Abstract
Patients with cancers have been severely affected by the COVID-19 pandemic. This is highlighted by the adverse outcomes in cancer patients with COVID-19 as well as by the impact of the COVID-19 pandemic on cancer care. Patients with cancer constitute a heterogeneous population that exhibits distinct mechanisms of immune dysfunction, associated with distinct systemic features of hot (T-cell-inflamed/infiltrated) and cold (Non-T-cell-inflamed and/or infiltrated) tumors. The former show hyper immune activated cells and a highly inflammatory environment while, contrastingly, the latter show the profile of a senescent and/or quiescent immune system. Thus, the evolution of SARS-CoV-2 infection in different types of cancers can show distinct trajectories which could lead to a variety of clinical and pathophysiological outcomes. The altered immunological environment including cytokines that characterizes hot and cold tumors will lead to different mechanisms of immune dysfunction, which will result in downstream effects on the course of SARS-CoV-2 infection. This review will focus on defining the known contributions of soluble pro- and anti-inflammatory mediators on immune function including altered T-cells and B-cells responses and as well on how these factors modulate the expression of SARS-CoV-2 receptor ACE2, TMPRSS2 expression, and lymph node fibrosis in cancer patients. We will propose immune mechanisms that underlie the distinct courses of SARS-CoV-2 infection in cancer patients and impact on the success of immune based therapies that have significantly improved cancer outcomes. Better understanding of the immune mechanisms prevalent in cancer patients that are associated to the outcomes of SARS-CoV-2 infection will help to identify the high-risk cancer patients and develop immune-based approaches to prevent significant adverse outcomes by targeting these pathways.
INTRODUCTION
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has at present, (November 2021) caused 257 million infections and more than 5.1 million deaths worldwide (New York Times, November 21, 2021). Studies have shown that, cancer patients are particularly more susceptible to SARS-CoV-2 infections (0.9% vs 0.29%).1, 2, 3, 4, 5 Besides that, the factors that have been most consistently linked with increased risk of severe COVID-19 disease and/or death are prevalent in cancer patients, and include but not limited to older age (≥ 60 years), a history of smoking, obesity, hypertension, cardiovascular disease, and diabetes1. Higher susceptibility of cancer patients to SARS-CoV-2 infection is either due to impaired immune responses that are characteristic of the cancer (and associated co-morbidities) or due to the anti-cancer treatments that alter immune homeostasis.1, 2, 3 To understand why cancer patients are at greater risk of complications and/or death associated with COVID-19 it is important to decipher immune responses that govern the development of specific cancers, associated therapies and comorbidities.6 , 7
The severity of COVID-19 disease in cancer patients is partly a function of the etiology, type (hot vs cold tumors, where hot tumors have an immunologically active microenvironment), stage and anatomical location of the tumor.4 , 8, 9, 10, 11, 12 These factors along with treatment regimens play a crucial role in diversifying the immune landscape of a malignancy.8 , 12 Indeed, it has been observed that COVID-19 patients with hot tumors (ie, lung cancer and hematological malignancies (HM)) are likely to develop severe COVID-19 disease.1 , 4 , 10 , 11 Recent reports have shown that adverse outcomes in COVID-19 infected cancer patients can result from: alterations in expression of host proteins that promote SARS-CoV-2 entry (ACE2 and TMPRSS2), an aberrant cytokine profile (mainly IL-1β, IL-2, IL-6, GM-CSF, IFNγ, TNF-α, and TGF-b), lymph node thrombosis, impaired T/B-cell responses, and impaired inflammasome response – especially the NLRP3 inflammasome.1 , 3 , 8 , 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 To decipher the mechanisms that drive these adverse outcomes in COVID-19 infected cancer patients, this review will assess the impact of “hot” vs “cold” immune and/or tumor environments (and associated therapies) on susceptibility and course of disease in subjects with these 2 types of tumors and infected with SARS-CoV-2. The mechanisms discussed below can help further research into the development of tailored approaches that promote anti-cancer responses while restraining COVID-19 disease severity.
COVID-19 AND ITS OUTCOMES
Coronaviruses are a diverse group of respiratory viruses that can infect humans and animals.23 In 2019, Wuhan (China) saw the emergence of a novel coronavirus “SARS-CoV-2″ that has been responsible for an unusual and highly transmissible viral pneumonia pandemic that was designated as coronavirus disease 2019 (COVID-19).24 SARS-CoV-2 enters the host respiratory epithelial cells by binding the angiotensin converting enzyme II (ACE2).25 , 26 Specifically, the C-terminal domain of the SARS-CoV-2 spike (S) protein, known as “receptor binding domain (RBD)”, binds ACE2 to aid viral entry into the host cell.27 , 28 Next, host proteases (Transmembrane Protease Serine Protease 2 (TMPRSS2), cathepsin L and furin), cleave the S protein of SARS-CoV-2 that is required to activate endocytic entry of SARS-CoV-2 and initiate infection.26 , 29 , 30 During the initial phase of the infection, susceptible epithelial cells in the nostrils allow for SARS-CoV-2 replication and subsequent transmission to lower respiratory tract epithelial cells and finally to alveolar epithelial cells (Fig 1 ).30 Rapid replication of SARS-CoV-2 in the respiratory tract can promote systemic proinflammatory cytokine production (known as the “Cytokine storm”), such as: IL-1β, IL-6, IL-7, IL-8, IL-9, IL-10, FGF, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1A, MIP1-B, PDGF, TNF-α, and VEGF, which subsequently results in dysregulation of immune functions, severe inflammation (including myocarditis) and multiple organ failure in some COVID-19 patients (Fig 1).21 , 22 , 31, 32, 33, 34 The most striking impact of this storm is observed in the respiratory tract, where pro-inflammatory cytokines drive the pathology of acute respiratory distress syndrome (ARDS) and ultimately respiratory failure - the main causes of death in COVID-19 patients.11 , 32 , 33 , 35
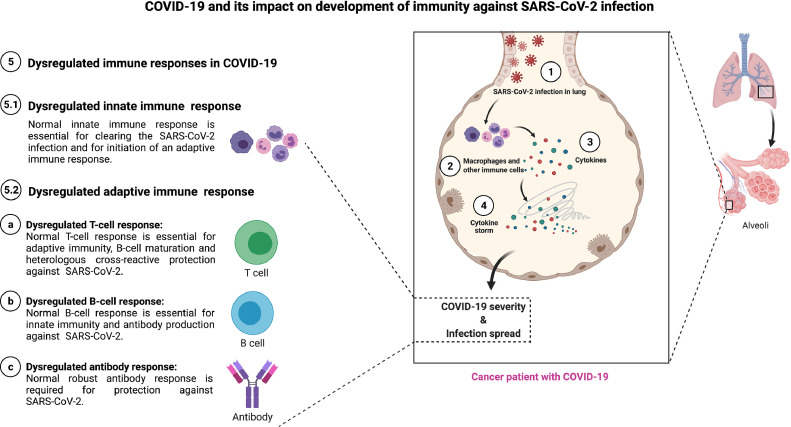
Fig 1.
