Abstract
OBJECTIVES:
The purpose of this research was to determine the effectiveness of Pentaglobin® as adjuvant therapy in the treatment of sepsis in preterm newborns.
MATERIALS AND METHODS:
It was a prospective, observational, randomized study for 272 premature neonates and very low birth weight (VLBW) that were diagnosed with sepsis carried at neonatal intensive care units. The patients randomized into control group who received standard sepsis antibiotic treatments, and an intervention group who received Pentaglobin® 5 ml/kg daily for 3 consecutive days as an adjunct therapy to a standard sepsis antibiotic treatment.
RESULTS:
Multiple organisms that isolated from culture specimens were Gram-negative bacteria, Gram-positive, and candida (56.25%, 42.28%, and 1.47%, respectively). The disease duration was distinctively longer in patients who were treated by the standard antibiotic protocol (mean ± standard deviation [SD]: 30.76 ± 3.97, odds ratio [OR]: 30.76, 95% confidence interval [CI]: 30.051, 31.473) comparing to the patients who received Pentaglobin adjuvant therapy (mean ± SD: 26.48 ± 5.55, OR: 26.48, 95% CI: 25.489, 27.477) (P < 0.000). Patients treated by standard antibiotic protocol were associated to a substantially increased risk of death (11.76%, hazard ratio 4.400, 95% CI: 1.432, 13.529, P = 0.009).
CONCLUSION:
Neonatal sepsis is more common in premature and VLBW newborns, and Pentaglobin® management of newborn nosocomial sepsis might be used in addition to other therapies.
Keywords: Immunoglobulin M-enriched immunoglobulin, neonate, premature, sepsis, very low birth weight
Introduction
Neonatal sepsis is a potentially fatal disease that occurs when a serious infection affects infants within the 1st month of life; it is a systemic disease defined by the systemic inflammatory response syndrome.[1] The presence of at least two clinical signs and/or two or more symptoms such as temperature waving, hypotension, reduced perfusion with paleness and spotted skin, acidic breath, arrhythmia, apnea, breathing difficulties, grunting, hypoxemia, restlessness, stupor, epilepsy, feeding intolerance, abdominal discomfort, jaundice, hemorrhage, and variability in white blood cells [WBCs] count).[2]
Despite using an effective antimicrobial therapy with advanced services provided in the intensive care unit (ICU), morbidity and mortality of neonatal sepsis remain high in immunocompromised premature neonates.[3] Many studies have established that preterm babies' immune systems are underdeveloped; moreover, numerous variables linked with preterm delivery may affect immune function. This can result in a significant risk of infection, which contributes to mortality in this population.[4] In fetus, B-lymphocytes are existent by the end of the first trimester, and there is a small production of active immunoglobulin since this process depends on exposure to antigens. The maternal Immunoglobulin G (IgG) antibodies, which are actively carried across the placenta from the maternal circulation in high numbers after 32 weeks of gestation, are a valuable mechanism that provides protection to the child.[5]
The purpose of this study is to assess the efficacy of Pentaglobin (IgM-enriched intravenous immunoglobulin [IgM-IVIG] product) as adjunctive pharmacotherapy of premature newborn sepsis, as well as the influence of pentaglobin on survival and hospitalization length.
Materials and Methods
A prospective, multi-center, randomized, controlled, open-labeled interventional study was carried out at neonatal intensive care unit (NICU) in a complex of several teaching hospitals at the Medical City of Baghdad, Baghdad, Iraq, between February 2019 to the end of June 2020. The study enrolled 272 preterm neonates' patients with gestational age (<37 completed weeks) in the 1st month of life and birth weight <1500 g (very low birth weight [VLBW]), who have late-onset neonatal sepsis and blood culture confirmed the infection in addition to systemic symptoms of infection.
Two hundred and seventy-two neonates had received a combination of empiric ampicillin plus gentamicin injection and were ordered a blood cultures. Antibiotics were chosen based on the sensitivity of the isolated organisms. The study excluded neonates with congenital abnormalities and toxoplasmosis, other (syphilis, varicella-zoster, parvovirus B19), rubella, cytomegalovirus, and Herpes illnesses are all the examples of TORCH infections.
