ABSTRACT.
Patients with diabetes mellitus (DM) are at greater risk of developing active tuberculosis and other intracellular bacterial infections, although the risk of acquiring infections from nontuberculous Mycobacterium (NTM) remains undefined. This study evaluated associations between DM and incidence of NTM infection-caused pulmonary and cutaneous diseases. Data for DM patients were extracted from the National Health Insurance Research Database of Taiwan. The DM cohort included 136,736 patients, and cases were matched randomly by age, gender, and index year with non-DM patients. Multivariate Cox proportional hazards regression was used to calculate adjusted hazard ratios of incident NTM-caused diseases in the DM cohort compared with non-DM control subjects. The frequency of incident NTM-caused diseases was significantly greater in DM patients (0.12%) than in non-DM patients (0.08%) (P < 0.05), including patients with type 1 DM (0.12%) and type 2 DM (0.12%) (all P < 0.05). Adjusted multivariate Cox regression analysis revealed that the incidence of NTM-caused diseases in DM patients was 1.43-fold greater than that in non-DM patients overall (P < 0.05), particularly in pulmonary (1.13-fold), other specific (excluding pulmonary, cutaneous, and disseminated diseases; 3.88-fold), and unspecific (atypical NTM infection; 1.54-fold) diseases (all P < 0.05). In conclusion, both type 1 DM and type 2 DM patients have high risk of NTM-caused diseases, suggesting that physicians need to pay more attention to this issue concerning the high risk of NTM-caused infection in DM patients.
INTRODUCTION
Diabetes mellitus (DM) is a multifactorial metabolic condition with a complex pathogenesis and various etiologies and presentations. The chronic hyperglycemic conditions in DM patients induce oxidative stress, inflammation, and immune dysfunction, all of which may contribute to complicated pathologies arising from poor glycemic control.1 In vivo and in vitro studies have identified multilevel immune system dysfunction in DM patients, which may lead eventually to increased occurrence of infectious diseases and poor outcomes.2,3 Consequently, patients with DM are shown to have greater risk of developing active tuberculosis (TB) and other intracellular bacterial infections such as listeriosis and melioidosis.4,5 Although DM ranked seventh among the leading causes of death in 2015, TB has been recognized as one cause of the mortality as a result of a bacterial infectious disease.6 Environmental exposure via water and soil to various species of nontuberculous Mycobacterium (NTM) has been suggested to cause a broad range of infections around the world,7 coinciding with pathogenicity of the organisms and susceptibility of the host.8 In a 2-year study of residents in a single U.S. state, Winthrop et al.9 noted that respiratory NTM isolates were increasingly common, although no correlation was shown with the prevalence of pulmonary disease. A fairly recent Australian study found that patients with DM represented greater than three times more NTM infections than the general population.3 Although pulmonary NTM disease is considered to be an emerging public health disease,9 the risk of DM patients acquiring NTM infections still remains undefined.2,10
More than 150 NTM species are recognized for causing disease in humans.11 Although TB is the most prevalent mycobacterial infection in DM patients, epidemiological evidence indicates an increasing incidence of pulmonary, cardiac, and soft tissue-related NTM infections in this patient population.12–14 The risk factors contributing to pulmonary infections are complicated and often involve patients with severe underlying parenchymal lung disease; hence, studies on patients with pulmonary infections, including those caused by NTM, may be confounded by a number of variables. However, in a study by Song et al.,15 high-resolution computed tomography revealed the extent of bronchiectasis, cellular or inflammatory bronchiolitis, and emphysema in NTM patients, and the findings of Mycobacterium avium complex (MAC) correlated with pulmonary function testing. In another study, similar high-resolution computed tomographic findings corresponded histopathologically to bronchiolectasis, and bronchiolar and peribronchiolar inflammation with or without granuloma formation.16 The results of these studies imply that NTM infection may present as small airway disease, as also shown by Fjallbrant et al.17 in hypersensitivity pneumonitis associated with MAC. MAC was also the most common organism, along with rapidly growing mycobacteria, in a 7-year retrospective study conducted from 1997 to 2003 in a university hospital in Taiwan among 412 patients with clinically significant illnesses.18 That longitudinal study demonstrated increased incidence (9–17%) of NTM disease across the 7-year time span, including pulmonary infection, pleurisy, skin and soft tissue infections, osteomyelitis, and lymphadenitis. In another report, NTM infection was a risk factor for chronic obstructive pulmonary disease19 and, in one case study, disseminated Mycobacterium marinum infection involved not only cutaneous tissue, but also the lung in a male patient with a previous history of DM, steroid injections, and working in the fisheries industry.20 Nevertheless, exposure to NTM pathogens, transmission routes, and risk factors remain to be elucidated before the etiopathology and underlying mechanisms can be fully understood.
