Abstract
Dairy workers experience a high degree of bioaerosol exposure, composed of an array of biological and chemical constituents, which have been tied to adverse health effects. A better understanding of the variation in the magnitude and composition of exposures by task is needed to inform worker protection strategies. To characterize the levels and types of exposures, 115 dairy workers grouped into three task categories on nine farms in the high plains Western United States underwent personal monitoring for inhalable dust, endotoxin, 3-hydroxy fatty acids (3-OHFA), muramic acid, ergosterol, and ammonia through one work shift. Eighty-nine percent of dairy workers were exposed to endotoxin at concentrations exceeding the recommended exposure guidelines (adjusted for a long work shift). The proportion of workers with exposures exceeding recommended guidelines was lower for inhalable dust (12%), and ammonia (1%). Ergosterol exposures were only measurable on 28% of samples, primarily among medical workers and feed handlers. Milking parlor workers were exposed to significantly higher inhalable dust, endotoxin, 3-OHFA, ammonia, and muramic acid concentrations compared to workers performing other tasks. Development of large modern dairies has successfully made progress in reducing worker exposures and lung disease prevalence. However, exposure to endotoxin, dust, and ammonia continues to present a significant risk to worker health on North American dairies, especially for workers in milking parlors. This study was among the first to concurrently evaluate occupational exposure to assayable endotoxin (lipid A), 3-hydroxy fatty acids or 3-OHFA (a chemical measure of cell bound and noncell-bound endotoxins), muramic acid, ergosterol, and ammonia among workers on Western U.S. dairies. There remains a need for cost-effective, culturally acceptable intervention strategies integrated in OHS Risk Management and production systems to further optimize worker health and farm productivity.
Keywords: 3-hydroxy fatty acid, bioaerosol, dairy, endotoxin, ergosterol, muramic acid, organic dust, task
Introduction
Dairy work has long been associated with inhalation hazards and an increased prevalence of respiratory diseases.[1] However, manifestation of symptoms and disease remains poorly understood. Additionally, increased scale of operations and task-specialization has altered patterns of exposure and susceptibility. The United States dairy industry has been steadily increasing herd sizes such that 63% of the milk supply in 2011 was sourced from operations with greater than 500 cows (35% with more than 2,000 cows).[2,3] This shift in production has required a greater reliance on workers with no prior farm experience or contact with animals.[4] As a result, increased susceptibility to exposure and illness among modern dairy workers is an emerging occupational health issue.[5,6]
Respiratory hazards on a dairy are complex and include organic and inorganic dust, as well as chemicals (e.g., ammonia). Exposure assessments have historically focused on the organic dust fraction due to its proinflammatory properties.[1] Prolonged exposure to organic dust may exceed a suggested occupational exposure limit (OEL) of 2.4 mg/m3, especially in tasks such as feeding, moving, and milking cows.[1,7–9] Endotoxin or lipopolysaccharides (LPS) an assayable biological marker for Gram-negative bacteria in organic dust has been measured specifically due to its adverse respiratory effects.[1] Endotoxin exposure on dairies is highly variable[1,5,6,10,11] and often exceeds recommended occupational exposure limits by several orders of magnitude.[7–9,12]
Uncertainty regarding etiology of lung disease among dairy workers has been in part due to limitations in the biological assay methods (e.g., Limulus Amebocyte Lysate, recombinant Factor C) used to measure endotoxin (specifically the cell-free Lipid A moiety of LPS) and limited measures of other relevant constituents. Evaluation of exposure to 3-hydroxy fatty acids (3-OHFA) can provide more refined chemical measures of cell-bound and noncell-bound endotoxin, with some studies indicating a stronger relationship between even-chain length 3-OHFA exposure and measures of health effects.[1,6,13–15] In addition, Gram-positive bacteria and fungi most likely contribute to the overall inflammatory response. Recent studies have shown that Gram-positive bacteria may be more abundant than other microbes in milking parlor aerosols.[16,17] Evidence from in vitro studies strongly suggests that muramic acid (a marker for Gram-positive bacteria) and ergosterol (a marker for fungi), in addition to endotoxin, may help explain disease mechanisms.[1,14,18] These markers have been shown to be present in high concentrations in agricultural environments, but remain inadequately studied in the context of exposure and health. Chemical constituents (i.e., ammonia) have been found in high levels at traditional dairies, but occupational exposures to ammonia at modern dairies have not been well-characterized.
Collectively, altered exposure patterns, uncertainty in bioaerosol composition and interaction between constituents drive the need to better characterize and further control respiratory hazards on modern dairies. This study builds on previous epidemiological studies of bioaerosol exposures and respiratory disease in the dairy industry. The primary objective of this study was to characterize task-based exposures to inhalable dust, microbial markers, and ammonia on large-scale modern dairies in the high plains Western United States.
Methods
Participant recruitment and data collection
Thirty dairies located in Colorado and Wyoming were identified and randomly recruited through the Colorado Livestock Association and the Colorado State University (CSU) Integrated Livestock Management program. Nine (30%) agreed to participate in the study. All study protocols were approved by the CSU Institutional Review Board. Data collection commenced in fall 2007 and was completed in summer 2011. Each dairy participant was sampled once during the study period.
Study population
Study enrollment was offered to all workers employed at participating dairies; the study population was based on willingness to participate and availability during sampling days. Participants were provided informed consent in either English or Spanish. Each worker completed a pre- and post-work questionnaire administered by an interviewer. The questionnaires were developed in collaboration with a companion study conducted at the University of California Davis (UCD).[10,19] The questionnaires collected information on 12 primary workday tasks and activities developed in consultation with the dairy industry. The tasks included: milking, breeding, birthing, medical care, mixing feed and feeding cows, moving cows, flushing manure, rebedding/scraping stalls, milking parlor maintenance, lagoon/waste maintenance, repairing pens/gates/corrals, and other. Additional data was collected on worker demographics and behaviors (e.g., tobacco use, environmental smoke exposure, alcohol consumption). Workers were asked about the primary and secondary tasks performed on the day they participated. Farm characteristics including milking parlor design, stall design, herd size, age of farm, and workplace activities were collected through a combination of walk-through surveys, public databases, and self-administered questionnaires distributed to farm managers.