COVID-19 and its impact on development of immunity against SARS-CoV-2 infection. SARS-CoV-2 infect lung epithelial cells (1) and is sensed by macrophages and other innate immune cells (2). Upon sensing the SARS-CoV-2 infection, innate immune cells express cytokines (3) which accelerate the production of more cytokines and lead to cytokine storm (4). The resulting cytokine storm lead to dysregulation of immune functions and/or responses (5): dysregulated innate immune response (5.1), dysregulated adaptive immune response (5.2) – dysregulated T-cell response (a), dysregulated B-cell response (b), and dysregulated antibody response (c).
SARS-CoV-2 INFECTION IN CANCER PATIENTS
Numerous studies have shown that COVID-19 with ‘pre-existing conditions’ especially cancer, have higher mortality rates than “healthy” population (16-fold higher risk) (FDA's Oncology Center of Excellence).3 , 8 , 36 , 37 The estimated 19 million new cancer cases (led by breast, lung, colorectal, prostate cancer, skin and stomach cancers), 10 million deaths in 2020 (GLOBOCAN), along with the increased infection risk, make it crucial to understand the immunological interplay between cancer and SARS-CoV-2 infection. Moreover, major signaling pathways impaired by SARS-CoV-2 infection are also upregulated in patients with cancer and COVID-19, and include: cytokine signaling, type-I interferon signaling, androgen receptor signaling, and immune checkpoint signaling (Fig 2 ).38 Untangling the complex relationships between the immune responses triggered by the many cancer types and SARS-CoV-2 infection has been an on-going challenge for the field, and will be further discussed in the following sections.
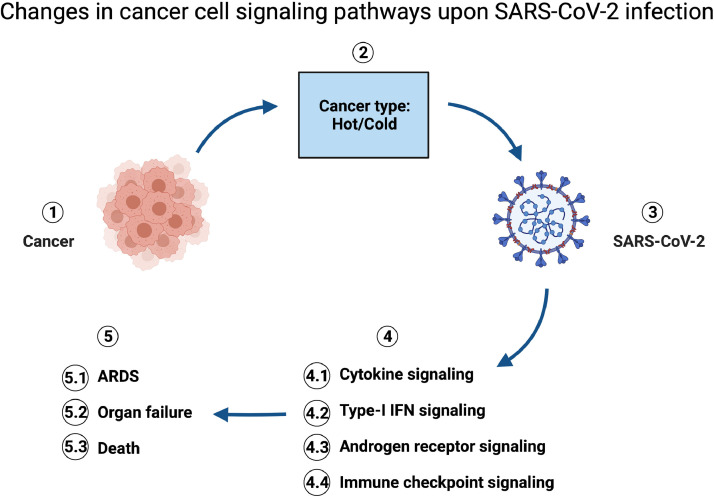
Fig 2.
Changes in cancer cell signaling pathways upon Sars2 infection. Cancer (1) is heterogenous depending on the tumor micro environment: hot or cold (2), and the susceptibility of patients with cancer to SARS-CoV-2 infections (3) is influenced by cancer type. Four major signaling pathways that are common and impaired in both diseases are: cytokine, type-I IFN, androgen receptor, and immune checkpoint signaling pathways (4). These impairments in signaling pathways lead to cytokine storm that consequently ends up on acute respiratory distress syndrome (ARDS), organ failure, and death (5).
Viral infections and the associated chronic inflammatory responses have often been associated with cancer presentation and/or progression.37 , 39 , 40 Among viruses, a group of viruses is associated with the incidence and/or progression of cancers and are known as oncogenic viruses, for example; HIV, HPV, HBV, HCV, EBV.37 , 41, 42, 43 The designation of SARS-CoV-2 as an “oncogenic virus” and its role in tumorigenesis remain subjects of ongoing research.37 , 44 Studies looking into the link between SARS-CoV-1 infection (a virus that shares 79.6% homology at genome level with SARS-CoV-2) and cancer have reported that this virus can interfere with signaling pathways, such as p53, EGFR, JAK/STAT, or MAPK signaling, that will promote carcinogenic transformation of cells.47, 48, 49 Moreover, pro-inflammatory cytokine production during SARS-CoV-2 infection (including IL-6: a typical feature of oncogenic viruses) could drive pro-tumorigenic activity.44, 45, 46 Even if SARS-CoV-2 does not play a direct role in cancer etiology, it still has the potential to alter the immune landscape and which would enhance adverse outcomes in patients with cancer.8 , 50 , 51 To understand the role of SARS-CoV-2 infection in tumor progression it is important to consider etiological differences among cancers that complicate the cancer immune landscape and drive adverse outcomes in SARS-CoV-2 infected cancer patients.
Recent epidemiological studies have reported that patients with hematological, lung or breast cancers are more likely to develop adverse outcomes that culminate in increasing the risk of hospitalization and deaths, during SARS-CoV-2 infection.36 , 52 , 53 Interestingly, these reported cancer types have very diverse immunopathology and hence are likely to mount distinct changes in the immune landscape during infection.54, 55, 56 Hematological malignancies and/or blood cancer are liquid (leukemias) or are localized to sites of development and/or primary ie, bone marrow or secondary lymphoid organs, unlike most solid tumors.57, 58, 59 These features make hematological malignancies highly available to interactions with immune cells and respond avidly to on-going systemic inflammatory responses (such as those observed during severe COVID-19). In this scenario, it is likely that pre-infection cancers associated inflammation synergizes with systemic immune responses seen during SARS-CoV-2 infection – resulting in a higher than usual inflammation and potentially higher mortality rates.
In contrast to hematological malignancies, lung and breast cancers are solid tumors that respectively fall under “hot” and “cold” tumor categories, respectively.12 , 60, 61, 62, 63 Similar to most hematological malignancies, a hot tumor (like melanoma, non-small cell lung cancer, and cancers of the liver, kidney, bladder, and head and neck) shows a high level of interfacing capacity with the immune system.63 , 64 Indeed, the tumor microenvironment (TME) of hot tumors includes several subsets of innate as well as adaptive immune cells that are endowed with a wide array of effector functions.63 , 65 Specifically, cytokines and chemokines (including but not limited to; CCL2, CCL3, CCL4, CCL5, CXCL9, CXCL10, CCL17/22) produced by these tumors allow for the migration of tumor specific T cells in the TME; these T cells are responsive to immune check point blocker therapies that rescue anti-tumor T cell effector function.12 , 63, 64, 65, 66, 67 In contrast, cold tumors (like ovarian, breast and pancreatic cancers) are characterized as “non-inflamed” or “immune-deserts” and present with a microenvironment that presents striking features of T-cell r exclusion from the TME.12 , 63, 64, 65 , 68, 69, 70 In the context of SARS-CoV-2 infection, it can be speculated that hot tumors (like lung cancer) that are immunologically active and anatomically primed will fuel the systemic inflammatory responses observed during SARS-CoV-2 infection (Fig 3 ). However, the exact mechanisms driving adverse outcomes with each cancer (with or without therapy) are yet to be elucidated.
