ABSTRACT.
Visible signs of disease can evoke stigma while stigma contributes to depression and mental illness, sometimes manifesting as somatic symptoms. We assessed these hypotheses among Ebola virus disease (EVD) survivors, some of whom experienced clinical sequelae. Ebola virus disease survivors in Liberia were enrolled in an observational cohort study starting in June 2015 with visits every 6 months. At baseline and 18 months later, a seven-item index of EVD-related stigma was administered. Clinical findings (self-reported symptoms and abnormal findings) were obtained at each visit. We applied the generalized estimating equation method to assess the bidirectional concurrent and lagged associations between clinical findings and stigma, adjusting for age, gender, educational level, referral to medical care, and HIV serostatus as confounders. When assessing the contribution of stigma to later clinical findings, we restricted clinical findings to five that were also considered somatic symptoms. Data were obtained from 859 EVD survivors. In concurrent longitudinal analyses, each additional clinical finding increased the adjusted odds of stigma by 18% (95% CI: 1.11, 1.25), particularly palpitations, muscle pain, joint pain, urinary frequency, and memory loss. In lagged associations, memory loss (adjusted odds ratio [AOR]: 4.6; 95% CI: 1.73, 12.36) and anorexia (AOR: 4.17; 95% CI: 1.82, 9.53) were associated with later stigma, but stigma was not significantly associated with later clinical findings. Stigma was associated with select symptoms, not abnormal objective findings. Lagged associations between symptoms and later stigma substantiate the possibility of a pathway related to visible symptoms identified by community members and leading to fear of contagion.
INTRODUCTION
Over half of the 28,616 individuals who were diagnosed with Ebola virus disease (EVD) survived during the 2013– 2016 West Africa outbreak.1,2 Many of these EVD survivors faced discriminatory and stigmatizing attitudes upon their return to the community, slowing the process of reintegration.3–5 Survivors suffered from social isolation, job loss, disruption of resources, chronic stress, and various hardships related to these stigmatizing attitudes.3,6–9 The challenges encountered by survivors support Goffman’s definition of stigma as discriminatory attitudes that are “deeply discrediting” and exclude individuals from full social acceptance.10 In addition to the social impact of stigma, existing evidence demonstrates its negative effect on physical and mental health outcomes,9,11 reinforcing the importance of better understanding stigma toward EVD survivors and the factors that allow it to persist.
As described by Jones et al., conditions accompanied by visual symptoms evoke stronger antisocial reactions than conditions that can be concealed; “visible concealability” is included as one of the most important psychological components of stigma.12,13 Conditions with apparent symptoms, such as leprosy and visible skin conditions, lead to higher levels of stigma.14–16 The disease avoidance model provides a practical framework for examining this relationship, positing that individuals and society avoid those with visible signs or labels that connotate the disease.17,18 The psychological processes that evolved to identify threats of disease can cause the misinterpretation of visible cues, leading to avoidance of and stigma toward those with signs of disease even when there is no true threat of contagion.17,18
Furthermore, existing qualitative studies suggest a potential bidirectional relationship between clinical findings and stigma toward EVD survivors, with possible explanations including the fear of contagion toward those with visible disease and the impact of stigma on mental and physical health.3,4,19 Ebola virus disease survivors with an acute illness may have experienced stigmatizing attitudes from community members because of the fear of infection, contributing to internalized stigma;3 conversely, perceived and internalized stigma may have contributed to depression and mental illness, manifesting as somatic symptoms.20,21 Such a relationship between stigma and clinical manifestations has been observed in other stigmatized diseases, such as HIV.22
Post-EVD clinical sequelae—such as uveitis, muscle plain, and memory loss—and stigma among EVD survivors have been independently reported to resolve over the same time period in Liberia, raising the question of whether these phenomena in EVD survivors are linked.23–27 Considering that visual symptoms experienced by EVD survivors had the potential to evoke stronger antisocial reactions, we hypothesized that the lack of “visible concealability” of post-EVD clinical sequelae will lead to stigma. Alternatively, EVD survivors faced depression, anxiety, and other poor mental health outcomes. We hypothesized that EVD survivors who perceived or internalized stigma will be at risk for these poor mental health outcomes, which have the potential to manifest as somatic symptoms.
METHODS
Study participants and procedures.
