ABSTRACT.
Scrub typhus is an acute febrile disease caused by Orientia tsutsugamushi, which is transmitted through chigger mites. Delayed treatment results in various complications and, in severe cases, death. Granzymes are secreted by cytotoxic T lymphocytes or natural killer cells and are known to play an important role in controlling intracellular pathogens. To date, few studies have been done on granzymes in patients with scrub typhus. In this study, granzymes A and B showed a significant increase during the acute stage of scrub typhus compared with healthy control subjects, and decreased sharply after treatment. In addition, granzymes A and B were significantly high in the moderately elevated liver enzyme group. In conclusion, it appears that the host during the acute phase of scrub typhus increases cytotoxic T-cell activity to control infection.
INTRODUCTION
Scrub typhus is an acute febrile illness caused by the intracellular pathogen Orientia tsutsugamushi, which is transmitted by the bite of infected chiggers.1 The clinical course of scrub typhus varies from a self-limiting to a fatal illness, with an estimated median mortality of 6.0% (range, 0–70.0%).2 It still remains a substantial public health problem in the Asia-Pacific region and is also an emerging problem in other regions, including the Middle East, South America, and Africa.3,4
Granzymes are a family of homologous serine proteases released by cytoplasmic granules within cytotoxic T lymphocytes and natural killer cells. Cytotoxic T lymphocytes and natural killer cells are important effector cells in cell-mediated immunity, and are involved in adaptive and innate responses. So far, five granzymes (A, B, H, K, and M) have been described in humans, but granzyme A (a tryptase) and granzyme B (an aspartase) have been best studied.5,6 Both granzymes A and B promote cytotoxic T lymphocyte-mediated eradication of intracellular pathogens via the induction of apoptotic cell death, which can also play a role in inflammation.7,8 Elevated levels of plasmatic granzymes have been detected in numerous diseases, including several viral, bacterial, and parasitic infections.5,9 Currently, one study has investigated the expression of granzymes in patients with scrub typhus in the acute phase;10 however, no studies have yet investigated the change of extracellular granzyme levels in patients with scrub typhus before and after treatment. Therefore, we carried out a prospective study to assess the extracellular levels of granzymes A and B, and their clinical implication in O. tsutsugamushi infection.
METHODS
Patients and data collection.
A total of 70 eligible febrile adult patients (≥ 18 years of age) who were clinically suspected of having scrub typhus were recruited prospectively on admission to a 1,200-bed tertiary hospital in South Kora between October 2016 and December 2018. Initial blood specimens were obtained within 24 hours of hospital admission, and follow-up blood specimens were acquired 1 or 2 weeks after appropriate treatment. Demographic and clinical information were collected retrospectively from electronic medical records. Normal aminotransferase values for our laboratory were as follows: aspartate aminotransferase (AST), 12 to 33 IU/L; alanine aminotransferase (ALT), 5–35 IU/L. Abnormal aminotransferase values were graded as mild (one to three times the upper reference limit) to moderate (more than three times the upper reference limit). Sixteen healthy control subjects (age, 18–60 years) were recruited from the local population. They were screened to ensure the absence of underlying disease and any infectious disease.
Microbiological study.
Laboratory diagnosis of scrub typhus was made according to one of the following criteria: (1) an increase in an indirect immunofluorescence assay (IFA) IgM titer ≥ 1:160 against O. tsutsugamushi, (2) an increase in an IFA IgG titer ≥ 1:256, (3) a ≥ 4-fold increase in an IFA titer in paired sera, and (4) a positive result from a nested polymerase chain reaction (PCR) targeting the 56-kDa gene of O. tsutsugamushi.
