Abstract
Context
Apigenin displays antioxidant and anti-inflammatory effects. However, effects of apigenin magnesium (AM) complex on these aspects remain unknown.
Objective
This study investigated the effects of AM complex on oxidative stress and inflammatory responses in hydrogen peroxide (H2O2)-induced rat hepatic stellate cells (HSCs).
Materials and methods
The antioxidant and anti-inflammatory effects of AM complex at concentrations of 0.625, 1.25, and 2.5 mg/mL were evaluated, comparing to HSCs treated by H2O2 alone. Cell viability, reactive oxygen species (ROS), the activity of superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA), nitric oxide (NO), interleukin 6 (IL-6), and nuclear factor-kappa B (NF-κB) levels were measured. Moreover, cell apoptosis, mRNA expression levels of transforming growth factor-β (TGF-β), NF-κB, and inducible nitric oxide synthase (iNOS) were assessed.
Results
AM complex significantly inhibited oxidative stress and inflammatory response at concentrations of 0.625, 1.25, and 2.5 mg/mL (IC50 = 1.679 mg/mL). AM complex elevated the survival rate of H2O2-treated HSCs and had no toxic effects on HSCs. AM complex also promoted SOD activity and GSH levels but suppressed ROS, MDA, and NO levels. Additionally, AM complex decreased IL-6 and NF-κB levels, gene expression of TGF-β, NF-κB, and iNOS, as well as induced apoptosis of HSCs.
Discussion and conclusions
Data indicated that AM complex mitigated oxidative stress and inflammatory responses on H2O2-treated HSCs, suggesting that AM complex is a possible candidate for anti-hepatic diseases. Additional efforts, both in vivo and in humans, are required to assess of AM complex as a potential therapeutic drug in liver diseases.
Keywords: MTT, redox system, proinflammatory cytokines, real-time PCR
Introduction
Hepatic diseases, ranging from early steatosis to severe hepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma (HCC), have a high incidence worldwide (Li et al. 2016). Hepatic disease affects more than 10% of the world population and its deadly end-stage usually occurs after cirrhosis and liver cancer. Various aetiologies rank the disease as the fourth or fifth leading cause of death worldwide. Hepatic diseases may be caused by various risk factors, such as obesity, virus, alcohol, drugs, and other toxins (Obert et al. 2015). Since the liver is the central organ for excreting toxins and metabolic nutrients, it is more vulnerable to oxidative stress and inflammation produced from toxins and metabolites in the body (Li et al. 2015). Substantial animal studies and clinical trials have shown that persistent oxidative stress and inflammation in the liver are crucial in the occurrence and development of hepatic illness, regardless of the aetiology. Both persistent oxidative stress and inflammation are regarded as vital factors in the pathogenesis of acute and chronic liver diseases. Oxidative stress refers to cellular or molecular damage caused by reactive oxygen species (ROS), due to the imbalance between ROS production and antioxidant defense responses. Oxidative stress causes hepatic damage through altering biological molecules, such as DNA, proteins, and lipids, and modulating biological pathways associated with gene transcription, protein expression, cell apoptosis, and hepatic stellate cell (HSC) activation (Li et al. 2015). Inflammation is an important part of the immune response, manifested in the fact that inflammatory cells penetrate mainly to the liver to combat pathogen invasion. However, once stimuli persist or are overwhelming, they cause cell damage and lipid accumulation, increasing the risk of severe liver diseases such as steatohepatitis, fibrosis and cancer (Seki and Schwabe 2015). A better understanding of the potential roles of oxidative stress and inflammation in hepatic disease pathogenesis may provide novel opportunities for therapeutic intervention to prevent progression to advanced disease.
Apigenin (4′, 5, 7-trihydroxyflavone), a natural plant flavone, is found in many plants, such as herbs, spices, citrus fruit, vegetables, and other foods (Sung et al. 2016; Liu et al. 2017). Experimental studies demonstrated that apigenin has some pharmacological activity, such as antioxidant (Zhou et al. 2017; Wang et al. 2018), anti-inflammatory (Kilani-Jaziri et al. 2016; Patil et al. 2016), and anticancer (Salmani et al. 2017; Madunic et al. 2018) activities. Our previous studies also have shown that apigenin has a protective effect on hepatic fibrosis induced by CCl4 and non-alcoholic fatty liver disease (NAFLD) induced by a high fat diet (Xi et al. 2014; Shao et al. 2015; Shi et al. 2015; Zhuang et al. 2016; Pan et al. 2017). The mechanism may be related to lipid peroxide inhibition, the clearance of free radicals, and the reduction of inflammatory factors (Xi et al. 2014; Shao et al. 2015; Zhuang et al. 2016). Research indicates that the unique pharmacological and biological effects of apigenin are mainly determined by three hydroxyl groups at its 4′, 5, 7 position and double bond at C2 and C3 (Figure 1). As a natural product with a high effect and low toxicity, apigenin has a great potential for development. However, due to poor hydrophilicity and poor lipophilicity, the clinical application of apigenin is greatly limited. Therefore, in our previous experiments, apigenin and magnesium carbonate in alkaline solution were used to form an apigenin magnesium (AM) complex to improve its solubility (Pan et al. 2014). Considering its partial acidity, strong super delocalization, large π bond conjugation systems of benzopyrone, and the strong coordination ability of the oxygen atoms, the spatial structure of apigenin is conducive to complex formation (Badea et al. 2011; Lu et al. 2011). Magnesium is an essential element in the normal life and metabolism of an organism. Combining apigenin and magnesium ions may form a new complex that improves physical and chemical properties and functions of apigenin. Given the potent antioxidant, free radical scavenging, and anti-inflammatory effects of apigenin, we herein hypothesized that AM complex may have similar or even better activities (Li et al. 2016; Medina et al. 2017).
Figure 1.
