To the Editor:
A better understanding of the mechanisms by which air pollutants influence the development of asthma is critically needed (1). Of particular interest is the role of ozone (O3) because of the persistence of high ambient O3 in population centers and the likelihood that levels will increase in the future due to climate change (2). Epidemiological studies provide evidence that exposure to O3 is associated with the development of asthma (1), most notably the nonatopic (nonallergic) subtype of asthma (3, 4). The biologic plausibility of O3-induced, nonatopic asthma is supported by data showing the development of key disease features in humans and animal models after O3 exposure. Examples of this include eosinophilic airway inflammation in children without atopy (5); goblet cell hyperplasia and airway hyperresponsiveness in nonhuman primates (6, 7); mucous cell metaplasia in rats chronically exposed to O3, which persisted for several weeks after O3 exposures ceased (7), and, lastly, eosinophilic upper and lower airway inflammation in mice repeatedly exposed to O3, which is dependent on type 2 innate lymphoid cells (7). The effects of repeated O3 exposure in mice were also shown to be strain-dependent (8). These strain-dependent effects are indicative of gene–environment interaction (GxE) and provide a means by which to discover genes that mediate the response to repeated O3 exposure.
Previous studies have examined GxE in response to a single O3 exposure using primarily classical inbred strains and derivatives thereof (see Table E1 in the data supplement). Identification of GxE using mouse models has become more powerful with the advent of new mouse genetic reference populations such as the Collaborative Cross (CC). The CC is a panel of recombinant inbred strains derived from eight inbred strains (9), including three wild-derived inbred strains (CAST/EiJ, PWK/PhJ, and WSB/EiJ), which in total captures ∼90% of known genetic variation present in laboratory mice (10) (>40 million single nucleotide variants as well as structural variants). In addition, new allelic combinations in the CC produce novel, “emergent” phenotypes; for example, CC strain CC011/Unc develops colitis spontaneously (11) and CC027/GeniUnc is the only strain shown to be susceptible to peanut-induced anaphylaxis after oral peanut exposure (12). Given these findings, we sought to exploit the genetic diversity in the CC to identify a new model of O3-induced nonatopic asthma. More specifically, our goal was to survey CC strains to identify one that exhibits heightened susceptibility to O3 compared with previously tested strains (8) and develops hallmark traits of asthma, including airway eosinophilia, mucous cell metaplasia, and airway hyperresponsiveness.
In this study, we exposed female mice from 12 CC strains to 0.8 ppm O3 (4 hours/day) for 9 days, based on a “repeated O3 exposure” model (Figures 1A and E1 and Table E2) developed by Harkema and colleagues (7, 8). We used female mice only for logistic reasons and because our prior study of acute O3 exposure-induced airway neutrophilia in five CC strains showed no demonstrable sex effects (13). Across the 12 strains evaluated here, repeated O3 exposure caused variable degrees and types of airway inflammation, as measured by BAL differential cell counts (Figure E2). Most notably, compared with the other CC strains tested or the classical inbred strains C57BL/6NTac and BALB/cNTac (8), CC002/Unc (hereafter referred to as “CC002”) exhibited extreme eosinophilic inflammation. BAL fluid from CC002 mice contained nearly 40% eosinophils versus 0–7% across the other strains (Figure 1A) and 0–2% in C57BL/6NTac and BALB/cNTac (8). Compared with eosinophils, neutrophils and lymphocytes accounted for a small fraction of the total number of leukocytes in BAL in CC002 (Figure E2). BAL protein, a marker of tissue injury, was also elevated after repeated O3 exposure in most strains and the magnitude of CC002’s response was second highest after CC025 (Figure E3). Overall, the exaggerated eosinophilic inflammation in CC002 in response to O3 suggested it may represent a new model of nonatopic asthma; thus, we focused our follow-up experiments on this strain.
Figure 1.
