ABSTRACT
The immunologic mechanisms underlying the improved serologic responses to heterologous prime-boost avian influenza vaccination are unclear. An exploratory analysis of the immune responses following 1 dose of influenza A/H7N9 inactivated vaccine in subjects who received an influenza A/H7N7 inactivated vaccine (N = 17) 8 years earlier or who were influenza A/H7-naïve (10) was performed. Plasma IL-6 and IL-21 concentrations by ELISA, the frequency of A/H7N7-specific memory B cells and antibody secreting cells by ELISpot, the frequency of circulating T follicular helper cells and the frequency of T cells expressing IL-6 and IL-21 by flow cytometry were assessed at baseline (D1), and 8 days (D9) and 28 days (D29) after vaccination. We assessed the correlation between these measurements and the D29 serologic responses to the boost vaccine. Plasma IL-6 concentration on D9 significantly correlated with the H7N7 and H7N9 hemagglutination inhibition (HAI) antibody levels (P = .03 and 0.02 respectively); and the percentage of T cells expressing IL-21 on D9 significantly correlated with H7N9 HAI antibody seroconversion (P < .001). Significant associations with other immunologic markers were not detected. We detected an association between plasma IL-6 and intracellular IL-21 and serologic responses to heterologous prime-boost avian influenza vaccination. A clarification of the role of these and additional immunologic markers requires larger clinical trials.
KEYWORDS: Prime-boost, avian influenza, vaccine
The zoonotic transmission of influenza A/H7N9 viruses to humans has resulted in 1568 cases over five epidemic waves and has raised concerns about the strain’s potential to cause a pandemic. Novel influenza hemagglutinins (HAs), such as the ones derived from influenza A/H5N1 and A/H7N9, are poorly immunogenic in humans when administered without adjuvants.1,2 The inclusion of an adjuvant and/or heterologous prime-boost vaccination have been demonstrated to improve immune responses to novel influenza vaccines.3,4 The latter approach does not require a match between the priming vaccine and the pandemic strain, which allows for the priming dose to be administered years in advance.5 Moreover, a heterologous prime-boost approach results in rapid and broad serologic responses.6,7 The immunologic mechanisms underlying the improved responses in heterologous prime-boost approaches are not fully elucidated. Nayak et al. demonstrated the recruitment of HA-specific CD4 + T cells upon boosting and the rise in CD4 + T cells to be associated with a rapid antibody response in persons who received a heterologous prime-boost influenza A/H5N1 compared to unprimed subjects.8 A subset of CD4 + T cells, the circulating T follicular helper (TFH) ICOS+CXCR3+CXCR5+ cells, were demonstrated to be associated with improved preexisting antibody responses but not with primary responses to the seasonal influenza vaccine.9 These ICOS+CXCR3+CXCR5+ CD4+ TFH cells expressed interleukin-2 (IL-2), IL-10 and IL-21 upon antigen stimulation. In another study evaluating immune responses following immunization with seasonal influenza vaccines, Mohanty et al. demonstrated a strong correlation between serologic responses to the vaccine and intracellular IL-6 production in monocytes on Days 2 and 6.10
We recently demonstrated that 65% and 41% of individuals who were primed with monovalent inactivated influenza vaccine A/H7N7 (IIV H7N7) developed a hemagglutination inhibition (HAI) antibody titer of 40 or more on Days 9 and 29, respectively, following boosting with IIV H7N9 approximately 8 years later.6 We performed an exploratory analysis to determine the correlation between the serologic responses to the boost vaccination (HAI and microneutralization, [MN], levels) and plasma and intracellular IL-6 and IL-21, the frequency of TFH cells, memory B cells (MBCs) and antibody secreting cells (ASCs).