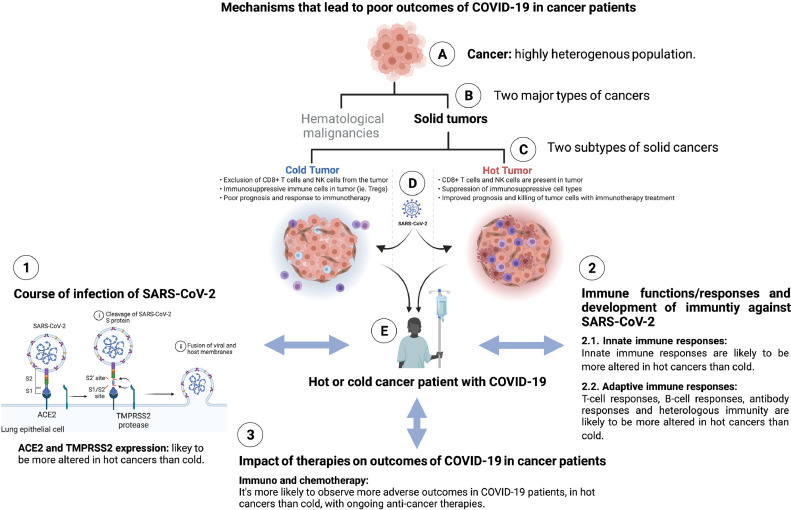
Fig 3.
Mechanisms that lead to poor outcomes of COVID-19 in cancer patients. Cancer patients are highly heterogeneous population (A) and are divided into two major categories; hematological malignancies and solid tumors (B). Solid tumors can be further divided into hot tumor and cold tumor groups (C), which can get infected by SARS-CoV-2 (D) and develop COVID-19 (E). Course of infection of SARS-CoV-2 (1) – influenced by differential expression of ACE2 and TMPRSS2 in hot vs cold cancers – can lead to poor outcome of COVID-19 in hot vs cold cancer patients. Immune responses and development of immunity against SARS-CoV-2 (2) – influenced by differential innate (2.1) as well as adaptive (2.2) immune responses in hot vs cold cancers – can lead to poor outcome of COVID-19 in hot vs cold cancer patients. Impact of therapies (3) – influenced by immunotherapy as well as chemotherapy in hot vs cold cancers – can lead to poor outcomes of COVID-19 in hot vs cold cancer patients.
IMMUNE MECHANISMS THAT DETERMINE THE ADVERSE OUTCOMES OF COVID-19 IN HOT AND COLD TUMORS
To understand the impact of these heterogenous cancer etiologies on COVID-19 outcomes, it is important to first elucidate their impact on SARS-CoV-2 entry and/or infection and existing or resulting immune cascades. Expression of ACE2 (the viral entry receptor) in lung epithelial was shown to be higher in older subjects, smokers and/or subjects suffering from smoking related disorders like chronic obstructive pulmonary disease (COPD).3 , 13 , 71, 72, 73 Moreover, expression of TMPRSS2, a membrane-bound serine protease known to synergize with ACE2 to promote SARS-CoV-2 entry, has also been observed to be highly expressed in prostate cancer (cold cancer), where it is upregulated by androgen receptor (AR).74, 75, 76 Interestingly, in two recent studies, prostate cancer (cold cancer) patients not treated with androgen deprivation therapy (ADT) were more likely to be infected by SARS-CoV-2, suggesting a possible relation of the increased expression of TMPRSS2 and the development of severe COVID-19 in patients with cold tumors like prostate cancer.17 , 77 Based on these data, it can be hypothesized that the increased expression of ACE2 and TMPRSS2, mainly due to pro-inflammatory conditions, in subjects with factors known to be associated with lung cancer incidence could lead to increased viral titers and development of severe COVID-19 (Fig 3).
Innate immune responses and development of severe SARS-CoV-2 in hot vs cold cancer
The innate immune response is the first line of defense against SARS-CoV-2 infection.78, 79, 80 SARS-CoV-2 infection is sensed by pathogen recognition receptors (PRRs) – mainly by RIG-I Like Receptors (RLRs); MDA5 and LGP2, and NOD Like Receptors (NLRs); NOD1 – in the lung epithelial cells, and innate immune response is initiated.79 , 80 Upon induction of innate immune response, cells in lung epithelium – epithelial cells as well as immune cells – produce proinflammatory cytokines, chemokines, interferons (IFNs); type-I and type-III, and IFN stimulated genes (ISGs).80, 81, 82, 83, 84 However, type-I and type-III IFN defined innate immune response in COVID-19 are dysregulated and its kinetics set the severity and future pathological outcomes of the disease.83 , 85 , 86 For example, the initial type-I and type-III IFNs response is higher in patients with mild and/or moderate COVID-19 while it is reduced in severe COVID-19 patients.83 , 85 , 86 In particular, COVID-19 patients in critical condition (severe COVID-19) exhibit increased frequencies of innate immune cells – mainly neutrophils, monocytes and macrophages – that produce increased level of cytokines which results in the cytokine storm. Cytokine storm induction is correlated with the severity of COVID-19 and its adverse outcomes.84 , 86 , 87
At present, very limited knowledge is available on SARS-CoV-2 specific innate immune responses in patients with cancers. Compromised innate immune response in patients with cancers make cancer patients more susceptible to SARS-CoV-2 infection.8 , 88, 89, 90 Higher frequencies of adverse outcomes of COVID-19 inflicted by the dysregulated innate immune responses are likely in patients with hot cancers than the patients with cold cancers, because of increased immune compromised environment of hot cancers than the cold cancers (Fig 3).91 Specifically, lung cancer (a hot cancer) has active but compromised and/or exhausted lung TME and thus has a disrupted innate immune response against SARS-CoV-2 infection.91 , 92 The immune activation profile of TME in lung cancer – infiltrated with immune cells and chronically inflamed – can fuel up the dysregulation of innate immune responses against SARS-CoV-2 in lungs.10 , 91 Moreover, cytokine storm could occur in tumor cells too, because of several underlying factors, and that can impact the innate immune responses mounted against SARS-CoV-2 infection as well as the severity of COVID-19.92 On the other hand, dysregulation of innate immune response in consequence of SARS-CoV-2 infection can facilitate the growth of lung cancer and/or other tumors.91
Adaptive immune responses and the development of severe SARS-CoV-2 in hot vs cold cancer
At present, very few studies have focused on deciphering SARS-CoV-2 specific adaptive immune responses in patients with cancers. In SARS-CoV-2 infection, classic anti-viral responses – where anti-viral cytotoxic CD8 T-cell responses (aided by a CD4 T-cell driven T-helper 1 response) limit viral persistence by killing virus infected host cells – are disrupted and a rapid depletion of CD4 T-cells, CD8 T-cells and B-cells is observed (Fig 3).87 Recently, Mansi et al. showed that although cancer patients failed to mount T-cell responses against SAR-CoV-2, no impact on the pre-established immune memory against common viruses in SARS-CoV-2 infected patients was observed, arguing that the impaired SARS-CoV-2 specific T-cell responses can be used as a determinant for adverse outcome of COVID-19 in cancer patients.93
As discussed above, the outcome of SARS-CoV-2 infection can be shaped by the immune microenvironment of a tumor. On the one hand, while hot TME is highly dynamic and allows for immune cell infiltration, cold tumors lack immune cell infiltration, suggesting that the impairment of pre-existing anti-tumor T-cells responses in cancer patients that acquire SARS-CoV-2 infection would be greater in hot cancers or hematological cancers that regularly interface with immune cells. In addition to pre-existing anti-tumor T-cells, heterologous immunity – a phenomenon of protection in which an individual develops pathogen-specific T-cells against an unencountered pathogen after being exposed to cross-reactive non-identical pathogens94, 95, 96, 97, 98 – is also observed in SARS-CoV-2 unexposed individuals.97 , 99, 100, 101 This is particularly true in individuals that have higher frequencies of T cells with responses to cross-reactive epitopes shared by common human coronaviruses (HCoV-OC43, HCoV-229E, HCoV-NL63, and HCoV-HKU1).101 , 102 Because of increased T-cell activity in hot or HM tumors, impairments in cross-reactive immunity may be expected. A recent study by Bilich et. al. showed reduced prevalence of pre-existing cross-reactive CD4 T-cell responses against SARS-CoV-2 in unexposed patients with HMs compared to patients with solid tumors.97 Moreover, the unexposed subjects with HMs presented signs of T-cell exhaustion (higher proportion of T cells expressing PD-1, LAG3, and TIM3) and reduced magnitude, diversity, and persistence (memory) of SARS-CoV-2 specific T-cell immunity.97 In addition to T-cells, B lymphocytes (B-cells) also play a critical role in protecting against SARS-CoV-2 infection by producing neutralizing anti-viral antibodies. Although B cells have not been extensively studied in cancer patients with COVID-19, Mansi et al. have shown that SARS-CoV-2 patients with solid tumors or HMs produced high titers of virus specific antibodies, which could compensate for an impaired T cell response.93