The patients were randomly divided into two groups (1:1): Group A and Group B. Group A (control group) involved 136 patients received standard antibiotic protocol for sepsis treatment according to their blood culture and Group B (interventional group) that involved 136 patients treated at the day of sepsis diagnosis with standard sepsis antibiotic protocol according to their blood culture plus pentaglobin® intravenously 5 ml/kg/day over 6 h for 3 consecutive days.
For all patients (272), complete blood count baseline values and 3 days after treatment total (WBCs cutoff <5000/μl or more 20000/μl) with differential, C-reactive protein (CRP cutoff = 6 mg/l), liver function test and kidney function test were recorded. Mortality rate (short-term mortality: Death between 7 and 21 days after therapy), duration of hospitalization, and outcome were evaluated. The randomization pattern and demographic characteristics of all patients in the two groups are illustrated in Table 1.
Table 1.
Demographic features of the patients in two groups
| Demographic characteristic | Group A (n=136) | Group B (n=136) | P |
|---|---|---|---|
| Age (days) | 12.12±3.49 | 13.04±4.26 | 0.067 |
| Gender, n (%) | |||
| Female (total number=138) | 67 (49.26) | 71 (52.21) | 0.716 |
| Male (total number=134) | 69 (50.74) | 65 (47.79) | |
| Gestational age (weeks) | 30.04±3.12 | 29.33±3.27 | 0.084 |
| Birth body weight (g) | 1333.82±127.38 | 1302.07±143.53 | 0.068 |
| Birth length (cm) | 40.66±4.57 | 39.62±5.11 | 0.092 |
| Head circumference (cm) | 28.87±1.47 | 28.46±1.95 | 0.063 |
| Mod of delivery, n (%) | |||
| Normal delivery | 75 (55.15) | 66 (48.53) | 0.332 |
| Cesarean section | 61 (44.85) | 70 (52.21) | 0.332 |
| PROAM >18 h, n (%) | 38 (27.94) | 49 (36.03) | 0.193 |
| Maternal UTI history, n (%) | 26 (19.12) | 19 (13.97) | 0.328 |
Data are presented in the form of mean, SD, and, n (%). UTI=Urinary tract infection, SD=Standard deviation, PROAM=Prolonged rupture of the amniotic membranes
Ethics statement
The research ethics committee of Baghdad Teaching Hospital, Medical City, Baghdad, Iraq, approved this study protocol (Identifier: 4658, date of registration January 19, 2019). In addition, parents of newborns completed formal consent agreement papers to participate in the study.
Statistical analysis
SPSS software (version 23.0; SPSS, Chicago, IL, USA) and Prism 8 (version 8.4.3 GraphicPad Software, LLC) for OS X were used for the statistical analysis and figures. The continuous variables were characterized using mean standard deviations and, where applicable, median interquartile ranges (IQR). To compare the mean values of the same group before and after therapy, a paired Student's t-test was employed. The length of stay in the NICU was compared using an independent sample t-test. The Chi-square test analyzed the categorical variables, and a 95% confidence interval has reported. In this study, we used Cox regression model to determine the possible influence of treatment on illness duration. The composite end-points were determined using a hazard ratio and a 95% confidence interval, and the result was evaluated using two binary levels. All statistical analyses were significant at P < 0.05.
Results
A total of 272 neonates were included with (median age of 12 days [IQR, 10–14; range 6–26 days]; 50.74% female). The demographic characteristics for patients, maternal and delivery characteristics are summarized in table I demonstrated adequate randomization of the studied patients that is supported by no significant difference between groups.
Bacterial isolates from blood culture
The patterns of multiple organisms were isolated from culture specimens for all patients were Gram-negative (56.25%), Gram-positive (42.28%), and less common types of candida infection (1.47%).
For Group A, the most common types of microorganism were Gram-positive bacteria (50.00%), Gram-negative (47.94%) and show less with candida pathogen (2.41%). For Group B, the most common types of microorganism were Gram-negative bacteria (64.71%), Gram-positive (34.56%), and isolated growth of candida in one case (0.74%) [Table 2]. There was no significant variation in pathogenic bacteria between the groups.
Table 2.