Personal exposure monitoring
Inhalable dust
Personal inhalable dust samples were collected through one full-work shift using SKC Button samplers and 25-mm PVC filters with a 5-μm pore size (SKC Inc., Eighty Four, PA). Each sampler was connected to a personal sampling pump (MSA, Cranberry, PA; SKC Inc., Eighty Four, PA) that was calibrated to a flow rate of 4 L/min using a DryCal calibrator (BIOS, Butler, NJ). Flow rates were recorded before and after each sampling event and considered acceptable if the difference was less than 5%. Filters were desiccated for 24 hr pre- and postsampling prior to gravimetric analysis with a Mettler MT5 balance (Mettler-Toledo, Columbus, OH). Field and laboratory blanks were analyzed in a similar manner. Field blanks were averaged for each sampling session and subtracted from sample results for that session before calculating airborne concentrations. The limit of detection (LOD) for inhalable dust samples was 6.8 ug/m3 using an average sampling time of 9 hr to match the predominant work shift. Subsequently, all filters were stored at −70°C prior to extraction and analysis for endotoxin, 3-OHFA, muramic acid, and ergosterol. Filters were extracted in pyrogene-free water with 0.05% Tween-20.
Endotoxin
Filter extracts were analyzed using the Recombinant Factor C (rFC) assay (Lonza) on a Biotek reader (Biotek Instruments FLX800TBIE, Winooski, VT) as previously described by Saito et al.[15] Quality assurance spiking assays were performed to assess matrix interference or enhancement. Endotoxin content was quantified in relation to United States Reference Standard EC-6 and reported as endotoxin units per cubic meter of air (EU/m3). The LOD for endotoxin was 0.05 Eu/m3. Three aliquots of each filter endotoxin extract were stored at −70°C for analysis of 3-OHFA, muramic acid, and ergosterol.
3-OHFA, muramic acid, and ergosterol: Gas chromatography–mass spectroscopy (GC–MS)
Sample analysis for 3-OHFA, muramic acid and ergosterol content was undertaken as previously described by Reynolds et al.[6,14] To prepare samples, aliquots were digested using HCl or KOH and solid-phase extracted (SPE). Samples were quantified on an Agilent 6890 Series gas chromatograph equipped with a DB-5MS column and Waters Quattro Micro mass spectrometer (Agilent Technologies, Santa Clara, CA and Waters Corporation, Milford, MA). The mass spectrometer was operated in multiple reactions (MRM) and single-ion monitoring (SIM) modes for the bacteria and fungi constituents, respectively.
3-OHFA and muramic acid
Following SPE, samples were derivatized in 50 μL BSTFA/1%TMCS and 5 μL pyridine (85°C for 30 min). Subsequently, samples were diluted to 100 μL with heptane and analyzed by GC–MS–MS. Different length 3-OHFA chains were separated by gas chromatography (GC) with an inlet temperature of 280°C, and final holding temperature of 290°C in the oven. The GC inlet temperature for analyzing muramic acid was 260°C with an oven holding temperature of 280°C. The mass spectrometer interface temperature was 300°C. The collision energy for 3-OHFA and muramic acid was 10 and 6 eV, respectively. The LOD was 0.5 ng/μL (23 ng/m3) and the limit of quantification (LOQ) was 1 ng/μL (45 ng/m3) for both markers. The 3-OHFA constituents with carbon chain lengths between 8 and 18 were quantified and reported as total carbon chain length 3-OHFA components (total 3-OHFA) and even-numbered and odd-numbered carbon chain lengths.
Ergosterol
Ergosterol samples recovered from SPE were derivatized in 50-μL 1:1 BSTFA/1%TMCS and hexane at 80°C for 30 min. Separation occurred at an inlet temperature of 280°C with a final holding temperature of 280°C. For quantification, m/z 363 and 365 were monitored for ergosterol and D-ergosterol, respectively. The LOD for the method was 0.5 ng/μL (11 ng/m3) and the LOQ was 1.0 ng/μL (22 ng/m3).
Ammonia
Personal exposure to ammonia was measured using direct-reading Pac 7000 monitors (Drager, Lubeck, Germany) with a resolution limit of 1 ppm. These monitors were set to data log every minute. The Pac 7000 detectors were calibrated prior to each monitoring session using standard gas. Ammonia data was downloaded with Drager Gas Detection software (Drager, Lubeck, Germany).
Environmental variables
Air temperature (°C), relative humidity (%RH), carbon dioxide (CO2), and carbon monoxide (CO) concentrations were recorded using a Q-Trak (Model 8554, TSI, Shoreview, MN). Measurements were recorded inside and outside the milking parlor at the beginning and end of each sampling campaign. Calibration with standard gases and a sling psychrometer was performed before and after sampling.
Data analysis
Task groups were developed for the questionnaires based on both previous studies and consultation with the dairy industry. The 12 task categories originally included in the post-work shift self-administered questionnaires were condensed into three categories due to small sample sizes for the majority of tasks. The final task groups were based on observation of working groups, potentially similar bioaerosol exposures, and on-site worker locations. Task categories were: milking and milking parlor maintenance; medical work, which included maternity/breeding, routine medical care, and birthing/calf rearing; and other, including feeding cows/moving feed, scraping of stalls and corrals (rebedding), maintenance of corrals and fences (maintenance), moving cows (moving), administration and supervision (administration), and workers undertaking multiple tasks (multiple).
Bioaerosol exposure measurements were lognormally distributed and were log transformed for statistical analysis. Bioaerosol exposures were first examined by calculation of descriptive measures (geometric means (GM), geometric standard deviations (GSD), and exposure distribution), as well as a comparison to the recommended occupational exposure limits (OEL). The OELs were adjusted to account for the average 54-hr workweek of the sample population (OELadj) using OSHA Model (PELadj = PEL x [40/54]). The adjusted OELS are: 1.8 mg/m3 for inhalable dust,[7–9] 67 EU/m3 for endotoxin (based on the Danish recommendation of 90 EU/m3),[12] and 5.6 ppm for ammonia.[7–9] Spearman’s rank correlation coefficient (rs) was used to analyze correlations between exposures and environment (temperature, humidity, and CO2). Muramic acid results below the LOD were substituted with half the LOD value (0.25 ng/mL). The ergosterol data were highly censored (72% <LOD) and were categorized dichotomously (above and below the LOD). The effect of task on bioaerosol exposure was evaluated by analysis of variance (ANOVA) with an alpha of 0.05. All statistical analyses were performed using SAS V.9.3 (SAS Institute, Inc., Cary, NC) with accompanying figures generated in Sigma Plot V.12.0 (Systat Software Inc., San Jose, CA).
