Abstract
Dental caries is still one of the most prevalent diseases worldwide. Research has shown that fluoride has a role in caries prevention. For many reasons there are concerns about young children using fluoride-containing oral care products. Consequently, there is a need to identify effective fluoride-free products. A large body of literature now exists on the use of biomimetic hydroxyapatite (HAP) as an active ingredient in oral care products to combat caries.
Aim:
To conduct a systematic review of the clinical evidence of the effects of HAP-based fluoride-free oral care products in caries reduction and conduct a meta-analysis of available randomized clinical trials (RCTs).
Methods:
Using the PICO question “In individuals of all ages (P), do fluoride-free oral care products containing HAP as the anti-caries agent (I), compared to products with fluoride or without caries control products (C), reduce the risk of dental caries (O)?” Ovid MEDLINE (PubMed), Scopus, EMBASE, and Web of Science databases were searched using the following keywords: apatite, hydroxyapatite, caries, dental decay, dentin(e), enamel, toothpaste, dentifrice, mouthwash, gels, biofilm, (dental) plaque, ero(de, ded, sion), (de, re)mineral(ise, ized, ised, ization, isation). Reviews, tooth whitening, tooth sensitivity, and in vitro studies were excluded. PRISMA was used for the search and GRADE was used to assess quality. Clinical trials were subjected to the Cochrane Risk of Bias assessment followed by meta-analysis.
Results:
291 studies were retrieved; 22 were suitable for systematic review, 5 were clinical caries trials and 4 were RCTs. A meta-analysis of 3 RCTs was possible showing HAP provided 17% protection against caries. The other 17 trials had simpler proxy outcomes for anticaries effects. Some trials showed non-inferior performance of HAP products compared to those with fluoride.
Conclusion:
There is good evidence that hydroxyapatite in oral care products in the absence of fluoride effectively reduces caries.
Keywords: caries, caries prevention, dentin, early childhood caries, enamel, hydroxyapatite, meta-analysis, primary teeth, randomized clinical trial, systematic review, toothpaste
Abstract
La carie dentaire demeure l’une des maladies les plus répandues dans le monde. La recherche a montré que le fluorure joue un rôle dans la prévention des caries. Pour plusieurs raisons, l’utilisation de produits de soins buccodentaires contenant du fluorure chez les jeunes enfants suscite des inquiétudes. Par conséquent, un besoin existe de cibler des produits efficaces sans fluorure. Un grand éventail de littérature existe maintenant sur l’utilisation d’hydroxyapatite biomimétique (HAP) comme ingrédient dans les produits de soins buccodentaires pour lutter contre la carie.
Objectif :
Mener une revue systématique des données probantes cliniques sur les effets des produits de soins buccodentaires sans fluorure à base d’HAP pour la réduction de caries et réaliser une méta-analyse d’essais cliniques randomisés (ECR) offerts.
Méthodes :
Des recherches ont été effectuées dans les bases de données Ovid MEDLINE (PubMed), Scopus, EMBASE et Web of Science avec la question PICO : « Les produits de soins de santé buccodentaires qui contiennent de l’HAP à titre d’agent anti-carie (I) réduisent-ils le risque de caries dentaires (O) chez les personnes de tous les âges (P) en comparaison aux produits contenant du fluorure ou n’ayant aucun produit de contrôle de la carie (C)? Les mots clés suivants ont aussi été utilisés : apatite, hydroxyapatite, caries, carie dentaire, dentin(e), émail, pâte dentifrice, dentifrice, bain de bouche, gels, biofilm, plaque (dentaire), éro (der, dée, sion), (de, re) minéral (iser, isée, isation). Les analyses documentaires, ainsi que les études sur le blanchiment des dents, la sensibilité dentaire, et les études in vitro ont été exclus. PRISMA a été utilisé pour la recherche et le système GRADE a été utilisé pour évaluer la qualité. Les essais cliniques ont été sujets à l’évaluation de risques de biais de Cochrane suivis par une méta-analyse.
Résultats :
291 études ont été repérées : 22 études étaient propices à la revue systématique, 5 étaient des essais cliniques sur les caries et 4 étaient des ECR. Une méta-analyse de 3 RCT a été possible montrant que l’HAP avait fourni une protection de 17 % contre la carie. Les 17 autres essais avaient des résultats de substitution plus simples pour les effets anti-carie. Certains essais ont montré une performance comparable des produits d’HAP par rapport à ceux contenant du fluorure.
Conclusion :
Il y a de bonnes preuves que l’hydroxyapatite dans les produits de soins buccodentaires, en l’absence de fluorure, réduit la carie de façon efficace.
PRACTICAL IMPLICATIONS OF THIS RESEARCH.
Studies show that biomimetic hydroxyapatite-containing, fluoride-free oral care products are effective in reducing dental decay.
Dental hygienists can recommend fluoride-free, hydroxyapatite-containing oral care products to their clients to effectively reduce their risk of dental decay.
For families seeking to limit their children’s exposure to fluoridated oral products, dental hygienists can recommend fluoride-free, hydroxyapatite-containing toothpastes specially formulated for toddlers and preschool children.
INTRODUCTION
Despite advances in general oral health over the last few decades, dental caries continues to be a major health problem in Canadians, especially in children. 1 While many children never experience dental decay before the eruption of their permanent teeth, primary teeth in general are more susceptible to dental decay. 2 When one or more carious lesions are present in the primary dentition this is known as early childhood caries (ECC), which can range from mild to severe. 3 Severe ECC (type II or III) or nursing bottle caries can be caused by bottle feeding of infants with sugary beverages or even milk at night time. 4
Caries is a worldwide problem occurring much too frequently in children with many etiological risk factors. 5 The number of day surgeries, which is between 8.8 and 24.3 per 100 children (ages 12 to 59 months) in Canadian hospitals due to ECC, is a good proxy for the prevalence of this disease. 6 While ECC is not associated with exposure to fluoride 7 there are some risk factors for ECC that can be mitigated. Improving oral hygiene habits, limiting sugar intake, and using new and effective toothpastes and toothbrushes, for example, are approaches that might improve the oral health of children as well as adults in Canada. 5
For decades, fluoride has been the pre-eminent ingredient in consumer products to prevent dental caries. The evidence that fluoridated toothpaste significantly reduces caries is well documented. 8 , 9 It is now widely accepted that fluoride prevents dental decay by encouraging topical remineralization of early white spot, incipient lesions. New non-fluoride remineralization strategies have emerged that seem to be promising. 10
