Abstract
BACKGROUND:
Colorectal cancer is the third leading to death type of cancer in the world. The therapeutic guideline varied between different methods. As the main therapeutic guideline is chemotherapy, recent studies had shown utilization of natural products in combination with conventional medication, elevate the efficiency of chemotherapeutic methods. Kombucha is a traditional beverage obtained from the fermentation of green tea as a rich source of flavonoid medicinal plant. This study aimed to evaluate the natural potential of combination therapy of this natural product with doxorubicin as a chemotherapeutic agent.
MATERIALS AND METHODS:
The study was performed as in vitro evaluation of biological activity of kombucha on HCT-116 cell line (human colon cancer cell line). The cytotoxic effect of different kombucha beverages (fermented green tea) in comparison with green tea extract was evaluated by dimethylthiazolyl tetrazolium bromide (MTT) assay. In the next step, anticancer activity of doxorubicin as a general guideline chemotherapeutic agent in combination with kombucha was evaluated by cell cycle analysis and apoptosis assay flow cytometry. Apoptotic genes expression pattern was determined using real-time polymerase chain reaction. The experiments were designed in three independent replications and statistically analyzed using SPSS software.
RESULTS:
The results show that kombucha compared with the green tea extract caused more (1.2 fold) early apoptosis induction and G0/G1 phase arrest. Moreover, kombucha increased the expression levels of p21, p53, and B-cell leukemia/lymphoma 2 (Bcl-2)-associated X protein genes (2, 2.5, and 1.5 fold, respectively) while it decreased Bcl-2 gene expression level (5–8 fold) compared with doxorubicin alone. Combination of kombucha with doxorubicin shows 2-fold increased G0/G1 phase compared with the doxorubicin treatment.
CONCLUSION:
This result indicated that kombucha caused boosted anticancer activity of doxorubicin agent. These findings suggest that kombucha may be has an assistor and useful role in colorectal cancer treatment align with chemotherapy.
Keywords: Apoptosis, cell cycle, colorectal cancer, doxorubicin, kombucha.
Introduction
One of the main worldwide causes of mortality is cancer and demonstrates a serious health problem. According to world health organization (WHO) reports, 17.5 million anticipated cancer deaths and 27 million new cancer cases will happen annually 2050.[1] Accounting to 8% of all cancer-related deaths annually, colorectal cancers are the third leading cause of cancer death in both men and women.[2,3] Asian lifestyle emerged by special dietary habits with higher intake of meat, fat, and total calories, along with increased life stresses, pressures, and expectancy, caused a remarkable increase in the burden of colorectal cancer prognosis.[2,4]
In Iran, colorectal cancer is one of the most common prognostic cancers. This type of cancer is the third most common cancer in Iranian men (standardized incidence: 8.1–8.3/100,000) and the fourth most common cancer in women with a standardized incidence of 6.5–7.5/100,000.[5,6,7] As known, this is a multifactorial disease. Low physical activity, high body mass index (BMI), high-fat diet, alcohol consumption, low intake of vegetables and fruit, tobacco smoking, a family history, and use of certain medications, including contraception pills and nonsteroidal anti-inflammatory drugs, are risk factors for cancer.[7,8,9]
Disruption of the apoptosis process, mutations in proto-oncogenes, mutations in repairing genes, and tumor suppressors are the main cause's of colorectal cancer .[10,11] Other than surgery, radiotherapy, endocrine therapy, and immunotherapy, chemotherapy is the main treatment for cancer.[12] However, conventional cancer chemotherapy is associated with high systemic toxicity and low therapeutic effectiveness. Therefore, reducing the side effects of chemotherapy has become a focus in the management of cancer.[13]
Natural product and medicinal herb use in combination with conventional medication is a new manner to help reduce the side effects of chemotherapy.[14] Green tea as a medicinal plant is one of the richest sources of flavonoids. Green tea is rich in flavonoids such as catechin, epicatechin, epigallocatechin, and epigallocatechin gallate, which have been recognized as anti-inflammatory, antioxidant, anticancer, and antimutagenic agents.[15] Based on practical studies, consumption of green tea can help weight loss, decreases BMI, and decreases insulin resistance.[16] Despite the huge evidence on the green tea benefits, this type of tea has very low popularity. Among the available black tea brands, the imported ones are more popular than the homemade products.[17]
Kombucha is a traditional beverage usually obtained from the fermentation of black or green tea (sweetened with 5%–8% of sugar) by a symbiotic microbial consortium, which is mainly composed of acetic acid bacteria and osmophilic yeasts.[16,17] Kombucha is known for its nutraceutical properties and consumed in Asia for thousands of years. History of preparation of this drink dating back to approximately 220 BC . In last decades, the market interest has spread to the whole world, and also, home brewing is now a common practice.[18,19] Today, kombucha is commonly consumed worldwide as a medicinal health-promoting beverage.[20,21] Many researches demonstrated that kombucha constitutes a potent therapeutic supplement that improved resistance against cancer, prevented cardiovascular diseases, promoted digestive functions, stimulated the immune system, and reduced inflammatory problems.[20,22,23] The beneficial effects of kombucha are attributed to the presence of polyphenols, gluconic acid, glucuronic acid, lactic acid, vitamins, amino acids, antibiotics, and a variety of micronutrients produced during fermentation.[24,25] The anticancer mechanisms of green tea polyphenols include inhibition of gene mutation, inhibition of cancer cell proliferation, induction of cancer apoptosis, and termination of metastasis.[24,26,27] In the traditional medicine, the anticancer activity of kombucha had reported with no scientific knowledge background. Recent studies as Jaya Balan group investigations, take a new overview for anticancer specification of kombucha especially in liver and renal carcinoma.[22] Hence, the present study aimed to evaluate the anticancer potential of kombucha on the HCT-116 cell line (human colon cancer cell line) and therapeutic effectiveness in combination with doxorubicin as a common chemotherapeutic agent.
