Abstract
Background
Huashibaidu Formula (HSBD) for the COVID-19 treatment has been supported by the China's Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. However, it is not clear whether HSBD can improve blood oxygen saturation and when it should be used with conventional therapies.
Purpose
To access the effect of HSBD combined with conventional treatment on blood oxygen saturation of COVID-19 patients.
Methods
A single-center retrospective cohort study was conducted to collect the confirmed severe COVID-19 patients’ information, treated by the National Traditional Chinese Medicine Medical Team at the Jinyintan hospital between January 24 and March 31, 2020. According to whether HSBD was used during hospitalization, participants were separated into the conventional treatment group and the HSBD group (HSBD and conventional treatment). The primary observation indicators included the time for relieving blood oxygen saturation and the improvement ratio of blood oxygen saturation in each group.
Results
Of 111 patients with severe COVID-19, 53.2% (59/111) received HSBD, and 46.8% (52/111) only received conventional treatment, respectively. No statistically significant difference was found in image, clinical symptoms, and past medical history between the two groups (p > 0.05). Notably, the median time for relieving blood oxygen saturation in the conventional treatment group was 11 days (IQR, 8–14.25), while that in the HSBD group was only 6 days (IQR, 3.25–10.75), which was significantly shortened by 4.09 days (95%CI, 2.07–6.13; p= 0.0001), compared with the conventional treatment group. After repeated measurement design analysis, the main effect within times (p< 0.001) and the main effect were significantly different under the oxygen saturation dimension between two groups (p= 0.004). However, time and group interaction were observed no significant difference (p= 0.094). After 14 days of treatment, the improvement ratio of the HSBD group over the conventional treatment group was 1.20 (95%CI, 0.89–1.61).
Conclusion
For severe COVID-19 patients, the HSBD has a tendency to shorten the time for relieving blood oxygen saturation. After taking a course of HSBD, the effect can be more obvious.
Keywords: COVID-19, Severe symptom, Blood oxygen saturation, Traditional Chinese medicine, Huashibaidu formula
Abbreviations: ACE2, angiotensin I converting enzyme 2; COVID-19, coronavirus disease 2019; HSBD, Huashibaidu Formula; IQR, Interquartile Range; IL-6, Interleukin-6; NHC, National Health Commission
Graphical abstract
Introduction
The coronavirus disease 2019 (COVID-19) as an acute respiratory infection has become a major global public health event (Lai et al., 2020; Richardson et al., 2020). COVID-19 is a mild infection with dry cough and malaise as primary symptoms (Huang et al., 2020) and it also has a good overall outcome (The Novel Coronavirus Pneumonia Emergency Response Epidemiology, 2020). However, severe patients may rapidly progress to acute respiratory distress syndrome (Chen et al., 2020), sepsis, acid-base imbalance, disturbance of blood coagulation, and multi-organ failure (Wang et al., 2020c; Zhou et al., 2020) in one week after onset, previous study exemplified a mortality rate of approximately 25.7% in COVID-19 patients admitted to hospitals in United Kingdom (Horby et al., 2021). Unlike non-severe cases, Patients with severe COVID-19 mainly present with long hospital stays and high mortality rates. Dramatically, finger oxygen saturation less than 93% on air inhalation rest is a diagnostic criterion for severe COVID-19. Therefore, identifying effective treatments to relieve the oxygen saturation of patients is specifically significant for treating severe COVID-19 infections.
Although no specific drug for COVID-19, the pandemic has been well contained in China. Traditional Chinese Medicine (TCM) represented by the “3-drugs-3-formulas" has played a crucial role in controlling the epidemic. As one of the representatives of "3-drugs-3-formulas", HSBD is a standard treatment for COVID-19 adopted by the National Traditional Chinese Medicine Medical Team at the Wuhan Jinyintan hospital. Previous studies found that it remarkably shortened the median duration of nucleic acid shedding and hospital stay of severe COVID-19 patients, improved clinical symptoms, and promoted the improvement of physical and chemical inspection indicators and pulmonary CT images (Shi et al., 2021; Wang et al., 2020b, 2021). HSBD was approved by the National Medical Products Administration for marketing in China on March 2, 2021, confirming a full recognition of the clinical efficacy of TCM in containing the outbreak. On April 15, 2021, the National Health Commission (NHC) and the National Administration of Traditional Chinese Medicine of China jointly released the HSBD as a therapeutic drug for severe COVID-19 in the "DTP for COVID-19 (Trial Version 8)". Although the mortality rate of severe COVID-19 varied across studies, ranging from 8.0 to 61.5%, it usually increases significantly among elderly patients who had severe pneumonia, compared with other age groups (Asselah et al., 2021; Bhatraju et al., 2020; Menzella et al., 2020; Wang et al., 2020a; Yang et al., 2020). Besides, oxygen saturation status is one of the most vital signs in COVID-19 patients, which directly affects the patients’ prognosis. HSBD could reduce the mortality of severe COVID-19 patients (Yang et al., 2020). Nonetheless, it remains unclear whether HSBD can improve blood oxygen saturation and when it should be used with conventional therapies. Therefore, we conducted a retrospective cohort study to access the impact of the HSBD on the oxygen saturation status of severe COVID-19 infections.
