic M, Jakubowicz T, Cron P, Ming X F, Han J W, Hemmings B A. Activation and phosphorylation of a pleckstrin homology domain containing protein kinase (RAC-PK/PKB) promoted by serum and protein phosphatase inhibitors. Proc Natl Acad Sci USA. 1996;93:5699–5704. doi: 10.1073/pnas.93.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonyak M A, Moscatello D K, Wong A J. Constitutive activation of c-Jun N-terminal kinase by a mutant epidermal growth factor receptor. J Biol Chem. 1998;273:2817–2822. doi: 10.1074/jbc.273.5.2817. [DOI] [PubMed] [Google Scholar]
- 5.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt P K. The akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci USA. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg K L, Carlberg K, Rohrschneider L R, Siminovitch K A, Stanley E R. The major SHP-1-binding, tyrosine-phosphorylated protein in macrophages is a member of the KIR/LIR family and an SHP-1 substrate. Oncogene. 1998;17:2535–2541. doi: 10.1038/sj.onc.1202203. [DOI] [PubMed] [Google Scholar]
- 7.Bignon J S, Siminovitch K A. Identification of PTP1C mutation as the genetic defect in motheaten and viable motheaten mice: a step toward defining the roles of protein tyrosine phosphatases in the regulation of hemopoietic cell differentiation and function. Clin Immunol Immunopathol. 1994;73:168–179. doi: 10.1006/clin.1994.1185. [DOI] [PubMed] [Google Scholar]
- 8.Bourette R P, Myles G M, Choi J L, Rohrschneider L R. Sequential activation of phosphatidylinositol 3-kinase and phospholipase C-gamma2 by the M-CSF receptor is necessary for differentiation signaling. EMBO J. 1997;16:5880–5893. doi: 10.1093/emboj/16.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen H E, Chang S, Trub T, Neel B G. Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1996;16:3685–3697. doi: 10.1128/mcb.16.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 12.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 13.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 14.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 15.Fang X, Yu S, Eder A, Mao M, Bast R C J, Boyd D, Mills G B. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene. 1999;18:6635–6640. doi: 10.1038/sj.onc.1203076. [DOI] [PubMed] [Google Scholar]
- 16.Fowles L F, Martin M L, Nelsen L, Stacey K J, Redd D, Clark Y M, Nagamine Y, McMahon M, Hume D A, Ostrowski M C. Persistent activation of mitogen-activated protein kinases p42 and p44 and ets-2 phosphorylation in response to colony-stimulating factor 1/c-fms signaling. Mol Cell Biol. 1998;18:5148–5156. doi: 10.1128/mcb.18.9.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 18.Gong J, Traganos F, Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem. 1994;218:314–319. doi: 10.1006/abio.1994.1184. [DOI] [PubMed] [Google Scholar]
- 19.Grillot D A, Gonzalez-Garcia M, Ekhterae D, Duan L, Inohara N, Ohta S, Seldin M F, Nunez G. Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J Immunol. 1997;158:4750–4757. [PubMed] [Google Scholar]
- 20.Gupta S, Barrett T, Whitmarsh A J, Cavanagh J, Sluss H K, Derijard B, Davis R J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 21.Jacob A, Cooney D, Tridandapani S, Kelley T, Coggeshall K M. FcgammaRIIb modulation of surface immunoglobulin-induced Akt activation in murine B cells. J Biol Chem. 1999;274:13704–13710. doi: 10.1074/jbc.274.19.13704. [DOI] [PubMed] [Google Scholar]
- 22.Jiao H, Berrada K, Yang W, Tabrizi M, Platanias L C, Yi T. Direct association with and dephosphorylation of Jak2 kinase by the SH2-domain-containing protein tyrosine phosphatase SHP-1. Mol Cell Biol. 1996;16:6985–6992. doi: 10.1128/mcb.16.12.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiao H, Yang W, Berrada K, Tabrizi M, Shultz L, Yi T. Macrophages from motheaten and viable motheaten mutant mice show increased proliferative responses to GM-CSF: detection of potential HCP substrates in GM-CSF signal transduction. Exp Hematol. 1997;25:592–600. [PubMed] [Google Scholar]
- 24.Jin D I, Jameson S B, Reddy M A, Schenkman D, Ostrowski M C. Alterations in differentiation and behavior of monocytic phagocytes in transgenic mice that express dominant suppressors of ras signaling. Mol Cell Biol. 1995;15:693–703. doi: 10.1128/mcb.15.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kallunki T, Su B, Tsigelny I, Sluss H K, Derijard B, Moore G, Davis R, Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 26.Kane L P, Shapiro V S, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 28.Kita Y, Kimura K D, Kobayashi M, Ihara S, Kaibuchi K, Kuroda S, Ui M, Iba H, Konishi H, Kikkawa U, Nagata S, Fukui Y. Microinjection of activated phosphatidylinositol-3 kinase induces process outgrowth in rat PC12 cells through the Rac-JNK signal transduction pathway. J Cell Sci. 1998;111:907–915. doi: 10.1242/jcs.111.7.907. [DOI] [PubMed] [Google Scholar]