Materials and Methods
Study design and setting
The study was performed as in vitro evaluation of biological activity of kombucha on HCT-116 cell line (human colon cancer cell line). Two different kombucha beverages (pasteurized fermented green tea) were purchased from Delestan Health Tea Factory: kombucha beverage was fermented for 2 weeks (kombucha 1) and 3 weeks (kombucha 2). For comparison, green tea extract was prepared from boiled green tea (12 g) for 15 min, then filtered through a sterile sieve, and cooled to room temperature. Doxorubicin was purchased from Sigma-Aldrich Chemicals (USA). The HCT-116 cell line was purchased from Pasteur Institute of Iran, Tehran, Iran.
Evaluation of cytotoxicity
Determination of half maximal inhibitory concentration (IC50)
The cytotoxic effect of different kombucha beverages (fermented green tea) in comparison with green tea extract was evaluated by dimethylthiazolyl tetrazolium bromide (MTT) assay. Briefly, HCT-116 cells were seeded in Dulbecco's Modified Eagle's Medium, supplemented with 10% fetal bovine serum, 100 μg/mL of penicillin, and 100 μg/mL of streptomycin in 96-well plate sat 10,000 cells per well for 24 h at 37°C in a humidified atmosphere of 95% air and 5% CO2. After 24 h, cells were exposed for 48 h at 37°C to known concentrations of the kombucha beverage, green tea extract, and doxorubicin. Then, the culture medium was removed and 200 μl of MTT reagent (diluted in culture medium, 0.5 mg/ml) was added. Following incubation for 4 h, the MTT/medium was removed and replaced with dimethyl sulfoxide (200 μl) to dissolve the formazan crystals. The absorbance of each well was recorded by EPOCH microplate spectrophotometer (BioTek, USA) at 570 nm. The cells without culture medium treatment were defined as a control group. The cell viability was calculated using the following formula:
Growth inhibition percent = A570 nm of treated cells/A570 nm of control cells × 100.[1,13]
Evaluation of anticancer activity
Cell cycle analysis
HCT-116 cells were seeded at a density of 2 × 105 cells into 24-well tissue culture plates. After culturing for 24 h, the medium was changed and the desired to concentrations of the kombucha beverages 1 (3.21 mg) and 2 (3.83 mg), combination of kombucha beverages 1 + doxorubicin (2.1 mg) and kombucha beverages 2 + doxorubicin (1.43 mg), green tea extract (1.9 mg), and doxorubicin (0.0018 mg) added. After 48 h of incubation, the cells were harvested and fixed gently with 80% ethanol in the freezer for 2 h and then treated with 0.25% Triton X-100 for 5 min in ice bath. Cells were resuspended in 150 μL of PBS containing 40 μg/mL propidium iodide (PI) and 0.1 mg/mL RNase and were incubated in a dark room for 20 min at room temperature. Cell cycle analysis was performed using a FACScan flow cytometer. The data were analyzed using FlowJo 9.0 software (Tree Star, Ashland, OR, USA). Untreated cells were used as a control group.[28,29]
Apoptosis assay
HCT-116 cells were seeded in 24-well tissue culture plates (2 × 105 cells/well). After 48 h, the medium was changed and treated with the desired concentrations as above. After 48 h, the cells floating in the medium were collected and added to the detached adherent cells with 0.05% trypsin. After gentle pipetting, the cells were centrifuged for 5 min at 1500 × g. The cell pellet was stained with annexin V-fluorescein isothiocyanate (FITC) and PI according to the manufacturer's instructions. Briefly, the supernatant was removed, and cell pellet was washed twice with ice-cold PBS (pH 7.4) and resuspended in 500 μL PBS. The cells were incubated with 5 μL of annexin V-FITC and 10 μL of PI in the dark for 20 min at room temperature. Finally, the stained cells were subjected to fluorescence intensity assay in FL-1 (FITC) and FL-2 (PI) channels, using FACScan flow cytometer (Mindray, China). Annexin V-FITC detects translocation of phosphatidylinositol from the inner to the outer cell membrane during early apoptosis, and PI can enter the cell in late apoptosis or necrosis. Untreated cells were used as a control for the double staining. The data were analyzed using FlowJo 9.0 software (Tree Star, Ashland, OR, USA).[28,29]
