ABSTRACT
This study was conducted to assess the nasopharyngeal (NP) carriage and acute otitis media (AOM) occurrence in Korean children who received pneumococcal conjugate vaccines (PCVs). The longitudinal study was conducted through four consecutive visits. At each visit, NP aspirates were obtained and subjects were asked to visit if AOM occurred. A total of 305 subjects were enrolled and received PCV13 (n = 182) or PCV10 (n = 123). In the PCV13 group, the NP carriage of Streptococcus pneumoniae at each visit was 2.7%, 14.8%, 18.7%, and 15.9%, respectively. Non-typeable Haemophilus influenzae (NTHi) was 3.3%, 2.7%, 2.7%, and 5.5%, and that of Moraxella catarrhalis was 1.1%, 9.3%, 4.9%, and 0.5%. In the PCV10 group, the NP carriage of S. pneumoniae at each visit was 3.3%, 7.3%, 6.5%, and 4.1%, respectively. That of NTHi was 2.4%, 4.1%, 1.6%, and 0.8%, and that of M. catarrhalis was 4.1%, 0.8%, 0.8%, and 0.0%. AOM occurrence in the PCV13 group observed after the primary dose and before booster dose was 20.9%, occurrence after booster dose was 11.0%, and the incidence of two or more AOM was 11.0%. In the PCV10 group, AOM occurrence was 9.8%, 7.3%, respectively, and the incidence of two or more AOM was 2.4%. The predominant S. pneumoniae isolated were non-vaccine type (10A, 15A, and 15B). In this study, AOM occurrence was lower in the PCV10 group than in the PCV13 group. This seems to be related to ecological changes that lead to differences in NP carriage, especially S. pneumoniae and NTHi.
KEYWORDS: nasopharyngeal carriage, acute otitis media, pneumococcal conjugate vaccine, children
Introduction
Nasopharyngeal pathogen colonization is known to be closely associated with the development of localized bacterial respiratory infections, including acute otitis media (AOM), sinusitis, and bronchopneumonia in infants and children aged 5 y or younger. Increased mucosal and T-cell immunity following vaccination with pneumococcal conjugate vaccine (PCV) can reduce nasopharyngeal colonization by vaccine strain pneumococci and prevent localized pneumococcal infections in children.1 Therefore, there is a great interest amongst clinical practitioners regarding the pneumococcal nasopharyngeal colonization effect and protective effect against nasopharyngeal colonization, which precede the development of AOM after PCV vaccination. In this regard, since first introducing PCV in the early 2000s, many clinical epidemiology studies on pneumococcal nasopharyngeal colonization were carried out worldwide, which reported a consistent finding that vaccine serotype-induced pneumococcal colonization is reduced after PCV vaccination.2-5 However, the time of PCV7 introduction, coverage rate, status of PCV10 and PCV13 introduction, and the pneumococcal epidemiology vary by region. Adding to this, nasopharyngeal colonization does not imply a diseased state and interpretation of nasopharyngeal colonization pattern requires consideration of many factors. In fact, according to the World Health Organization (WHO) position paper, serotypes included in PCV10 and PCV13 are known to have an effect of reducing pneumococcal nasopharyngeal colonization, while the quantification of the effects of each vaccine is difficult because vaccination schedule, epidemiology, and the effects of previous PCV7 vaccination vary by region.6
In Korea, AOM is one of the most common acute respiratory tract infections in children, and antibiotic prescription and development of resistance to antibiotics are very serious problems. However, there has been no epidemiological study on the preventive effect of PCV in Korean children. In this regard, we conducted the present study to obtain epidemiological data on the impact of PCVs on AOM prevention and nasopharyngeal bacterial colonization by assessing nasopharyngeal flora carriage and AOM occurrence, in subjects receiving PCV10 or PCV13, as part of the Korean National Immunization Program (NIP).
Materials and methods
The study was conducted in healthy infants and children who visited one of the eight hospitals (Seoul St. Mary’s Hospital, St. Vincent’s Hospital, Incheon St. Mary’s Hospital, Changwon Fatima Hospital, Chonbuk National University Children’s Hospital, Dongsan Medical Center, Wonju Severance Christian Hospital, and Hanil General Hospital) in Korea from November 2014 to May 2019 for a routine checkup and vaccination visits using the method described below. This study was an open-label study.
Study subjects
All study subjects included in this study were infants under 2 months old, who visited the above institutions for PCV10 or PCV13 vaccinations and whose guardians agreed to their participation in the present study. The subjects voluntarily participated in the study, and there was no advertisement for the enrollment. Guardians were compensated as per the pre-defined protocol after each completed visit. However, infants excluded from the study included those under the control and protection of a group, organization or a protective facility, a court of law, or the government, medically if they received immunosuppressants or other immunomodulators for 14 d or longer, those who were suspected of immunodeficiency based on medical history and physical examination, those who were suffering from AOM or had a history of AOM at the time of enrollment or those who had congenital malformation, or were suffering from serious chronic diseases. PCV10 contains ten serotypes of pneumococcus (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F) which are conjugated to a carrier protein. PCV13 contains 13 serotypes of pneumococcus (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) which are conjugated to carrier protein. In Korea, both PCV10 and PCV13 are included in NIP. The possibility of selection bias was minimized by enabling physicians to offer both vaccines and letting guardians voluntarily choose either one. Certain sites did not prescribe specific vaccines. The study was conducted in compliance with the Declaration of Helsinki and the International Committee on Harmonization Guidelines for Good Clinical Practice. The protocol was reviewed and approved by the Institutional Review Boards (KC120IMI0878). Written informed consent was obtained from all parents or legal authorized representatives prior to enrolling the subjects in the study.