Even given the previous reports of NTM infection in DM patients, and increased incidence of NTM disease among hospitalized patients in general, to the best of our knowledge, few studies have yet investigated correlations between DM and the incidence of NTM-related diseases. Therefore, our study aimed to evaluate associations between DM and the incidence of NTM infection-caused pulmonary and cutaneous diseases.
PATIENTS AND METHODS
Data source.
All data for our study were extracted from the database of the universal National Health Insurance program in Taiwan, which was formed in March 1995 from the merger of 13 existing insurance-related systems. As of 2008, the National Health Insurance program covered more than 99% of the Taiwanese population (23 million people)21 and is therefore a nationally representative population. In the National Health Insurance Research Database (NHIRD), each data file has a unique identifier that is used to link different data files. In our study, the details of inpatient orders data sets were used to retrieve patient data collected between January 1, 2000, and December 12, 2015. Diseases were diagnosed according to the International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) codes.
Ethical considerations.
To protect patient privacy, all personal patient information from the NHIRD is encrypted before being released to the public. Therefore, all data for our study were confirmed to be deidentified and were analyzed anonymously, precluding signed informed consent. The study protocol was approved by the Ethics Review Board of the Tri-Service General Hospital (no. TSGH-B-109-32).
Study design and sample.
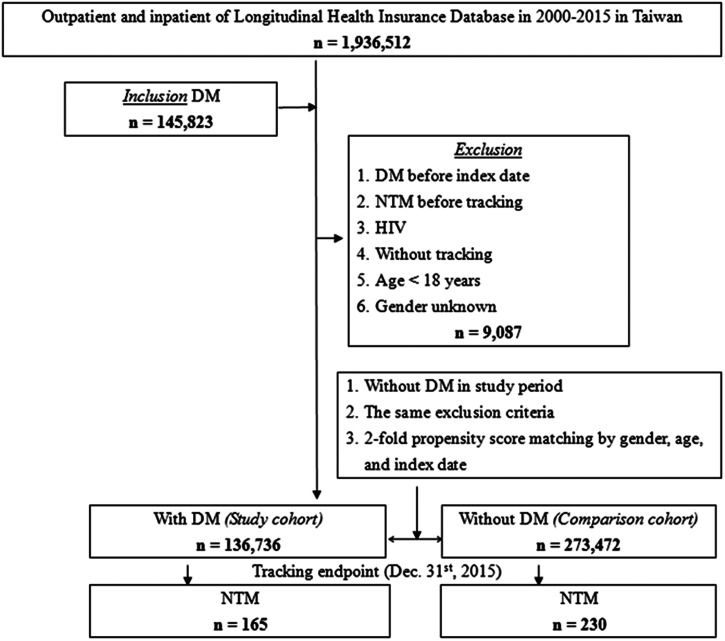
This study used a retrospective population-based cohort design. The flowchart in Figure 1 indicates how the study population was selected. After exclusions, a cohort of DM patients (N = 136,736) diagnosed (ICD-9-CM 250) from 2000 to 2015 was established for comparison with a matched non-DM cohort. Patients younger than 18 years (adult cases), DM patients diagnosed before the index date, patients without tracking or with NTM-caused diseases diagnosed before tracking, HIV patients, DM patients who had repeat NTM infections, and those with gender unknown were excluded. From the reimbursement claims data files, 273,472 people without DM were selected randomly as the reference cohort and were matched according to gender, age, index year, and occupation according to a 1:2 ratio of DM to non-DM patients.
Figure 1.
The flowchart for selection of study population. DM = diabetes mellitus; NTM = nontuberculous Mycobacterium.
Main outcomes.
The principal outcome was NTM-caused diseases (ICD-9-CM 031.0, 0.31.1, 031.2, 031.8, and 0.31.9), which included pulmonary (ICD-9-CM 031.0), cutaneous (ICD-9-CM 031.1), disseminated (ICD-9-CM 031.2), other specific (ICD-9-CM 031.8) (excluding pulmonary, cutaneous, and disseminated diseases), and unspecific (ICD-9-CM 038.9) (atypical NTM infection) diseases. In Taiwan, pulmonary disease resulting from NTM is diagnosed when clinical, radiological, and microbiological characteristics of the patient meet the criteria for NTM lung disease established in 1997 by the American Thoracic Society (ATS),22 as reported in recent study in which 55% of patients with NTM isolates met the ATS criteria for NTM disease.23 Based on patients’ ICD-9 codes, pleurisy resulting from NTM is diagnosed when NTM species are isolated from a pleural effusion specimen but not from other body sites. Disseminated disease is diagnosed when NTM isolates are recovered from more than one body site, or from blood or bone marrow. Lymphadenitis resulting from NTM is diagnosed when culture of a biopsy specimen or discharge of a lymph node yields an NTM isolate. Last, skin/soft tissue disease or osteomyelitis is diagnosed when NTM isolates are found in the culture of wound discharge or biopsy specimen of a lesion involving the skin, subcutaneous tissue, muscle, synovium, or bone.18 Follow-up appointments were terminated when NTM-caused diseases were diagnosed or at the end of December 31, 2015.