Methods
Subjects
Between January 24, 2020, and March 31, 2020, data were retrospectively collected from severe COVID-19 patients treated by the National Chinese Medical Team at Jinyintan hospital. Inclusion criteria: (1) Meeting the diagnostic criteria of severe COVID-19; (2) 18–95 years old; (3) Those with complete follow-up information. Exclusion criteria: (1) Incomplete clinical data; (2) Those with additional severe acute and chronic diseases significantly affecting the treatment and prognosis, including severe heart attack and cerebral infarction, liver and kidney insufficiency, and psychiatric diseases.
Study design: a single-center, retrospective cohort research
Data collection: We retrospectively collected data including demographic characteristics, epidemiological information, past medical history, clinical symptoms, and laboratory findings including local arterial oxygen pressure, oxygen saturation, complete blood count (leukocyte, lymphocyte, monocyte count, eosinophil count, neutrophil, lymphocyte ratio, hemoglobin level, and platelet count), serum biochemical tests (liver and renal function and lactate dehydrogenase), and coagulation indices.
Additionally, we carefully evaluated the patient's hospital history, including cardiovascular diseases, gastrointestinal diseases, endocrine diseases, malignancies, neurological diseases, and respiratory diseases. Vital signs included body temperature, respiratory rate, heart rate, and blood pressure. Clinical symptoms included fever, chest pain, palpitations, dyspnea, cough, wheezing, fatigue, dry mouth, nausea and vomiting, diarrhea, anorexia, and medication. Medication included antivirals, antibiotics, immune intervention, and hormone usage.
The criteria for the timing of oxygen therapy were used to train doctors according to the DTP for COVID-19. Additionally, the physicians came from the same ward. Moreover, the instruments to test blood oxygen saturation were uniform.
All data information is obtained from the hospital inpatient medical record data information system and the laboratory test results database system. and reviewed by two physicians. If problems arise during the data verification, the information center would immediately be contacted for data source verification. This ensures the accuracy and completeness of data.
Definition
Severe COVID-19: according to the DTP for COVID-19 (Trial Version 7) issued by the NHC of the People's Republic of China, severe cases should meet any of the following criteria: 1) shortness of breath, respiratory rate ≥ 30 beats/min; 2) during resting-state, oxygen saturation without oxygen uptake ≤ 93%; 3) arterial partial pressure of oxygen (PaO2)/oxygen concentration ≤ 300 mmHg (1 mmHg = 0.133 kPa); 4) worsening progressive of clinical symptoms, lung imaging findings showed rapid progress > 50% during the past 24–48 h.
Oxygen saturation status by 6-point method (Wang et al., 2020d): 5 points - necessary to invasive ventilator; 4 points - necessary to high-flow humidified oxygen/high-frequency oxygen/non-invasive ventilator; 3 points - necessary to high-flow mask (> 5 l); 2 points - necessary to low-flow (1 l-5 l) mask/nasal cannula oxygen; 1 point-oxygen saturation above 93% without oxygen intaking; 0 points - discharge.
Time for relieving blood oxygen saturation (Wang et al., 2020d): The duration to decrease the oxygen saturation status score by 2 or more points.
The improvement ratio between groups: Group A to group B:
Treatment and outcome measures
In the present study, the use of HSBD was defined as receiving this medication for no less than six days during the hospitalization. HSBD comprises 14 herbs of Chinese medicine, including Raw ephera, Agastache, Raw gymsum, Almond, Rhizoma Pinellinae Praeparata, Magnolia Officinalis, Rhizoma atractylodis, Amomum tsao-ko, Poria cocos, Radix Astragali, Radix Paeoniae Rubra, lepidium seed, rhubarb, and Liquorice. Two or four formula a day, orally or by nasal feeding, at least 14 days for one course. We separated the patients into two groups according to the treatment they received: one group receiving HSBD (HSBD and conventional treatment group) and the other only receiving conventional treatment group.
Treatment regimens. (1) Conventional treatment group: medicines of conventional treatment were, according to DTP for COVID-19 (Trial Version 7), except for using HSBD, the use of antibiotics, antiviral drugs, hormones, other proprietary Chinese medicine preparations were all acceptable; (2) HSBD group: The treatment method combining HSBD with conventional treatment.
