Abstract
Context
Socheongryongtang is a traditional Korean medical prescription used to treat pulmonary diseases.
Objective
This study investigated the therapeutic mechanism of socheongryongtang for pulmonary diseases.
Materials and methods
Seventy BALB/c mice were used: control, 0.8 mg/kg/study LPS intranasal instillation, 1 mg/kg/day Spiriva oral administration for five days, two socheongryongtang groups (150 or 1500 mg/kg/day orally treatment for five days). To illuminate the anti-COPD mechanism, several factors were evaluated such as WBC and differential counts in BALF and IgE in serum, morphological changes, and changes of COPD-related cytokines (TNF-α, IFN-γ, TGF-β) and chemokines (CXCL1, CCL-2, CCR2) in the lung. In order to confirm the statistical significance, all results were compared under p < 0.01 and p < 0.05.
Results
LPS induced a high level of WBC, neutrophils and eosinophils in our in vivo study. Additionally, COPD related cytokines and chemokines such as TNF-α, IFN-γ, TGF-β, CXCL1, CCL-2 and CCR2 were induced by LPS. Compared to the LPS treatment group, socheongryongtang significantly controlled the level of WBC, neutrophils and eosinophils as well as the level of IgE. It effectively down-regulated the morphological changes, such as fibrosis near bronchoalveolar spaces, small airway destruction (emphysema), etc. It also inhibited the levels of COPD-related cytokines (TNF-α, IFN-γ, TGF-β) and chemokines (CXCL1, CCL-2, CCR2) compared to the LPS treatment group. In particular, socheongryongtang significantly down-regulated the levels of TNF-α, IFN-γ, and CCR2.
Conclusions
Socheongryongtang controlled COPD, but as it has been used as a prescription for respiratory disease, we should additionally evaluate the therapeutic effects against various pulmonary diseases.
Keywords: Traditional prescription, chronic obstructive pulmonary disease, TNF-α, IFN-γ
Introduction
According to a report by the World Health Organization (WHO) in 2017, chronic obstructive pulmonary disease (COPD) was the third most common cause of death among the top 10, accounting for about 5% of all deaths. The representative pulmonary changes in COPD patients are the infiltration of inflammatory cells near small airway spaces (Franklin et al. 1956; Ludwig et al. 1985), hypersecretion of mucous (Forbes et al. 2009), fibrosis and alveolar wall destruction (emphysema) (Pesci et al. 1998; O’Donnell et al. 2004; Hopkinson and Polkey 2009), etc.
COPD has various causes, including tobacco smoke, occupational factors and environmental pollution, as these factors simulate inflammation on the pulmonary system (O’Byrne 2007; Nowrin et al. 2014; Ramos et al. 2014; Bagdonas et al. 2015). In particular, the main cause of COPD occurrence is tobacco smoke, and the most vulnerable age groups to COPD are those aged 40 or older (O’Byrne 2007; Bagdonas et al. 2015; WHO 2017). When COPD severity increases, patients may experience difficulty freely exchanging gas (O2/CO2) between the lung and the small vessels. This situation causes apnoea and eventually leads to death (O’Donnell et al. 2004). In the early stage of COPD, patients just cough, while in the next stage, pathological changes in the lung can be accompanied by morphological changes of goblet cells and smooth muscles, fibrogenesis, alveolar capping by mucous, etc. (Hogg et al. 2004; Hogg and Timens 2009), and in the final stage, apnoea in patients can lead to death (Butler et al. 2018).
The relationship between COPD and cytokines/chemokines has been reported, including cytokines such as interleukin (IL)-8, tumour necrosis factor (TNF)-α, interferon (IFN)-γ, transforming growth factor (TGF)-β, CC-chemokine ligand (CCL)-2, CC-chemokine receptor (CCR)-2, CXC-chemokine ligand (CXCL)1, CXCL9, CXCL10, CXCL11, etc. (Keatings et al. 1996; de Boer et al. 2000; Barnes 2008, 2009). TGF-β induces pulmonary system fibrosis and IFN-γ stimulates the release of various cytokines and chemokines (Barnes 2009). TNF-α induces neutrophil proliferation, which is stimulated by IL-9, and increased neutrophils could destroy small airway walls (Keatings et al. 1996; Barnes 2008). Chemokines are one of the important factors that induce pulmonary inflammation in COPD patients. CCR2 is the receptor of CCL-2, and CCL-2 stimulates the activation of monocytes and T cells in the lungs of COPD patients, then accumulates macrophages to the lung (de Boer et al. 2000), and finally, CXCL1, which is released by macrophages, induces emphysema via neutrophil proliferation (Barnes 2009).
As the respiratory systems in COPD patients are affected by fibrosis and emphysema, it is extremely difficult to completely cure COPD (Rabe et al. 2007), hence anti-COPD drugs usually only aim to suppress the symptoms of COPD, such as mucous hypersecretion, cough, etc. (Donnelly and Barnes 2006). The more serious problem is that the drugs which have been used have serious adverse effects (McEvoy and Niewoehner 1997; Kim et al. 2014), and because of this, there have recently been trials to develop anti-COPD candidates without side effects that are more effective.
