Abstract
Neurotrophins promote multiple actions on neuronal cells including cell survival and differentiation. The best-studied neurotrophin, nerve growth factor (NGF), is a major survival factor in sympathetic and sensory neurons and promotes differentiation in a well-studied model system, PC12 cells. To mediate these actions, NGF binds to the TrkA receptor to trigger intracellular signaling cascades. Two kinases whose activities mediate these processes include the mitogen-activated protein (MAP) kinase (or extracellular signal-regulated kinase [ERK]) and phosphoinositide 3-kinase (PI3-K). To examine potential interactions between the ERK and PI3-K pathways, we studied the requirement of PI3-K for NGF activation of the ERK signaling cascade in dorsal root ganglion cells and PC12 cells. We show that PI3-K is required for TrkA internalization and participates in NGF signaling to ERKs via distinct actions on the small G proteins Ras and Rap1. In PC12 cells, NGF activates Ras and Rap1 to elicit the rapid and sustained activation of ERKs respectively. We show here that Rap1 activation requires both TrkA internalization and PI3-K, whereas Ras activation requires neither TrkA internalization nor PI3-K. Both inhibitors of PI3-K and inhibitors of endocytosis prevent GTP loading of Rap1 and block sustained ERK activation by NGF. PI3-K and endocytosis may also regulate ERK signaling at a second site downstream of Ras, since both rapid ERK activation and the Ras-dependent activation of the MAP kinase kinase kinase B-Raf are blocked by inhibition of either PI3-K or endocytosis. The results of this study suggest that PI3-K may be required for the signals initiated by TrkA internalization and demonstrate that specific endocytic events may distinguish ERK signaling via Rap1 and Ras.
Neurotrophins have long been recognized for their role in regulating neuronal survival, cell growth, differentiation, and neuronal plasticity. The archetypal neurotrophin, nerve growth factor (NGF), elicits most of these effects by binding and activating the receptor tyrosine kinase (RTK), TrkA, which leads to the activation of several well-defined signaling cascades. Of these, the phosphoinositide 3-kinase (PI3-K) and extracellular signal-regulated kinase (ERK) pathways are two of the most extensively studied. PI3-Ks have been implicated in multiple biological responses including membrane trafficking, proliferation, differentiation, and survival (67). These kinases consist of a family of proteins which phosphorylate phosphatidylinositol (PI) at the D3 position and have been categorized into three classes based on their lipid substrate specificity in vitro (96). The lipid products of PI3-Ks {PI 3-phosphate [PI(3)P], PI(3,4)P, PI(3,5)P, and PI(3,4,5)P} are known to act as second messengers and mediate most of the known functions of PI3-Ks in cells (45).
The mitogen-activated protein kinase family members, ERK1 and ERK2, can also be activated by a wide variety of stimuli to promote a diverse array of cellular functions (77). In addition to their established role in mitogenesis, recent advances have identified both novel mechanisms of activation and novel functions of ERKs in neurons (26). Following growth factor stimulation of neuronal cells, ERK is phosphorylated and activated by the dual-specificity kinase, MEK, which is phosphorylated and activated by members of the Raf serine/threonine kinase family. The Raf family of protein kinases consists of Raf-1, B-Raf, and A-Raf. Neurons lack A-Raf but express the ubiquitous Raf-1 and the neuronal isoform B-Raf. Although Raf-1 is generally considered the classic upstream activator of MEKs in nonneuronal cells (3), Raf-1 is not a major MEK kinase in neuronal tissue (8). Furthermore, in the neuronal model system, PC12 cells, Raf-1 may contribute less than 5% of the total MEK kinase activity following NGF treatment (110), whereas B-Raf has been shown to be the major Raf isoform activated by NGF in these cells (6, 35, 36). These studies emphasize the need to examine B-Raf regulation in order to understand ERK signaling in PC12 cells and neurons.
Activation of the B-Raf–ERK cascade is linked to RTK signaling by members of the Ras superfamily of small GTPases. We have previously shown that NGF can activate B-Raf and ERKs via two distinct pathways utilizing Ras and the related Ras family member, Rap1 (106). The significance of these two pathways is that the engagement of Rap1-dependent signaling by NGF, but not epidermal growth factor (EGF), affords specificity to growth factor signaling. Ras-dependent signaling to ERKs is transient, whereas Rap1-dependent signaling to ERKs is sustained (106). The sustained activation of ERKs via Rap1 has been proposed to participate in NGF-dependent PC12 cell differentiation, and a role for Rap1 in the induction of electrical excitability and NGF-dependent gene expression has been shown (90, 106). Like all small GTPases, Ras and Rap1 are activated by specific guanine nucleotide exchange factors which stimulate the exchange of bound GTP for GDP. The association of B-Raf with either Ras-GTP or Rap1-GTP is an essential step in B-Raf activation. Binding to its upstream small GTPase activator alone, however, is not sufficient for full B-Raf activation, suggesting that other factors are required (48, 51, 105).
While PI3-K and ERKs are well-studied downstream targets of NGF, prevailing models have them existing as two distinct pathways with PI3-K and its target kinase Akt controlling cell survival and ERK signaling controlling cell growth or differentiation (18, 52). As such, no one has looked extensively at the cross-talk between these two cascades in NGF signaling. Indeed, the role of PI3-K in ERK signaling in general is not completely clear. For example, overexpression of a constitutively active p110-α, a PI3-K isoform, has been shown to activate the Ras-ERK pathway in at least one system (31), but not in others (22, 37, 42, 47). In addition, many studies have demonstrated a requirement of PI3-K activity for ERK activation by multiple diverse stimuli (13, 16, 17, 25, 40, 47, 76, 86, 89, 91), while other reports failed to show a sensitivity of some of these same stimuli to PI3-K inhibitors (59, 78, 85, 92). One explanation for these apparent discrepancies may be that the ability of PI3-K inhibitors to block ERK activation is dependent on the cell type, type of stimuli, and strength of the signal (17, 100). However, the mechanisms through which PI3-K facilitates ERK activation are not known, and the role of PI3-K in regulating B-Raf activity has not been established.
The major goal of this study was to examine the role of PI3-K in NGF signaling to ERKs. We show here that PI3-K inhibitors block the activation of B-Raf and ERK by NGF in primary sensory neurons and PC12 cells. We demonstrate that PI3-K inhibitors block the activation of Rap1, but not Ras, and suggest that this may be due to the ability of these inhibitors to block TrkA internalization. In addition to these actions upstream of Rap1, we identify a requirement of PI3-K downstream of Ras. Both actions contribute to NGF-dependent signaling to ERKs in PC12 cells.
MATERIALS AND METHODS
Materials.
PC12-GR5 cells were kindly provided by R. Nishi, Oregon Health Sciences University, Portland. Forskolin, PD98059, and LY294002 (LY) were purchased from Cal Biochem (Riverside, Calif.). Monodansylcadaverine (MDC), wortmannin, and 3-isobutyl-1-methylxanthine (IBMX) were purchased from Sigma (St. Louis, Mo.). NGF and EGF were from Boehringer Mannheim (Indianapolis, Ind.). Phosphorylation-specific mouse monoclonal antibodies (MAb) which recognize phosphorylated ERK1 (pERK1) and ERK2 (pERK2) at residues threonine 183 and tyrosine 185, as well as anti-ERK1/2 polyclonal antibodies which recognize ERK1 and ERK2 independent of phosphorylation on residues 183 and 185, were purchased from New England Biolabs (Beverly, Mass.). Phosphorylation-specific antibodies which recognize TrkA phosphorylated on tyrosines 674 and 675 (pTrkA) and Akt phosphorylated on serine 308 (pAkt) were also purchased from New England Biolabs, as were phosphorylation state-independent Akt antibodies. Polyclonal antibodies to ERK2 (C14-AC), B-Raf (C19), Rap1 (also called Krev-1), and TrkA (C14) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Anti-Flag (M2) antibody was purchased from Sigma. Anti-Ras MAb and anti-PI3-K (p85) rabbit polyclonal antibodies were from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). Anti-myc MAb (9E10) was kindly provided by Andrey Shaw, Washington University, St. Louis, Mo. Nickel agarose (Ni-nitrilotriacetic acid [NTA]-agarose) was purchased from Qiagen Inc. (Chatsworth, Calif.) and radioisotopes were from NEN-DuPont (Boston, Mass.). All other reagents were from Sigma.
Cell culture.
PC12 cells were maintained in DMEM (Dulbecco modified Eagle medium) plus 10% horse serum and 5% fetal calf serum on 100-mm-diameter plates to 60 to 70% confluence at 37°C in 5% CO2 prior to harvesting. For immune complex assays and Western blotting, PC12 cells were maintained in DMEM containing 0.1% horse serum and 0.5% fetal calf serum for 16 h at 37°C in 5% CO2 prior to treatment with various reagents. Adult dorsal root ganglion (DRG) neurons were cultured as previously described (12). Briefly, ganglia were dissected from 250- to 300-g Sprague-Dawley rats, dissociated enzymatically, and plated on polylysine- and laminin-coated chamber slides for immunocytochemistry or 35-mm-diameter plates for B-Raf assays. Cells were maintained in F12 medium with 10% fetal calf serum. DRG cultures were serum starved in F12 medium for 4 to 6 h prior to treatment. The following drug concentrations were used to treat both DRG cultures and PC12 cells, unless otherwise stated: NGF (50 ng/ml), EGF (50 ng/ml), forskolin (10 μM), IBMX (100 μM), LY (20 μM), wortmannin (200 nM), MDC (100 μM), and PD98059 (40 μM). All inhibitors were added 10 min prior to treatment.
Transfections.
PC12 cells that were 60% confluent were cotransfected with the indicated cDNAs using Superfect from Qiagen Inc. according to the manufacturer's instructions. The vector pcDNA3 (Invitrogen Corp.) was added to each set of transfections to ensure that each plate received the same amount of DNA. Following 24 h of recovery, cells were starved overnight in DMEM containing 0.1% horse serum and 0.5% fetal calf serum before treatment and harvest.
Western blotting.
Cell lysates were prepared as described previously (98). Protein concentrations were assessed by the Bradford protein assay. Cell lysates containing equal amounts of protein per treatment condition were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by transfer onto polyvinylidine difluoride membranes. Membranes were blocked in 5% milk and probed with primary antibodies per the manufacturer's instructions followed by the appropriate horseradish peroxidase-conjugated anti-rabbit or anti-mouse monoclonal secondary antibody (Amersham). Proteins were detected by enhanced chemiluminescence.
Immune complex assays.