The outcome measures included the time for relieving blood oxygen saturation, blood oxygen saturation trend over time, and the improvement ratio between groups after 14 days of treatment (a course of Chinese medicine).
Statistical analysis
Categorical variables were described as frequencies (percentage); Continuous variables were described as mean(SD)or median (IQR), based on whether they conformed to a normal distribution. Means for continuous variables were compared in groups using the t-test (normal distribution) or Wilcoxon rank-sum test (not normal distribution). Moreover, categorical variables were compared using the χ2 test, when the data were limited the Fisher exact test was used. A repeated-measures design was used to count the status of oxygen saturation over the course of treatment. The data were analyzed by using the R software (3.6.2), invoking the ggplot2 package to graph the violin plots and the fit plots of locally estimated scatterplot smoothing (LOESS) regression. The test level α was 0.05, and the result was statistically significant when the p-value was less than 0.05.
Results
Study participants
307 cases were enrolled totally in the present study, 196 non-severe including mild and moderate cases were excluded. 111 cases were included in our analysis, of which 52 cases in the conventional treatment group, and 59 cases in the HSBD group. The mean age was 61.81 years, respectively 61.29 years in the conventional treatment group and 62.27 years in the HSBD group. A total of 22 women were in the conventional treatment group while 14 were in the HSBD group. The past medical history was dominated by cardiovascular diseases (44.1%). No statistically significant difference was noted in age and past medical history between the two groups (p > 0.05). In terms of clinical symptoms, fever (27.9%), cough (60.0%), and shortness of breath (34.2%) were predominant, whereas, there was no statistical difference between the two groups (p > 0.05). In the baseline oxygen saturation status score, no statistical difference was also observed between the two groups (p > 0.05) (Table 1 ).
Table 1.
Characteristics between the two groups of severe patients.
| Total (n = 111) | Conventional treatment group (n = 52) | Huashibaidu group (n = 59) | p | |
|---|---|---|---|---|
| Gender(Female, No., %) | 36 (32.4%) | 22 (42.3%) | 14 (23.7%) | 0.037 |
| Age(year, ) | 61.81 (12.71) | 61.29 (12.60) | 62.27 (12.89) | 0.686 |
| Symptoms(No., %) | ||||
| Fever | 31 (27.9%) | 13 (25.0%) | 18(30.5%) | 0.519 |
| Cough | 66 (60.0%) | 27 (51.9%) | 39 (67.2%) | 0.102 |
| Tachypnea | 38 (34.2%) | 12 (23.1%) | 26 (44.1%) | 0.020 |
| Coma | 1 (0.9%) | 0 (0.0%) | 1 (1.7%) | >0.999 |
| Sore throat | 1 (0.9%) | 1 (1.9%) | 0 (0.0%) | 0.473 |
| Diarrhea | 2 (1.8%) | 0 (0.0%) | 2 (3.4%) | 0.497 |
| Nausea and vomiting | 1 (0.9%) | 0 (0.0%) | 1 (1.7%) | >0.999 |
| Comorbidity (No., %) | ||||
| Cardiovascular diseases | 49 (44.1%) | 25 (48.1%) | 24 (40.7%) | 0.433 |
| Digestive system disease | 14 (12.6%) | 6 (11.5%) | 8 (13.6%) | 0.749 |
| Endocrine system disease | 19 (17.1%) | 8 (15.4%) | 11 (18.6%) | 0.649 |
| Malignant tumor | 6 (5.4%) | 2 (3.8%) | 4(6.8%) | 0.683 |
| Nervous system disease | 2 (1.8%) | 0 (0.0%) | 2 (3.4%) | 0.497 |
| Respiratory system disease | 8 (7.2%) | 2 (3.8%) | 6 (10.2%) | 0.279 |
| Vital signs | ||||
| Body temperature (°C, ) | 36.90 (0.70) | 36.95 (0.79) | 36.86 (0.61) | 0.669 |
| Heart rate (beats/min, ) | 90.91 (11.96) | 89.85 (12.06) | 91.85 (11.89) | 0.382 |