Bacterial isolates from blood culture in the two groups
| Microorganisms | Group A (n=136) | Group B (n=136) | Total number in two groups | P |
|---|---|---|---|---|
| Gram - negative bacteria, n (%) | ||||
| Escherichia coli | 21 (15.44) | 28 (20.59) | 49 | 0.344 |
| Klebsiella pneumonia | 13 (9.56) | 19 (13.97) | 32 | 0.347 |
| Enterobacter spp. | 12 (8.82) | 15 (11.03) | 27 | 0.686 |
| Pseudomonas aeruginosa | 9 (6.62) | 8 (5.88) | 17 | 1 |
| Serratia marcescens | 5 (3.68) | 11 (8.09) | 16 | 0.126 |
| Acinetobacter baumannii | 5 (3.68) | 4 (2.94) | 9 | 1 |
| Burkholderia cepacia | 0 | 3 (2.21) | 3 | 0.247 |
| Total gram - negative bacteria, n (%) | 65 (47.94) | 88 (64.71) | 153 (56.25) | |
| Gram - positive bacteria, n (%) | ||||
| Staphylococcus epidermidis | 23 (16.91) | 19 (13.97) | 42 | 0.615 |
| Staphylococcus aureus | 17 (12.50) | 12 (8.82) | 29 | 0.432 |
| Enterococcus | 17 (12.5) | 8 (5.88) | 25 | 0.092 |
| Listeria monocyte | 6 (4.41) | 5 (3.68) | 11 | 1 |
| Group B streptococcus | 2 (1.47) | 1 (0.74) | 3 | 1 |
| α - hemolytic streptococci streptococcus pneumonia | 3 (2.21) | 2 (1.47) | 5 | 1 |
| Total gram - positive bacteria, n (%) | 68 (50.00) | 47 (34.56) | 115 (42.28) | |
| Candida, n (%) | 3 (2.21) | 1 (0.74) | 4 (1.47) | 1 |
Data is presented in the form of n (%)
Laboratory parameters
In this study, we analyzed the effect of treatment regimen on laboratory parameter from the baseline to the end of 3 days therapy for both groups. For Group A and Group B, WBC and CRP were significantly reduced at the end of therapy (P < 0.000). In contrast, despite a slight reduction in absolute neutrophil count (ANC) and an increase in platelet count in both groups, there were no significant changes found in both groups [Table 3].
Table 3.
Comparison of laboratory parameters from baseline to the end of treatment in Group A and Group B
| Variables | Group A |
Group B |
||||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | P | Before treatment | After treatment | P | |
| WBC | 24,338.71±2625.35 | 14,083.87±1262.36 | <0.000 | 24,725.41±2436.04 | 13,143.44±1116.59 | <0.000 |
| ANC | 1200.69±231.85 | 1212.38±224.16 | 0.687 | 1188.68±232.05 | 1204.07±220.87 | 0.596 |
| Platelet count | 99,975.23±179.98 | 100,007.70±105.26 | 0.084 | 99,983.37±135.51 | 100,013.38±115.26 | 0.064 |
| CRP | 50.10±12.37610 | 22.05±4.42 | 0.000 | 46.57±13.09 | 4.71±1.82 | <0.000 |
Data is presented in the form of mean, SD. WBCs=White blood cells, ANC=Absolute neutrophil count, CRP=C-reactive protein, SD=Standard deviation
Outcome of the treatment
Tables 4 and 5, together with Figures 1–4, demonstrated the impact of Pentaglobin as adjuvant treatment on hospitalization length, mortality rate, and composite end-points.
Table 4.
Outcome of treatment
| Variables | Group A (n=136) | Group B (n=136) | OR (95% CI) | P |
|---|---|---|---|---|
| Hospitalization (days) | 30.76±3.97 | 26.48±5.55 | A: 30.76 (30.051-31.473) B: 26.48 (25.489-27.477) |
<0.000 |
| Mortality rate, n (%) | 16 (11.76) | 4 (2.94) | HR (95% CI) | 0.009 |
| 4.400 (1.432-13.529) |
Data are presented in the form of mean, SD, and n (%).OR=Odd ratio, CI=Confidence interval, HR=Hazard ratio, SD=Standard deviation
Table 5.