Results
Farm characteristics and worker demographics
A total of 115 workers participated in the study. Not all workers provided data for every variable. The participating farms varied in the number of employees sampled (mean: 12.8, SD: 12.1), herd size (mean: 2,019 cows, SD: 994), and age of the farm (mean: 8.3 years, SD: 6.3) (Table 1). Both the parallel and the herringbone milking parlor styles were represented. Work shifts ranged from 2 to 12 hr, averaging 9 hr (Table 1). The most common tasks among the participants were milking and milking parlor maintenance (n = 38, 33.0%), medical (n = 28, 24.3%), and other (n = 22, 19.1%) workers. The majority of dedicated milking parlor and medical workers were located at Farms 5 and 7, while multitasking workers predominated at the other dairies.
Table 1.
Characteristics of sampled dairy farms in high plains U.S. monitored 2007–2011.
| Farm 1 | Farm 2 | Farm 3 | Farm 4 | Farm 5 | Farm 6 | Farm 7 | Farm 8 | Farm 9 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Approximate year farm established | 1999 | 2004 | 1987 | 1999 | 2006 | 1996 | 2006 | 2004 | 2009 | |
| Milking cows | 2,200 | 3,000 | 680 | 1,000 | 2,100 | 2,400 | 3,500 | 791 | 2,500 | |
| Milking stalls | 56 | 70 | 16 | 38 | 40 | 60 | 40 | 34 | 80 | |
| Parlor type | Parallel | Parallel | Herring | Parallel | Herring | Herring | Parallel | Herring | Parallel | |
| Location | WY | CO | CO | CO | CO | CO | CO | CO | CO | |
| Season | Fall | Fall | Spr | Spr-Fall | Spr-Sum | Sum | Sum-Spr | Win | Spr | |
| Year monitored | 2008 | 2008 | 2009 | 2009–10 | 2009–10 | 2009 | 2009–10 | 2011 | 2011 | |
| Total approx. work force | 15 | 40 | 30 | 23 | 26 | 38 | 80 | 21 | 28 | 301 |
| No. employees monitored | 5 | 5 | 4 | 11 | 20 | 8 | 42 | 6 | 14 | 115 |
| Work shift (hours) | (12) | (10–12) | (8–10) | (8–10) | (2–9) | (10–11) | (8–9) | (8–9) | (7–10) | |
| Tasks monitored | ||||||||||
| Milking | 0 | 0 | 2 | 1 | 9 | 1 | 18 | 1 | 6 | 38 (33.0%) |
| Medical | 0 | 0 | 0 | 3 | 6 | 4 | 12 | 1 | 2 | 28 (24.3%) |
| Multiple | 4 | 5 | 2 | 3 | 1 | 0 | 3 | 3 | 1 | 22 (19.1%) |
| Rebedding | 0 | 0 | 0 | 3 | 1 | 2 | 3 | 0 | 0 | 9 (7.8%) |
| Feeding | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 1 | 6 (5.2%) |
| Other | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 5 (4.3%) |
| Moving | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 4 (3.5%) |
| Maintenance | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 3 (2.6%) |
The study population was largely nonsmoking Hispanic males aged 25 to 45 years (Table 2). Almost half the workers had only primary school education while 31.2% completed high school (Table 2). Female workers accounted for 11% of the population. Seventy-three percent of workers were born in Mexico, with 11% from Guatemala, 8% from the United States, and others from El Salvador, Honduras, Puerto Rico, Ecuador, and Burundi. Workers with less than one year of dairy work experience (i.e., new workers) comprised approximately 16% of the study population. Approximately 16% of workers were current smokers, of which 39% smoked during sampling.
Table 2.
Demographics for dairy worker population monitored on high plains U.S. dairies 2007–2011.
|
Population characteristics |
Total n (%) | Male n (%) | Female n (%) |
|---|---|---|---|
| Gender (n = 115) | 115 | 102 (88.9%) | 13 (11.1%) |
| Age distribution (n = 115) | |||
| 18–24years | 32 (27.9%) | 30 (29.4%) | 2 (15.4%) |
| 25–40years | 55 (47.8%) | 50 (49.0%) | 5 (38.5%) |
| 41–72years | 28 (24.3%) | 22 (21.6%) | 6 (46.2%) |
| Ethnicity (n = 115) | |||
| Hispanic or Latino | 91 (79.1%) | 82 (80.4%) | 9 (69.2%) |
| Not Hispanic or Latino | 9 (7.8%) | 7 (6.9%) | 2 (15.4%) |
| Don’t know | 15 (13.1%) | 13 (12.7%) | 2 (15.4%) |
| Country of origin | |||
| United States | 9 (8%) | ||
| Mexico | 82 (72.6%) | ||
| Guatemala | 12 (10.6%) | ||
| Other | 10 (8.8%) | ||
| Education (English or Spanish) (n = 109) | |||
| Primary | 51 (46.8%) | 44 (45.4%) | 7 (58.3%) |
| Middle | 12 (11.0%) | 11 (11.3%) | 1 (8.3%) |
| High | 34 (31.2%) | 34 (35.1%) | 0 |
| University | 11 (10.1%) | 7 (7.2%) | 4 (33.3%) |
| Don’t know | 1 (0.9%) | 1 (1.0%) | 0 |
| Dairy work experience (n = 114) | |||
| New worker (≤ 1year) | 19 (16.7%) | 16 (15.8%) | 3 (23.1%) |
| Experienced worker (> 1year) | 95 (83.3%) | 85 (84.2%) | 10 (76.9%) |
| Current smoker (n = 113) | |||
| No | 95 (84.1%) | 83 (83.0%) | 12 (92.3%) |
| Yes | 18 (15.9%) | 17 (17.0%) | 1 (7.7%) |
| Smoked during monitoring (n = 114) | |||
| No | 107 (93.9%) | 94 (93.1%) | 13 (100.0%) |
| Yes | 7 (6.1%) | 7 (6.9%) | 0 |
Worker exposure to airborne contaminants
The exposures among workers for each bioaerosol constituent exhibited wide variability, which indicates a potentially nonhomogenous exposure group. The cross-shift time-weighted average (TWA) geometric mean (GM) for inhalable dust exposures was 0.67 mg/m3 (range: 0.02–6.82).