Peripheral blood mononuclear cells were collected from acute-phase blood samples from scrub typhus patients. DNA from peripheral blood mononuclear cells was were purified using the QIAamp DNA Blood Mini Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s protocol. Nested PCR for the 56-kDa gene of O. tsutsugamushi was performed as described previously by Lee et al.11 The amplified PCR products were confirmed by 1.2% agarose gel electrophoresis, purified using a QIAquick gel extraction kit (QIAGEN), and were then sent to COSMO Genetech (Seoul, Korea) for sequencing. IFA for O. tsutsugamushi was examined by Green Cross Reference Laboratory (Yongin, Korea).
Acute- and convalescent-phase sera granzymes A and B were measured by ELISA using the human granzyme A and B ELISA Kit (RayBiotech, Norcross, GA) at the Green Cross Reference Laboratory.
Statistical analyses.
Descriptive statistics are expressed as numbers and percentages, means and ranges, or medians and interquartile ranges. Statistical analyses were performed using the Kruskal-Wallis test, the Mann-Whitney test, and Spearman’s correlation test with MedCalc for Windows (v. 19.3; MedCalc Software, Mariakerke, Belgium). In all analyses, a two-tailed P value of < 0.05 was considered significant.
Ethics statement.
This study was approved by the institutional review board of Jeonbuk National University Hospital, and all patients provided written informed consent (registration no. 2019-09-021-004).
RESULTS
During the study period, 70 scrub typhus patients were enrolled. Seven patients with co-infection of scrub typhus and other infectious disease were excluded, resulting in a total of 63 patients. The mean age of the patients was 67.6 ± 12.6 years; 44 (69.8%) were female. The majority of patients presented with a skin rash (n = 44, 69.8%) and eschar (n = 55, 87.3%). In laboratory findings, most patients showed mild to moderate elevation of liver function tests despite no underlying liver diseases, and more than half the patients had thrombocytopenia on admission. However, the abnormal laboratory findings had normalized on the second visit. In addition, the most commonly identified genotypes of O. tsutsugamushi were the Boryong strain (n = 50, 82.0%), which is the predominant strain throughout South Korea. All patients were treated successfully with either oral doxycycline or intravenous azithromycin. The demographic and clinical characteristics of the enrolled patients are summarized in Table 1.
Table 1.
The demographic and clinical characteristics and laboratory findings of scrub typhus patients
| Characteristics | Scrub typhus (N = 63) |
|---|---|
| Age, y; mean ± SD | 67.6 ± 12.6 |
| Gender, n (%) | |
| Male | 19 (30.2) |
| Female | 44 (69.8) |
| Comorbidities, n (%) | |
| Cardiovascular disease* | 11 (17.5) |
| Cerebrovascular disease | 13 (20.6) |
| Pulmonary disease† | 3 (4.8) |
| Connective tissue disease | 0 (0.0) |
| Liver disease | 2 (3.2) |
| Chronic kidney disease | 0 (0.0) |
| Diabetes mellitus | 23 (36.5) |
| Solid tumor | 5 (7.9) |
| Clinical signs and symptoms, n (%) | |
| Headache | 30 (47.6) |
| Dyspepsia | 30 (47.6) |
| Nausea/vomiting | 19 (30.2) |
| Abdominal pain | 13 (20.6) |
| Fever | 63 (100.0) |
| Myalgia | 35 (55.6) |
| Lymphadenopathy | 21 (33.3) |
| Rash | 44 (69.8) |
| Eschar | 55 (87.3) |
| Laboratory values, median (IQR) | |
| WBC count, ×1,000/mm3 | 7.9 (5.8–10.7) |
| Platelet count, ×1,000/mm3 | 128.0 (101.3–174.5) |
| Total bilirubin, mg/dL | 0.7 (0.5–0.9) |
| Albumin, g/dL | 3.5 (3.0–3.9) |
| AST, IU/L | 88.0 (54.3–148.5) |
| ALT, IU/L | 74.0 (39.0–115.3) |
| Lactate dehydrogenase | 859.5 (687.0–986.0) |
| Creatinine, mg/dL | 0.8 (0.6–1.1) |
| hs-CRP, mg/dL | 104.3 (62.5–161.1) |
| Genotype, n (%); N = 61 | |
| Boryong strain | 50 (82.0) |
| Karp strain | 9 (14.8) |
| Gilliam strain | 1 (1.6) |
| Kawasaki strain | 1 (1.6) |
| Treatment, n (%) | |
| Doxycycline | 56 (88.9) |
| Azithromycin | 4 (6.3) |
| Azithromycin or doxycycline | 3 (4.8) |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; hs-CRP = high-sensitivity C-reactive protein; IQR = interquartile range; WBC = white blood cell.
