ABSTRACT.
Streptococcus agalactiae serotype distribution and its antibiotic susceptibility affect disease prevention strategies, but the serotype distribution varies among patient groups. The objectives of this study were to establish the group B Streptococcus (GBS) serotype distribution in patients from Egypt and to assess antibiotic sensitivity of invasive GBS isolates. A total of 490 patients participated in this multicenter study; 160 had urinary tract infection, 115 complained of diabetic foot ulcers, 125 men had genital tract infections, and 30 women females had genital tract infections. Others had bronchopneumonia, otitis media, synovitis, or meningitis. Serotyping of the isolated GBS was performed at the CDC in the United States. Antibiotic sensitivity patterns were determined using the disk diffusion method. In men, the most common serotypes were II, III, and V, whereas types Ia, II, III, and V were isolated from women. Macrolides (erythromycin) resistance occurred in 4.1% of the isolates; 10.2% were resistant to both clindamycin and inducible resistance of macrolides, lincomycin, and streptogramin; 17.3% were resistant to quinolones; and 95.9% were resistant to tetracyclines. GBS primarily infected the urinary tract, skin, soft tissue, and genital tract in both genders. Isolates were sensitive to beta-lactam drugs, vancomycin, and linezolid; 14.0% were resistant to macrolides with or without clindamycin. Only 6.0% of the strains were sensitive to tetracyclines. Although GBS causes invasive infections in Egyptian adults, it rarely causes neonatal meningitis or sepsis. Future studies should determine whether GBS isolates are transmitted sexually, by performing a follow-up study of the partner of the infected patient.
INTRODUCTION
Streptococcus agalactiae [group B Streptococcus (GBS)] is within the phylum Firmicutes.1 Optional, fastidious, beta-hemolytic cocci, GBS can be found in normal genitourinary, rectal, and oral flora.2 Based on the cell wall capsular polysaccharide component, GBS is classified into 10 serotypes: Ia, Ib, II, III, IV, V, VI, VII, VIII, and IX.3
Epidemiological studies conducted in East African countries showed GBS colonization rates ranging from 3.0% to 28.8%; Central Africa, 20.0%; western Africa, 2.5% to 34.2%; southern Africa, 1.8% to 48.2%; and northern Africa, 17.0% to 26.5%.4 Furthermore, most patients with GBS invasive infection have at least one chronic debilitating disease—in particular, diabetes mellitus, which is probably a result of its associated leukocyte dysfunction.5
Invasive disease resulting from GBS infection has greatly increased in non-pregnant adults during the past decade and includes bacteremia, endocarditis, and infections of soft tissue, bones, and joints.2 There are considerable differences among countries in the distribution of GBS serotypes. Recent research has focused on the molecular and epidemiological qualities of invasive GBS isolates.6–8
To our knowledge, no published data exist on the incidence of invasive GBS disease and its antimicrobial susceptibility. This study aimed to determine the serotype and antibiotic susceptibility of GBS isolates from Egyptian patients to determine their serotype and antibiotic susceptibility.
MATERIALS AND METHODS
Study design.
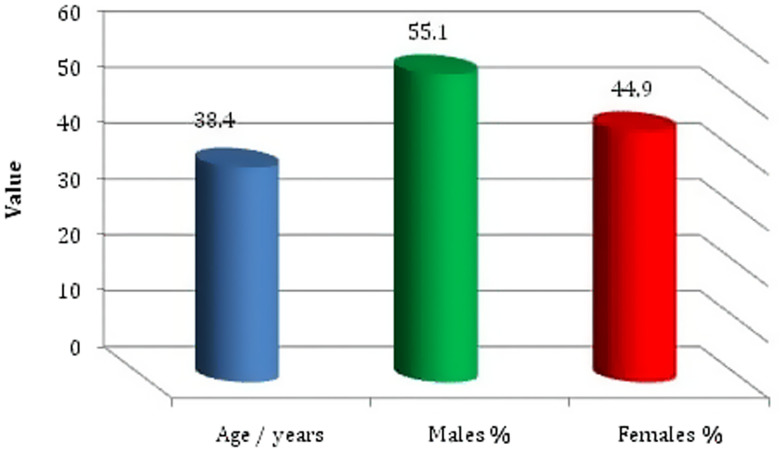
Between September 2016 and December 2019, 490 patients age 24 to 53 years old were recruited for the prospective observational multicenter study. All patients were Egyptians and came from rural areas. A total of 160 patients had urinary tract infections with or without diabetes mellitus (120 women and 40 men); 115 patients complained of diabetic foot ulcers (skin and soft tissue infection; 60 women and 55 men); 125 men had genital tract infections in the form of prostatitis, urethritis, and pyospermia; and 30 women had genital tract infections manifested by increased white blood cells in their vaginal discharge. Forty patients had bronchopneumonia (10 women and 30 men), otitis media (10 men), synovitis (five men), or meningitis (five men) (Table 1, Figure 1).