Fourteen percent (n = 16) of the dairy workers sampled had personal inhalable dust exposure measurements that exceeded the recommended OELadj of 1.8 mg/m3. As with similar studies, dust exhibited the least variability based on a geometric standard deviation (GSD) of 2.5.[6,11] Eighty-nine percent of worker endotoxin exposures exceeded the recommended OELadj of 67 EU/m3 (Table 3). The GM for endotoxin exposure was 438 EU/m3 (range: LOD–4430). It should be noted that not all analytes have the same number of samples. In the case of ammonia, smaller numbers are due to failures of the Pac7000 samplers. Aliquots of extractions for GC–MS analysis were not available for the initial sample sets. The GM of 3-OHFA and muramic acid were 356 ng/m3 and 9.6 ng/m3, respectively. Ergosterol exposures were extremely low; 72% were below the LOD. An extreme exception was an ergosterol concentration of 536.9 ng/m3 for a medical worker. Ammonia concentrations were also typically very low with a GM of 0.27 ppm and a single sample exceeding the recommended OELadj of 5.6 ppm (Table 3). Across the whole study population, ammonia concentrations were considered to be extremely variable with a GSD of 5.3, followed by muramic acid (4.7) and endotoxin (3.9) (Table 3).
Table 3.
Summary of the bioaerosol exposures measured in high plains U.S. dairies, 2007–2011.
| Bioaerosols | n | GM | GSD | Min-Max | Upper 95thPercentile | OELadja | Number of Measurements Exceeding OELadja |
|---|---|---|---|---|---|---|---|
| Inhalable dust (mg/m3) | 114 | 0.67 | 2.5 | 0.02–6.82 | 3.08 | 1.8mg/m3b | 16 (14%) |
| Endotoxin (EU/m3) | 114 | 438 | 3.9 | LOD–4430 | 3686 | 67EU/m3c | 101 (89%) |
| 3-OHFA (ng/m3) | 93 | 356 | 2.6 | 35–2112 | 1674 | ||
| Muramic acid (ng/m3) | 82 | 9.6 | 4.7 | LOD–250.0 | 101.7 | ||
| Ergosterol (ng/m3)d | 83 | LOD–536.9 | |||||
| Ammonia (ppm) | 98 | 0.27 | 5.3 | 0.01–12.0 | 4.2 | 5.6e | 1 (1%) |
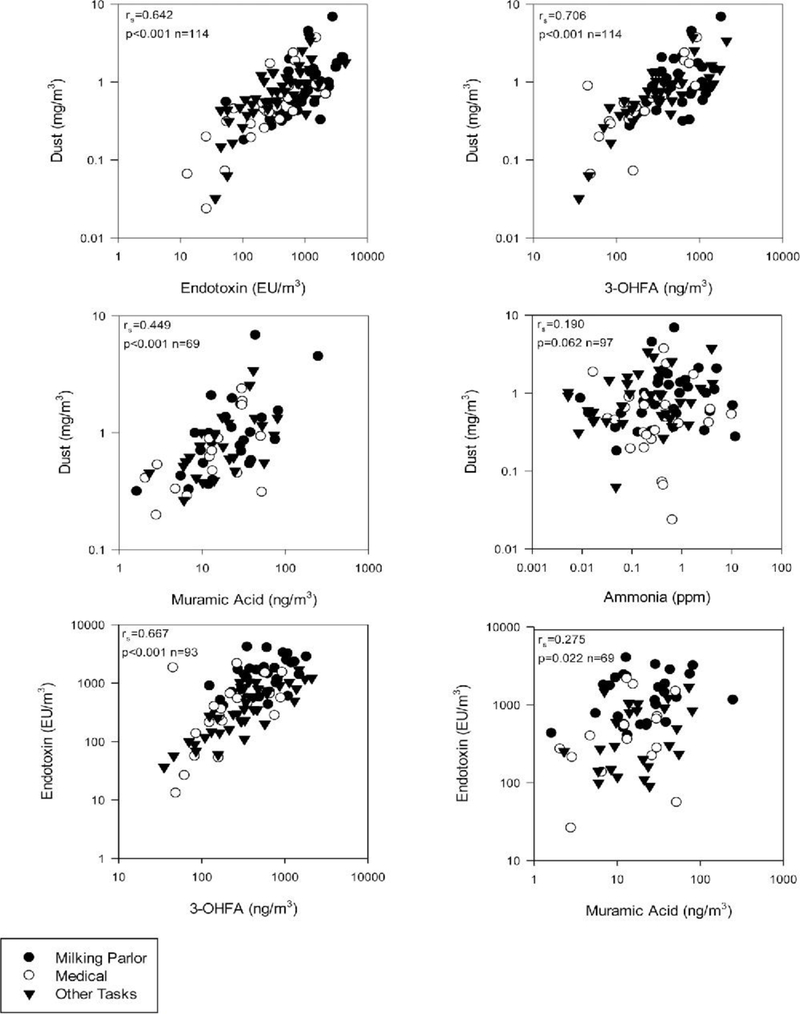
There were strong correlations for the entire study population among dust, endotoxin, and 3-OHFA, and a moderate correlation for dust and muramic acid (Figure 1). Ammonia did not correlate with any bioaerosol constituents (Figure 1). Correlation patterns were similar for the three major task groups.
Figure 1.
Relationships between types of exposures on high plains Western U.S. dairies 2007–2011.
In the milking parlor, there was a moderate positive correlation between temperature, endotoxin, and 3-OHFA. In addition there was a moderate negative correlation between humidity and dust, endotoxin, and 3-OHFA (Table 4). There was no other notable correlation between ambient measurements (CO2, temperature, and humidity) and measured contaminants for the remaining task groups (data not shown).
Table 4.
Spearman rank correlation of milking parlor concentrations of inhalable dust, endotoxin, 3-OHFA, ammonia, and muramic acid to CO2, temperature and humidity.