In order to find the safe and effective anti-COPD drug, many studies have been reported that evaluated the therapeutic effects from natural products using various animal models (Ghorani et al. 2017; Boskabadi et al. 2018, 2019; Saadat et al. 2019). Socheongryongtang is a traditional prescription for respiratory diseases described in Donguibogam, which was written by Jun Huh, a clinician (Huh 2002). Donguibogam is the encyclopaedia on traditional Korean medicine that was published about 600 years ago in Korea, and in it there are several remedies for pulmonary diseases such as macmoondongtang, socheongryongtang, chungsangbohwahwan, samsoyum, etc. Socheongryongtang, one of these, is made of several herbs’ leaves and roots extracts such as Pinellia ternata, Ephedra sinica, Paeonia lactiflora, Schisandra chinensis Baill, Zinger rhizome, Glycyrrhiza radix, Cinnamomi ramulus and Anotogaster sieboldii. Although to date there has been no evidence of their therapeutic mechanisms, they have been recently and broadly used as pulmonary disease medicines in Korea.
This study elucidates the anti-COPD mechanism of socheongryongtang and also confirms the therapeutic potency of socheongryongtang against COPD.
Materials and methods
Socheongryongtang and animal experiment
Socheongryongtang is one of the over-the-counter medicines (OTCs) manufactured by Hankuk Inspharm, Ltd. (Jeonnam, Korea), and it was produced by the prescription of socheongryongtang in the Korean Traditional Medical Encyclopaedia, Donguibogam (Huh 2002). The animal schedule employed herein was based on the modified Kobayashi’s method (Kobayashi et al. 2013), the COPD model could be established by 72 h after LPS intranasal instillation (Lee et al. 2018). The same study was conducted twice for repeatability and 35 mice were used per study. Seventy female BALB/c mice (4 weeks old) were purchased from Samtako (Osan, Korea), and they were acclimated for seven days after they arrived in the animal facility. All animals were classified into five groups (14 mice per group) depending on the agent treatment, such as saline for five days (control) without LPS treatment, 0.8 mg/kg LPS intranasal instillation (induction) once for the study, 1 mg/kg Spiriva (NDC 0597-0075-41, Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT) for five days (positive control) with LPS treatment, 150 mg/kg socheongryongtang for five days with LPS treatment, and 1500 mg/kg socheongryongtang for five days with LPS treatment. The dose of socheongryongtang given to the animals was determined based on the recommended therapeutic dose for humans. The route of administration for all agents was oral, except for LPS treatment (intranasal instillation). At day 3, after each agent treatment, 0.8 mg/kg LPS was instilled through the intranasal route, and at day 6, all animals were anaesthetized with 50 mg/kg Zoletil (Virbac, Carros, France).
Ethics statement
All experiments were approved by the Institutional Animal Care and Use Committee at Dongshin University (animal study approval no. 2014-08-01-1).
Bronchoalveolar fluid (BALF) and serum analysis
BALF and serum analysis were conducted based on our previous study (Lee et al. 2018). The same animal study was conducted twice for the verification of repeatability. Three mice in the first study were used for BALF and serum evaluation, and the other four mice were used for the other studies, such as morphological analysis (H&E stain and Masson’s trichrome stain) and specific protein expression, which were related with COPD occurrence (IHC). On schedule, BALF was collected from all mice after they were anaesthetized with 50 mg/kg Zoletil (Virbac, Carros, France) and their tracheas were cannulated with disposable animal feeding needles. Lavages were performed with three 0.4 mL aliquots of cold phosphate-buffered saline (PBS). BALF samples were collected and immediately centrifuged at 3000 rpm for 5 min (Sorvall Legend Micro 17R, Thermo Fisher Scientific, Inc., Waltham, MA). The cell pellets were resuspended in PBS for total and differential cell counts. The numbers of total cells and differential cells were counted with a Hemavet Multi-Species Hematology System (Drew Scientific Inc., Waterbury, CT). Some animals after collecting cells (as well as the others which were not used for cell collection) were sacrificed with an additional Zoletil injection. The IgE levels in the serum were measured using a specific mouse IgE ELISA kit (BD Bioscience, catalog number 555248, San Jose, CA) according to the manufacturer’s protocols.
Histopathological analysis
Histopathological analysis was conducted as previously described (Lee et al. 2018). Lung tissues were fixed in 10% (v/v) formaldehyde solution, dehydrated in a graded ethanol series (99.9%, 90%, 80% and 70%), and embedded in paraffin. A total of eight animals from two studies were used for histological analysis which was compared with the histopathological changes between five groups, with four animals selected from each group. Lung tissues were sectioned (4 µm) longitudinally and stained with H&E and Masson’s trichrome.
Real-time polymerase chain reaction (RT-PCR)
In order to evaluate the changes of cDNA levels of TNF-α, IFN-γ, TGF-β, CXCL1, CCL-2 and CCR2 (Lee et al. 2018), which are related to COPD occurrence. Total RNA was extracted from the lung using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Total RNA (100 ng) was used as a template for the reaction. Primers were synthesized for RT-PCR, as shown in Table 1. The RT-PCR cycles consisted of denaturation at 95 °C for 5 s and annealing/extension at 65 °C for 30 s for 40 cycles using QTOWER2.2 (Analytik Jena AG, Jena, Germany).