Specimen collection and analyses
Nasopharyngeal aspirate specimens were collected from the study subjects through nasopharyngeal aspiration at four time points: before 2 months old, prior to PCV vaccination (first visit; pre-vaccination); 1 month after the three primary PCV doses, between 7 and 9 months old (second visit; post-primary vaccination); before the booster dose, between 12 and 15 months old (third visit; pre-booster vaccination); 1 month after the booster dose, between 16 and 18 months old (fourth visit; post-booster vaccination). Completion of four PCV vaccinations was not a condition for participation in the study. In Korea, the full vaccination rate of PCVs is high at 97.2% (as of 2018) as PCVs are included in NIP. While vaccination rates have not been measured in this study, the full vaccination rate has remained over 95% since 2014 indicating that a similar vaccination rate could be predicted throughout the study.
In addition to routine visits, subjects who had developed AOM were asked to re-visit for NP aspirate specimen collection. The collected NP aspirate specimens were inoculated onto 5% sheep blood agar, chocolate agar, Columbia CNA media plate, and AAV BAP agar, and incubated overnight at 35°C in 5% CO2. The cultured strains were transferred to BHI medium supplemented with glycerol (BHI medium 98 mL + hematin 1 mL (1 mg/mL) + NAD 1 mL (1 mg/mL) + 10% glycerol), and the strains were identified by standard methods. H. influenzae were isolated and identified based on colony morphology and growth on chocolate agar supplemented with bacitracin, factor V, and X. In addition, the isolated serotypes of S. pneumoniae and the genotypes of H. influenzae were each analyzed by PCR. All specimen analyses were carried out at Seegene Research Center and Vaccine Bio Research Institute of the Catholic University of Korea.
AOM follow-up
AOM monitoring period was equal to the follow-up period, and the parents of the subjects were recommended to bring their child to the hospital for examination and to test if their child exhibited AOM symptoms, including fever, crying, and otalgia. Also, in order to minimize loss of follow-up, an automated phone text message was sent each month to remind the parents or guardians to bring their child to the hospital if their child exhibited these AOM symptoms. The clinical diagnosis of AOM was defined as findings of redness, bulging, or loss of light reflex of the tympanic membrane on physical examination, or presence of effusion in the middle ear with two or more of the following symptoms in the past 2 d: fever, otalgia and crying, otorrhea, hearing loss, and fatigue. Recurrent AOM was defined as a re-occurrence of AOM of at least 2 weeks and at most 4-week post-AOM treatment.
Statistical analysis
The main purpose of the present study was to identify the incidence rate in an open cohort. As this was a descriptive longitudinal epidemiological study and the P-value cannot be used for statistical difference, Chi-square was used to compare bacterial isolates from the NP aspirates at each routine visit and AOM and recurrent AOM visits between the two groups.
Results
The study was a follow-up hospital-based epidemiological study in which 312 infants who met the inclusion criteria and visited one of the eight hospitals. A total of 305 subjects (182 subjects in the PVC 13 group and 123 subjects in the PVC 10 group) completed the study, and the results of specimen analysis are given below.
S. pneumoniae carriage distribution and rates in both groups
In the PCV10 group, S. pneumoniae was detected in nasopharyngeal aspirate specimen in 3.3% (4/123) at the first visit, 7.3% (9/123) at the second visit, 6.5% (8/123) at the third visit, and 4.1% (5/123) at the fourth (final) visit, whereas, in the PCV13 group, S. pneumoniae detection for each visit was 2.7% (5/182), 14.8% (27/182), 18.7% (34/182), and 15.9% (29/182), respectively. As such, with the exception of the first visit before the first vaccination, significantly more S. pneumoniae were isolated at the second, third, and final visit in the PCV13 group than in the PCV10 group (Figure 1).
Figure 1.