| Dust | Endotoxin | 3-OHFA | Muramic Acid | Ammonia | |
|---|---|---|---|---|---|
| Parlor temperature | 0.060 | 0.371 | 0.395 | −0.143 | 0.322 |
| p = 0.0761 | p = 0.052 | p = 0.051 | p = 0.495 | p = 0.102 | |
| n = 28 | n = 28 | n = 25 | n = 25 | n = 27 | |
| Parlor humidity | −0.409 | −0.615 | −0.571 | −0.135 | −0.359 |
| p = 0.031 | p = 0.001 | p = 0.003 | p = 0.519 | p = 0.066 | |
| n = 28 | p = 28 | n = 25 | n = 25 | n = 27 | |
| Parlor carbon dioxide | −0.214 | −0.172 | −0.308 | −0.128 | −0.169 |
| p = 0.274 | p = 0.382 | p = 0.134 | p = 0.541 | p = 0.401 | |
| n = 28 | n = 28 | n = 25 | n = 25 | n = 27 |
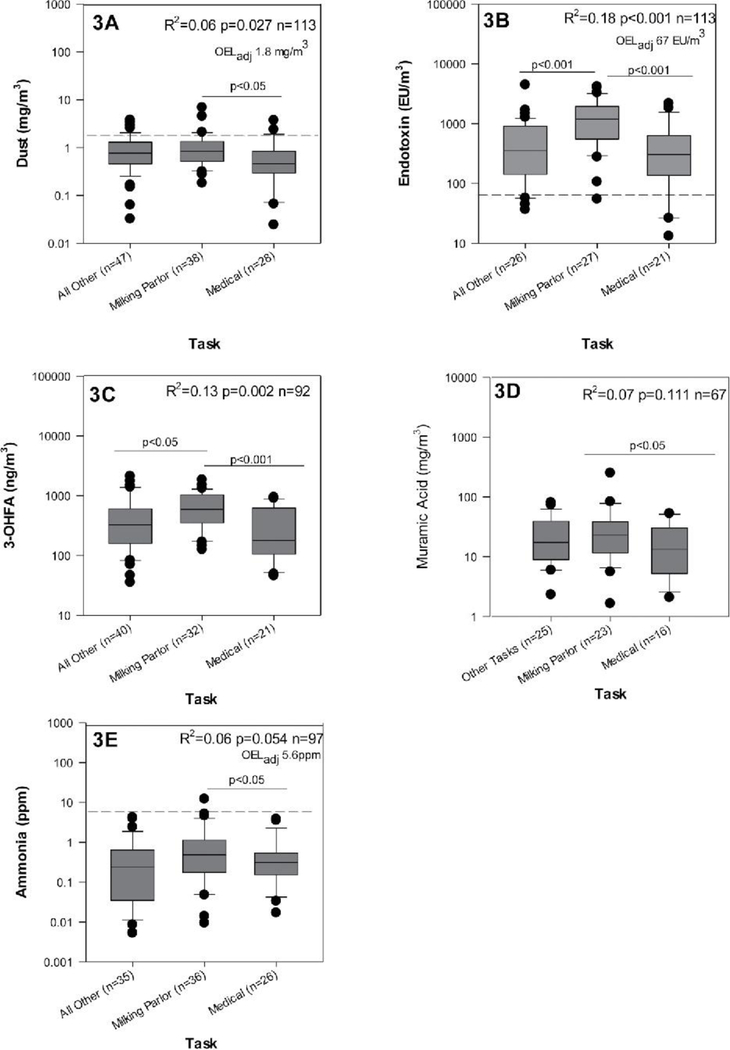
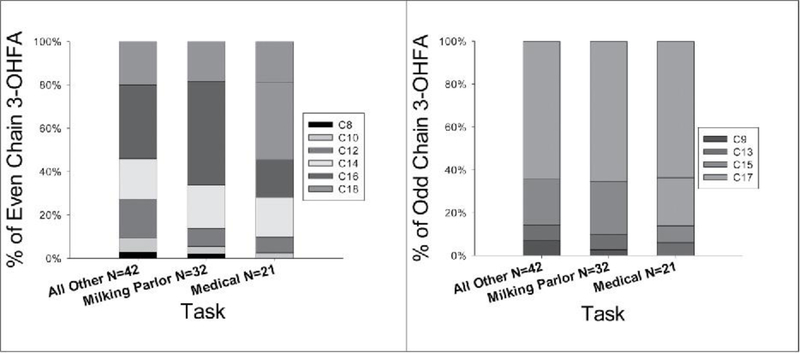
The task performed by the workers at the dairy had a meaningful impact on measured bioaerosol exposure. Milkers encountered higher exposure to dust, endotoxin, 3-OHFA, and muramic acid in comparison to the medical group and higher exposure than the other task group for endotoxin, 3-OHFA, and ammonia (Figure 2). Within each task there was a large variation in all exposures. Given this, it is still noteworthy that the vast majority of endotoxin exposures exceeded the recommended OEL. In conjunction with these results, the profiles of even and odd chains of 3-OHFA were consistent across each task (Figure 3). The most abundant even and odd carbon chain lengths were C16 and C17, respectively. The milking parlor task exhibited a slightly higher amount of C16 compared to the medical and other tasks.
Figure 2.
Task based variation in dust (3A), endotoxin (3B), 3-OHFA (3C), ammonia (3D), and muramic acid (3E) exposures in high plains U.S. dairies 2007–2011. Dashed line in dust, endotoxin, and ammonia figures represents OELadj. R2 interpretation: Task explains x% of variation between these groups.
Figure 3.
Comparison of 3-OHFA chain profiles by task 2006–2011.
The milking parlor showed the same 3-OHFA concentrations as the Other task, and more than twice the medical amount, but had a higher percentage of odd chain fatty acids at 26% vs. the 20 and 19% of the other tasks, respectively (Figure 3).
Discussion
Dairy workers are exposed to high levels of bioaerosols, which has been linked to adverse respiratory health effects. In this study, we explored differences in exposure by common tasks on modern dairy farms. We benchmarked findings with common markers (e.g., dust, endotoxin, and ammonia) and included several novel markers: 3-OHFA, muramic acid, and ergosterol. These findings can help inform research on relevant etiologic agents associated with respiratory health and the development of appropriate interventions.
Exposures
The range of endotoxin exposures in this study was consistent with previous studies on dairy farms.[1,6,10,11,13,20–23] However, we observed an extremely high proportion of workers (89%) with inhalable endotoxin exposures exceeding the recommended OELadj. The geometric mean exposure of 438 EU/m3 is almost seven times the recommended OELadj for the average 54-hr workweek. Inhalable dust, 3-OHFA, and ammonia exposures among the dairy workers were also similar to previous studies.[6,10,13,24] The geometric mean dust concentration of 0.67 mg/m3 was below the recommended OELadj of 1.8 mg/m3. However, 12% of TWA inhalable dust measurements exceeded the OELadj and 37% of measurements exceeded the 50% action level. In this study, only one TWA measurement exceeded the OELadj for ammonia of 5.6 ppm; nine measurements were above the action level. The geometric mean of muramic acid was 9.6 ng/m3. This is one of the first studies to measure occupational exposure to muramic acid as a marker for Gram-positive bacteria in dairies. There are no OELs and a minimal amount of published data on dairy exposures to muramic acid.[25]
The variation in exposure of dairy workers to bioaerosols and ammonia, as evident by the high GSD, indicates that these workers cannot be considered a homogenous exposure group. While the variation in tasks at the start of the project identified twelve task groups based on workplace inspections, the small sample sizes for the majority of tasks required that they be combined into three categories for more robust analysis: milking parlor workers; medical (routine veterinary, calves, breeding, and maternity); and all other (feeding, rebedding, moving, maintenance, and other) tasks.