Methods
Study design. We previously reported the primary results from DMID 13–0044.6 This was a Phase 2 open-label study of the safety and immunogenicity of 1 intramuscular dose of IIV1 A/H7N9 at the 45 µg HA dosage level given to non-pregnant healthy subjects, 19–50 years of age, who had received two doses of IIV1 A/H7N7 at any dosage level 8 years earlier in DMID 07–0023 (NCT00546585), or who were IIV A/H7-naïve subjects. The DMID 07–0023 study vaccine (prime) was an A/H7N7 egg-based, subvirion formalin inactivated reassortant IIV1 produced by Sanofi Pasteur. The HA donor virus was A/mallard/Netherlands/12/2000 (H7N3), the NA donor virus was A/mallard/Netherlands/2/2000 (H10N7), and the internal genes were influenza A/H1N1-derived (Puerto Rico 8/34 (PR8) and A/Johannesburg/82/96).11 The DMID 13–0044 study vaccine (boost) was IIV1 A/H7N9 vaccine manufactured by Sanofi Pasteur using the same manufacturing process as the seasonal IIV, except for a minor modification in the phosphate buffered saline (PBS) diluent in the formulation step. The boost vaccine (lot number UD16397) was derived from the influenza A/Shanghai/2/2013 virus (1st wave strain). Venous blood for immunogenicity assays was drawn from subjects on Day 1 (D1 prior to the boost), Day 9 and Day 29 (D9, D29) post boost vaccination in DMID 13–0044. Stored plasma and peripheral blood mononuclear cells (PBMCs) from DMID 07–0023 drawn 28 days after the second prime vaccine (D56 prime) were also utilized in the immunologic assays except when residual blood volume was limiting (Figure 1). All specimen testing was performed simultaneously in 2018 on samples from both studies.
Figure 1.

Schematic of the two clinical trials and immunologic parameters tested relative to receipt of the vaccines
Ethics statement. The DMID 07–0023 (NCT00546585) and DMID 13–0044 (NCT02586792) studies were reviewed and approved by the Baylor College of Medicine Institutional Review Board. All subjects provided written informed consent prior to study participation. Subjects in DMID 07–0023 on whose stored samples testing was performed provided written consent for future research use of stored samples.
Enzyme-linked immunosorbent assay (ELISA) for plasma IL-6 and IL-21 quantification. Plasma samples were thawed at 37°C, and IL-6 and IL-21 concentrations were determined using commercially available kits (Catalog numbers 88-7066-22 and BMS2043, respectively; ThermoFisher, Waltham, MA USA). Optical Density readings for each sample were compared against standard curves constructed using recombinant standards and converted into protein concentrations through interpolation. The lower limit of detection (LLOD) of each ELISA was 2 pg/ml.
Flow cytometry assays. The percentage of circulating ICOS+CXCR3+ CXCR5+ CD4+ TFH cells and the percentage of T cells expressing intracellular cytokines (IL-6 and/or IL-21) were determined using flow cytometry. The following gating strategy was used to identify TFH cells. First lymphocytes were gated, then singlets, then viable cells, then CD3 + T cells, then CD4 + T cells. TFH cells were identified as ICOS+, CXCR3+, CXCR5+, CD4 + T cells by Boolean logic on the 3 markers: ICOS, CXCR3, and CXCR5 to quantify the triple positive population. PBMCs were stimulated for 24 hours with influenza A antigens (0.125 µg/well for HA proteins and 0.085 µg/well of N7 (influenza A/H7N7 or H7N9-specific)). Antigen-specific responses were compared with negative control (unstimulated cells); anti-CD3 (0.25 µg/mL; Tonbo Biosciences) + anti-CD28 (0.25 µg/mL; BD Biosciences), and PMA (10 ng/mL; Sigma) + ionomycin (2 µg/mL; Sigma) as positive controls. Each well had a total final volume of 200 µL. Following overnight (24 h) stimulation, cells were stained with an amine reactive viability dye (Ghost Dye V-510; Tonbo Biosciences), and surface antibodies (CD4, CXCR3, CXCR5, CD14, CD19, CD56; BD Biosciences). For percent cytokine expression on T cells, the gating strategy was the same as above: lymphocytes, then singlets, then viable cells, then CD3 + T cells. Percent T cells expressing cytokines were determined using fluorescence-minus-one (FMO) controls for each cytokine. For the detection of intracellular cytokines, Golgi Plug and Golgi Stop protein transport inhibitors (1 µg each; BD Biosciences) were added during the final 8 hours of the stimulation period. The antibodies used for intracellular cytokine production were CD3, IL-21, IL-17, and IL-6 (BD Biosciences). A minimum of 70% viability was the threshold for credible data. Cells were acquired on a BD LSR II Fortessa equipped with 4 lasers and 20 detectors (BD Biosciences). All reported values have been corrected for background by subtracting the non-stimulated responses.