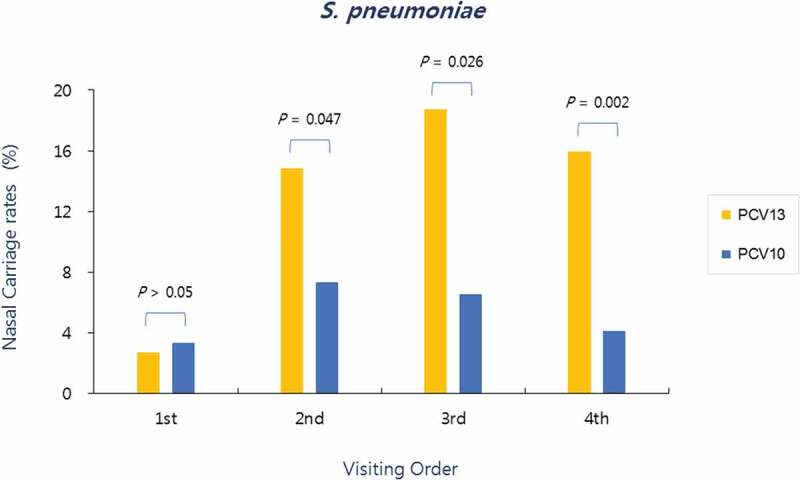
Nasopharyngeal carriage rates of S. pneumoniae according to the visiting order in both groups. Significantly more S. pneumoniae were isolated at the second (post-primary vaccination), third (pre-booster vaccination), and fourth visits (post-booster vaccination) in the PCV13 group than in the PCV10 group
Non-typeable H. influenzae (NTHi) carriage distribution and rates in both groups
In the PCV10 group, NTHi was detected in nasopharyngeal aspirate specimen in 2.4% (3/123) at the first visit, 4.1% (5/123) at the second visit, 1.6% (2/123) at the third visit, and 0.8% (1/123) at the fourth (final) visit, whereas, in the PCV13 group, NTHi detection for each visit was 3.3% (6/182), 2.7% (5/182), 2.7% (5/182), and 5.5% (10/182), respectively. As such, significantly more NTHi were isolated at the final visit in the PCV13 group than in the PCV10 group (Figure 2).
Figure 2.
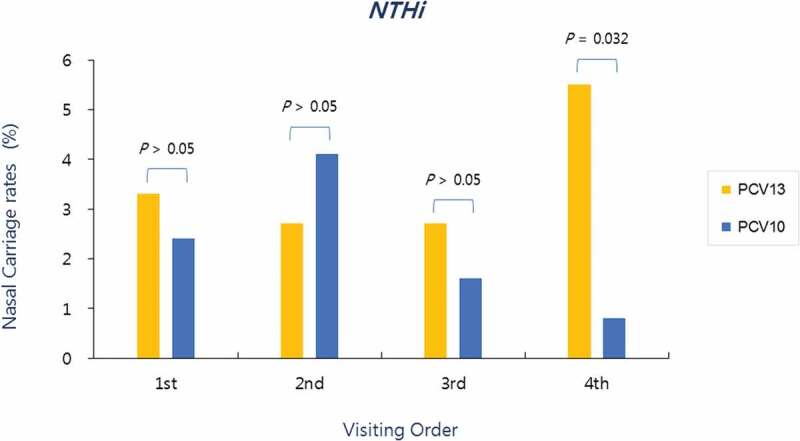
Nasopharyngeal carriage rates of NTHi according to the visiting order in both groups. Significantly more NTHi isolated at the fourth visit (post-booster vaccination) in the PCV13 group than in the PCV10 group
M. catarrhalis carriage distribution and rates in both groups
In the PCV10 group, M. catarrhalis was detected in nasopharyngeal aspirate specimen in 4.1% (5/123) at the first visit, 0.8% (1/123) at the second visit, 0.8% (1/123) at the third visit, and 0.0% (0/123) at the fourth visit, whereas, in the PCV13 group, M. catarrhalis detection for each visit was 1.1% (2/182), 9.3% (17/182), 4.9% (9/182), and 0.5% (1/182), respectively. As such, significantly more M. catarrhalis were isolated at the second and third visits in the PCV13 group than in the PCV10 group (Figure 3).
Figure 3.
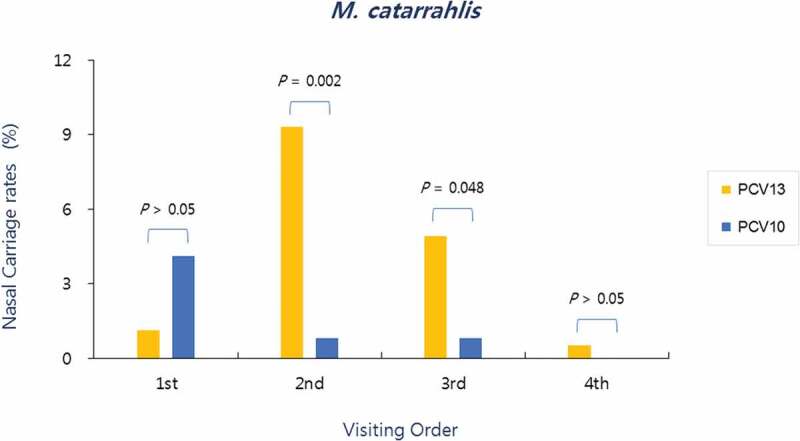
Nasopharyngeal carriage rates of M. catarrhalis according to the visiting order in both groups. Significantly more M. catarrhalis were isolated at the second (post-primary vaccination) and third visits (pre-booster vaccination) in the PCV13 group than in the PCV10 group
AOM occurrence rates according to the visiting time in both groups
In the PCV10 group, percentages of institution visits due to AOM occurrence were 4.1% (5/123) between the first visit and second visit, 5.7% (7/123) between the second and third visits, and 7.3% (9/123) between the third and fourth visits, and incidence of two or more AOM recurrence was 2.4% (3/123). In the PVC 13 group, percentages of institution visits due to AOM occurrence were 6.6% (12/182), 14.3% (26/182), and 11.0% (20/182) for the same time periods, and incidence of two or more AOM recurrence was 11.0% (20/182).