Impaired T and B-cell responses can result from damage to specific organs, like the lymph nodes (LNs).103, 104, 105 Indeed, SARS-CoV-2 infection causes swelling and inflammation of lymph nodes, which can progress to LN fibrosis, prevent germinal center formation of and consequently disrupt healthy virus specific T-cell responses and neutralizing antibody production.18 , 19 , 106 Lymph nodes of COVID-19 patients with lung carcinoma metastatic cancer – a cancer known to have compromised innate (such as, IFN-I and IFN-III) and T-cell (memory TH1) responses in mediastinal lymph nodes – were found to be enlarged with tissue structure disruption and immune cell dysregulation, including macrophage accumulation and lymphopenia.18 , 19 , 106 Although little is known about lymphedema in SARS-CoV-2 infected cancer patients, it can be postulated that like in HIV infection, increased concentrations of lymphatic TGF-b and IL-1b might play a major role in the development of LN fibrosis107 , 108 which would impact on adaptive immune responses. Further studies to clarify the decipher the impact of these LN fibrosis inducing cytokines on priming adaptive cellular and humoral responses to SARS-CoV-2 infection need to be conducted.
IMPACT OF CANCER THERAPIES ON COVID-19 OUTCOMES IN HOT VS COLD CANCER
Anti-cancer therapies
Stratifying groups of cancer patients based on the type of treatment, stage of treatment, dosage of treatment etc. could provide better insights into the outcomes of COVID-19 in infected patients. Studies have shown that the immune system of patients with cancer undergoes diverse alterations due to the various treatment regimens they receive.3 , 45 Traditional anti-cancer therapies like chemotherapy cause bone marrow suppression which leads to thrombocytopenia and neutropenia, while DNA damage to lymphocytes caused by radiation therapy can cause lymphopenia.3 , 13 , 45 , 109 , 110 The depletion of leukocytes in conjunction with the use of corticosteroids and other immunosuppressive therapeutics impair immune responses against even the most common bacterial and viral pathogens and this compromised immunity could contribute to increased incidence of COVID-19 related adverse outcomes in cancer patients (Fig 3).13 , 45 , 110 Epidemiological studies have reported mixed results on the association between chemotherapies and COVID-19 associated adverse outcomes. In a Japanese seroprevalence study, Ab levels against SARS-CoV-2 nucleocapsid (but not spike) protein were lower in cancer patients who received chemotherapy treatment within 1 month compared with those who did not receive it.111 A study from Israel also reported that IgG levels were significantly lower in patients who received combined chemotherapy with immunotherapy (commonly administered in specific cancers like lung cancer (hot cancer), triple-negative breast cancer (cold cancer)).112 , 113 Unlike chemotherapy, immunotherapies (immune check inhibitors, adaptive cell therapies, cancer vaccines etc.) that help prime the immune system to kill cancer cells, could be advantageous in this scenario and could help in mounting anti-SARS-CoV-2 responses.114
Cancer is often characterized by a state of systemic chronic inflammation. However, several tumors require interventions that rejuvenate immune responses within the host TME. These therapies include the use of immune checkpoint inhibitors (ICIs), cytokine-based therapies, chimeric antigen receptor T (CAR-T) cell therapy, bispecific T cell engagers (BiTEs), and allogeneic stem cell transplantation.3 , 45 , 115, 116, 117 Although these therapies drive efficacious anti-tumor responses they also induce systemic inflammation and the cytokine storm that can harm normal healthy tissues (eg, pneumonitis) and predispose the patient to adverse effects that are associated with SARS-CoV-2 infection.3 , 45 , 118 , 119 Most commonly, the use of immunotherapies, such as CAR-T and CTLA-4 therapies, can promote systemic immune hyperactivation that results in the clinical manifestation of cytokine release syndrome.45 , 115 , 118 , 120 Indeed, it has been observed that cytokines that mediate efficacious ICI responses during CAR-T cell and BiTE therapy (IFN-γ, TNF-α, IL-2 and GM-CSF) are also some of the prime conductors of cytokine storm.3 , 121, 122, 123 However, a recent report showed that the prior anti-PD-1therapy – an ICI that is widely used to treat lung cancer – does not appear to impact the severity of COVID-19 in patients with lung cancers. That is contrary to the assumptions that the prior use of ICIs in cancer patients can dampen the inflammation and severity of COVID-19 disease.52 Clinical studies aimed at elucidating the impact of immunotherapies on the manifestation of adverse outcomes in COVID-19 patients have showed conflicting results.6 , 124 , 125
Vaccine and alternate therapies for efficacious response to SARS-CoV-2 infection in cancer patients
Impairments of B-cell responses contribute to proper but delayed COVID-19 vaccine responses in patients with cancers. However, the extent to which cancer heterogeneity contributes to this impairment remains unclear. COVID-19 vaccine recipients with solid tumors were observed to have humoral immune responses; anti-SARS-CoV-2 spike (S) IgG), comparable to those observed in healthy subjects; of note low cellular responses were monitored in these patients.126 Whereas patients with HMs elicited suboptimal humoral and cellular immune responses.126 In addition, antibody titers sharply decreased within 3 months of vaccination for most of cancer patients (most prominently for patients with HMs).126 In another controlled mRNA vaccine immunization study, Shroff et al., 2021, it was found that neutralizing antibodies were produced in 67% and 80% of cancer patients after the primary and secondary vaccine shots, respectively127 as compared to healthy controls. A further 3-fold increase in median titers was observed upon booster immunization.127 Quantification of antigen (RBD and Spike S1) specific memory B-cell subsets, revealed that frequencies of spike-specific memory B-cells required two immunizations in cancer patients vs a single immunization in healthy subjects127 further indicating that a third vaccine dose might help enhance antibody responses in patients with cancers. This lower or non-responsiveness to COVID-19 vaccine by cancer patients is further confirmed in a recent study from US which reported that 46% of the patients with HMs – 31 out of 67 patients – did not produce detectable anti-SARS-CoV-2 spike antibodies following two shots of the Pfizer-BioNTech COVID-19 vaccine. These 31 patients were considered “non-responders” to the COVID-19 vaccine.128 Another study showed that the seroconversion – the time from vaccination to the availability of virus specific antibodies in the blood – rate for CVOID-19 was only 55% in cancer patients following one dose of Pfizer-BioNTech, though it reached 100% in the control group (25 subjects).129 That being said, these promising observations have been confounded by reports that some cancer patients fail to respond to the SARS-CoV-2 vaccine at all (Fig 4 ). These poor vaccine specific antibody responses could result from suboptimal T/B-cell priming and collaboration that is a consequence of the fibrosis (driven by excessive TGF-β) which disrupts the LN architecture. Further studies to understand the mechanisms that underlie poor immune response to vaccine in SARS-CoV-2 infected cancer patients are still needed.