Ex vivo ELIspot assay. The frequency of H7N7-specific MBCs, frequency of H7N9-specific ASCs, and frequency of A/H7N7-specific ASCs were measured using the ELIspot assay. Cryopreserved PBMC were thawed, rested at least for 6 hours in R10 (RPMI 1640 with 10% fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 10 mM HEPES, 100 IU of penicillin/ml, and 100 μg of streptomycin/ml), to a final concentration 1 ~ 2 million cells/ml. For MBCs, the cells were stimulated with resiquimod (R848, 1 µg/ml) and recombinant human IL-2 (10 ng/ml) and incubated for 96 hours at 37°C. Each well had a total final volume of 200 µL. The plates were coated with H7N9- or H7N7-specific HA (0.125 µg/well) and NA (0.085 µg/well, HA from an influenza A/H3N2 strain (positive control) and 3 negative control conditions: i) coating antigen + no PBMC, ii) no coating antigen + PBMC, and iii) no coating antigen + no PBMC (only medium and detection antibodies). The plates were then washed 5 times with PBS, and blocked with R10 medium. Resuspended cells were incubated with the above experimental conditions for 16–24 hours. After washing the plates 6 times, biotinylated anti-human IgG antibody was added at a 1:2000 dilution in a volume of 100 µl/well. The liquid was discarded, and the plates were washed with PBS (200 µl/well) for 8 times. The streptavidin-ALP enzyme was added at a dilution of 1:5000 in a volume of 100 µl/well. The excess liquid was then discarded and the plates were washed 12 times. BCIP-NBT (nitro-blue tetrazolium chloride and 5-bromo-4-chloro-3ʹ-indolyphosphate p-toluidine) was added at a volume of 100 µl/well. The reaction was stopped with running water wash 5 times. The plates were dried in darkness and the spots counted with a plate reader (Immunospot® Analyzer, Cellular Technology Limited). The detection of ASCs was performed as described above minus the stimulation with resiquimod and IL-2.
Statistical Analyses. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Comparisons were conducted to evaluate differences in responses between study groups or by seroconversion status using a non-parametric Wilcoxon test for all response types. Correlation coefficients were determined by the Spearman rank-order correlation method. Statistical significance was considered at a level of α = 0.05 and all tests were two-sided. Given the exploratory nature of the analyses, multiple comparisons were not considered, and because missing data were minimal, imputation was not performed.
Results
A total of 27 healthy adult subjects (Group 1: 17 subjects with prior receipt of 2 doses of the A/H7N7 vaccine; and Group 2: 2 placebo recipients from DMID 07–0023, and an additional 8 subjects who were influenza A/H7-naïve) were enrolled between the dates of 12 January 2016 and 01 July 2016. The percentage of subjects with HAI titers ≥40 on D9 and D29 post boosting, respectively, was 65% and 41% in primed subjects and 10% and 0% in unprimed subjects. A detailed description of the serologic responses was reported previously.6
Plasma and intracellular IL-6 and IL-21 measurement. Plasma concentration of IL-6 and IL-21 stayed approximately constant on D56 prime, D1 and D9. Most T cells where cytokine expression was detected expressed a single cytokine. On average, the mean of IL-6+IL-21− and IL-6−IL-21+ ranged from 6.26–7.99% across all time points in both groups. We observed no specific trend in the IL-6+ or IL-21+ T cells on D56 prime, D1, and D9. We detected a correlation between D9 plasma IL-6 concentrations and H7N7 HAI and MN antibody titers (r = 0.42, p = .030 and r = 0.43, p = .026), and a weaker correlation between plasma IL-6 concentrations and H7N9 HAI and MN antibody titers on D9 (r = 0.38, p = .05 and r = 0.35, p = .07; Figure 2). We found no such correlation between plasma IL-21 concentrations and serologic responses to the vaccine (Figure 2). Persons who had higher percentages of T cells expressing IL-21 were more likely to have D9 HAI seroconversion to H7N9 (P = .006) (Figure 3A). There was no observed relationship between T cells expressing IL-6 and H7N9 HAI seroconversion on D9 (p = .17 and 0.282, respectively) (Figure 3B). Similar findings were observed using the H7N7 HA antigen (data not shown).
Figure 2.