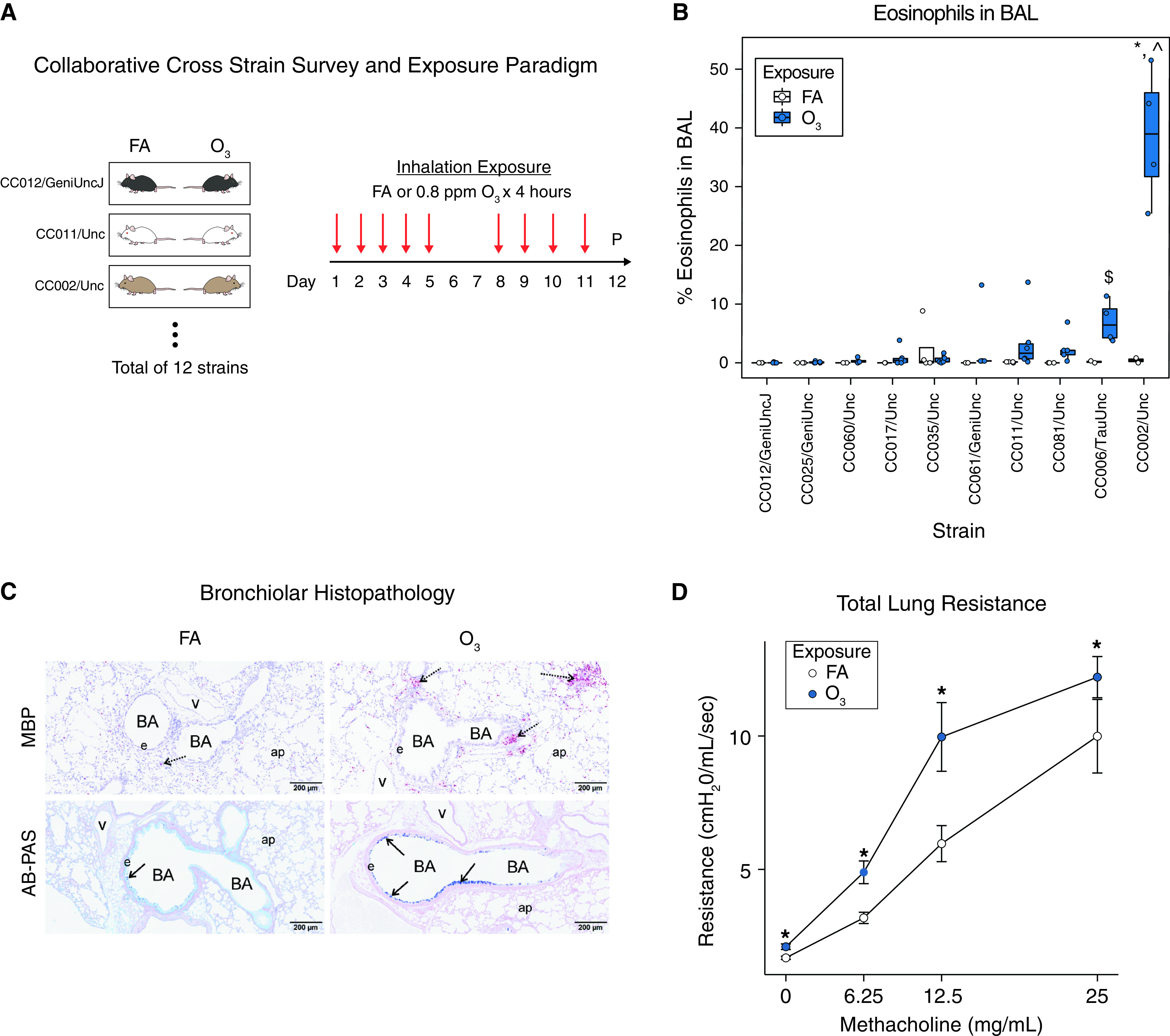
Repeated O3 exposure causes eosinophilic inflammation, airway mucous cell metaplasia, and airway hyperresponsiveness in CC002 mice. (A) Experimental design involving repeated exposure to O3 or filtered air (FA) across 12 Collaborative Cross (CC) strains. Mice were phenotyped 21 hours after last exposure. (B) Variation in O3-induced airway eosinophils in BAL fluid in 10 out of 12 CC strains tested. See Supplement for data on other cell types and CC strains. *P < 0.05 and $P < 0.01 for O3 versus FA; ⁁P < 0.05 versus other strains. FA, N = 2–4 per strain; O3, N = 4–6 per strain. (C) Representative histological sections of FA controls and O3 exposed CC002 mice showing (top) peribronchial eosinophilic inflammation, detected by immunohistochemical staining for major basic protein (MBP, red stain), and (bottom) mucous cell metaplasia in bronchiolar epithelium as detected by Alcian blue–periodic acid–Schiff (AB-PAS) staining. Scale bars, 200 μm. BA = bronchial airway, e = epithelium, ap = alveolar parenchyma, v = blood vessel. Arrows denote regions of airways featuring eosinophils or mucous cells. (D) Total lung resistance at baseline and after escalating doses of methacholine in O3 exposed (vs. FA control) CC002 mice in a separate experiment. N = 7 per group. *P < 0.05 versus FA control.