This investigation used data from the observational cohort study of EVD survivors implemented by the Partnership for Research on Ebola Virus in Liberia (PREVAIL III: Ebola Natural History Study). Starting in June 2015, PREVAIL III used a proactive recruitment strategy to identify eligible EVD survivors and their close contacts in the seven highly EVD-affected counties.24 Ebola virus disease survivors of any age were considered eligible for enrollment if they were listed in the national registry provided by the Ministry of Health. The current data analysis included EVD survivors who met the eligibility criteria and were seropositive to Ebola virus (EBOV)-specific anti-glycoprotein (GP); it excluded seronegative survivors.
Study visits occurred every 6 months during which trained Liberian health providers conducted a medical history, review of symptoms, and physical examination. Questions on EVD-related stigma were included in the questionnaire at baseline and 18 months after baseline. These questions were directed to EVD survivors who were 12 years and above. More details about the parent PREVAIL III study can be found elsewhere.24
Data collection and measurements.
We used the review of systems and physical examination to assess for abnormal clinical findings. Clinical findings were defined as the presence of self-reported symptoms (yes/no) or as abnormal findings (yes/no). The severity of symptoms and findings were not recorded. See Supplemental Tables 1 and 2 for more details.
To focus our investigation on the association of clinical findings and stigma and reduce the likelihood of Type I errors, we chose to examine previously identified clinical findings associated with post-EVD clinical sequelae, or clinical findings identified in the random forests as having greater variable importance than the most significant association (and endorsed in focus groups). As published elsewhere,24 clinical data from a 1-year assessment of PREVAIL III were used to compare the prevalence of symptoms and abnormal findings among survivors and their close contacts. Other than uveitis, abnormal findings were collapsed into systems because of low prevalence. Ebola virus disease survivors had a higher prevalence of the following symptoms and systems of abnormal findings than close contacts: fatigue, headache, muscle pain, joint pain, urinary frequency, memory loss, chest findings, abdominal findings, musculoskeletal findings, neurologic findings, and uveitis (all with P < 0.0001).24 In the direction assessing stigma and later clinical findings, we restricted our use of the clinical findings to those that overlapped with somatic symptoms given our hypothesized underlying pathways.
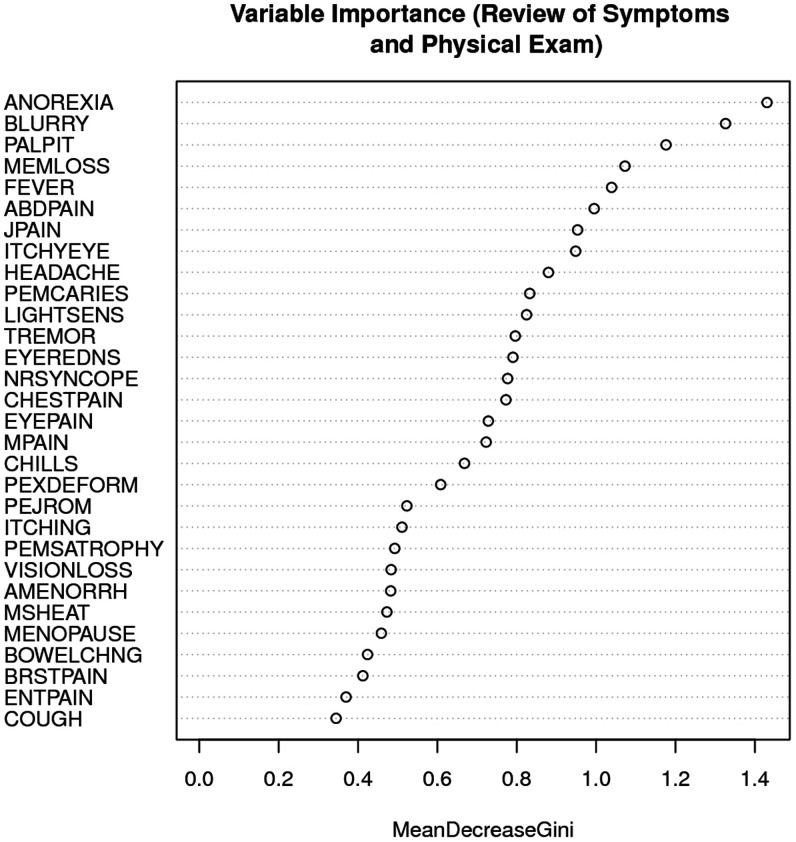
Given the low prevalence of stigma at 18 months, we used random forests to identify additional clinical findings (beyond those more common among survivors than contacts) relevant to the potential association with stigma. A random forest uses a multitude of regression trees to predict an outcome. These analyses had stigma at 18 months as the outcome and all symptoms (94 items) and abnormal findings (62 items) at 12 months as potential predictors. We used a variable importance metric, mean decrease accuracy, to assess the relevance of various symptoms and abnormal findings. Mean decrease accuracy measures the amount of prediction accuracy is lost when each variable is excluded. The predictors we found to have greater importance than the other common findings among survivors were included in analyses. We identified anorexia, blurry vision, and palpitations as variables with greater importance assigned to them than memory loss. We discussed these additional variables in a focus group. Anorexia was potentially relevant to the direction considering clinical findings associated with later stigma, whereas palpitations was potentially relevant to the direction considering stigma associated with later clinical findings. Thus, we included palpitations and anorexia in our investigation.