Table 1.
Primer sequences for RT-PCR.
| Genes | Primer sequences | |
|---|---|---|
| TNF-α | Forward | 5o-CTGAGTTCTGCAAAGGGAGAG-3C |
| Reverse | 5e-CCTCAGGGAAGAATCTGGAAAG-3C | |
| IFN-γ | Forward | 5o-GGCCATCAGCAACAACATAAG-3G |
| Reverse | 5e-GTTGACCTCAAACTTGGCAATAC-3G | |
| TGF-β | Forward | 5o-CTTCAGCTCCACAGAGAAGAACTGC-3C |
| Reverse | 5e-CACAATCATGTTGGACAACTGCTCC-3C | |
| CXCL 1 | Forward | 5o-ATCCAGAGCTTGAAGGTGTTG-3A |
| Reverse | 5e-GTCTGTCTTCTTTCTCCGTTACTT-3G | |
| CCL-2 | Forward | 5o-AACTCTCACTGAAGCCAGCTCT-3A |
| Reverse | 5e-CGTTAACTGCATCTGGCTGA-3C | |
| CCR2 | Forward | 5o-ACACCCTGTTTCGCTGTAGG-3A |
| Reverse | 5e-GATTCCTGGAAGGTGGTCAA-3G | |
| GAPDH | Forward | 5o-GTGGAGTCATACTGAACATGTAG-3G |
| Reverse | 5e-AATGGTGAAGGTCGGTGTG-3A | |
Immunohistochemical (IHC) analysis
IHC analysis was conducted as previously described (Lee et al. 2018). Deparaffinized tissue sections were treated with 3% hydrogen peroxide in methanol for 10 min in order to remove endogenous peroxidase. Antigen retrieval was carried out with sodium citrate buffer (0.1 M) using the microwave method. Next, the slides were incubated with normal serum so as to block non-specific binding, then incubated for 1 h with primary antibodies (diluted 1:100 to 1:200) such as TNF-α (MyBioSource, San Diego, CA), IFN-γ (sc-74104, Santa Cruz Biotechnology, Dallas, TX), TGF-β (MBS462142, MyBioSource, San Diego, CA), CXCL1 (PAB8798, Abnova, Taipei, Taiwan), CCL-2 (PAB16617, Abnova, Taipei, Taiwan) and CCR2 (Biorbyt, orb137093, Cambridge, UK). The slides were incubated for 10 min with biotinylated secondary antibodies (PK-7800, Vector Laboratories, Burlingame, CA) and horseradish peroxidase-conjugated streptavidin. Finally, signals were detected using the 3,3-diaminobenzidine tetrahydrochloride substrate chromogen solution, and the cells were counterstained with Mayer’s haematoxylin.
Statistical analysis
Results are expressed as mean ± standard deviations (SDs). Group differences were evaluated by one-way analysis of variance, followed by Dunnett’s multiple comparisons test. p < 0.01 and p < 0.05 were considered to be statistically significant.
Results
Socheongryongtang suppressed the proliferations of both WBC and inflammatory cells in BALF
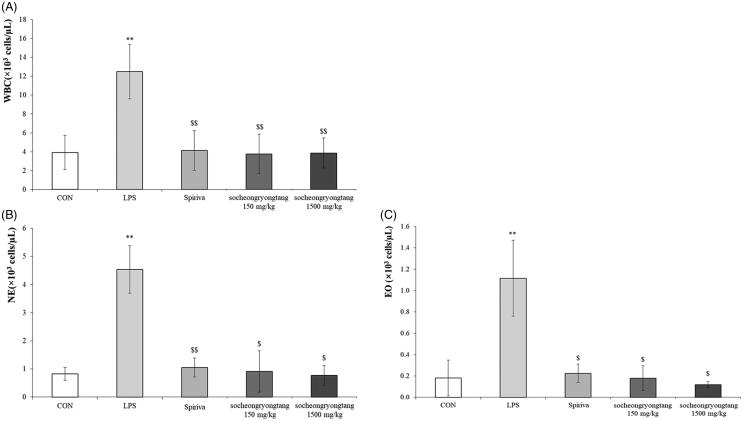
Neutrophil is one of the most important biomarkers for COPD (Pesci et al. 1998), as the neutrophil population in COPD typically surges. In the LPS-induced COPD animal model, the level of WBC was increased by about three times compared to that in the control group (p < 0.001), and the population of WBC in the Spiriva treatment group was similar to those in the socheongryongtang treated groups (Figure 1(A), p < 0.001). The level of neutrophils in the LPS treatment group was about five times higher than those in the control group (p < 0.001), Spriva treatment group (p < 0.001) or socheongryongtang treatment groups (Figure 1(B), p < 0.05), and the level of eosinophils was similar to that of neutrophils (Figure 1(C), p < 0.05). Socheongryongtang dramatically suppressed the LPS-induced proliferations of WBC, neutrophils and eosinophils in BALF.
Figure 1.