First, in a replication experiment involving more mice per exposure group, we again observed marked eosinophilia in O3-exposed CC002 mice (Figure E4), demonstrating reproducibility. We then examined the histopathology of CC002 airways and found marked peribronchiolar and perivascular inflammatory cell infiltrates (Figure E5). Immunohistochemical staining for major basic protein confirmed the predominantly eosinophilic nature of this inflammation in large airways (Figure 1C). Alcian blue–periodic acid–Schiff–stained lung sections indicated that repeated O3 also caused mucous cell metaplasia (Figure 1C) that on average was mild in small diameter bronchioles (Figure E6A). Morphometric assessment of Alcian blue–periodic acid–Schiff–stained mucosubstances in respiratory epithelium lining the mid-axial bronchiolar airway correlated with the semiquantitative severity scoring of mucous cell metaplasia throughout the lung lobe of exposed CC002 mice (Figure E6B). Consistent with this histopathology, Muc5ac (mucin 5ac) gene expression was significantly elevated in O3-exposed CC002 mice, as was Clca1 (chloride channel accessory 1) (Figure E7). We also observed increased expression of Chil4 (chitinase-like 4), which is often upregulated in type 2 airway inflammation (14) (Figure E8). In addition, we found that repeated O3 exposure caused an increase in total serum IgE (Figure E8), which is perhaps not surprising given that increased expression of total IgE has been observed after repeated O3 exposure previously (15). Finally, we determined that repeated O3 exposure increased total lung resistance at baseline and in response to methacholine challenge in CC002 mice (Figure 1D).
Our study demonstrates the effectiveness of using the CC to identify new mouse models of susceptibility to air pollutant-induced respiratory disease phenotypes. The discovery of CC002’s exaggerated eosinophilic response to repeated O3 exposure adds to the growing knowledge about gene-by-ozone interactions (16); our results also show that O3 can cause hallmark asthma phenotypes, including airway hyperresponsiveness in less than two weeks in this strain. We note that because we used female mice only, we cannot rule out the possibility of sex effects in this exposure paradigm; this should be addressed in future studies (17). In total, CC002’s unique response to repeated O3 provides an exciting opportunity to illuminate novel mechanisms that underlie the association between O3 exposure and nonatopic asthma and motivate the use of quantitative trait locus mapping approaches to identify the genetic variants that underlie CC002’s susceptibility. Because the CC002 genome contains alleles and allelic combinations not previously studied in the context of O3 exposure, we expect this approach will likely lead to the identification of genes that have not been previously implicated in response to air pollutants.
Acknowledgments
Acknowledgment
The authors thank Amy Porter and the Michigan State University (MSU) Histopathology Laboratory for technical assistance with histology and immunohistochemistry, and Ashleigh Tindle at MSU for morphometric analysis.
Footnotes
Supported by National Institute of Environmental Health Sciences grants ES024965, ES024965-S1, R21ES032089, a T32 training grant ES007126-35, and a Leon and Bertha Golberg Postdoctoral Fellowship from the University of North Carolina Curriculum in Toxicology and Environmental Medicine.