When comparing these data in terms of the status of primary and booster vaccination, 12 subjects (9.8%) in the PCV10 group and 38 subjects (20.9%) in the PCV13 group made visits to the institutions due to AOM between the primary dose and the booster dose, and 9 subjects (7.3%) in the PCV10 group and 20 subjects (11.0%) in the PCV13 group made visits to the institutions due to AOM after the booster dose. The AOM occurrence and recurrence in the PCV13 group were all significantly higher than those of the PCV10 group for all vaccination status (Figure 4).
Figure 4.
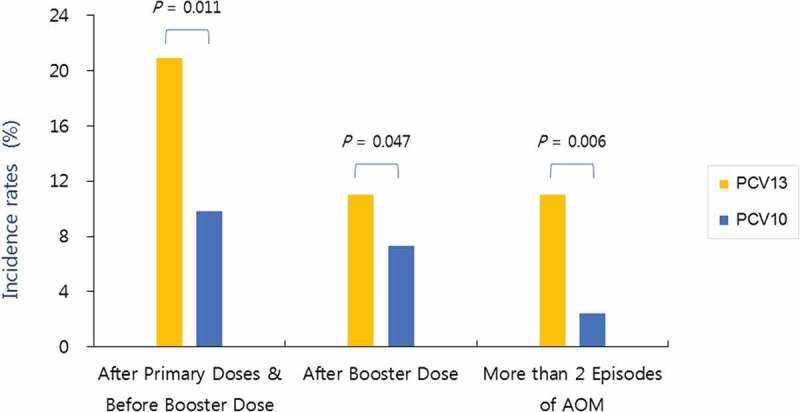
Acute otitis media (AOM) incidence and recurrence rates according to the occurrence time in both groups. The AOM incidence and recurrence in the PCV13 group were all significantly higher than those of the PCV10 group for all vaccination status
Distributions of S. pneumoniae serotypes
In the PCV13 group, 95 strains of S. pneumoniae were detected; in the PCV10 group, 26 strains of S. pneumoniae were detected. In the PCV13 group, 20 strains (21.1%) were vaccine serotypes (4 (7), 6A (5), 6B (5), 19A (2), and 23F (1)), and 42 strains (44.2%) were non-vaccine serotypes (10A (18), 15A (10), 34 (5), 15B (5), 11A (1), 22F (1), 35B (1), and 35F (1)). In the PCV10 group, 2 strains (7.7%) were vaccine serotypes (6B (2)), and 6 strains (23.1%) were non-vaccine serotypes (10A (1), 6A (2), 11A (2), and 19A (1)). In addition, a high number of non-typeable strains were isolated, with 33 non-typeable strains (34.7%) in the PCV13 group and 18 non-typeable strains (69.2%) in the PCV10 group (Table 1.)
Table 1.
Distributions of S. pneumoniae serotypes in both groups
| Serotypes | PCV13 group | PCV10 group |
|---|---|---|
| 10A | 18/95 | 1/26 |
| 15A | 10/95 | |
| 4 | 7/95 | |
| 6A | 5/95 | 2/26 |
| 34 | 5/95 | |
| 15B | 5/95 | |
| 6B | 5/95 | 2/26 |
| 19A | 2/95 | 1/26 |
| 11A | 1/95 | 2/26 |
| 23F | 1/95 | |
| 22F | 1/95 | |
| 35B | 1/95 | |
| 35F | 1/95 | |
| Non-typeable | 33/95 | 18/26 |
Distributions of NTHi genotypes
Twenty-six and eleven strains of NTHi were detected in the PCV13 and PCV10 groups, respectively. The NTHi genotypes detected in the PCV13 group included 15 BLPACR (β-lactamase positive amoxicillin clavulanate resistant) strains, 9 BLNAR (β-lactamase negative ampicillin-resistant) strains, 1 BLPAR (β-lactamase positive ampicillin-resistant) strain, and 1 BLANAS (β-lactamase negative ampicillin susceptible) strain. The NTHi genotypes detected in the PCV10 group included 9 BLPACR strains and 2 BLNAR strains, and with the exception of 1 strain in the PCV13 group; all the isolated strains were resistant in both groups (Table 2).
Table 2.
Distributions of NTHi resistant types in both groups
| Resistant types | PCV13 | PCV10 |
|---|---|---|
| BLANAS | 1/26 | 0 |
| BLNAR | 9/26 | 2/11 |
| BLPAR | 1/26 | 0 |
| BLPACR | 15/26 | 9/11 |
BLANAS (beta-lactamase negative ampicillin susceptible).
BLNAR (beta-lactamase negative ampicillin-resistant).
BLPAR (beta-lactamase positive ampicillin-resistant).
BLPACR (beta-lactamase positive amoxicillin clavulanate resistant).