Socheongryongtang significantly controlled the number of white blood cells as well as the populations of both neutrophils and eosinophils in bronchoalveolar fluid. (A) Socheongryongtang completely inhibited the level of WBC, which had been increased by LPS intranasal infiltration, and this inhibition was on a similar level as that by Spiriva treatment. The proliferation of (B) neutrophil and (C) eosinophil were thoroughly down-regulated by socheongryongtang treatment. Each bar represents the mean ± SD (N = 6). **p < 0.01 vs. control group; $p < 0.05 vs. LPS intranasal instillation group; $$p < 0.01 vs. LPS intranasal instillation group.
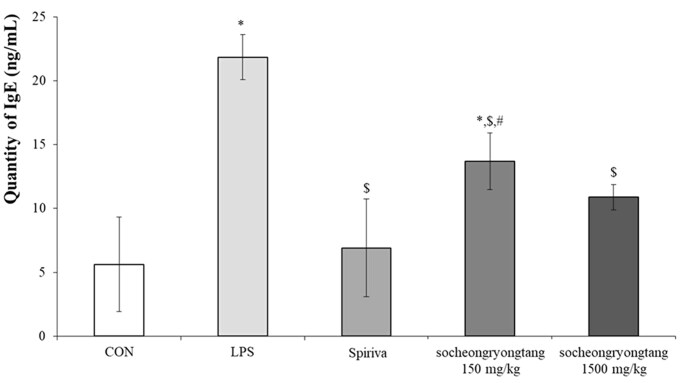
Socheongryongtang dose-dependently inhibited the increment of IgE in serum
One of the key factors which could help reach a verdict regarding COPD is IgE, as a surge of IgE is usually observed in COPD patients (Bożek and Rogala 2018). In order to measure the change of IgE, the level of IgE in serum was measured (Figure 2). In the LPS-induced COPD group, the quantity of IgE (21.9 ± 1.76 ng/mL of serum) increased by about five times compared to that in the control group (5.6 ± 3.70 ng/mL of serum, p < 0.05), while the level of that in the Spiriva treatment group (6.9 ± 3.83 ng/mL of serum) was similar to that in the control group. In the 150 mg/kg socheongryongtang group, the quantity of IgE (13.7 ± 2.21 ng/mL of serum) was significantly and down-regulated (p < 0.05), but in the 1500 mg/kg socheongryongtang group, it (10.9 ± 0.99 ng/mL of serum, p < 0.05) was similar to that in the Spiriva treatment group.
Figure 2.
Socheongryongtang suppressed the IgE level in serum. Each bar represents the mean ± SD (N = 6). *p < 0.05 vs. control group; $p < 0.05 vs. LPS intranasal instillation group; #p < 0.05 vs. Spiriva treatment group.
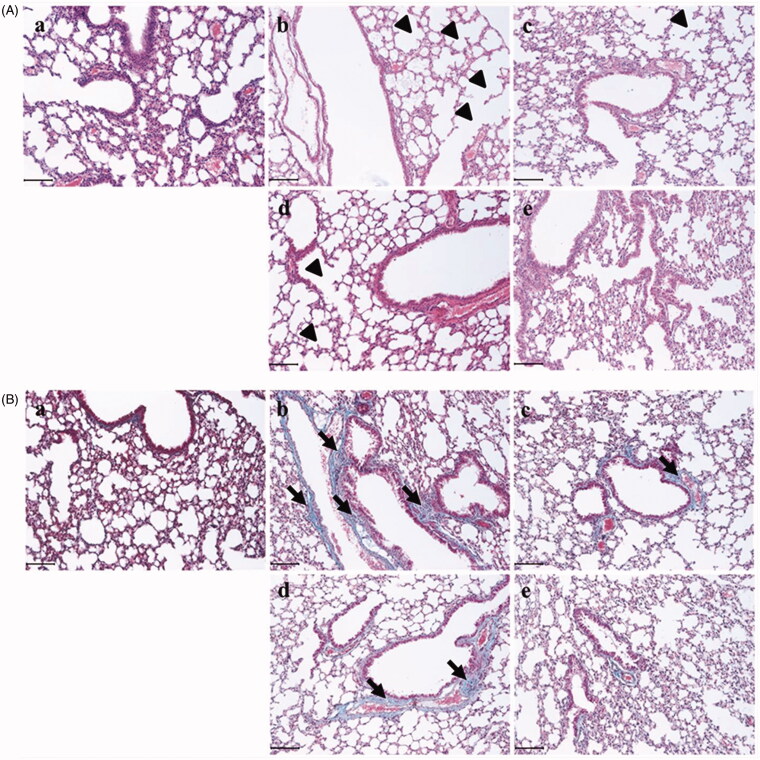
Socheongryongtang effectively inhibited the typical changes of COPD in the respiratory system
In order to evaluate the anti-COPD effect of socheongryongtang, the morphological changes in the pulmonary system were measured using H&E staining (Figure 3(A)) and Masson’s trichrome staining (Figure 3(B)). In the LPS-induced COPD group, alveolar wall destructions (Figure 3(Ab)) and fibrosis (Figure 3(Bb)) were observed at several points, but in the Spiriva treatment group, these changes were recovered (Figure 3(Ac, Bc)). Socheongryongtang treatment significantly inhibited the LPS-induced changes in the lung (Figure 3(Ad, Ae, Bd, Be)).