Apoptotic gene expression assay
The HCT-116 cells were seeded in 6-well plates at 1 × 106 cells per well for 24 h and then treated as previous. After incubation for 48 h of incubation, total RNA was extracted using RNX-Plus reagent (RNX-Plus™ CinnaGen Co). Total extracted RNA was quantified by optical density measurement (A260/A280 ratio) with NanoDrop 2000c Spectrophotometer. The cDNA synthesis was performed using QuantiTect Reverse Transcription Kit (Qiagen, USA). The cDNA was utilized as a template for subsequent quantitative polymerase chain reaction (qPCR) amplification using primers specific for B-cell leukemia/lymphoma 2 (Bcl-2)-associated X protein (Bax), Bcl-2, p21, p53, and β-actin genes. The β-actin gene was used as an internal control gene [Table 1]. The primer sequences were designed using Gene Runner software version 6.4.08 Beta (Hastings Software Inc., NY, US).
Table 1.
Primer sequences used for evaluation of apoptotic gene expression by quantitative polymerase chain reaction
| Gene name | Gene sequence (5'→3') | Product length (bp) |
|---|---|---|
| Bax | ||
| Forward | CCCGAGAGGTCTTTTTCCGAG | 144 |
| Reverse | TGGTTCTGATCAGTTCCGGC | |
| Bcl-2 | ||
| Forward | GTTCCGCGTGATTGAAGA | 191 |
| Reverse | CCCGGTTATCGTACCCT | |
| p21 | ||
| Forward | GGCACCCTAGTTCTACCTCA | 245 |
| Reverse | CTCCTTGTTCCGCTGCTAAT | |
| p53 | ||
| Forward | CGTGTGGAGTATTTGGATGAC | 101 |
| Reverse | TTGTAGTGGATGGTGGTACAGTC | |
| β-actin | ||
| Forward | AAAACTGGAACGGTGAAGGT | 130 |
| Reverse | AACAACGCATCTCATATTTGGAA |
q-PCR reaction was carried out on step one real-time PCR system (Thermo Scientific, USA( using SYBR Green PCR Master Mix. The reaction mixture consisted of 1 μl of cDNA, 5.75 μM of each primer, and 12.5 μ M of 1X qPCR master mix. The qPCR program performed with following steps: The first step was initial denaturation at 95°C for 2 min, followed by 35-cycle denaturation at 95°C for 30 s, annealing at 58°C for 30 s, extension at 72°C for 40 s, and the final extension at 72°C for 7 min. The results were expressed as 2−ΔΔCq.[30,31]
Data collection tool and technique
All of the experiments were in a minimum of three independent replicates. The results were expressed as mean ± standard deviation. Differences between groups were assessed by one-way ANOVA. The significance of difference between particular treatment groups was analyzed using SPSS software (version 20.0; IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant.
Results
Cell viability
The cell viability assay was assessed by MTT method. After evaluation of half-maximal inhibitory concentration (IC50) concentration of each agent individually, HCT-116 cells were exposed for 48 h at 37°C to known serial concentrations of kombucha beverage, green tea extract, and doxorubicin. The IC50values of each one are presented in Table 2. According to IC50 concentration, the treatment groups were selected as follows: kombucha beverage 1 (3.21 mg), kombucha beverage 2 (3.83 mg), kombucha beverage 1 + doxorubicin (2.1 mg), kombucha beverage 2 + doxorubicin (1.43 mg), green tea extract (1.9 mg), and doxorubicin (0.0018 mg). The results show that kombucha beverages could significantly increase the cytotoxicity of doxorubicin, in this state, the IC50of doxorubicin diminished to ~1.5-fold in HCT-116 cells.
Table 2.