The primary concern with fluoridated toothpastes used by children under age 6 is the increased risk of dental fluorosis from fluoride ingestion. 9 , 11 It was established years ago that children under age 3 are unable to expectorate toothpaste efficiently and they tend to swallow significant proportions of the toothpaste placed in their mouth. 12 A recent update by the Centers for Disease Control and Prevention in the United States showed that preschoolers and toddlers were still being exposed to greater than recommended amounts of fluoridated toothpaste early in life. 12 Ingestion of fluoride during development of the permanent teeth (birth to age 6 years) results in varying degrees of dental fluorosis. 13 , 14 Dental professionals routinely advise their clients that fluoridated toothpaste use should be limited to a pea-sized amount in 3 year olds and even less for babies and toddlers. 15 , 16 A pea-sized amount is approximately 0.25 g of toothpaste but children still use more toothpaste, and the majority of those ages ≤3 years use it 2 times a day or more often. 17 There is no direct evidence that these smaller amounts of toothpaste can prevent cavities: the “rice-sized smear” may even be ineffective in preventing caries formation. 18 There is not only concern about dental fluorosis from, too much fluoride ingestion from all sources in early developmental ages, but recent studies indicate that there may also be concern about fluoride’s potential neurotoxicity on developing brains. 19– 21
Dental manufacturers are continually improving dental products for use by consumers at home. Old formulations are refined and new formulations developed with the goal of improving the oral health of the population. Toothpaste manufacturers have reformulated and rebranded their products to focus on trends in cosmetic and preventive oral health care. These include antiplaque, antitartar, antigingivitis, remineralization, whitening, breath freshening, antisensitivity, and anti-erosion claims. 22 However, most of these products continue to contain fluoride for basic caries prevention. Products validated by the Canadian Dental Association are listed on the Canadian Dental Association’s website. 23
Hydroxyapatite (HAP) is the primary calcium phosphate mineral in human mineralized tissues, i.e., teeth and bones. Calcium phosphate crystallites, including HAP, have been extensively studied and determined to be biocompatible in humans. They are non-toxic when swallowed, at least in doses that are normally applied during toothbrushing. 24 HAP has been successfully used as a biocompatible (biomimetic) active mineral to encourage better bone healing 25 and implant placement. 26 Biomimetic means that the synthesized material exhibits chemical–physical features close to those found in the human body. Hydroxyapatite crystals can be synthesized to the same formulation as found in the mineralized tissues of dentin and enamel and can be made to resemble the same crystal structure. However, not all hydroxyapatite-based materials are biomimetic. 27 Since HAP generally is biocompatible with and beneficial to mineralized tissues it would seem logical to add it to toothpaste to benefit tooth enamel and dentin. 28 After thorough testing, HAP-containing toothpastes were first approved for sale in Japan in the 1980s to treat dentin hypersensitivity and for caries prevention. 29
One of the roles of dental hygienists is to provide evidence-based recommendations in their practice to improve oral health. The purpose of this systematic review is to highlight the extent of the literature and, in particular, the state of the evidence from clinical trials on the oral health benefits of biomimetic hydroxyapatite. In addition, this review is the first meta-analysis of clinical caries trials on HAP toothpaste. As fluoride has been determined to be a suitable anticaries agent for all ages, this study sought to examine the literature on HAP’s anticaries effects in clients of any age to determine its universal application. Of particular interest, though, was the evidence for its benefit in children so that fluoride-free oral care products specifically formulated for young children (toddlers, preschoolers, and children in grade school) might be recommended.
METHODOLOGY
The PICO framework
The PICO framework was used to guide the focus of this review.
P ( Patient, Problem, Population ): clients of all ages, with primary, mixed or permanent dentitions.
I ( Intervention ): the introduction of one of the following oral care products containing biomimetic hydroxyapatite as an active ingredient: toothpaste, mouthwash or gel.
C ( Comparison, Control ): hydroxyapatite free oral care products versus fluoride-containing products, placebo or no intervention.
O ( Outcome ): a measurable oral health effect that is either a direct measurement of reduced dental decay or a suitable proxy for reduced caries risk.
Databases searched
Following the PRISMA guidelines for literature searches 30 (Supplemental Table S1, available at cjdh.ca), Ovid MEDLINE (PubMed), EMBASE, Scopus, and Web of Science were chosen as the primary databases. The University of Toronto Library provided the databases and the electronic journals from which full texts and supplementary information were extracted for each publication. FM and JE independently collected full papers from their literature searches and all authors agreed on the final list of selected publications. Google Scholar was also searched to check for any publications that were missed on the broad search. HL scanned the titles of all papers in all the databases; FM and JE searched PubMed independently and produced almost the same list of publications to screen. The search was not limited to English-language publications. Any foreign language publication was included if it was considered relevant to the review. FM and JE translated the full texts that were in German, and the publications in Korean and Russian were translated by HL with the help of Google Translate.
The authors searched the literature up to and including March 15, 2021, using the following inclusion and exclusion criteria. Studies on animals were excluded from the review. Those conducted in vitro were extracted and read in full to understand proof of concept for HAP as an effective anticaries agent for use in the oral cavity. However, these studies were also excluded from the systematic analysis. All studies that were conducted in vivo in humans, which had any implication of an anticaries effect on the dentition were included in the qualitative synthesis. Studies that focused on tooth whitening and desensitization of teeth, although showing important tooth enamel interactions, did not meet the inclusion criteria. Clinical trials and in vivo experiments examining effects of HAP on dental biofilm and enamel surfaces were considered to have sufficient proxy for anticaries effects. Studies conducted in situ in humans that tested for remineralization of enamel blocks imbedded in prostheses were considered similar in terms of clinical anticaries evidence to clinical trials.
Table 1.
Summary of clinical trials of anticaries effects of hydroxyapatite (HAP) with GRADE assignments
|
Study author (country) |
Subjects |
HAP product |
Controls |
Study design & length |
Experimental conditions |
|
Blinding |
Examiner calibration |
Conclusions |
Comments |
Quality of evidencea |
GRADE graphica |
|
Paszynska et al. 202136 (Poland) |
177 children, ages 3 to 7 years |
Kinder Karex (10% HAP) |
Elmex Kinder Zahnpasta (500 ppm fluoride) |
Randomized clinical caries trial one year |