Predictors of mortality
| Variables | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Treatment (B versus A) | 0.227 (0.073-0.699) | 0.010 | 0.231 (0.057-0.789) | 0.021 |
| Age | 0.867 (0.746-1.007) | 0.062 | 0.841 (0.697-1.014) | 0.069 |
| Gestational age | 0.771 (0.610-0.975) | 0.030 | 0.847 (0.654-1.097) | 0.208 |
| Birth weight | 0.996 (0.993-0.999) | 0.009 | 0.997 (0.992-1.001) | 0.094 |
| Mode of delivery (CS versus VD) | 0.553 (0.213-1.438) | 0.224 | - | - |
| Hospitalization period | 1.089 (0.993-1.196) | 0.071 | 1.065 (0.951-1.191) | 0.276 |
| WBC at baseline | 1.000 (1.000-1.000) | 0.069 | - | - |
| ANC at baseline | 1.004 (0.999-1.009) | <0.001 | 0.996 (0.994-0.998) | <0.001 |
| Platelet | 1.209 (0.981-1.489) | 0.075 | ||
| CRP | 0.996 (0.960-1.032) | 0.821 | - | - |
Binary logistic regression analysis expressed in OR (95% CI). R2 (Cox and Snell) =0.150 (which determine the overall accuracy of the module). OR=Odd ratio, CI=Confidence intervals, WBCs=White blood cells, ANC=Absolute neutrophil count, CRP=C-reactive protein, CS=Cesarean section, VD=Vaginal delivery
Figure 1.
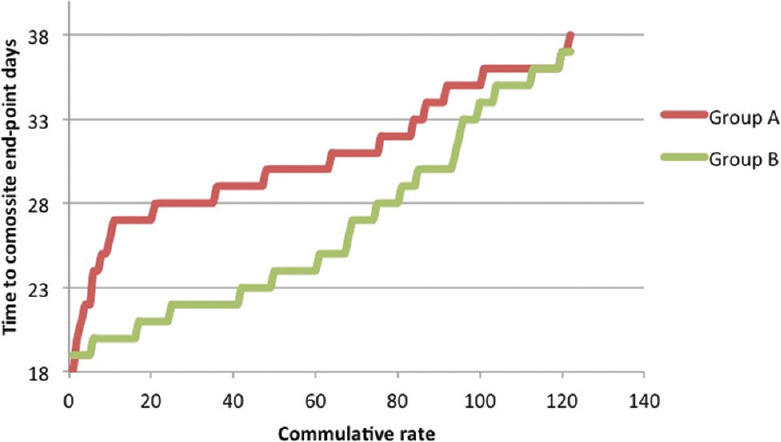
The time-dependent effect of reaching the composite end-points
Figure 4.
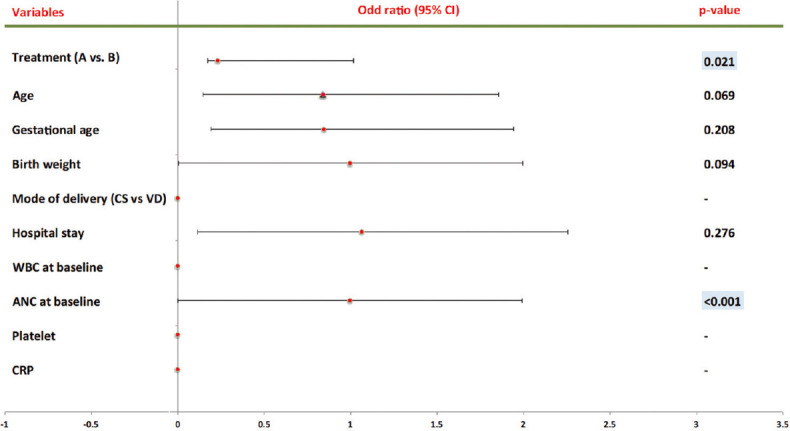
Predictors of Mortality Multivariate
The Cox regression model was utilized to describe the possible influence of Pentaglobin on hospitalization length. Patients treated with conventional antibiotic regimen had a substantially longer illness duration (mean ± standard deviation [SD]: 30.76 ± 3.97, OR: 30.76, 95% CI: 30.051, 31.473) comparing to patients who received Pentaglobin adjuvant therapy (mean ± SD: 26.48 ± 5.55, OR: 26.48, 95% CI: 25.489, 27.477) (P < 0.000) [Table 4 and Figure 1]. Moreover, a patient treated by standard antibiotic protocol (Group A) has a significantly higher risk for mortality compared with patients received Pentaglobin adjuvant therapy (Group B) (11.76%, HR 4.400, 95% CI: 1.432,13.529, P = 0.009) [Table 4 and Figure 2].
Figure 2.