The IC50 values of kombucha beverage 1 (3.21 mg), kombucha beverage 2 (3.83 mg), kombucha beverage 1+doxorubicin (2.1 mg), kombucha beverage 2+doxorubicin (1.43 mg), green tea extract (1.9 mg), and doxorubicin (0.0018 mg) on HCT-116 cells
| Treatment | IC50 value (mg.ml) |
|---|---|
| Kombucha beverage 2+doxorubicin | 0.586±0.46 |
| Kombucha beverage 1+doxorubicin | 0.615±0.28 |
| Doxorubicin | 0.879±0.38 |
| Kombucha beverage 2 | 0.937±0.19 |
| Kombucha beverage 1 | 0.964±0.29 |
| Green tea extract | 1.24±0.1 |
Data represent the mean±SD. SD=Standard deviation
Cell cycle
The cell cycle analysis of treated HCT-116 cells with each group is shown in Table 3. The results showed that administration of kombucha compared with the green tea extract significantly increased the G0/G1 phase compared with green tea extract (i.e., 60.5% and 55.7% at kombucha 2 and kombucha 1 vs. 55.6% green tea, respectively), with a concomitant decrease in S phase cells. Therefore, during kombucha treatment, transition of the cells to S phase could not performed and they remain in G0/G1 phase of the cell cycle. Moreover, the co-administration of kombucha with doxorubicin increased the G0/G1 phase compared with the doxorubicin alone (i.e., 71.2% vs. 61.5%, respectively) [Table 3].
Table 3.
Cell cycle analysis results of kombucha beverage 1 (3.21 mg), kombucha beverage 2 (3.83 mg), kombucha beverage 1+doxorubicin (2.1 mg), kombucha beverage 2+doxorubicin (1.43 mg), green tea extract (1.9 mg), and doxorubicin (0.0018 mg) on HCT-116 cells
| Cell cycle stage | Kombucha beverage 2+doxorubicin | Kombucha beverage 1+doxorubicin | Doxorubicin | Kombucha beverage 2 | Kombucha beverage 1 | Green tea extract | Control |
|---|---|---|---|---|---|---|---|
| G0/G1 | 71.2 | 66.1 | 61.5 | 60.5 | 55.7 | 55.6 | 51.2 |
| S | 12.4 | 12.7 | 13 | 13.2 | 14.4 | 14.8 | 20.9 |
| G2/M | 8.92 | 14 | 22.5 | 22.7 | 28.3 | 28.5 | 29.3 |
Apoptosis
Apoptosis progress was analyzed using double-staining annexin V-FITC/PI in the treated HCT-116 cells using flow cytometry. The scatter plot of double-variable flow cytometry illustrated living cells in the Q1 quadrant (FITC−/PI−), early apoptotic cells in the Q2 quadrant (FITC+/PI−), late apoptotic cells in the Q3 quadrant (FITC+/PI+), and necrotic cells in the Q4 quadrant (FITC−/PI+). As shown in Figure 1, treatment with kombucha compared with the green tea extract caused significantly increasing in the rate of early apoptosis [i.e., 15.6% and 10% at kombucha 2 and kombucha 1 vs. 6.08% green tea, Figure 1a-f, respectively]. Moreover, the co-administration of kombucha with doxorubicin drug increased the cells in the early phase apoptosis rate compared with the doxorubicin individually (i.e., 20.1% vs. 17.4%, respectively).
Figure 1.
Apoptosis assay using flow cytometry following the treatment of cells for 48 h. (a) Kombucha beverage 2 + doxorubicin (1.43 mg); (b) Kombucha beverage 1 + doxorubicin (2.1 mg); (c) doxorubicin (1.43 mg); (d) Kombucha beverage 2 (3.83 mg); (e) Kombucha beverage 1 (3.21 mg); (f) Green tea extract (1.9 mg); (g) Control
Apoptotic genes expression
Expression of genes that involved in cell survival and apoptosis (Bax, Bcl-2, p21, and p53) evaluated by real-time PCR. As illustrated in Figures 2-5, the administration of kombucha compared with the green tea extract increased the expression levels of p21, p53, and Bax while it decreased the expression level of Bcl-2 in HCT-116 cells during 48 h. Moreover, the treatment HCT-116 cells with kombucha compared with the green tea extract increased the ratio of Bax/Bcl-2 expression. Moreover, the co-administration of kombucha with doxorubicin increased the p21, p53, Bax, and the ratio of Bax/Bcl-2 expression while it decreased the expression level of Bcl-2 compared with the doxorubicin treatment alone [Table 4].
Figure 2.