The independent variables were age, gender, insured premium, season, location of residence, urbanization level, hospital level, and the revised Charlson comorbidity index (CCI_R). CCI_R was defined as CCI that removed DM. The Charlson comorbidity index, as adapted by Deyo et al.,24 was used to assess the level of general comorbid conditions.
Statistical analysis.
The χ2 test and Student’s t-test were used to examine baseline demographic variables of patients with DM and non-DM. Categorical variables were compared using the χ2 test, and continuous variables were compared using Student’s t-test. Possible intervening variables (P < 0.05) among baseline variables listed in Table 1 were adjusted when subsequent statistical analyses were performed. Multivariate Cox proportional hazards regression analysis was used to estimate the hazard ratios and 95% CIs of NTM in the DM cohort compared with the non-DM cohort. Kaplan-Meier analysis was used to measure the cumulative NTM incidence for both study cohorts, and the log-rank test was used to evaluate differences between the two cumulative incidence curves. All statistical analyses were carried out using IBM SPSS statistical software (version 22 for Windows; IBM Corp., Armonk, NY). A P value of less than 0.05 in two-tailed tests was considered to be statistically significant.
Table 1.
Baseline study characteristics
| Characteristic | Total | DM | Non-DM | P value* |
|---|---|---|---|---|
| (N = 410,208) | (n = 136,736) | (n = 273,472) | ||
| Gender, n (%) | – | – | – | 1.00 |
| Male | 202,764 (49.43) | 67,588 (49.43) | 135,176 (49.43) | – |
| Female | 207,444 (50.57) | 69,148 (50.57) | 138,296 (50.57) | – |
| Age, y; mean (sd) | 63.38 (13.79) | 63.43 (12.48) | 63.35 (14.55) | 0.13 |
| Age group, y; n (%) | – | – | – | 1.00 |
| 18–44 | 36,840 (8.98) | 12,280 (8.98) | 24,560 (8.98) | – |
| 45–64 | 161,166 (39.29) | 53,722 (39.29) | 107,444 (39.29) | – |
| ≥ 65 | 212,202 (51.73) | 70,734 (51.73) | 141,468 (51.73) | – |
| Insured premium, NT$; n (%) | – | – | – | < 0.01† |
| < 18,000 | 405,604 (98.88) | 135,620 (99.18) | 269,984 (98.72) | – |
| 18,000–34,999 | 3,843 (0.94) | 991 (0.72) | 2,852 (1.04) | – |
| ≥ 35,000 | 761 (0.19) | 125 (0.09) | 636 (0.23) | – |
| CCI_R, mean (sd) | 0.91 (1.73) | 0.93 (2.04) | 0.90 (1.55) | < 0.01† |
| Season, n (%) | – | – | – | 1.00 |
| Spring (March–May) | 108,972 (26.57) | 36,324 (26.57) | 72,648 (26.57) | – |
| Summer (June–August) | 95,559 (23.30) | 31,853 (23.30) | 63,706 (23.30) | – |
| Autumn (September–November) | 85,833 (20.92) | 28,611 (20.92) | 57,222 (20.92) | – |
| Winter (December–February) | 119,844 (29.22) | 39,948 (29.22) | 79,896 (29.22) | – |
| Location, n (%) | – | – | – | < 0.01† |
| Northern Taiwan | 160,099 (39.03) | 51,698 (37.81) | 108,401 (39.64) | – |
| Middle Taiwan | 112,065 (27.32) | 36,684 (26.83) | 75,381 (27.56) | – |
| Southern Taiwan | 110,805 (27.01) | 39,701 (29.03) | 71,104 (26.00) | – |
| Eastern Taiwan | 25,275 (6.16) | 7,984 (5.84) | 17,291 (6.32) | – |
| Outlying islands | 1,964 (0.48) | 669 (0.49) | 1,295 (0.47) | – |
| Urbanization level, n (%) | – | – | – | 0.37 |
| 1 (the highest) | 138,062 (33.66) | 46,084 (33.70) | 91,978 (33.63) | – |
| 2 | 174,336 (42.50) | 58,222 (42.58) | 116,114 (42.46) | – |
| 3 | 29,970 (7.31) | 10,035 (7.34) | 19,935 (7.29) | – |
| 4 (the lowest) | 67,840 (16.54) | 22,395 (16.38) | 45,445 (16.62) | – |
| Level of care, n (%) | – | – | – | < 0.01† |
| Hospital center | 130,297 (31.76) | 41,422 (30.29) | 88,875 (32.50) | – |
| Regional hospital | 127,680 (31.13) | 43,893 (32.10) | 83,787 (30.64) | – |
| Local hospital | 152,231 (37.11) | 51,421 (37.61) | 100,810 (36.86) | – |
CCI_R = Charlson comorbidity index revised (CCI removed DM); DM = diabetes mellitus; NT$ = national Taiwanese dollars.