Uveitis was considered a negative control in this study because a relationship with stigma was not expected and participants were unaware that they had uveitis at the time of interview. Moreover, most cases of uveitis were inactive at the time of diagnosis (baseline eye visit) and manifested only by scarring (only 5% of participants had active uveitis). The median visual acuity of participants with uveitis was near normal (20/25). In addition, the ophthalmology group of PREVAIL III who cared for survivors observed that a diagnosis or clinical findings consistent with EVD-associated uveitis did not prompt reports of stigma. This was substantiated by the literature.28
Ebola virus disease–related stigma was measured by a seven-item index adapted from the People Living with HIV (PLHIV) Stigma Index.29 More details about the development of the EVD-related stigma index can be found elsewhere.23 Briefly, the PLHIV Stigma Index has been administered to over 100,000 PLHIV in more than 90 countries, including Liberia in 2013. During the EVD outbreak in Liberia, PREVAIL III study staff used a combination of focus groups of EVD survivors and mental health and EVD experts to select, adapt, and test stigma items that were specifically relevant to the experience of EVD survivors. Of the seven stigma items, two are related to discriminatory and stigmatizing attitudes from other people, two are related to access to work and social services, and three are related to internalized stigma. The sum of affirmative responses to each yes/no item was defined as the EVD-related stigma index.
The investigator team for this study, led by M. Badio and J. Kelly, considered expert opinion and other evidence to identify potential confounders from the questionnaire administered at study baseline.23 These covariates included age, sex, educational level, referral to partnering health facilities for medical care (not related to ophthalmology), and HIV serostatus.
Statistical analyses.
Clinical findings were dichotomized and considered the dependent variables. Stigma was a variable based on seven items and considered the independent variable. We assessed concurrent and lagged associations between stigma and clinical findings using data from baseline to 18 months. Stigma data were only collected at baseline and 18 months, and these available data shaped the study visits used for our analyses. In contrast, clinical findings data were available at each study visit. To assess concurrent associations for each clinical finding and stigma, we used pooled data from baseline and 18 months. In addition, we summed the clinical findings and assessed the relationship with stigma. For lagged associations, data used from study visits depended on the direction of the relationship being assessed. The temporal lag was 6 months because of the time period between study visits. To assess stigma associated with later clinical findings, we used baseline stigma and month 6 clinical findings. To assess clinical findings associated with later stigma, we used month 12 clinical findings and month 18 stigma. See Supplemental Table 6 for more details on the PREVAIL III measurement timeline and available data for analysis.
We applied the generalized estimating equation (GEE) method with an exchangeable correlation structure to account for correlated responses from the same participant. Each clinical finding was assessed with a multivariate statistical model. Analyses with data from ophthalmic exams and semen collection was limited because of the dyssynchronous timing of these substudy visits. To control the overall Type I error rate with multiple hypothesis testings, a P value of <0.01 was considered to be statistically significant and used for all of our analyses.