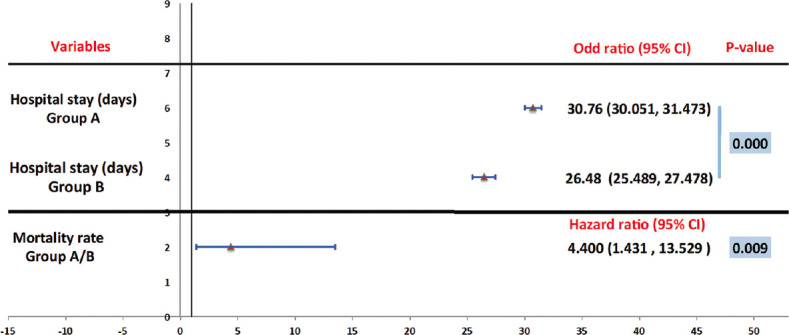
Hospital stay and mortality rate between groups
When binary logistic regression analysis was used to estimate the risk of death, the results revealed that the risk of mortality was lower in Group B when compared to Group A (Univariate P = 0.010, Multivariate P = 0.021) and the inverse relationship of mortality rate with the addition of Pentaglobin to the standard antibiotic protocol [Table 5, Figures 3 and 4]. Furthermore, an inverse relationship has been founded between the gestational age (univariate P = 0.030), birth weight (univariate P = 0.009), and ANC (Univariate P ≤ 0.001, multivariate P ≤ 0.001) with mortality rate [Table 5 and Figure 3]. In contrast, there were no links between the ages of mums, mode of delivery, hospitalization period, WBC baseline, platelet count, and CRP with mortality rate.
Figure 3.
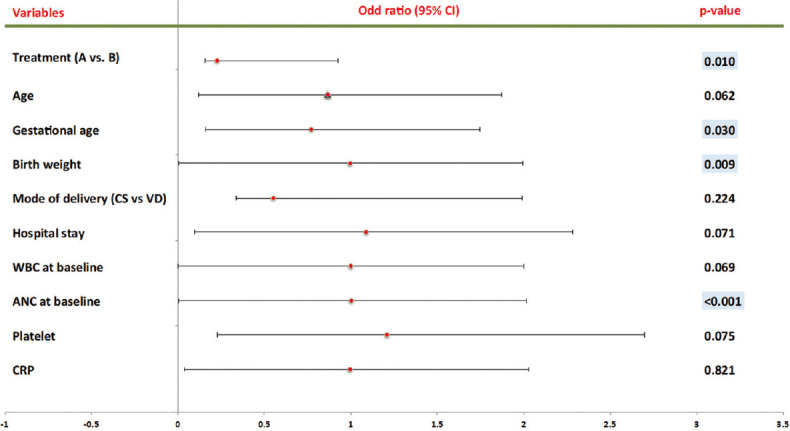
Predictors of Mortality Univariate
Discussion
Despite antibiotic therapy for preterm neonates in NICUs, neonatal nosocomial sepsis considered to be a significant cause of mortality and morbidity.[6] The newborn immune system's immaturity, slow production of endogenous immunoglobulin, absence of maternal transplacental transport of IgG, and defects in the complement system all support the use of effective immunotherapy as adjuvant treatment for neonatal sepsis.[7] Therefore, Pentaglobin gained great interests in clinical work as adjuvant therapies.[8] Immunoglobulin is one of the main interconnectable components of the immune system that permit a host to defend against microbial infection.[9] Despite variable in vitro mechanisms have been explained, the immunomodulatory effects of IVIG in in vivo are poorly understood. Meanwhile, IgM-enriched IVIG (Pentaglobin®) in neonates was studied and confirmed a significant improvement in the immunological functions by modifying complement activation, cytokine production can be redirected toward an anti-inflammatory profile., and neutralization of toxins.[10] Furthermore, IVIG act by opsonization (enhance phagocytosis by resulting C3b), complement fixation (activation), antibody dependent-cytotoxicity, and neutrophil chemoluminescence.[11]
The higher antimicrobial and opsonization activity of IgM/IgA-enriched (Pentaglobin®) due to its pentameric structure improves the activation of the complement system in the neonate. This property confirms the use of IgM-enriched IVIG (Pentaglobin®) as adjuvant treatment in sepsis superior to the use of standard-IVIG.[12] Furthermore, Boonsopa et al. concluded that the use of IGM-enriched IVIG result in reduction in morbidity and improvement of clinical parameters of neonate with sepsis and hypertension.[13] Moreover, an improved outcomes in in neonates treated with IVIG was confirmed by Jenson et al.[14] These findings were confirmed by another finding of combining results of 7 studies conducted as a systematic review, which has considered the association between IVIG therapy and the variable causes of mortality during hospitalization in premature infants, was resulted in a reduction in all causes of mortality with IVIG treatment in cases of culture-proven infection.[15] Another research, done by Bayry et al., confirmed that modest dosages of IVIG in vitro may stimulate immunoglobulin production from B-cells of CVID patients.[16] In contrast, other studies did not achieve moderate improvements in the outcome that postulated with intravenous immune globulin.[17] It might be argued that these studies did not exclude the benefit with enriched immune globulin preparations, not randomized, placebo-controlled, or blinded.