Figure 3.
Socheongryongtang prevented the LPS-induced morphological changes in the pulmonary system. (A) In H&E staining, socheongryongtang suppressed the morphological changes related to COPD, such as inflammatory cell infiltration near the small airway and airway wall destruction (emphysema). (B) In Masson’s trichrome staining, socheongryongtang decreased the fibrogenicity near the bronchoalveolar area. N = 6. Scale bar: 100 µm. (a) Control; (b) 0.8 mg/kg LPS intranasal instillation; (c) 1 mg/kg Spiriva treatment for five days after LPS instillation; (d) 150 mg/kg socheongryongtang treatment for five days after LPS instillation; (e) 1500 mg/kg socheongryongtang treatment for five days after LPS instillation. Arrow head, emphysema; arrow, fibrosis. Magnification, ×200.
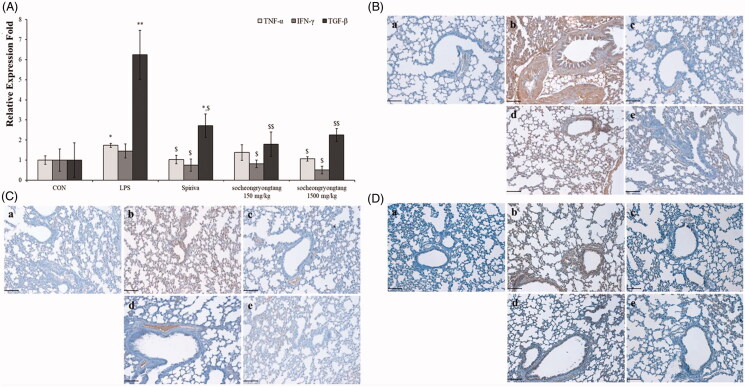
Socheongryongtang effectively decreased the levels of cytokines -related to COPD
In our previous study, we reported that the changes in cytokines and chemokines could be related to COPD severity (Lee et al. 2018). In order to determine the relationship between cytokines and COPD severity, gene levels and proteins expression such as TNF-α, IFN-γ and TGF-β were measured using q-PCR (Figure 4(A)) and IHC (Figure 4(B–D)). All of the gene levels which were raised by LPS intranasal instillation were suppressed by Spiriva treatment or socheongryongtang treatment, excluding 150 mg/kg socheongryongtang treatment on the level of TNF-α (Figure 4(A), p < 0.001 or p < 0.05). The levels of suppression of three genes by socheongryongtang treatment were similar to those by Spiriva treatment, and 150 mg/kg socheongryongtang treatment particularly inhibited their expression. Socheongryongtang down-regulated the level of TNF-α (Figure 4(B)) and significantly and effectively inhibited the levels of IFN-γ (Figure 4(C)) and TGF-β (Figure 4(D)), even with 150 mg/kg socheongryongtang treatment. The down-regulated levels of IFN-γ and TGF-β by 150 or 1500 mg/kg socheongryongtang treatment (Figure 4(Cd–Ce, Dd–De)) were similar to those by Spiriva treatment (Figure 4(Cc, Dc)).
Figure 4.
Socheongryongtang completely inhibited the changes of COPD-related cytokines such as TNF-α, IFN-γ and TGF-β by LPS instillation in the respiratory system. (A) Socheongryongtang thoroughly controlled the DNA levels of cytokines such as TNF-α, IFN-γ and TGF-β to a similar extent as Spiriva treatment. (B) Socheongryongtang suppressed the expression level of TNF-α. (C) Socheongryongtang significantly decreased IFN-γ expression, even with 150 mg/kg socheongryongtang treatment, to an extent similar to that by Spiriva treatment. (D) Similar to the change in IFN-γ expression, socheongryongtang effectively down-regulated TGF-β expression. In other words, the TGF-β level in 150 mg/kg socheongryongtang treatment was strongly suppressed. (a) Control; (b) 0.8 mg/kg LPS intranasal instillation; (c) 1 mg/kg Spiriva treatment for five days after LPS instillation; (d) 150 mg/kg socheongryongtang treatment for five days after LPS instillation; (e) 1500 mg/kg socheongryongtang treatment for five days after LPS instillation. Each bar represents the mean ± SD (N = 8). Arrow, positive cells. *p < 0.05 vs. control group; **p < 0.01 vs. control group; $p < 0.05 vs. LPS intranasal instillation group; $$p < 0.01 vs. LPS intranasal instillation group. Magnification, ×200.