For ERK and PI3-K assays, treated and untreated cells were lysed in a buffer containing 1% Nonidet P-40 (NP-40), 10% sucrose, 20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg of leupeptin per ml, 1 mM sodium orthovanadate, and 10 mM sodium fluoride. The lysates were spun at low speed to remove nuclei, and the supernatant was assayed for kinase activity. ERK activity was assayed as described previously (98) using myelin basic protein (MBP) and [γ-32P]ATP as substrates with equal amounts of protein per treatment condition. For PI3-K assays, supernatants containing 500 μg of total protein were incubated with anti-PI3-K (p85) antibodies overnight at 4°C. A 50% slurry of protein G-Sepharose beads (20 μl) was then added for 45 min. Beads containing immunoprecipitates were washed once with lysis buffer, twice with 1% NP-40 in phosphate-buffered saline (PBS), and once with distilled water. PI3-K activity was measured using PI as a substrate. PI (10 μg/sample) was dried under nitrogen stream, resuspended in 10 μl of 20 mM HEPES, and sonicated for 3 min. Washed samples were preincubated with sonicated lipid substrate for 10 min on ice prior to the addition of 40 μl of a kinase reaction mixture containing 20 mM HEPES, 30 mM MgCl2, 20 μM ATP, and 10 μCi of [γ-32P]ATP per sample. After 15 min of incubation at room temperature, the reactions were terminated by the addition of 80 μl of 1 M HCl. Lipids were extracted with a 1:1 chloroform-methanol solution and separated by thin-layer chromatography on silica gel 60 plates in a solvent containing chloroform-methanol-water-ammonium hydroxide (45:35:2.6:7.4 [vol/vol]). The incorporation of 32P into PI was visualized using a PhosphorImager (Molecular Dynamics). To determine the location of phosphorylated PI, unlabeled phosphatidylinositol phosphate was run in parallel and visualized using an I2 vapor chamber.
For B-Raf assays, untreated and treated cells were lysed in 1% NP-40 buffer containing 10 mM Tris (pH 7.4), 5 mM EDTA, 50 mM NaCl, and 1 mM PMSF. Immune complex kinase assays were performed as described previously (98) using MEK-1 and [γ-32P]ATP as substrates with lysates containing equal amounts of protein per treatment condition. The reaction products of all kinase assays were resolved by SDS-PAGE and analyzed with a PhosphorImager (Molecular Dynamics).
Luciferase reporter gene assays.
Following transfection of GAL4-CREB (7), and 5XGal-E1b-TATA-luciferase (Gal-luciferase) (88), PC12 cells were treated with the appropriate stimuli for 4 to 5 h. Cells were then lysed, and equal protein amounts of lysate per condition were assayed for luciferase activity as previously described (98). All experiments were performed with at least three independently treated plates per condition.
In vivo Rap1 and Ras activation assays.
Activated Rap1 was isolated from cell lysates using a protocol adapted from that of Franke et al. (21). Treated cells from three 70% confluent 100-mm-diameter plates were lysed in 400 μl of ice-cold Rap1 lysis buffer (10% glycerol, 1% NP-40, 50 mM Tris-HCl [pH 8.0], 200 mM NaCl, 5 mM MgCl2, 1 mM PMSF, 1 μM leupeptin, 10 μg of soybean trypsin inhibitor per ml, 10 mM NaF, 0.5 mM aprotinin, 1 mM Na3 VO4). Lysates were clarified by low-speed centrifugation, and supernatants containing 2 mg of total protein were incubated with 40 μg of glutathione S-transferase (GST)-RalGDS fusion protein (gift of J. L. Bos, Utrecht University, Utrecht, The Netherlands) coupled to glutathione agarose beads for 1 h at 4°C. Beads were pelleted and rinsed three times with lysis buffer, and protein was eluted from the beads with Laemmli buffer. Activated Ras was isolated from stimulated cell lysates using agarose-coupled GST-Raf1-Ras binding domain (RBD) provided in the Ras Activation Assay Kit (Upstate Biotechnology, Inc.) following the manufacturer's recommended protocol. The amount of Rap1 or Ras bound to beads was detected by Western blotting.
Nickel affinity chromatography.
For studies examining polyhistidine-tagged Rap1 (His-Ras) or polyhistidine-tagged Rap1 (His-Rap1), transfections were performed using Superfect. Cells were lysed in a buffer containing 1% NP40, 10% glycerol, 10 mM Tris (pH 8.0), 20 mM NaCl, 30 mM MgCl2, 1 mM PMSF, 1 μg of leupeptin per ml, 0.5 mM aprotinin, and 1 mM Na3 VO4, and supernatants were prepared. Transfected His-tagged proteins were precipitated from supernatants containing equal amounts of protein using Ni-NTA agarose and washed with 10 mM imidazole in lysis buffer and eluted with PBS containing 500 mM imidazole and 5 mM EDTA. Eluates containing His-tagged proteins were separated by SDS-PAGE, and B-Raf protein was detected by Western blotting. Equal amounts of each eluate were immunoprecipitated with B-Raf antisera, and B-Raf activity was measured by immune complex assay. Equal amounts of His-Ras or His-Rap1 in the eluates was confirmed by Western blotting.
Cell surface biotinylation.
PC12 cells were grown in 35-mm-diameter six-well plates to 60 to 70% confluence prior to treatment. Treated cells were quickly washed on ice with ice-cold PBS. N-hydroxy-succinimide–biotin (Pierce) (1.5 mg/ml) was added to cells followed by a 30-min incubation with gentle motion at 0 to 4°C. Cells were rinsed three times with 0.1 M glycine in PBS to quench unreacted biotin prior to lysis in a solution of 1% NP-40, 20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM PMSF, 1 μg of leupeptin per ml, 1 mM sodium orthovanadate, and 10 mM sodium fluoride. Cell lysates were incubated with 100 μl of a 50% suspension of UltraLink Immobilized NeutrAvidin (Pierce) for 2 to 4 h at 4°C. The beads were pelleted and washed twice with a high-salt wash buffer (0.1% Triton X-100, 500 mM NaCl, 5 mM EDTA, 50 mM Tris [pH 7.5]) and once with 50 mM Tris (pH 7.5). Biotinylated proteins were eluted from the beads by incubating at 80°C for 5 min in 2× Laemmli buffer. The amount of TrkA bound to the beads was detected by Western blotting.
Immunocytochemistry.
Cultures were fixed for 10 min in PBS containing 3% paraformaldehyde and 15% picric acid, followed by 10 min in cold 100% methanol. They were then rinsed in PBS and incubated for 30 min in blocking buffer consisting of 1.5% normal goat serum, 1% porcine gelatin, and 0.2% Triton X-100 in PBS. Primary and secondary antibodies were diluted in the same solution. Cells were incubated in primary antibody overnight, washed with PBS, and incubated with secondary antibody for 30 min (cyanine dye 3-conjugated donkey anti-rabbit or cyanine dye 2-conjugated goat anti-mouse diluted 1:200; Jackson Immunoresearch Laboratories, West Grove, Pa.). As a negative control, cells were processed without a primary antibody. Primary antibodies were used at the following dilutions: pERK, 1:1,000; Rap1/Krev-1, 1:500; B-Raf, 1:500; and Ras, 1:500. Cells were examined by epifluorescence and confocal microscopy. For pERK1/2 immunostaining in DRG cultures, the signal intensity in neuron cell bodies was quantitated from at least 100 cells per treatment condition using NIH Image.
Electron microscopy.
PC12 cells were fixed in 4% paraformaldehyde and 0.05% glutaraldehyde in 100 mM phosphate buffer (pH 7.2) for 1 h at ambient temperature. Cells were rinsed in 100 mM phosphate buffer (pH 7.2), scraped off tissue culture dishes with a rubber policeman, and microcentrifuged into a pellet. The fixed cell pellets were infused with polyvinylpyrrolidone and sucrose (93) and prepared for cryosectioning (27). Cryosections were immunolabeled as described previously (62). Diluted antibodies were microcentrifuged prior to use. Following incubation with anti-Rap1/Krev-1 primary antibody (1:50 to 1:100 dilution), the sections were rinsed and incubated with protein A-gold (1:10) (Amersham Life Sciences, Inc.). Controls included substitution of primary antibody with purified rabbit immunoglobulin G or irrelevant antibodies.
RESULTS
PI3-K inhibitors block B-Raf and ERK activation by NGF in DRG sensory neurons.
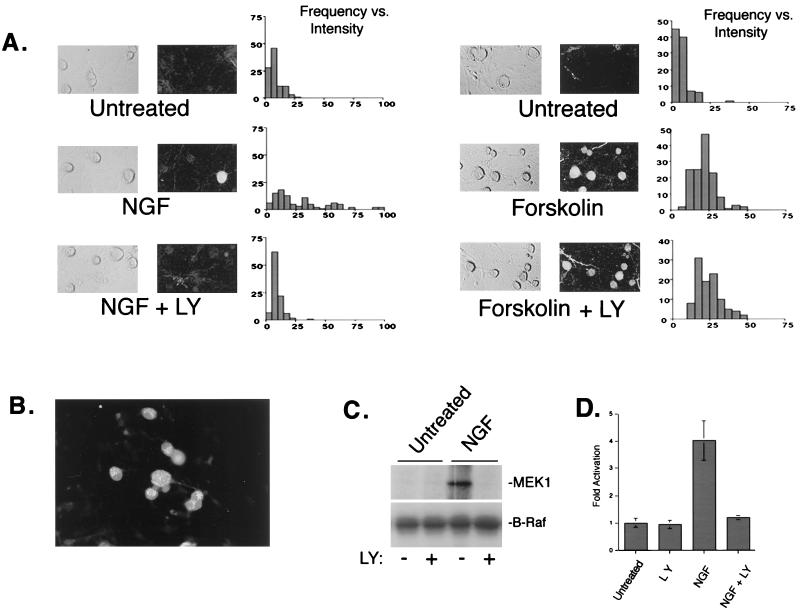
Neurotrophins are known to promote the survival and differentiation of sensory neurons of the DRG during development. In adult sensory neurons, NGF acts as a potent survival factor following injury and is required for maintenance of the differentiated phenotype. Here, we examined the ability of NGF to regulate ERKs in cultures of adult rat DRG neurons. ERK activation was monitored by immunocytochemistry with a widely used phospho-specific ERK antibody which recognizes the phosphorylation of the two sites known to be responsible for ERK activation. NGF treatment of DRG cultures resulted in increased pERK staining in nearly half of the sensory neurons present in the DRG culture, consistent with previous studies showing TrkA expression in 40 to 50% of neurons in the ganglia (2, 57). No pERK staining was observed in glial cells, which do not express TrkA (44). The increase in ERK activation by NGF was completely abolished in the presence of the PI3-K inhibitor, LY (20 μM) (Fig. 1A, left panels). Wortmannin (200 nM), a fungal metabolite which inhibits PI3-K activity through an independent mechanism (60), also blocked NGF-induced pERK staining in these cells (data not shown). ERK activation by cyclic AMP (cAMP)-dependent signals was elicited by treatment with forskolin, an activator of adenylyl cyclases. LY had no effect on forskolin stimulation of ERKs (Fig. 1A, right panels). Interestingly, forskolin stimulated ERK activation in all of the sensory neurons in DRG cultures but did not stimulate ERKs in glia. In cultures of DRG cells, B-Raf, an upstream activator of ERKs, was expressed exclusively in neurons (Fig. 1B). Together, these results are consistent with previous reports (19, 53) and support a model in which B-Raf expression accounts for the cell type-specific actions of cAMP on ERK activation (19, 81, 98).