Table 1.
Group B Streptococcus serotype distribution between females and males with multisystem affectation (N = 490)
| GBS serotype | UTI | Diabetic foot | GTI | Bronchopneumonia | Otitis media | Synovitis | Meningitis | n | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female (n = 120) | Male (n = 40) | Female (n = 60) | Male (n = 55) | Female (n = 30) | Male (n = 125) | Female (n = 10) | Male (n= 30) | Female (n = 0) | Male (n = 10) | Female (n = 0) | Male (n = 5) | Female (n = 0) | Male (n = 5) | ||
| Ia | 40 (33.3%) | 5 (12.5%) | 15 (25.0%) | 10 (18.2%) | 5 (16.7%) | 15 (12.0%) | – | 5 (16.7%) | – | – | – | – | – | – | 95 |
| Ib | 10 (8.3%) | – | – | 5 (9.1%) | – | 5 (4.0%) | – | – | – | – | – | – | – | – | 20 |
| II | 20 (16.7%) | 25 (62.5%) | 10 (16.7%) | 15 (27.3%) | 5 (16.7%) | 15 (12.0%) | 5 (50.0%) | 5 (16.7%) | – | – | – | – | – | – | 100 |
| III | 30 (25.0%) | 5 (12.5%) | 5 (8.3%) | – | 10 (33.3%) | 50 (40.0%) | 5 (16.7%) | – | – | – | – | – | – | 105 | |
| IV | – | – | – | – | – | – | – | 5 (16.7%) | – | – | – | – | – | – | 5 |
| V | 10 (8.3%) | 5 (12.5%) | 25 (41.7%) | 20 (36.3%) | 35 (28.0%) | 5 (50.0%) | 5 (16.7%) | – | 5 (50.0%) | – | – | – | – | 110 | |
| VI | 10 (8.3%) | – | – | 5 (9.1%) | 5 (16.7%) | 5 (4.0%) | – | – | – | – | – | – | – | – | 25 |
| VII | – | – | 5 (8.3%) | – | 5 (16.7%) | – | – | – | – | 5 (50.0%) | – | 5 (100%) | – | 5 (100%) | 25 |
| VIII | – | – | – | – | – | – | – | 5 (16.7%) | – | – | – | – | – | – | 5 |
GBS = group B Streptococcus; GTI = genital tract infection; UTI = urinary tract infection.
Figure 1.
Age and gender distribution of the enrolled patients. This figure appears in color at www.ajtmh.org.
Before the study was conducted, the purpose and objectives of the study were described to students and the study participants or guardians. Prior to any procedure in specialized treatment centers (including the Al-Hussein University Hospital in Cairo), all participants provided informed consent. Study practices were consistent with good clinical practice guidelines and the Declaration of Helsinki. The study also received approval from the National Liver Institute Institutional Review Board (IRB no. 00003545, University of Menoufia). All participant information was kept confidential.
Diabetes mellitus was diagnosed according to the WHO and the American Diabetes Association as fasting blood glucose ≥ 126 mg/dL or 2-hour post prandial blood glucose ≥ 200 mg/dL.9
Data regarding age, gender, residence, education, job position, past medical history, current symptoms, and other risk factors were recorded and collected through a written questionnaire adapted from the Exposure Prevention Information Network (http://www.healthsystem.virginia.edu/internet/epinet).
Exclusion criteria.
Patients treated with antibiotics 2 weeks before recruitment or with a complex past or present history of pregnancy (abortion, premature delivery, premature rupture of membranes) were excluded from the study.
Sample collection and bacterial identification.
Excessive secretions were removed from vaginal specimens, and mucus from the lowest third of the vagina was removed without a speculum, as recommended by the CDC (2010).60 Clean-catch voided urine and genital samples were subjected to direct microscopic examination for the presence of white blood cells, and all samples were stained with Gram and Giemsa stains. Sterile swabs were taken from foot ulcers of diabetic patients, bronchoalveolar lavage fluid from patients with bronchopneumonia, ear swab and pipette-aspirated specimens from patients with otitis media, blood specimens from patients with endocarditis, and cerebrospinal fluid from patients with meningitis.
Specimens were cultivated on double-layer sheep blood agar plates to isolate GBS by the beta-hemolytic activity on red blood cells. GBS colonies appear gray or whitish gray and are surrounded by a weak zone of hemolysis in blood culture media. Bacterial isolates were also characterized using the coagulase and catalase tests. Latex agglutination tests confirmed the presence of GBS.
Serotyping.
Samples were sent to the CDC in Atlanta, Georgia, for serotyping of GBS isolates. Samples were transported using a single rabbit anti-bulb kit (Denka Seiken, Tokyo, Japan) according to the manufacturer’s protocol.
Antimicrobial susceptibility testing.
