Abstract
Rationale: Outdoor air pollution contributes to asthma development and exacerbations, yet its effects on airway pathology have not been defined in children.
Objectives: To explore the possible link between air pollution and airway pathology, we retrospectively examined the relationship between environmental pollutants and pathological changes in bronchial biopsy specimens from children undergoing a clinically indicated bronchoscopy.
Methods: Structural and inflammatory changes (basement membrane [BM] thickness, epithelial loss, eosinophils, neutrophils, macrophages, mast cells, and lymphocytes) were quantified in biopsy specimens by using immunohistochemistry. The association between exposure to particulate matter less than 10 μm in aerodynamic diameter (PM10), SO2 and NO2 and biopsy findings was evaluated by using a generalized additive model with Gamma family to allow for overdispersion, adjusted for atmospheric pressure, temperature, humidity, and wheezing.
Results: Overall, 98 children were included (age 5.3 ± 2.9 yr; 53 with wheezing/45 without wheezing). BM thickness increased with prolonged exposure to PM10 (rate ratio [RR], 1.29; 95% confidence interval [CI], 1.09–1.52), particularly in children with wheezing. Prolonged exposure to PM10 was also associated with eosinophilic inflammation in children with wheezing (RR, 3.16; 95% CI, 1.35–7.39). Conversely, in children without wheezing, increased PM10 exposure was associated with a reduction of eosinophilic inflammation (RR, 0.12; 95% CI, 0.02–0.6) and neutrophilic inflammation (RR, 0.36; 95% CI, 0.14–0.89). Moreover, NO2 exposure was also linked to reductions in neutrophil infiltration (RR, 0.57; 95% CI, 0.34–0.93) and eosinophil infiltration (RR, 0.33; 95% CI, 0.14–0.77).
Conclusions: Different patterns of association were observed in children with wheezing and in children without wheezing. In children without wheezing, exposure to PM10 and NO2 was linked to reduced eosinophilic and neutrophilic inflammation. Conversely, in children with wheezing, prolonged exposure to PM10 was associated with increased BM thickness and eosinophilic inflammation, suggesting that it might contribute to asthma development by promoting airway remodeling and inflammation.
Keywords: asthma, wheezing, air pollution, particulate matter
Asthma is a heterogeneous disease, usually characterized by a range of respiratory symptoms that vary over time across the life course of individual patients (1, 2). Asthma is a serious global health problem affecting all age groups, and its prevalence is increasing, especially among children, imposing an unacceptable burden on healthcare systems and society (1).
The pathological hallmarks of the disease encompass both chronic airway inflammation, usually eosinophilic, and airway remodeling. This includes thickening of the subepithelial basement membrane (BM), shedding of the epithelial layer, an increased smooth muscle area, increased mucus production, and neoangiogenesis. Our group has previously demonstrated that some of these features, particularly BM thickening, are early events in the natural history of the disease, being already present in early infancy, even in children with the mildest forms of asthma and without atopy (3–5).
Despite the growing burden of asthma, causes and pathophysiological mechanisms underlying the disease remain to be established. Asthma is a multifactorial disorder caused by the complex interaction between genetic and environmental factors (2, 6, 7).
Several epidemiological studies demonstrated that outdoor air pollution is an important contributing factor to both asthma exacerbations and, possibly, new-onset cases (2, 8–11).
A recent statement from the American Thoracic Society concluded that there is now enough epidemiological evidence to indicate a causal link between long-term exposure to outdoor air pollution and new cases of incident asthma in children (10). Of note, Khreis and colleagues (11) have recently estimated the incidence of asthma related to air pollution by using a validated and harmonized European land-use regression model: they suggested that matching nitric dioxide, particulate matter, and black carbon minimum concentrations may prevent as much as 33% of the incident cases of asthma in children. Of note, outdoor air pollution is associated with impaired lung function, with increased hyperreactivity and airway inflammation being measured indirectly through fractional exhaled nitric oxide (12–15). These observations highlight the urgent need to reduce children’s exposure to outdoor air pollution.
Despite such evidence, the pathophysiological mechanisms linking air pollution and asthma development have not been fully understood. In particular, no study has addressed the influence of long-term outdoor air pollution exposure on inflammatory and structural changes in the airways of children with wheezing.
In this study, we sought to examine the relationship between previous exposure to nitrogen dioxide (NO2), particulate matter less than 10 μm in aerodynamic diameter (PM10), and sulfur dioxide (SO2) levels and the pathological traits typical of asthma in a cohort of children living in Northeast Italy, one of the most polluted regions in Europe.