FIG. 1.
Role of PI3-K in neuronal ERK activation. (A) ERK activity was assessed by immunostaining fixed cells with a pERK1/2 antibody. Adult rat DRG cultures were treated for 20 min with either 50 ng of NGF per ml (left) or 10 μM forskolin (right) in the presence or absence of LY (20 μM), a specific PI3-K inhibitor. For each pair of photos, light-field micrographs are shown on the left and fluorescence micrographs showing pERK1/2 immunoreactivity are shown on the right. The signal intensity in neurons was quantitated for each treatment condition using NIH Image. Histograms represent the distribution of fluorescent intensities as a function of frequency (n = 100 cells). LY completely abolished ERK activation by NGF but had no effect on forskolin-stimulated ERKs. (B) Immunofluorescence localization of B-Raf expression in adult rat DRG cultures. Note that expression is detected only in neurons. (C) Biochemical examination of B-Raf in cultured DRG neurons. B-Raf activity was measured by immune complex kinase assay using anti-B-Raf antisera and recombinant MEK-1 as a substrate. A representative gel is shown (n = 3). (D) Average values from three experiments as shown in panel C, presented as mean fold activation with standard errors indicated by the error bars.
Biochemical examination of ERKs in cultured DRG neurons was complicated by the large number of glial cells which also express ERKs. However, the selective expression of B-Raf in DRGs (Fig. 1B) allowed us to directly evaluate B-Raf activity in sensory neurons by immune complex kinase assay in mixed cell cultures. Using this assay, we observed an increase in B-Raf kinase activity following NGF treatment which was inhibited by the presence of LY (Fig. 1C and D).
PI3-K inhibitors block ERK activation by NGF in PC12 cells.
To understand the mechanism through which PI3-K inhibitors block ERK activation by NGF, we examined this pathway in the neuronal model system, PC12 cells. In these cells, NGF activation of ERKs at both 5 and 40 min was blocked by LY (20 μM), as assessed by both Western blotting with pERK antibodies and by immune complex kinase assay (Fig. 2A and B). Raising intracellular cAMP levels by treatment with forskolin and the phosphodiesterase inhibitor IBMX stimulated ERK activation, as expected (98). This activation by forskolin-IBMX was not blocked by LY (Fig. 2A and B) at concentrations that blocked NGF-stimulated PI3-K activity (Fig. 2C). We further examined the role of PI3-K in NGF signaling by monitoring a physiological downstream target of ERKs. Previous studies have shown that NGF-induced gene expression mediated by the transcription factor, CREB, occurs via ERK-dependent mechanisms (15, 24, 84, 104). To determine whether PI3-K played a role in NGF's regulation of physiological targets of ERKs in PC12 cells, we examined CREB-dependent transcription using a GAL4-CREB–Gal-luciferase reporter system. NGF treatment of PC12 cells stimulated CREB-dependent transcription which was blocked by LY (Fig. 2D, left panel). The magnitude of inhibition by LY was similar to that seen with the MEK inhibitor, PD98059 (Fig. 2D, right panel). These studies suggest that the PI3-K-dependent regulation of ERKs in PC12 cells is important in controlling the activity of downstream ERK targets as well.
FIG. 2.
Requirement of PI3-K for ERK activation by NGF in PC12 cells. PC12 cells were left untreated (Untr. and U) or treated with forskolin plus IBMX (F/I) for 20 min or NGF for either 5 min (5′) or 40 min (40′), as indicated. (A) Phosphorylation of ERKs was used as a measure of ERK activation in cell lysates monitored by Western blotting using pERK1/2 antibodies. (B) ERK activation was measured in cell lysates by immune complex kinase assay using an anti-ERK2 antibody and MBP as a substrate. In both assays, LY blocked ERK activation by NGF but not by forskolin. (C) (left) PI3-K assay showing blockade of NGF-induced PI3-K activity by LY (20 μM). (Right) Average values from three assays are presented as fold activation with standard errors indicated by the error bars. (D) LY inhibition of the transcription factor CREB, an ERK-dependent target of NGF. Following transfection with GAL4-CREB (2 μg) and Gal-luciferase (2 μg), cells were left untreated or treated with NGF, LY, or the MEK inhibitor PD98059 (PD) (40 μM) as indicated. CREB-dependent transcription was monitored by luciferase assay and reported as fold increase in luciferase activity. CREB activation by NGF was sensitive to LY as well as PD.
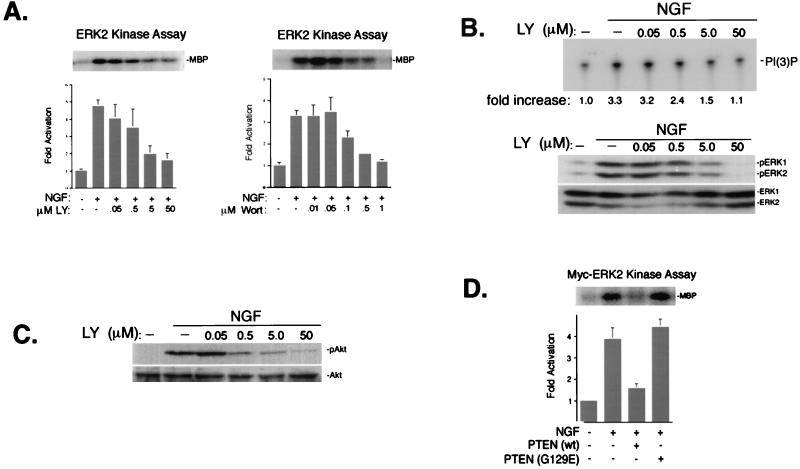
To confirm the specificity of the PI3-K inhibitors, we compared the actions of LY and wortmannin on ERK with their actions on PI3-K. Both LY and wortmannin blocked NGF-induced ERK2 activation in a dose-dependent manner (Fig. 3A). The ability of LY to inhibit NGF's activation of PI3-K (Fig. 3B), as well as the inhibition of the PI3-K-dependent phosphorylation of Akt (Fig. 3C), displayed a similar dose dependence, as did ERK inhibition (Fig. 3A and B). NGF-induced PI3-K and ERK signals also displayed a similar dose response to wortmannin (data not shown). We independently confirmed the role of PI3-K signals in NGF activation of ERKs by attenuating the action of PI3-K activity via the expression of lipid phosphatases which remove the phosphate from the D3 position. Transfection of cDNA encoding PTEN, a PI3-K antagonizing PI(3,4,5)P phosphatase (49), abolished the ability of NGF to activate cotransfected myc-tagged ERK2 (myc-ERK2) (Fig. 3D), suggesting that the lipid products of PI3-K were required for ERK activation. This was confirmed in experiments expressing a mutant PTEN cDNA (PTEN-G129E) encoding a single amino acid substitution which specifically ablates its lipid phosphatase activity (58, 66). PTEN-G129E had no effect on NGF-induced myc-ERK2 activation (Fig. 3D). Together, these experimental results suggest that the ability of LY and wortmannin to inhibit ERK activation by NGF was a function of their specific inhibition of the lipid kinase activity of PI3-K.
FIG. 3.
PI3-K inhibitors block NGF activation of PI3-K and ERK. (A) Dose-dependent action of LY and wortmannin on ERK activation by NGF (15 min). Cells were treated with NGF and either LY (0.05, 0.5, 5.0, and 50 μM) or wortmannin (Wort) (0.01, 0.05, 0.1, 0.5, and 1 μM), as indicated. ERK activation was measured in cell lysates by immune complex kinase assay using an anti-ERK2 antibody and MBP as a substrate. A representative gel is shown (upper panels) along with the average values from three experiments, with standard errors indicated by the error bars (bar graphs). ERK activity is presented as fold activation over basal levels. (B) Dose dependency of LY's inhibition of PI3-K activity. Cells were treated with NGF and LY (0.05, 0.5, 5.0, and 50 μM), and PI3-K activity was measured as described in Materials and Methods (upper panel). Fold increase in PI3-K activity is shown below the top panel. Lower panels show the action of LY on NGF-induced phosphorylation of ERK in the same cell lysates, as measured by Western blotting using pERK antibodies (middle panel). The expression of total ERKs is shown as a loading control (bottom panel). (C) Dose dependency of LY inhibition of pAkt. Cells were treated with NGF and LY (0.05, 0.5, 5.0, and 50 μM), lysates were prepared, and the levels of pAkt were visualized by Western blotting (upper panel). The expression of total Akt is shown (bottom panel). (D) Inhibition of NGF activation of ERK by PTEN. PC12 cells were cotransfected with myc-ERK2 and wild-type (wt) PTEN or a mutated PTEN [PTEN (G129E)] which lacks lipid phosphatase activity. myc-ERK2 activation was measured in cell lysates by immune complex kinase assay using an anti-myc antibody (9E10) and MBP as a substrate. A representative gel is shown (upper panel) along with the average values from three experiments, with standard errors indicated by the error bars (bar graph). myc-ERK2 activity is presented as fold over basal levels.
PI3-K inhibitors block activation of Rap1, but not Ras, by NGF.