| Respiratory rate (breaths/min, IQR) | 22.00 (20.50, 23.00) | 22.00 (20.50, 23.00) | 22.00 (22.00, 22.50) | 0.126 |
| Systolic blood pressure (mmHg, ) | 130.59 (16.59) | 126.44 (19.02) | 135.88 (14.06) | 0.022 |
| Diastolic blood pressure (mmHg, ) | 81.86 (10.38) | 78.75 (9.83) | 85.24 (10.18) | 0.008 |
| Laboratory results | ||||
| White blood cell count (/l, IQR) | 6.43 (5.04, 9.00) | 6.56 (5.00, 8.88) | 6.42 (5.04, 9.03) | 0.951 |
| Neutrophil cell count (/l, IQR) | 5.08 (3.56, 7.49) | 5.10 (3.53, 7.50) | 4.89 (3.57, 7.40) | 0.939 |
| Lymphocyte count (/l, IQR) | 0.90 (0.60, 1.29) | 0.92 (0.65, 1.09) | 0.84 (0.60, 1.33) | 0.866 |
| Immature granulocyte count (/l, IQR) | 0.03 (0.01, 0.12) | 0.03 (0.01, 0.12) | 0.03 (0.01, 0.09) | 0.929 |
| monocytes count (/l, IQR) | 0.41 (0.26, 0.53) | 0.40 (0.30, 0.50) | 0.41 (0.26, 0.57) | 0.880 |
| Eosinophils count (/l, IQR) | 0.02 (0.00, 0.08) | 0.01 (0.00, 0.06) | 0.04 (0.01, 0.08) | 0.141 |
| Hemoglobin (g/l, ) | 123.19 (17.39) | 121.12 (16.64) | 125.02 (17.97) | 0.238 |
| Platelet count (/l, IQR) | 217.00 (161.50, 280.00) | 221.50 (154.75, 290.50) | 215.00 (168.50, 271.00) | 0.848 |
| IL-6 (pg/ml, IQR) | 8.99 (6.99, 12.80) | 8.84 (6.55, 13.65) | 9.19 (7.26, 11.90) | 0.927 |
| AmyloidA(mg/l, IQR) | 179.00 (78.80, 245.64) | 186.92 (146.75, 253.92) | 172.10 (45.95, 225.10) | 0.123 |
| α- Rockulosidase (U/l, IQR) | 22.00 (20.00,28.00) | 22.00 (19.25, 26.00) | 23.00 (20.00, 29.00) | 0.298 |
| Albumin (g/l, ) | 30.43 (3.78) | 29.64 (3.61) | 31.10 (3.82) | 0.042 |
| Prealbumin (mg/l, IQR) | 131.00 (84.00, 192.00) | 120.00 (85.25, 163.50) | 137.00 (81.00, 221.00) | 0.335 |
| Aminotransferase alanine (U/l, IQR) | 38.50 (22.50, 54.50) | 41.00 (24.00, 54.00) | 38.00 (21.00, 58.00) | 0.721 |
| Aminotransferase aspartate (U/l, IQR) | 37.00 (29.00, 56.00) | 39.00 (30.50, 55.00) | 34.00 (26.00, 56.00) | 0.591 |
| Total bilirubin (μmol/l, IQR) | 11.95 (9.15, 15.90) | 11.10 (8.75, 13.75) | 12.90 (9.80, 16.75) | 0.178 |
| Indirect bilirubin (μmol/l, IQR) | 7.70 (5.62, 10.55) | 7.50 (5.95, 9.25) | 8.10 (5.05, 11.50) | 0.708 |
| Direct bilirubin (μmol/l, IQR) | 4.10 (3.20, 5.40) | 3.90 (3.00, 4.65) | 4.45 (3.32, 5.88) | 0.072 |
| Lactate dehydrogenase (U/l, IQR l) | 339.00 (261.00, 451.00) | 350.00 (275.75, 447.25) | 304.00 (254.00, 449.00) | 0.371 |
| Uric acid ((μmol/l, IQR) | 225.00 (182.25, 299.50) | 207.00 (168.50, 282.50) | 233.00 (189.50, 335.50) | 0.148 |
| Creatinine (μmol/l, IQR) | 70.50 (59.20, 83.57) | 70.90 (56.85, 81.35) | 70.10 (60.40, 90.80) | 0.474 |
| Blood urea nitrogen (mmol/l, IQR) | 4.85 (3.92, 6.57) | 5.00 (3.65, 6.65) | 4.70 (4.20, 5.75) | 0.895 |
| D-dimer (μg/ml, IQR) | 1.10 (0.56, 3.44) | 1.08 (0.55, 1.95) | 1.46 (0.64, 7.91) | 0.118 |
| Prothrombin time activity (%, IQR) | 96.20 (78.30, 118.30) | 96.20 (81.40, 116.50) | 93.50 (76.75, 120.80) | 0.511 |
| Baseline oxygen saturation status (No., %) | ||||
| 1 | 90 | 45 | 45 | 0.575 |
| 2 | 1(1.1%) | 1(2.2%) | 0(0%) | |
| 3 | 3(3.3%) | 2(4.4%) | 1(2.2%) | |
| 4 | 8(8.9%) | 38(84.4%) | 40(88.9%) | |
| 4(8.9) | 4(8.9%) | |||
| Medication (No., %) | ||||
| Antivirals | 63(57.3%) | 40 (78.4%) | 23 (39.0%) | <0.001 |
| Antibiotics | 79 (71.8%) | 46(90.2%) | 33 (55.9%) | <0.001 |
| Immune intervention IFN-γ | 45 (17.1%) | 18 (35.3%) | 27 (45.8%) | 0.318 |
| Hormone usage | 33 (30.0%) | 18 (35.3%) | 15 (25.4%) | 0.365 |
Notes:P values were calculated by Pearson's chiquared test or fisher's exact test for Count Data, and t-test or Mann-Whitney test, as appropriate. P values denoted the comparison among Conventional treatment group and Huashibaidu group.