- 29.Kops G J, de Ruiter N D, De Vries-Smits A M, Powell D R, Bos J L, Burgering B M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 30.Kovarik J, Kuntz L, Ryffel B, Borel J F. The viable motheaten (me-v) mouse—a new model for arthritis. J Autoimmun. 1994;7:575–588. doi: 10.1006/jaut.1994.1042. [DOI] [PubMed] [Google Scholar]
- 31.Krautwald S, Büscher D, Kummer V, Buder S, Baccarini M. Involvement of the protein tyrosine phosphatase SHP-1 in Ras-mediated activation of the mitogen-activated protein kinase pathway. Mol Cell Biol. 1996;16:5955–5963. doi: 10.1128/mcb.16.11.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minshall C, Arkins S, Dantzer R, Freund G G, Kelley K W. Phosphatidylinositol 3′-kinase, but not S6-kinase, is required for insulin-like growth factor-I and IL-4 to maintain expression of Bcl-2 and promote survival of myeloid progenitors. J Immunol. 1999;162:4542–4549. [PubMed] [Google Scholar]
- 33.Roach T I, Slater S E, White L S, Zhang X, Majerus P W, Brown E J, Thomas M L. The protein tyrosine phosphatase SHP-1 regulates integrin-mediated adhesion of macrophages. Curr Biol. 1998;8:1035–1038. doi: 10.1016/s0960-9822(07)00426-5. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 35.Rohrschneider L R, Bourette R P, Lioubin M N, Algate P A, Myles G M, Carlberg K. Growth and differentiation signals regulated by the M-CSF receptor. Mol Reprod Dev. 1997;46:96–103. doi: 10.1002/(SICI)1098-2795(199701)46:1<96::AID-MRD15>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Rommel C, Clarke B A, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos G D, Glass D J. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 37.Scheid M P, Schubert K M, Duronio V. Regulation of BAD phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J Biol Chem. 1999;274:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- 38.Sevilla L, Aperlo C, Dulic V, Chambard J C, Boutonnet C, Pasquier O, Pognonec P, Boulukos K E. The Ets2 transcription factor inhibits apoptosis induced by colony-stimulating factor 1 deprivation of macrophages through a Bcl-xL-dependent mechanism. Mol Cell Biol. 1999;19:2624–2634. doi: 10.1128/mcb.19.4.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shultz L D, Schweitzer P A, Rajan T V, Yi T, Ihle J N, Matthews R J, Thomas M L, Beier D R. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 40.Sizemore N, Leung S, Stark G R. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stacey K J, Fowles L F, Colman M S, Ostrowski M C, Hume D A. Regulation of urokinase-type plasminogen activator gene transcription by macrophage colony-stimulating factor. Mol Cell Biol. 1995;15:3430–3441. doi: 10.1128/mcb.15.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timms J F, Carlberg K, Gu H, Chen H, Kamatkar S, Nadler M J S, Rohrschneider L R, Neel B G. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol Cell Biol. 1998;18:3838–3850. doi: 10.1128/mcb.18.7.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timokhina I, Kissel H, Stella G, Besmer P. Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 1998;17:6250–6262. doi: 10.1093/emboj/17.21.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsui H W, Siminovitch K A, de Souza L, Tsui F W. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 45.van den Berg W B, van Lent P L. The role of macrophages in chronic arthritis. Immunobiology. 1996;195:614–623. doi: 10.1016/S0171-2985(96)80026-X. [DOI] [PubMed] [Google Scholar]
- 46.Veillette A, Thibaudeau E, Latour S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J Biol Chem. 1998;273:22719–22728. doi: 10.1074/jbc.273.35.22719. [DOI] [PubMed] [Google Scholar]
- 47.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 48.Wu H, Moulton K, Horvai A, Parik S, Glass C K. Combinatorial interactions between AP-1 and ets domain proteins contribute to the developmental regulation of the macrophage scavenger receptor gene. Mol Cell Biol. 1994;14:2129–2139. doi: 10.1128/mcb.14.3.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang B-S, Hauser C A, Henkel G, Colman M S, Van Beveren C, Stacey K J, Hume D A, Maki R A, Ostrowski M C. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol. 1996;16:538–547. doi: 10.1128/mcb.16.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi T, Ihle J N. Association of hematopoietic cell phosphatase with c-Kit after stimulation with c-Kit ligand. Mol Cell Biol. 1993;13:3350–3358. doi: 10.1128/mcb.13.6.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]