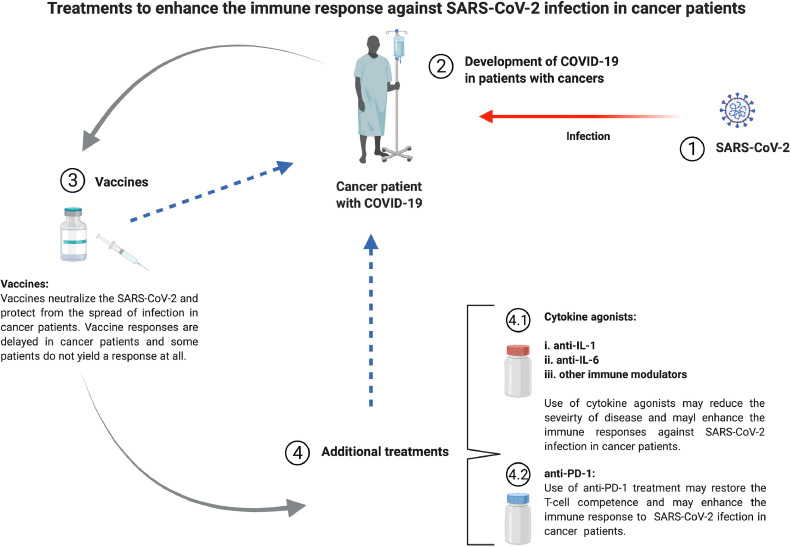
Fig 4.
Treatments to enhance the immune response against SARS-CoV-2 infection in cancer patients. SARS-CoV-2 infect individuals with cancers (1) and SARS-CoV-2 infected individuals develop COVID-19 (2). COVID-19 vaccine is the first strategy to protect immunocompromised individuals, particularly cancer patients, from SARS-CoV-2 infection (3). Additional treatments are required for the cancer patients who cannot acquire protection, against COVID-19, through vaccine (4). Additional treatments include cytokine agonists (4.1): anti-IL-1 (a), anti-IL-6 (b), other immune modulators (c), and anti-PD-1 (4.2).
Given the poor COVID-19 vaccine efficacy in cancer patients, it becomes increasingly important to find alternate means of treating SARS-CoV-2 infected cancer patients (Fig 4). Alternate treatments that modulate the immune system by reducing aberrant inflammation and improve T-cell function (cytokine blockers and ICIs) could benefit this population.130, 131, 132 Two primary systemic innate immune cytokines that mediate the pathology of cytokine release syndrome are IL-1 and IL-6.133 , 134 IL-1, the master orchestrator of inflammatory responses in COVID-19, promotes innate immune activation and drives the production of proinflammatory molecules.133 IL-1 inhibitors, approved by the Food and Drug Administration (FDA) – already available in the market – either bind directly to IL-1 (Rilonacept and Canakinumab) or block IL-1 binding to the IL-1 receptor (Anakinra).130 , 135 Like IL-1, elevated IL-6 levels in patients with severe COVID-19 identifies can also be targeted by existing FDA approved agents that either directly target IL-6 (Clazakizumab, Siltuximab, Sirukumab and Olokizumab), IL-6 cognate receptor (Sarilumab and Tocilizumab) or block IL-6 trans-signaling (Olamkicept) by blocking the soluble IL-6 receptor (sIL-6R).134 Although preliminary studies have yielded conflicting results in patients with mild to moderate COVID-19, the use of IL-6 in patients with severe COVID-19 is still seen as a viable treatment option.136 , 137 Other compounds that may have therapeutic potential in severe COVID-19 include inhibitors against: interferons (α, β, γ), kinases (JAK, MAPK, P13K), GM-CSF, CCR, NF-kB and JAK/STATs.138 In fact, the JAK inhibitor “baricitinib” has been approved by FDA for emergency use in combination with “remdesivir” (FDA).139 Further investigation into mechanisms that drive these pro-inflammatory responses must be conducted in order to identify therapeutics for cancer patients with severe COVID-19 infections.
Another promising option to treat the COVID-19 patients with cancers is the use of PD-1 inhibitors (Fig 4). While PD-1 blockade has improved the survival rate of patients with multiple incurable cancers, the potential beneficial therapeutic impact of PD-1 blockade is unknown in context of COVID-19 patients with cancers.140 Like cancer patients, increased frequencies of PD-1 expressing T-cells are observed in COVID-19 patients.141 It can be argued that, although on the one end therapies like PD-1/PD-L1 blockade (that restore T-cell competence in cancer and chronic viral infections) may enhance detrimental hyperimmune response in COVID-19 patients, they could provide much needed immunological control of viral infections.142 Current clinical trials aimed at evaluating the efficacy of anti-PD-1 antibody administration to both cancer and non-cancer patients with COVID-19 are underway, and may help in understanding whether restoring the competence of PD-1 expressing T-cells can efficaciously control SARS-CoV-2 infection.140
RELEVANCE TO CANCER CARE
The impact of COVID-19 pandemic on cancer patients is far reaching. Early in the pandemic, there was a great need to divert health care resources to address a rapidly growing numbers of COVID-19 patients, as well as to protect healthy individuals from SARS-CoV-2 infection by suspending non-urgent health care (American Cancer Society). Clinical trials for finding new cures have also been affected by the COVID-19 pandemic, with 60% of research programs suspending screening and/or enrollment of subjects for clinical trials.143 A large portion of research funds have been invested in COVID-19 clinical trials; more than 6442 ongoing studies are listed in clinicaltrials.gov, as of September 2021. While these measures were essential, delays in cancer screening, diagnosis, and treatment due to the restricted access to care will most likely result in missed diagnoses and an increase in late-stage diagnoses and preventable cancer deaths.143, 144, 145, 146, 147, 148 The adverse impact of the pandemic on cancer care can be expected to translate into increased cancer mortality over the coming years.
PERSPECTIVES
Caveats of these studies include, but are not limited to: (1) short duration studies, (2) the small sample size for cancer patients with COVID-19, (3) the very heterogeneous profile of the cancer patient cohorts in terms of cancer type, status of the cancer, nature of treatment, status of treatment, (4) lack focus on defining the mechanisms involved in adverse outcomes for cancer patients with COVID-19. Given the complexity and heterogeneity of the cancer population in context of SARS-CoV-2 infection, an unbiased systems biology approach would be very useful to understand mechanisms and identify therapeutic targets that can aid in improving outcomes in SARS-CoV-2 infected cancer patients.