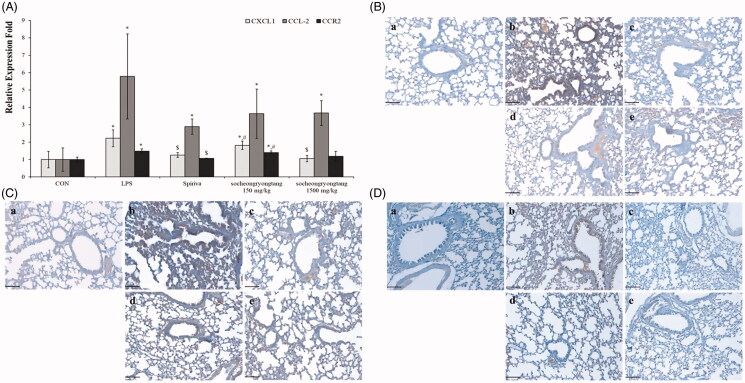
Socheongryongtang effectively inhibited the expression levels of LPS-induced chemokines in the lung
The expression level of CXCL1 was decreased by socheongryongtang treatment, while the DNA levels of CCL-2 and CCR2 were not decreased (Figure 5(A), p < 0.05). However, the levels of all of their proteins CXCL1, CCL-2 and CCR2 were effectively suppressed by socheongryongtang treatment (Figure 5(B–D)). Socheongryongtang significantly inhibited the expression of CXCL1, even with 150 mg/kg socheongryongtang treatment (Figure 5(Bd, Be)), but the level of modulation by Spiriva treatment was higher than those by socheongryongtang treatment (Figure 5(Bc)). The down-regulated levels of CCL-2 expression were similar between 150 and 1500 mg/kg socheongryongtang treatment (Figure 5(Cd, Ce)), and the downregulation of CCL-2 expression level did not differ between Spiriva treatment (Figure 5(Cc)) and socheongryongtang treatment (Figure 5(Cd, Ce)). In the case of CCR2 expression, the suppressed levels by socheongryongtang treatment were similar to that by Spiriva treatment (Figure 5(D)), even with 150 mg/kg socheongryongtang treatment (Figure 5(Dd)).
Figure 5.
Socheongryongtang down-regulated the expression of CXCL1, CCL-2 and CCR2. (A) Socheongryongtang statistically significantly inhibited the DNA level of CXCL1 and trended towards suppressing the levels of CCL-2 and CCR2. (B) Socheongryongtang significantly suppressed the CXCL1 expression with 150 mg/kg treatment to an extent similar to that by Spiriva treatment. Socheongryongtang down-regulated the expression of CCL2 (C) and CCR2 (D). Each bar represents the mean ± SD (N = 8). Arrow, positive cells. *p < 0.05 vs. control group; $p < 0.05 vs. LPS intranasal instillation group; #p < 0.05 vs. Spiriva treatment group. Magnification, ×200.
Discussion
In 2017, the WHO reported that COPD was the third most common cause of death in 2015, and it was projected to remain as the third most common cause of death by 2030 worldwide. Thus, COPD is an important disease to study, not only because it has high mortality, but also because the morbidity and severity in older populations are higher than those in the young, and over time most societies are moving towards aging societies (WHO 2017). In our environment, there are many inducers/irritants of COPD, such as tobacco smoke, air pollutions, occupational dusts and fumes, etc., and with the further modernization of society, opportunities to contact these inducers/irritants will likely increase (Franklin et al. 1956; Ludwig et al. 1985; Forbes et al. 2009; Hopkinson and Polkey 2009). As it cannot be completely recovered from, COPD is a very severe disease (Rabe et al. 2007), and the medicines currently prescribed to patients are only for relieving symptoms, such as the depression of inhalation, and are not for completely curing COPD. The categories of disease drugs currently used are classified as bronchodilators, corticosteroids, and so on, but they have several adverse effects (McEvoy and Niewoehner 1997; Donnelly and Barnes 2006; Kim et al. 2014).
There are various outcomes that may occur in COPD patients, from just cough to death caused by mucous hypersecretion, fibrosis, alveolar wall destruction (emphysema), etc. (Franklin et al. 1956; Ludwig et al. 1985; Pesci et al. 1998; O’Donnell et al. 2004; Forbes et al. 2009; Hopkinson and Polkey 2009). This disease occurrence is particularly related with cytokines (TNF-α, IFN-γ, TGF-β, IL-1β, IL-6, etc.), chemokines (CXCL1, CCL-2, CCR2, etc.) and collagenase/gelatinase (MMP-9, MMP-12, etc.) (Barnes 2009). TNF-α stimulates the release of IL-8, which increases the number of neutrophils, and finally destroys the alveolar wall (Keatings et al. 1996; Barnes 2008). The other function of TNF-α induces collagenase/gelatinase (MMP-9, MMP-12, etc.) and destroys the alveolar wall (Linder et al. 2015). IFN-γ activates alveolar macrophages, which then release a variety of factors related to COPD, such as cytokines and chemokines (Bodisco et al. 1992). TGF-β, which is made from respiratory epithelial cells, leads to pulmonary fibrosis, and the fibrosis of small airways is one of the major causes of apnoea (Barnes 2008).
The level of CXCL1, which is expressed on the surfaces of neutrophils and monocytes in COPD patients’ sputum or BALF, is substantially higher than that in normal persons, and induces the severity of COPD through CCR2 (Davies et al. 2015). CCL-2 stimulates the attraction of monocytes in the small airway, changes from monocytes to macrophages for the release of CXCL1, then the released CXCL1 activates neutrophils to destroy alveolar wall by elastase (de Boer et al. 2000; Barnes 2009). CCR2 is a G protein-coupled receptor that is activated by CCL-2 binding. It has a double-edged-sword-like function: a pro-inflammatory effect which is modulated by antigen presenting cells and T cells as well as an anti-inflammatory effect which is regulated by regulatory T cells (Traves et al. 2002). Particularly in COPD, CCR2 induced airway wall destruction via CCL-2 binding (Zhang et al. 2010).