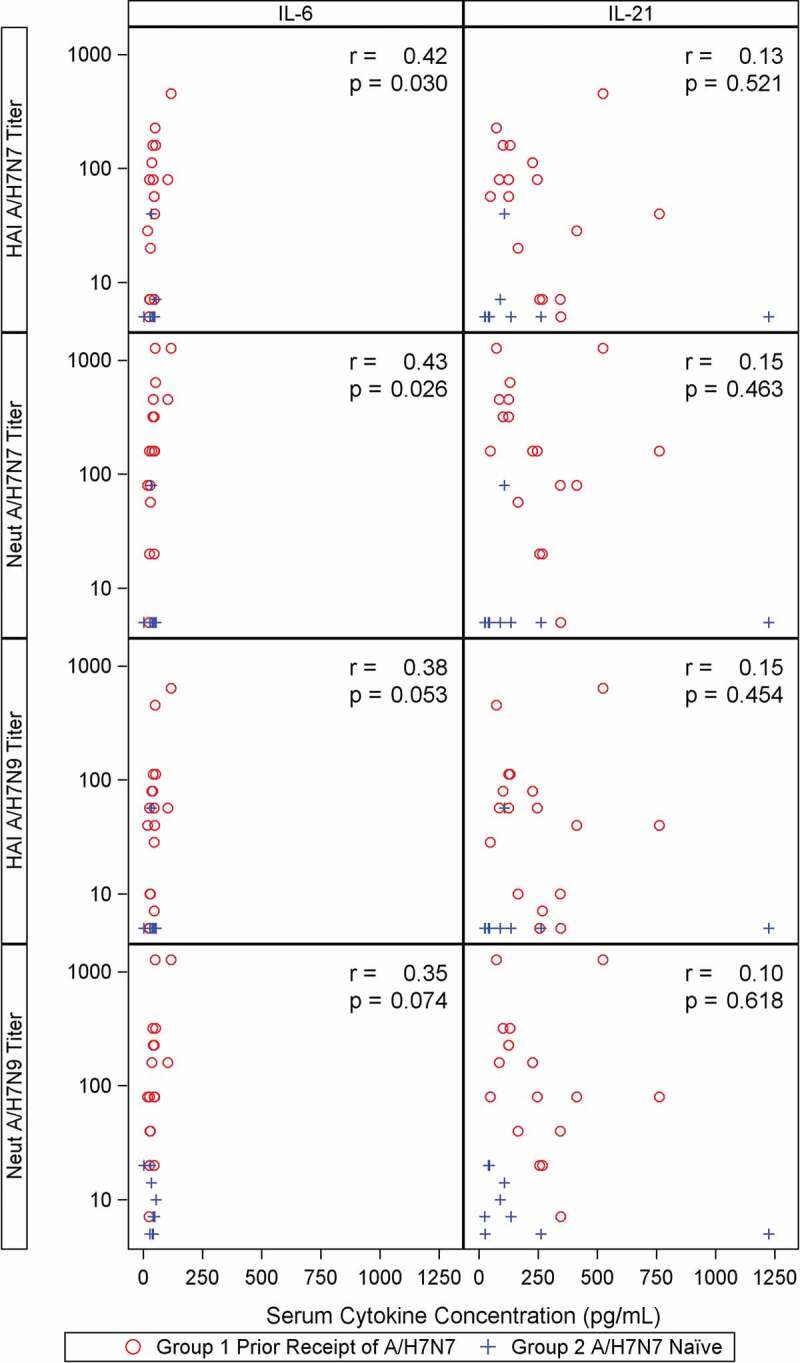
Correlation of Day 9 plasma IL-6 and IL-21 concentrations with HAI and MN antibody titers
Figure 3.
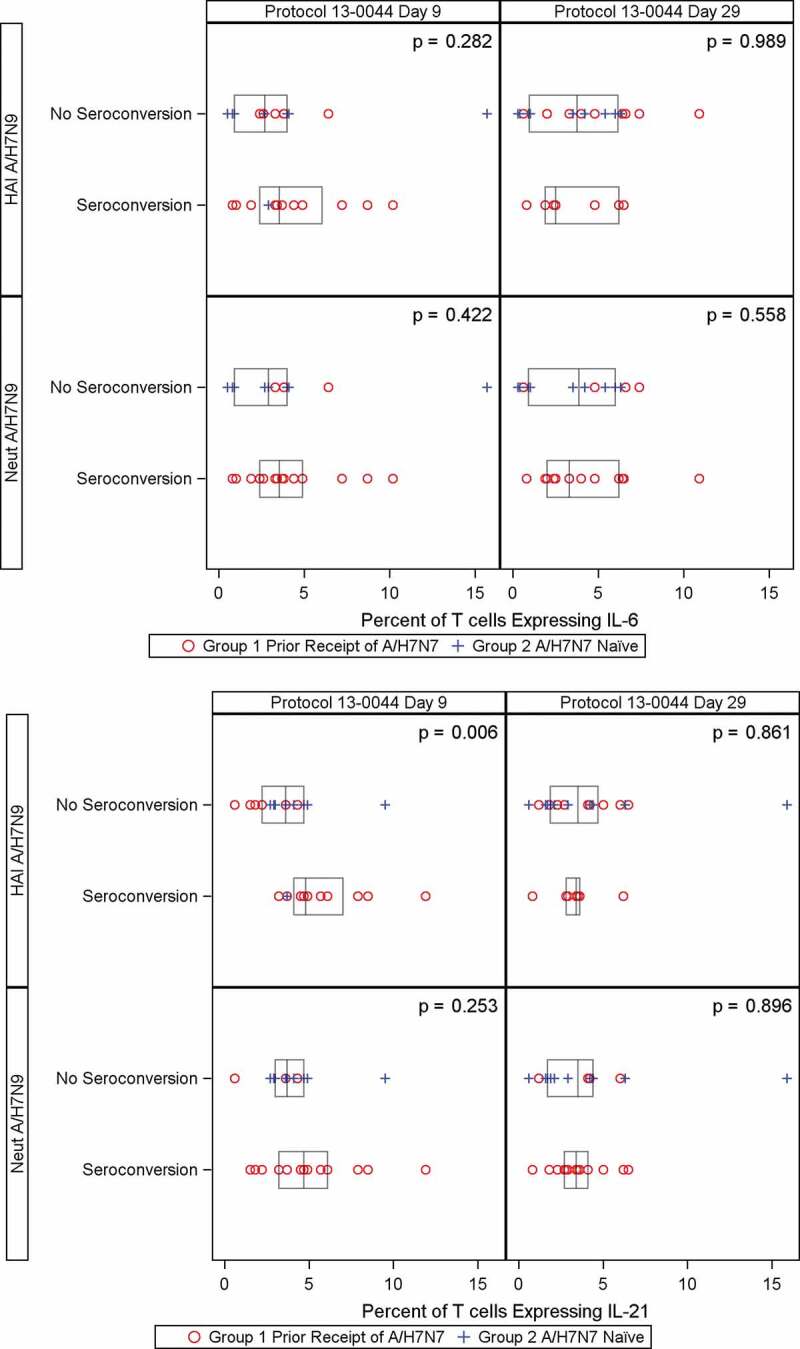
Percent of T-cells expressing IL-21 (Panel A) and percent of T-cells expressing IL-6 (Panel B) on Day 9 and Day 29 by seroconversion of H7N9 HAI and MN antibodies
Frequency of A/H7N7-specific MBCs. We detected no difference in the frequency of circulating H7N7-specific MBCs as measured by ELISpot between Groups 1 and 2 on D56 prime, D1, D9 or D29. There was no correlation between the frequency of circulating H7N7-specific MBCs and H7N9 HAI antibody titers on D1, D9 and D29 (Figure 4A). Similar findings were demonstrated with H7N9 MN antibody titers, and H7N7 HAI antibody titers (data not shown).
Figure 4.
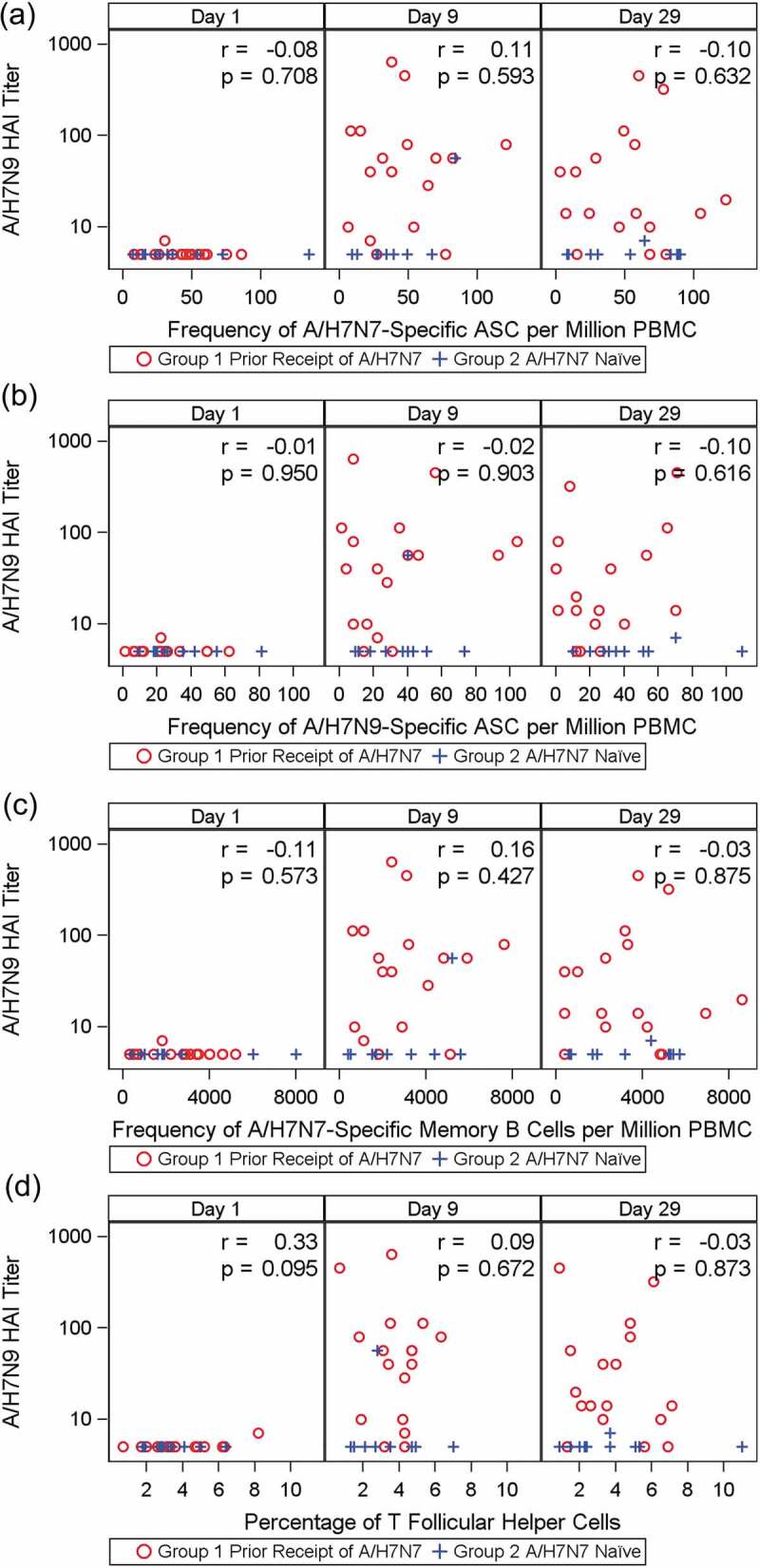
Correlation between the frequency of H7N7-specific MBCs (as number of spot-forming antigen-specific memory B cells million PBMCs, Panel A), H7N7-specific ASCs (number of spot-forming H7N7-specific ASCs per million PBMCs, Panel B), H7N9-specific ASCs (number of spot-forming H7N9-specific ASCs per million PBMCs, Panel C) and circulating TFH cells (Panel D) and H7N9 HAI titers on Day 9 and Day 29