We conducted several sensitivity analyses. We grouped individual symptoms by body system and detected a similar magnitude of associations when we reran the analyses (Supplemental Table 3). We evaluated the distribution of missing data by study visit (e.g., participants who had data at baseline but not at 18 months, and vis-versa) and found no significant predictors of missing data. Analyses were performed using STATA/IC (Version 13.1, STATA Corporation, College Station, TX) and R (Version 3.2.3, R Foundation for Statistical Computing, Vienna, Austria) including packages xtable, gee, and RandomForest.
Ethics statement.
The National Research Ethics Board of Liberia and the National Institute of Allergy and Infectious Diseases Institutional Review Board (IRB) at the United States National Institutes of Health approved the PREVAIL III study protocol. Before any study-related procedures were conducted, participants signed or marked the approved informed consent form, and parents or guardians provided this consent on behalf of all child participants, while adolescents provided assent as appropriate.
RESULTS
Partnership for Research on Ebola Virus in Liberia III enrolled 1,145 EVD survivors. In these analyses, we excluded participants who did not have antibodies to Ebola virus and were under the age of 12. There were 859 remaining EVD survivors (75%). Of these seropositive participants, we obtained a follow-up measurement of EVD-related stigma from 740 EVD survivors at 18 months. The baseline visit was a median of 352 days (interquartile range: 306, 402) after discharge from an Ebola treatment unit. From baseline to 18 months, EVD survivors who reported at least one item from the EVD-related stigma index declined from 63% to 5%. Characteristics of clinical findings and EVD-related stigma measured at each study visit can be found in Supplemental Tables 4 and 5.
At study baseline, there was a broad distribution of ages, which were as follows: age 12–19, 141 (16.4%); age 20–29, 245 (28.5%); age 30–39, 231 (26.9%); age 40–49, 144 (16.8%); age 50 or older, 98 (11.4%). The minority (44.0%) was male. One-fifth (20.6%) of survivors had not completed any formal education. Nearly half (41.2%) were referred to partnering health facilities for medical care. The HIV seropositivity among survivors (1.4%). These characteristics were similar at 18 months (Table 1) with the exception of fewer referrals to medical care (12%). There were 77 men who were positive for Ebola virus in their semen before 18 months. Only nine (11.7%) of these men endorsed any stigma at 18 months.
Table 1.
Participant characteristics and study baseline and visit 4
| Baseline (%) | Visit 4 (18 months; %) | P value | |
|---|---|---|---|
| N = 859 | N = 740 | ||
| Age 12–19 | 16.4 | 16.2 | – |
| Age 20–29 | 28.5 | 29.1 | – |
| Age 30–39 | 26.9 | 27.2 | 0.998 |
| Age 40–49 | 16.8 | 16.4 | – |
| Age 50+ | 11.4 | 11.2 | – |
| Male | 44 | 43 | 0.716 |
| Education (No formal education) | 20.6 | 19.3 | – |
| Education (Primary, junior high or vocational) | 38 | 38.2 | 0.807 |
| Education (High school or beyond) | 41.4 | 42.4 | – |
| Referred to medical care | 41.2 | 12 | < 0.001 |
| HIV-positive | 1.4 | 2 | 0.444 |
Concurrent associations.
Each additional clinical finding increased the adjusted odds of stigma by 18% (95% CI: 1.11, 1.25). We found concurrent associations with palpitations (adjusted odds ratio [AOR]: 1.22; 95% CI: 1.10, 1.35), muscle pain (AOR: 1.22; 95% CI: 1.10, 1.35), joint pain (AOR: 1.19; 95% CI: 1.08, 1.31), urinary frequency (AOR: 1.32; 95% CI: 1.18, 1.48), and memory loss (AOR: 1.22; 95% CI: 1.10, 1.35) (Table 2). The associations between concurrent abnormal findings observed on examination and stigma did not reach statistical significance.
Table 2.