In order to evaluate the therapeutic effect of socheongryongtang on COPD and its mode of action, an LPS-induced COPD murine model was orally applied with socheongryongtang, and the anti-asthmatic effects were measured based on several points such as the functional/anatomical and histopathological alterations as well as the changes of cytokines/chemokines. Socheongryongtang controlled the LPS-induced representative COPD changes such as the down-regulations of WBC, neutrophils and eosinophils in BALF, the suppression of IgE level in serum, the inhibition of emphysema and fibrosis in the pulmonary system, and the decreases of cytokines (IFN-γ, TGF-β and TNF-α) and chemokines (CCR2, CXCL1 and CCL-2). In particular, the change in the level of blood cells by socheongryongtang of at least 150 mg/kg, which is 1/10 the dosage for human application, sufficiently inhibited their proliferations and also completely controlled the expression levels of IFN-γ, TGF-β and CCR2. Taken of all, we found that 150–1500 mg/kg dosage clearly suppressed COPD severity in our animal model. In general, we considered oral intake of socheongryongtang of 0.73–7.3 g daily for the treatment of COPD. The oral dose of mouse is 150–1500 mg/kg/day. The conversion factor between human and mouse is 12.33 (Shin et al. 2010). Therefore, if the effective dose for mice is 150 mg/kg/day, the human equivalent dose is 730 mg/60 kg/day as socheongryongtang. Official daily intake of socheongryongtang for human is 9 g. Thus, the optimal dose of COPD treatment is less than the daily intake. We concluded that oral intake of 7.3 g of socheongryongtang is beneficial to suppress the possibility of the occurrence of COPD.
Conclusions
Based on the results, we concluded that socheongryongtang suppressed COPD severity, and although it regulated almost all COPD-related factors, the therapeutic mechanism of socheongryongtang was based on the regulation of blood cells in BALF, IgE in serum, two cytokines (IFN-γ and TGF-β) and one chemokine (CCR2). Socheongryongtang is one of the prescriptions for the suppression of COPD severity.
Disclosure statement
The authors declare that they have no competing interests.
References
- Bagdonas E, Raudoniute J, Bruzauskaite I, Aldonyte R.. 2015. Novel aspects of pathogenesis and regeneration mechanisms in COPD. Int J COPD. 10:995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. 2008. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 8(3):183–192. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. 2009. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 41(6):631–638. [DOI] [PubMed] [Google Scholar]
- Bodisco T, Vecchiarelli A, Dottorini M, Eslami A, Bertotto A, Massucci M, Crupi S, Cenci E, Arcangeli C, Fabietti PG, et al. 1992. Interferon-gamma (IFN-γ) induced activation of alveolar macrophages (AM) from allergic patients with chronic obstructive pulmonary disease (COPD). J Biol Regul Homeost Agents. 6(3):87–92. [PubMed] [Google Scholar]
- Boskabadi J, Askari VR, Hosseini M, Boskabady MH.. 2019. Immunomodulatory properties of captopril, an ACE inhibitor, on LPS-induced lung inflammation and fibrosis as well as oxidative stress. Inflammopharmacology. 27(3):639–647. [DOI] [PubMed] [Google Scholar]
- Boskabadi J, Mokhtari-Zaer A, Abareshi A, Khazdair MR, Emami B, Mohammadian Roshan N, Hosseini M, Boskabady MH.. 2018. The effect of captopril on lipopolysaccharide-induced lung inflammation. Exp Lung Res. 44(4–5):191–200. [DOI] [PubMed] [Google Scholar]
- Bożek A, Rogala B.. 2018. IgE-dependent sensitization in patients with COPD. Ann Agric Environ Med. 25(3):417–420. [DOI] [PubMed] [Google Scholar]
- Butler A, Walton GM, Sapey E.. 2018. Neutrophilic inflammation in the pathogenesis of chronic obstructive pulmonary disease. COPD. 15(4):1–13. [DOI] [PubMed] [Google Scholar]
- Davies C, Rhodes JA, Barnes P, Donnelly L.. 2015. Elevated CCL2 response in COPD and attenuation by selective chemokine receptor antagonists. Eur Respir J. 46(59):PA3900. [Google Scholar]
- de Boer WI, Sont JK, van Schadewijk A, Stolk J, van Krieken JH, Hiemstra PS.. 2000. Monocyte chemoattractant protein 1, interleukin 8, and chronic airways inflammation in COPD. J Pathol. 190(5):619–626. [DOI] [PubMed] [Google Scholar]
- Donnelly LE, Barnes PJ.. 2006. Chemokine receptors as therapeutic targets in chronic obstructive pulmonary disease. Trends Pharmacol Sci. 27(10):546–553. [DOI] [PubMed] [Google Scholar]
- Forbes LJL, Kapetanakis V, Rudnicka AR, Cook DG, Bush T, Stedman JR, Whincup PH, Strachan DP, Anderson HR.. 2009. Chronic exposure to outdoor air pollution and lung function in adults. Thorax. 64(8):657–663. [DOI] [PubMed] [Google Scholar]