Frequency of A/H7N7-specific ASCs and A/H7N9-specific ASCs. There was no difference in the frequencies of circulating H7N7-specific ASCs as measured by ELISpot between Groups 1 and 2 on D56 prime, D1, D9 and D29, and no statistically significant correlation between the frequencies of circulating A/H7N7-specific ASCs or circulating A/H7N9-specific ASCs and H7N9 HAI antibody titers on D9 and D29. (Figure 4B and 4C).Similar findings were demonstrated with H7N7 HAI antibody titer (data not shown).
Frequency of circulating TFH cells (ICOS+CXCR3+ CXCR5+ CD4+). The frequency of TFH cells was low at all tested time points and did not increase with time with a mean (standard deviation) ranging between 3.32% (1.76) and 4.10% (1.95). We found no correlation between the frequency of TFH cells and H7N9 HAI antibody titers on D9 and D29 (Figure 4D). Similar findings were demonstrated with H7N9 MN antibody titers, and H7N7 HAI antibody titers (data not shown).
Discussion
In this report we present data on the association between cytokine and cellular immune responses and the improved serologic responses observed with heterologous prime boost influenza A H7N7-H7N9 vaccination. We found a significant correlation between the levels of circulating IL-6 levels on D9 and HAI antibody levels; and the frequency of T cells with intracellular IL-21 expression and seroconversion, but detected no significant differences between the primed and unprimed groups’ frequencies of antigen-specific MBCs, circulating TFH cells and antigen- specific ASCs, despite the marked difference in the serologic response.
Evidence of the association between IL-6 levels and improved serologic vaccine responses has been previously suggested albeit not specifically in the setting of a prime-boost heterologous influenza vaccination. For example, it has been demonstrated that following vaccination with seasonal IIV there is a rise in monocyte-driven intracellular IL-6 expression, which is associated with serologic vaccine responses.10 Oil-in-water adjuvanted influenza A/H5N1 inactivated vaccines are associated with a more robust rise in serologic responses when compared to unadjuvanted vaccines.12 Studies have shown that an early rise in serum IL-6 concentration is part of the differential immune response between recipients of adjuvanted vs. unadjuvanted vaccines.13,14 In our study we found no increase in the serum concentration of IL-6 on D9 after vaccination in comparison to baseline. A study suggested that the rise in plasma IL-6 levels following immunization with adjuvant is early and transient.15 The frequency of monocytes expressing IL-6 was not evaluated in our study. Moreover, it is possible that we missed the change in levels by assessing the IL-6 levels on D9, or that in fact our small sample size led to an overestimation of the correlation.