Concurrent associations between clinical findings and stigma
| Clinical finding | Odds ratio (95% CI) | P value |
|---|---|---|
| Fatigue | 1.14 (1.02, 1.27) | 0.02 |
| Muscle pain | 1.22 (1.1, 1.35) | <0.001 |
| Joint pain | 1.19 (1.08, 1.31) | <0.001 |
| Headache | 1.02 (0.93, 1.12) | 0.692 |
| Urinary frequency | 1.32 (1.18, 1.48) | <0.001 |
| Memory loss | 1.22 (1.1, 1.35) | <0.001 |
| Anorexia | 1.1 (0.98, 1.23) | 0.105 |
| Palpitations | 1.24 (1.1, 1.4) | <0.001 |
| Chest exam findings | 1.05 (0.84, 1.31) | 0.647 |
| Neurological exam findings | 1.03 (0.84, 1.27) | 0.767 |
| Abdominal exam findings | 1.14 (1.01, 1.29) | 0.029 |
| Musculoskeletal exam findings | 0.99 (0.82, 1.21) | 0.937 |
Lagged associations.
We found lagged associations with memory loss (AOR: 4.6; 95% CI: 1.73, 12.36) and anorexia (AOR: 4.17; 95% CI: 1.82, 9.53) and later stigma. Palpitations had 2.9 times the odds of later stigma, but the association did not reach statistical significance. No abnormal findings were associated with later stigma. Uveitis was our negative control and not found to be associated with later stigma (AOR: 0.99; 95% CI: 0.46, 2.14) (Table 3). We found no lagged associations with stigma and later clinical findings (Table 4).
Table 3.
Lagged associations between clinical findings and later stigma
| Clinical finding | Lagged association | Lagged association |
|---|---|---|
| Odds Ratio (95% CI) | P value | |
| Fatigue | 1.09 (0.24, 4.89) | 0.907 |
| Anorexia | 4.17 (1.82, 9.53) | 0.001 |
| Headache | 1.32 (0.64, 2.7) | 0.454 |
| Palpitations | 2.87 (1.04, 7.93) | 0.042 |
| Muscle pain | 1.3 (0.52, 3.27) | 0.573 |
| Joint pain | 1.54 (0.74, 3.19) | 0.25 |
| Urinary frequency | 0 (0, Inf) | 0.988 |
| Memory loss | 4.63 (1.73, 12.36) | 0.002 |
| Uveitis | 0.99 (0.46, 2.14) | 0.989 |
| Chest exam findings | 0 (0, Inf) | 0.991 |
| Abdominal exam findings | 0.4 (0.09, 1.75) | 0.226 |
| Musculoskeletal exam findings | 2.03 (0.43, 9.57) | 0.37 |
| Neurological exam findings | 2.14 (0.26, 17.98) | 0.482 |
Table 4.
Lagged associations between stigma and later clinical findings
| Clinical finding | Lagged association | Lagged association |
|---|---|---|
| Odds ratio (95% CI) | P value | |
| Fatigue | 0.95 (0.8, 1.14) | 0.599 |
| Headache | 1.06 (0.96, 1.18) | 0.275 |
| Palpitations | 1.11 (0.94, 1.31) | 0.225 |
| Muscle pain | 1.08 (0.94, 1.23) | 0.283 |
| Joint pain | 1.04 (0.93, 1.15) | 0.513 |
Random forest plots.
This analysis largely recapitulated our findings from the concurrent associations because statistically significant clinical findings, particularly memory loss, muscle pain, joint pain, and headache, were among the important variables identified in the random forest plot (Figure 1). Memory loss was the variable of highest importance among statistically significant clinical findings.
Figure 1.
Variable importance of clinical findings and later stigma.
DISCUSSION
In a large cohort of Ebola survivors, we found that stigma was strongly associated with select symptoms but not abnormal findings on examination. Lagged associations between clinical symptoms and later stigma substantiate the possibility of a pathway related to visible symptoms identified by community members and leading to fear of contagion. Other symptoms, most of which were also apparent to community members (e.g., muscle and joint pains), were only identified in concurrent associations, suggesting that greater power at 12 months and 18 months may have identified more lagged associations and generated stronger evidence that select clinical symptoms predict stigma. Additional clinical symptoms contributing to stigma are supported by other qualitative studies.3,4,19 Although lagged analyses did not provide additional evidence to support underlying pathways related to mental health or healthcare behaviors, the temporal lag of 6 months was long enough that survivors could receive psychosocial services, resolve personal stressors, or notice a change in healthcare provider attitudes. Thus, the temporal lag may have been too long to detect associations with stigma and later clinical symptoms but short enough to detect associations within clinical symptoms and later stigma. This may explain why some concurrent longitudinal associations (e.g., muscle pain) were not attributed to either lagged analysis.