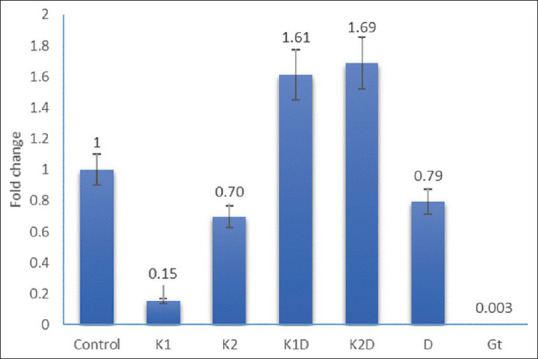
Expression of B-cell leukemia/lymphoma 2-associated X protein gene following the treatment of cells for 48 h. Control; K1 = Kombucha beverage 1 (3.21 mg), K2 = Kombucha beverage 2 (3.83 mg), K1D = Kombucha beverage 1 + doxorubicin (2.1 mg), K2D = Kombucha beverage 2 + doxorubicin (1.43 mg), D = doxorubicin (1.43 mg), GT = Green tea extract (1.9 mg)
Figure 5.
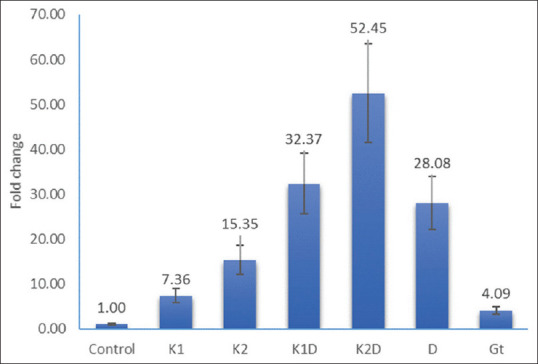
Expression of p21 gene following the treatment of cells for 48 h. Control; K1 = Kombucha beverage 1 (3.21 mg), K2 = Kombucha beverage 2 (3.83 mg), K1D = Kombucha beverage 1 + doxorubicin (2.1 mg), K2D = Kombucha beverage 2 + doxorubicin (1.43 mg), D = Doxorubicin (1.43 mg), GT = Green tea extract (1.9 mg)
Table 4.
Relative expression of Bax/Bcl2 in HCT-116 human colorectal cancer cell treated with kombucha beverage 1 (3.21 mg), kombucha beverage 2 (3.83 mg), kombucha beverage 1+doxorubicin (2.1 mg), kombucha beverage 2+doxorubicin (1.43 mg), green tea (1.9 mg), and doxorubicin (0.0018 mg) on expression levels of Bax, Bcl-2, p21, and p53
| Type of treatment | Kombucha beverage 2+doxorubicin | Kombucha beverage 1+doxorubicin | Doxorubicin | Kombucha beverage 2 | Kombucha beverage 1 | Green tea extract | Control |
|---|---|---|---|---|---|---|---|
| Ratio Bax/Bcl2 | 0.326 | 0.197 | 0.019 | 0.016 | 0.002 | 0.000000083 | 1 |
Figure 3.
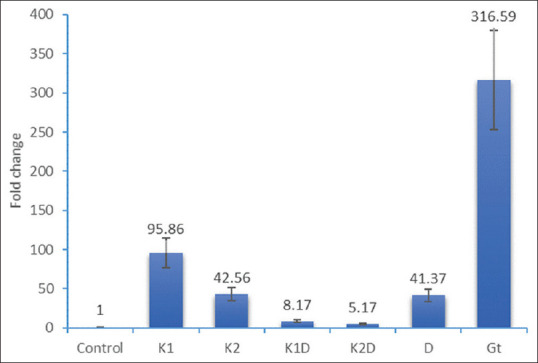
Expression of B-cell leukemia/lymphoma 2 gene following the treatment of cells for 48 h. Control; K1 = Kombucha beverage 1 (3.21 mg), K2 = Kombucha beverage 2 (3.83 mg), K1D = Kombucha beverage 1 + doxorubicin (2.1 mg), K2D = Kombucha beverage 2 + doxorubicin (1.43 mg), D = Doxorubicin (1.43 mg), GT = Green tea extract (1.9 mg)
Figure 4.