FINAL SUMMARY
The impact of the ongoing COVID-19 pandemic is being felt in subjects that have a pre-disposed immunocompromised profile (eg, cancer patients). Due to the immense heterogeneity (type and treatment of tumors) among the cancer populations, conflicting observations of adverse outcomes to SARS-CoV-2 infection have been reported. Mechanistic understanding of how infections progress in homogenous cancer populations will help develop therapeutics that can restrain adverse outcomes and promote healthy recovery.
ACKNOWLEDGMENTS
Conflict of Interest: All authors have read the journal's policy on disclosure of potential conflicts of interest and have none to declare.
Manuscript was drafted by MBL. Figures were created by MBL using BioRender at BioRender.com. AAS and RPS edited the manuscript and provided rigorous intellectual input. SS, PMDRE, SPR revised and approved the final version. Authors have read the authorship agreement of the journal. This work was funded by NIH and/or NCI grant 1U54CA260563-01.
Thanks to Malvika Chaudhary and Mohamed Salah Abdel-Hakeem for proofreading the manuscript.
Footnotes
Rafick Pierre Sekaly and Ashish Arunkumar Sharma contributed equally to this work.
References
- 1.Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannakoulis VG, Papoutsi E, Siempos II. Effect of Cancer on Clinical Outcomes of Patients With COVID-19: A Meta-Analysis of Patient Data. JCO Glob Oncol. 2020;6:799–808. doi: 10.1200/GO.20.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakouny Z, Hawley JE, Choueiri TK, et al. COVID-19 and Cancer: Current Challenges and Perspectives. Cancer Cell. 2020;38:629–646. doi: 10.1016/j.ccell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C, Zhao Y, Okwan-Duodu D, Basho R, Cui X. COVID-19 in cancer patients: risk, clinical features, and management. Cancer Biol Med. 2020;17:519–527. doi: 10.20892/j.issn.2095-3941.2020.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nature Medicine. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derosa L, Melenotte C, Griscelli F, et al. The immuno-oncological challenge of COVID-19. Nature Cancer. 2020;1:946–964. doi: 10.1038/s43018-020-00122-3. [DOI] [PubMed] [Google Scholar]
- 9.Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10:589–597. doi: 10.1016/S1470-2045(09)70069-5. [DOI] [PubMed] [Google Scholar]
- 10.Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicenter, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicenter, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan Q, Zhang H, Zheng J, Zhang L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer. 2020;6:605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and Cancer: a Comprehensive Review. Curr Oncol Rep. 2020;22:53. doi: 10.1007/s11912-020-00934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JC, Sausville EL, Girish V, et al. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev Cell. 2020;53:514–529. doi: 10.1016/j.devcel.2020.05.012. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 16.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montopoli M, Zumerle S, Vettor R, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Annals of Oncology. 2020;31:1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q, Shi Y, Cai J, et al. Pathological changes in the lungs and lymphatic organs of 12 COVID-19 autopsy cases. National Science Review. 2020;7:1868–1878. doi: 10.1093/nsr/nwaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabary M, Khanmohammadi S, Araghi F, Dadkhahfar S, Tavangar SM. Pathologic features of COVID-19: A concise review. Pathol Res Pract. 2020;216 doi: 10.1016/j.prp.2020.153097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albiges L, Foulon S, Bayle A, et al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: results from the Gustave Roussy cohort. Nature Cancer. 2020;1:965–975. doi: 10.1038/s43018-020-00120-5. [DOI] [PubMed] [Google Scholar]
- 21.Quagliariello V, Bonelli A, Caronna A, et al. SARS-CoV-2 infection and cardioncology: from cardiometabolic risk factors to outcomes in cancer patients. Cancers. 2020;12:3316. doi: 10.3390/cancers12113316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quagliariello V, Bonelli A, Caronna A, et al. SARS-CoV-2 infection: NLRP3 inflammasome as plausible target to prevent cardiopulmonary complications? Eur Rev Med Pharmacol Sci. 2020;24:9169–9171. doi: 10.26355/eurrev_202009_22867. [DOI] [PubMed] [Google Scholar]
- 23.Hu B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19. Nature Reviews Microbiology. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature Communications. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao XH, Li TY, He ZC, et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Mao B, Liang S, et al. Association between age and clinical characteristics and outcomes of COVID-19. Eur Respir J. 2020;55 doi: 10.1183/13993003.01112-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rugge M, Zorzi M, Guzzinati S. SARS-CoV-2 infection in the Italian Veneto region: adverse outcomes in patients with cancer. Nature Cancer. 2020;1:784–788. doi: 10.1038/s43018-020-0104-9. [DOI] [PubMed] [Google Scholar]
- 37.Jyotsana N, King MR. The Impact of COVID-19 on Cancer Risk and Treatment. Cellular and Molecular Bioengineering. 2020;13:285–291. doi: 10.1007/s12195-020-00630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Molecular Cancer. 2021;20:76. doi: 10.1186/s12943-021-01363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an important role in acute lung infection-enhanced lung tumor metastasis. Clin Cancer Res. 2013;19:4706–4716. doi: 10.1158/1078-0432.CCR-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooksley CD, Avritscher EB, Bekele BN, Rolston KV, Geraci JM, Elting LS. Epidemiology and outcomes of serious influenza-related infections in the cancer population. Cancer. 2005;104:618–628. doi: 10.1002/cncr.21203. [DOI] [PubMed] [Google Scholar]
- 42.Kohlhapp FJ, Huelsmann EJ, Lacek AT, et al. Non-oncogenic Acute Viral Infections Disrupt Anti-cancer Responses and Lead to Accelerated Cancer-Specific Host Death. Cell Rep. 2016;17:957–965. doi: 10.1016/j.celrep.2016.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman JH, Zloza A. Infection: a Cause of and Cure for Cancer. Curr Pharmacol Rep. 2017;3:315–320. doi: 10.1007/s40495-017-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stingi A, Cirillo L. SARS-CoV-2 infection and cancer: Evidence for and against a role of SARS-CoV-2 in cancer onset. Bioessays. 2021;43 doi: 10.1002/bies.202000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han HJ, Nwagwu C, Anyim O, Ekweremadu C, Kim S. COVID-19 and cancer: From basic mechanisms to vaccine development using nanotechnology. Int Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhardwaj K, Liu P, Leibowitz JL, Kao CC. The coronavirus endoribonuclease Nsp15 interacts with retinoblastoma tumor suppressor protein. J Virol. 2012;86:4294–4304. doi: 10.1128/JVI.07012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geisslinger F, Vollmar AM, Bartel K. Cancer Patients Have a Higher Risk Regarding COVID-19 - and Vice Versa? Pharmaceuticals (Basel) 2020;13:143. doi: 10.3390/ph13070143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geisslinger F, Vollmar AM, Bartel K. Cancer Patients Have a Higher Risk Regarding COVID-19–and Vice Versa? Pharmaceuticals. 2020;13:143. doi: 10.3390/ph13070143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizutani T. Signal transduction in SARS-CoV-infected cells. Ann N Y Acad Sci. 2007;1102:86–95. doi: 10.1196/annals.1408.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Souchelnytskyi S, Nera A, Souchelnytskyi N. COVID-19 engages clinical markers for the management of cancer and cancer-relevant regulators of cell proliferation, death, migration, and immune response. Scientific Reports. 2021;11:5228. doi: 10.1038/s41598-021-84780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGonagle D, Ramanan AV, Bridgewood C. Immune cartography of macrophage activation syndrome in the COVID-19 era. Nature Reviews Rheumatology. 2021;17:145–157. doi: 10.1038/s41584-020-00571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 Blockade on Severity of COVID-19 in Patients with Lung Cancers. Cancer Discov. 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye L, Creaney J, Redwood A, Robinson B. The Current Lung Cancer Neoantigen Landscape and Implications for Therapy. Journal of Thoracic Oncology. 2021;16:922–932. doi: 10.1016/j.jtho.2021.01.1624. [DOI] [PubMed] [Google Scholar]