Time for relieving blood oxygen saturation
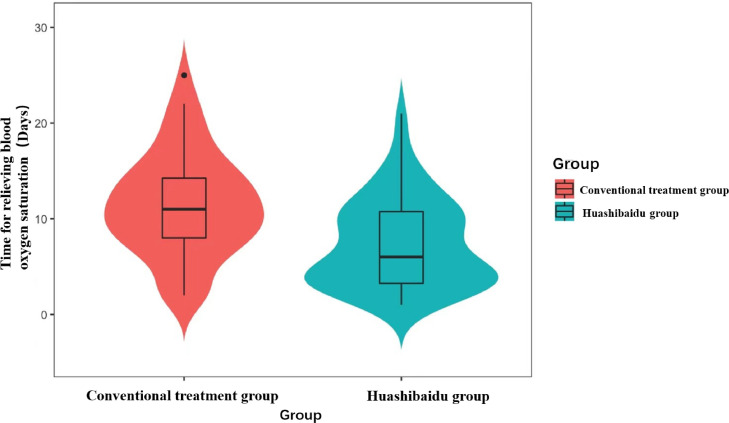
Among 111 patients, the time for relieving blood oxygen saturation was 11 days (IQR, 8–14.25) in the conventional treatment group and 6 days (IQR, 3.25–10.75) in the HSBD group. The time for relieving blood oxygen saturation was 4.09 days reduced in the HSBD group (95%CI, 2.07–6.13; p= 0.0001), which was statistically significant (Fig. 1 ).
Fig. 1.
Time for relieving blood oxygen saturation.
Blood oxygen saturation trend over time and the improvement ratio between groups
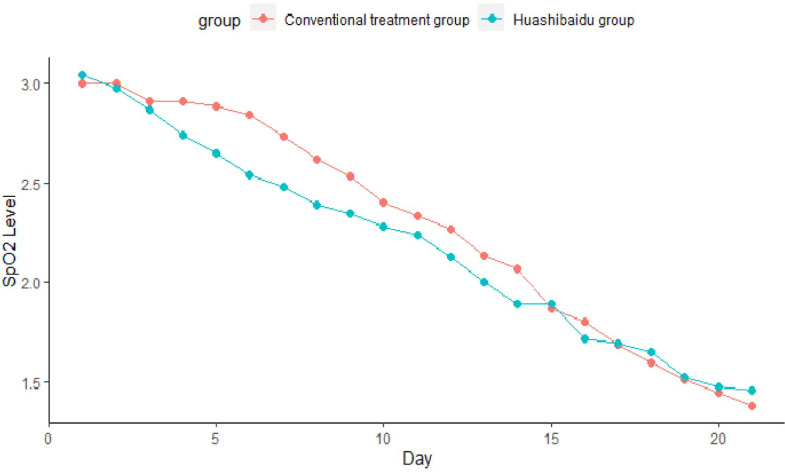
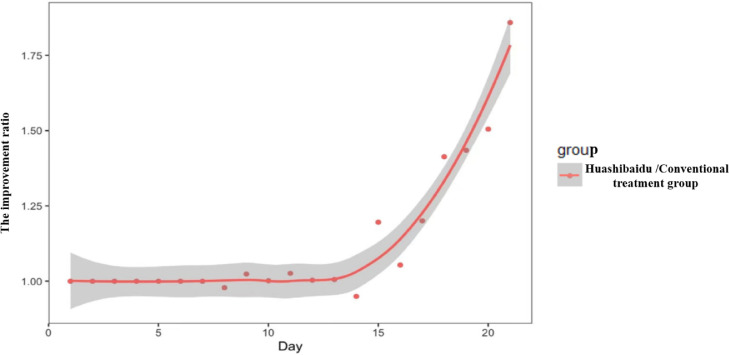
In severe patients, after repeated measurements, the main effect within times (p < 0.001) and the main effect between the two groups (p= 0.004) were significantly different under the oxygen saturation dimension. However, time and group interaction were no significant difference (p= 0.094) (Fig. 2 ). After one course of medication (14 days), the improvement ratio was 1.20 (95%CI, 0.89–1.61) in the HSBD over the Conventional treatment group (Fig. 3 ).
Fig. 2.
Trend of blood oxygen saturation over time in two groups.
Fig. 3.
The improvement ratio between groups.