Disentangling the directionality of the relationship between clinical findings and stigma may inform the extent to which these associations are the result of social process, pathophysiological mechanisms, mental illness, or a combination. Our evidence that memory loss and anorexia may have caused stigma supports the concept that these cause–effect relationships may be the result of a societal process. During the West African outbreak, studies of the natural history of EVD characterized clinical sequelae and documented that EBOV RNA can persist over time, particularly in the semen.26,27,30,31 Community members perceived EVD survivors to be contagious, particularly when they were sick (e.g., clinical sequelae).3 These perceptions may have contributed to discriminatory and stigmatizing attitudes toward survivors, even though EBOV viral persistence rapidly declined and transmission was rarely observed to occur.32–34 These attitudes led some survivors to deny being ill.19 At the end of the West African outbreak, the WHO updated their advice on several issues, including sexual transmission of EVD and clinical care for survivors.35,36 These updates and other scientific advances in our knowledge of EBOV research, including transmission, were slow to be disseminated to West African communities, if they were disseminated at all. Public health campaigns about lessons learned from EVD research may be considered as part of outbreak preparedness strategies and have the potential to benefit response teams and EVD survivors during current and future EVD outbreaks.
Post-EVD clinical sequelae have been described as a collection of self-reported symptoms and abnormal findings that may be the result of various pathophysiological mechanisms.24,37 Ebola virus viral persistence has been linked to pathological mechanisms that can cause uveitis and meningoencephalitis.38,39 Notably, these were extraordinary cases of expatriated EVD responders who were critically ill and received a higher level of care than available in West Africa. For the overwhelming majority of EVD survivors who experienced at least one post-EVD clinical sequela, the underlying pathophysiological mechanism is unknown. Some of these symptoms may be linked to somatic symptoms, and mental illness such as posttraumatic stress or depression may be part of the contributing pathway.3 We did not find strong evidence, however, that stigma may explain somatic symptoms, which could be conflated with other pathophysiological mechanisms such as viral persistence.
This study had limitations. EVD-related stigma was rare at 18 months, and this limited the power of our analyses to determine the impact of clinical findings on later stigma, as witnessed in wide CIs. The 6-month temporal lag was long and therefore the lagged association may not be sensitive enough to detect concurrent associations observed with certain clinical findings. Participants were enrolled on average about 1 year post-EVD, and community members may have feared contagion for shorter periods of time. Certain relationships between post-EVD clinical sequelae and stigma may have occurred and resolved before the PREVAIL III study. Furthermore, stigma was only assessed at baseline and 18 months, which limited our ability to assess the relationship closer to survival. When the PREVAIL III study began, it enrolled the largest cohort of EVD survivors in West Africa and, although this was not a population-based sample, the sample was largely representative of those highly EVD-affected Liberian counties. Checks on missing data between baseline and 18 months suggested that we did not observe selection bias during the follow-up period. Although there were large amounts of data collected on participating EVD survivors, a complete medical history was difficult to obtain because of poor medical record-keeping in Liberia, so there may have been some unmeasured confounding. For uveitis, we did not find an association with stigma, as expected, which served as a negative control and gave additional validity to our findings. Although these findings may provide some evidence of temporality and support the potential of a causal relationship, we were unable to unequivocally determine causality.
This study established a quantitative and directional relationship in which select symptoms contributed to stigma among EVD survivors. Distrust and stigma have been reported during the subsequent EVD outbreaks in the Democratic Republic of the Congo, which offer opportunities for intervention development and additional longitudinal studies of the causal pathways as more EVD cases survive and face the possibility of being stigmatized, particularly when they become sick and their symptoms are visible to community members. The public health community has a responsibility to eliminate stigmatizing and discriminatory attitudes against survivors in every way possible.
Supplemental Material
ACKNOWLEDGMENTS
We want to thank the Ebola virus disease (EVD) survivors for their participation and the PREVAIL team for making this study possible. We appreciate the input and support of M. Maria Glymour and H. Clifford Lane.
Note: Supplemental file appears at www.ajtmh.org.
REFERENCES
- 1.Regional Office for Africa of World Health Organization, 2021. Health Topics. Ebola Virus Disease. Situation Reports from 2014--2016. Available at: https://www.afro.who.int/health-topics/ebola-virus-disease. Accessed September 20, 2021.