The disk diffusion method was used to assess antibiotic sensitivity patterns of the GBS isolates (Kirby and Bauer method).61 The types of antibiotics and their concentrations were used per Clinical and Laboratory Standard Institute guidelines:10 ampicillin; amoxicillin; penicillins; cefotaxime; linezolid; vancomycin; erythromycin; clindamycin; inducible macrolides, lincomycin, and streptogramin (iMLS); quinolones; and tetracyclines. Streptococcus pneumoniae ATCC 49619 was used as a quality control strain.
Statistical analysis.
SPSS (v. 22.0; IBM, Armonk, NY) was used for all data analyses. Descriptive statistics include mean, percentage, and SD.
RESULTS
GBS strains were isolated from all 490 patients [age range, 24–53 years; 220 women (44.9%) and 270 men (55.1%)].
Isolation of invasive GBS.
GBS strains were isolated from all 490 patients and identified by colony characteristics, type of hemolysis, Gram stain, and catalase test results. The final diagnosis was confirmed by the latex agglutination test (Oxoid). GBS caused urinary tract, soft tissue, and genital tract infections in more than 80% of the male and female patients in our study. Women with diabetes were more prone to GBS urinary tract infection, especially those in their childbearing years as opposed to their menopausal years.
Serotyping of GBS.
All nine GBS serotypes were isolated from all samples from male and female patients. Serotypes Ia, II, III, and V were isolated most isolated, representing 19.4%, 20.4%, 21.5%, and 22.4%, respectively, of all isolates. In the male patients, the most common GBS serotypes, in descending order, were V, II, III, and Ia; in females, the most common isolates were Ia, III, II, and V (Table 1).
There was a marked difference between the GBS serotypes isolated from male and female patients with urinary tract infections. In descending order, Ia, III, II, Ib, V, and VI were isolated from female patients, whereas types II, Ia, III, and V were isolated from male patients (Table 1). GBS serotypes isolated from male genital tract infections were III, V, Ia, II, Ib, and VI, in descending order. In female patients, the GBS serotypes were III, Ia, II, VI, and VII, in descending order (Table 1). Serotypes Ia, II, and III were isolated from female urinary tract (75%) and genital tract (67%) infections of other patients (Tables 1). GBS serotypes were more or less similar in diabetic and non-diabetic female patients with urinary tract infections (Table 2). A marked difference was detected between the serotypes isolated from patients with prostatitis (serotypes III, V, II, Ia, and Ib), urethritis (III, V, Ia, and II), and pyospermia (Ia, III, V, and VI) (Table 3).
Table 2.
Group B Streptococcus types of urinary tract infection in female patients with or without diabetes (N = 120)
| GBS serotype | Group | |||
|---|---|---|---|---|
| Diabetes (n = 50) | No diabetes (n = 70) | |||
| n | % | n | % | |
| Ia | 10 | 20 | 30 | 42.9 |
| Ib | 5 | 10 | 5 | 7.1 |
| II | 10 | 20 | 10 | 14.3 |
| III | 10 | 20 | 20 | 28.6 |
| V | 5 | 10 | 5 | 7.1 |
| VI | 10 | 20 | 0 | 0 |
GBS = group B Streptococcus.
Table 3.
Group B Streptococcus types isolated from male genital tract infection (N = 125)
| GBS serotype | Group | |||||
|---|---|---|---|---|---|---|
| Prostate (n = 70) | Urethral discharge (n = 35) | Semen (n = 20) | ||||
| n | % | n | % | n | % | |
| Ia | 5 | 7.1 | 5 | 14.3 | 5 | 25.0 |
| Ib | 5 | 7.1 | 0 | 0.0 | 0 | 0.0 |
| II | 10 | 14.3 | 5 | 14.3 | 0 | 0.0 |
| III | 30 | 42.9 | 15 | 42.9 | 5 | 25.0 |
| V | 20 | 28.6 | 10 | 28.6 | 5 | 25.0 |
| VI | 0 | 0.0 | 0 | 0.0 | 5 | 25.0 |
GBS = group B Streptococcus.
GBS serotypes isolated from pus collected from diabetic feet in female and male patients were, in descending order, V, Ia, II, III, and VII in women, and serotypes V, II, Ia, Ib, and VI in men (Table 1). GBS serotypes II and V were isolated from bronchopneumonia samples of both genders, whereas types Ia, III, IV, and VIII were isolated from men only (Table 1). In 10 male patients with otitis media, serotypes V and VII were isolated; serotype VII was isolated from five male patients with synovitis and five men with meningitis (Table 1).
Antibiotic susceptibility test for the isolated GBS.
All GBS serotypes isolated were sensitive to beta-lactam drugs (ampicillin, amoxicillin, penicillins, and cephalosporins), linezolid, and vancomycin. Although 4.08% were resistant to the macrolides (erythromycin) and 10.2% were resistant to clindamycin and iMLS, 17.3% were resistant to quinolones and 95.9% were resistant to tetracyclines.