This letter has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Thurston GD, Balmes JR, Garcia E, Gilliland FD, Rice MB, Schikowski T, et al. Outdoor air pollution and new-onset airway disease. An official American Thoracic Society workshop report. Ann Am Thorac Soc. 2020;65:387–398. doi: 10.1513/AnnalsATS.202001-046ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cromar KR, Gladson LA, Ewart G. Trends in excess morbidity and mortality associated with air pollution above American Thoracic Society-recommended standards, 2008–2017. Ann Am Thorac Soc. 2019;65:836–845. doi: 10.1513/AnnalsATS.201812-914OC. [DOI] [PubMed] [Google Scholar]
- 3. McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;65:386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 4. Nishimura KK, Iwanaga K, Oh SS, Pino-Yanes M, Eng C, Keswani A, et al. Early-life ozone exposure associated with asthma without sensitization in Latino children. J Allergy Clin Immunol. 2016;65:1703–1706.e1. doi: 10.1016/j.jaci.2016.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frischer T, Studnicka M, Halmerbauer G, Horak F, Jr, Gartner C, Tauber E, et al. Ambient ozone exposure is associated with eosinophil activation in healthy children. Clin Exp Allergy. 2001;65:1213–1219. doi: 10.1046/j.1365-2222.2001.01155.x. [DOI] [PubMed] [Google Scholar]
- 6. Flayer CH, Larson ED, Joseph A, Kao S, Qu W, Van Haren A, et al. Ozone-induced enhancement of airway hyperreactivity in rhesus macaques: effects of antioxidant treatment. J Allergy Clin Immunol. 2020;65:312–323. doi: 10.1016/j.jaci.2019.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harkema JR, Wagner JG. Innate lymphoid cell-dependent airway epithelial and inflammatory responses to inhaled ozone: a new paradigm in pathogenesis. Toxicol Pathol. 2019;65:993–1003. doi: 10.1177/0192623319873872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harkema JR, Hotchkiss LA, Vetter NA, Jackson-Humbles DN, Lewandowski RP, Wagner JG. Strain differences in a murine model of air pollutant-induced nonatopic asthma and rhinitis. Toxicol Pathol. 2017;65:161–171. doi: 10.1177/0192623316674274. [DOI] [PubMed] [Google Scholar]
- 9. Srivastava A, Morgan AP, Najarian ML, Sarsani VK, Sigmon JS, Shorter JR, et al. Genomes of the mouse collaborative cross. Genetics. 2017;65:537–556. doi: 10.1534/genetics.116.198838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts A, Pardo-Manuel de Villena F, Wang W, McMillan L, Threadgill DW. The polymorphism architecture of mouse genetic resources elucidated using genome-wide resequencing data: implications for QTL discovery and systems genetics. Mamm Genome. 2007;65:473–481. doi: 10.1007/s00335-007-9045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rogala AR, Morgan AP, Christensen AM, Gooch TJ, Bell TA, Miller DR, et al. The Collaborative Cross as a resource for modeling human disease: CC011/Unc, a new mouse model for spontaneous colitis. Mamm Genome. 2014;65:95–108. doi: 10.1007/s00335-013-9499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orgel K, Smeekens JM, Ye P, Fotsch L, Guo R, Miller DR, et al. Genetic diversity between mouse strains allows identification of the CC027/GeniUnc strain as an orally reactive model of peanut allergy. J Allergy Clin Immunol. 2019;65:1027–1037.e7. doi: 10.1016/j.jaci.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tovar A, Crouse WL, Smith GJ, Thomas JM, Keith BP, McFadden KM, et al. Integrative phenotypic and genomic analyses reveal strain-dependent responses to acute ozone exposure and their associations with airway macrophage transcriptional activity. bioRxiv. 2021 doi: 10.1152/ajplung.00237.2021. https://www.biorxiv.org/content/10.1101/2021.01.29.428733v1 [DOI] [PMC free article] [PubMed]
- 14. Ong CB, Kumagai K, Brooks PT, Brandenberger C, Lewandowski RP, Jackson-Humbles DN, et al. Ozone-induced type 2 immunity in nasal airways. Development and lymphoid cell dependence in mice. Am J Respir Cell Mol Biol. 2016;65:331–340. doi: 10.1165/rcmb.2015-0165OC. [DOI] [PubMed] [Google Scholar]
- 15. Neuhaus-Steinmetz U, Uffhausen F, Herz U, Renz H. Priming of allergic immune responses by repeated ozone exposure in mice. Am J Respir Cell Mol Biol. 2000;65:228–233. doi: 10.1165/ajrcmb.23.2.3898. [DOI] [PubMed] [Google Scholar]
- 16. Romieu I, Moreno-Macias H, London SJ. Gene by environment interaction and ambient air pollution. Proc Am Thorac Soc. 2010;65:116–122. doi: 10.1513/pats.200909-097RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silveyra P, Al Housseiny H, Rebuli ME. Sex-Based Differences in Lung Physiology. Springer; 2021. Sex and gender differences in the susceptibility to environmental exposures; pp. 251–290. [Google Scholar]