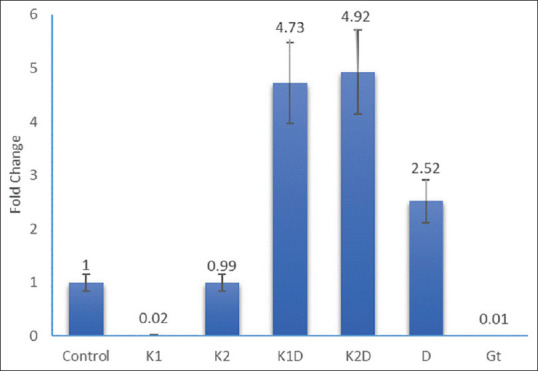
Expression of p53 gene following the treatment of cells for 48 h. Control; K1 = Kombucha beverage 1 (3.21 mg), K2 = Kombucha beverage 2 (3.83 mg), K1D = Kombucha beverage 1 + doxorubicin (2.1 mg), K2D = Kombucha beverage 2 + doxorubicin (1.43 mg), D = Doxorubicin (1.43 mg), GT = Green tea extract (1.9 mg)
Discussion
In the traditional medicine according to epidemiological studies, green tea is known as strong protective against certain human cancers. The main constructors of chemical composition of green tea are catechin polyphenols that demonstrated inhibition activity against proliferation of colon carcinoma, lung carcinoma, breast carcinoma, melanoma, and leukemic cells. Chemoprevention using a combination of dietary phytochemicals with diverse mechanisms has proposed as a successful approach to control different types of cancer with fewer side effects.[22,32]
The anticancer and antiapoptotic effect of kombucha beverage prepared from green tea on human colon cancer cells had not studied yet. Hence, in the present study, we evaluated the effects of kombucha on the carcinogenic factors in HCT-116 cells (human colorectal cancer cell line) as in vitro model. The study was performed in comparative mode between kombucha beverage, green tea extract, and doxorubicin drug individually or combined. As many researches have investigated the antiproliferative properties of kombucha, the current study demonstrated that kombucha has a significant antiproliferative activity in vitro, against HCT-116 human colon cancer cells. This effect suggested that correlated to the kombucha rich matrix of polyphenols, D-saccharic acid-1,4-lactone, vitamins, gluconic acid, glucuronic acid, and lactic acid.[33] On the other hand, catechin that was found in green tea has antioxidant, anticancer, antidiabetic, and anti-atherosclerosis properties, which were better exerted under an environment containing acetic acid and glucuronic acid. The increase of anticancer effects of catechin available in green tea may be because of the existence of acetic acid and gluconic acid in kombucha.[15,34]
In the recent study, the estimation of anticancer activity, evaluated by cell cycle analysis in HCT-116 cells treated with the desired concentrations of the kombucha beverages, kombucha beverage + doxorubicin, green tea extract, and doxorubicin, demonstrated that the co-administration of kombucha with doxorubicin drug increased the cells in the G0/G1 phase compared with the doxorubicin treatment alone. Therefore, in other words, kombucha beverages enhanced activity of antitumor agents that is new sighing anti-colorectal activity of in vitro studies and provides the possibility that kombucha beverage acts as a biochemical modulator. It could expect that kombucha beverages promote cancer chemotherapy.
A regulated form of cellular suicide is apoptosis that occurs in multicellular organisms due to endogenous and exogenous death stimuli, including death ligands such as tumour necrosis factor alpha (TNF-α), FasL/CD95/Apo1, and TNF-related apoptosis-inducing ligand, as well as chemotherapeutic agents such as cisplatin, doxorubicin, and 5′FU.[35,36,37] Ability of cellular mechanisms to suppress the tumor formation and response to many types of cancer therapy is due to p53 proteins that induce apoptosis. Following cellular stresses, p53 protein was stabilized and binds to DNA as a tetramer, in a sequence-specific manner that results in the transcriptional activation of a series of apoptosis-associated proteins including p53-upregulated modulator of apoptosis, Bax, Bcl-2 antagonist killer, and Bcl-2. By keeping a balance between pro- and antiapoptotic members, Bcl-2 proteins (located on the mitochondrial membrane) regulate apoptosis.[37,38] In this study evaluation of apoptosis progress by annexin V-FITC, staining in HCT-116 cells treated with the desired concentrations of the kombucha beverages revealed a significant increase in early-phase apoptosis rate when doxorubicin administrated simultaneously with kombucha beverage in comparison with the doxorubicin individually.
Targeted induction of apoptosis in cancer cells is an important strategy for cancer prevention and therapy. The present study findings demonstrated that kombucha caused early apoptosis in HCT-116 human colorectal cancer cell line and enhanced the effect of doxorubicin as a chemotherapeutic agent. In addition, real-time PCR analysis showed a significant increase of Bax, p21, and p53 gene expression levels when doxorubicin administrated simultaneously with kombucha beverages versus doxorubicin alone. Hence, according to the finding of this study, kombucha beverage could be discussed as an assistor or booster of antitumor activity of doxorubicin as a chemotherapeutic agent.
Conclusion
As previous studies revealed that green tea had a great potential in cancer therapy, a recent study suggests that kombucha beverage inhibited antiapoptotic function of Bcl-2 and induced the cascade expression of Bax, p21, and p53 genes more strongly. Therefore, kombucha beverages prepared from green tea possess interesting antiproliferative properties associated with significant antiapoptotic activity in cellular and molecular scales. Furthermore, these findings declared a new potential of natural therapy alone or align to chemotherapy to elevate the efficiency of general therapeutic guidelines and decreased their side effects. On the other hand, this study reviled the impact of natural product manufacturing. As known, natural product therapy is one of the hopeful aims as therapeutic or therapeutic assistor in cancer medication. Use as nutrition or as drug in two ways had a unique goal in cancer therapy domain. As reported in the traditional documents the anticancer use of kombucha, this study demonstrated this biological activity against colorectal cancer in the in vitro model. In addition, this study reviled a promiseful evidence for use of kombucha combined with chemotherapeutic guidelines to elevate the efficiency of general therapeutic medicines. Therefore, this study suggested that future researches will aimed to define new practical guidelines more efficient with less adverse effects assisted with natural products. Moreover, this study demonstrated that kombucha beverages cause boosted activity of doxorubicin antitumor agent in colorectal cancer treatment. Therefore, this study recommended the more focus on biological activity of kombucha beverage as a less known natural product in cancer therapy especially in the practical models.