DISCUSSION
GBS is one of the primary causes of neonatal sepsis and meningitis, and is a potentially invasive infectious agent of pregnant and non-pregnant adults.11,12 The serological reaction against the polysaccharide capsule of GBS9 or multiplex polymerase chain reaction results can identify 10 serotypes (Ia, Ib, and II–IX) into 10 different serotypes.13
The serotype isolated most commonly from patients enrolled in our study was type V, followed by types III, II, and Ia, representing 22.4%, 21.5%, 20.4%, and 19.4%, respectively, of all samples. These results are consistent with another study conducted in Egypt by Shabayek et al.14 In Kuwait, Boswihi et al.15 reported that serotype V was isolated from 38.5% of 143 GBS isolates, followed in frequency by serotypes III (20.9%), Ia (7.7%), and II (11.2%). Serotypes Ib and IV each accounted for only 3.5%, and VI and VII constituted 2.8% and 0.6%, respectively.15
In Europe and North America, serotype III is a common colonizer in Canada compared with our study.16–20 In Malaysia, serotype IV strains predominated and were the second most common isolate in adults with skin infections.21,22 In Gabon, serotype V was the most common, and in the Republic of The Gambia, serotype VI was the most common.23,24 The most common serotypes in Taiwan were III and VI; and in Japan, VI and VIII.25–27 Serotype IX was recently reported from Denmark.25 The distribution and prevalence of certain serotypes can change over time.
Four serotypes (Ia, II, III, and V) represented 83.7% of all isolates in our study. Similarly, Ji et al.28 found that six serotype III serotypes represented 54.9% of 153 GBS isolates. The contribution (Ia, Ib, III) of total serotypes, representing 85.6%, is greater than that found in a study conducted in Beijing (75.1% of all serotypes). Lu et al.29–35 found that serotype II (7.0% of total serotypes) was also greater (69.2%).
The distribution and resistance of GBS serotypes shifted between serotype V and serotype Ia as the dominant GBS serotype of infected women. Although the most prevalent serotype found in our study, Ia, is of increasing concern in Egypt, serotype V GBS isolates most often led to invasive inflammation in patients, adults, and neonates in the United States and Canada,36,37 and Australia and New Zealand.38
In the male patients, the most common SGB type was V, followed by II, III, Ia, and VII, whereas type Ia was isolated most commonly from the female patients (27.3%). Type V was isolated from only 18.2% of women. Suara et al.39 (1994) found that of 32 infected pregnant women, 12 (30%) were colonized with serotype V. Serotype V has been identified as globally important in neonatal and adult colonization disease and serotype disease.31,40,41
In our study, 125 of 160 isolates (78.1%) from patients with urinary tract infections were GBS serotypes Ia, II, and III.14,42,43 These results are consistent with one of two previous studies conducted in Egypt (and were higher than those reported in the second study).44,45 The most prevalent serotypes in the study by Ulett et al.46 were in the 62 cases of GBS urinary tract infections, with serotypes Ia, III, and V representing 76% of the cases. The remaining 24% of the cases were isolates of serotypes Ib, II, and IV.
Global GBS serotype variability is linked to different sociocultural, geographic, climatic, biological, and methodological factors, emphasizing the importance of individualization, local infection rates, and preventive strategies.
GBS-attributed skin and soft tissue infections can manifest as cellulitis, abscesses, foot infections, or ulcers. Diabetes mellitus is a common underlying disease in patients infected with GBS and with soft tissue infections.47
Severe invasive disease has been linked to serotypes Ia, Ib, II, III, and, most recently, V.1 In our study, GBS serotypes isolated from pus collected from diabetic feet in both female and male patients were, in decreasing prevalence, serotypes V, Ia, II, III, and VII, and serotypes V, II, Ia, Ib, and VI, respectively. Consistent with our study, Lee et al.35 reported types V, II, and Ia to be the most common GBS serotypes causing soft tissue infections; 82.6% of these serotypes caused diabetes foot in both genders.
The most common isolated serotype isolated from genital infections was III in both genders, representing 38.7% (60 of 155 isolates), followed by isotype V with 22.6% (35 of 155 isolates). Serotype III predominated (50.9%), followed by serotype II, in a study of Malaysian 19 adult and neonate patients with genitourinary infections. Serotype III is related to a greater risk of newborn infection with more persistent colonization.48
GBS serotypes II and V were isolated from bronchopneumonia specimens from both genders, whereas types Ia, III, IV, and VIII were isolated from the men only. Foster-Nyarko et al.49 had similar results when evaluating the infection rates, serotypes, and antibiotic GBS susceptibility in 1,200 2-month-old children. They found that serotypes V and II were the dominant GBS serotypes. Nine GBS serotypes, most frequently V (37.6%) and II (34.6%), composed approximately 72% of all identified GBS serotypes. The other GBS serotypes were Ia (7.5%), IV (6.0%), Ib (5.3%), VII (3.8%), VIII (0.8%), III (0.8%), and VI (0.8%). Deng and Yang50–52 found serotypes Ia, III, and II were the most prevalent, particularly in cases of identified neonatal fatal pneumonia.