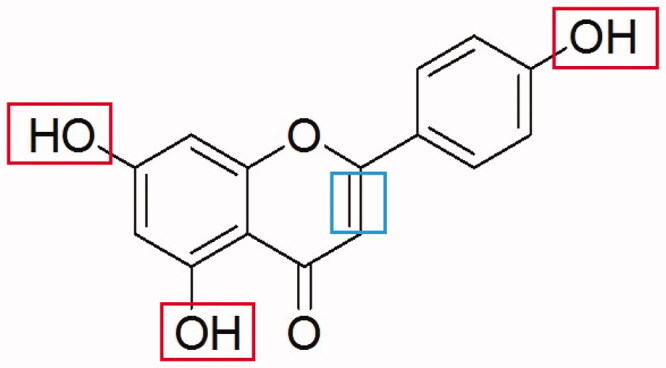
Structural formula of apigenin. The three rectangles indicate the three hydroxyl groups. The square indicates the double bonds at C2 and C3.
Hepatic fibrosis is one of the common pathological bases of all chronic hepatic diseases, and the key factor affecting hepatic fibrosis is the activation of HSCs and its transformation into myofibroblasts (Lucey et al. 2013). Many factors, such as inflammatory factors, changes in extracellular matrix, growth factors, and oxidative stress, can stimulate the activation of HSCs (Tsuchida & Friedman 2017; Wang et al. 2017; Cheng et al. 2019; Yan et al. 2019). For these reasons, HSCs were used to establish the oxidative stress cellular model induced by hydrogen peroxide (H2O2). We used this model to examine the protective effects of AM complex on oxidative stress and inflammatory responses.
Materials and methods
Chemical compounds and reagents
AM complex was prepared as previously reported (Pan et al. 2014) and authenticated with UV, IR, and elemental analysis. The main process was as follows: To a solution of apigenin (1.35 mg, 5 mmol) in EtOH (100.0 ml) was added Mg(Ac)2 (0.71 mg, 5 mmol) in EtOH (100.0 mL). The mixture was adjusted pH to 9.0 with NaOH (0.05 mol/L), then stirred at 50 °C for 1 h, 1000 rpm. The reaction mixture was cooled to room temperature, the residue was washed with EtOH 50 mL twice, thereafter, washed with acetone/acetic acid, and filtered and concentrated at 50 °C, under reduced pressure to give a target compound (AM complex). N-acetylcysteine (NAC), dimethyl sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modification of Eagle’s medium (DMEM) was purchased from Hyclone (Logan, UT, USA). Collagenase Type I, Collagenase Type II, and trypsin were purchased from Worthington Biochemical Corporation (Lakewood, NJ, USA). Enzyme-linked immunosorbent assay (ELISA) kits for detecting interleukin 6 (IL-6) and nuclear factor-kappa B (NF-κB) level were purchased from Elabscience (Wuhan, China). Kits for detecting nitric oxide (NO) levels and 0.25% pancreatic enzymes were purchased from Shanghai Biyuntian Biotechnology Co., Ltd (Shanghai, China). Kits for detecting superoxide dismutase (SOD) activity, glutathione (GSH), and malondialdehyde (MDA) content were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). A Hoechst 33258 stain was purchased from KeyGen Biotech Co., Ltd. (Jiangsu, China). TRIzol reagent was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Separation, culture and treatments of primary HSCs
The liver of a male Sprague-Dawley (SD) rat was perfused with enzymes at two steps for the digestion of collagen, hepatic parenchyma cells, and their released DNA, in situ perfusion with pre-perfusion fluid, and ex vivo perfusion with enzyme-containing perfusion fluid. HSCs were harvested after removing confounding cells by centrifugation (450 g, 5 min). Cell viability was determined after trypan blue staining. HSCs were passaged when in a logarithmic growth period after 0.25% trypsin digestion. After amplification, the cell concentration was adjusted to 6 × 104 cells/mL. HSCs were inoculated on a culture plate and cultured in a humidified incubator in an atmosphere of 95% air and 5% CO2 at 37 °C. Cells incubated with culture medium alone were used as a negative control. HSCs were pre-treated with various concentrations of AM complex (0.625, 1.25, and 2.5 mg/mL) and NAC (0.82 mg/mL) for 0.5 h, and exposed to H2O2 (100 μM) for another 48 h. AM complex and NAC were dissolved in DMEM in all experiments. Each experiment was repeated three times.
Evaluation of toxicity of AM complex in HSCs
HSCs were seeded in 96-well plates (6 × 103/well) and cultured in DMEM with 10% foetal bovine serum (FBS) for 24 h. Cells were treated with AM complex at concentrations of 0, 0.625, 1.25, 2.5, 5.0, and 10 mg/mL for 0.5 h. Then 15 μL of MTT (5 mg/mL) were added to each well and incubated at 37 °C for 4 h. Next, 200 µL of DMSO were added to dissolve the crystals. The spectrophotometric absorbance was measured at 492 nm. The cell survival rate equaled [1−(testOD value−blankOD value)/(controlOD value−blankOD value)] × 100%.
Evaluation of AM complex protection of HSCs against oxidative injury
HSCs were seeded in 96-well plates (6 × 103/well) and cultured in DMEM with 10% FBS for 24 h. HSCs were then treated with AM complex/NAC and H2O2 at indicated concentrations as described above. Cell viability was evaluated using the MTT assay as described above to evaluate AM complex protection against oxidative injury in HSCs.
ROS assessment
The intracellular ROS was determined using a dihydroethidium (DHE) probe (Shanghai Biyuntian Biotechnology Co., Ltd, Shanghai, China). HSCs were seeded in 96-well plates and cultured in DMEM with 10% FBS for 24 h, and then treated with various concentrations of AM complex/NAC and H2O2 as described above. Then, the culture medium was removed, and cells were incubated with DHE solutions (10 μM) for 20 min. After that, cells were washed three times to thoroughly remove excess DHE. Micrographs were taken under a fluorescence microscope (Olympus, Tokyo, Japan). The excitation and emission wavelengths were set at 300 and 610 nm, respectively.