New caries measured using ICDASb |
|
Double blinded |
Kappa = 0.91 to 0.93 |
Caries progression in primary teeth was slowed by the fluoride control toothpaste; the HAP toothpaste was not inferior to the fluoride control toothpaste |
A well-conducted head-to-head RCT of good length using a state-of-the-art caries measurement (ICDAS) Subjects were young children with primary dentitions |
HIGH |
• • • • |
|
Grocholewicz et al. 202037 (Poland) |
92 subjects Age ranging from 20 to 30 years Mean age 23.3 years |
ApaCare Repair (10% HAP gel) |
1. Ozone (OzonyTron application) 2. HAP gel + ozone No non-treatment control |
Randomized clinical caries trial 2 years |
Interproximal digital radiography of incipient caries remineralization |
|
Single blinded |
Not reported |
HAP gel provided significant remineralization effects (reversed caries), which was enhance by ozone treatment |
A well-conducted RCT of good length using a superior measurement of reversal of proximal caries |
HIGH |
• • • • |
|
Badiee et al. 202038 (Iran) |
50 subjects after orthodontics 10 years to 35 years old (173 teeth) |
6.7% HAP toothpaste formulated for the trial |
Fluoride toothpaste positive control |
Randomized clinical caries trial 6 months |
Remineralization of white spot lesions measured using ICDAS, DIAGNOdent, photographic pixel changes |
|
Single blinded |
Not reported |
Both pastes significantly reduced enamel white spot lesions HAP toothpaste outperformed the fluoride toothpaste in remineralizing white spot lesions |
A well-conducted RCT with unique measurements for incipient caries reversal Subjects included some children |
HIGH |
• • • • |
|
Schlagenhauf et al. 201939 (Germany) |
150 subjects ages 12 to 25 years |
Karex (10% HAP) |
Toothpaste containing 1400 ppm fluoride (amine fluoride + stannous fluoride) |
Randomized clinical trial 6 months |
New caries measured using ICDAS |
|
Double blinded |
Kappa = 0.80 |
Karex with HAP works as well as regular strength fluoride toothpaste in preventing progression of caries in high risk clients |
Head-to-head RCT against fluoride using an improved caries monitoring system (ICDAS) Subjects included some children |
HIGH |
• • • • |
|
Kani et al. 198940 (Japan) |
181 children in grade school |
Apato toothpaste (5% HAP) |
Placebo (Kirara; HAP and fluoride free) |
Clinical placebo-controlled trial comparing groups Randomization of individuals not possible 3 years |
Teacher supervised after-lunch tooth brushing No extra preventive care instructions given |
|
Not reported |
Not reported |
Significant reduction in DMFTc by HAP toothpaste after 3 years |
Longest trial, measured caries progression using DMFT Subjects were children in grade school |
MODERATE |
• • • |
aThe quality of the evidence and grade graphics are based on Richards 32
bICDAS: International Caries Detection and Assessment System (see ref. 36)
cDMFT: decayed missing filled teeth
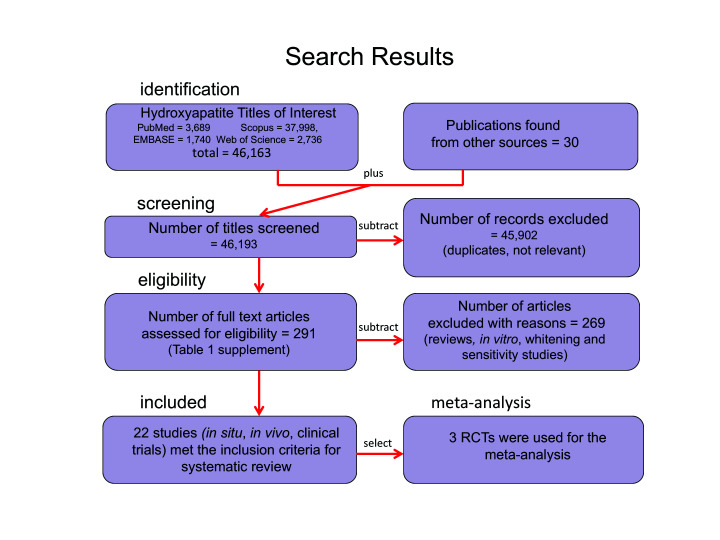
Figure 1.
Flow diagram summary of systematic review search strategy and results
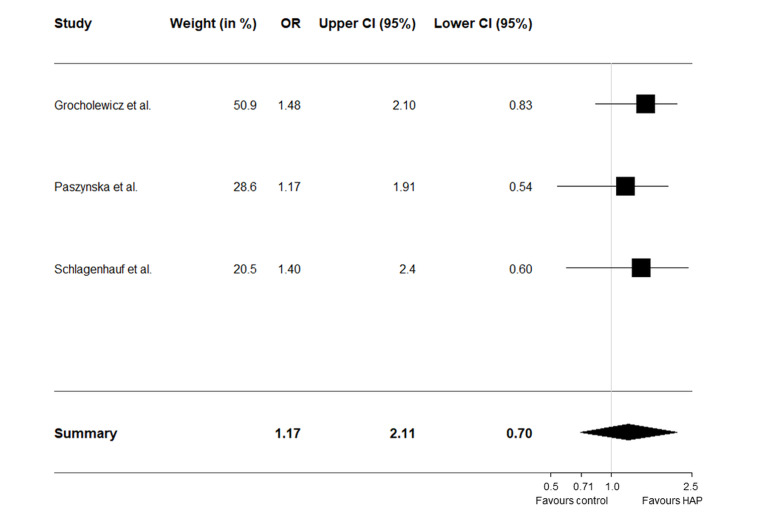
Figure 2.
Forest plot of the meta-analysis results of 3 RCTs of hydroxyapatite toothpaste
OR: odds ratio; CI: confidence interval. The diamond indicates the overall magnitude of the combined trials showing that caries reduction favours HAP toothpaste.
Qualitative synthesis
Qualitative synthesis was conducted on the included studies. The caries clinical trials were suitable for Cochrane Risk of Bias (RoB) analysis and meta-analysis. For the RoB analysis, study authors used the methods of Sterne 31 and the guidance and graphics provided by Richards. 32
Quantitative synthesis and meta-analysis
For the meta-analysis, caries incidence was used to calculate a weighted odds ratio on the caries preventive effect of the hydroxyapatite group. The null hypothesis tested in the clinical trials was that HAP-containing toothpaste did not affect the incidence of caries compared to the control. The ratios were weighted based on the number of participants included in the respective study. The odds ratio was used for the meta-analysis. Both odds ratio and meta-analysis were calculated using the open source software R, version 3.6.3. In addition to the standard R packages, this study used the packages oddsratio and forestplot. 33 , 34
RESULTS
There were 12,042 studies that mentioned “hydroxyapatite” and “caries”; 23,024 mentioned “hydroxyapatite” and “enamel.” After the search protocol was applied and duplicates and irrelevant papers were excluded, the authors identified 291 publications relevant to HAP in oral care products in the prevention of dental diseases. The details of the search are provided in Supplemental Table S2; Supplemental Table S3 lists all 291 publications found as well as the focus and experimental design of each study (both are available at cjdh.ca). All 291 studies were read in full. The authors determined that 269 publications were not suitable for this systematic review because they were either reviews or their focus was on tooth whitening or desensitization, rather than specific HAP anticaries mechanisms. A large proportion of the studies were experiments conducted in vitro showing that HAP interacts favourably with tooth surfaces, suggesting HAP could be a useful anticaries agent. While all 291 publications are relevant for understanding how HAP interacts with enamel and why it may have an anticaries effect on human teeth, only 22 addressed the PICO framework of the current study. Figure 1 summarizes t,he search results and criteria used to extract the studies for the systematic review.
PubMed, EMBASE, and Web of Science produced different search results that ranged from 1,740 (EMBASE) to 2,736 (Web of Science) to 3,689 (PubMed/MEDLINE). The Scopus search, in contrast, produced 10-fold more publications than PubMed of potentially relevant papers (37,998 titles) when the search was conducted for “all fields.” After screening all 4 data sources and eliminating duplicates, as well as adding 30 publications that were not found in the databases, 291 publications clearly indicated an anticaries role for HAP in oral care products. Many open access publications in peer-reviewed journals not listed in PubMed were found in Scopus and Web of Science databases, indicating that these sources are important to screen for relevant publications. Administering the inclusion criteria, the 291 publications were further reduced to 22 studies. This smaller group of studies showed that HAP in oral care products has antibacterial properties or interferes with dental biofilm accumulation, has enamel and dentin remineralization effects, prevents tooth surface demineralization or erosion, has favourable deposition on tooth surfaces and biofilm, and provides calcium and phosphate deposits or release of these ions in biofilm and saliva. Among those 22 studies, 5 clinical trials reported the direct measurement of anticaries effects in young adults and children after exposure to HAP toothpaste. Four of those trials were well-conducted, randomized controlled clinical trials (RCTs). The 5 trials were used in the RoB analysis; 3 of them reported enough information and were similar in clinical design to permit a quantitative synthesis (meta-analysis) using the R-statistics program as described in the Methods.