- 2. WHO Ebola Response Team , 2016. After Ebola in West Africa–unpredictable risks, preventable epidemics. N Engl J Med 375: 587–596. [DOI] [PubMed] [Google Scholar]
- 3. Rabelo I, Lee V, Fallah MP, Massaquoi M, Evlampidou I, Crestani R, Decroo T, Van den Bergh R, Severy N, 2016. Psychological distress among Ebola survivors discharged from an Ebola treatment unit in Monrovia, Liberia—a qualitative study. Front Public Health 4: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tambo E, Chengho CF, Ugwu CE, Wurie I, Jonhson JK, Ngogang JY, 2017. Rebuilding transformation strategies in post-Ebola epidemics in Africa. Infect Dis Poverty 6: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Focus 1000, US Centers for Disease Control and Prevention, UNICEF, 2015. Study of Public Knowledge, Attitudes, and Practices Relating to Ebola Virus Disease Prevention and Medical Care in Sierra Leone (Ebola KAP-4), Final Report. Freetown, Sierra Leone: Focus1000. Available at: http://focus1000.org/index.php/downloads-resources/send/4-ebola-kap-study/32-kap-4-preliminary-findings. Accessed December 15, 2019.
- 6. Jagadesh S, Sevalie S, Fatoma R, Sesay F, Sahr F, Faragher B, Semple MG, Fletcher TE, Weigel R, Scott JT, 2018. Disability among Ebola survivors and their close contacts in Sierra Leone: a retrospective case-controlled cohort study. Clin Infect Dis 66: 131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis N, Alva S, Son Y, 2017. Comprehensive Program for Ebola Survivors: Baseline Assessment. Arlington, VA: Advancing Partners & Communities. [Google Scholar]
- 8. Lee-Kwan SH, DeLuca N, Adams M, Dalling M, Drevlow E, Gassama G, Davies T, Centers for Disease Control and Prevention (CDC) , 2014. Support services for survivors of ebola virus disease—Sierra Leone, 2014. MMWR Morb Mortal Wkly Rep 63: 1205–1206. [PMC free article] [PubMed] [Google Scholar]
- 9. Link BG, Phelan JC, 2006. Stigma and its public health implications. Lancet 367: 528–529. [DOI] [PubMed] [Google Scholar]
- 10. Goffman E, 1963. Stigma: Notes on the Management of Spoiled Identity. Englewood Cliffs, NJ: Prentice-Hall, Inc. [Google Scholar]
- 11. Hatzenbuehler ML, Phelan JC, Link BG, 2013. Stigma as a fundamental cause of population health inequalities. Am J Public Health 103: 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park JH, Faulkner J, Schaller M, 2003. Evolved disease-avoidance processes and contemporary anti-social behavior: prejudicial attitudes and avoidance of people with physical disabilities. J Nonverbal Behav 27: 65–87. [Google Scholar]
- 13. Jones EE, 1984. Social Stigma: The Psychology of Marked Relationships. New York, NY: WH Freeman. [Google Scholar]
- 14. Van Brakel WH, Sihombing B, Djarir H, Beise K, Kusumawardhani L, Yulihane R, Kurniasari I, Kasim M, Kesumaningsih KI, Wilder-Smith A, 2012. Disability in people affected by leprosy: the role of impairment, activity, social participation, stigma and discrimination. Glob Health Action 5: 18394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brieger WR, Oshiname FO, Ososanya OO, 1998. Stigma associated with onchocercal skin disease among those affected near the Ofiki and Oyan Rivers in western Nigeria. Soc Sci Med 47: 841–852. [DOI] [PubMed] [Google Scholar]
- 16. Vlassoff C, Weiss M, Ovuga E, Eneanya C, Nwel PT, Babalola SS, Awedoba AK, Theophilus B, Cofie P, Shetabi P, 2000. Gender and the stigma of onchocercal skin disease in Africa. Soc Sci Med 50: 1353–1368. [DOI] [PubMed] [Google Scholar]
- 17. Park JH, 2003. Evolved disease-avoidance processes and contemporary anti-social behavior: prejudicial attitudes and avoidance of people with physical disabilities. J Nonverbal Behav 27: 65–87. [Google Scholar]
- 18. Oaten M, Stevenson RJ, Case TI, 2011. Disease avoidance as a functional basis for stigmatization. Philos Trans R Soc Lond B Biol Sci 366: 3433–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karafillakis E. et al. , 2016. ‘Once there is life, there is hope’ Ebola survivors’ experiences, behaviours and attitudes in Sierra Leone, 2015. BMJ Glob Health 1: e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pers YM, Sow MS, Taverne B, March L, Izard S, Étard JF, Barry M, Touré A, Delaporte E, 2017. Characteristics of the musculoskeletal symptoms observed among survivors of Ebola virus disease in the Postebogui cohort in Guinea. Rheumatology (Oxford) 56: 2068–2072. [DOI] [PubMed] [Google Scholar]