Among the three task groups, milking parlor workers had the highest exposures for dust, endotoxin, 3-OHFA, muramic acid, and ammonia, but not for ergosterol. This finding is similar to that reported by Basinas et al.[26] even though the sizes of the milking herds were much larger in this current study. Other studies (primarily in California) have reported that the preparation and distribution of feed and rebedding had higher dust exposures compared to milking.[10,22,27] Garcia et al.[10] found endotoxin levels highest for moving cows and medical work.
The strong influence of task on 3-OHFA exposures was further supported by the wide variation in the 3-OHFA chain profiles for the various tasks. Interestingly, the even-chain 3-OHFA profiles were very similar for milking parlor staff, as were the profiles for the medical and other groups. This suggests that these groups may be exposed to similar Gram-negative bacteria. There are no OELs for 3-OHFA, but exposure has been associated with both increased inflammatory markers and decreased pulmonary function among dairy workers.[6,13]
Overall, 70% of ergosterol measurements were below the LOD of 0.5 ng/m3. In the milking parlor, only 7% of samples were above the LOD. Detectable ergosterol concentrations were most frequently found for the combined Other task category (63%), followed by medical workers (52%). Medical tasks had the highest single exposure of 577 ng/m3. These low measurements are surprising considering that fecal biomass is estimated to be composed of more than 40% fungi and that dried fecal matter may contain an average of 9.4 μg/g ergosterol.[28] In addition, task was also an important explanatory variable for ammonia. This is supported by studies by Mutlu et al.[29] and Leytem et al.[30] who found that dairy ammonia exposures could vary considerably across various dairy locations.
Relevance of exposures to worker health
Exposure levels found in this study have been linked to adverse respiratory outcomes in previously published literature. The 95th percentile of endotoxin exposures (3686 EU/m3) are above the range linked to acute bronchoconstriction (1000–2000 EU/m3) and mucous membrane irritation (200–500 EU/m3).[31] Measurements of 3-OHFA in this study are comparable to the ranges reported in previous dairy worker studies, which have demonstrated an association between 3-OHFA and health effects including increased proinflammatory cytokines and measurable respiratory restriction.[13] Furthermore, evidence from cell studies using settled dust by Poole et al. suggests that Gram-positive bacteria and muramic acid may be an important etiological factor or marker for respiratory disease.[14] There are currently no published exposure standards for ergosterol and limited health information, although increases in ergosterol loading in dust have been associated with increased asthma symptoms.[32] TWA concentrations of 7.5 ppm for ammonia have been associated with a ≥3% cross-shift decline in forced expiratory volume in the first second (FEV1) among swine and poultry workers.[8,9] Ammonia has also been identified as a causal factor in the development of chronic obstructive respiratory disease among atopic farmers.[33] Exposure to ammonia at higher concentrations appears likely to be episodic on dairy farms since it is associated with activities such as flushing manure and summer season.[22,34–37] Collectively, these exposure levels may place dairy workers at risk for respiratory health effects.
The study population was predominantly Hispanic (79%). These worker demographics are similar to those in both the companion study in California where 91% of the workers were Latino[5] and a previous Colorado study where 94–100% of study participants on dairies were Hispanic.[6] The questionnaire results indicate a number of factors that may contribute to increased risk for health effects on large-scale dairies. These factors include the high percentage of new workers with less than 1 year of work experience on farms (16.7%), smoking (15.9%), and exposure to secondhand smoke (SHS) (13.2%), long work shifts averaging 9.5 hr, shift-work on 24-hr dairies (7%), and the very low use of respiratory protection devices (0.8%). Workers with limited farm experience and exposure are at greater risk of proinflammatory lung effects upon initial exposure to organic dust and its constituents.[1,38–41] Smoking has been identified as a potential effect modifier exacerbating the severity of pulmonary effects associated with exposure to organic dusts.[6,42,43] The extended work shifts of up to 12 hr (average 9 hr) require that recommended exposure limits be adjusted when developing occupational health and safety programs for dairies. Recommended exposure guidelines are typically based on the exposure of a “healthy” worker through an 8-hr workday and 40-hr workweek.
Implications
Review of recent publications shows a consistent relationship between respiratory disease and exposure to bioaerosols on dairies. There is a need for development and evaluation of culturally appropriate and cost effective intervention strategies using the hierarchy of control.[4,44–48] Based on this study, it is recommended that occupational health and safety strategies initially focus on the milking parlor. This is because new (immunologically susceptible) workers are typically assigned to the milking parlor,[44,49] in which high dust, endotoxin, 3-OHFA, and muramic acid exposures exist.
The correlations between dust and the bioaerosols measured in this and other studies suggests that controlling dust exposure will likely reduce exposure to microorganisms and associated toxins and proinflammatory cell wall constituents.[6,20,21] Potential control strategies for reducing bioaerosol exposures in the milking parlor could include the regular cleaning and maintenance of the existing ventilation systems and automated floor flushing systems, as well as introduction of footbaths and increased frequency of flushing.[17,20,50] There is also limited evidence that parlor design and milking stall formation can influence aerosol concentrations.[10,17,20,26] Changes could be made in the types of bedding materials used in the animal housing areas to reduce the bioaerosol exposures of staff who undertake the cleaning and rebedding of stalls. Sand can inhibit growth of Escherichia coli 0157:H7[51] and could be applied to reduce the prevalence of enteric bacteria in free stalls, maternity and medical barns, and calf hutches. Alternatively, sawdust bedding could be replaced with compost, which is reported to be a lower emitter of dust, endotoxin, and β (1–3)-glucan.[52]
Application of bactericides and sanitizers could be explored as potential controls for biofilms on floors and in pipes in the milking parlor. However, this would add to employee exposure to potentially hazardous substances in the workplace, and routine thorough cleaning with soap and water may circumvent the need for chemical inhibitors. It is worth noting that the use of pesticide/herbicides has been identified as an effect modifier on the interaction between endotoxin exposure and lung function.[6]
Limitations
The current study was limited by the small data set, with fewer than 10 employees for some tasks and farms. The nesting of factors such as workers within farms should be addressed in future epidemiological studies. These studies could focus on a particular high-risk workplace location or employee activities such as milking cows. The study was also limited by the use of one cross-shift exposure monitoring period per worker; more information about daily or longer-term variability would add to our knowledge regarding typical worker exposures. The structure of this data set did not lend itself to controlling for season. In a related study, we did not see any difference in exposures by season.[53]
Conclusions and recommendations
This study was among the first to concurrently evaluate occupational exposure to active endotoxin (lipid A), total endotoxin (three hydroxyl fatty acids; 3-OHFA), muramic acid, ergosterol, and ammonia among workers on Western dairies. The high endotoxin exposures measured in this study indicate that employees on large-scale dairies are at increased risk of adverse respiratory effects, especially workers in the milking parlors. Research to design and evaluate potential interventions should focus on efficacy, cost, and acceptability for reducing worker exposures to the broad range of bioaerosol and chemical constituents found in dairies. Intervention strategies need to be tailored to specific farm tasks and locations and should be part of a comprehensive OHS Risk Management program integrated into production systems to further optimize worker health and farm productivity.
