ABSTRACT.
Congenital Chagas disease (CCD) has become a global health problem. Historically, the diagnosis of CCD has been carried out using parasitological methods and traditional serological techniques, however, new serological techniques such as chemiluminescent microparticle immunoassays (CMIA) have been developed in the last few years with many advantages compared with traditional serological tests. A total of 75 children born to 72 Latin American Chagas-infected mothers were consecutively enrolled and studied by CMIA and indirect immunofluorescence (IIF) at 0–2, 6, 9, and 12 months of age. At the end of the follow-up, 74 out of 75 children were considered uninfected and one child was diagnosed with CCD. Our study emphasizes the need to carry out serological follow-up on every newborn from a mother with Chagas disease and shows that CMIA assay is a great diagnostic tool as a single serological test at 9 months of age to rule out CCD or to identify possible transmission.
INTRODUCTION
Chagas disease (CD) is a parasitic zoonosis caused by the protozoan Trypanosoma cruzi. This disease affects around 6 million people worldwide, most of them in Latin American countries where this disease is endemic.1,2 As a result of globalization, CD has been spread around the world. It has been estimated that the majority of CD cases in nonendemic countries are located in the United States and Europe with > 300,000 and > 80,000 infected individuals, respectively.3
In nonendemic countries, given the absence of vectors and the existence of directives that regulate the screening of CD in blood transfusions and organ donors, congenital transmission is the main route of T. cruzi infection, and it has emerged as a public health problem and a great challenge to deal with. Taking into account that the majority of congenital Chagas disease (CCD) cases are asymptomatic or present nonspecific signs,4 it has become necessary to perform T. cruzi screening in every newborn of an infected mother to rule out mother-to-child transmission. Screening strategies to diagnose Chagas disease in pregnant women have already been officially implemented in some European countries, like Spain and Italy.5–7
Historically, CCD diagnosis has been carried out using parasitological methods such as microhaematocrit and haemoculture in the first months of life, however, the sensitivity of these techniques has proven to be low.8,9 Recently, molecular methods have been developed allowing an early diagnosis with higher sensitivity.8,10–13 Regardless of these techniques, serological studies should be performed to confirm complete clearance of maternal antibodies and the absence of infection at 10 months of life according to WHO criteria.14 Indirect immunofluorescence (IIF), indirect hemagglutination (IHA), and ELISA have traditionally been used for this purpose. New serological techniques such as chemiluminescent microparticle immunoassays (CMIA) and electrochemiluminescence immunoassays (ECLIA) have been developed in the last few years with many advantages compared with traditional serological tests. The automation and use of recombinant antigens of these new techniques have proved to be more sensitive and have less cross-reaction than crude antigen-based assays.15 However, these techniques remain logistically and economically prohibitive for resource-limited settings.3
The aim of this study was to evaluate the usefulness of CMIA as a single technique for the diagnosis of CCD in a cohort of children born in a nonendemic country, with the main objective of establishing the optimal time period for performing serological tests.
MATERIALS AND METHODS
Patients and sample collection.
The study was carried out on 75 children born to 72 Latin American Chagas-infected mothers, who attended the Tropical Medicine Unit of Virgen de la Arrixaca Hospital in Murcia (Spain) from September 2011 to February 2019. The diagnosis of CD in mothers was carried out using two serological tests according to the WHO criteria.1 The majority of them came from Bolivia (97%) and ranged in age from 21 to 43 (mean ± SD age, 32.5 ± 5.1). One mother gave birth to twins and two mothers became pregnant twice during the study period. All children were born in Spain so we can rule out all routes of transmission other than congenital ones.
The protocol followed for CCD diagnosis has been previously reported by Simón and others.9 Briefly, cord blood (8 mL) at birth and peripheral blood (2 mL) at 1 month of age were collected. From these samples, 0.5 mL of blood was used for microhaematocrit tests. The remaining blood was immediately mixed with an equal volume of lysis buffer containing 6 M guanidine hydrochloride and 200 mM ethylenediaminetetraacetic acid (EDTA) pH 8 and was stored at 4°C (for at least 48 hours) for genomic DNA isolation. In reference to serology testing, the protocol includes a sample at 0–2 months, 6 months, 9 months, and 12 months of age. Two milliliter of serum samples from children were collected at each of these time points.