All tested isolates were sensitive to beta-lactam drugs (ampicillin, amoxicillin, penicillins, and cephalosporins), linezolid, and vancomycin. Although 4.08% were resistant to the macrolides (erythromycin) and 10.2% were resistant to clindamycin and iMLS, 17.3% were resistant to quinolones and 95.9% were resistant to tetracyclines. A study in Nigeria reported an extremely high level of penicillin and ampicillin resistance (100%).53–55 The authors considered the high resistance rate the consequence of the ease by which antibiotics are procured in developing countries. They concluded that there was widespread therapeutic antibiotic use, and this practice may be justified to a certain extent. Some women with penicillin allergies require other GBS prophylactic antibiotics, such as clindamycin, erythromycin, or vancomycin.
Alternative antibiotics, including clindamycin, erythromycin, or vancomycin, are administered to pregnant women with penicillin allergies. Multiple studies have shown the resistance to clindamycin and erythromycin is increasing.14,42,51–54
Shabayek et al.40 found that 13.15% of GBS isolates were erythromycin resistant and 23.7% were clindamycin resistant. In the study of Shabayek et al., erythromycin, and clindamycin resistance rates were much lower than those reported in Syria by Dunia et al.56 However, these antibiotics are at higher rates than those reported for erythromycin.57
No isolates studied by Sadaka et al.43 were resistant to penicillins such as penicillin and ampicillin. Moreover, all isolates have ceftriaxone, cefepime, vancomycin, linezolid, and cefotaxime. Sadaka et al.43 found the resistance to levofloxacin, azithromycin, erythromycin, and clindamycin was 43.4%, 28.3%, 22.6%, and 15%, respectively. Eleven GBS isolates (20.8%) were intermediately tetracycline susceptible.43
In a recent Egyptian study conducted by Foad,58 (2016), GBS was very vulnerable to ampicillin, benzylpenicillin, cefotaxime, ceftriaxone, linezolid, trimethoprim/sulfamethoxazole, and vancomycin. In isolates from urinary tract and vaginal infections, levofloxacin demonstrated good susceptibility (82.1% and 78.9%, respectively). Erythromycin and clindamycin, however, demonstrated a moderate urinary and vaginal resistance (53.6% and 68.4%, respectively) and (57.1% and 63.2%, respectively) to clindamycin. The rate of resistance in urinary and vaginal infections was very high (96.4% and 94.7%, respectively).58
Penicillin and ampicillin, both first-line treatments for pregnant women and neonates, have been found to be efficient in the treatment and prevention of GBS.59 Therefore, penicillin is less likely to select resistant organisms and has a narrow range of antimicrobial activity. Penicillin, ceftriaxone, or vancomycin were susceptible to 100% of isolates. Cefazoline, clindamycin, and erythromycin may be useful alternatives for women allergic to penicillin. Ji et al.28 found 64.9% were resistant to erythromycin, 52.4% to clindamycin, and 25.9% to levofloxacin. Clindamycin and levofloxacin resistance has increased significantly during the 6 years of monitoring. However, in the past 20 years, the proportions of GBS isolates resistant to erythromycin, clindamycin, or levofloxacin have increased considerably.60 Most previous studies show GBS isolates had a high rate of resistance to tetracycline.
CONCLUSION
Although GBS causes invasive infections in Egyptian adults, it rarely causes neonatal meningitis or sepsis. GBS infects mainly the urinary tract and skin and soft tissue in both genders. However, the genital tract was more infected in men than in women in our study. Diabetic young females (of childbearing age) are more likely to be infected with GBS than older women.
Many GBS isolates were found to be sensitive to beta-lactam drugs, vancomycin, and linezolid, whereas only 14% were resistant to macrolides with or without clindamycin, 17.3% were resistant to quinolone, and only 4% of the strains were sensitive to tetracyclines.
Future studies should determine whether GBS isolates are transmitted sexually, by performing a follow-up study of the partner of the infected patient. Furthermore, it is essential to assess whether GBS may cause autoinfection in patients who may have a GBS focus elsewhere in their body. The most significant limitation of our study was that pregnant women could not follow the positive GBS culture to determine premature diabetes and infant infection rates.