Acknowledgments
The authors thank the dairy producers and workers who participated in this study.
Funding
This work was funded through the High Plains Intermountain Center for Agricultural Health and Safety by a grant from the CDC NIOSH 5U54OH008085 and the Western Center for Agricultural Health and Safety through NIOSH grant number OH007550–06.
References
- 1.Reynolds SJ, Nonnenmann MW, Basinas I, et al. : Systematic review of respiratory health among dairy workers. J. Agromed 18(3):219–243 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Douphrate DI, Hagevoort GR, Nonnenmann MW, et al. : The dairy industry: A brief description of production practices, trends, and farm characteristics around the world. J. Agromed 18(3):187–197 (2013). [DOI] [PubMed] [Google Scholar]
- 3.National Agricultural Statistics Service: Farms, Land in Farms, and Livestock Operations 2011 Summary. 2012. [Google Scholar]
- 4.Hagevoort GR, Douphrate DI, and Reynolds SJ: A review of health and safety leadership and managerial practices on modern dairy farms. J. Agromed 18(3):265–273 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Eastman C, Schenker MB, Mitchell DC, Tancredi DJ, Bennett DH, and Mitloehner FM: Acute pulmonary function change associated with work on large dairies in California. J. Occup. Environ. Med 55(1):74–79. doi: 10.1097/JOM.1090b1013e318270d318276e318274 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Reynolds S, Clark ML, Koehncke N, et al. : Pulmonary function reductions among potentially susceptible subgroups of agricultural workers in Colorado and Nebraska. J. Occup. Environ. Med 54(5)632–641. doi: 10.1097/JOM.0b013e31824d2e1c (2012). [DOI] [PubMed] [Google Scholar]
- 7.Donham KJ, Cumro D, S.J. R, and A J. Merchant: Dose-response relationships between occupational aerosol exposures and cross-shift declines of lung function in poultry workers: Recommendations for exposure limits. J. Occup. Environ. Med 42(3):260–269 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Reynolds SJ, Donham KJ, Whitten P, Merchant JA, Burmeister LF, and Popendorf WJ : Longitudinal evaluation of dose-response relationships for environmental exposures and pulmonary function in swine production workers. Am. J. Ind. Med 29(1):33–40 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Donham KJ, Reynolds SJ, Whitten P, Merchant JA, Burmeister L, and Popendorf WJ: Respiratory dysfunction in swine production facility workers: Dose-response relationships of environmental exposures and pulmonary function. Am. J. Ind. Med 27(3):405–418 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Garcia J, Bennett DH, Tancredi D, et al. : Occupational exposure to particulate matter and endotoxin for California dairy workers. Int. J. Hyg. Environ. Health 216(1):56–62 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Basinas I, Sigsgaard T, Erlandsen M, et al. : Exposure-affecting factors of dairy farmers’ exposure to inhalable dust and endotoxin. Ann. Occup. Hyg 58(6):707–723 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Dutch Expert Committee on Occupational Safety: Endotoxins: Health-Based Recommended Occupational Exposure Limit. Den Haag: Health Council of the Netherlands, 2010. [Google Scholar]
- 13.Burch JB, Svendsen E, Siegel PD, et al. : Endotoxin exposure and inflammation markers among agricultural workers in Colorado and Nebraska. J. Toxicol. Environ. Health, A 73(1):5–22 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Poole JA, Dooley GP, Saito R, et al. : Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J. Toxicol. Environ. Health, A 73(10):684–700 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito R, Cranmer BK, Tessari JD, et al. : Recombinant factor C (rFC) assay and gas chromatography/mass spectrometry (GC/MS) analysis of endotoxin variability in four agricultural dusts. Ann. Occup. Hyg 53(7):713–722 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Blais Lecours P, Veillette M, Marsolais D, and Duchaine C.: Characterization of bioaerosols from dairy barns: Reconstructing the puzzle of occupational respiratory diseases by using molecular approaches. Appl. Environ. Microbiol 78(9):3242–3248 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funk S.: “Evaluation of Seasonal Ventilation Changes and Their Effect on Ambient Dust, Endotoxin and Bioaerosol Concentrations in a Dairy Parlor.” MS thesis, Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, CO, 2011. [Google Scholar]
- 18.Poole JA, and Romberger DJ: Immunological and inflammatory responses to organic dust in agriculture. Curr. Opin. Allergy Clin. Immunol 12(2):126–132 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel C, Garcia J, Wu D, et al. : Activation of inflammatory responses in human U937 macrophages by particulate matter collected from dairy farms: An in vitro expression analysis of pro-inflammatory markers. Environ. Health 11(1):17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia J, Bennett DH, Tancredi DJ, et al. : Characterization of endotoxin collected on California dairies using personal and area-based sampling methods. J. Occup. Environ. Hyg 9(10):580–591 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Saito R.: “Analyses and Exposure Assessment of Bacterial Endotoxin in Agricultural Environments.” PhD diss., Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, CO, 2008. [Google Scholar]
- 22.Lester B.: “Comparison of Occupational and Environmental Exposures at Colorado Dairies.” PhD diss., Environmental and Radiological Health Sciences, Colorado State University, Fort Collins, CO, 2007. [Google Scholar]
- 23.Smit LAM, Heederik D, Doekes G, Blom C, van Zweden I, and Wouters IM: Exposure–response analysis of allergy and respiratory symptoms in endotoxin-exposed adults. Eur. Resp. J 31(6):1241–1248 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Reynolds SJ, Nakatsu J, Tillery M, et al. : Field and wind tunnel comparison of four aerosol samplers using agricultural dusts. Ann. Occup. Hyg 53(6):585–594 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nonnenmann MW, Gimeno D. de Porras Ruiz, Levin J, et al. : Pulmonary function and airway inflammation among dairy parlor workers after exposure to inhalable aerosols. Am. J. Ind. Med 60:255–263. doi: 10.1002/ajim.22680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basinas I, Schlunssen V, Takai H, et al. : Exposure to inhalable dust and endotoxin among Danish pig farmers affected by work tasks and stable characteristics. Ann. Occup. Hyg 57(8):1005–1019 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Nieuwenhuijsen MJ, Noderer KS, Schenker MB, Vallyathan V, and Olenchock S.: Personal exposure to dust, endotoxin and crystalline silica in California agriculture. Ann. Occup. Hyg 43(1):35–42 (1999). [PubMed] [Google Scholar]