- 55.Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L. The crosstalk between tumor cells and the immune microenvironment in breast cancer: implications for immunotherapy. Frontiers in Oncology. 2021;11:11. doi: 10.3389/fonc.2021.610303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun C, Pleyer C, Wiestner A. COVID-19 vaccines for patients with haematological conditions. Lancet Haematol. 2021;8:e312–e3e4. doi: 10.1016/S2352-3026(21)00073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hus I, Salomon-Perzyński A, Tomasiewicz K, Robak T. The management of hematologic malignancies during the COVID-19 pandemic. Expert Opin Pharmacother. 2021;22:565–582. doi: 10.1080/14656566.2020.1849143. [DOI] [PubMed] [Google Scholar]
- 58.Shimada A. Hematological malignancies and molecular targeting therapy. Eur J Pharmacol. 2019;862 doi: 10.1016/j.ejphar.2019.172641. [DOI] [PubMed] [Google Scholar]
- 59.Holstein SA, Lunning MA. CAR T-Cell Therapy in Hematologic Malignancies: A Voyage in Progress. Clin Pharmacol Ther. 2020;107:112–122. doi: 10.1002/cpt.1674. [DOI] [PubMed] [Google Scholar]
- 60.Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nature Reviews Disease Primers. 2021;7:3. doi: 10.1038/s41572-020-00235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cersosimo RJ. Lung cancer: A review. American Journal of Health-System Pharmacy. 2002;59:611–642. doi: 10.1093/ajhp/59.7.611. [DOI] [PubMed] [Google Scholar]
- 62.Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nature Reviews Disease Primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 63.Maleki Vareki S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer. 2018;6:157. doi: 10.1186/s40425-018-0479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin Oncol. 2015;42:663–671. doi: 10.1053/j.seminoncol.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zemek RM, De Jong E, Chin WL, et al. Sensitization to immune checkpoint blockade through activation of a STAT1/NK axis in the tumor microenvironment. Sci Transl Med. 2019:11. doi: 10.1126/scitranslmed.aav7816. [DOI] [PubMed] [Google Scholar]
- 66.Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kohli K, Pillarisetty VG, Kim TS. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Therapy. 2021 doi: 10.1038/s41417-021-00303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strasner A, Karin M. Immune Infiltration and Prostate Cancer. Front Oncol. 2015;5:128. doi: 10.3389/fonc.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinez-Bosch N, Vinaixa J, Navarro P. Immune Evasion in Pancreatic Cancer: From Mechanisms to Therapy. Cancers (Basel) 2018;10:10. doi: 10.3390/cancers10010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu JC, Khodadadi H, Malik A, et al. Innate Immunity of Neonates and Infants. Front Immunol. 2018;9:1759. doi: 10.3389/fimmu.2018.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36:453–471. doi: 10.1016/s0959-8049(99)00319-6. [DOI] [PubMed] [Google Scholar]
- 73.Muus C, Luecken MD, Eraslan G, et al. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. bioRxiv. 2020:2020.04.19.049254.
- 74.Lucas JM, Heinlein C, Kim T, et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention? Cancer Discov. 2020;10:779–782. doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baratchian M, McManus JM, Berk MP, et al. Androgen regulation of pulmonary AR, TMPRSS2 and ACE2 with implications for sex-discordant COVID-19 outcomes. Scientific Reports. 2021;11:11130. doi: 10.1038/s41598-021-90491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel VG, Zhong X, Liaw B, et al. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann Oncol. 2020;31:1419–1420. doi: 10.1016/j.annonc.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Latif MB, Raja R, Kessler PM, Sen GC. Relative Contributions of the cGAS-STING and TLR3 Signaling Pathways to Attenuation of Herpes Simplex Virus 1 Replication. J Virol. 2020;94:94. doi: 10.1128/JVI.01717-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang H, Lyu Y, Hou F. SARS-CoV-2 infection and the antiviral innate immune response. Journal of Molecular Cell Biology. 2020;12:963–967. doi: 10.1093/jmcb/mjaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin X, Riva L, Pu Y, et al. MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Reports. 2021;34 doi: 10.1016/j.celrep.2020.108628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lazear HM, Schoggins JW, Diamond MS. Shared and Distinct Functions of Type I and Type III Interferons. Immunity. 2019;50:907–923. doi: 10.1016/j.immuni.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stanifer ML, Pervolaraki K, Boulant S. Differential Regulation of Type I and Type III Interferon Signaling. Int J Mol Sci. 2019;20:20. doi: 10.3390/ijms20061445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim Y-M, Shin E-C. Type I and III interferon responses in SARS-CoV-2 infection. Experimental & Molecular Medicine. 2021;53:750–760. doi: 10.1038/s12276-021-00592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perlman S, Dandekar AA. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol. 2005;5:917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J, Subbarao K. The Immunobiology of SARS*. Annu Rev Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- 87.García LF. Immune Response, Inflammation, and the Clinical Spectrum of COVID-19. Frontiers in Immunology. 2020;11:11. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bora VR, Patel BM. The Deadly Duo of COVID-19 and Cancer! Frontiers in Molecular Biosciences. 2021;8 doi: 10.3389/fmolb.2021.643004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sinha S, Kundu CN. Cancer and COVID-19: Why are cancer patients more susceptible to COVID-19? Med Oncol. 2021;38:101. doi: 10.1007/s12032-021-01553-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai M, Liu D, Liu M, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malkani N, Rashid MU. SARS-COV-2 infection and lung tumor microenvironment. Mol Biol Rep. 2021;48:1925–1934. doi: 10.1007/s11033-021-06149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moujaess E, Kourie HR, Ghosn M. Cancer patients and research during COVID-19 pandemic: a systematic review of current evidence. Crit Rev Oncol Hematol. 2020;150 doi: 10.1016/j.critrevonc.2020.102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mansi L, Spehner L, Daguindau E, et al. Study of the SARS-CoV-2-specific immune T-cell responses in COVID-19-positive cancer patients. Eur J Cancer. 2021;150:1–9. doi: 10.1016/j.ejca.2021.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vali B, Tohn R, Cohen MJ, et al. Characterization of cross-reactive CD8+ T-cell recognition of HLA-A2-restricted HIV-Gag (SLYNTVATL) and HCV-NS5b (ALYDVVSKL) epitopes in individuals infected with human immunodeficiency and hepatitis C viruses. J Virol. 2011;85:254–263. doi: 10.1128/JVI.01743-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petrova G, Ferrante A, Gorski J. Cross-reactivity of T cells and its role in the immune system. Crit Rev Immunol. 2012;32:349–372. doi: 10.1615/critrevimmunol.v32.i4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bilich T, Roerden M, Maringer Y, et al. Preexisting and Post-COVID-19 Immune Responses to SARS-CoV-2 in Patients with Cancer. Cancer Discov. 2021;11:1982–1995. doi: 10.1158/2159-8290.CD-21-0191. [DOI] [PubMed] [Google Scholar]