Children were considered uninfected when two serology tests were negative at any time, and infected, if both remained positive at 12 months of age or parasitological results were positive at birth and at 1 month of age. If CCD infection was detected, children were treated with benznidazole 10 mg/kg body weight per day for 60 days.
Serological methods.
Both the mother and child’s serum samples were tested for the presence of T. cruzi antibodies by CMIA and IIF assay.
The CMIA Architect Chagas assay (ARCHITECT Chagas®, Abbott, IL) is based on recombinant proteins FP3, FP6, FP10, and TcF. These four proteins include 14 different antigenic regions and represent the three morphologies of the parasite (amastigote, trypomastigote, and epymastigote). Chemiluminescence is measured in relative light units (RLUs). The assay results are presented as a ratio of the specimen signal (in RLUs) to the cut-off value (S/CO), where S/CO values of < 0.8 are considered negative, values of ≥ 1 positive, and the gray zone was from ≥ 0.8 to < 1.
The IIF test (CHAGAS IFI IgG+IgM®, Vircell Microbiologist, Spain) uses T. cruzi epymastigotes as antigens. Sera were analyzed at two dilutions: 1:80 and 1:160, and titers equal to or above 1:80, were considered positive.
Both assays were performed according to the manufacturer’s instructions.
Parasitological methods.
Microhaematocrit testing was performed as described by Feilij and others.15 For DNA detection, the guanidine-EDTA blood mixture was boiled for 15 minutes and DNA extraction was carried from a 400-µL blood sample using the Maxwell 16 Blood DNA Purification Kit (Promega Biotech Iberica, Madrid, Spain), according to the manufacturer’s instructions. Polymerase chain reaction (PCR) detection of the T. cruzi kDNA using primers 121 (5′-AAATAATGTACGGGKGAGATGCATGA-3′) and 122 (5′-GTTCGATTGGGGTTGGTGTAATATA-3′) was carried out under the previously reported conditions.16
Statistical analyses.
The results were analyzed using IBM SPSS Statistics 23.0. Differences in means () among groups were examined using Student’s t test. P values < 0.05 were considered to be statistically significant.
Bioethical criteria.
The study was reviewed and approved by the Ethical Committee of the Virgen de la Arrixaca Hospital. Informed consent forms were signed by parents after detailed interviews.
RESULTS
Seventy-two infected mothers and 75 newborns were studied by CMIA at delivery. Their mean S/CO values were 8.78 and 8.74, respectively. No statistically significant difference was found between the mean values of the mothers and their children (P = 0.84).
At the end of the follow-up, 74 out of 75 children were considered uninfected (PCR, microhaematocrit, CMIA, and IIF tests were negative) and one child was diagnosed with CCD (the CMIA and IIF tests were positive at 10 months of age and PCR was positive at 14 months of age).
Uninfected children were classified into three groups according to the time of seronegativization by CMIA: 1) patients with a negative CMIA result at 6 months; 2) patients with a negative CMIA result at 9 months; and 3) patients with a negative CMIA result at 12 months (Table 1).
Table 1.
Mean values of serological follow-up of uninfected children and their mothers measured by CMIA and number of samples for each group according to the time of seronegativization
| Age | Mothers | 0–2 months | 6 months | 9 months | 12 months |
|---|---|---|---|---|---|
| (S/CO ± SD) | (S/CO ± SD) | (S/CO ± SD) | (S/CO ± SD) | (S/CO ± SD) | |
| Group A | 7.06 ± 2.09 | 6.64 ± 1.99 | 0.43 ± 0.32 | * | * |
| (N = 18) | |||||
| Group B | 9.47 ± 1.28 | 9.44 ± 2.27 | 2.77 ± 1.25 | 0.40 ± 0.25 | * |
| (N = 24) | |||||
| Group C | 9.98 ± 2.41 | 9.61 ± 2.35 | 3.68 ± 2.60 | 1.70 ± 0.76 | 0.14 ± 0.19 |
| (N = 32) | (N = 24) | (N = 10) |
CMIA = chemiluminescent microparticle immunoassays.