Ethical considerations
The authors would like to thank the Iran University of Medical Sciences for funding this study as a PhD thesis. This study has been approved by Ethics Committee of IUMS ( ethic code : IR.IUMS.FMD.REC.1396.92234775204).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Alemi A, Farrokhifar M, Zare-Zardini H, Karamallah MH. A comparison between the anticancer activities of free paclitaxel and paclitaxel-loaded niosome nanoparticles on human acute lymphoblastic leukemia cell line Nalm-6. Iran J Pediatr Hematol Oncol. 2018;8:153–60. [Google Scholar]
- 2.Bishehsari F, Mahdavinia M, Vacca M, Malekzadeh R, Mariani-Costantini R. Epidemiological transition of colorectal cancer in developing countries: Environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014;20:6055–72. doi: 10.3748/wjg.v20.i20.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 4.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–78. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 5.Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13:143–6. [PubMed] [Google Scholar]
- 6.Moghimi-Dehkordi B, Safaee A, Zali MR. Prognostic factors in 1,138 Iranian colorectal cancer patients. Int J Colorectal Dis. 2008;23:683–8. doi: 10.1007/s00384-008-0463-7. [DOI] [PubMed] [Google Scholar]
- 7.Rafiemanesh H, Pakzad R, Abedi M, Kor Y, Moludi J, Towhidi F, et al. Colorectal cancer in Iran: Epidemiology and morphology trends. EXCLI J. 2016;15:738–44. doi: 10.17179/excli2016-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosseini SV, Izadpanah A, Yarmohammadi H. Epidemiological changes in colorectal cancer in Shiraz, Iran: 1980-2000. ANZ J Surg. 2004;74:547–9. doi: 10.1111/j.1445-2197.2004.03064.x. [DOI] [PubMed] [Google Scholar]
- 9.Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24:1207–22. doi: 10.1007/s10552-013-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sever R, Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5:a006098. doi: 10.1101/cshperspect.a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang LH, Wu CF, Rajasekaran N, Shin YK. Loss of tumor suppressor gene function in human cancer: An overview. Cell Physiol Biochem. 2018;51:2647–93. doi: 10.1159/000495956. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Lv L, Yang K. Chemotherapy targeting cancer stem cells. Am J Cancer Res. 2015;5:880–93. [PMC free article] [PubMed] [Google Scholar]
- 13.Alemi A, Reza JZ, Haghiralsadat F, Jaliani HZ, Karamallah MH, Hosseini SA, et al. Paclitaxel and curcumin coadministration in novel cationic PEGylated niosomal formulations exhibit enhanced synergistic antitumor efficacy. J Nanobiotechnol. 2018;16:28. doi: 10.1186/s12951-018-0351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang BL, Shen YM, Zhang QW, Li YL, Luo M, Liu Z, et al. Codelivery of curcumin and doxorubicin by MPEG-PCL results in improved efficacy of systemically administered chemotherapy in mice with lung cancer. Int J Nanomed. 2013;8:3521–31. doi: 10.2147/IJN.S45250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosseini S, Gorjian M, Rasouli L, Shirali S. A comparison between the effect of green tea and kombucha prepared from green tea on the weight of diabetic rat. Biomed Pharmacol J. 2015;12:141–6. [Google Scholar]
- 16.Tehrani HG, Allahdadian M, Zarre F, Ranjbar H, Allahdadian F. Effect of green tea on metabolic and hormonal aspect of polycystic ovarian syndrome in overweight and obese women suffering from polycystic ovarian syndrome: A clinical trial. J Educ Health Promot. 2017;6:36. doi: 10.4103/jehp.jehp_67_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezaee E, Mirlohi M, Hassanzadeh A, Fallah A. Factors affecting tea consumption pattern in an urban society in Isfahan, Iran. J Educ Health Promot. 2016;5:13. doi: 10.4103/2277-9531.184568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barati F, Javanbakht J, Adib-Hashemi F, Hosseini E, Safaeie R, Rajabian M, et al. Histopathological and clinical evaluation of Kombucha tea and Nitrofurazone on cutaneous full-thickness wounds healing in rats: An experimental study. Diagn Pathol. 2013;8:120. doi: 10.1186/1746-1596-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Gaggìa F, Baffoni L, Galiano M, Nielsen DS, Jakobsen RR, Castro-Mejía JL, et al. Kombucha beverage from green, black and rooibos teas: A comparative study looking at microbiology, chemistry and antioxidant activity. Nutrients. 2018;11:1. doi: 10.3390/nu11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dufresne C, Farnworth E. Tea, kombucha, and health: A review. Food Res Int. 2000;33:409–21. [Google Scholar]