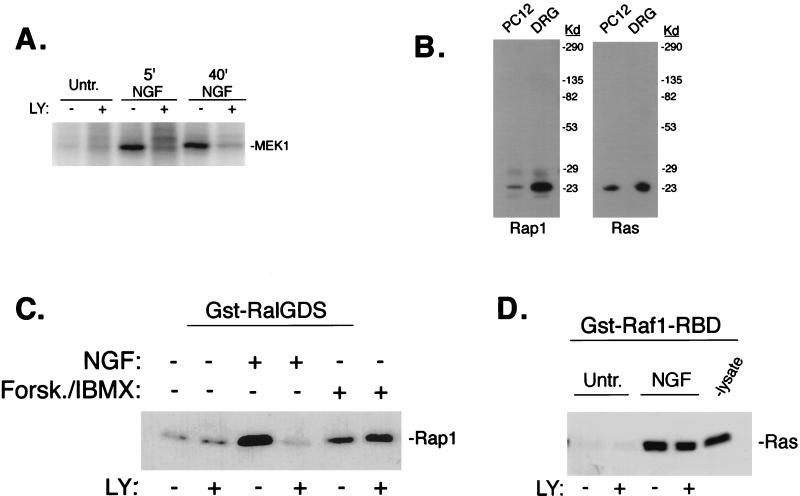
To determine if PI3-K was required for the activation of upstream activators of ERKs in PC12 cells, we analyzed endogenous B-Raf activity by immune complex kinase assay following NGF treatment. Inhibition of PI3-K activity by LY completely blocked B-Raf activation at both early (5 min) and late (40 min) time points (Fig. 4A). Our lab has previously shown that NGF activates B-Raf and ERKs via Ras and Rap1 (106). Figure 4B shows the relative expression of Ras and Rap1 in both PC12 cells and DRGs. To determine the effect of PI3-K inhibition on Ras and Rap1, we monitored their activation by widely used affinity purification protocols. Rap1 activation was examined using GST-RalGDS and Ras activation was examined using GST-Raf1-RBD. Interestingly, pretreatment with LY completely blocked the ability of NGF to activate Rap1 but had no effect on NGF's activation of Ras (Fig. 4C and D). Similar effects were observed with wortmannin (data not shown). In these same cell lysates, the PI3-K-dependent phosphorylation of Akt by NGF was completely inhibited in the presence of LY (data not shown), demonstrating that LY was inhibiting PI3-K in these experiments. As with ERK activation, forskolin-stimulated Rap1 activity was not blocked by LY pretreatment (Fig. 4C). The ability of LY to inhibit NGF activation of Rap1, but not Ras, was also observed using GTP loading assays (data not shown).
FIG. 4.
LY inhibition of Rap1 and B-Raf activation. (A) LY inhibition of NGF-induced B-Raf activation. PC12 cells were left untreated (Untr.) or treated with NGF and LY as indicated, and B-Raf activity was measured by immune complex kinase assay using anti-B-Raf antisera and recombinant MEK-1 as a substrate. 5′ and 40′, 5 and 40 min, respectively. (B) Western blot showing expression of Rap1 and Ras in PC12 cells and DRG cells, as indicated. The positions of molecular mass markers (in kilodaltons) are indicated to the right of the gels. (C) LY inhibition of NGF-induced Rap1 activation. PC12 cells were either left untreated or treated with NGF, forskolin plus IBMX (Forsk./IBMX) and LY as indicated. Lysates were incubated with GST-RalGDS and precipitated with glutathione beads. The amount of active Rap1 bound to the beads was measured by SDS-PAGE followed by Western blotting using anti-Rap1 antibodies. (D) Lack of LY inhibition of NGF-induced Ras activation. Lysates were either left untreated (Untr.) or treated as indicated. Lysates were prepared and incubated with GST-Raf1-RBD and precipitated with glutathione beads. The amount of active Ras bound to the beads was measured by SDS-PAGE followed by Western blotting using anti-Ras antibodies.
Active Ras also requires PI3-K to couple to B-Raf and ERK activation.
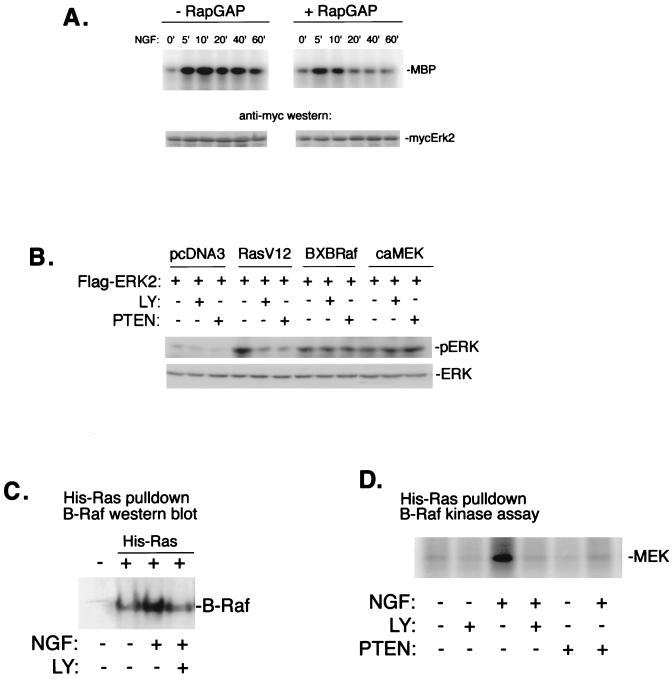
Previous results have suggested that ERK activation 5 min after NGF treatment is independent of Rap1 (106). To confirm this, we have measured ERK activation by NGF in the absence or presence of the specific enzymatic inhibitor of Rap1, Rap1GAP1. As shown in Fig. 5A, the expression of Rap1GAP1 completely inhibited the late phase of ERK activation by NGF (>20 min) with no effect on the early phase (5 min). At 10 min, we observed a partial inhibition of ERK which is consistent with the contribution of both Ras- and Rap1-dependent signals to ERK activation at this time (106). PI3-K inhibitors blocked B-Raf and ERK activation following 5 min of NGF treatment, without affecting Ras activity. This finding suggests that PI3-K activity might also be required at a step between Ras and B-Raf activation. To test this, we examined the requirement of PI3-K for Ras-GTP to activate ERK2. Transfection of cDNA encoding constitutively active Ras (RasV12) induced Flag-ERK2 activation, as assessed by anti-Flag immunoprecipitation followed by pERK Western blotting. This activation was blocked by either cotransfection of PTEN or treatment with LY, while neither PTEN nor LY blocked ERK activation by constitutively active Raf (BXBRaf) or constitutively active MEK (caMEK) (Fig. 5B). These data demonstrate that PI3-K was acting downstream of Ras and upstream of B-Raf. To directly examine the ability of Ras to couple to B-Raf, we monitored the recruitment of B-Raf to Ras upon NGF treatment. NGF stimulation markedly increased the association of B-Raf with Ras. This association was blocked by LY (Fig. 5C). In addition, both LY and PTEN blocked the ability of NGF to stimulate Ras-associated B-Raf kinase activity (Fig. 5D). LY also blocked the ability of B-Raf to associate with NGF-stimulated His-Rap1 (data not shown), as expected, since LY blocked Rap1 activation, as shown in Fig. 4C.
FIG. 5.
Requirement of PI3-K for Ras coupling to downstream kinases. (A) Independence of Rap1 for early activation of ERK by NGF. PC12 cells were transfected with myc-ERK2 in the absence (−) or presence (+) of cotransfected Rap1GAP1 (RapGAP), and treated with NGF for the indicated times (in minutes). myc-ERK2 activation was measured in cell lysates by immune complex kinase assay using an anti-myc antibody and MBP as a substrate. The amount of myc-ERK2 within each lysate is shown in the lower panels. A representative gel is shown (n = 3). (B) PC12 cells were cotransfected with cDNA encoding constitutively active Ras (RasV12), constitutively active Raf (BXBRaf), or constitutively active MEK (caMEK), along with Flag-ERK2, and treated with LY or cotransfected with PTEN, as indicated. Flag-ERK2 activity was assessed by anti-Flag immunoprecipitation followed by pERK Western blotting. The amount of total ERK is shown in the lower panel. (C) Recruitment of B-Raf to Ras upon NGF treatment. PC12 cells were transfected with His-Ras and treated with NGF in the absence or presence of LY as indicated. His-Ras and associated proteins were precipitated from lysates using nickel-NTA agarose, associated proteins were resolved by SDS-PAGE, and B-Raf was detected by Western blotting. The position of B-Raf (95 kDa) is shown. (D) NGF stimulation of Ras-associated B-Raf kinase activity. PC12 cells were transfected with His-Ras and/or PTEN and treated with NGF or LY as indicated. B-Raf activity within His-Ras eluates was measured by immune complex kinase assay using MEK-1 (MEK) as a substrate.
PI3-K inhibition blocks TrkA internalization.
In sensory neurons, PI3-K has been shown to be required for the retrograde transport of 125I-labeled NGF from nerve terminals (70), but whether this action involved endocytosis or subsequent transport steps has not been established. As shown in Fig. 6A and B, the cell surface expression of TrkA in PC12 cells was reduced by NGF, presumably via endocytosis into vesicles containing activated TrkA (28, 29). This effect was largely inhibited in the presence of LY. We detected a reduction in TrkA cell surface expression as early as 5 min of NGF treatment with a peak at 10 min. LY blocked this effect at all time points examined (data not shown). LY completely blocked phosphorylation of Akt, but not TrkA (Fig. 6C and D), demonstrating that LY's actions were specific for targets downstream, but not upstream, of PI3-K. This suggests that PI3-K facilitates the endocytosis of TrkA, similarly to its action on the platelet-derived growth factor receptor (65).
FIG. 6.
Facilitation of NGF-induced TrkA internalization by PI3-K. (A) PC12 cells were left untreated (Untr.) or treated with NGF in the presence (+) or absence (−) of LY, as indicated. Cell surface proteins were biotinylated as described in Materials and Methods. Biotinylated proteins were recovered using UltraLink Immobilized NeutrAvidin (Pierce), and the amount of TrkA recovered was assessed by Western blotting. A representative blot with the position of TrkA is shown. (B) Bar graph showing the averages from three biotinylation experiments as in panel A. The data are presented as percentages of maximal stimulation, with standard errors indicated by the error bars. (C) Phosphorylation of Akt is completely blocked by LY. Parallel plates of PC12 cells were treated as in panel A and examined for pAkt expression by Western blotting. The position of pAkt (upper panel) is shown, along with total Akt (lower panel) as a loading control. (D) Phosphorylation of TrkA is not affected by LY. Cells were treated with LY and NGF as indicated, and lysates were examined for pTrkA expression by Western blotting. A representative blot is shown, and the position of pTrkA is indicated (n = 3).
Rap1 is associated with submembraneous vesicles in PC12 cells.