End of follow-up.
Serological follow-up of uninfected children.
Serological follow-up of 74 uninfected children showed a sustained decrease in maternal antibodies until negativization. The CMIA assay showed seronegativization in 18 children at the age of 6 months (Group A), 24 at the age of 9 months (Group B), and 32 at the age of 12 months (Group C). The mean values of CMIA for each group at different points of time are shown in Table 1. In the case of group C, CMIA values were calculated using data for 24 children at 6 months and 10 children at 9 months because of the absence of serum samples.
The three groups of children were compared according to the mean value of CMIA at different time points (0–2, 6, 9, and 12 months of age). At birth and at 6 months, the mean S/CO of children from group A was lower than in group B and C (P < 0.05) and there were no statistically significant differences between group B and C (P = 0.76). Nonetheless, at 9 months of age a statistically significant difference was detected between the mean S/CO of children from group B and C (P < 0.05).
Our results show that 27% (18/66) of children at 6 months no longer had specific T. cruzi antibodies according to CMIA, increasing to 81% (42/52) at 9 months, and none of them (74/74) at 12 months. The IIF test was carried out in parallel showing that 84% (47/56) of children were negative at 6 months and all children were negative at 9–12 months of age.
Congenital Chagas case.
The infected child and her mother were studied by CMIA at delivery, with S/CO values of 10.97 and 10.11, respectively. The patient was diagnosed with an S/CO value of 6.74 and an IIF titer higher than 1:160 at 10 months of age. The child was born at term and did not show any clinical manifestations of symptomatic CCD. Microhaematocrit and PCR at birth and at 1 month of age were negative. After diagnosis, the complementary tests performed (hemogram, biochemical, and cardiovascular studies) did not show any alterations.
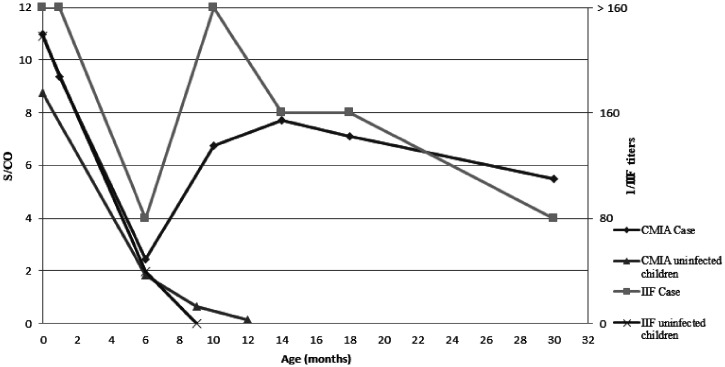
The serological follow-up of the infected child revealed a sustained decrease in antibodies from birth to the test at 6 months; an unexpected increase in the CMIA value and IIF titer at the age of 9 months was observed with an increment, which continued until 12 months (Figure 1). At 6 months of age, there was no difference between the infected child (S/CO 2.44) and uninfected children from group B and C (S/CO 3.19). However, a clear difference was observed at 9 months between infected and uninfected children (S/CO 6.74 and S/CO 0.61, respectively).
Figure 1.
Evolution of CMIA values (S/CO) and IIF values (1/titer) of congenital Chagas case and uninfected children.
After diagnosis, the child was treated and no side effects were observed. Serological follow-up was performed before treatment and at 1 year posttreatment with an S/CO value of 7.11 and 5.48 and an IIF titer of 1:160 and 1:80, respectively.