Treatment regimens and outcomes
Regarding medication, 23 (39.0%) and 40 (78.4%) patients used antivirals in the HSBD group and the conventional treatment group respectively (p < 0.001). A total of 33 (55.9%) and 46 (90.2%) patients used antibiotics in the HSBD group and the conventional treatment group, respectively (p< 0.001). Additionally, 111 patients were discharged from the hospital and no critically ill cases were reported during hospitalization.
Discussion
In the study, we found that the HSBD appeared to improve oxygen saturation status and relieve clinical symptoms compared to conventional treatment. Remarkably, a dramatic decrease in lung function for gas exchange (Yang et al., 2020) causes exacerbation and even death of patients with severe COVID-19. Also, it could decrease oxygen saturation and oxygen partial pressure in patients relying on oxygen or ventilator-assisted gas exchange to sustain life. Moreover, autopsy analysis confirmed that the typical pathological features of severe COVID-19 included ischemic-hypoxic damage to pulmonary vascular endothelium (Buja et al., 2020). Therefore, protecting the ventilatory function of patients and improving their oxygen saturation status is vital to their treatment. The HSBD demonstrated that it alleviated oxygen saturation in COVID-19 patients. Latest studies indicated that Chinese medicine treatment represented by HSBD significantly alleviated the clinical symptoms and improved quality of life in patients with COVID-19 (Yang et al., 2020). Besides, it can also improve the immunity of the patients and liver function, thereby improving the prognosis of patients to a significant degree (Fu et al., 2020; Hu et al., 2021; Yina et al., 2021)
HSBD has remarkable advantages in relieving blood oxygen saturation, which is consistent with its efficacy in other aspects including virus shedding time (Shi et al., 2021). Based on the improvement ratio, from day 1 to day 14, no significant difference was found between the HSBD and the conventional treatment group. Nevertheless, when the HSBD was used for one cycle (14 days), the improvement rate of the HSBD dramatically increased. This indicates that the TCM is a process that takes time, with an accumulation from quantitative changes to qualitative changes. Periodic use of HSBD combined with conventional treatment improves the ventilation function of patients and relieves respiratory distress. Nonetheless, the functional mechanism of the HSBD and how it synergistically functions with conventional treatments warrants additional investigations. Besides, the timing of its use and the presence of additional side effects should be confirmed by in-depth pharmacokinetic tests and molecular biological experiments.
HSBD is a core prescription summarized by the National Traditional Chinese Medicine Medical Team based on the early national recommended treatment plan combined with the clinical practice of Jinyintan hospital. In basic studies, the HSBD contains various active ingredients including quercetin, lignan, and kaempferol (Yizi et al., 2020). These ingredients potentially facilitate the reduction of excessive inflammatory response and oxidative stress. Moreover, these ingredients also lower ACE2 expression, ultimately alleviating clinical symptoms in severe patients (Xun et al., 2020; Yao et al., 2020). Previous researches demonstrated that the severity of SARS-CoV-2 infection in pulmonary bronchial epithelial cells was attached to the level of angiotensin I converting enzyme 2 (ACE2) expression (Jia et al., 2005), and a lack of ACE2 expression prevented SARS-CoV-2 infection (Hoffmann et al., 2020).
Previous studies focused on the role of HSBD in relieving clinical symptoms including fever, cough, sputum, chest tightness, negative conversion, and improving patients' lung CT performance (Han et al., 2020; Liu et al., 2021; Luo et al., 2021; Wang and Qi, 2020; Wang et al., 2021; Zhao et al., 2021). In this study, the main observation index was the time for relieving blood oxygen saturation, which is the most direct manifestation of COVID-19 patient and best reflects the severity of the patient's lung inflammation. Moreover, it is also the index most directly related to mortality (Lacasse et al., 2020; Spinner et al., 2020; Xie et al., 2020). Compared with other studies based on conventional therapies, this study focused on the performance of blood oxygen saturation after the combination of conventional treatment and HSBD, which represented the efficacy of HSBD and provided a foundation for further clinical trials.
There are several limitations in the present study. Firstly, our study was retrospective, and the completeness of medical records may affect the quality of data. Secondly, the relatively small sample size of 111 patients might cause biases on the conclusions. Thus, large-sample, multicenter, prospective studies should be conducted to further confirm our findings. Despite above limitations, there are some notable strengths. To the best of our knowledge, this is the first study to explore the impact of HSBD in blood oxygen saturation. We revealed statistically significant difference in the time for relieving blood oxygen saturation in the HSBD compared to the conventional treatment. Besides, the highest improvement ratio was observed after one course (14 days) of HSBD treatment, which was consistent with what was observed clinically. A pronounced improvement was noted in oxygen saturation with the combination of HSBD and conventional treatment. Our findings provided a reference for clinicians in treatment of severe COVID-19.