An additional study that used a questionnaire to ask clients about the subjective improvements to their oral health after using HAP toothpaste was found but it 35 was deemed unsuitable for the systematic analysis. Table 1 summarizes the details of the 5 clinical caries trials that were included in the RoB and meta-analysis.
Four of the five clinical studies were well-conducted, randomized, single or double blinded clinical trials on HAP-containing toothpaste. All were conducted in children or young adults. They were rated according to the GRADE graphics ratings following the recommendations of Richards 32 for use in reviews in dentistry. The ratings were determined on the basis of the power of the trial design (e.g., sample size), method of randomization, the level of blinding, length of the trial, placebo controls, positive controls (comparison to the known positive benefits of fluoride toothpaste), whether the differences were statistically significant (strength of the findings), and whether the outcome supported the mechanism of reduction in dental caries.
The RoB analysis based on Sterne et al 31 of the 5 clinical trials indicated that 4 of the 5 clinical trials had low risk of bias (Table 2). The Japanese placebo-controlled trial by Kani et al 40 could not have been blinded and did not provide enough information so it was deemed to have high risk of bias.
A meta-analysis was performed on 3 of the 4 well-conducted clinical trials. The trial conducted by Badiee et al 38 did not provide enough data to estimate an odds ratio and was therefore excluded. The meta-analysis of 3 HAP toothpaste anticaries RCTs is shown in Figure 2.
In vivo evidence
Studies in vivo and in situ of benefits of HAP-containing oral care products on the reduction of risk of dental decay are summarized in Supplemental Table S4 (available at cjdh.ca).
The qualitative synthesis comprised 17 human studies. There were 8 in vivo trials, 5 of which showed antiplaque properties of HAP. Four of the studies were RCTs. One in vivo trial showed how HAP improves the calcium/phosphate deposits in biofilm. One in vivo trial showed how HAP protects against acid erosion, and another showed favourable HAP deposits on enamel surfaces. There were 9 trials conducted in situ; 2 showed biofilm reduction on tooth blocks worn in situ and 7 measured the effects of HAP on the remineralization of enamel or dentin blocks exposed in the oral cavity or the researchers measured the protection against acid erosion. Three in vivo trials (biofilm reduction) were conducted on children, and 3 studies examined human primary enamel blocks imbedded in appliances worn in situ by adult volunteers . Except for one study that used human enamel from adult teeth, the remaining in situ studies involved bovine enamel and dentin blocks worn in adults as well as other blocks made from materials commonly found in the oral cavity, such as titanium.
This variety of experimental designs made it difficult to conduct a quantitative synthesis of the data. The heterogeneity, however, did not diminish the evidence from the individual studies in Supplemental Table S4 for anticaries properties of the active ingredient HAP in the oral care products under investigation. Each study was assessed for the quality of the trial and whether the results supported the conclusions presented.
DISCUSSION
The studies on the benefits of HAP toothpaste have been largely ignored in the United States and Canada perhaps because of a lack of available toothpastes and funding for research. In 2015, Oral Science’s X-Pur Remin ™ was the first Canadian fluoride-free HAP adult toothpaste to be approved for sale for the purpose of reducing dental decay. 58 A handful of others listed with Health Canada containing the ingredient calcium phosphate hydroxide (a different way of saying hydroxyapatite) have been approved since then. No HAP toothpaste has been formulated specifically targeting children under the age of 2 years.
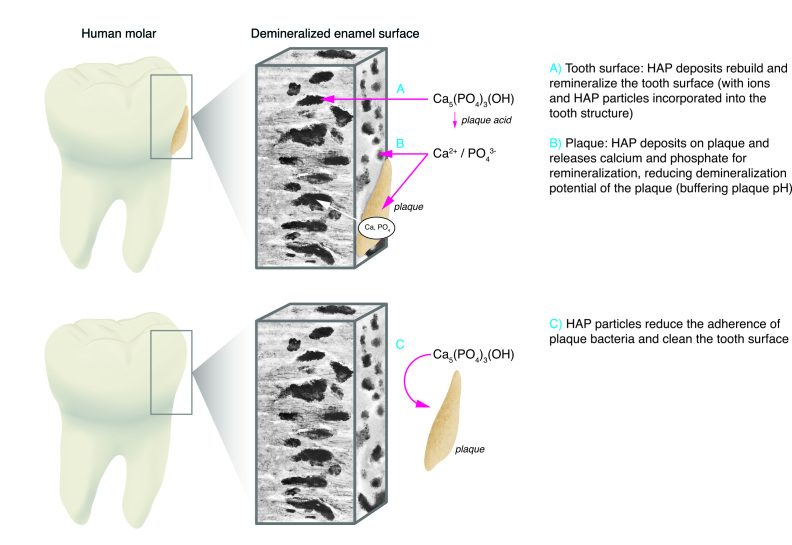
Figure 3.
Diagrammatic summary of how hydroxyapatite functions to reduce the risk of caries
A) HAP particles penetrate enamel defects on the surface and below the surface to adhere to existing enamel structure. B) The ions produced as a result of biofilm acid dissolution of some HAP particles also contribute to the remineralization of enamel as it repairs after the acid attack is over. Some of the acid is buffered by HAP. In addition, those ions deposited in biofilm contribute to the rebuilding of tooth structure. C) HAP particles have been shown to bind to biofilm bacteria, inhibit their activity, and act as an abrasive to prevent biofilm accumulation. All 3 mechanisms serve to reduce the risk of caries progression in the dentition.
Table 2.
Risk of Bias (RoB) analysis of the clinical trials on hydroxyapatite from Table 1
|
Bias domain and signalling question |
Paszynska et al. 202136 |
Grocholewicz et al. 202037 |
Badiee et al. 201938 |
Schlagenhauf et al. 201939 |
Kani et al. 198940 |
|
RANDOMIZATION | |||||
|
Was the allocation sequence random? |
Y |
Y |
Y |
Y |
Y |
|
Was the allocation sequence concealed until the participants were assigned to the intervention? |
PY |
PY |
NI |
PY |
N |
|
Did the baseline difference suggest a problem with the randomization process? |
N |
PN |
NI |
N |
PY |
|
Risk of bias judgementa |
+ |
+ |
? |
+ |
- |
|
DEVIATIONS FROM INTENDED INTERVENTIONS | |||||
|
Were participants aware of the assigned intervention? |
N |
Y |
N |
N |
PN |
|
Were people delivering interventions aware of the participants' assigned intervention? |
N |
Y |
PN |
N |
Y |
|
If yes, were there deviations from the intended intervention that arose because of trial context? |
|
N |
|
|
Y |
|
If yes, were these deviations likely to have affected the outcome? |
|
|
|
|
|
|
If yes, were these deviations balanced between the groups? |
|
|
|
|
N |
|
Was an appropriate analysis used to estimate the effect of assignment to intervention? |
|
|
|
|
|
|
If no, was there potential for substantial impact on the result? |
Y |
Y |
Y |
Y |
PN |
|
Risk of bias judgement |
+ |
? |
+ |
+ |
- |
|
MISSING OUTCOME DATA | |||||
|
Were data for this outcome for all or nearly all participants randomized? |
Y |
Y |
PY |
Y |
N |
|
If no, is there evidence that the result was not biased by missing outcome data? |
|
|
|
|
NI |
|
If no, could the absence of outcome data depend on its true value? |
|
|
|
|
NI |
|
Risk of bias judgement |
+ |
+ |
+ |
+ |
- |
|
MEASUREMENT OF THE OUTCOME | |||||
|
Was the method of measuring the outcome appropriate? |
Y |
Y |
Y |
Y |
Y |
|
Could measurement of the outcome have differed between intervention groups? |
N |
N |
N |
N |
PN |
|
If no, were outcome assessors aware of the intervention received by the study participants? |
N |
Y |
|
N |
Y |
|
If yes, could assessment of the outcome have been influenced by the knowledge of if the intervention was received? |
|
|
|
|
|
|
If yes, is it likely that this occurred? |
|
N |
|
|
PN |
|
Risk of bias judgement |
+ |
+ |
+ |
+ |
? |
|
SELECTION OF THE REPORTED RESULT | |||||
|
Were the data that produced the results analysed in accordance to the prespecified analysis plan that was finalized before unblinded outcome data were available for analysis? |
Y |
NI |
NI |
Y |
NI |
|
Is the numerical result being assessed likely to have been selected, on the basis of the results, from: | |||||
|
a) multiple eligible outcome measurements? |
N |
N |
N |
N |
NI |
|
b) multiple eligible analyses of the data? |
N |
N |
N |
N |
NI |
|
Risk of bias judgement |
+ |
+ |
+ |
+ |
- |
|
OVERALL BIAS |
+ |
+ |
+ |
+ |
- |
aRisk of bias judgement was based on RoB2 by Sterne et al.31
Y: yes; N: no; PY: probably yes; PN: probably no; NI: not indicated; Low risk: + ; questionable risk: ? ; high risk: -.