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1. Raabe VN, Shane AL, Group B, 2019. Streptococcus (Streptococcus agalactiae). Microbiol Spectr 7: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Björnsdóttir ES. et al. , 2016. Changing epidemiology of group B streptococcal infections among adults in Iceland: 1975–2014. Clin Microbiol Infect 22: 379.e9–379.e16. [DOI] [PubMed] [Google Scholar]
- 3. Lee CC, Hsu JF, Janapatla RP, Chen C, Zhou YL, Lien R, Chiu CH, 2019. Clinical and microbiological characteristics of group B Streptococcus from pregnant women and diseased infants in intrapartum antibiotic prophylaxis era in Taiwan. Sci Rep 9: 13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gizachew M, Tiruneh M, Moges F, Tessema B, 2019. Streptococcus agalactiae maternal colonization, antibiotic resistance and serotype profile in Africa: a metanalysis. Ann Clin Microbiol Antimicrob 18: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Batista RP, Ferreira CR, 2015. Streptococcus agalactiae septicemia in a patient with diabetes. Autops Case Rep 5: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kwatra G, Adrian PV, Shiri T, Buchmann EJ, Cutland CL, Madhi SA, 2015. Natural acquired humoral immunity against serotype-specific group B Streptococcus rectovaginal colonization acquisition in pregnant women. Clin Microbiol Infect 21: 568.e13–e568.e21. [DOI] [PubMed] [Google Scholar]
- 7. Medugu N. et al. , 2017. Group B streptococcal colonization and transmission dynamics in pregnant women and their newborns in Nigeria: implications for prevention strategies. Clin Microbiol Infect 23: 673.e9–673.e16. [DOI] [PubMed] [Google Scholar]
- 8. Metcalf BJ. et al. , 2017. Short-read whole genome sequencing for determination of antimicrobial resistance mechanisms and capsular serotypes of current invasive Streptococcus agalactiae recovered in the USA. Clin Microbiol Infect 23: 574.e7–574.e14. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association , 2019. Standard of medical care in diabetes 2019. Diabetes Care 42: S4–S6. [DOI] [PubMed] [Google Scholar]
- 10. Clinical and Laboratory Standards Institute , 2015. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-fifth Informational Supplement. CLSI document M100-S25, vol. 35. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 11. Teatero S, Ferrieri P, Martin I, Demczuk W, McGeer A, Fittipaldi N, 2017. Serotype distribution, population structure, and antimicrobial resistance of group B Streptococcus strains recovered from colonized pregnant women. J Clin Microbiol 55: 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Slotved HC, Kong F, Lambertsen L, Sauer S, Gilbert GL, 2007. Serotype IX, a proposed new Streptococcus agalactiae serotype. J Clin Microbiol 45: 2929–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao K, Poulsen K, Maione D, Rinaudo CD, Baldassarri L, Telford JL, Sorensen UB, Kilian M, 2013. Capsular gene typing of Streptococcus agalactiae compared to serotyping by latex agglutination. J Clin Microbiol 51: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shabayek S, Abdalla S, Abouzeid AM, 2014. Serotype and surface protein gene distribution of colonizing group B Streptococcus in women in Egypt. Epidemiol Infect 142: 208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boswihi SS, Udo EE, Al-Sweih N, 2012. Serotypes and antibiotic resistance in group B Streptococcus isolated from patients at the Maternity Hospital, Kuwait. J Med Microbiol 61: 126–131. [DOI] [PubMed] [Google Scholar]
- 16. Ippolito DL. et al. , 2010. Group B Streptococcus serotype prevalence in reproductive-age women at a tertiary care military medical center relative to global serotype distribution. BMC Infect Dis 10: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamagni TL. et al. , 2013. Emerging trends in the epidemiology of invasive group B streptococcal disease in England and Wales, 1991–2010. Clin Infect Dis 57: 682–688. [DOI] [PubMed] [Google Scholar]
- 18. Melin P, Efstratiou A, 2013. Group B streptococcal epidemiology and vaccine needs in developed countries. Vaccine 31: D31–D42. [DOI] [PubMed] [Google Scholar]
- 19. Florindo C. et al. , 2014. Epidemiological surveillance of colonising group B Streptococcus epidemiology in the Lisbon and Tagus Valley regions, Portugal (2005 to 2012): emergence of a new epidemic type IV/clonal complex 17 clone. Euro Surveill 19: 20825. [DOI] [PubMed] [Google Scholar]
- 20. Fabbrini M. et al. , 2016. The protective value of maternal group B Streptococcus antibodies: quantitative and functional analysis of naturally acquired responses to capsular polysaccharides and pilus proteins in European maternal sera. Clin Infect Dis 63: 746–753. [DOI] [PubMed] [Google Scholar]