Discussion
PCV7 was introduced in Korea in 2003 and has been utilized with a high vaccination rate of 75%. Seven years later, in 2010, PCV10 and PCV13 were introduced, and since 2014 these two vaccines have been given as part of the NIP for infants and children of 2 to 59 months of age. Since the first introduction of PCV7 to date, all PCV vaccinations in Korea are given on a 3+1 schedule, in which children receive a series of three primary doses, starting at 2 months of age, at a 2-month interval between doses and a booster dose between 12 and 15 months of age. Based on the Immunization Registry Information System data from the Korea Centers for Disease Control and Prevention, the vaccination rate of PCV is very high in Korea, with 78% completing 4 doses and 90% receiving at least three doses. In this descriptive prospective study, we assessed whether there are differences in NP colonization by commonly implicated bacteria S. pneumonia, NTHi, M. catarrhalis flora, and AOM occurrence depending on the type of PCV received. In particular, the study was conducted on the condition that the two groups had no AOM occurrence before the PCV vaccination, as the exclusion criteria were children suffering from AOM or with a history of AOM. AOM occurrence was studied in two time frames: from after the primary dose to before the booster dose and from after the booster dose to a post-dose 6-month monitoring period.
In the PCV13 group, the pneumococcal nasopharyngeal carriage rate ranged from 2.7% to 18.7%, depending on the visiting time, whereas in the PCV10 group, the pneumococcal nasopharyngeal carriage rate ranged from 3.3% to 7.3%, and the rates were significantly lower than those of PCV13 group for all visits after the primary vaccination until after the booster vaccination (Figure 1). Independent of the type of vaccine received during the study period, pneumococcal nasopharyngeal carriage rate in all subjects was 3.0%, 11.8%, 13.8%, and 11.1%, in the order of visiting time. Considering the pneumococcal nasopharyngeal carriage rate in Korean infants and children before the introduction of PCV7, which exceeded 30%,7,8 and carriage rate immediately before the introduction of PCV10 and PCV13, which was found to be 28.6%,4 the pneumococcal nasopharyngeal carriage rate has been relatively reduced while maintaining a PCV vaccination rate of ≥90% with the introduction of PCV10 and PCV13 and implementation of NIP, even when compared to the highest carriage rate (13.8%) found in our study at the third visit. With respect to differences in carriage rate by visiting time, S. pneumonia carriage rate increased until the third visit and decreased at the fourth visit in the PCV13 group, whereas the carriage rate continued to decrease at the third and fourth visits, following the highest rate at the second visit, in the PCV10 group. Thus, it was assumed that S. pneumonia carriage starts to decrease earlier in the PCV10 group relative to the PCV13 group. For pneumococcal serotype distribution, 10A and 15A serotypes were the most common in the PCV13 group. Non-vaccine types (including non-typeable strains) were predominant serotypes in both the PCV13 group, with 78.9%, and PCV10 group, with 92.3% (Table 1). With an increasing vaccination rate of PCV worldwide, studies on changes in pneumococcal nasopharyngeal carriage over time showed a pattern of reduction in vaccine serotypes and replacement by non-vaccine serotypes.9,10 This pattern was also observed in the present study. On the other hand, a study by Korea Centers for Disease Control and Prevention, which was conducted around the same time as the present study, analyzed pneumococcal serotypes in children and adolescents in Korea and found that 83.2% of the pneumococcal serotypes isolated from invasive infections were identified to be non-vaccine types, of which 10A and 15A were the most common. This showed that the distribution pattern of invasive infections and nasopharyngeal serotype carriage found in our study were similar within Korea.11
When NTHi nasopharyngeal carriage rate was analyzed by visiting time, there was no significant change in the first three visits, but the rate increased to 5.5% in the final visit in the PCV13 group. Whereas the carriage rate increased (4.1%) compared to before vaccination but gradually decreased to 0.8% at the final visit in the PCV10 group. The NTHi nasopharyngeal carriage rate of the two groups was different for each visit, and although no significant difference was found for the first three visits, the NTHi carriage rate in the PCV13 group was significantly higher than the PCV10 group at the last visit, which was between 16 and 18 months of age (Figure 2). We presume that this was due to the inclusion of H. influenzae protein D as one of the carrier proteins in PCV10. However, we cannot conclude on a direct relationship based on the results from studies conducted in this regard. A study in New Zealand, which analyzed the pathogen density in the nasopharyngeal and middle ear specimens of 217 individuals in the PCV7 group and 240 individuals in the PCV10 group, showed no difference in the NTHi density between the two groups.12 In a study that investigated nasopharyngeal carriage rate at 1 month and 6 months after the third dose in infants who received PCV10 or PCV13 at 1, 2, and 3 months of age found no difference between the two groups.13 A study in Australia, which analyzed bronchoalveolar lavage (BAL) fluids and NP swabs of 543 children who underwent bronchoscopy for chronic cough, found that of the 262 children in the PCV7 group, 53 in the PCV10 group, and 166 in the PCV13 group, 89 (34%), 9 (17%), and 47 (28%) children had NTHi airway infection, respectively. Children who received at least two doses of PCV10 had significantly less NTHi-induced lower airway infection compared to children vaccinated with other types of PCV, but no difference was found in NTHi NP carriage among the PCV types. The investigators presumed that this result was due to the effect of PCV10 on enhancing systemic immunity but not nasal mucosal immunity.14 A similar result was found in a study on otitis media, which reported that the rate of NTHi culture from the ear discharge was lower in the group that had received PCV10 compared with groups that received other PCV but did not find reduced NTHi NP carriage.15 In a previous study by the authors, a significant difference in the NTHi carriage rate was observed after the booster dose, and differences in AOM occurrence were also found after the vaccination with the primary dose. Since no tests were performed on the causative agents of AOM in this study, we believe that it is not possible to conclude on the correlation between NTHi carriage rate and NTHi-induced AOM. Therefore, in order to make this correlation, a well-designed study is needed in the future. All but one NTHi isolated in the two groups had resistant genotypes (Table 2), which was similar to the findings from NTHi resistance studies conducted in Korea.16,17 However, our results showed a high rate of expression of the BLPACR strain, and we believe that the resistance of this strain is evolving in Korea. As such, continuous follow-up epidemiological studies are needed.