*Includes myocardial infarction, congestive heart failure, and peripheral vascular disease.
Includes chronic obstructive pulmonary disease and asthma.
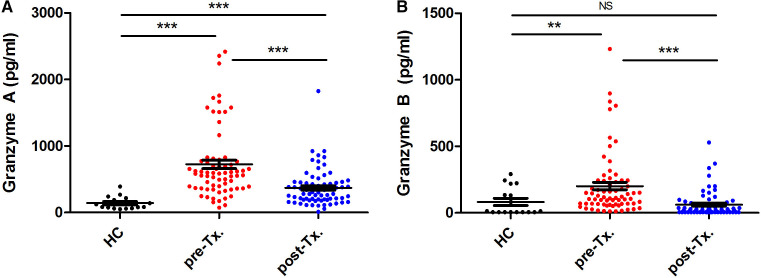
To establish the presence of circulating granzymes during the acute and convalescent phases of scrub typhus, we measured granzymes A and B in the plasma of 63 patients with scrub typhus. Granzymes A and B in the plasma of 16 healthy control subjects were also measured. Both granzymes A and B were elevated in the plasma of patients during the acute phase of scrub typhus compared with healthy control subjects (median, 601.7 versus 104.5 pg/mL, P < 0.001; and 130.0 versus 9.6 pg/mL, P = 0.002, respectively) (Figure 1A and B). Plasma levels of both granzymes A and B decreased significantly when patients improved clinically compared with the acute phase (Figure 1A and B). Although granzyme B levels appeared to decrease relatively quickly and reached base levels upon convalescence, granzyme A levels were still significantly enhanced compared with healthy control subjects (Figure 1A, post-treatment versus healthy control subjects, P < 0.001; and Figure 1B, post-treatment versus healthy control subjects, not significant). During the acute phase in scrub typhus patients, a strong to moderate correlation was seen between granzyme A and B levels (Spearman’s rho, r = 0.66; P < 0.001).
Figure 1.
Extracellular levels of granzyme A and B in scrub typhus patients and healthy control subjects (HCs). Granzyme A (A) and granzyme B (B) are presented. Medians with interquartile range are shown. Significance was determined via Mann-Whitney tests. ***P < 0.001; **P = 0.002. NS = not significant; Tx. = treatment. This figure appears in color at www.ajtmh.org.
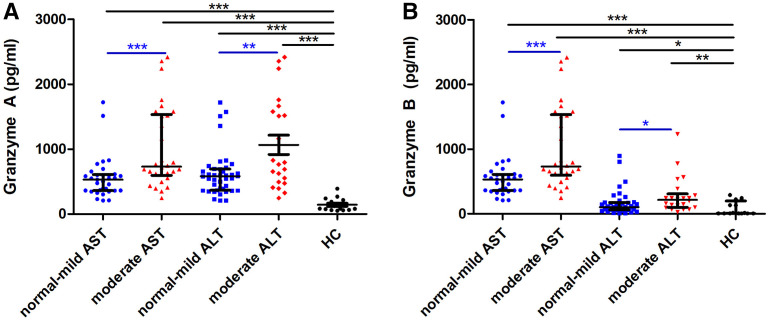
Elevated liver enzyme levels observed in approximately 80% to 90% of the patients could be a result of immunopathology rather than tissue damage via the bacteria themselves. Therefore, we analyzed further whether granzyme A and B levels correlated with liver enzyme levels as a measure for liver damage. Both granzyme A and B levels were significantly greater in moderately elevated AST and ALT groups than normal–mild AST and ALT groups (granzyme A in moderate AST group: median, 546.1 pg/mL, P = 0.005; granzyme A in moderate ALT group: 548.2 pg/mL, P < 0.05; granzyme B in moderate AST group: 99.96 pg/mL, P = 0.01; granzyme B in moderate ALT group: 109.8 pg/mL, P = 0.03) (Figure 2).