While both Ras and Rap1 are tightly associated with membranes (4, 56, 103), several studies have shown that they are localized to different subcellular regions. In neuronal cells, Ras and Rap1 display distinct subcellular distributions (38). In other cell types, Ras proteins are known to localize to the plasma membrane (4, 103), while Rap1 expression has been detected in intracellular compartments including the Golgi complex (4, 102) and late endosomes (64). Here, we examined the subcellular localization of Ras and Rap1 using immunofluorescence techniques. In both DRG neurons and PC12 cells, Ras displayed a plasma membrane distribution, while Rap1 was localized to intracellular structures (Fig. 7A). To further characterize these Rap1-containing structures, we examined the subcellular localization of Rap1 in PC12 cells by immunogold electron microscopy. Rap1 expression was not detected in the plasma membrane but was found associated with structures which resemble endocytic vesicles (Fig. 7B). This observation, combined with the ability of PI3-K inhibitors to block TrkA internalization, suggests that one mechanism through which PI3-K inhibitors block NGF-induced Rap1-ERK activation might be through the inhibition of receptor-mediated endocytosis.
FIG. 7.
Subcellular localization of Ras and Rap1 in PC12 cells. (A) Immunofluorescence detection of Ras and Rap1. DRG cells (upper panels) or PC12 cells (lower panels) were fixed and incubated with monoclonal antibodies to Ras and polyclonal antibodies to Rap1. Anti-Ras (left panels) and Anti-Rap1 (right panels) were visualized by confocal microscopy. (B) Immunoelectron microscopy of Rap1. Using immunogold electron microscopy and anti-Rap1 antibodies, immunogold particles were detected on sections of fixed PC12 cells. Two adjacent PC12 cells are shown with their plasma membranes indicated. The nucleus of one cell can be seen. Gold particles are clustered within vesicular structures. Magnification, ×48,000. The insert shows vesicular structures with gold particles.
Blocking endocytosis mimics the effects of PI3-K inhibition on NGF-induced Ras, Rap1, and ERK activation.
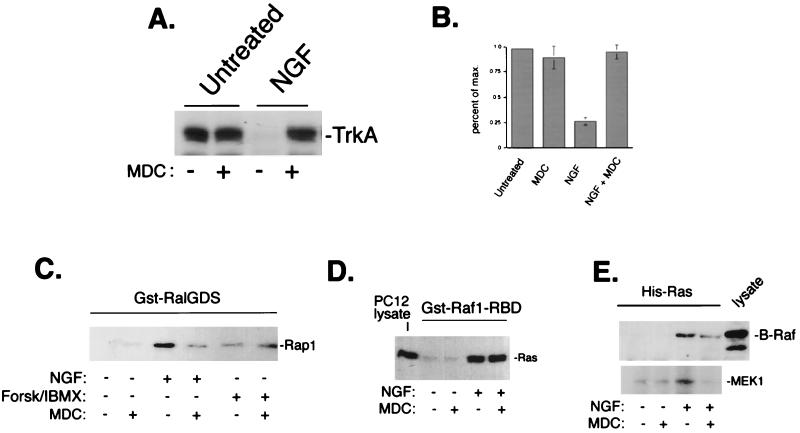
Clathrin-mediated endocytosis is required for ERK activation by EGF (54, 63, 97) and insulin-like growth factor (10). TrkA has also been shown to undergo endocytosis via a clathrin-mediated process (29). Once internalized into endocytic vesicles, TrkA remains phosphorylated and continues to signal to downstream effectors (5, 20, 28, 71, 94, 109), suggesting that internalization may be important for TrkA signaling as well. We employed the primary amine MDC, which is known to block clathrin-mediated endocytosis (68, 80, 82), to examine Ras and Rap1 activation in the absence of TrkA internalization. As expected, MDC pretreatment blocked NGF-mediated endocytosis of TrkA (Fig. 8A and B). At 10 min following NGF treatment of PC12 cells, when both Ras and Rap1 are active, pretreatment with MDC (100 μM) largely inhibited Rap1 activation by NGF but did not inhibit Ras activation (Fig. 8C and D). As seen with LY (Fig. 4B), activation of Rap1 by forskolin plus IBMX was not blocked by MDC (Fig. 8C).
FIG. 8.
MDC inhibition of NGF signaling to ERK. (A) MDC blocks TrkA internalization. PC12 cells were left untreated or treated with NGF in the presence (+) or absence (−) of a 10-min pretreatment with MDC as indicated. Cell surface proteins were biotinylated and precipitated as described in the legend to Fig. 6, and the amount of TrkA recovered was assessed by Western blotting. A representative blot with the position of TrkA is shown. (B) Bar graph showing the averages from three independent experiments as shown in panel A, with standard errors indicated by the error bars. (C) Inhibition of NGF-induced Rap1 activation by MDC. PC12 cells were treated with NGF or forskolin plus IBMX (Forsk/IBMX) for 10 min in the presence or absence of MDC as indicated. Rap1 activation was measured using Gst-RalGDS “pull-down” as described in the legend to Fig. 4. The position of Rap1 is shown. (D) Lack of inhibition of NGF-induced Ras activation by MDC. Cells were treated with NGF and MDC as indicated, and Ras activation was measured using Gst-Raf1-RBD pull-down as described in the legend to Fig. 4. The position of Ras in PC12 lysates is shown. (E) Inhibition of Ras-dependent recruitment and activation of B-Raf by MDC. Cells were transfected with His-Ras and treated with NGF in the presence or absence of MDC as indicated. His-Ras and associated proteins were precipitated from lysates using nickel-NTA agarose, and associated proteins were eluted from His-Ras as described in Materials and Methods. Eluates were split and assayed for associated B-Raf protein by Western blotting (upper panel) or for associated B-Raf kinase activity (lower panel), as measured by immune complex assay using MEK1 as a substrate (n = 3). The position of MEK1 is shown. The position of B-Raf within PC12 lysates is shown in the upper panel (lysate).
Ras recruitment and activation of B-Raf were also blocked by MDC (Fig. 8E). This suggests that endocytosis may be required for ERK activation at two sites, one site upstream of Rap1 activation and a second site downstream of Ras activation. Indeed, examination of ERKs revealed that MDC completely blocked ERK activation at 10 min (Fig. 9A), presumably by blocking both sites. As seen with MDC's inhibition of Rap1, MDC blocked phosphorylation of ERKs by NGF, but not forskolin plus IBMX (Fig. 9A). This inability of LY or MDC to block activation of Rap1 and ERK by forskolin plus IBMX may be due to the nature of the intracellular signal, cAMP, that is generated by forskolin. Unlike NGF, cAMP appears to be able to activate intracellular pathways upstream of Rap1 independently of endocytosis. NGF's activation of ERKs at 10 min was completely abolished following expression of an interfering mutant of dynamin (K44E), known to block clathrin-mediated endocytosis (1, 23, 101) (Fig. 9B and C). This result is consistent with recent evidence demonstrating that dynamin is required for TrkA internalization in PC12 cells (109). As seen in PC12 cells, blocking endocytosis with MDC also blocked ERK activation in DRG neurons, as measured by pERK immunofluorescence (Fig. 9D).
FIG. 9.
Requirement of endocytosis for ERK activation by NGF in PC12 and DRG cells. (A) PC12 cells were treated with NGF or forskolin plus IBMX (Forsk./IBMX) for 10 min in the presence (+) or absence (−) of MDC, as indicated. Activation of ERK was measured in cell lysates by Western blotting using pERK1/2 antibodies. The positions of pERKs (pErk1 and pErk2) are shown. The amount of total ERKs in each lysate is shown in the lower panel. (B) PC12 cells were transfected with myc-ERK2 in the presence or absence of either wild-type dynamin (Dyn-WT) or a mutated dynamin (Dyn-K44E), and cells were treated with NGF (N) or EGF (E) or left untreated (U). myc-ERK2 activation was measured in cell lysates by immune complex kinase assay using an anti-myc antibody and MBP as a substrate. A representative gel is shown (upper panel) and the amount of myc-ERK2 within each lysate is shown below (lower panel). (C) Data from three independent experiments as performed in panel B are represented as fold activation, with standard errors indicated by the error bars. (D) Adult rat DRG cultures were treated for 20 min with NGF in the presence or absence of MDC. Fluorescence micrograph showing pERK1/2 immunoreactivity (upper panels) and light-field micrographs (lower panels) are shown.
DISCUSSION
In this study, we show that NGF activation of B-Raf and ERK signaling in both DRG cultures and PC12 cells is blocked by inhibition of PI3-K. Multiple methods of inhibiting PI3-K, using both pharmacological and molecular agents, all had the same effect. Furthermore, this requirement of PI3-K was specific for NGF; the ability of cAMP to activate ERKs in both DRGs and PC12 cells did not require PI3-K.
We have previously demonstrated that both Ras and Rap1 pathways contribute to the activation of ERKs by NGF. Indeed, the co-ordinated signaling via these two pathways accounts for the ability of NGF to induce both sustained activation of ERKs and neuronal differentiation (106). Interestingly, in this study, we demonstrate that the requirement for PI3-K in ERK activation by NGF may reflect distinct actions on signaling via Ras and Rap1. Thus, PI3-K inhibition blocked activation of Rap1 but not Ras. However, PI3-K did block the ability of activated Ras to couple to B-Raf and ERKs. Both actions of PI3-K prevent NGF from utilizing either Ras- or Rap1-dependent signals to activate ERKs.
Our data demonstrating the requirement for PI3-K in the activation of Rap1 may reflect a role in the endocytosis of activated TrkA receptors. We found that inhibition of PI3-K activity blocked TrkA internalization after NGF stimulation. Other blockers of endocytosis had the same effect as PI3-K inhibitors, preventing activation of Rap1 but not Ras. Taken together, we propose a model in which TrkA activation can stimulate Ras at the plasma membrane but requires PI3-K-dependent internalization to activate Rap1 (Fig. 10).
FIG. 10.
Model of NGF signaling to ERKs. In PC12 cells, NGF activates Ras and Rap1 to mediate the rapid and sustained activation of ERKs, respectively. Both TrkA internalization and Rap1 activation require PI3-K. Clathrin-mediated endocytosis is also required for Rap1 activation and the sustained activation of ERK. In contrast, Ras activation by NGF is independent of both PI3-K activity and endocytosis. This may reflect the distinct localizations of Ras at the plasma membrane and Rap1 within endosomal compartments. PI3-K-dependent endocytosis may regulate ERK signaling at a second site downstream of Ras, since both rapid ERK activation by NGF and Ras activation of B-Raf are blocked by both PI3-K inhibitors and inhibitors of endocytosis.