In our study, the patterns of multiple organisms were isolated from culture specimens for all patients were Gram-negative (56.25%), Gram-positive (42.28%), and less frequent candida infection (1.47%). This finding was similar with a recent research, which found that Gram-negative and Gram-positive bacteria caused more than 70% of newborn bloodstream infections.[18] Meanwhile, the frequency and microorganism profiles of neonatal sepsis are varied widely from different countries and centers.[19] Besides, in the developing countries, neonatal sepsis found to be more frequently caused by Gram-negative bacteria.[20] In this study, the relatively high incidence of Gram-negative bacteria infection in preterm neonates could be attributed to prematurity, VLBW, endotracheal intubation or mechanical ventilation, and a prolonged duration of hospital stay.[21]
The effect of using pentaglobin in this study accompanied by a reduction in WBCs count and CRP indicates the suppression of infection and an improvement in the immunological functions.[22] The nonsignificant increase in ANC in both groups may be due to longer recovery duration of neutropenia in the neonate with low gestational age and VLBW compared to full-term neonate.[23] Moreover, low birth weight and gestational age are significantly associated with the development of neutropenia in neonates due to delayed immune system maturation and/or nutritional insufficiency. Consequently, neutropenia is a commonly diagnosed condition in premature and critically ill neonates.[24] In addition, the slight, nonsignificant, elevation in platelets counts may be related to more frequent Gram-negative organisms. The previous study conducted by Bhat Y. R et al. has reported that the causative organisms could affect the duration of thrombocytopenia associated with sepsis.[23] Gram-negative and fungal infections exhibited thrombocytopenia for a longer period of time (2–8 days) than Gram-positive infections (0.4–2.8 days).[25] These observations have recommended the requirement for platelet count monitoring in neonates with sepsis.[26]
The hospitalization periods were significantly shorter for Group B compared to A (P 0.009). This related to the rapid recovery of the patients and the infection treatment regime.
Because the circulation half-life of external IgM in healthy VLBW newborns is around 1 week with a large variety of kinetics, and the impact of this form of therapy cannot be studied over a lengthy period of time, death after discharge has been chosen as the primary goal.[27]
In this study, the mortality rate was considerably less in neonates received Pentaglobin adjuvant therapy compared to those using the standard antibiotic protocol (P ≤ 0.05) [Tables 4, 5 and Figures 2, 3]. This finding was consistent with a recent research that found IgM-IVIG to be more effective than antibiotics alone in reducing short-term mortality in extremely low birth weight infants with confirmed sepsis.[28]
Many studies analyzed different factors that could predict the risk of mortality in the premature neonate with sepsis. The findings corroborate the effect of low ANC, young gestational age, and low birth weight on high mortality rate among neonate with sepsis.[29] Moreover, a considerable correlation was found between gestational age and birth weight with immune system maturation, nutrient deprivation, and neutropenia. These studies are symmetrical with our result using binary logistic regression analysis for predicted risk of mortality [Table 5 and Figures 3, 4].
In multivariate analysis, Group B predicts lower mortality rate in which offer 76.9% reduction in mortality compared to Group A and its effect was independent, ([1–0.231] ×100 = 0.769 × 100 = 76.9%). Besides, at baseline, the probability of death was increased inversely with ANC (for every one ANC decrease there is 0.4% increase probability of death).