- Franklin W, Lowell FC, Michelson AL, Schiller IW.. 1956. Chronic obstructive pulmonary emphysema; a disease of smokers. Ann Intern Med. 45(2):268–274. [DOI] [PubMed] [Google Scholar]
- Ghorani V, Boskabady MH, Khazdair MR, Kianmeher M.. 2017. Experimental animal models for COPD: a methodological review. Tob Induc Dis. 15:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. 2004. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 350(26):2645–2653. [DOI] [PubMed] [Google Scholar]
- Hogg JC, Timens W.. 2009. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 4:435–459. [DOI] [PubMed] [Google Scholar]
- Hopkinson NS, Polkey MI.. 2009. Chronic obstructive pulmonary disease in non-smokers. Lancet. 374(9706):1965–1966. [DOI] [PubMed] [Google Scholar]
- Huh J. 2002. Donguibogam. Seoul, Korea: Bubinmunhwasa. [Google Scholar]
- Keatings VM, Collins PD, Scott DM, Barnes PJ.. 1996. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 153(2):530–534. [DOI] [PubMed] [Google Scholar]
- Kim V, Desai P, Newell JD, Make BJ, Washko GR, Silverman E, Crapo JD, Bhatt SP, Criner GJ.. 2014. Airway wall thickness is increased in COPD patients with bronchodilator responsiveness. Respir Res. 15(1):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Fujinawa R, Ota F, Kobayashi S, Angata T, Ueno M, Maeno T, Kitazume S, Yoshida K, Ishii T, et al. 2013. A single dose of lipopolysaccharide into mice with emphysema mimics human chronic obstructive pulmonary disease exacerbation as assessed by micro-computed tomography. Am J Respir Cell Mol Biol. 49(6):971–977. [DOI] [PubMed] [Google Scholar]
- Lee SY, Cho JH, Cho SS, Bae CS, Kim GY, Park DH.. 2018. Establishment of a chronic obstructive pulmonary disease mouse model based on the elapsed time after LPS intranasal instillation. Lab Anim Res. 34(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder R, Ronmark E, Pourazar J, Behndig A, Blomberg A, Lindberg A.. 2015. Serum metalloproteinase-9 is related to COPD severity and symptoms – cross-sectional data from a population based cohort-study. Respir Res. 16:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig PW, Schwartz BA, Hoidal JR, Niewoehner DE.. 1985. Cigarette smoking causes accumulation of polymorphonuclear leukocytes in alveolar septum. Am Rev Respir Dis. 131(6):828–830. [DOI] [PubMed] [Google Scholar]
- McEvoy CE, Niewoehner DE.. 1997. Adverse effects of corticosteroid therapy for COPD. A critical review. Chest. 111(3):732–743. [DOI] [PubMed] [Google Scholar]
- Nowrin K, Sohal SS, Peterson G, Patel R, Walters EH.. 2014. Epithelial–mesenchymal transition as a fundamental underlying pathogenic process in COPD airways: fibrosis, remodeling and cancer. Expert Rev Respir Med. 8(5):547–559. [DOI] [PubMed] [Google Scholar]
- O’Byrne PM. 2007. Exacerbations of asthma and COPD: definitions, clinical manifestations and epidemiology. Contrib Microbiol. 14:1–11. [DOI] [PubMed] [Google Scholar]
- O’Donnell RA, Peebles C, Ward JA, Daraker A, Angco G, Broberg P, Pierrou S, Lund J, Holgate ST, Davies DE.. 2004. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax. 59:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesci A, Majori M, Cuomo A, Borciani N, Bertacco S, Cacciani G, Gabrielli M.. 1998. Neutrophils infiltrating bronchial epithelium in chronic obstructive pulmonary disease. Respir Med. 92(6):863–870. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. 2007. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 176(6):532–555. [DOI] [PubMed] [Google Scholar]
- Ramos FL, Krahnke JS, Kim V.. 2014. Clinical issues of mucus accumulation in COPD. Int J COPD. 9:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S, Beheshti F, Askari VR, Hosseini M, Mohamadian Roshan N, Boskabady MH.. 2019. Aminoguanidine affects systemic and lung inflammation induced by lipopolysaccharide in rats. Respir Res. 20(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JW, Seol IC, Son CG.. 2010. Interpretation of animal dose and human equivalent dose for drug development. J Korean Med. 31(3):1–7. [Google Scholar]
- Traves SL, Sulpitt S, Russell RE, Barnes PJ, Donnelly LE.. 2002. Increased levels of the chemokines GROalpha and MCP-1 in sputum samples from patients with COPD. Thorax. 57(7):590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization [WHO] . 2017. Dec 1. Chronic obstructive pulmonary disease (COPD). Fact sheet. Geneva (Switzerland): WHO. [Google Scholar]
- Zhang J, Patel L, Pienta KJ.. 2010. Chapter 3: targeting chemokine (C-C motif) ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog Mol Biol Transl Sci. 95:31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]