Author contributions
H.L.Q., L.C. and X.Y.B. designed the study. T.Y.X. carried out the statistical analysis. X.Y.B. and M.Y. wrote the manuscript. L.B. conducted data extraction. R.L.G and the members of the National Traditional Chinese Medicine Medical Team recruited patients. L.C. and H.L.Q revised the final paper. All data were generated in house, and no paper mill was used. All authors agree to be account able for all aspects of work ensuring integrity and accuracy.
Ethical approval
The study was approved by the ethical committee of Institute of the Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences (Beijing, China) and Jinyintan hospital (Wuhan, China). The study was designed by the China center for Evidence-Based Traditional Chinese Medicine and implemented in partnership with Jinyintan hospital in Wuhan (CACMS-IRB2020–002–1).
Grant support
This work was supported by National Science and Technology Major Project (2018ZX10101001–005) , China Academy of Chinese Medical Sciences Project (No. 2020YFC0841500), the Fundamental Research Funds for the Central public welfare research institutes” (Z0727) .
Disclosures: All authors have nothing to disclose.
Declaration of Competing Interest
All authors declare that no support from any organization for the submitted work; no financial relationships with any organization that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work. The authors declare no competing interests.
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. No pharmaceutical companies provided the study medication and financing for this study.
Acknowledgments
The authors thank all doctors in Wuhan Jinyintan hospital for his/her great help in treating patients with COVID-19.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.phymed.2021.153868.
Appendix. Supplementary materials
References
- Asselah T., Durantel D., Pasmant E., Lau G., Schinazi R.F. COVID-19: discovery, diagnostics and drug development. J. Hepatol. 2021;74:168–184. doi: 10.1016/j.jhep.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L., Kritek P.A., West T.E., Luks A., Gerbino A., Dale C.R., Goldman J.D., O'Mahony S., Mikacenic C. Covid-19 in critically ill patients in the seattle region - case series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buja L.M., Wolf D.A., Zhao B., Akkanti B., McDonald M., Lelenwa L., Reilly N., Ottaviani G., Elghetany M.T., Trujillo D.O., Aisenberg G.M., Madjid M., Kar B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020;48 doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Wu L., Ma Y., Liang Q. The efficacy and safety of Xuebijing injection for corona virus disease 2019: a protocol for a systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e23401. doi: 10.1097/MD.0000000000023401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Wang Y., Hu K., Tang Z., Song X. The therapeutic efficacy of Huashi Baidu Formula combined with antiviral drugs in the treatment of COVID-19: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e22715. doi: 10.1097/MD.0000000000022715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., McCray P.B.., J.r. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacasse Y., Sériès F., Corbeil F., Baltzan M., Paradis B., Simão P., Abad Fernández A., Esteban C., Guimarães M., Bourbeau J., Aaron S.D., Bernard S., Maltais F. Randomized trial of nocturnal oxygen in chronic obstructive pulmonary disease. N. Engl. J. Med. 2020;383:1129–1138. doi: 10.1056/NEJMoa2013219. [DOI] [PubMed] [Google Scholar]
- Lai C.C., Ko W.C., Lee P.I., Jean S.S., Hsueh P.R. Extra-respiratory manifestations of COVID-19. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Yang W., Liu Y., Lu C., Ruan L., Zhao C., Huo R., Shen X., Miao Q., Lv W., Li H., Shi H., Hu L., Yang Z., Zhang L., Wang B., Dong G., Xian Y., Li B., Zhou Z., Xu C., Chen Y., Bian Y., Guo J., Yang J., Wang J., Qi W., Chen S., Chen Y., Yan B., Wang W., Li J., Xie X., Xu M., Jiang J., Wang G., Cong X., Zhu H., Shi J., Leng L., Li D., Guo L., Huang L. Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): a single-center, open-label, randomized controlled trial. Phytomedicine. 2021;91 doi: 10.1016/j.phymed.2021.153671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Yang M., Tang Q.L., Hu X.Y., Willcox M.L., Liu J.P. Characteristics of registered clinical trials on traditional Chinese medicine for coronavirus disease 2019 (COVID-19): a scoping review. Eur. J. Integr. Med. 2021;41 doi: 10.1016/j.eujim.2020.101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzella F., Fontana M., Salvarani C., Massari M., Ruggiero P., Scelfo C., Barbieri C., Castagnetti C., Catellani C., Gibellini G., Falco F., Ghidoni G., Livrieri F., Montanari G., Casalini E., Piro R., Mancuso P., Ghidorsi L., Facciolongo N. Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation. Crit. Care. 2020;24:589. doi: 10.1186/s13054-020-03306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N., Guo L., Liu B., Bian Y., Chen R., Chen S., Chen Y., Chen Y., Cong X., Dong G., Guo J., Hu L., Jiang J., Leng L., Li B., Li D., Li H., Li J., Li L., Liu J., Lu C., Lv W., Miao Q., Qi W., Shi Z., Shi J., Shi H., Tian Y., Wang B., Wang G., Wang J., Wang W., Xian Y., Xie X., Xiong Y., Xu C., Xu M., Yan B., Yang J., Zhang L., Zhou Z., Zhu H., Huang L. Efficacy and safety of Chinese herbal medicine versus Lopinavir-Ritonavir in adult patients with coronavirus disease 2019: a non-randomized controlled trial. Phytomedicine. 2021;81 doi: 10.1016/j.phymed.2020.153367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., Ogbuagu O., Malhotra P., Mullane K.M., Castagna A., Chai L.Y.A., Roestenberg M., Tsang O.T.Y., Bernasconi E., Le Turnier P., Chang S.C., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wang H., Gaggar A., Brainard D.M., McPhail M.J., Bhagani S., Ahn M.Y., Sanyal A.J., Huhn G., Marty F.M. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology, T. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) - China, 2020. China CDC Wkly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Qi F. Traditional Chinese medicine to treat COVID-19: the importance of evidence-based research. Drug Discov. Ther. 2020;14:149–150. doi: 10.5582/ddt.2020.03054. [DOI] [PubMed] [Google Scholar]
- Wang J.B., Wang Z.X., Jing J., Zhao P., Dong J.H., Zhou Y.F., Yang G., Niu M., Zhao X., Jiang T.J., Bi J.F., Xu Z., Zhang P., Wu D., Bai Z.F., Guo Y.M., Yu S.M., Sun Y.Q., Zhang Z.T., Zhan X.Y., Li P.Y., Ding J.B., Zhao P.F., Song X.A., Tang J.Y., He D.C., Chen Z., Qin E.Q., Wang R.L., Xiao X.H. Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin J. Integr. Med. 2020;26:648–655. doi: 10.1007/s11655-020-3426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lu C., Li H., Qi W., Ruan L., Bian Y., Shi H., Song H., Tu S., Zhang Y., Bai T., Cao R., Hong K., Li H., Liu L., Lu S., Rong N., Liu Y., Fang J., Shi J., Yang W., Zhao B., Yang Y., Zhao Y., Li S., Fan T., Rong P., Huang L. Efficacy and safety assessment of severe COVID-19 patients with Chinese medicine: a retrospective case series study at early stage of the COVID-19 epidemic in Wuhan. China. J. Ethnopharmacol. 2021;277 doi: 10.1016/j.jep.2021.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lu X., Li Y., Chen H., Chen T., Su N., Huang F., Zhou J., Zhang B., Yan F., Wang J. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020;201:1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Covassin N., Fan Z., Singh P., Gao W., Li G., Kara T., Somers V.K. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin. Proc. 2020;95:1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun S., Jialei T., Shaoju X., Bin Y. Exploring the molecular mechanism of HSBDF for the treatment of COVID-19 based on network pharmacology. Chin. Mater. Med. 2020;08:2050–2055. doi: 10.13863/j.issn1001-4454.2020.08.048. [DOI] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L., Yin B., Jin Z., Bao G., Li Y. Analysis of Chinese medicine theory and modern pharmacological mechanism of HSBDF in the treatment of severe COVID-19. J. Hainan Med. Coll. 2020;26(16):1209–1213. doi: 10.13210/j.cnki.jhmu.20200729.002. [DOI] [Google Scholar]
- Yizi X., Caiting Z., Shuliang J., Yi H.B., Huiting H., Feng Z.S., Hong L.X. Exploring the molecular mechanism of HSBDF for the treatment of COVID-19 based on network pharmacology and molecular docking technology. Chin. Pharmacol. Clin. 2020;36(03):28–35. doi: 10.13412/j.cnki.zyyl.20200602.001. [DOI] [Google Scholar]
- Zhao C., Li L., Yang W., Lv W., Wang J., Guo J., Dong Y., Shi N., Lu C., Li Z., Shi Z., Chen R., Huo R., Che Q., Tian Y., Xiang X., Wang J., Zhou J., Bian Y., Chen S., Chen Y., Chen Y., Cong X., Dong G., Hu L., Jiang J., Leng L., Li B., Li D., Li H., Li J., Qi W., Miao Q., Shi H., Shi J., Wang B., Wang G., Wang W., Xian Y., Xie X., Xu C., Xu M., Yan B., Yang J., Zhang L., Zhou Z., Zhu H., Xiong Y., Liu B., Huang L. Chinese medicine formula huashibaidu granule early treatment for mild COVID-19 patients: an unblinded, Cluster-Randomized Clinical Trial. Front. Med. (Lausanne) 2021;8 doi: 10.3389/fmed.2021.696976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.