χ2/Fisher exact tests on category variables and t-test on continue variables.
P < 0.05
RESULTS
Patients’ baseline characteristics.
A total of 136,736 DM patients and 273,472 non-DM patients older than 18 years were included in this study (Figure 1 and Table 1). No significant differences were found in age, gender, and urbanization between the two cohorts at baseline or the end point (all P > 0.05, Table 1 and Supplemental Table S1). However, significant differences were found between the groups in insured premium, CCI_R, location of residence, and hospital level (all P < 0.05, Table 1 and Supplemental Table S1).
Associations between patient characteristics and incidence of NTM-caused diseases.
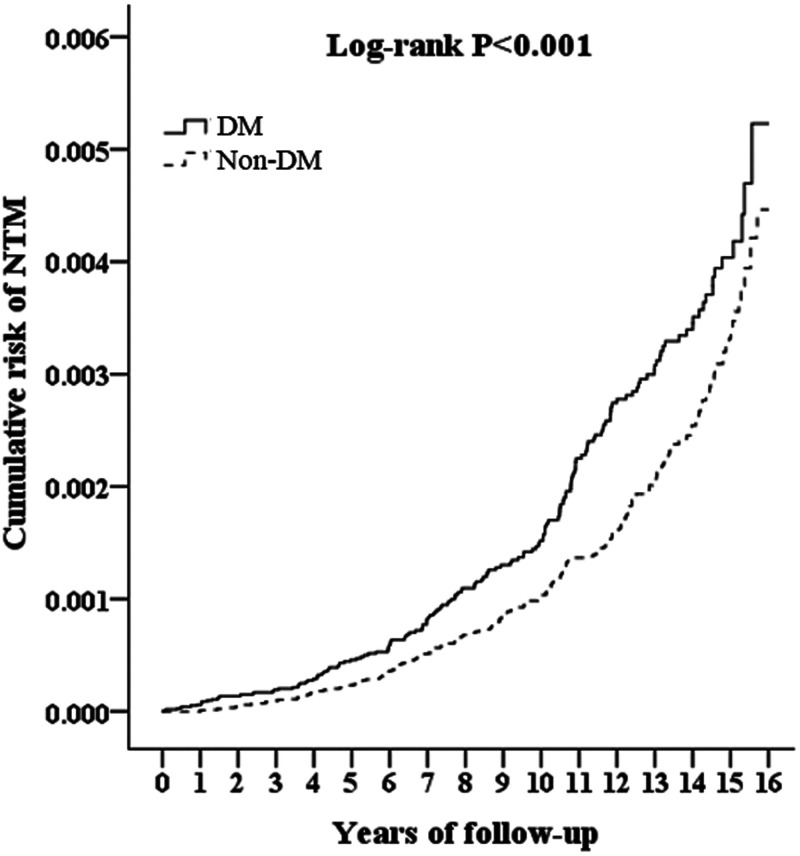
Table 2 shows the frequency of incident NTM-caused diseases, which was significantly increased in DM patients (0.12%) compared with non-DM patients (0.08%) (P < 0.05, Supplemental Table S1). Multivariate Cox regression analysis, after adjusting for possible confounding variables in Table 1, shows that the incidence of NTM-caused diseases in DM patients was approximately 1.43-fold (95% CI, 1.17–1.74) higher than in non-DM patients (P < 0.05, Table 2). High risk of incident NTM-caused diseases was also observed in patients with type 1 DM (T1DM), type 2 DM (T2DM), or a longer DM history (frequency of DM and interval from first to last DM diagnosis) compared with non-DM patients (all P < 0.05, Supplemental Table S2). Kaplan-Meier plot and log-rank tests showed that DM patients also had a high risk of incident NTM-caused diseases compared with non-DM patients across all years of tracking (all P < 0.005, Figure 2 and Supplemental Table S3). In addition, a high insured premium, high urbanization level, and high level of care also were associated with incident NTM-caused diseases (all P < 0.05, Table 2). After stratifying by the variables (age, gender, season, urbanization level, or level of care) listed in Table 2 and analyzing using multivariable Cox regression adjusted for these variables, each subgroup among DM patients had a greater risk of NTM-caused diseases than subgroups in non-DM patients (all P < 0.05, Supplemental Table S4), suggesting there were no interactions between these variables and DM for the risk of NTM-caused diseases.
Table 2.