When nasopharyngeal carriage rate of M. catarrhalis, which is the third important causative agent of AOM occurrence, was analyzed by visiting time, the carriage rate increased from the first to third visit, and markedly decreased at the fourth visit in the PCV13 group. In contrast, a significant decrease in carriage rate was found following the first visit in the PCV10 group, with no carriage found at the fourth visit (Figure 3). This result was similar to the finding that S. pneumoniae and M. catarrhalis in the nasal cavity are synergistically correlated in healthy children.18 However, M. catarrhalis carriage rate was, overall, very low in the two groups relative to S. pneumonia carriage rate.
With respect to differences in AOM occurrence between the PCV10 and PCV13 groups, AOM occurrence was significantly lower in the PCV10 group at all visiting times, and the same result was found for two or more AOM recurrence. The effect of PCV on AOM is not as clear as its effect on invasive pneumococcal infection or pneumonia and somewhat more complicated. This is because AOM caused by non-vaccine serotypes and bacterial infections from bacteria other than S. pneumonia, H influenza, and M. catarrhalis may also play a role. Considering the changes in NP carriage, it may be more appropriate to view it as a modification of the AOM occurrence pattern rather than a simple reduction in AOM occurrence.19
Studies on the AOM occurrence and PCV types found varying results. A study that compared AOM and AOM-related diagnosis in areas using PCV13 and PCV10 in Sweden from 2005 to 2014 reported that AOM incidence and ventilation tube insertion rates significantly decreased in areas that used PCV10 compared to areas that used PCV13.20 Furthermore, a Cochrane review on the effect of PCV on preventing AOM noted that PCV reduced S. pneumonia nasopharyngeal carriage, which led to reduced AOM, and that there is a need to monitor the shift toward AOM caused by non-vaccine serotypes and other bacteria. This review also reported that the analysis of 11 trials of PCVs containing 7 to 11 types showed PCV7 and PCV10 were associated with 20% and 53% relative risk reduction (RRR) in pneumococcal AOM, respectively. The effects of these two vaccines on all-cause AOM were not clear.21 A 3-year study conducted in Belgium after the PCV13-to-PCV10 switch found that nasopharyngeal carriage of PCV13 serotypes was decreased, whereas carriage of non-vaccine serotypes was maintained at a high level. Also, of the serotypes included in PCV13, 19F, and 19A, they found predominant serotypes in all groups that received PCV13, PCV10, or a mix of both vaccines and concluded that surveillance on these serotypes is important.22 However, our study did not find increased 19F and 19A serotypes in both groups (Table 1). Studies have shown that the earlier the age of initial AOM is associated with a higher risk of AOM recurrences and persistent otitis media effusion in children.23,24 A cohort study conducted in the Netherlands with three groups, pre-PCV (2004 to 2006), PCV7 (2006 to 2011), and PCV10 (2011 to 2015) groups, reported that time-to-first AOM was significantly longer in the PCV10 cohort compared with the other cohorts.25 In our study, AOM occurrence was analyzed for two time frames: from after the first vaccination to before the booster vaccination and from after the booster vaccination to a 6-month follow-up monitoring period. During the time frame from after the first vaccination to before the booster vaccination, the rate of AOM occurrence was higher in the PCV13 group (20.9%) than the PCV10 group (9.8%). A rate of more than 2 episodes of AOM during the entire study period was also higher in the PCV13 group (11.0%) than the PCV10 group (2.4%). We believe that these results are related to the significant differences in nasopharyngeal flora carriage rate at each visiting time between the two groups.
In conclusion, this study is significant in the field of clinical practice for several reasons. First, we investigated epidemiological data on nasopharyngeal carriage and AOM in the group vaccinated after the implementation of NIP of the two vaccines, PCV10 and PCV13, in 2014. Results of this study may be compared to the epidemiological data before the introduction of PCV7 and after the PCV7 introduction and before the PCV10 and PCV13 introduction. Second, we collected data by prospectively following the subjects at several time points for the assessment of AOM occurrence. To date, the status of AOM occurrence in Korea was reported by retrospective analysis of the data from the Health Insurance Review & Assessment Service, although limitations in the accuracy of the data were apparent due to antibiotic prescription issues in Korea. Third, our study compared the effects of the two vaccines currently used in Korea on nasopharyngeal carriage and AOM.