Table 3.
Fluoride-free toothpaste brandsa suitable for children, their availability, and their ingredients
|
|
Availability in Canada |
Active ingredient |
Sweeteners |
Biofilm disrupters |
Other ingredients |
|
Fluoride-free toothpastes with HAP marketed to children | |||||
|
Kinder Karex (Dr. Kurt Wolff GmbH & Co. KG, Johanneswerkstr. 34-36, 33611 Bielefeld, Germany) |
International online import |
10% hydroxyapatite (HAP) |
xylitol |
silica HAP SMCT (sodium methyl cocoyl taurate) |
cellulose gum aroma 1,2-hexanediol caprylyl glycol hydrogenated starch hydrolysate sorbitol |
|
Risewell Kids (Risewell. 82 Beaver Street New York, NY 10005 USA) |
Online import from the USA |
hydroxyapatite (HAP) |
xylitol stevia rebaudiana extract erythritol sorbitol |
silica calcium carbonate Echinacea Purpurea extract |
glycerin propanediol vanilla planifolia fruit extract potassium cocoate cellulose gum gluconate xanthan gum |
|
Biorepair™ Kids 0/6 Age with Peach Extract (COSWELL SPA Via P. Gobetti n. 4 – 40050 Funo di Argelato (BO), Italy) |
Online import |
15% hydroxyapatite (HAP) |
sorbitol sodium saccharin |
silica sodium myristoyl sarcosinate sodium methyl cocoyl taurate |
glycerin zinc PEG-32 cellulose gum aroma (fragaria vesca juice, anethole, menthol, mentha piperita oil) citric acid sodium benzoate potassium sorbate phenoxyethanol benzyl alcohol |
|
Fluoride-free calcium-based toothpaste | |||||
|
X-Pur ReminTM (Oral Science. 9575-C, Ignace Street Brossard, QC J4Y 2P3) |
Drug stores (Shoppers Drug Mart, London Drugs by request) Online shipping from the manufacturer |
10% hydroxyapatite (HAP) |
xylitol |
zeolite itanium oxide cetylpyridinium chloride |
dimethyl silicic anhydride PEG 400 polyvinylpyrrolidine glycyrrthenic acid glycerin castor oil sodium lauroyl glutamate carragenan ethanol flavour CMC |
|
Fluoride-free calcium-based toothpaste | |||||
|
MI Paste (GC America Inc. 3737 W 127th St, Alsip, IL 60803, USA) Only “MI Paste Plus” with fluoride is currently a registered trademark (TM) in Canada |
Dental offices Order directly from the USA |
casein phosphoprotein-amorphous calcium phosphate (CPP-ACP) |
D-sorbitol Na-Saccharin xylitol |
CPP-ACP Ti, Zn, Mg oxides |
glycerol guar gum carboxymethyl cellulose propylene glycol propyl, butyl p-hydroxybenzoate |
|
Non-HAP fluoride-free “training toothpaste” | |||||
|
ColgateTM fluoride-free toothpaste (Colgate-Palmolive Canada Inc. 2 Morneau Shepell Centre, 6th Floor, 895 Don Mills Road, Toronto, ON M3C 1W3) |
Drug stores Grocery stores Online orders |
green tea extract baking soda |
xylitol |
papain silica sodium lauryl sulfate |
polyethylene glycol-12 cellulose gum benzyl alcohol |
|
OrajelTM fluoride-free toothpaste (Church & Dwight Canada 5485 Ferrier St, Mount Royal, QC H4P 1M6) |
Drug stores Grocery stores Online orders |
none |
sodium saccharin sorbitol |
poloxamer 407 |
simethicone propylene glycol glycerin cellulose gum citric acid aroma |
|
CrestTM Baby Training Toothpaste (Procter & Gamble Inc. PO Box 355, Station A Toronto, ON M5W 1C5) |
Drug stores Grocery stores Online orders |
none |
xylitol |
none |
glycerin propylene glycol carbomer sodium benzoate sodium hydroxide flavour |
|
Burt’s BeesTM Baby Fluoride-Free Toothpaste (The Burt’s Bees Products Co. 1221 Broadway, Oakland, CA 94612, USA) |
Drug stores Grocery stores Online orders |
none |
stevia rebaudiana extract |
hydrated silica titanium dioxide |
glycerin sodium cocodyl glutamate aroma xanthan gum carrageenen |
aProducts listed with a trademark symbol (™) have registered their names in Canada.
Mechanism of action of HAP
Previously Enax et al 59 proposed the mechanisms of action between HAP and the tooth surface. Figure 3 shows how HAP prevents demineralization of enamel, promotes remineralization, and interferes with dental biofilm.
Based on the evidence published to date one can conclude the following:
Biomimetic HAP particles rebuild tooth mineral.
HAP reverses and remineralizes early carious lesions by providing the ions (calcium and phosphate) required for remineralization.
HAP provides additional calcium and phosphate in saliva and biofilm for improved remineralization conditions in the oral cavity. Adding calcium and phosphate to biofilm is an important mechanism for remineralization. 60 , 61
HAP works differently from fluoride and works at least as well as fluoride in preventing dental decay in the primary and secondary dentitions.
Fluoride-free oral care products containing HAP as an active ingredient can be obtained in Canada but none are sold over the counter, whereas fluoridated toothpastes dominate the toothpaste aisles. Table 3 lists a selection of fluoride-free toothpaste brands that can be obtained in Canada. There are other fluoride-free toothpastes with HAP available internationally by online order but they are not formulated for children under 2 years. There are also many fluoride-free toothpastes for adults and children that have natural ingredients but they have not been tested against fluoride toothpastes for caries reduction in clinical trials. Some fluoride-free training toothpastes sold in Canadian stores for use in babies and toddlers are included in the table for comparison but none have been shown to be effective against dental decay.