DISCUSSION
Traditionally, serological diagnosis of CCD has been performed using two different assays (ELISA, IHA, or IIF) considering a child to be infected if both assays were positive at 10 months of age or later.4 Nowadays, it is still necessary to perform these two conventional serological tests to confirm Chagas disease as a result of cross reactivity of these techniques with other parasites and the absence of optimal diagnostic sensitivity. Recently, fully automated serological assays with higher sensitivity and high throughput have been developed.17–19 These new assays are based on recombinant antigens and use chemiluminescence or electrochemiluminescence technology to detect T. cruzi antibodies in serum samples. ARCHITECT® Chagas (ABBOT), Chagas LIAISON® (DIASORIN), and Elecsys® Chagas (ROCHE) are the most commonly used assays in nonendemic countries, showing higher sensitivity than those based on crude antigen.17,18
The use of CMIA to diagnose both mothers and children has recently been recommended by the WHO Technical Group on “Prevention and Control of Congenital Transmission and Case Management of Congenital Infections with Trypanosoma cruzi” for screening as a stand-alone test,14 even though positive results must be confirmed by conventional serological tests. Some studies about the utility of CMIA for the diagnosis of CCD have already been published;18,19 however, studies concerning its usefulness for the diagnosis of CCD are scarce.20 In this study, we want to evaluate CMIA assay performance for the screening of CCD.
The results obtained using CMIA show that children who clear out maternal antibodies at 6 months had lower CMIA mean values at birth (6.64) than those who were still positive until 9–12 months of age (9.44 and 9.61, respectively). It is reasonable to assume that the lower the CMIA values children have at birth, the faster the maternal antibodies will disappear. Therefore, the CMIA values of children at birth could be used for predicting the time at which uninfected children will clear maternal antibodies.
According to other studies, maternal antibodies can be detected by CMIA assay for a longer period of time than by the IIF test.18 Approximately 73% (48 out of 66) of uninfected children had a positive CMIA assay at 6 months of age and 19% (10/52) at 9 months although S/CO values were close to cut-off point (1.7 ± 0.76). On the other hand, only 16% (9/56) of uninfected children had a positive IIF result at 6 months and at 9 months of age all IIF results were negative.
Because of CMIA high sensitivity, its use for the diagnosis of CCD has been controversial. It has been reported that maternal antibodies are detected for a longer period of time with CMIA assay; therefore, to observe complete clearance of antibodies, this assay should be performed at a later age than IIF studies.20
Our study shows that a drop in IgG antibodies measured by CMIA and IIF occurs in both groups of children, infected and uninfected, from birth to 6 months of life. Trypanosoma cruzi antibodies measured by CMIA were similar for uninfected children and the infected child at 6 months (S/CO ± SD 3.19 ± 2.60 and S/CO 2.44, respectively). It is at the 9th month of life when an increase in the specific antibody titers in the infected child (S/CO 6.74) and a decrease (S/CO ± SD 1.70 ± 0.76) or negativization of antibodies in uninfected children are both observed. These data support our diagnostic algorithm: if serological studies were performed at 6 months of age to distinguish between infected and uninfected children, it would be nearly impossible to make the distinction since maternal antibodies are still present and could lead us into a false positive result. Therefore, our recommendation would be to perform a CMIA test at 9 months of age; if negative, the child does not have CCD; and if positive, an IIF test should be performed to confirm the diagnosis.
One of the limitations of this study was that only one infected child was followed up by CMIA, and no other cases have been described previously because of the majority of children being diagnosed with less than 1 year by direct methods and these are treated to avoid loss of follow-up.9 In the present study, a large number of uninfected children were studied and, to our knowledge, this is the first infected child follow-up by CMIA from birth to 12 months of age, which represents a step forward in the knowledge of the evolution of antibodies measured by CMIA. In future, however, it would be necessary to study a greater number of infected children to corroborate these findings.
Although this article focuses on serological techniques, the diagnosis algorithm used in our hospital includes the performance of direct techniques (microhaematocrit, PCR) at birth and at 1 month of life. These techniques have less sensitivity than serological methods and false negative results have already been published.21 In our congenital Chagas case, PCR at birth and at 1 month of age were negative, so the diagnosis was made at 10 months of age by CMIA and by IIF.
Congenital infection could be acquired at any stage of pregnancy; so these false negative results by PCR may be resulting from a later acquisition of the disease, near to childbirth or even as a result of perinatal transmission, which might modify parasitemia.22 With that in mind, performing serological studies on every newborn at risk is highly recommended to avoid increasing undiagnosed infected children.