Limitations of this include lack of analysis for causative agent through ear discharge or myringotomy, rather than from nasopharyngeal aspirate specimens in AOM patients, and lack of consideration of risk factors when analyzing carriage and AOM occurrence rate. And, there is a possibility that a few number of AOM cases might be not reported due to the study limitation. Also, further analyses on non-typeable serotypes were not performed.
We believe that future studies would use our data as a framework. These studies would require a longer period for observation of AOM occurrence patterns and examination of antibiotic-resistance patterns of the predominant non-vaccine serotypes found in nasopharyngeal carriage over time after the introduction of PCVs. Furthermore, studies are required to also determine the effects of PCV10 on NTHi carriage and NTHi infections. Moreover, randomized-controlled trials to compare the effectiveness of these two types of vaccines are additionally needed.
Acknowledgments
The authors would like to thank the infants and their families for participating in the study. The authors would also like to thank the research workers of the Vaccine Bio Research Institute of the Catholic University of Korea for supporting this study.
Funding Statement
This work was supported by the GlaxoSmithKline Biologicals SA under Grant [Study# 117029].
Disclosure of potential conflicts of interest
GlaxoSmithKline Biologicals SA was provided the opportunity to review a preliminary version of this manuscript for factual accuracy but the authors are solely responsible for final content and interpretation. The authors received no financial support or other forms of compensation related to the development of the manuscript.
References
- 1.Ramirez KA, Peters TR.. Streptococcus pneumoniae (Pneumococcus). In: Kliegman RM, St Geme JW, editors. Nelson textbook of pediatrics. 21st ed. Philadelphia (PA): Elsevier; 2020. p. 1436–40. [Google Scholar]
- 2.Wang L, Fu J, Liang Z, Chen J. Prevalence and serotype distribution of nasopharyngeal carriage of Streptococcus pneumoniae in China: a meta-analysis. BMC Infect Dis. 2017;17(1):765. doi: 10.1186/s12879-017-2816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wouters I, Van Heirstraeten L, Desmet S, Blaizot S, Verhaegen J, Goossens H, Van Damme P, Malhotra-Kumar S, Theeten H. Nasopharyngeal s. pneumoniae carriage and density in Belgian infants after 9 years of pneumococcal conjugate vaccine programme. Vaccine. 2018;36(1):15–22. doi: 10.1016/j.vaccine.2017.11.052. [DOI] [PubMed] [Google Scholar]
- 4.Lee EK, Jun JK, Choi UY, Kwon HJ, Kim KH, Kang JH. Nasopharyngeal carriage rate and serotypes of Streptococcus pneumoniae and antimicrobial susceptibility in healthy Korean children younger than 5 years old: focus on influence of pneumococcal conjugate vaccination. Infect Chemother. 2013;45(1):76–84. doi: 10.3947/ic.2013.45.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Linden M, Imöhl M, Busse A, Rose M, Adam D. Bacterial spectrum of spontaneously ruptured otitis media in the era of pneumococcal conjugate vaccination in Germany. Eur J Pediatr. 2015;174(3):355–64. doi: 10.1007/s00431-014-2409-0. [DOI] [PubMed] [Google Scholar]
- 6.WHO . Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – february 2019. Wkly Epidemiol Rec. 2019;94:85–104. [accessed 2019 Aug 25]. https://apps.who.int/iris/bitstream/handle/10665/310968/WER9408.pdf?ua=1. [Google Scholar]
- 7.Kim SM, Hur JK, Lee KY, Shin YK, Park SE, Ma SH, Ahn YM, Kang JH. Epidemiology study of pneumococcal nasal carriage and serotypes among Korea children. Kor J Pediatr. 2004;47:611–16. [Google Scholar]
- 8.Kim KH, Lee JE, Whang IT, Ryu KH, Hong YM, Kim GH, Lee K, Kang ES, Hong KS. Serogroup and antimicrobial resistance of Streptococcus penumoniae isolated from oropharynx in children attending day care center. Kor J Pediatr. 2002;45:346–52. [Google Scholar]
- 9.Hau I, Levy C, Caeymaex L, Cohen R. Impact of pneumococcal conjugate vaccines on microbial epidemiology and clinical outcomes of acute otitis media. Paediatr Drugs. 2014;16(1):1–12. doi: 10.1007/s40272-013-0044-2. [DOI] [PubMed] [Google Scholar]
- 10.Cohen R, Varon E, Doit C, Schlemmer C, Romain O, Thollot F, Béchet S, Bonacorsi S, Levy C. A 13-year survey of pneumococcal nasopharyngeal carriage in children with acute otitis media following PCV7 and PCV13 implementation. Vaccine. 2015;33(39):5118–26. doi: 10.1016/j.vaccine.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Lee HJ Serotype distribution of pneumococci isolated from invasive infections in Korean children (2 year study). Cheongju (Korea): KCDC; 2018. Report No: 11-1352159-000725-01. [accessed 2019 Aug 25]. https://nip.cdc.go.kr/irgd/index.html. [Google Scholar]