There have been many studies on the benefits of calcium and phosphates added to fluoride toothpastes and professional fluoride products, but this review focused on hydroxyapatite added to oral care products without fluoride. Some of the evidence in support of the anticaries effects of HAP oral care products was obtained in adults. Because of the concern for fluoride toxicity in infants, toddlers, preschoolers, and children who still have most of their primary teeth, the US Food and Drug Administration (FDA) ,required warning labels on toothpaste starting in 1997. The American Dental Association took this into consideration by lowering the recommended amount of toothpaste on the toothbrushes for toddlers under age 3 years. 16 A simple and convenient solution to the problem is to substitute fluoride in toothpastes targeted for young children with an effective and safe anticaries agent. Some fluoride-free toothpaste formulations have been tested in school-based, once-a-day exposure clinical trials and not surprisingly, due to the absence of any active ingredient or avoidance of fluoridated products at home, they did not show much of an additional benefit. 62
In a recent review of the non-fluoride formulations for caries prevention in the primary dentition, Wang et al. 63 reviewed arginine, chlorhexidine, triclosan, and xylitol, but did not mention any study on HAP. Some of the evidence reviewed was on dental products other than toothpaste (e.g., xylitol wipes, arginine-containing mint confections). Their conclusion was that there is a need for high-quality randomized controlled trials to make any recommendations. The same conclusion was reached by Philip 64 who published a review on non-fluoride enamel remineralization in which it was mentioned that there was some evidence that HAP by itself had anticaries properties. However, the author also noted that RCTs were lacking. In fact, the original placebo-controlled 3-year clinical trial that was published in 1989 by Kani et al 40 was not mentioned because it is not found in PubMed.
In a 2018 review by Epple et al. 24 , the safety of calcium phosphates, including biomimetic HAP, was reviewed and it was concluded that HAP can be safely swallowed when used in oral care products. Hydroxyapatite is an active biomimetic crystallite that has been shown to prevent caries in the primary dentition with similar results to fluoridated toothpaste. HAP-containing toothpastes specially formulated for babies, toddlers, and young children have been available in Europe and in the US by online order. None have been approved in the US for the “anticaries” claim that is afforded the fluoride toothpastes sold in that country. However, in Canada, X-Pur Remin ™ toothpaste by Oral Science has a Health Canada-approved anticaries claim. 58 Although this toothpaste is approved for use in children over 2 years of age, it is not specifically formulated for toddlers ages 6 months to 2 years.
The Canadian market is ready for additional HAP toothpastes for use in babies after their first primary teeth erupt (age 6 months), in toddlers who swallow toothpaste, in pre-schoolers, and in young children (see Table 3 for a list of toothpastes for toddlers). HAP toothpastes will reduce the risk of dental fluorosis and other potential health complications from ingestion of too much fluoride at a very young age. In addition, HAP has been shown to be safe if accidentally swallowed, reassuring parents of babies and toddlers that toothpastes made with this active ingredient are safe to use as soon as the primary teeth erupt. Having a toothpaste that is not only safe to use but also as effective as a fluoride toothpaste is a good way to encourage oral hygiene and at the same time protect newly erupted primary teeth from dental decay. There are clinical trials showing that HAP added to fluoride-containing toothpaste improves the anticaries effectiveness of fluoride but these were not included in this systematic review. The current study was interested in only those trials showing that HAP independently had anticaries effects. Oral health professionals should now feel more comfortable recommending fluoride-free oral care products containing just HAP as the active ingredient. Even so, more RCTs comparing HAP with fluoride as anticaries agents are needed to bolster the support that dental hygienists and dentists may already have for HAP as an alternative to fluoride for reducing caries. This systematic analysis provides an up-to-date, thorough review of the evidence that HAP is an effective anticaries agent in oral care products and provides the first meta-analysis of clinical trials on the anticaries effects of HAP toothpaste.
Included in this systematic review were studies that used Biorepair ™ , a HAP-containing toothpaste that is supplemented with zinc. Although HAP itself has antiplaque properties, it should be noted that zinc adds to the antibacterial properties when combined with HAP in toothpaste and mouthwash. The studies by Wierichs, 42 Bossù, 45 Al Asmari, 47 Hagenfeld, 48 Kensche, 50 Harks, 51 Hegazy, 52 Lelli, 55 and Hannig 56 (Supplemental Table S4) used Biorepair ™ in their experiments.
Limitations
This systematic review was based on the assumption that caries evaluation was consistent across the studies, as sources of heterogeneity were difficult to identify. Thus, an analysis of heterogeneity was not performed due to the limited number of similar studies. In addition, there were inherent difficulties with statistical analysis given that the studies themselves were the unit of analysis, and even when appropriate weights were used, the authors were unable to determine how random effects might influence the meta-analysis.
CONCLUSION AND RECOMMENDATION
Based on this systematic review and meta-analysis, it can be concluded that biomimetic hydroxyapatite-containing, fluoride-free oral care products are effective in reducing dental decay, especially in children. Additional RCTs similar to those included in this review would strengthen this conclusion and validate the extent of the effect.
CONFLICTS OF INTEREST
Dr. Kurt Wolff GmbH & Co. KG, Bielefeld, Germany, provided financial support for this review in the form of a small grant for computer expenses. JE and FM are paid senior scientists at Dr. Kurt Wolff and authors of numerous scientific publications in the field of dental care.
Supplementary Tables
Acknowledgments
The authors wish to thank Kevin Limeback, BSc, BEd, for help with the design and artwork of Figure 3.