We found the percent of T cells expressing intracellular IL-21 was significantly higher in persons who had seroconversion than in those who did not, but we detected no difference in serum IL-21 concentration between primed and unprimed subjects and between subjects who demonstrated seroconversion and those who did not. These seemingly discrepant findings are explained by the fact that we are measuring two different compartments of the immune system and are in line with the literature: circulating IL-21 levels were not consistently found to correlate with vaccine seroresponse, but the upregulation of IL-21 in CD4 T cells following vaccination has been found to correlate with vaccine seroresponse in several studies.16–19 We detected no trend in the frequency of T-cells expressing IL-6 or IL-21 pre- and post-vaccination boosting. This may be due to our small sample size, or to a nonspecific nature of this finding.
We detected no significant differences in the percentage of antigen-specific MBCs, ASCs and TFH cells between primed and unprimed subjects and between responders and non-responders. Of note, most of the abovementioned measurements were numerically higher in responders compared to non-responders. This raises the possibility that differences could not be detected due to the study’s small sample size, or at least partially because of the poor viability of the plasmablasts following a cycle of freezing and thawing The other possibility is that the lack of difference in the examined cellular components between primed and unprimed subjects is accurate and an alternate unexamined mechanism underlies the difference in immunogenicity. We did not detect MBC responses following the boost vaccination on Day 29, when these responses are expected to occur. The prime vaccination in our study was performed with inactivated unadjuvanted vaccine, and in a heterologous prime-boost study, Galli et al. demonstrated that MBC responses in individuals primed with unadjuvanted inactivated influenza A/H5N1 vaccine following the boost were comparable to those who are not primed despite a robust antibody response. However, those primed with an adjuvanted vaccine did demonstrate a robust MBC response following the boost.20
In conclusion, we found evidence for a correlation between circulating IL-6 levels and the percent of T cells expressing IL-21 and serologic responses following a heterologous prime-boost vaccination with inactivated influenza A/H7N7-H7N9, but no significant associations between the serologic responses and the percentage of antigen-specific MBCs, ASCs and TFH cells between primed and unprimed subjects and between responders and non-responders, likely due to small sample size or due to alternate mechanisms. A larger clinical trial (NCT03738241) evaluating the associations between these immunological markers, as well as additional markers, and serologic responses to a heterologous prime boost influenza A/H7N9 is currently being performed.
Acknowledgments
We would like to thank our study subjects (DMID 07-0023, DMID 13-0044), members of the Safety Monitoring Committee (Dr. Hoonmo Lee Koo, Dr. Jose Serpa-Alvarez, Dr. Jeanne S. Sheffield, Dr. Richard Webby, Dr. Maria Deloria Knoll), Yu Li, Nima Baghaei, The Division of Microbiology and Infectious Diseases at the National Institutes of Health team: Wendy Buchanan, Suzanne Murray, Soju Chang, Valerie Riddle, Teresa Hauguel and Chris Roberts. We are grateful for the manuscript review expertise provided by the following colleagues at BARDA: Corrina Pavetto, MS, Bai Yeh, MBA, Vittoria Cioce, PhD, Christine Oshansky, PhD, and James King, MD. These individuals were not specifically compensated for these contributions.