- 12.de Gier C, Granland CM, Pickering JL, Walls T, Bhuiyan M, Mills N, Richmond PC, Best EJ, Thornton RB, Kirkham LS. PCV7- and PCV10-vaccinated otitis-prone children in New Zealand have similar pneumococcal and Haemophilus influenzae densities in their nasopharynx and middle ear. Vaccines (Basel). 2019;7(1):14. doi: 10.3390/vaccines7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pomat WS, AHJ VDB, Wana S, Francis JP, Solomon V, Greenhill AR, Ford R, Orami T, Passey M, Jacoby P, et al. Safety and immunogenicity of pneumococcal conjugate vaccines in a high-risk population: a randomized controlled trial of 10-valent and 13-valent pneumococcal conjugate vaccine in Papua New Guinean infants. Clin Infect Dis. 2019;68(9):1472–81. doi: 10.1093/cid/ciy743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hare KM, Smith-Vaughan HC, Leach AJ, Pizzutto SJ, McCallum GB, Chang AB. Reduced nontypeable Haemophilus influenzae lower airway infection in children with chronic endobronchial suppuration vaccinated with the 10-valent pneumococcal H. influenzae protein D conjugate vaccine. Vaccine. 2018;36(13):1736–42. doi: 10.1016/j.vaccine.2018.02.054. [DOI] [PubMed] [Google Scholar]
- 15.Leach AJ, Wigger C, Hare K, Hampton V, Beissbarth J, Andrews R, Chatfield M, Smith-Vaughan H, Morris PS. Reduced middle ear infection with non-typeable Haemophilus influenzae, but not Streptococcus pneumoniae, after transition to 10-valent pneumococcal non-typeable H. influenzae protein D conjugate vaccine. BMC Pediatr. 2015;15:162. doi: 10.1186/s12887-015-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim IS, Ki CS, Kim S, Oh WS, Peck KR, Song JH, Lee K, Lee NY. Diversity of ampicillin resistance genes and antimicrobial susceptibility patterns in Haemophilus influenzae strains isolated in Korea. Antimicrob Agents Chemother. 2007;51(2):453–60. doi: 10.1128/AAC.00960-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park C, Kim KH, Shin NY, Byun JH, Kwon EY, Lee JW, Kwon HJ, Choi EY, Lee DG, Sohn WY, et al. Genetic diversity of the ftsI gene in β-lactamase-nonproducing ampicillin-resistant and β-lactamase-producing amoxicillin-/clavulanic acid-resistant nasopharyngeal Haemophilus influenzae strains isolated from children in South Korea. Microb Drug Resist. 2013;19(3):224–30. doi: 10.1089/mdr.2012.0116. [DOI] [PubMed] [Google Scholar]
- 18.Xu Q, Almudervar A, Casey JR, Pichichero ME. Nasopharyngeal bacterial interactions in children. Emerg Infect Dis. 2012;18(11):1738–45. doi: 10.3201/eid1811.111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagan R. The potential effect of widespread use of pneumococcal conjugate vaccines on the practice of pediatric otolaryngology: the case of acute otitis media. Curr Opin Otolaryngol Head Neck Surg. 2004;12(6):488–94. doi: 10.1097/01.moo.0000145958.12395.ec. [DOI] [PubMed] [Google Scholar]
- 20.Gisselsson-Solen M. Trends in otitis media incidence after conjugate pneumococcal vaccination: a national observational study. Pediatr Infect Dis J. 2017;36(11):1027–31. doi: 10.1097/INF.0000000000001654. [DOI] [PubMed] [Google Scholar]
- 21.Fortanier AC, Venekamp RP, Boonacker CW, Hak E, Schilder AG, Sanders EA, Damoiseaux RA. Pneumococcal conjugate vaccines for preventing acute otitis media in children. Cochrane Database Syst Rev. 2019;5:CD001480. doi: 10.1002/14651858.CD001480.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wouters I, Desmet S, Van Heirstraeten L, Blaizot S, Verhaegen J, Van Damme P, Malhotra-Kumar S, Theeten H; NP carriage Study Group . Follow-up of serotype distribution and antimicrobial susceptibility of Streptococcus pneumoniae in child carriage after a PCV13-to-PCV10 vaccine switch in Belgium. Vaccine. 2019;37(8):1080–86. doi: 10.1016/j.vaccine.2018.12.068. [DOI] [PubMed] [Google Scholar]
- 23.Kvaerner KJ, Nafstad P, Hagen JA, Mair IW, Jaakkola JJ. Recurrent acute otitis media: the significance of age at onset. Acta Otolaryngol. 1997;117(4):578–84. doi: 10.3109/00016489709113441. [DOI] [PubMed] [Google Scholar]
- 24.MLA DH, Fortanier AC, Smit HA, Uiterwaal CPM, van der Ent CK, Schilder A, Damoiseaux RMJ, Venekamp RP, Bruijning-Verhagen P. Impact of early-onset acute otitis media on multiple recurrences and associated health care use. J Pediatr. 2016;177:286–91. doi: 10.1016/j.jpeds.2016.06.066. [DOI] [PubMed] [Google Scholar]
- 25.Fortanier AC, Venekamp RP, Hoes AW, Schilder AGM. Does pneumococcal conjugate vaccination affect onset and risk of first acute otitis media and recurrences? A primary care-based cohort study. Vaccine. 2019;37(11):1528–32. doi: 10.1016/j.vaccine.2019.01.064. [DOI] [PubMed] [Google Scholar]