Multivariate Cox regression analysis of patient characteristics associated with infection of nontuberculous Mycobacterium
| Variables | HR (95%CI) | P value | aHR* (95%CI) | P value |
|---|---|---|---|---|
| Diabetes mellitus | ||||
| Without | Ref. | – | Ref. | – |
| With | 1.44 (1.16–1.73) | < 0.01† | 1.43 (1.17–1.74) | < 0.01† |
| Gender | ||||
| Male | 1.34 (1.10–1.63) | < 0.01† | 1.36 (1.12–1.66) | < 0.01† |
| Female | Ref. | – | Ref. | – |
| Age group (y) | ||||
| 18–44 | Ref. | – | Ref. | – |
| 45–64 | 1.016 (0.57–1.75) | 0.96 | 1.08 (0.56–1.73) | 0.91 |
| ≥ 65 | 1.060 (0.62–1.81) | 0.85 | 1.14 (0.66–1.95) | 0.66 |
| Insured premium (NT$) | ||||
| < 8,000 | Ref. | – | Ref. | – |
| 18,000–34,999 | 1.18 (0.02–2.21) | 0.07 | 1.09 (0.03–1.29) | 0.09 |
| ≥ 35,000 | 1.23 (0.17–8.78) | 0.83 | 1.22 (0.17–8.71) | 0.84 |
| CCI_R | 1.18 (1.01–1.22) | < 0.01† | 1.07 (1.02–1.11) | 0.04† |
| Season | ||||
| Spring | Ref. | – | Ref. | – |
| Summer | 0.94 (0.72–1.24) | 0.62 | 0.95 (0.72–1.24) | 0.63 |
| Autumn | 0.82 (0.63–1.08) | 0.14 | 0.82 (0.63–1.08) | 0.14 |
| Winter | 0.86 (0.65–1.14) | 0.26 | 0.87 (0.65–1.15) | 0.29 |
| Location | ||||
| Northern Taiwan | Ref. | – | – | – |
| Middle Taiwan | 0.83 (0.65–1.06) | 0.12 | – | – |
| Southern Taiwan | 1.07 (0.85–1.35) | 0.60 | – | – |
| Eastern Taiwan | 0.20 (0.09–1.47) | 0.20 | – | – |
| Outlying islands | 0.53 (0.07–3.77) | 0.52 | – | – |
| Urbanization level | ||||
| 1 (the highest) | 2.82 (1.83–4.35) | < 0.01† | 2.79 (1.81–4.31) | < 0.01† |
| 2 | 2.43 (1.70–3.47) | < 0.01† | 1.77 (1.19–2.63) | < 0.01† |
| 3 | 1.84 (1.29–2.61) | < 0.01† | 1.51 (1.04–2.20) | 0.03† |
| 4 (the lowest) | Ref. | – | Ref. | – |
| Level of care | ||||
| Hospital center | 1.49 (1.15–1.93) | < 0.01† | 1.46 (1.08–1.97) | 0.02† |
| Regional hospital | 1.22 (1.05–1.35) | 0.02† | 1.14 (1.05–1.27) | 0.02† |
| Local hospital | Ref. | – | Ref. | – |
aHR = adjusted hazard ratio; CCI_R = Charlson comorbidity index revised (CCI removed DM); HR = hazard ratio; NT$ = national Taiwanese dollars.
Adjusted variables listed in the table.
P < 0.05.
Figure 2.
Kaplan-Meier plot for cumulative incidence of nontuberculous Mycobacterium (NTM)-caused diseases among patients with diabetes mellitus (DM) (solid line) and among those with non-DM (dashed line).
Associations between DM and incidence of specific NTM-caused disease.
As shown in Table 3, after adjusting for the variables listed in Table 2, DM patients exhibited a 1.13-fold (95% CI, 1.03–1.53), 3.88-fold (95% CI, 1.34–11.22), and 1.54-fold (95% CI, 1.13–2.11) greater risk of developing NTM-caused pulmonary and other specific (excluding pulmonary, cutaneous, and disseminated diseases) and unspecific (atypical NTM infection) diseases, respectively, than non-DM patients (all P < 0.05). In addition, DM patients had a 1.28-fold (95% CI, 1.05–1.60) and 1.82-fold (95% CI, 1.50–2.23) greater risk for developing single and repeated NTM-caused diseases, respectively that non-DM patients (all P < 0.05).
Table 3.