Footnotes
CDHA Research Agenda categories: risk assessment and management; capacity building of the profession
References
- Pierce A , Singh S , Lee J , Grant C , Cruz de Jesus V , Schroth RJ The burden of early childhood caries in Canadian children and associated risk factors Front Public Health 2019 ; 7 : 328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch RJ The primary and mixed dentition, post-eruptive enamel maturation and dental caries: a review Int Dent J 2013 ; 63 ( Suppl 2 ): 3 – 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyne AH Early childhood caries: nomenclature and case definition Community Dent Oral Epidemiol 1999 ; 27 ( 5 ): 313 – 315 [DOI] [PubMed] [Google Scholar]
- Veerkamp JS , Weerheijm KL Nursing-bottle caries: the importance of a development perspective ASDC J Dent Child 1995 ; 62 ( 6 ): 381 – 386 [PubMed] [Google Scholar]
- Meyer F , Enax J Early childhood caries: Epidemiology, aetiology, and prevention Int J Dent 2018 ; 2018 : 1415873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth RJ , Quiñonez C , Shwart L , Wagar B Treating early childhood caries under general anesthesia: a national review of Canadian data J Can Dent Assoc 2016 ; 82 : g20 [PubMed] [Google Scholar]
- Tiberia MJ , Milnes AR , Feigal RJ , Morley KR , Richardson DS , Croft WG , et al. Risk factors for early childhood caries in Canadian preschool children seeking care Pediatr Dent 2007 ( 3 ): 201 – 208 [PubMed] [Google Scholar]
- Young DA , Nový BB , Zeller GG , Hale R , Hart TC , Truelove EL , American Dental Association Council on Scientific Affairs The American Dental Association Caries Classification System for clinical practice: a report of the American Dental Association Council on Scientific Affairs J Am Dent Assoc 2015 ; 146 ( 2 ): 79 – 86 [DOI] [PubMed] [Google Scholar]
- Wright JT , Hanson N , Ristic H , Whall CW , Estrich CG , Zentz RR Fluoride toothpaste efficacy and safety in children younger than 6 years: a systematic review J Am Dent Assoc 2014 ; 145 ( 2 ): 182 – 189 [DOI] [PubMed] [Google Scholar]
- González-Cabezas C , Fernández CE Recent advances in remineralization therapies for caries lesions Adv Dent Res 2018 ; 29 ( 1 ): 55 – 59 [DOI] [PubMed] [Google Scholar]
- Bentley EM , Ellwood RP , Davies RM Fluoride ingestion from toothpaste by young children Br Dent J 1999 ; 186 ( 9 ): 460 – 462 [DOI] [PubMed] [Google Scholar]
- Thornton-Evans G , Junger ML , Lin M , Wei L , Espinoza L , Beltran-Aguilar E Use of toothpaste and toothbrushing patterns among children and adolescents—United States, 2013-2016 MMWR Morb Mortal Wkly Rep 2019 ; 68 ( 4 ): 87 – 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira AP , Lawrence HP , Limeback H , Sampaio FC , Grynpas M A visual analog scale for measuring dental fluorosis severity J Am Dent Assoc 2005 ; 136 ( 7 ): 895 – 901 [DOI] [PubMed] [Google Scholar]
- Neurath C , Limeback H , Osmunson B , Connett M , Kanter V , Wells CR Dental fluorosis trends in US oral health surveys: 1986 to 2012 JDR Clin Trans Res 2019 ; 4 ( 4 ): 298 – 308 [DOI] [PubMed] [Google Scholar]
- Canadian Dental Association. CDA Position on Fluoride [Internet]. Approved February 2021 [cited 2021 Apr 6]. Available from: https://www.cda-adc.ca/en/about/position_statements/fluoride/
- American Dental Association Council on Scientific Affairs Fluoride toothpaste use for young children J Am Dent Assoc 2014 ; 145 ( 2 ): 190 – 191 Available from: https://jada.ada.org/article/S0002-8177(14)60226-9/pdf [DOI] [PubMed] [Google Scholar]
- Creeth J , Bosma ML , Govier K How much is a “pea-sized amount”? A study of dentifrice dosing by parents in three countries Int Dent J 2013 ; 63 ( Suppl 2 ): 25 – 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva MF , Delbem ACB , Danelon M , Nagata ME , Moraes FRN , Coclete GEG , et al. Fluoride concentration and amount of dentifrice influence enamel demineralization in situ J Dent 2017 ; 66 : 18 – 22 [DOI] [PubMed] [Google Scholar]
- Farmus L , Till C , Green R , Hornung R , Martinez-Mier EA , Ayotte P , et al. Critical windows of fluoride neurotoxicity in Canadian children Environ Res 2021 ; 26 : 111315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services (National Toxicology Program). Systematic review of fluoride exposure and neurodevelopment and cognitive health effects [draft NTP monograph]. Bethesda, MD : : NIH, US Department of Health and Human Services ; ; 2019 . Available from: https://www.asdwa.org/wp-content/uploads/2019/10/draft_fluoride_monograph_20190906_5081.pdf [Google Scholar]
- Till C , Green R Controversy: The evolving science of fluoride: when new evidence doesn’t conform with existing beliefs Pediatr Res 2020 . May 22 doi: 10 1038/s41390-020-0973-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert F An introduction to toothpaste—Its purpose, history and ingredients Monogr Oral Sci 2013 ; 23 : 1 – 14 [DOI] [PubMed] [Google Scholar]
- Canadian Dental Association. Seal Products [Internet]. 2021 [cited 2021 Apr 6]. Available from: https://www.cda-adc.ca/en/oral_health/seal/products/
- Epple M Review of potential health risks associated with nanoscopic calcium phosphate Acta Biomater 2018 ; 77 : 1 – 14 [DOI] [PubMed] [Google Scholar]
- Oliveira HL , Da Rosa WLO , Cuevas-Suárez CE , Carreño NLV , da Silva AF , Guim TN , et al. Histological evaluation of bone repair with hydroxyapatite: a systematic review Calcif Tissue Int 2017 : 101 ( 4 ): 341 – 354 [DOI] [PubMed] [Google Scholar]
- Yazdani J , Ahmadian E , Sharifi S , Shahi S , Maleki Dizaj S A short view on nanohydroxyapatite as coating of dental implants Biomed Pharmacother 2018 ; 105 : 553 – 557 [DOI] [PubMed] [Google Scholar]
- Gómez-Morales J , Iafisco M , Delgado-López JM , Sarda S , Drouet C Progress on the preparation of nanocrystalline apatites and surface characterization: overview of fundamental and applied aspects Prog Crystal Growth Charact Mater 2013 ; 59 : 1 – 46 [Google Scholar]
- Roveri N , Batistella E , Bainchi CL , Fortran I , Foresti E , Iafisco M , et al. Surface enamel remineralization: Biomimetic apatite nanocrystals, and fluoride ions different effects J Nanomat 2009 : 746383 [Google Scholar]
- Manchery N , John J , Nagappan N , Subbiah GK , Premnath P Remineralization potential of dentifrice containing nanohydroxyapatite on artificial carious lesions of enamel: a comparative in vitro study Dent Res J (Isfahan) 2019 ; 16 ( 5 ): 3107 [PMC free article] [PubMed] [Google Scholar]
- Rethlefsen ML Kirtley S Waffenschmidt S Ayala AP Moher D Page MJ PRISMA-SGroup et al. ; . PRISMA-S: an extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews Syst Rev 2021 ; 10 ( 1 ): 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC , Savović J , Page MJ , Elbers RG , Blencowe NS , Boutron I , et al. RoB 2: a revised tool for assessing risk of bias in randomised trials BMJ 2019 ; 366 : l4898 [DOI] [PubMed] [Google Scholar]
- Richards D Rating the quality of evidence in evidence-based dentistry Evid Based Dent 2019 ; 20 ( 1 ): 32 – 33 [DOI] [PubMed] [Google Scholar]
- Schratz PR. Package “oddsratio”: Odds ratio calculation for GAM(M)s & GLM(M)s [Internet]. 2020 [cited 2021 Mar 22]. Available from: https://zenodo.org/record/3842657#.YIGY5BNKibU
- Gordon M, Lumley T. Advanced forest plot using “grid” graphics [Internet]. 2020 [cited 2021 Mar 22]. Available from: https://cran.r-project.org/web/packages/forestplot/forestplot.pdf