- 21. Karunakaran R, Raja NS, Hafeez A, Puthucheary SD, 2009. Group B Streptococcus infection: epidemiology, serotypes, and antimicrobial susceptibility of selected isolates in the population beyond infancy (excluding females with genital tract- and pregnancy-related isolates) at the University Malaya Medical Centre, Kuala Lumpur. Jpn J Infect Dis 62: 192–194. [PubMed] [Google Scholar]
- 22. Dhanoa A, Karunakaran R, Puthucheary SD, 2010. Serotype distribution and antibiotic susceptibility of group B streptococci in pregnant women. Epidemiol Infect 138: 979–981. [DOI] [PubMed] [Google Scholar]
- 23. Belard S. et al. , 2015. Streptococcus agalactiae serotype distribution and antimicrobial susceptibility in pregnant women in Gabon, Central Africa. Sci Rep 5: 17281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Doare K, Jarju S, Darboe S, Warburton F, Gorringe A, Heath PT, Kampmann B, 2016. Risk factors for group B Streptococcus colonisation and disease in Gambian women and their infants. J Infect 72: 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsai M, Hsu JF, Lai MY, Lin LC, Chu SM, Huang HR, Fu MR, Lu JJ, 2019. Molecular characteristics and antimicrobial resistance of group B Streptococcus strains causing invasive disease in neonates and adults. Front Microbiol 10: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsubara K, Katayama K, Baba K, Nigami H, Harigaya H, Sugiyama M, 2002. Seroepidemiologic studies of serotype VIII group B Streptococcus . Jpn J Infect Dis 186: 855–858. [DOI] [PubMed] [Google Scholar]
- 27. Lin HC. et al. , 2016. Clonal dissemination of invasive and colonizing clonal complex 1 of serotype VI group B Streptococcus in central Taiwan. J Microbiol Immunol Infect 49: 902–909. [DOI] [PubMed] [Google Scholar]
- 28. Ji W. et al. , 2017. Colonization prevalence and antibiotic susceptibility of group B Streptococcus in pregnant women over a 6-year period in Dongguan, China. PLoS One 12: e0183083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu B, Li D, Cui Y, Sui W, Huang L, Lu X, 2014. Epidemiology of group B Streptococcus isolated from pregnant women in Beijing, China. Clin Microbiol Infect 20: O370–O373. [DOI] [PubMed] [Google Scholar]
- 30. Kwatra G, Adrian PV, Shiri T, Buchmann EJ, Cutland CL, Madhi SA, 2014. Serotype-specific acquisition and loss of group B Streptococcus recto-vaginal colonization in late pregnancy. PLoS One 9: e98778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davies HD. et al. , 2001. Antibodies to capsular polysaccharides of group B Streptococcus in pregnant Canadian women: relationship to colonization status and infection in the neonate. J Infect Dis 184: 285–291. [DOI] [PubMed] [Google Scholar]
- 32. Kunze M, Ziegler A, Fluegge K, Hentschel R, Proempeler H, Berner R, 2011. Colonization, serotypes and transmission rates of group B streptococci in pregnant women and their infants born at a single university center in Germany. J Perinat Med 39: 417–422. [DOI] [PubMed] [Google Scholar]
- 33. Madhi SA. et al. , 2013. Considerations for a phase-III trial to evaluate a group B Streptococcus polysaccharide–protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young infants. Vaccine 31: D52–D57. [DOI] [PubMed] [Google Scholar]
- 34. Ueno H, Yamamoto Y, Yamamichi A, Kikuchi K, Kobori S, Miyazaki M, 2012. Characterization of group B Streptococcus isolated from women in Saitama City, Japan. Jpn J Infect Dis 65: 516–521. [DOI] [PubMed] [Google Scholar]
- 35. Lee BK. et al. , 2010. Epidemiology of group B Streptococcus in Korean pregnant women. Epidemiol Infect 138: 292–298. [DOI] [PubMed] [Google Scholar]
- 36. Castor ML. et al. , 2008. Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect Dis Obstet Gynecol 2008: 727505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dogan B, Schukken YH, Santisteban C, Boor KJ, 2005. Distribution of serotypes and antimicrobial resistance genes among Streptococcus agalactiae isolates from bovine and human hosts. J Clin Microbiol 43: 5899–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao Z, Kong F, Zeng X, Gidding HF, Morgan J, Gilbert GL, 2008. Distribution of genotypes and antibiotic resistance genes among invasive Streptococcus agalactiae (group B Streptococcus) isolates from Australasian patients belonging to different age groups. Clin Microbiol Infect 14: 260–267. [DOI] [PubMed] [Google Scholar]
- 39. Suara RO, Adegbola RA, Baker CJ, Secka O, Mulholland EK, Greenwood BM, 1994. Carriage of group B streptococci in pregnant Gambian mothers and their infants. J Infect Dis 170: 1316–1319. [DOI] [PubMed] [Google Scholar]