A role for endocytosis in NGF signaling is consistent with the emerging view that internalization of active TrkA receptors into signaling vesicles is required for the downstream actions of neurotrophins (109). For example, the ability of both NGF and TrkA to be transported in a retrograde fashion from the nerve terminal to the cell body has been demonstrated by many groups (5, 71, 83, 95). Recent studies demonstrate that this retrograde transport process delivers active TrkA to the cell body which may be required for the stimulation of gene expression by NGF (5, 71, 94). These conclusions are supported by studies in which signaling-competent vesicles containing active NGF-TrkA complexes were isolated from PC12 cells following clathrin-mediated endocytosis of TrkA (28, 29, 32). Furthermore, these data are consistent with those of studies examining other growth factor receptors, as well as G-protein-coupled receptors, where clathrin-mediated endocytosis has been implicated in ERK activation (10, 14, 54, 63, 97). Therefore, receptor trafficking may serve an important signaling function in addition to simply mediating receptor down-regulation via lysosomal degradation. It is possible that the sorting determinants and mechanisms for targeting proteins to signaling vesicles versus lysosomes may be differentially regulated for different ligand-receptor complexes (46, 99, 107, 108). Whether such sorting events impart specificity in the ability of growth factors to activate Rap1-dependent pathways remains to be determined.
A proposed role for endocytosis is to bring activated receptors to the location of downstream signaling molecules (9). Accordingly, the localization of Ras and Rap1 to distinct membrane compartments may account for the differential roles of PI3-K and endocytosis in Ras and Rap1 activation. Our data showing that Rap1 resides with vesicular membranes are consistent with the localization of Rap1 to endosomal compartments. In contrast, Ras is at the plasma membrane itself and can be activated by TrkA without additional endocytic events. Membrane targeting of Ras-like molecules is determined by postranslational modifications of their C termini. Differences in the C-terminal motifs found in Rap1 and Ras may account for their different membrane distribution (11, 50, 69). Importantly, these differences in membrane distribution of Ras family members influence downstream signaling actions (69). Our data suggest that their location may also determine how these small G proteins become activated.
PI3-K-dependent TrkA internalization may control differential activation of Ras versus Rap1 signaling to dictate the specificity of NGF action. Our lab has previously shown that in PC12 cells Rap1 is activated by NGF (106), but not EGF (98). Furthermore, we have shown that Rap1-dependent signals contribute to growth factor specificity by mediating both the sustained phase of ERK activation by NGF and aspects of neuronal differentiation (106). Differences in the kinetics of receptor internalization have also been proposed to impart specificity to NGF versus EGF signaling in PC12 cells (32). In this study, Huang et al. have shown that TrkA remains associated with caveola-like membranes following NGF treatment, whereas EGF treatment results in the rapid depletion of the EGF receptor from this membrane population. Consistent with these results, we have observed a more rapid internalization of the EGF receptor compared to TrkA following ligand stimulation (data not shown).
The slower internalization of TrkA following NGF treatment may allow for the assembly of signaling complexes which subsequently lead to Rap1 activation within the mature signaling endosome. It has been shown that the phosphorylation of the adapter molecule FRS2 recruits additional adapter molecules that contribute to the sustained ERK activation seen following treatment with NGF (43). One of these adapter molecules which binds phosphorylated FRS2 is Crk (55). Crk has been shown to contribute to Rap1 activation via its association with C3G, the Rap1-specific exchanger (33, 61, 106). Accelerating TrkA internalization by incubating PC12 cells with an NGF-antibody complex results in a transient activation of ERKs similar to that seen with EGF treatment (75). Interestingly, acceleration of TrkA internalization resulted in a TrkA signaling complex that lacked critical phosphorylations, including phosphorylation of FRS2, that are required for sustained activation of ERKs. Here, we propose that the contributions of Rap1 signaling and endocytosis to sustained ERK activation are intimately connected; Rap1 participates in the late phase of ERK activation by NGF because Rap1 activation by NGF requires endocytic events.
One finding of this study was that the ability of Ras to couple to its downstream effector, B-Raf, was also blocked by inhibitors of PI3-K. This action explains the contribution of PI3-K to the early (Ras-dependent) activation of ERKs. This inhibition of Ras-dependent signaling downstream of Ras activation may also reflect a requirement of PI3-K for endocytic events. B-Raf is localized to vesicles within DRG neurons and PC12 cells, and this localization did not appear to change following NGF treatment (data not shown). Interestingly, Grimes and coworkers (29) have shown that NGF treatment leads to the redistribution of TrkA immunostaining to a pattern similar to that seen for B-Raf. Therefore, it is possible that endocytosis and subsequent vesicular fusion are required to bring Ras in contact with B-Raf, consistent with the ability of MDC to disrupt Ras–B-Raf complexes. This model is consistent with recent evidence suggesting that Ras is internalized into endocytic vesicles following growth factor stimulation (72). The requirement of PI3-K in Ras-dependent activation of B-Raf may also depend on a second action of PI3-K. For example, recent studies suggest that phosphorylation of Raf-1 by kinases downstream of PI3-K may stimulate Raf kinase activity (74, 87). In contrast, the direct phosphorylation of Raf-1 by the PI3-K target, Akt, has been shown to be inhibitory in some cells (79, 111). However, this ability of Akt to inhibit Raf-1 appears to depend on the cell type and cellular context (73). A similar study examining B-Raf has also been reported (30), although no studies have been performed with neuronal cells. In any case, the data presented here demonstrate that PI3-K has indirect actions that enhance signaling through B-Raf.
The major significance of this study is that we show PI3-K is required for NGF activation of ERKs through its specific action in regulating TrkA internalization. Both PI3-K and ERKs are well-studied targets downstream of NGF. A prevailing view is that they represent the end points of distinct linear pathways and trigger distinct physiological actions. However, recent reports suggest that these pathways may be interconnected (34, 39, 41). Here, we provide a biochemical basis for a model that these two pathways share overlapping functions within neurons and suggest that the regulation of endocytosis by PI3-K may provide an important mechanism to modulate ERK signaling cascades.
ACKNOWLEDGMENTS
We are grateful to Johannes Bos and Michael Gold for providing reagents. We thank William Mobley for helpful scientific discussions and sharing unpublished data. We are grateful to William Sellers for helpful discussions and providing PTEN and PTEN mutants for this study. We thank Michelle Bobo for technical assistance and Lorene Langeberg and John Scott for help with confocal microscopy. We also thank Kendall Carey for technical and scientific support.
P.J.S.S. was supported in part by funds from the National Cancer Institute.
REFERENCES
- 1.Altschuler Y, Barbas S M, Terlecky L J, Tang K, Hardy S, Mostov K E, Schmid S L. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J Cell Biol. 1998;143:1871–1881. doi: 10.1083/jcb.143.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Averill S, McMahon S B, Clary D O, Reichardt L F, Priestley J V. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avruch J, Zhang X-F, Kyriakis J M. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 4.Beranger F, Goud B, Tavitian A, de Gunzburg J. Association of the Ras-antagonistic Rap1/Krev-1 proteins with the Golgi complex. Proc Natl Acad Sci USA. 1991;88:1606–1610. doi: 10.1073/pnas.88.5.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharyya A, Watson F L, Bradlee T A, Pomeroy S L, Stiles C D, Segal R A. Trk receptors function as rapid retrograde signal carriers in the adult nervous system. J Neurosci. 1997;17:7007–7016. doi: 10.1523/JNEUROSCI.17-18-07007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busca R, Abbe P, Mantoux F, Aberdam E, Peyssonnaux C, Eychene A, Ortonne J P, Ballotti R. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000;19:2900–2910. doi: 10.1093/emboj/19.12.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardinaux J R, Notis J C, Zhang Q, Vo N, Craig J C, Fass D M, Brennan R G, Goodman R H. Recruitment of CREB binding protein is sufficient for CREB-mediated gene activation. Mol Cell Biol. 2000;20:1546–1552. doi: 10.1128/mcb.20.5.1546-1552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catling A D, Reuter C W, Cox M E, Parsons S J, Weber M J. Partial purification of a mitogen-activated protein kinase kinase activator from bovine brain. Identification as B-Raf or a B-Raf-associated activity. J Biol Chem. 1994;269:30014–30021. [PubMed] [Google Scholar]
- 9.Ceresa B P, Schmid S L. Regulation of signal transduction by endocytosis. Curr Opin Cell Biol. 2000;12:204–210. doi: 10.1016/s0955-0674(99)00077-0. [DOI] [PubMed] [Google Scholar]
- 10.Chow J C, Condorelli G, Smith R J. Insulin-like growth factor-I receptor internalization regulates signaling via the Shc/mitogen-activated protein kinase pathway, but not the insulin receptor substrate-1 pathway. J Biol Chem. 1998;273:4672–4680. doi: 10.1074/jbc.273.8.4672. [DOI] [PubMed] [Google Scholar]
- 11.Choy E, Chiu V K, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov I E, Philips M R. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 12.Cook S P, Rodland K D, McCleskey E W. A memory for extracellular Ca2+ by speeding recovery of P2X receptors from desensitization. J Neurosci. 1998;18:9238–9244. doi: 10.1523/JNEUROSCI.18-22-09238.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross D A, Watt P W, Shaw M, van der Kaay J, Downes C P, Holder J C, Cohen P. Insulin activates protein kinase B, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett. 1997;406:211–215. doi: 10.1016/s0014-5793(97)00240-8. [DOI] [PubMed] [Google Scholar]