Figure 2.
Extracellular levels of granzyme A (A) and B (B) in the acute phase of scrub typhus patients according to level of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Medians with interquartile range are shown. Significance was determined via Mann-Whitney tests. ***P < 0.001, **P < 0.01, *P < 0.05. This figure appears in color at www.ajtmh.org.
DISCUSSION
We investigated the extracellular levels of granzymes A and B in 63 patients with scrub typhus over a 3-year period. Based on our study, increased levels of granzymes A and B in scrub typhus patients compared with healthy control subjects were observed. In addition, longitudinal plasma samples from scrub typhus patients showed increased levels of granzymes A and B during the acute phase of the infection, and a subsequent decline during its resolution.
Elevated levels of granzymes have been reported in other infectious diseases.5 In line with earlier studies, we also noted elevated plasma concentrations of granzymes A and B during the acute phase of scrub typhus.
Increases in liver enzymes in patients with scrub typhus have been reported in previous studies.12,13 Previous studies revealed that scrub typhus hepatitis caused mild portal inflammation and lobular activities without triggering intense interface hepatitis or intralobular hepatocyte death resulting from focal direct liver injury.12,14 In our study, we have observed that 90% and 81% of scrub typhus patients showed abnormal AST and ALT levels, respectively.
Apart from the benefit of defense against intracellular pathogens, granzymes have also been implicated in immunopathology in other infectious diseases.15 Indeed, both granzyme A and B levels were significantly greater in the group with elevated AST and ALT levels in our study. These findings may be explained by the activity of CD8+ T cells that release cytotoxic granules containing granzymes, which has recently been shown to contribute to histopathology in an animal model of O. tsutsugamushi infection.16,17
Liver function impairment in patients with scrub typhus improves with appropriate treatment, and elevated liver enzyme levels alone are not associated with severe scrub typhus.12,18 Increased granzyme levels are associated with disease severity in several infectious diseases.19–21 However, we could not demonstrate a correlation between elevated granzyme levels and severity of illness because no severe cases were available for our study.
We are aware of a few limitations of our study. Because it is a single-center study, there needs to be caution in interpreting results, and the results should not be generalized. In addition, the results may be biased by the large proportion of patients with mild to moderate illnesses resulting from early diagnosis and treatment.
In conclusion, we demonstrate that plasma levels of granzymes A and B increased significantly during the acute phase of scrub typhus. Enhanced levels of both granzymes A and B correlate with liver pathology in patients and subsequently decline after appropriate treatment.