- 40. Edmond KM. et al. , 2012. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet 379: 547. [DOI] [PubMed] [Google Scholar]
- 41. Meehan M, Cunney R, Cafferkey M, 2014. Molecular epidemiology of group B streptococci in Ireland reveals a diverse population with evidence of capsular switching. Eur J Clin Microbiol Infect Dis 33: 1155–1162. [DOI] [PubMed] [Google Scholar]
- 42. Shabayek SA, Abdalla SM, Abouzeid AM, 2009. Vaginal carriage and antibiotic susceptibility profile of group B Streptococcus during late pregnancy in Ismailia. Egypt. J Infect Public Health 2: 86–90. [DOI] [PubMed] [Google Scholar]
- 43. Sadaka SM, Aly HA, Meheissen MA, Orief YI, Arafa BM, 2018. Group B streptococcal carriage, antimicrobial susceptibility, and virulence related genes among pregnant women in Alexandria, Egypt. Alex J Med 54: 69–76. [Google Scholar]
- 44. Wali IE, Sorour AE, Abdalla MAH, 2007. Assessment of different methods for detection of group B streptococci carriage among pregnant females. Egypt J Med Microbiol 16: 593–598. [Google Scholar]
- 45. Elbaradie SMY, Mahmoud M, Farid M, 2009. Maternal and neonatal screening for group B streptococci by SCP B gene based PCR: a preliminary study. Int J Med Microbiol 27: 17–21. [PubMed] [Google Scholar]
- 46. Ulett KB, Benjamin WH, Zhuo F, Xiao M, Kong F, Gilbert GL, Schembri MA, Ulett GC, 2009. Diversity of group B Streptococcus serotypes causing urinary tract infection in adults. J Clin Microbiol 47: 2055–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sendi P, Johansson L, Norrby-Teglund A, 2008. Invasive group B streptococcal disease in non-pregnant adults: a review with emphasis on skin and soft-tissue infections. Infection 36: 100–111. [DOI] [PubMed] [Google Scholar]
- 48. Madrid L. et al. , 2018. Maternal carriage of group B Streptococcus and Escherichia coli in a district hospital in Mozambique. Pediatr Infect Dis J 37: 1145–1153. [DOI] [PubMed] [Google Scholar]
- 49. Foster-Nyarko E. et al. , 2016. Associations between nasopharyngeal carriage of group B Streptococcus and other respiratory pathogens during early infancy. BMC Microbiol 16: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Deng J, Yang Y, 2008. Detection and molecular serotyping of group B Streptococcus in fatal neonatal pneumonia in China. Pediatrics 121 (Suppl 2): S127. [Google Scholar]
- 51. Quiroga M, Pegels E, Oviedo P, Pereyra E, Vergara M, 2008. Antibiotic susceptibility patterns and prevalence of group B Streptococcus isolated from pregnant women in Misiones, Argentina. Braz J Microbiol 39: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Allam AA, Bahgat MA, 2006. Phenotype and genotype of some clinical group B Streptococcus isolates resistant to erythromycin in Egypt. Egypt J Med Microbiol 15: 71–77. [Google Scholar]
- 53. Abdelmoaty T, Wafaa Z, Kawthar M, 2009. Prevalence and antibiotic susceptibility of anogenital group B streptococci colonization in pregnant women. Egypt J Med Lab. 18: 105–111. [Google Scholar]
- 54. Joachim A, Matee MI, Massawe FA, Lyamuya EF, 2009. Maternal and neonatal colonization of group B Streptococcus at Muhimbili National Hospital in Dares Salaam, Tanzania: prevalence, risk factors and antimicrobial resistance. BMC Public Health 9: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Onipede A, Adefusi O, Adeyemi A, Adejuyigbe E, Oyelese A, Ogunniyi T, 2012. Group B Streptococcus carriage during late pregnancy in Ile-ife, Nigeria. Afr J Clin Exp Microbiol 13: 135–143. [Google Scholar]
- 56. Dunia R, Kowatli K, Abutouk A, Rosa-Fraile M, 2006. Epidemiology of group B Streptococcus in pregnant women in Syria. Arab J Pharm Sci 3: 81–88. [Google Scholar]
- 57. Al-Sweih N, Hammoud M, Al-Shimmiri M, Jamal M, Neil L, Rotimi V, 2005. Serotype distribution and mother-to-baby transmission rate of Streptococcus agalactiae among expectant mothers in Kuwait. Arch Gynecol Obstet 272: 131–135. [DOI] [PubMed] [Google Scholar]
- 58. Foad MF, 2016. Urinary tract and vaginal infections caused by group B Streptococcus and the macrolide-inducible resistance to clindamycin in non-pregnant females. Int J Curr Microbiol Appl Sci 5: 486–496. [Google Scholar]
- 59. Schrag S, Phil D, Zell ER, Stat M, Lynfield R, Roome A, 2002. A population based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med 347: 233–239. [DOI] [PubMed] [Google Scholar]
- 60. Verani JR, McGee L, Schrag SJ, 2010. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, 2010. MMWR Morbid Mortal Wkly Rep 19: 1–36. [PubMed] [Google Scholar]
- 61. Dunkelberg WE, 1981. Kirby-Bauer disk diffusion method. Am J Clin Path 75: 273.