Multivariate Cox regression analysis of diabetes mellitus associated with infection of the nontuberculous Mycobacterium subgroup
| NTM subgroup | DM | Non-DM | DM vs. Non-DM | |||||
|---|---|---|---|---|---|---|---|---|
| Events | PYs | Rate (per 105 PYs) | Events | PYs | Rate (per 105 PYs) | aHR* (95%CI) | P value | |
| Overall | 165 | 1,754,087.97 | 9.41 | 230 | 3,524,118.09 | 6.53 | 1.43 (1.17–1.74) | < 0.01† |
| Pulmonary | 70 | 1,754,087.97 | 3.99 | 119 | 3,524,118.09 | 3.38 | 1.13 (1.03–1.53) | 0.01† |
| Cutaneous | 3 | 1,754,087.97 | 0.17 | 5 | 3,524,118.09 | 0.14 | 1.01 (0.20–4.23) | 0.89 |
| Disseminated | 5 | 1,754,087.97 | 0.29 | 12 | 3,524,118.09 | 0.34 | 0.65 (0.21–2.06) | 0.45 |
| Other specified‡ | 12 | 1,754,087.97 | 0.68 | 5 | 3,524,118.09 | 0.14 | 3.88 (1.34–11.22) | < 0.01† |
| Unspecified§ | 75 | 1,754,087.97 | 4.28 | 89 | 3,524,118.09 | 2.53 | 1.54 (1.13–2.11) | < 0.01† |
| Infection | ||||||||
| Single | 100 | 1,754,087.97 | 5.70 | 159 | 3,524,118.09 | 4.51 | 1.28 (1.05–1.60) | < 0.01† |
| Repeated | 65 | 1,759,806.22 | 3.69 | 71 | 3,537,121.14 | 2.01 | 1.82 (1.50–2.23) | < 0.01† |
aHR = adjusted hazard ratio; DM = diabetes mellitus; NTM = nontuberculous Mycobacterium; PYs = person-years.
Adjusted for the variables listed in Table 2.
P < 0.05.
NTM-caused diseases excluding pulmonary, cutaneous, and disseminated diseases
Atypical NTM infectious disease.
DISCUSSION
Our study is the first to evaluate associations between DM and the incidence of NTM-caused diseases. Results of this study have demonstrated that DM patients have a high risk of incident NTM-caused pulmonary disease and other specific and unspecific diseases compared with non-DM patients. Among the patients with DM, those with T1DM and T2DM had a similar high risk of NTM-caused diseases. In addition, as defined by ICD-9-CM codes, we found that NTM-caused pulmonary disease in DM patients was primarily a result of infection by M. avium, M. intracellulare, or M. kansasii. DM patients in the study cohort had more frequent NTM species-induced pulmonary diseases than non-DM patients. We suggest that DM is associated with NTM-caused disease, although further study is needed for confirmation because many risk factors are involved and there are many differences in the clinical status of DM patients.
NTM infections are reported to have four main clinical manifestations: pulmonary disease, lymphadenitis, skin/soft tissue and disseminated disease (as in blood infections or sepsis), of which pulmonary NTM appears to occur most commonly.23 We found that pulmonary disease had the greatest incidence in both the DM and non-DM groups during 15 years of follow-up. Furthermore, we demonstrated that the risk of incident NTM-caused pulmonary and other specific diseases was significantly greater in DM patients than in non-DM patients. In the “other specific diseases” category, NTM-caused syndromes excluded pulmonary, cutaneous, and disseminated diseases. Taken together, these results suggest that DM may be associated with the incidence of multiple NTM-caused diseases apart from cutaneous and disseminated diseases, and further investigation is clearly warranted.
Previous studies indicate that the distribution of NTM species varies geographically.13 MAC infections are the most common and have been reported in pulmonary NTM infections in North America and Asia, including in Taiwan.9,25–28 In contrast, a recent study by Zhang et al.23 showed that M. abscessus-chelonae infection was the most prevalent in pulmonary NTM disease among patients in Singapore, consistent with results of another Singapore study.29 The greater prevalence of M. abscessus in the Singapore setting was suggested by Zhang et al.23 to be attributed possibly to the combined effects of its tropical climate, densely populated urban living, and TB history, especially because M. chelonae is seldom isolated in chronic lung disease. Further studies are required to determine whether DM patients in tropical climates indeed have a greater risk of M. abscessus-caused pulmonary disease. Meanwhile, M. kansasii, which was demonstrated in our study and is noted to be especially virulent,28 has been considered rare in Asia, even though it was the fourth most common isolate in the recent Singapore study just mentioned.23 Huang et al.27 also reported that M. kansasii was a prominent contributor to the increase in the incidence of NTM infections in southern Taiwan in the 5-year period from 2010 to 2014.
Much also remains to be learned about the characteristics of NTM subspecies and related host susceptibility, as well as molecular mechanisms and possible target genes for treating drug-resistant NTM organisms. The pathogenicity of a particular NTM species is believed to interact with the host immune system to determine whether the host is susceptible.8 Both human leukocyte antigen phenotypes and genetic defects may be involved in pulmonary infections with different NTM species, such as the significant association shown between the NRAMP1 gene and NTM-caused nodular bronchiectasis.28 An association also was shown between the M. abscessus subspecies, the macrolide-resistant erm gene, greater disease severity, and poorer outcomes.29 Mycobacterium abscessus is reported to be the most pathogenic and drug-resistant NTM, contributing to complications and long, costly, largely ineffective antimicrobial treatment in infected patients.30 Antibiotic resistance of the NTM species associated most frequently with pulmonary manifestations, MAC and M. abscessus, are increasing interest in the molecular mechanisms of drug resistance.