- 98.Friberg H, Burns L, Woda M, et al. Memory CD8+ T cells from naturally acquired primary dengue virus infection are highly cross-reactive. Immunol Cell Biol. 2011;89:122–129. doi: 10.1038/icb.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Braun J, Loyal L, Frentsch M, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 100.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 101.Nelde A, Bilich T, Heitmann JS, et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat Immunol. 2021;22:74–85. doi: 10.1038/s41590-020-00808-x. [DOI] [PubMed] [Google Scholar]
- 102.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nature Reviews Immunology. 2008;8:675–684. doi: 10.1038/nri2379. [DOI] [PubMed] [Google Scholar]
- 104.CL Willard-Mack. Normal Structure, Function, and Histology of Lymph Nodes. Toxicologic Pathology. 2006;34:409–424. doi: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]
- 105.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 106.Song Z, Bao L, Yu P, et al. SARS-CoV-2 causes a systemically multiple organs damages and dissemination in Hamsters. Frontiers in Microbiology. 2021;11:11. doi: 10.3389/fmicb.2020.618891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang L, Deng J, Xu W, et al. CD8+ T cells with high TGF‑β1 expression cause lymph node fibrosis following HIV infection. Mol Med Rep. 2018;18:77–86. doi: 10.3892/mmr.2018.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huot N, Bosinger SE, Paiardini M, Reeves RK. Müller-Trutwin M. Lymph Node Cellular and Viral Dynamics in Natural Hosts and Impact for HIV Cure Strategies. Frontiers in immunology. 2018;9:780. doi: 10.3389/fimmu.2018.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 110.Hijano DR, Maron G, Hayden RT. Respiratory Viral Infections in Patients With Cancer or Undergoing Hematopoietic Cell Transplant. Front Microbiol. 2018;9:3097. doi: 10.3389/fmicb.2018.03097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yazaki S, Yoshida T, Kojima Y, et al. Difference in SARS-CoV-2 antibody status between patients with cancer and health care workers during the COVID-19 pandemic in Japan. JAMA Oncology. 2021;7:1141–1148. doi: 10.1001/jamaoncol.2021.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncology. 2021;7:1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun L, Warner JL, Parikh RB. Immune Responses to SARS-CoV-2 Among Patients With Cancer: What Can Seropositivity Tell Us? JAMA Oncology. 2021;7:1123–1125. doi: 10.1001/jamaoncol.2021.2096. [DOI] [PubMed] [Google Scholar]
- 114.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3:436–443. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol. 2015;33:2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Echeverry G, Fischer GW, Mead E. Next Generation of Cancer Treatments: Chimeric Antigen Receptor T-Cell Therapy and Its Related Toxicities: A Review for Perioperative Physicians. Anesthesia & Analgesia. 2019;129:434–441. doi: 10.1213/ANE.0000000000004201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 119.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nature Reviews Immunology. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Berner F, Bomze D, Diem S, et al. Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol. 2019;5:1043–1047. doi: 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berraondo P, Sanmamed MF, Ochoa MC, et al. Cytokines in clinical cancer immunotherapy. British Journal of Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 123.Mangalmurti N, Hunter CA. Cytokine Storms: Understanding COVID-19. Immunity. 2020;53:19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee LYW, Cazier JB, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ehmsen S, Asmussen A, Jeppesen SS, et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39:1034–1036. doi: 10.1016/j.ccell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shroff RT, Chalasani P, Wei R, et al. Immune Responses to COVID-19 mRNA Vaccines in Patients with Solid Tumors on Active, Immunosuppressive Cancer Therapy. medRxiv. 2021 doi: 10.1038/s41591-021-01542-z. [DOI] [Google Scholar]
- 128.Agha M, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv. 2021 doi: 10.1093/ofid/ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Palich R, Veyri M, Marot S, et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021;32:1051–1053. doi: 10.1016/j.annonc.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Geng J, Wang F, Huang Z, Chen X, Wang Y. Perspectives on anti-IL-1 inhibitors as potential therapeutic interventions for severe COVID-19. Cytokine. 2021;143 doi: 10.1016/j.cyto.2021.155544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pearce L, Davidson SM, Yellon DM. The cytokine storm of COVID-19: a spotlight on prevention and protection. Expert Opin Ther Targets. 2020;24:723–730. doi: 10.1080/14728222.2020.1783243. [DOI] [PubMed] [Google Scholar]
- 132.Burrage DR, Koushesh S, Sofat N. Immunomodulatory Drugs in the Management of SARS-CoV-2. Frontiers in immunology. 2020;11:1844. doi: 10.3389/fimmu.2020.01844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. 2013;25:469–484. doi: 10.1016/j.smim.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jones SA, Hunter CA. Is IL-6 a key cytokine target for therapy in COVID-19? Nature Reviews Immunology. 2021;21:337–339. doi: 10.1038/s41577-021-00553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39:12. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cavalli G, Larcher A, Tomelleri A, et al. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. The Lancet Rheumatology. 2021;3:e253–ee61. doi: 10.1016/S2665-9913(21)00012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cavalli G, Dagna L. The right place for IL-1 inhibition in COVID-19. Lancet Respir Med. 2021;9:223–224. doi: 10.1016/S2213-2600(21)00035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Snow TAC, Singer M, Arulkumaran N. Immunomodulators in COVID-19: Two Sides to Every Coin. Am J Respir Crit Care Med. 2020;202:1460–1462. doi: 10.1164/rccm.202008-3148LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vivarelli S, Falzone L, Torino F, et al. Immune-checkpoint inhibitors from cancer to COVID‑19: a promising avenue for the treatment of patients with COVID‑19 (Review) Int J Oncol. 2021;58:145–157. doi: 10.3892/ijo.2020.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Diao B, Wang C, Tan Y, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Frontiers in immunology. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med. 2020;217 doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Waterhouse DM, Harvey RD, Hurley P, et al. Early Impact of COVID-19 on the Conduct of Oncology Clinical Trials and Long-Term Opportunities for Transformation: Findings From an American Society of Clinical Oncology Survey. JCO Oncology Practice. 2020;16:417–421. doi: 10.1200/OP.20.00275. [DOI] [PubMed] [Google Scholar]
- 144.Mitchell EP. Declines in Cancer Screening During COVID-19 Pandemic. J Natl Med Assoc. 2020;112:563–564. doi: 10.1016/j.jnma.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 Pandemic on Cancer-Related Patient Encounters. JCO Clinical Cancer Informatics. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the Number of US Patients With Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic. JAMA Netw Open. 2020;3:e2017267. doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sharpless NE. COVID-19 and cancer. Science. 2020;368:1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]