- 21.Kapp JM, Sumner W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann Epidemiol. 2019;30:66–70. doi: 10.1016/j.annepidem.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Aloulou A, Hamden K, Elloumi D, Ali MB, Hargafi K, Jaouadi B, et al. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement Altern Med. 2012;12:63. doi: 10.1186/1472-6882-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutta D, Gachhui R. Nitrogen-fixing and cellulose-producing Gluconacetobacter kombuchae sp. nov., isolated from kombucha tea. Int J Syst Evol Microbiol. 2007;57:353–7. doi: 10.1099/ijs.0.64638-0. [DOI] [PubMed] [Google Scholar]
- 24.Jayabalan R, Marimuthu S, Thangaraj P, Sathishkumar M, Binupriya AR, Swaminathan K, et al. Preservation of kombucha tea-effect of temperature on tea components and free radical scavenging properties. J Agric Food Chem. 2008;56:9064–71. doi: 10.1021/jf8020893. [DOI] [PubMed] [Google Scholar]
- 25.Murugesan GS, Sathishkumar M, Jayabalan R, Binupriya AR, Swaminathan K, Yun SE. Hepatoprotective and curative properties of kombucha tea against carbon tetrachloride-induced toxicity. J Microbiol Biotechnol. 2009;19:397–402. doi: 10.4014/jmb.0806.374. [DOI] [PubMed] [Google Scholar]
- 26.Abou-Taleb K, Ebeed N, Soheir S, El-Salam SS, Amin S. Antimicrobial and antiproliferative, pro-apoptotic actions of kombucha fermented solutions against colon and heptao cancer cell lines. World Journal of Pharmaceutical and Life Sciences. 2017;3:120–32. [Google Scholar]
- 27.Leal JM, Suárez LV, Jayabalan R, Oros JH, Escalante-Aburto A. A review on health benefits of kombucha nutritional compounds and metabolites. CyTA – Journal of Food. 2018;16:390–9. [Google Scholar]
- 28.Chen L L, Zhang HY. Cancer preventive mechanisms of the green tea polyphenol (-)-epigallocatechin-3-gallate. Molecules. 2007;12:946–57. doi: 10.3390/12050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecumberri E, Dupertuis YM, Mirahbell R, Pichard C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin Nutr. 2013;32:894–903. doi: 10.1016/j.clnu.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Du GJ, Zhang Z, Wen XD, Yu C, Calway T, Yuan CS, et al. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012;4:1679–91. doi: 10.3390/nu4111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thangapazham RL, Singh AK, Sharma A, Warren J, Gaddipati JP, Maheshwari RK. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007;245:232–41. doi: 10.1016/j.canlet.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Huang CY, Han Z, Li X, Xie HH, Zhu SS. Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol Lett. 2017;14:3623–7. doi: 10.3892/ol.2017.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moradzadeh M, Hosseini A, Erfani S, Rezaei H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol Rep. 2017;69:924–8. doi: 10.1016/j.pharep.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Jayabalan R, Malbasa RV, Loncar ES, Vitas JS, Sathishkumar M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr Rev Food Sci Food Saf. 2014;13:538–50. doi: 10.1111/1541-4337.12073. [DOI] [PubMed] [Google Scholar]
- 35.Deghrigue MC, Battikh H, Abid K, Bakhrouf A. Antiproliferative and antimicrobial activities of kombucha tea. Afr J Microbiol Res. 2013;7:3466–70. [Google Scholar]
- 36.Beigmohammadi F, Karbasi A, Beigmohammadi Z. Production of high glucuronic acid level in kombucha beverage under the influence environmental condition. Journal of Food Technology and Nutrition. 2010;7:30–38. [Google Scholar]
- 37.Bai WK, Shen E, Hu B. The induction of the apoptosis of cancer cell by sonodynamic therapy: A review. Chin J Cancer Res. 2012;24:368–73. doi: 10.3978/j.issn.1000-9604.2012.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houghton JA. Apoptosis and drug response. Curr Opin Oncol. 1999;11:475–81. doi: 10.1097/00001622-199911000-00008. [DOI] [PubMed] [Google Scholar]