- 28.Jost DI, Indorf C, Joergensen RG, and Sundrum A.: Determination of microbial biomass and fungal and bacterial distribution in cattle faeces. Soil Biol. Biochem 43(6):1237–1244 (2011). [Google Scholar]
- 29.Mutlu A, Mukhtar S, Capareda SC, et al. : “A Process Based Approach for Ammonia Emission Measurements at a Free-stall Dairy.” 2004ASAE/CSAE Annual International Meeting 1–4 August. Ottawa, Ontario, Canada, 2004. http://agrilife.org/cafoaq/files/2012/01/PU01204_3.pdf [Google Scholar]
- 30.Leytem AB, Dungan RS, and Bjorneberg DL: Case study: Seasonal and spatial distribution of ambient ammonia concentrations measured at a large open-lot dairy. Prof. Anim. Scientist 25(6):786–793 (2009). [Google Scholar]
- 31.Schenker M.: Respiratory health hazards in agriculture. Am. J. Respir. Crit. Care. Med 158(Supplement 1):S1–S76 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Mendell MJ, Mirer AG, Cheung K, Tong M, and Douwes J.: Respiratory and allergic health effects of dampness, mold, and dampness-related agents: A review of the epidemiologic evidence. Environ. Health. Perspect 199(6):748–756 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eduard W, Pearce N, and Douwes J.: Chronic bronchitis, COPD, and lung function in farmers. Chest. 136(3):716–725 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Powell JM, Broderick GA, and Misselbrook TH: Seasonal diet affects ammonia emissions from tie-stall dairy barns. J. Dairy Sci 91(2):857–869 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Pinder RW, Pekney NJ, Davidson CI, and Adams PJ: A process-based model of ammonia emissions from dairy cows: Improved temporal and spatial resolution. Atmosph. Environ 38(9):1357–1365 (2004). [Google Scholar]
- 36.Mukhtar S, Mutlu A, Capareda SC, and Parnell CB: Seasonal and spatial variations of ammonia emissions from an open-lot dairy operation. J. Air Waste Mgmt. Assoc 58(3):369–376 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Ngwabie NM, Jeppsson KH, Gustafsson G, and Nimmermark S.: Effects of animal activity and air temperature on methane and ammonia emissions from a naturally ventilated building for dairy cows. Atmosph. Environ. 45(37):6760–6768 (2011). [Google Scholar]
- 38.Heederik D, Sigsgaard T, Thorne PS, et al. : Health effects of airborne exposures from concentrated animal feeding operations. Environ. Health Persp 115(2):298–302 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bakirci N, Kalaca S, Francis H, et al. : Natural history and risk factors of early respiratory responses to exposure to cotton dust in newly exposed workers. J. Occup. Environ. Med 49(8):853–861. 810.1097/JOM.1090b1013e3180dca1598 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Dosman JA, Lawson JA, Kirychuk SP, Cormier Y, Biem J, and Koehncke N.: Occupational asthma in newly employed workers in intensive swine confinement facilities. Eur. Resp. J 24(4):698–702 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Larsson B-M, Larsson K, Malmberg P, Mårtensson L, and Palmberg L.: Airway responses in naive subjects to exposure in poultry houses: Comparison between cage rearing system and alternative rearing system for laying hens. Am. J. Ind. Med 35(2):142–149 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Bünger J, Schappler-Scheele B, Hilgers R, and Hallier E.: A 5-year follow-up study on respiratory disorders and lung function in workers exposed to organic dust from composting plants. Int. Arch. Occup. Environ. Health 80(4):306–312 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Gainet M, Thaon I, Westeel V, et al. : Twelve-year longitudinal study of respiratory status in dairy farmers. Eur. Resp. J 30(1):97–103 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Baker D, and Chappelle D.: Health status and needs of Latino dairy farmworkers in Vermont. J. Agromed 17(3):277–287 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Schenker M, and Gunderson P.: Occupational health in the dairy industry needs to focus on immigrant workers, the new normal. J. Agromed 18(3):184–186 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Villarejo D, McCurdy SA, Bade B, Samuels S, Lighthall D, and Williams D.: The health of California’s immigrant hired farmworkers. Am. J. Ind. Med 43(4):387–397 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Hansen E, and Donohoe M.: Health issues of migrant and seasonal farmworkers. J. Health Care Poor Underserv 14(2):153–164 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Villarejo D.: The health of U.S. hired farm workers. Ann. Rev. Public Health 24(1):175–193 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Douphrate DI, Rosecrance JC, Stallones L, Reynolds SJ, and Gilkey DP: Livestock-handling injuries in agriculture: An analysis of Colorado workers’ compensation data. Am. J. Ind. Med 52(5):391–407 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choudhry AH, Reynolds SJ, Mehaffy J, et al. : Evaluation of parlor cleaning as an intervention for decreased occupational exposure to dust and endotoxin among dairy parlor workers—A pilot study. J. Occup. Environ. Hyg 9(7):D136–D140 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Westphal A, Williams ML, Baysal-Gurel F, LeJeune JT, and McSpadden Gardener BB: General suppression of Escherichia coli O157:H7 in sand-based dairy livestock bedding. Appl. Environ. Microbiol 77(6):2113–2121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samadi S, van Eerdenburg FJCM, Jamshidifard A-R, et al. : The influence of bedding materials on bio-aerosol exposure in dairy barns. J. Expos. Sci. Environ. Epidemiol 22(4):361–368 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Schaeffer JW, Reynolds S, Magzamen S, et al. : Size, composition, and source profiles of inhalable bioaerosols from Colorado dairies. Environ. Sci. Technol 51(11):6430–6440. doi: 10.1021/acs.est.7b00882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]