Early diagnosis of CCD, particularly before the first year of life, is crucial because the treatment is well tolerated with few or no side effects and its effectiveness is nearly 100%;4,23,24 when the treatment is delayed beyond the first age of life, the time of seronegativization is longer, and the disease progresses decreasing the treatment tolerance and the cure rate.25
Regarding the infected child, the treatment was delayed after diagnosis because of the time taken to contact their parents and the bureaucracy needed to get benznidazol. Despite all these difficulties, the patient was treated at 14 months decreasing his S/CO value from 7.11 to 5.48 and his IIF titer from 1:320 to 1:80 in 1 year, which indicates an adequate therapeutic response and the potential elimination of the parasite.
CONCLUSION
Our study emphasizes the need to carry out serological follow-up on every newborn from a mother with CD, and suggests that CMIA assay could be a useful diagnostic tool as a single serological test at 9 months of age to rule out CCD or to identify possible transmission; although, further evaluations should be necessary to confirm and validate the results.
ACKNOWLEDGMENTS
We would like to thank Fuensanta Franco for her technical assistance.
REFERENCES
- 1. WHO , 2012. Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. World Health Organization technical report series/Geneva, Switzerland: World Health Organization/Special Programme for Research and Training in Tropical Diseases (TDR), 2012(975): v–xii, 1–100. [PubMed]
- 2. PAHO , 2006. Estimación Cuantitativa de la Enfermedad de Chagas en las Américas. Montevideo PAHO; Report No: OPS/HDM/CD/425-06.
- 3. Coura JR, Viñas PA, 2010. Chagas disease: a new worldwide challenge. Nature 465: S6–S7. [DOI] [PubMed] [Google Scholar]
- 4. Torrico F, Alonso-Vega C, Suarez E, Rodriguez P, Torrico MC, Dramaix M, Truyens C, Carlier Y, 2004. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg 70: 201–209. [PubMed] [Google Scholar]
- 5. Requena-Méndez A, Albajar-Viñas P, Angheben A, Chiodini P, Gascón J, Muñoz J, 2014. Health policies to control Chagas disease transmission in European countries. PLoS Negl Trop Dis 8: e3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Consiglio Regionale Regione Toscana , 2012. Programma Regionale per la Prevenzione e il Controllo della malattia di Chagas Congenita: Indicazioni per l’assistenza in Gravidanza. Servizio Sanitario della Toscana, Regione Toscana, Florence, Italy. Available at: http://servizi.salute.toscana.it/csr/img/getfile_img1.php?id=24147. Accessed November 11, 2020.
- 7. Generalitat Valenciana , 2009. Enfermedad de Chagas Importada, Protocolo de actuación en la Comunitat Valenciana. Valencia, Spain: Conselleria de Sanitat, Generalitat Valenciana. Available at: http://publicaciones.san.gva.es/publicaciones/documentos/V-5243-2008.pdf. Accessed November 14, 2020.
- 8. Mora MC, Sanchez Negrette O, Marco D, Barrio A, Ciaccio M, Segura MA, Basombrío MA, 2005. Early diagnosis of congenital Trypanosoma cruzi infection using PCR, hemoculture, and capillary concentration, as compared with delayed serology. J Parasitol 91: 1468–1473. [DOI] [PubMed] [Google Scholar]
- 9. Simón M, Gil-Gallardo LJ, Iborra MA, Carrilero B, López MC, Romay-Barja M, Murcia L, Thomas MC, Benito A, Segovia M, 2019. An observational longitudinal study to evaluate tools and strategies available for the diagnosis of congenital Chagas Disease in a non-endemic country. Acta Trop 199: 105127. [DOI] [PubMed] [Google Scholar]
- 10. Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin MJ, Freilij H, 2003. Aetiological treatment of congenital Chagas’ disease diagnosed and monitored by the polymerase chain reaction. J Antimicrob Chemother 52: 441–449. [DOI] [PubMed] [Google Scholar]