ELISA analysis
HSCs were seeded in 96-well plates and cultured in DMEM with 10% FBS for 24 h. Cells were then treated with AM complex/NAC and H2O2 at indicated concentrations as described above. The cell supernatant was collected to analyse NO, IL-6, and NF-κB levels. The cells were lysed with cell lysate for 1 h and centrifuged at 13200 g for 15 min. Subsequently, the supernatant was collected to detect SOD, GSH, and MDA levels. All measurements were performed with an ELISA kit according to the kit instructions.
Cell apoptosis assay
HSCs were added in 96-well plates and cultured in DMEM with 10% FBS for 24 h. HSCs were then treated with various reagents at indicated concentrations as described above. The morphology of apoptotic HSCs was evaluated using a Hoechst33258 staining kit according to the protocol. Photographs were taken under a fluorescence microscope (Olympus, Tokyo, Japan).
Real-time polymerase chain reaction (PCR) analysis
HSCs were added in 96-well plates and cultured in DMEM with 10% FBS for 24 h. HSCs were then treated with various reagents at indicated concentrations as described above. Total RNA was extracted from HSCs using TRIzol reagent after digestion. The mRNA expressions of TGF-β, NF-κB, and iNOS were detected by real-time PCR. The RNA content was analysed by measuring the optical density at 260 nm. Prime Script RT Master Mix kit was used in cDNA synthesis. According to the protocol provided by the manufacturer, SYBR green premix was used in real-time PCR. Relative expression of target genes was normalized to actin. Results were given as a ratio compared with the vehicle control. The primer sequences used in this study are listed in Table 1.
Table 1.
List of primers for real-time polymerase chain reaction (PCR).
| Target | Full gene name | Primer | Sequence |
|---|---|---|---|
| TGF-β | transforming growth factor β | RP | tgctcgctttgtacaacagc |
| FP | cccgaatgtctgacgtattg | ||
| NF-κB | nuclear factor kappa B | RP | gcatccaccatggaagacaa |
| FP | gctaagtgtaggacactgtc | ||
| iNOS | inducible nitric oxide synthase | RP | gcttacctgggaatagcacc |
| FP | cacggagaacagcagagttg |
Statistical analysis
All data were analysed using SPSS version 19 software. All results were expressed as mean ± standard deviation (SD). Significant differences were evaluated by one-way ANOVA followed by an LSD test, and p < 0.05 indicated a statistically significant result.
Results
Protective effects of AM complex on H2O2-induced oxidative injury in HSCs
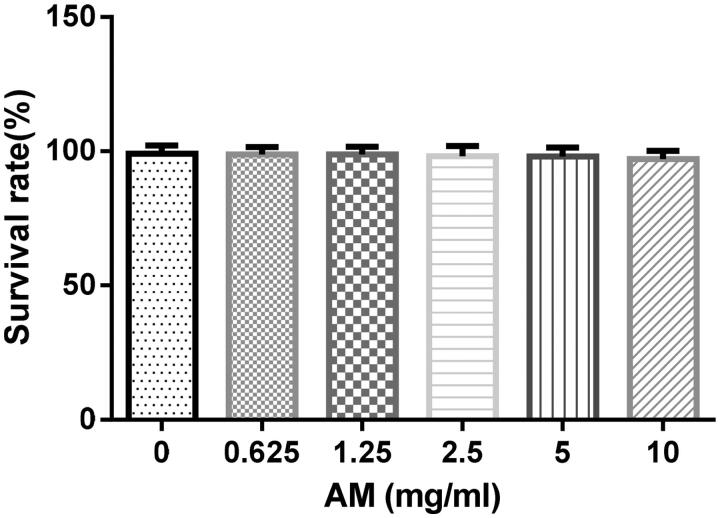
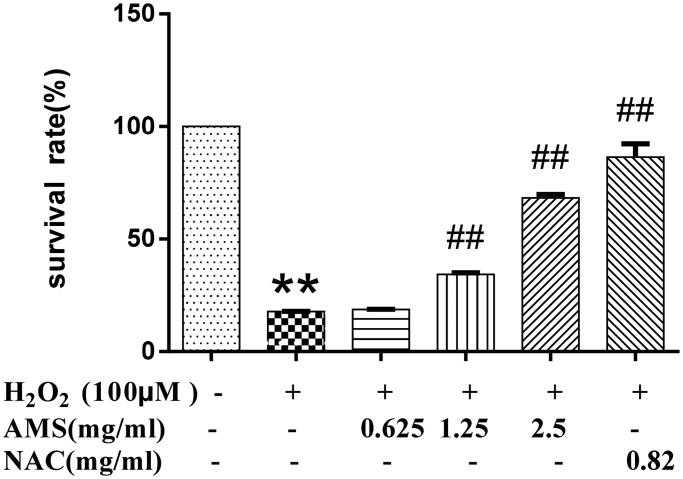
The results showed that AM complex at concentrations up to 10.0 mg/mL did not significantly affect HSCs viability (IC50 = 1.56 × 105 mg/mL), suggesting that AM complex might not be toxic to these cells (Figure 2). In addition, we found that H2O2 at 100 μM caused a significant reduction in HSC survival rate (p < 0.01). Accordingly, H2O2 of 100 μM was used to induce oxidative injury in HSCs for subsequent experiments. Next, we observed that AM complex increased cell survival rate in a concentration-dependent manner in HSCs exposed to H2O2, where concentrations of 1.25 and 2.5 mg/mL were statistically significant (p < 0.01) (Figure 3). In the H2O2-treated proliferation experiment, the IC50 of AM complex was 1.679 mg/mL. Overall, these data collectively indicated that AM complex protected HSCs against H2O2-induced oxidative injury.
Figure 2.
Toxicity of apigenin magnesium (AM) on hepatic stellate cells (HSCs). Values are presented as mean ± SD from three samples and were analysed using one-way ANOVA followed by an LSD test. There was no difference between groups.