Structural lung diseases (e.g., cystic fibrosis) and a history of TB are shown to predispose individuals to pulmonary NTM infection,9,23 along with advanced age (including older adult white women), male gender, lower body mass index, and immune system abnormalities.8 About 50% of patients with pulmonary NTM infection had chronic obstructive pulmonary disease (COPD) or bronchiectasis, with male patients having a greater incidence of COPD, and more often presenting with recurring cough and sputum production, whereas female patients have more frequent bronchiectasis and haemoptysis.23 These findings are consistent with those of other investigators.8 Similarly, data from our study show that men or patients with comorbidities (quantified by CCI_R) are at risk of incident NTM-caused diseases, although the specific comorbidities were not included in analysis. However, after stratifying by gender, male and female DM patients in our study had a similar risk of incident NTM-caused diseases. In addition, after adjusting for gender and comorbidities, DM patients still had a high risk of incident NTM-caused disease overall and pulmonary disease in particular. On the basis of these findings, we suggest that DM is an independent risk factor for the incidence of NTM-caused diseases, particularly pulmonary disease, and is not associated with gender or comorbidities.
Multiple NTM species isolated in a single geographic location or within a given time period is a characteristic of NTM lung infection, and the odds of isolating multiple species are especially high in COPD patients.27,31 In the recent study by Zhang et al.,23 about 30% of patients with pulmonary NTM had two or more species identified, especially in older adults. This may suggest that similar mechanisms, perhaps involving chronic inflammation and airway remodeling, underlie both COPD and bronchiectasis, and predispose patients to NTM infection. Because our study did not identify the proportion of comorbid COPD in DM-related, NTM-caused pulmonary diseases, or pulmonary disease caused by multiple NTM species, further investigation is needed to evaluate whether COPD is a major DM-associated, NTM-caused pulmonary disease; determine whether the DM-related incidence of pulmonary diseases is a result of infection of multiple NTM species; and determine whether chronic inflammation and remodeling of airways in comorbid COPD may play a role in the increased susceptibility of DM patients to NTM infection.
STRENGTHS AND LIMITATIONS
Our study was strengthened by the use of the NHIRD, which represents a nationwide population in Taiwan. This population-based longitudinal cohort study of the risk of incident NTM-caused disease in DM patients may thus be generalized to the general population of Taiwan, although it may not be generalized to other populations. Limitations of our study also must be considered when interpreting the findings. The NHIRD, for example, does not provide detailed information on possible DM-related factors such as smoking, body mass index, chest wall deformity, purified protein derivative skin tests, steroid use, antibiotic use, or glucose control (HbA1c), which could be potential confounding factors.
CONCLUSION
DM is associated with NTM-caused diseases, which may include pulmonary and other specific and unspecific diseases. Both T1DM and T2DM patients have a high risk of NTM-caused diseases. Increased patient, physician, and public awareness of the high risk of NTM-caused infection in DM may help to reduce risk, and anti-NTM therapy may be the most effective strategy by which to reduce the impact of NTM-caused diseases in DM patients at high risk of these infections. NTM-caused infection in DM patients warrants further study.
Supplemental Material
ACKNOWLEDGMENTS
We thank the National Defense Medical Center team for support.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1. Baker RG, Hayden MS, Ghosh S, 2011. NF-kappaB, inflammation, and metabolic disease. Cell Metab 13: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Viswanathan V, Vigneswari A, Selvan K, Satyavani K, Rajeswari R, Kapur A, 2014. Effect of diabetes on treatment outcome of smear-positive pulmonary tuberculosis: a report from South India. J Diabetes Complications 28: 162–165. [DOI] [PubMed] [Google Scholar]
- 3. Bridson T, Matthiesson A, Owens L, Govan B, Norton R, Ketheesan N, 2015. Diabetes: a contributor to tuberculosis in tropical Australia. Am J Trop Med Hyg 93: 547–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin YH, Chen CP, Chen PY, Huang JC, Ho C, Weng HH, Tsai YH, Peng YS, 2015. Screening for pulmonary tuberculosis in type 2 diabetes elderly: a cross-sectional study in a community hospital. BMC Public Health 15: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar Nathella P, Babu S, 2017. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology 152: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO , 2017. World Health Organization (WHO): Global Tuberculosis Report 2017. Geneva, Switzerland: World Healther Organization. Available at: https://www.who.int/tb/publications/global_report/en/. Accessed September 8, 2021.