Funding Statement
Funding for this project was provided by the National Institutes of Health, contracts HHSN272201300015I, HHSN272200800002C, HHSN272201500002C, and HHSN272201400006C. The 2013 H7N9 vaccine was provided by the US Department of Health and Human Services (HHS) Biomedical Advanced Research and Development Authority (BARDA) from the National Prepandemic Influenza Vaccine Stockpile and was manufactured by Sanofi Pasteur.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, Höschler K, Zambon MC. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367:1657–64. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 2.Jackson LA, Campbell JD, Frey SE, Edwards KM, Keitel WA, Kotloff KL, Berry AA, Graham I, Atmar RL, Creech CB, et al. Effect of varying doses of a monovalent H7N9 influenza vaccine with and without AS03 and MF59 adjuvants on immune response: a randomized clinical trial. JAMA. 2015;314(3):237–46. doi: 10.1001/jama.2015.7916. [DOI] [PubMed] [Google Scholar]
- 3.Atmar RL, Keitel WA.. Adjuvants for pandemic influenza vaccines. Curr Top Microbiol Immunol. 2009;333:323–44. doi: 10.1007/978-3-540-92165-3_16. [DOI] [PubMed] [Google Scholar]
- 4.Goji NA, Nolan C, Hill H, Wolff M, Noah DL, Williams TB, Rowe T, Treanor JJ. Immune responses of healthy subjects to a single dose of intramuscular inactivated influenza A/Vietnam/1203/2004 (H5N1) vaccine after priming with an antigenic variant. J Infect Dis. 2008;198:635–41. doi: 10.1086/590916. [DOI] [PubMed] [Google Scholar]
- 5.Goodman JL. Investing in immunity: prepandemic immunization to combat future influenza pandemics. Clin Infect Dis. 2016;62:495–98. doi: 10.1093/cid/civ957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Sahly HM, Atmar RL, Patel SM, Bellamy A, Liu L, Hong W, Zhu H, Guan Y, Keitel WA, DMID 13-0033 Vaccine Study Group. Safety and immunogenicity of an 8 year interval heterologous prime-boost influenza A/H7N7-H7N9 vaccination. Vaccine. 2019;37:2561–68. doi: 10.1016/j.vaccine.2019.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babu TM, Levine M, Fitzgerald T, Luke C, Sangster MY, Jin H, Topham D, Katz J, Treanor J, Subbarao K, et al. Live attenuated H7N7 influenza vaccine primes for a vigorous antibody response to inactivated H7N7 influenza vaccine. Vaccine. 2014;32(50):6798–804. doi: 10.1016/j.vaccine.2014.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayak JL, Fitzgerald TF, Richards KA, Yang H, Treanor JJ, Sant AJ. CD4+ T-cell expansion predicts neutralizing antibody responses to monovalent, inactivated 2009 pandemic influenza A(H1N1) virus subtype H1N1 vaccine. J Infect Dis. 2013;207:297–305. doi: 10.1093/infdis/jis684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5:176ra32. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohanty S, Joshi SR, Ueda I, Wilson J, Blevins TP, Siconolfi B, Meng H, Devine L, Raddassi K, Tsang S, et al. Prolonged proinflammatory cytokine production in monocytes modulated by interleukin 10 after influenza vaccination in older adults. J Infect Dis. 2015;211(7):1174–84. doi: 10.1093/infdis/jiu573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couch RB, Patel SM, Wade-Bowers CL, Nino D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS One. 2012;7:e49704. doi: 10.1371/journal.pone.0049704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–89. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 13.Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–73. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Sobolev O, Binda E, O’Farrell S, Lorenc A, Pradines J, Huang Y, Duffner J, Schulz R, Cason J, Zambon M, et al. Adjuvanted influenza-H1N1 vaccination reveals lymphoid signatures of age-dependent early responses and of clinical adverse events. Nat Immunol. 2016;17(2):204–13. doi: 10.1038/ni.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard LM, Hoek KL, Goll JB, Samir P, Galassie A, Allos TM, Niu X, Gordy LE, Creech CB, Prasad N, et al. Cell-based systems biology analysis of human AS03-adjuvanted H5N1 avian influenza vaccine responses: a phase i randomized controlled trial. PLoS One. 2017;12(1):e0167488. doi: 10.1371/journal.pone.0167488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pallikkuth S, Parmigiani A, Silva SY, George VK, Fischl M, Pahwa R, et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012;120:985–93. doi: 10.1182/blood-2011-12-396648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Ruiz M, Humar A, Baluch A, Keshwani S, Husain S, Kumar D. Baseline serum interleukin-6 to interleukin-2 ratio is associated with the response to seasonal trivalent influenza vaccine in solid organ transplant recipients. Vaccine. 2015;33(51):7176–82. doi: 10.1016/j.vaccine.2015.10.134. [DOI] [PubMed] [Google Scholar]
- 18.Spensieri F, Borgogni E, Zedda L, Bardelli M, Buricchi F, Volpini G, Fragapane E, Tavarini S, Finco O, Rappuoli R, et al. Human circulating influenza-CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc Natl Acad Sci U S A. 2013;110(35):14330–35. doi: 10.1073/pnas.1311998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pallikkuth S, Pilakka Kanthikeel S, Silva SY, Fischl M, Pahwa R, Pahwa S. Upregulation of IL-21 receptor on B cells and IL-21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV-infected persons on combination antiretroviral therapy. J Immunol. 2011;186(11):6173–81. doi: 10.4049/jimmunol.1100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert AK, et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A. 2009;106(10):3877–82. doi: 10.1073/pnas.0813390106. [DOI] [PMC free article] [PubMed] [Google Scholar]