Figure 3.
Effects of AM on HSC survival rate in the model of hydrogen peroxide (H2O2, 100 µM) induced oxidative stress. Values are presented as mean ± SD from three samples and were analysed using one-way ANOVA followed by an LSD test. *p < 0.05 and **p < 0.01 compared to the control group, #p < 0.05 and ##p < 0.01 compared with the HSCs that received H2O2 alone.
Antioxidative properties of AM complex in H2O2-treated HSCs
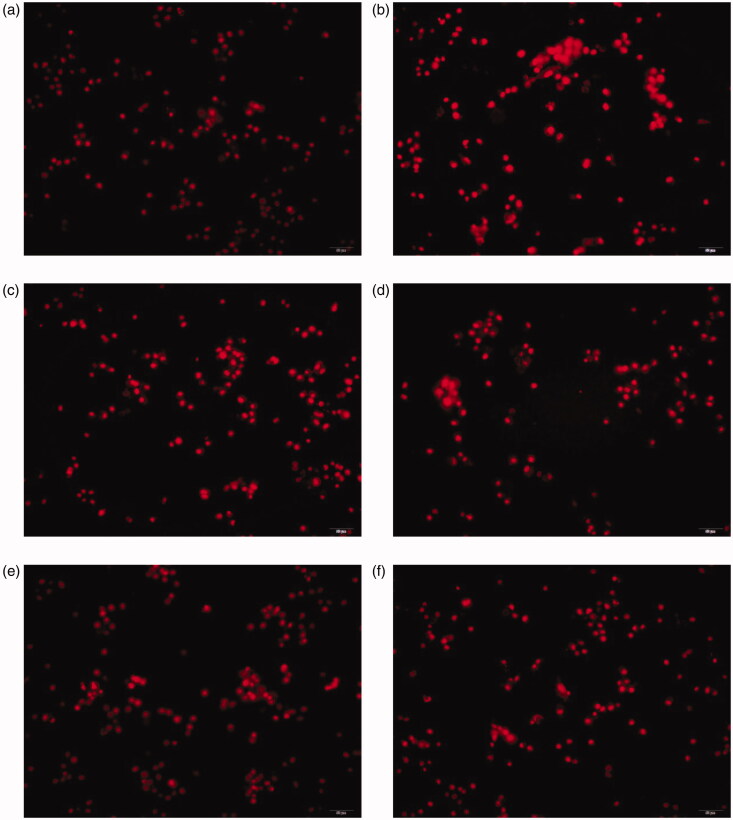
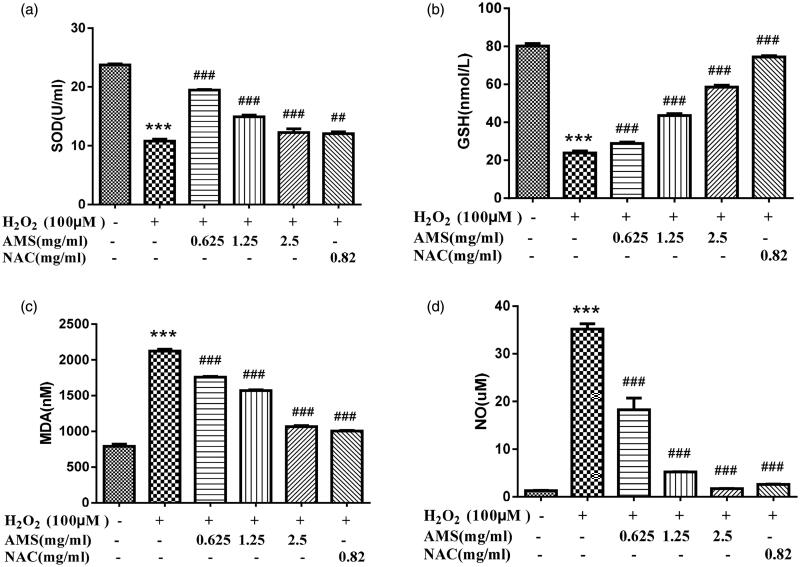
We evaluated the protective effects of AM complex against oxidative stress damage in H2O2-treated HSCs. Fluorescence microscope analyses demonstrated that H2O2-treated HSCs had significantly high intracellular levels of ROS, but AM complex decreased ROS production (Figure 4). Additionally, we measured the classical phase II metabolizing enzyme activity of SOD in H2O2-treated HSCs. The results showed that SOD activity was significantly reduced in H2O2-treated HSCs, but AM complex of 0.625, 1.25, and 2.5 mg/mL all significantly restored SOD activity (p < 0.001, p < 0.001, and p < 0.001, respectively). However, the effects of AM complex on SOD were reduced as the concentration increased. Moreover, the levels of GSH, MDA, and NO, three parameters indicative of oxidative status within cells, were determined. As shown in Figure 5, HSCs challenged with 100 μM H2O2 exhibited significantly reduced GSH levels (p < 0.001) but significantly elevated MDA and NO levels (p < 0.001 and p < 0.001, respectively) compared with the control group levels. However, pretreating the cells with AM complex (0.625, 1.25, and 2.5 mg/mL) for 0.5 h restored GSH levels (p < 0.001) and decreased MDA and NO levels, which were previously significantly increased (p < 0.001 and p < 0.001, respectively). The effects of AM complex on GSH, MDA and NO levels were in concentration-dependent manners. Altogether, these results suggested that AM complex exerted great antioxidative properties in H2O2-treated HSCs.
Figure 4.
Effect of AM complex on intracellular superoxide levels after H2O2 exposure in HSC. (a) control; (b) model; (c) AM complex (0.625 mg/mL); (d) AM complex (1.25 mg/mL); (e) AM complex (2.5 mg/mL); (f) N-acetylcysteine (NAC, 0.82 mg/mL).
Figure 5.