- Steinert S , Zwanzig K , Doenges H , Kuchenbecker J , Meyer F , Enax J Daily application of a toothpaste with biomimetic hydroxyapatite and its subjective impact on dentin hypersensitivity, tooth smoothness, tooth whitening, gum bleeding, and feeling of freshness Biomimetics (Basel) 2020 ; 5 ( 2 ): 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszynska E , Pawinska M , Gawriolek M , Kaminska I , Otulakowska-Skrzynska J , Marczuk-Kolada G , et al. Impact of a toothpaste with microcrystalline hydroxyapatite on the occurrence of early childhood caries: a 1-year randomized clinical trial Sci Rep 2021 ; 11 ( 1 ): 2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grocholewicz K , Matkowska-Cichocka G , Makowiecki P , Droździk A , Ey-Chmielewska H , Dziewulska A , et al. Effect of nano-hydroxyapatite and ozone on approximal initial caries: a randomized clinical trial Sci Rep 2020 ; 10 : 11192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiee M , Jafari N , Fatemi S , Ameli N , Kasraei S , Ebadifar A Comparison of the effects of toothpastes containing nanohydroxyapatite and fluoride on white spot lesions in orthodontic patients: a randomized clinical trial Dent Res J (Isfahan) 2020 ; 17 ( 5 ): 354 – 359 [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf U , Kunzelmann K-H , Hannig C , May TW , Hösl H , Gratza M , et al. Impact of a non-fluoridated microcrystalline hydroxyapatite dentifrice on enamel caries progression in highly caries-susceptible orthodontic patients: a randomized, controlled 6-month trial J Invest Clin Dent 2019 ; 10 : e12399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kani K , Kani M , Isozaki A , Shintani H , Ohashi T , Tokumoto T Effect of apatite-containing dentifrices on dental caries in school children J Dent Health 1989 ; 19 : 104 – 109 [Google Scholar]
- Sudradjat H , Meyer F , Loza K , Epple M , Enax J In vivo effects of a hydroxyapatite-based oral care gel on the calcium and phosphorus levels of dental plaque Eur J Dent 2020 ; 14 : 206 – 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierichs RJ , Musiol J , Erdwey D , Esteves-Oliveira M , Apel C , Meyer-Lueckel H Re- and demineralization characteristics of dentin depending on fluoride application and baseline characteristics in situ J Dent 2020 ; 94 : 103305 [DOI] [PubMed] [Google Scholar]
- Nobre CMG , Pütz N , König B , Rupf S , Hannig M Modification of in situ biofilm formation on titanium by a hydroxyapatite nanoparticle-based solution Front Bioeng Biotechnol 2020 ; 8 : 598311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre CMG , Pütz N , Hannig M Adhesion of hydroxyapatite nanoparticles to dental materials under oral conditions Scanning 2020 ; 2020 : 6065739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossù M , Saccucci M , Salucci A , Giorgio GD , Bruni E , Uccelletti D , et al. Enamel remineralization and repair results of biomimetic hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste J Nanobiotech 2019 ; 17 : 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaechi BT , AbdulAzees PA , Alshareif DO , Shehata MA , Lima PPdCS , Abdollahi A , et al. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children BDJ Open 2019 ; 5 : 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Asmari D , Almutairi A Clinical evaluation of zinc-carbonate hydroxyapatite nanocrystals mouthwash in controlling plaque induced gingivitis: a randomized clinical trial IP Int J Periodontol Implantol 2019 ; 4 : 98 – 102 [Google Scholar]
- Hagenfeld D , Prior K , Harks I , Jockel-Schneider Y , May TW , Harmsen D , et al. No differences in microbiome changes between anti-adhesive and antibacterial ingredients in toothpastes during periodontal therapy J Periodontal Res 2019 ; 54 ( 4 ): 435 – 443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensche A , Holder C , Basche S , Tahan N , Hannig C , Hannig M Efficacy of a mouthrinse based on hydroxyapatite to reduce initial bacterial colonisation in situ Arch Oral Biol 2017 ; 80 : 18 – 26 [DOI] [PubMed] [Google Scholar]
- Kensche A , Pötschke S , Hannig C , Richter G , Hoth-Hannig W , Hannig M Influence of calcium phosphate and apatite containing products on enamel erosion Scientific World J 2016 ; 2016 : 1 – 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harks I , Jockel-Schneider Y , Schlagenhauf U , May TW , Gravemeier M , Prior K , et al. Impact of the daily use of a microcrystal hydroxyapatite dentifrice on de novo plaque formation and clinical/microbiological parameters of periodontal health. A randomized trial PLoS One 2016 ; 11 : e0160142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy SA , Salama IR Antiplaque and remineralizing effects of Biorepair mouthwash: a comparative clinical trial Pediatr Dent J 2016 ; 26 : 89 – 94 [Google Scholar]
- Makeeva IM , Polyakova MA , Avdeenko OE , Paramonov YO , Kondrat’ev SA , Pilyagina AA Effect of long-term application of toothpaste Apadent Total Care Medical nano-hydroxyapatite Stomatologia 2016 ; 95 : 34 – 36 [DOI] [PubMed] [Google Scholar]
- Souza BM , Comar LP , Vertuan M , Fernandes C , Buzalaf MA , Magalhaes AC Effect of an experimental paste with hydroxyapatite nanoparticles and fluoride on dental demineralisation and remineralisation in situ Caries Res 2015 ; 49 : 499 – 507 [DOI] [PubMed] [Google Scholar]
- Lelli M , Marchetti M , Foltran I , Roveri N , Putignano A , Procaccini M , et al. Remineralization and repair of enamel surface by biomimetic Zn-carbonate hydroxyapatite containing toothpaste: a comparative in vivo study Front Physiol 2014 ; 5 : 333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannig C , Basche S , Burghardt T , Al-Ahmad A , Hannig M Influence of a mouthwash containing hydroxyapatite microclusters on bacterial adherence in situ Clin Oral Investig 2013 ; 17 : 805 – 814 [DOI] [PubMed] [Google Scholar]
- Najibfard K , Ramalingam K , Chedjieu I , Amaechi BT Remineralization of early caries by a nano-hydroxyapatite dentifrice J Clin Dent 2011 ; 22 : 139 – 143 [PubMed] [Google Scholar]
- Government of Canada. Licensed Natural Health Products [Internet]. Available from: https://health-products.canada.ca/lnhpd-bdpsnh/search-recherche.do
- Enax J , Fabritius H-O , Fabritius-Vilpoux K , Amaechi BT , Meyer F Modes of action and clinical efficacy of particulate hydroxyapatite in preventive oral health care − state of the art Open Dent J 2019 ; 13 : 274 – 287 [Google Scholar]
- Shaw L , Murray JJ , Burchell CK , Best JS Calcium and phosphorus content of plaque and saliva in relation to dental caries Caries Res 1983 ; 17 : 543 – 548 [DOI] [PubMed] [Google Scholar]
- Whitford GM , Wasdin JL , Schafer TE , Adair SM Plaque fluoride concentrations are dependent on plaque calcium concentrations Caries Res 2002 ; 36 ( 4 ): 256 – 265 [DOI] [PubMed] [Google Scholar]
- Hujoel PP , Hujoel MLA , Kotsakis GA Personal oral hygiene and dental caries: a systematic review of randomised controlled trials Gerodontology 2018 ; 35 ( 4 ): 282 – 289 [DOI] [PubMed] [Google Scholar]
- Wang Y , Li J , Sun W , Li H , Cannon RD , Mei L Effect of non-fluoride agents on the prevention of dental caries in primary dentition: a systematic review PLoS One 2017 ; 12 ( 8 ): e0182221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N State of the art enamel remineralization systems: The next frontier in caries management Caries Res 2019 ; 53 ( 3 ): 284 – 295 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.