- 14.Daaka Y, Luttrell L M, Ahn S, Della Rocca G J, Ferguson S S, Caron M G, Lefkowitz R J. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- 15.Deak M, Clifton A D, Lucocq L M, Alessi D R. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desbois-Mouthon C, Blivet-Van Eggelpoel M J, Auclair M, Cherqui G, Capeau J, Caron M. Insulin differentially regulates SAPKs/JNKs and ERKs in CHO cells overexpressing human insulin receptors. Biochem Biophys Res Commun. 1998;243:765–770. doi: 10.1006/bbrc.1998.8181. [DOI] [PubMed] [Google Scholar]
- 17.Duckworth B C, Cantley L C. Conditional inhibition of the mitogen-activated protein kinase cascade by wortmannin. Dependence on signal strength. J Biol Chem. 1997;272:27665–27670. doi: 10.1074/jbc.272.44.27665. [DOI] [PubMed] [Google Scholar]
- 18.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 19.Dugan L L, Kim J S, Zhang Y, Bart R D, Sun Y, Holtzman D M, Gutmann D H. Differential effects of cAMP in neurons and astrocytes. Role of B-raf. J Biol Chem. 1999;274:25842–25848. doi: 10.1074/jbc.274.36.25842. [DOI] [PubMed] [Google Scholar]
- 20.Ehlers M D, Kaplan D R, Price D L, Koliatsos V E. NGF-stimulated retrograde transport of trkA in the mammalian nervous system. J Cell Biol. 1995;130:149–156. doi: 10.1083/jcb.130.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke B, Akkerman J W, Bos J L. Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 1997;16:252–259. doi: 10.1093/emboj/16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frevert E U, Kahn B B. Differential effects of constitutively active phosphatidylinositol 3-kinase on glucose transport, glycogen synthase activity, and DNA synthesis in 3T3-L1 adipocytes. Mol Cell Biol. 1997;17:190–198. doi: 10.1128/mcb.17.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gagnon A W, Kallal L, Benovic J L. Role of clathrin-mediated endocytosis in agonist-induced down-regulation of the beta2-adrenergic receptor. J Biol Chem. 1998;273:6976–6981. doi: 10.1074/jbc.273.12.6976. [DOI] [PubMed] [Google Scholar]
- 24.Ginty D D, Bonni A, Greenberg M E. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 25.Grammer T C, Blenis J. Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene. 1997;14:1635–1642. doi: 10.1038/sj.onc.1201000. [DOI] [PubMed] [Google Scholar]
- 26.Grewal S S, York R D, Stork P J. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths G, McDowall A, Back R, Dubochet J. On the preparation of cryosections for immunocytochemistry. J Ultrastruct Res. 1984;89:65–78. doi: 10.1016/s0022-5320(84)80024-6. [DOI] [PubMed] [Google Scholar]
- 28.Grimes M L, Beattie E, Mobley W C. A signaling organelle containing the nerve growth factor-activated receptor tyrosine kinase, TrkA. Proc Natl Acad Sci USA. 1997;94:9909–9914. doi: 10.1073/pnas.94.18.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimes M L, Zhou J, Beattie E C, Yuen E C, Hall D E, Valletta J S, Topp K S, LaVail J H, Bunnett N W, Mobley W C. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan K L, Figueroa C, Brtva T R, Zhu T, Taylor J, Barber T D, Vojtek A B. Negative regulation of the serine/threonine kinase B-Raf by Akt. J Biol Chem. 2000;275:27354–27359. doi: 10.1074/jbc.M004371200. [DOI] [PubMed] [Google Scholar]
- 31.Hu Q, Klippel A, Muslin A J, Fantl W J, Williams L T. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science. 1995;268:100–102. doi: 10.1126/science.7701328. [DOI] [PubMed] [Google Scholar]
- 32.Huang C S, Zhou J, Feng A K, Lynch C C, Klumperman J, DeArmond S J, Mobley W C. Nerve growth factor signaling in caveolae-like domains at the plasma membrane. J Biol Chem. 1999;274:36707–36714. doi: 10.1074/jbc.274.51.36707. [DOI] [PubMed] [Google Scholar]
- 33.Ichiba T, Kuraishi Y, Sakai O, Nagata S, Groffen J, Kurata T, Hattori S, Matsuda M. Enhancement of guanine-nucleotide exchange activity of C3G for Rap1 by the expression of Crk, CrkL, and Grb2. J Biol Chem. 1997;272:22215–22220. doi: 10.1074/jbc.272.35.22215. [DOI] [PubMed] [Google Scholar]
- 34.Jackson T R, Blader I J, Hammonds-Odie L P, Burga C R, Cooke F, Hawkins P T, Wolf A G, Heldman K A, Theibert A B. Initiation and maintenance of NGF-stimulated neurite outgrowth requires activation of a phosphoinositide 3-kinase. J Cell Sci. 1996;109:289–300. doi: 10.1242/jcs.109.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaiswal R K, Moodie S A, Wolfman A, Landreth G E. The mitogen-activated protein kinase cascade is activated by B-Raf in response to nerve growth factor through interaction with p21ras. Mol Cell Biol. 1994;14:6944–6953. doi: 10.1128/mcb.14.10.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaiswal R K, Weissinger E, Kolch W, Landreth G E. Nerve growth factor-mediated activation of the mitogen-activated protein (MAP) kinase cascade involves a signaling complex containing B-Raf and HSP90. J Biol Chem. 1996;271:23626–23629. doi: 10.1074/jbc.271.39.23626. [DOI] [PubMed] [Google Scholar]
- 37.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Mizoguchi A, Kikuchi A, Takai Y. Tissue and subcellular distributions of the smg-21/rap1/Krev-1 proteins which are partly distinct from those of c-ras p21s. Mol Cell Biol. 1990;10:2645–2652. doi: 10.1128/mcb.10.6.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura N, Hayafuji C, Kimura N. Characterization of 17-β-estradiol-dependent and -independent somatostatin receptor subtypes in rat anterior pituitary. J Biol Chem. 1989;264:7033–7040. [PubMed] [Google Scholar]
- 40.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kita Y, Kimura K D, Kobayashi M, Ihara S, Kaibuchi K, Kuroda S, Ui M, Iba H, Konishi H, Kikkawa U, Nagata S, Fukui Y. Microinjection of activated phosphatidylinositol-3 kinase induces process outgrowth in rat PC12 cells through the Rac-JNK signal transduction pathway. J Cell Sci. 1998;111:907–915. doi: 10.1242/jcs.111.7.907. [DOI] [PubMed] [Google Scholar]
- 42.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kouhara H, Hadari Y R, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 44.Ladiwala U, Lachance C, Simoneau S J, Bhakar A, Barker P A, Antel J P. p75 neurotrophin receptor expression on adult human oligodendrocytes: signaling without cell death in response to NGF. J Neurosci. 1998;18:1297–1304. doi: 10.1523/JNEUROSCI.18-04-01297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leevers S J, Vanhaesebroeck B, Waterfield M D. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 46.Lenferink A E, Pinkas-Kramarski R, van de Poll M L, van Vugt M J, Klapper L N, Tzahar E, Waterman H, Sela M, van Zoelen E J, Yarden Y. Differential endocytic routing of homo- and hetero-dimeric ErbB tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 1998;17:3385–3397. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez-Llasaca M, Crespo P, Pellicci P G, Gutkind J S, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase γ. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 48.MacNicol M C, Muslin A J, MacNicol A M. Disruption of the 14-3-3 binding site within the B-Raf kinase domain uncouples catalytic activity from PC12 cell differentiation. J Biol Chem. 2000;275:3803–3809. doi: 10.1074/jbc.275.6.3803. [DOI] [PubMed] [Google Scholar]
- 49.Maehama T, Dixon J E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 50.Magee T, Marshall C. New insights into the interaction of Ras with the plasma membrane. Cell. 1999;98:9–12. doi: 10.1016/S0092-8674(00)80601-7. [DOI] [PubMed] [Google Scholar]
- 51.Marais R, Light Y, Paterson H F, Mason C S, Marshall C J. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272:4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 52.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 53.Martin K C, Michael D, Rose J C, Barad M, Casadio A, Zhu H, Kandel E R. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 54.Maudsley S, Pierce K L, Zamah A M, Miller W E, Ahn S, Daaka Y, Lefkowitz R J, Luttrell L M. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem. 2000;275:9572–9580. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- 55.Meakin S O, MacDonald J I, Gryz E A, Kubu C J, Verdi J M. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J Biol Chem. 1999;274:9861–9870. doi: 10.1074/jbc.274.14.9861. [DOI] [PubMed] [Google Scholar]
- 56.Mineo C, Anderson R G, White M A. Physical association with ras enhances activation of membrane-bound raf (RafCAAX) J Biol Chem. 1997;272:10345–10348. doi: 10.1074/jbc.272.16.10345. [DOI] [PubMed] [Google Scholar]
- 57.Molliver D C, Radeke M J, Feinstein S C, Snider W D. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- 58.Myers M P, Pass I, Batty I H, Van der Kaay J, Stolarov J P, Hemmings B A, Wigler M H, Downes C P, Tonks N K. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci USA. 1998;95:13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura K, Zhou C J, Parente J, Chew C S. Parietal cell MAP kinases: multiple activation pathways. Am J Physiol. 1996;271:G640–G649. doi: 10.1152/ajpgi.1996.271.4.G640. [DOI] [PubMed] [Google Scholar]
- 60.Nakamura M, Nakashima S, Katagiri Y, Nozawa Y. Effect of wortmannin and 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) on N-formyl-methionyl-leucyl-phenylalanine-induced phospholipase D activation in differentiated HL60 cells: possible involvement of phosphatidylinositol 3-kinase in phospholipase D activation. Biochem Pharmacol. 1997;53:1929–1936. doi: 10.1016/s0006-2952(97)00169-x. [DOI] [PubMed] [Google Scholar]
- 61.Nosaka Y, Arai A, Miyasaka N, Miura O. CrkL mediates ras-dependent activation of the Raf/ERK pathway through the guanine nucleotide exchange factor C3G in hematopoietic cells stimulated with erythropoietin or interleukin-3. J Biol Chem. 1999;274:30154–30162. doi: 10.1074/jbc.274.42.30154. [DOI] [PubMed] [Google Scholar]
- 62.Pestina T I, Jackson C W, Stenberg P E. Abnormal subcellular distribution of myosin and talin in Wistar Furth rat platelets. Blood. 1995;85:2436–2446. [PubMed] [Google Scholar]
- 63.Pierce K L, Maudsley S, Daaka Y, Luttrell L M, Lefkowitz R J. Role of endocytosis in the activation of the extracellular signal-regulated kinase cascade by sequestering and nonsequestering G protein-coupled receptors. Proc Natl Acad Sci USA. 2000;97:1489–1494. doi: 10.1073/pnas.97.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pizon V, Desjardins M, Bucci C, Parton R G, Zerial M. Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the Golgi complex. J Cell Sci. 1994;107:1661–1670. doi: 10.1242/jcs.107.6.1661. [DOI] [PubMed] [Google Scholar]