Effects of AM on superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA), and nitric oxide (NO) cellular levels in the model of H2O2 (100 µmol/L) induced oxidative stress. (a) SOD; (b) GSH; (c) MDA; (d) NO. Values are presented as mean ± SD from three samples and were analysed using one-way ANOVA followed by an LSD test. **p < 0.01 and ***p < 0.001 compared to the control group, ##p < 0.01 and ###p < 0.001 compared with the HSCs that received H2O2 alone.
Inhibition of secretion of pro-inflammatory cytokines in HSCs
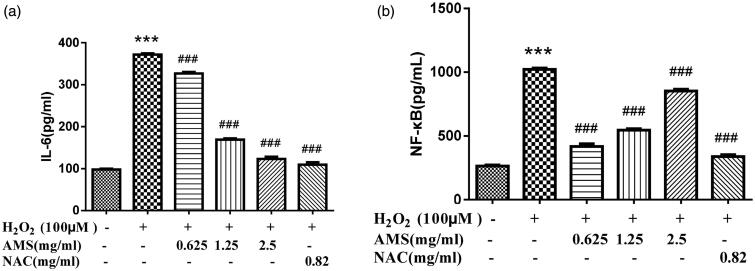
Changing levels of inflammatory factors IL-6 and NF-κB by pretreating with AM complex were determined in H2O2-stimulated HSCs (Figure 6). As expected, H2O2-treatment significantly increased IL-6 and NF-κB production. However, AM complex significantly inhibited IL-6 and NF-κB production at different concentrations (p < 0.001 and p < 0.001, respectively). Specifically, inhibitory effects of AM complex on IL-6 decreased with increasing AM complex concentration, with the strongest effect calculated at 0.625 mg/mL. In contrast, inhibitory effects on NF-κB increased with increasing AM complex concentration, with the strongest effect found at 2.5 mg/mL.
Figure 6.
Effects of AM on interleukin 6 (IL-6) and nuclear factor-kappa B (NF-κB) cellular levels in the model of H2O2 (100 µmol/L) induced oxidative stress. (a) IL-6; (b) NF-κB. Values are presented as mean ± SD from three samples and were analysed using one-way ANOVA followed by an LSD test. **p < 0.01 and ***p < 0.001 compared to the control group, ##p < 0.01 and ###p < 0.001 compared with the HSCs that received H2O2 alone.
Results of cell apoptosis
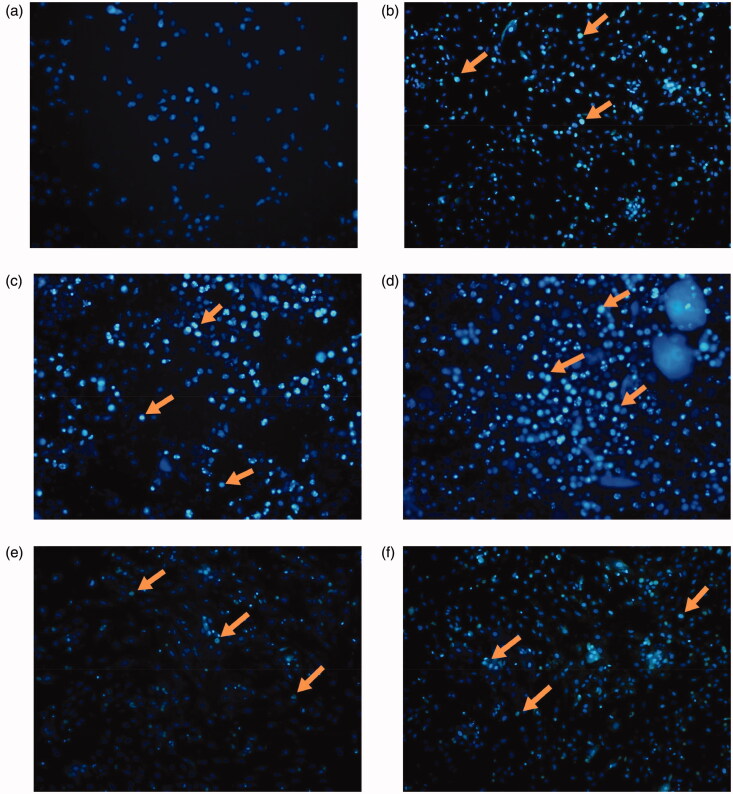
When the effects of AM complex on HSCs apoptosis were examined, our study indicated that HSCs treated with AM complex exhibited significant DNA condensation and fragmentation with brilliant blue staining (Figure 7). The effects on apoptosis rates are associated with concentration, with the strongest effects showing at 1.25 mg/mL.
Figure 7.
Effect of AM complex on apoptosis of HSCs after exposure to H2O2. The arrows indicate apoptotic cells. (a) control; (b) model; (c) AM complex (0.625 mg/mL); (d) AM complex (1.25 mg/mL); (e) AM complex (2.5 mg/mL); (f) NAC (0.82 mg/mL).