- 11. Velázquez EB, Rivero R, De Rissio AM, Malagrino N, Esteva MI, Riarte AR, Ruiz AM, 2014. Predictive role of polymerase chain reaction in the early diagnosis of congenital Trypanosoma cruzi infection. Acta Trop 137: 195–200. [DOI] [PubMed] [Google Scholar]
- 12. Bua J, Volta BJ, Perrone AE, Scollo K, Velázquez EB, Ruiz AM, De Rissio AM, Cardoni RL, 2013. How to improve the early diagnosis of Trypanosoma cruzi infection: relationship between validated conventional diagnosis and quantitative DNA amplification in congenitally infected children. PLoS Negl Trop Dis 10: e2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bua J, Volta BJ, Velázquez EB, Ruiz AM, Rissio AM, Cardoni RL, 2012. Vertical transmission of Trypanosoma cruzi infection: quantification of parasite burden in mothers and their children by parasite DNA amplification. Trans R Soc Trop Med Hyg 106: 623–628. [DOI] [PubMed] [Google Scholar]
- 14. Carlier Y, Altcheh J, Angheben A, Freilij H, Luquetti AO, Schijman AG, Segovia M, Wagner N, Albajar P, 2019. Congenital Chagas disease: updated recommendations for prevention, diagnosis, treatment, and follow-up of newborns and siblings, girls, women of childbearing age, and pregnant women. PLoS Negl Trop Dis 13: e0007694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feilij H, Muller L, Gonzalez Cappa SM, 1983. Direct micromethod for diagnosis of acute and congenital Chagas’ disease. J Clin Microbiol 18: 327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murcia L, Carrilero B, Muñoz MJ, Iborra MA, Segovia M, 2010. Usefulness of PCR for monitoring benznidazole response in patients with chronic Chagas’ disease: a prospective study in a non-disease-endemic country. J Antimicrob Chemother 65: 1759–1764. [DOI] [PubMed] [Google Scholar]
- 17. Flores-Chavez MD, Sambri V, Schottstedt V, Higuera-Escalante FA, Roessler D, Chaves M, Laengin T, Martinez A, Fleischer B, 2018. Evaluation of the Elecsys Chagas assay for detection of Trypanosoma cruzi-specific antibodies in a multicenter study in Europe and Latin America. J Clin Microbiol 56: e01446–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. I, borra-Bendicho MA, Albert-Hernández M, Márquez-Contreras C, Segovia-Hernández M, 2012. ARCHITECT Chagas(®): a new diagnostic tool in Chagas disease. Enferm Infecc Microbiol Clin 30: 463–465. [DOI] [PubMed] [Google Scholar]
- 19. Praast G, Herzogenrath J, Bernhardt S, Christ H, Sickinger E, 2011. Evaluation of the Abbott ARCHITECT Chagas prototype assay. Diagn Microbiol Infect Dis 69: 74–81. [DOI] [PubMed] [Google Scholar]
- 20. Abras A. et al. , 2017. Towards a new strategy for diagnosis of congenital Trypanosoma cruzi infection. J Clin Microbiol 55: 1396–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Volta BJ, Perrone AE, Rivero R, Scollo K, Bustos PL, Bua J, 2018. Some limitations for early diagnosis of congenital Chagas infection by PCR. Ped 141: S451–S455. [DOI] [PubMed] [Google Scholar]
- 22. Luquetti AO, Tavares SB, Siriano LR, de Oliveira RA, Campos DE, de Morais CA, de Oliveira EC, 2015. Congenital transmission of Trypanosoma cruzi in central Brazil. A study of 1,211 individuals born to infected mothers. Mem Inst Oswaldo Cruz 110: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freilij H, Altcheh J, 1995. Congenital Chagas’ disease: diagnostic and clinical aspects. Clin Infect Dis 21: 551–555. [DOI] [PubMed] [Google Scholar]
- 24. Altcheh J, Moscatelli G, Moroni S, Garcia-Bournissen F, Freilij H, 2011. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics 127: e212–e218. [DOI] [PubMed] [Google Scholar]
- 25. Moscatelli G, Moroni S, García-Bournissen F, González N, Ballering G, Schijman A, Corral R, Bisio M, Freilij H, Altcheh J, 2019. Longitudinal follow up of serological response in children treated for Chagas disease. PLoS Negl Trop Dis 13: e0007668. [DOI] [PMC free article] [PubMed] [Google Scholar]