The absolute risk regression (ARR) for each 1-week reduction in GA there is a probability of 22.9% increase in mortality risk (1.0–0.771 = 0.229 × 100 = 22.9%). Furthermore, there is a probability of 4% rise in the risk of mortality for each 10 g reduction in birth weight (1.0–0.996 = 0.004 × 100 = 0.4%; ARR for 1 g for 10 g = 4%). The result of this study was consistent with the previous study.[30]
Conclusion
Premature and/or VLBW neonates have a higher incidence of sepsis among neonates. Furthermore, the treatment of the neonatal sepsis with Pentaglobin as adjuvant therapy could have a positive response in reducing mortality rate and hospital stays.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Medical City Medical in Baghdad, Iraq, as well as all research participants, for their assistance in conducting this study.
References
- 1.Chiesa C, Panero A, Osborn JF, Simonetti AF, Pacifico L. Diagnosis of neonatal sepsis: A clinical and laboratory challenge. Clin Chem. 2004;50:279–87. doi: 10.1373/clinchem.2003.025171. [DOI] [PubMed] [Google Scholar]
- 2.Parthasarathy A, Menon P, Nair M. IAP Textbook of Pediatrics. New Delhi: JP Medical Ltd; 2019. [Google Scholar]
- 3.Cortese F, Scicchitano P, Gesualdo M, Filaninno A, De Giorgi E, Schettini F, et al. Early and late infections in newborns: Where do we stand? A review. Pediatr Neonatol. 2016;57:265–73. doi: 10.1016/j.pedneo.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Zhivaki D, Lo-Man R. Unique aspects of the perinatal immune system. Nat Rev Immunol. 2017;17:495–507. doi: 10.1038/nri.2017.54. [DOI] [PubMed] [Google Scholar]
- 5.Mahon JL, Stiller CR. The immunocompromised patient. Can Fam Physician. 1987;33:349–59. [PMC free article] [PubMed] [Google Scholar]
- 6.Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390:1770–80. doi: 10.1016/S0140-6736(17)31002-4. [DOI] [PubMed] [Google Scholar]
- 7.Capasso L, Maddaluno S, Coppola C, Dolce P, di Cola G. S., Sierchio, et al. “Do isolates from pharyngeal and rectal swabs match blood culture bacterial pathogens in septic VLBW infants? A pilot, cross-sectional study.”. European Journal of Pediatrics. 2021;180(3):799–806. doi: 10.1007/s00431-020-03788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotlan B, Stroncek DF, Marincola FM. Intravenous immunoglobulin-based immunotherapy: An arsenal of possibilities for patients and science. Immunotherapy. 2009;1:995–1015. doi: 10.2217/imt.09.67. [DOI] [PubMed] [Google Scholar]
- 9.Capasso L, Borrelli A, Cerullo J, Pisanti R, Figliuolo C, Izzo F, et al. Role of immunoglobulins in neonatal sepsis. Transl Med UniSa. 2015;11:28–33. [PMC free article] [PubMed] [Google Scholar]
- 10.Mitrevski M, Marrapodi R, Camponeschi A, Cavaliere FM, Lazzeri C, Todi L, et al. Intravenous immunoglobulin and immunomodulation of B-cell – In vitro and in vivo effects. Front Immunol. 2015;6:4. doi: 10.3389/fimmu.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson DG, Foreman O, Peterson A, Wen X, Scales SJ. Animal Model for Nephropathy and Agents for Treating the Same. Google Patents. 2017 [Google Scholar]
- 12.Esen F, Tugrul S. IgM-enriched immunoglobulins in sepsis. Intensive Care Medicine: Springer Verlag. 2009:102–10. [Google Scholar]
- 13.Boonsopa C, Poonnarattanakul W, Srijuntongsiri S. Comparison of adjunctive treatment with IgM-enriched IVIG and antibiotics alone in treatment of neonatal sepsis. Siriraj Med J. 2021;73:84–91. [Google Scholar]
- 14.Jenson, Hal B. Seminars in perinatology. W.B. Saunders Ltd; 1998. The role of intravenous immunoglobulin for the prevention and treatment of neonatal sepsis; pp. 50–63. [DOI] [PubMed] [Google Scholar]