- 21. Turan B, Hatcher AM, Weiser SD, Johnson MO, Rice WS, Turan JM, 2017. Framing mechanisms linking HIV-related stigma, adherence to treatment, and health outcomes. Am J Public Health 107: 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akena D, Musisi S, Joska J, Stein DJ, 2012. The association between aids related stigma and major depressive disorder among HIV-positive individuals in Uganda. PLoS One 7: e48671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kelly JD. et al. , 2019. Ebola virus disease-related stigma among survivors declined in Liberia over an 18-month, post-outbreak period: an observational cohort study. PLoS Negl Trop Dis 13: e0007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sneller MC. et al. , 2019. A longitudinal study of Ebola sequelae in Liberia. N Engl J Med 380: 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hereth-Hebert E. et al. , 2017. Ocular complications in survivors of the Ebola outbreak in Guinea. Am J Ophthalmol 175: 114–121. [DOI] [PubMed] [Google Scholar]
- 26. Etard JF. et al. , 2017. Multidisciplinary assessment of post-Ebola sequelae in Guinea (Postebogui): an observational cohort study. Lancet Infect Dis 17: 545–552. [DOI] [PubMed] [Google Scholar]
- 27. Mattia JG. et al. , 2016. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis 16: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. James PB, Wardle J, Steel A, Adams J, 2019. Post-Ebola psychosocial experiences and coping mechanisms among Ebola survivors: a systematic review. Trop Med Int Health 24: 671–691. [DOI] [PubMed] [Google Scholar]
- 29. National AIDS Commission, 2013 The People Living with HIV Stigma Index: Liberia PLHIV Stigma Index Report. Available at: https://www.medbox.org/document/the-people-living-with-hiv-stigma-index-liberia-november-2013#GO and report. Accessed September 20, 2021.
- 30. Tiffany A, Vetter P, Mattia J, Dayer JA, Bartsch M, Kasztura M, Sterk E, Tijerino AM, Kaiser L, Ciglenecki I, 2016. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin Infect Dis 62: 1360–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deen GF. et al. , 2017. Ebola RNA persistence in semen of Ebola virus disease survivor—final report. N Engl J Med 377: 1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mate SE. et al. , 2015. Molecular evidence of sexual transmission of Ebola virus. N Engl J Med 373: 2448–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dokubo EK. et al. , 2018. Persistence of Ebola virus after the end of widespread transmission in Liberia: an outbreak report. Lancet Infect Dis 18: 1015–1024. [DOI] [PubMed] [Google Scholar]
- 34. Diallo B. et al., 2016. Resurgence of Ebola virus disease in guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis 63: 1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization, 2016 Sexual and Reproductive Health. Interim Advice on Sexual Transmission of the Ebola Virus Disease. Available at: https://www.who.int/reproductivehealth/topics/rtis/ebola-virus-semen/en/. Accessed September 20, 2021.
- 36. World Health Organization, 2016 Clinical care for Survivors of Ebola Virus Disease. Interim Guidance. Available at: http://apps.who.int/iris/bitstream/10665/204235/1/WHO_EVD_OHE_PED_16.1_eng.pdf?ua=1. Accessed September 20, 2021.
- 37. Bausch DG, 2015. Sequelae after Ebola virus disease: even when it’s over it’s not over. Lancet Infect Dis 15: 865–866. [DOI] [PubMed] [Google Scholar]
- 38. Varkey JB. et al. , 2015. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med 372: 2423–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jacobs M. et al. , 2016. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 388: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.