mRNA expressions of TGF-β, NF-κB, and iNOS
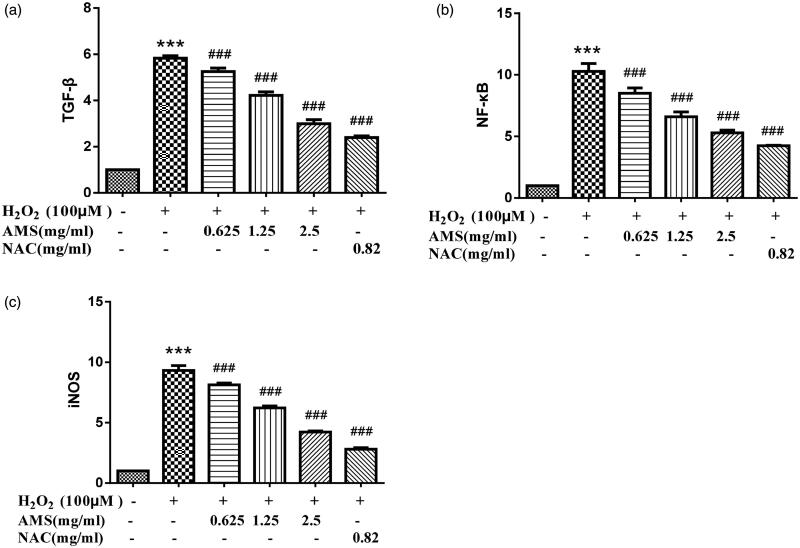
We next examined the roles of TGF-β, NF-κB, and iNOS signalling in AM complex protection of HSCs. Data indicated that AM complex could inhibit these pathways in H2O2-treated HSCs. As shown in Figure 8, mRNA expression of TGF-β, NF-κB, and iNOS increased in H2O2-treated HSCs (p < 0.001, p < 0.001, and p < 0.001, respectively) compared to the control’s mRNA expression. The increased mRNA expressions of TGF-β, NF-κB, and iNOS were reduced in a concentration dependent manner (p < 0.001, p < 0.001, and p < 0.001, respectively) in HSCs pre-treated with AM complex. Collectively, these data suggested that AM complex may inhibit TGF-β, NF-κB, and iNOS expression to protect against H2O2-induced inflammatory responses in HSCs.
Figure 8.
Effects of AM on mRNA expression of transforming growth factor-β (TGF-β), nuclear factor-kappa B (NF-κB), and inducible nitric oxide synthase (iNOS) in the model of H2O2 (100 µmol/L) induced oxidative stress. (a) TGF-β; (b) NF-κB; (c) iNOS. Values are presented as mean ± SD from three samples and were analysed using one-way ANOVA followed by an LSD test. **p < 0.01 and ***p < 0.001 compared to the control group, ##p < 0.01 and ###p < 0.001 compared with the HSCs that received H2O2 alone.
Discussion
Studies have indicated that apigenin exerts a protective effect against oxidative stress (Han et al. 2012; Huang et al. 2013). Apigenin has been shown to attenuate oxidative injury in ARPE-19 cells through activation of the Nrf2 pathway (Xu et al. 2016). Further studies show that apigenin inhibits lipid peroxidation and significantly increases the enzyme antioxidant defense mechanisms in paracetamol-induced hepatotoxicity in rats (Rašković et al. 2017). Additionally, many studies have demonstrated the anti-inflammatory activity of apigenin under a range of pathophysiological conditions. Apigenin promotes different anti-inflammatory pathways, including the p38/MAPK and PI3K/Akt pathways, as well as prevent IKB degradation and nuclear translocation of NF-κB, and reduce COX-2 activity (Huang et al. 2010). Moreover, apigenin may function as a potent anti-inflammatory and antifibrotic agent against bleomycin-induced lung fibrosis (Chen and Zhao 2016). These conclusions were consistent with our previous research (Xi et al. 2014; Shao et al. 2015; Zhuang et al. 2016). However, the effect of AM complex on oxidative stress and inflammatory response remained unknown.
In the present study, the protective effects of AM complex on oxidative stress and inflammatory responses were investigated on H2O2-induced rat HSCs. H2O2 exposure resulted in significant reductions in SOD activity and GSH levels and elevation of ROS, NO, MDA, IL-6, TGF-β, NF-κB, and iNOS levels in HSCs. However, AM complex treatment attenuated the reduction of SOD activity and GSH levels, and decreased ROS, NO, MDA, IL-6, TGF-βGFNF-κB, and iNOS levels in rat HSCs. Collectively, the present results suggest that AM complex exerts an obvious protective effect against H2O2-induced oxidative stress and inflammatory responses in HSCs.
Elucidation of the underlying effect of AM complex on oxidative stress and inflammatory responses is important for developing AM complex as a promising anti-hepatic disease candidate. Oxidative stress is an imbalance between ROS levels and antioxidant capacity in cells. ROS and reactive nitrogen species (RNS) are now being used to describe free radicals derived from molecular oxygen and oxidants derived from NO•, respectively (Turrens 2003; Zorov et al. 2014). SOD is a superoxide scavenger and one of the most important protective enzymes, providing the first antioxidant defense system in various organs and tissues, including the liver. GSH is a key ROS scavenger to protect liver cells from oxidative stress, and GSH depletion in hepatocytes may endanger the antioxidant defense system leading to ROS accumulation. SOD and GSH play an important role in the antioxidant defense system, while MDA is considered to be a main marker of lipid peroxidation in tissues. In addition, all liver cells are capable of producing NO, including hepatocytes, hepatic macrophages, hepatic sinusoidal endothelial cells, and HSCs. NO is a free radical with high toxicity. Studies have shown that NO reacts with O2 to form superoxide nitrite anion ONOO−. ONOO − is a strong oxidant of protein and non-protein sulfhydryl groups, which is 50–1000 times stronger than NO. ONOO − attacks HSCs to initiate a chain reaction of lipid peroxidation. Therefore, NO amplifies the damage effect of O2 and is one of the main free radicals of oxidative stress injury (Thuy et al. 2017). As shown in Figure 5, AM complex inhibited the H2O2-induced increase of ROS through elevating SOD activity and GSH content, and decreasing MDA and NO levels. AM complex thus provides a protective effect against H2O2-induced oxidative stress. The increased SOD and decreased MDA effect of AM complex is better than apigenin (Pan et al. 2017), which is consistent with our assumption. It is possible that the formation of AM complex changes apigenin’s physicochemical property and increases its solubility.