- 7. Vaerewijck MJ, Huys G, Palomino JC, Swings J, Portaels F, 2005. Mycobacteria in drinking water distribution systems: ecology and significance for human health. FEMS Microbiol Rev 29: 911–934. [DOI] [PubMed] [Google Scholar]
- 8. Chan ED, Iseman MD, 2013. Underlying host risk factors for nontuberculous mycobacterial lung disease. Semin Respir Crit Care Med 34: 110–123. [DOI] [PubMed] [Google Scholar]
- 9. Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, Saulson A, Hedberg K, 2010. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med 182: 977–982. [DOI] [PubMed] [Google Scholar]
- 10. Hodgson KA, Morris JL, Feterl ML, Govan BL, Ketheesan N, 2011. Altered macrophage function is associated with severe Burkholderia pseudomallei infection in a murine model of type 2 diabetes. Microbes Infect 13: 1177–1184. [DOI] [PubMed] [Google Scholar]
- 11. Orme IM, Ordway DJ, 2014. Host response to nontuberculous mycobacterial infections of current clinical importance. Infect Immunol 82: 3516–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drummond WK, Kasperbauer SH, 2019. Nontuberculous mycobacteria: epidemiology and the impact on pulmonary and cardiac disease. Thorac Surg Clin 29: 59–64. [DOI] [PubMed] [Google Scholar]
- 13. Hoefsloot W. et al. , 2013. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J 42: 1604–1613. [DOI] [PubMed] [Google Scholar]
- 14. Jackson E, Stewart A, Maguire EJ, Norton RE, 2007. Mycobacterial soft tissue infections in North Queensland. ANZ J Surg 77: 368–370. [DOI] [PubMed] [Google Scholar]
- 15. Song JW, Koh WJ, Lee KS, Lee JY, Chung MJ, Kim TS, Kwon OJ, 2008. High-resolution CT findings of Mycobacterium avium intracellulare complex pulmonary disease: correlation with pulmonary function test results. AJR Am J Roentgenol 191: W160–W166. [DOI] [PubMed] [Google Scholar]
- 16. Jeong YJ, Lee KS, Koh WJ, Han J, Kim TS, Kwon OJ, 2004. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology 231: 880–886. [DOI] [PubMed] [Google Scholar]
- 17. Fjallbrant H, Akerstrom M, Svensson E, Andersson E, 2013. Hot tub lung: an occupational hazard. Eur Respir Rev 22: 88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ding LW, Lai CC, Lee LN, Hsueh PR, 2006. Disease caused by non-tuberculous mycobacteria in a university hospital in Taiwan, 1997–2003. Epidemiol Infect 134: 1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yeh JJ, Wang YC, Sung FC, Chou CY, Kao CH, 2014. Nontuberculosis Mycobacterium disease is a risk factor for chronic obstructive pulmonary disease: a nationwide cohort study. Lung 192: 403–411. [DOI] [PubMed] [Google Scholar]
- 20. Oh TH, Kim UJ, Kang SJ, Jang HC, Park KH, Jung SI, Ahn JH, 2018. Disseminated invasive Mycobacterium marinum infection involving the lung of a patient with diabetes mellitus. Infect Chemother 50: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Health Insurance Administration , 2020. Universal Health Coverage in Taiwan. Available at: https://www.nhi.gov.tw/english/Content_List.aspx?n=8FC0974BBFEFA56D&topn=ED4A30E51A609E49. Accessed September 8, 2021.
- 22. American Thoracic Society , 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med 156: S1–S25. [DOI] [PubMed] [Google Scholar]
- 23. Zhang ZX, Cherng BPZ, Sng LH, Tan YE, 2019. Clinical and microbiological characteristics of non-tuberculous mycobacteria diseases in Singapore with a focus on pulmonary disease, 2012–2016. BMC Infect Dis 19: 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deyo RA, Cherkin DC, Ciol MA, 1992. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 25. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR, 2012. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 185: 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brode SK, Marchand-Austin A, Jamieson FB, Marras TK, 2017. Pulmonary versus nonpulmonary nontuberculous mycobacteria, Ontario, Canada. Emerg Infect Dis 23: 1898–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang HL, Cheng MH, Lu PL, Shu CC, Wang JY, Wang JT, Chong IW, Lee LN, 2017. Epidemiology and predictors of NTM pulmonary infection in Taiwan: a retrospective, five-year multicenter study. Sci Rep 7: 16300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koh WJ, Kwon OJ, Lee KS, 2005. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci 20: 913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lim AYH, Chotirmall SH, Fok ETK, Verma A, De PP, Goh SK, Puah SH, Goh DEL, Abisheganaden JA, 2018. Profiling non-tuberculous mycobacteria in an Asian setting: characteristics and clinical outcomes of hospitalized patients in Singapore. BMC Pulm Med 18: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim HJ, Park CM, Park YS, Lee J, Lee SM, Yang SC, Yoo CG, Kim YW, Han SK, Yim JJ, 2011. Isolation of multiple nontuberculous mycobacteria species in the same patients. Int J Infect Dis 15: e795–e798. [DOI] [PubMed] [Google Scholar]
- 31. Luthra S, Rominski A, Sander P, 2018. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front Microbiol 9: 2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.