- 15.Haque KN, Remo C, Bahakim H. Comparison of two types of intravenous immunoglobulins in the treatment of neonatal sepsis. Clin Exp Immunol. 1995;101:328–33. doi: 10.1111/j.1365-2249.1995.tb08359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayry J, Fournier EM, Maddur MS, Vani J, Wootla B, Sibéril S, et al. Intravenous immunoglobulin induces proliferation and immunoglobulin synthesis from B cells of patients with common variable immunodeficiency: A mechanism underlying the beneficial effect of IVIg in primary immunodeficiencies. J Autoimmun. 2011;36:9–15. doi: 10.1016/j.jaut.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 17.INIS Collaborative Group. Brocklehurst P, Farrell B, King A, Juszczak E, Darlow B, et al. Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med. 2011;365:1201–11. doi: 10.1056/NEJMoa1100441. [DOI] [PubMed] [Google Scholar]
- 18.Mutlu M, Aslan Y, Saygin B, Yilmaz G, Bayramoğlu G, Köksal I. Neonatal sepsis caused by gram-negative bacteria in a neonatal Intensive Care Unit: A six years analysis. HK J Paediatr (new series) 2011;16:253–7. [Google Scholar]
- 19.Nayak S, Rai R, Kumar VK, Sanjeev H, Pai A, Ganesh H. Distribution of microorganisms in neonatal sepsis and antimicrobial susceptibility patterns in a tertiary care hospital. Arch Med Health Sci. 2014;2:136. [Google Scholar]
- 20.Staude B, Oehmke F, Lauer T, Behnke J, Göpel W, Schloter M, et al. The microbiome and preterm birth: A change in paradigm with profound implications for pathophysiologic concepts and novel therapeutic strategies. Biomed Res Int. 2018;2018:7218187. doi: 10.1155/2018/7218187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y, Zhuang S, Du M. Risk factors of nosocomial infection with extended-spectrum beta-lactamase-producing bacteria in a neonatal Intensive Care Unit in China. Infection. 2007;35:339–45. doi: 10.1007/s15010-007-6356-9. [DOI] [PubMed] [Google Scholar]
- 22.Tschaikowsky K, Hedwig-Geissing M, Schiele A, Bremer F, Schywalsky M, Schüttler J. Coincidence of pro- and anti-inflammatory responses in the early phase of severe sepsis: Longitudinal study of mononuclear histocompatibility leukocyte antigen-DR expression, procalcitonin, C-reactive protein, and changes in T-cell subsets in septic and postoperative patients. Crit Care Med. 2002;30:1015–23. doi: 10.1097/00003246-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Bhat R, Kousika P, Lewis L, Purkayastha J. Prevalence and severity of thrombocytopenia in blood culture proven neonatal sepsis: A prospective study. Arch Pediatr Infect Dis. 2018;6:e12471. [Google Scholar]
- 24.Carbone F, Montecucco F, Sahebkar A. Current and emerging treatments for neonatal sepsis. Expert Opin Pharmacother. 2020;21:549–56. doi: 10.1080/14656566.2020.1721464. [DOI] [PubMed] [Google Scholar]
- 25.Lai MY, Tsai MH, Lee CW, Chiang MC, Lien R, Fu RH, et al. Characteristics of neonates with culture-proven bloodstream infection who have low levels of C-reactive protein (≤10 mg/L) BMC Infect Dis. 2015;15:320. doi: 10.1186/s12879-015-1069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts I, Murray NA. Neonatal thrombocytopenia: Causes and management. Arch Dis Child Fetal Neonatal Ed. 2003;88:F359–64. doi: 10.1136/fn.88.5.F359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shime N, Kawasaki T, Saito O, Akamine Y, Toda Y, Takeuchi M, et al. Incidence and risk factors for mortality in paediatric severe sepsis: Results from the national paediatric intensive care registry in Japan. Intensive Care Med. 2012;38:1191–7. doi: 10.1007/s00134-012-2550-z. [DOI] [PubMed] [Google Scholar]
- 28.Capasso L, Borrelli AC, Pirozzi MR, Bucci L, Albachiara R, Ferrara T, et al. IgM and IgA enriched polyclonal immunoglobulins reduce short term mortality in extremely low birth weight infants with sepsis: A retrospective cohort study. Minerva Pediatr (Torino) 2021;73:3–7. doi: 10.23736/S2724-5276.18.04850-8. [DOI] [PubMed] [Google Scholar]
- 29.Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal sepsis and neutrophil insufficiencies. Int Rev Immunol. 2010;29:315–48. doi: 10.3109/08830181003792803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rawat S, Neeraj K, Preeti K, Prashant M. A review on type, etiological factors, definition, clinical features, diagnosis management and prevention of neonatal sepsis. J Sci Innov Res. 2013;2:802–13. [Google Scholar]