- 65.Rakhit S, Pyne S, Pyne N J. The platelet-derived growth factor receptor stimulation of p42/p44 mitogen-activated protein kinase in airway smooth muscle involves a G-protein-mediated tyrosine phosphorylation of Gab1. Mol Pharmacol. 2000;58:413–420. doi: 10.1124/mol.58.2.413. [DOI] [PubMed] [Google Scholar]
- 66.Ramaswamy S, Nakamura N, Vazquez F, Batt D B, Perera S, Roberts T M, Sellers W R. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rameh L E, Cantley L C. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 68.Ray E, Samanta A K. Dansyl cadaverine regulates ligand induced endocytosis of interleukin-8 receptor in human polymorphonuclear neutrophils. FEBS Lett. 1996;378:235–239. doi: 10.1016/0014-5793(95)01462-4. [DOI] [PubMed] [Google Scholar]
- 69.Reuther G W, Der C J. The Ras branch of small GTPases: Ras family members don't fall far from the tree. Curr Opin Cell Biol. 2000;12:157–165. doi: 10.1016/s0955-0674(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 70.Reynolds A J, Bartlett S E, Hendry I A. Signalling events regulating the retrograde axonal transport of 125I-beta nerve growth factor in vivo. Brain Res. 1998;798:67–74. doi: 10.1016/s0006-8993(98)00396-5. [DOI] [PubMed] [Google Scholar]
- 71.Riccio A, Pierchala B A, Ciarallo C L, Ginty D D. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 72.Rizzo M A, Shome K, Watkins S C, Romero G. The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid and is independent of association with Ras. J Biol Chem. 2000;275:23911–23918. doi: 10.1074/jbc.M001553200. [DOI] [PubMed] [Google Scholar]
- 73.Rommel C, Clarke B A, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos G D, Glass D J. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 74.Sajan M P, Standaert M L, Bandyopadhyay G, Quon M J, Burke T R, Jr, Farese R V. Protein kinase C-zeta and phosphoinositide-dependent protein kinase-1 are required for insulin-induced activation of ERK in rat adipocytes. J Biol Chem. 1999;274:30495–30500. doi: 10.1074/jbc.274.43.30495. [DOI] [PubMed] [Google Scholar]
- 75.Saragovi H U, Zheng W, Maliartchouk S, DiGugliemo G M, Mawal Y R, Kamen A, Woo S B, Cuello A C, Debeir T, Neet K E. A TrkA-selective, fast internalizing nerve growth factor-antibody complex induces trophic but not neuritogenic signals. J Biol Chem. 1998;273:34933–34940. doi: 10.1074/jbc.273.52.34933. [DOI] [PubMed] [Google Scholar]
- 76.Sarbassov D D, Peterson C A. Insulin receptor substrate-1 and phosphatidylinositol 3-kinase regulate extracellular signal-regulated kinase-dependent and -independent signaling pathways during myogenic differentiation. Mol Endocrinol. 1998;12:1870–1878. doi: 10.1210/mend.12.12.0205. [DOI] [PubMed] [Google Scholar]
- 77.Schaeffer H J, Weber M J. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scheid M P, Duronio V. Phosphatidylinositol 3-OH kinase activity is not required for activation of mitogen-activated protein kinase by cytokines. J Biol Chem. 1996;271:18134–18139. doi: 10.1074/jbc.271.30.18134. [DOI] [PubMed] [Google Scholar]
- 79.Scheid M P, Woodgett J R. Protein kinases: six degrees of separation? Curr Biol. 2000;10:R191–R194. doi: 10.1016/s0960-9822(00)00349-3. [DOI] [PubMed] [Google Scholar]
- 80.Schlegel R, Dickson R B, Willingham M C, Pastan I H. Amantadine and dansylcadaverine inhibit vesicular stomatitis virus uptake and receptor-mediated endocytosis of alpha 2-macroglobulin. Proc Natl Acad Sci USA. 1982;79:2291–2295. doi: 10.1073/pnas.79.7.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schmitt J M, Stork P J S. β2-Adrenergic receptor activates extracellular signal-regulated kinases (ERKs) via the small G protein Rap1 and the serine/threonine kinase B-Raf. J Biol Chem. 2000;275:25342–25350. doi: 10.1074/jbc.M003213200. [DOI] [PubMed] [Google Scholar]
- 82.Schutze S, Machleidt T, Adam D, Schwandner R, Wiegmann K, Kruse M L, Heinrich M, Wickel M, Kronke M. Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling. J Biol Chem. 1999;274:10203–10212. doi: 10.1074/jbc.274.15.10203. [DOI] [PubMed] [Google Scholar]
- 83.Senger D L, Campenot R B. Rapid retrograde tyrosine phosphorylation of trkA and other proteins in rat sympathetic neurons in compartmented cultures. J Cell Biol. 1997;138:411–421. doi: 10.1083/jcb.138.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shaywitz A J, Greenberg M E. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 85.Shepherd P R, Nave B T, Rincon J, Haigh R J, Foulstone E, Proud C, Zierath J R, Siddle K, Wallberg-Henriksson H. Involvement of phosphoinositide 3-kinase in insulin stimulation of MAP-kinase and phosphorylation of protein kinase-B in human skeletal muscle: implications for glucose metabolism. Diabetologia. 1997;40:1172–1177. doi: 10.1007/s001250050803. [DOI] [PubMed] [Google Scholar]
- 86.Suga J, Yoshimasa Y, Yamada K, Yamamoto Y, Inoue G, Okamoto M, Hayashi T, Shigemoto M, Kosaki A, Kuzuya H, Nakao K. Differential activation of mitogen-activated protein kinase by insulin and epidermal growth factor in 3T3-L1 adipocytes: a possible involvement of PI3-kinase in the activation of the MAP kinase by insulin. Diabetes. 1997;46:735–741. doi: 10.2337/diab.46.5.735. [DOI] [PubMed] [Google Scholar]
- 87.Sun H, King A J, Diaz H B, Marshall M S. Regulation of the protein kinase raf-1 by oncogenic ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak. Curr Biol. 2000;10:281–284. doi: 10.1016/s0960-9822(00)00359-6. [DOI] [PubMed] [Google Scholar]
- 88.Sun P, Enslen H, Myung P S, Maurer R A. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- 89.Sutor S L, Vroman B T, Armstrong E A, Abraham R T, Karnitz L M. A phosphatidylinositol 3-kinase-dependent pathway that differentially regulates c-Raf and A-Raf. J Biol Chem. 1999;274:7002–7010. doi: 10.1074/jbc.274.11.7002. [DOI] [PubMed] [Google Scholar]
- 90.Swanson D J, Zellmer E, Lewis E J. AP1 proteins mediate the cAMP response of the dopamine beta-hydroxylase gene. J Biol Chem. 1998;273:24065–24074. doi: 10.1074/jbc.273.37.24065. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtani T, Yamanaka Y, Nishida K, Nakajima K, Hibi M, Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takuwa N, Fukui Y, Takuwa Y. Cyclin D1 expression mediated by phosphatidylinositol 3-kinase through mTOR-p70S6K-independent signaling in growth factor-stimulated NIH 3T3 fibroblasts. Mol Cell Biol. 1999;19:1346–1358. doi: 10.1128/mcb.19.2.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tokuyasu K T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989;21:163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- 94.Tsui-Pierchala B A, Ginty D D. Characterization of an NGF-P-TrkA retrograde-signaling complex and age-dependent regulation of TrkA phosphorylation in sympathetic neurons. J Neurosci. 1999;19:8207–8218. doi: 10.1523/JNEUROSCI.19-19-08207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ure D R, Campenot R B. Retrograde transport and steady-state distribution of 125I-nerve growth factor in rat sympathetic neurons in compartmented cultures. J Neurosci. 1997;17:1282–1290. doi: 10.1523/JNEUROSCI.17-04-01282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vanhaesebroeck B, Leevers S J, Panayotou G, Waterfield M D. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 97.Vieira A V, Lamaze C, Schmid S L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 98.Vossler M R, Yao H, York R D, Pan M G, Rim C S, Stork P J S. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 99.Waterman H, Sabanai I, Geiger B, Yarden Y. Alternative intracellular routing of ErbB receptors may determine signaling potency. J Biol Chem. 1998;273:13819–13827. doi: 10.1074/jbc.273.22.13819. [DOI] [PubMed] [Google Scholar]
- 100.Wennström S, Downward J. Role of phosphoinositide 3-kinase in activation of Ras and mitogen-activated protein kinase by epidermal growth factor. Mol Cell Biol. 1999;19:4279–4288. doi: 10.1128/mcb.19.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Whistler J L, von Zastrow M. Dissociation of functional roles of dynamin in receptor-mediated endocytosis and mitogenic signal transduction. J Biol Chem. 1999;274:24575–24578. doi: 10.1074/jbc.274.35.24575. [DOI] [PubMed] [Google Scholar]
- 102.Wienecke R, Maize J C, Jr, Shoarinejad F, Vass W C, Reed J, Bonifacino J S, Resau J H, de Gunzburg J, Yeung R S, DeClue J E. Colocalization of the TSC2 product tuberin with its target Rap1 in the Golgi apparatus. Oncogene. 1996;13:913–923. [PubMed] [Google Scholar]
- 103.Wu C, Butz S, Ying Y, Anderson R G. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. J Biol Chem. 1997;272:3554–3559. doi: 10.1074/jbc.272.6.3554. [DOI] [PubMed] [Google Scholar]
- 104.Xing J, Kornhauser J M, Zhengui X, Thiele E A, Greenberg M E. Nerve growth factor activates extracellular signal-related kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamamori B, Juroda S, Shimizu K, Fukui K, Ohtsuka T, Takai Y. Purification of a Ras-dependent mitogen-activated protein kinase kinase kinase from bovine brain cytosol and its identification as a complex of B-Raf and 14-3-3 proteins. J Biol Chem. 1995;270:11723–11726. doi: 10.1074/jbc.270.20.11723. [DOI] [PubMed] [Google Scholar]
- 106.York R D, Yao H, Dillon T, Ellig C L, Eckert S P, McCleskey E W, Stork P J. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 107.Zapf-Colby A, Hsu D, Olefsky J M. Comparison of the intracellular itineraries of insulin-like growth factor-I and insulin and their receptors in Rat-1 fibroblasts. Endocrinology. 1994;134:2445–2452. doi: 10.1210/endo.134.6.8194471. [DOI] [PubMed] [Google Scholar]
- 108.Zapf-Colby A, Olefsky J M. Nerve growth factor processing and trafficking events following TrkA-mediated endocytosis. Endocrinology. 1998;139:3232–3240. doi: 10.1210/endo.139.7.6122. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y, Moheban D B, Conway B R, Bhattacharyya A, Segal R A. Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci. 2000;20:5671–5678. doi: 10.1523/JNEUROSCI.20-15-05671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng C F, Ohmichi M, Saltiel A R, Guan K L. Growth factor induced MEK activation is primarily mediated by an activator different from c-raf. Biochemistry. 1994;33:5595–5599. doi: 10.1021/bi00184a031. [DOI] [PubMed] [Google Scholar]
- 111.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]