Investigations focussing on the relationship and interaction of oxidative stress and inflammation have attracted great attention as accumulated evidence indicates that they are tightly correlated and orchestrated to drive the pathophysiological pathway of liver diseases (Biswas 2016; Gao and Tsukamoto 2016; Lam et al. 2016). Many ROS or RNS can augment proinflammatory gene expression by provoking an intracellular signalling cascade. In addition, inflammatory cells could produce more ROS/RNS, resulting in exaggerated oxidative stress as an inflammatory lesion. In the present investigation, H2O2-induced oxidative stress caused an inflammatory response by elevating IL-6 and NF-κB levels, and mRNA expression of TGF-β, NF-κB, and iNOS. The production of iNOS, one of the three NO synthetases inside the body, does not depend on the increase of Ca2+ concentration and is expressed only when the cells are stimulated by various cytokines, inflammation, and pathological immune responses and not under normal conditions. It has been reported that TGF-β, IL-6, and NF-κB can induce the mRNA expression of iNOS, thereby producing a large amount of NO (Chen et al. 2003; Vital et al. 2003). These were consistent with our findings in the H2O2-induced oxidative stress model. AM complex can also exhibit anti-inflammatory properties, which may be associated with its protective effect against oxidative stress.
Conclusions
AM complex exerts an obvious protective effect on H2O2-induced oxidative stress and inflammatory response in rat HSCs. Hence, we suggest that AM complex may be developed as a promising hepatoprotective agent. Due to the good results found in this study, additional efforts both in vivo and in humans, are required to assess of AM complex as a potential therapeutic drug in liver disease.
Funding Statement
This work was supported by Zhejiang Administration of Traditional Chinese Medicine [Grant number 2012ZA109]; Hangzhou Health Bureau [Grant number 2012Z011]; and Hangzhou Administration of Science and Technology [Grant number 20170533B79].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Badea M, Olar R, Uivarosi V, Marinescu D, Aldea V, Barbuceanu SF, Nitulescu GM.. 2011. Thermal behavior of some vanadyl complexes with flavone derivatives as potential insulin-mimetic agents. J Therm Anal Calorim. 105:559–564. [Google Scholar]
- Biswas SK. 2016. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016:5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CW, Lee ST, Wu WT, Fu WM, Ho FM, Lin WW.. 2003. Signal transduction for inhibition of inducible nitric oxide synthase and cyclooxygenase-2 induction by capsaicin and related analogs in macrophages. Br J Pharmacol. 140:1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhao W.. 2016. Apigenin protects against bleomycin-induced lung fibrosis in rats. Exp Ther Med. 11:230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Li C, Yang CF, Zhong YJ, Wu D, Shi L, Chen L, Li YW, Li L.. 2019. Methyl ferulic acid attenuates liver fibrosis and hepatic stellate cell activation through the TGF-β1/Smad and NOX4/ROS pathways. Chem Biol Interact. 299:131–139. [DOI] [PubMed] [Google Scholar]
- Gao B, Tsukamoto H.. 2016. Inflammation in alcoholic and nonalcoholic fatty liver disease: friend or foe? Gastroenterology. 150:1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JY, Ahn SY, Kim CS, Yoo SK, Kim SK, Kim HC, Hong JT, Oh KW.. 2012. Protection of apigenin against kainate-induced excitotoxicity by anti-oxidative effects. Biol Pharm Bull. 35:1440–1446. [DOI] [PubMed] [Google Scholar]
- Huang CH, Kuo PL, Hsu YL, Chang TT, Tseng HI, Chu YT, Kuo CH, Chen HN, Hung CH.. 2010. The natural flavonoid apigenin suppresses Th1- and Th2-related chemokine production by human monocyte THP-1 cells through mitogen-activated protein kinase pathways. J Med Food. 13:391–398. [DOI] [PubMed] [Google Scholar]
- Huang CS, Lii CK, Lin AH, Yeh YW, Yao HT, Li CC, Wang TS, Chen HW.. 2013. Protection by chrysin, apigenin, and luteolin against oxidative stress is mediated by the Nrf2-dependent up-regulation of heme oxygenase 1 and glutamate cysteine ligase in rat primary hepatocytes. Arch Toxicol. 87:167–178. [DOI] [PubMed] [Google Scholar]
- Kilani-Jaziri S, Mustapha N, Mokdad-Bzeouich I, El Gueder D, Ghedira K, Ghedira-Chekir L.. 2016. Flavones induce immunomodulatory and anti-inflammatory effects by activating cellular anti-oxidant activity: a structure-activity relationship study. Tumour Biol. 37:6571–6579. [DOI] [PubMed] [Google Scholar]
- Lam P, Cheung F, Tan HY, Wang N, Yuen MF, Feng YB.. 2016. Hepatoprotective effects of Chinese medicinal herbs: a focus on anti-inflammatory and anti-oxidative activities. Int J Mol Sci. 17:465–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Tan HY, Wang N, Zhang ZJ, Lao LX, Wong CW, Feng YB.. 2015. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 16:26087–26124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HX, Wen LJ, Zhong X, Wu L.. 2016. Synthesis, characterization and antioxidant activity of the Cu (II) metal complex with 2-p-hydroxy benzoic-4-p-tolyl imino-5, 7-2 hydroxy benzopyran as a ligand derived from apigenin. Lat Am J Pharm. 35:330–336. [Google Scholar]
- Liu R, Ji P, Liu B, Qiao H, Wang X, Zhou L, Deng T, Ba Y.. 2017. Apigenin enhances the cisplatin cytotoxic effect through p53-modulated apoptosis. Oncol Lett. 13:1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HF, Chie YJ, Yang MS, Lu KW, Fu JJ, Yang JS, Chen HY, Hsia TC, Ma CY, Ip SW, et al. . 2011. Apigenin induces apoptosis in human lung cancer H460 cells through caspase- and mitochondria-dependent pathways. Hum Exp Toxicol. 30:1053–1061. [DOI] [PubMed] [Google Scholar]
- Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW.. 2013. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 19:3–26. [DOI] [PubMed] [Google Scholar]
- Madunic J, Madunic IV, Gajski G, Popic J, Garaj-Vrhovac V.. 2018. Apigenin: a dietary flavonoid with diverse anticancer properties. Cancer Lett. 413:11–22. [DOI] [PubMed] [Google Scholar]
- Medina JJM, Naso LG, Pérez AL, Rizzi A, Okulik NB, Ferrer EG, Williams P.. 2017. Apigenin oxidovanadium (IV) cation interactions. Synthesis, spectral, bovine serum albumin binding, antioxidant and anticancer studies. J Photoch Photobio A. 344:84–100. [Google Scholar]
- Obert J, Cave M, Marsano L.. 2015. Liver diseases. World Rev Nutr Diet. 111:146–150. [DOI] [PubMed] [Google Scholar]
- Pan XW, Tong YX, Zhuang RX, Wang FG, Fang HY, Huang WW, Huang HJ, Yang DH, Pan HY.. 2017. [Protective effect of apigenin nanoemulsion on CCl4-induced hepatic fibrosis in rats]. Chin Arch Trad Chin Med. 35:1482–1484. Chinese. [Google Scholar]
- Pan HY, Wang FG, Zhuang RX, Zhang J, Fang HY.Xi JJ. 2014. [Study on preparation of apigenin-magnesium complex]. Chin Arch Trad Chin Med. 33:337–339. Chinese. [Google Scholar]
- Patil RH, Babu RL, Naveen Kumar M, Kiran Kumar KM, Hegde SM, Nagesh R, Ramesh GT, Sharma SC.. 2016. Anti-inflammatory effect of apigenin on LPS-induced pro-inflammatory mediators and AP-1 factors in human lung epithelial cells. Inflammation. 39:138–147. [DOI] [PubMed] [Google Scholar]
- Rašković A, Gigov S, Čapo I, Kusturica MP, Milijasevic B, Kojic-Damjanov S, Martic N.. 2017. Antioxidative and protective actions of apigenin in a paracetamol-induced hepatotoxicity rat model. Eur J Drug Metab Pharmacokinet. 42:849–856. [DOI] [PubMed] [Google Scholar]
- Salmani JMM, Zhang XP, Jacob JA, Chen BA.. 2017. Apigenin’s anticancer properties and molecular mechanisms of action: recent advances and future prospectives. Chin J Nat Med. 15:321–329. [DOI] [PubMed] [Google Scholar]
- Seki E, Schwabe RF.. 2015. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 61:1066–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao YD, Zhao YM, Pan JM, Jiang XJ, Zhuang RX, Shi TT, Bao JF, Fan XH.. 2015. [Effect of apigenin on inflammatory response and insulin of L02 cell nonalcoholic steatohepatitis model]. Chin J Mod Appl Pharm. 32:1309–1312. Chinese. [Google Scholar]
- Shi TT, Zhuang RX, Zhou HP, Wang FG, Shao YD, Cai ZB.. 2015. [Effect of apigenin on protein expression of PPARs in liver tissues of rats with nonalcoholic steatohepatitis]. Chin J Hepatol. 23:124–129. Chinese. [DOI] [PubMed] [Google Scholar]
- Sung B, Chung HY, Kim ND.. 2016. Role of apigenin in cancer prevention via the induction of apoptosis and autophagy. J Cancer Prev. 21:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuy TTV, Thuy LTT, Yoshizato K, Kawada N.. 2017. Possible involvement of nitric oxide in enhanced liver injury and fibrogenesis during cholestasis in cytoglobin-deficient mice. Sci Rep. 7:41888–41901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T, Friedman SL.. 2017. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 14:397–411. [DOI] [PubMed] [Google Scholar]
- Turrens JF. 2003. Mitochondrial formation of reactive oxygen species. J Physiol (Lond). 552:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital AL, Goncalo M, Cruz MT, Figueiredo A, Duarte CB, Lopes MC.. 2003. Dexamethasone prevents granulocyte-macrophage colony-stimulating factor-induced nuclear factor-kappaB activation, inducible nitric oxide synthase expression and nitric oxide production in a skin dendritic cell line. Mediators Inflamm. 12:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhang H, Wang YY, Song FX, Yuan YF.. 2017. Inhibitory effects of quercetin on the progression of liver fibrosis through the regulation of NF-кB/IкBα, p38 MAPK, and Bcl-2/Bax signaling. Int Immunopharmacol. 47:126–133. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhou RJ, Zhao X, Ye H, Xie ML.. 2018. Apigenin inhibits ethanol-induced oxidative stress and LPS-induced inflammatory cytokine production in cultured rat hepatocytes. J Appl Biomed. 16:75–80. [Google Scholar]
- Xi JJ, Zhuang RX, Wang FG, Shao YD, Fang HY, Cai ZB, Pan HY.. 2014. [Therapeutic efficacy and mechanisms of apigenin on non-alcoholic fatty liver in rats]. Chin Arch Trad Chin Med. 32:1636–1638. Chinese. [Google Scholar]
- Xu XR, Li M, Chen WW, Yu HT, Yang Y, Hang L.. 2016. Apigenin attenuates oxidative injury in ARPE-19 cells thorough activation of Nrf2 pathway. Oxid Med Cell Longev. 2016:4378461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan JY, Shen S, He YT, Li ZQ.. 2019. TLR5 silencing reduced hyperammonaemia-induced liver injury by inhibiting oxidative stress and inflammation responses via inactivating NF-κB and MAPK signals. Chem Biol Interact. 299:102–110. [DOI] [PubMed] [Google Scholar]
- Zhou X, Wang F, Zhou RJ, Song XM, Xie ML.. 2017. Apigenin: a current review on its beneficial biological activities. J Food Biochem. 41:e12376. [Google Scholar]
- Zhuang RX, Pan XW, Fang HY, Wang FG, Shao YD, Shi TT, Xi JJ, Pan HY.. 2016. [Effects of apigenin nanoemulsion on hepatic fibrosis and tissue levels of MMP-1,TIMP-1 in rats]. Chin J Clin Pharm Therap. 21:1122–1125. Chinese. [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ.. 2014. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 94:909–950. [DOI] [PMC free article] [PubMed] [Google Scholar]