REFERENCES
- 1. Tamura A Ohashi N Urakami H Miyamura S , 1995. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int J Syst Bacteriol 45: 589–591. [DOI] [PubMed] [Google Scholar]
- 2. Taylor AJ Paris DH Newton PN , 2015. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis 9: e0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charles D. Ericsson MJ, Fournier PE, Raoult D, 2004. Rickettsioses and the international traveler. Clin Infect Dis 39: 1493–1499. [DOI] [PubMed]
- 4. Weitzel T Dittrich S Lopez J Phuklia W Martinez-Valdebenito C Velasquez K Blacksell SD Paris DH Abarca K , 2016. Endemic scrub typhus in South America. N Engl J Med 375: 954–961. [DOI] [PubMed] [Google Scholar]
- 5. Buzza MS Bird PI , 2006. Extracellular granzymes: current perspectives. Biol Chem 387: 827–837. [DOI] [PubMed] [Google Scholar]
- 6. Bots M Medema JP , 2006. Granzymes at a glance. J Cell Sci 119: 5011–5014. [DOI] [PubMed] [Google Scholar]
- 7. Wargnier A Legrosmaida S Bosselut R Bourge JF Lafaurie C Ghysdael J Sasportes M Paul P , 1995. Identification of human granzyme-B promoter regulatory elements interacting with activated T-cell-specific proteins: implication of ikaros and cbf binding-sites in promoter activation. Proc Natl Acad Sci USA 92: 6930–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mellor-Heineke S Villanueva J Jordan MB Marsh R Zhang K Bleesing JJ Filipovich AH Risma KA , 2013. Elevated granzyme B in cytotoxic lymphocytes is a signature of immune activation in hemophagocytic lymphohistiocytosis. Front Immunol 4: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Daalen KR Reijneveld JF Bovenschen N , 2020. Modulation of inflammation by extracellular granzyme A. Front Immunol 11: 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Fost M, Chierakul W, Pimda K, Dondorp AM, White NJ, Van der Poll T, 2005. Activation of cytotoxic lymphocytes in patients with scrub typhus. Am J Trop Med Hyg 72: 465–467. [PubMed]
- 11. Lee YM Kim DM Lee SH Jang MS Neupane GP , 2011. Phylogenetic analysis of the 56 kDa protein genes of Orientia tsutsugamushi in southwest area of Korea. Am J Trop Med Hyg 84: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu ML Liu JW Wu KL Lu SN Chiou SS Kuo CH Chuah SK Wang JH Hu TH Chiu KW , 2005. Abnormal liver function in scrub typhus. Am J Trop Med Hyg 73: 667–668. [PubMed] [Google Scholar]
- 13. Basnyat B Belbase RH Zimmerman MD Woods CW Reller LB Murdoch DR , 2006. Clinical features of scrub typhus. Clin Infect Dis 42: 1505–1506. [DOI] [PubMed] [Google Scholar]
- 14. Chung JH Lim SC Yun NR Shin SH Kim CM Kim DM , 2012. Scrub typhus hepatitis confirmed by immunohistochemical staining. World J Gastroenterol 18: 5138–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim TS Shin EC , 2019. The activation of bystander CD8+ T cells and their roles in viral infection. Exp Mol Med 51: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hauptmann M Kolbaum J Lilla S Wozniak D Gharaibeh M Fleischer B Keller CA , 2016. Protective and pathogenic roles of CD8+ T lymphocytes in murine Orientia tsutsugamushi infection. PLoS Negl Trop Dis 10: e0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zloza A , 2006. Evaluating the Role of CD4 (Dim) CD8 (Bright) T Cells in Viral Immunity . Chicago, IL: Rush University. [Google Scholar]
- 18. Kim DM Kim SW Choi SH Yun NR , 2010. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis 10: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lauw FN Simpson AJ Hack CE Prins JM Wolbink AM van Deventer SJ Chaowagul W White NJ van der Poll T , 2000. Soluble granzymes are released during human endotoxemia and in patients with severe infection due to Gram-negative bacteria. J Infect Dis 182: 206–213. [DOI] [PubMed] [Google Scholar]
- 20. Kaminski L Riehn M Abel A Steeg C Yar DD Addai-Mensah O Aminkiah F Owusu-Dabo E Jacobs T Mackroth MS , 2019. Cytotoxic T cell-derived granzyme B is increased in severe Plasmodium falciparum malaria. Front Immunol 10: 2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Napoli AM Fast LD Gardiner F Nevola M Machan JT , 2012. Increased granzyme levels in cytotoxic T lymphocytes are associated with disease severity in emergency department patients with severe sepsis. Shock 37: 257–262. [DOI] [PubMed] [Google Scholar]