Some of these results have been published in the form of an abstract (16).
Methods
Study Population
Children were recruited at the Department of Woman and Child Health, University of Padova, Padova, Italy, from 2002 to 2014. The study was performed according to the Declaration of Helsinki and was approved by the local ethics committee. Written consent was obtained from children’s parents. All children underwent bronchoscopy (with bronchoalveolar lavage [BAL] and bronchial biopsy) on the basis of appropriate clinical indications according to the European Respiratory Society and American Thoracic Society guidelines (17, 18), as summarized in Table E1 in the online supplement. An additional endobronchial biopsy for research purposes was performed with approval of the ethics committee and consent from the parents. Fiberoptic bronchoscopy was well tolerated by all children. Before bronchoscopy, all patients were evaluated by a respiratory pediatrician. The pediatrician collected a detailed clinical history, examined the child, and administered parental interviews focused on the presence of respiratory symptoms, frequency of respiratory tract infections (RTIs) in the previous year, and on-course treatment focused on asthma medications (inhaled corticosteroids [ICSs] or oral corticosteroids). The presence of wheezing with a pattern suggestive of asthma was based on reporting of repeated episodes of wheezing in the previous year, which was often associated with cough and dyspnea, particularly at night or in the early morning. Furthermore, wheezing had to be present even apart from colds (multitrigger) and had to be responsive to prescribed bronchodilators. Wheezing frequency was defined on a scale from 0 (no episodes) to 6 (daily episodes). At baseline, all children underwent routine blood tests, including a complete white blood cell count (total leukocytes, neutrophils, lymphocytes, monocytes, eosinophils, and basophils) and testing for total/specific IgE. As previously reported (5), the presence of atopy was defined by an increase in the total (higher than the age-related normal levels) and specific IgE (>0.35 kU/L; ImmunoCap, Phadia). In particular, specific IgEs for the following aeroallergens were investigated in all children: house dust mite (Dermatophagoides pteronyssinus and D. farinae), molds (Alternaria alternate), and cat dander and grass pollens (Lolium perenne, Poa pratensis, Phleum pratense, Dactylis glomerata, and Cynodon dactylon).
Air Pollution Exposure Evaluation
Clinical and pathological data were combined with information collected from the regional air pollution monitoring system.
Data from 2002 onward on daily concentrations of PM10, NO2, and SO2, together with meteorological data on temperature, relative humidity, and atmospheric pressure, were retrieved from the monitoring stations of the Environmental Protection and Prevention Agency of the Veneto Region. Children were linked to the data of the monitoring station nearest to their residence (with a maximum distance set at 20 km) (19). The distribution of pollutants in the Veneto region is highly homogeneous because of its particular morphological condition. The Po valley, of which Veneto occupies the eastern part, is a vast flat area in northern Italy surrounded by mountains and a shallow sea. This particular morphological structure leads to a very low wind speed and facilitates air pollutants’ stagnation.
Methods and technologies used to measure air pollutant concentrations were those designated by national and international consensus (20). Moreover, air pollution concentrations were compared with the 2005 World Health Organization air quality guidelines cutoffs (21) of 50 μg/m3 for PM10 and 40 μg/m3 for NO2. None of the children changed residential addresses during the study.
Bronchial Biopsy
Full details on the bronchoscopy, bronchial biopsy, and BAL procedures have been previously described (3–5). Briefly, bronchoscopy with endobronchial biopsy and BAL was performed by using a flexible bronchoscope with an external diameter of 4.9 mm. Bronchial biopsy specimens were taken by using Olympus FB 19 C-1 bronchial forceps, which were inserted through the service channel of the bronchoscope (2-mm diameter). Patients with insufficient or low-quality biopsy specimens were excluded from the study. Biopsy specimens were gently extracted, fixed in 4% formaldehyde, and dehydrated through alcohol series. They were embedded in paraffin wax and processed for histochemical and immunohistochemical analysis. Analysis of epithelial loss and reticular BM thickness was performed on sections stained with hematoxylin and eosin.
Inflammatory cells (eosinophils, neutrophils, mast cells, macrophages, and CD4+ T lymphocytes) were quantified in the area extending 50 μm beneath the BM and were expressed as the number of positive cells per square millimeter of subepithelium. Briefly, mouse monoclonal antibodies were used as previously detailed (3, 5) for identification of eosinophils (antieosinophil cationic protein, Diagnostic Developments), neutrophils (antielastase M752), mast cells (antitryptase M7052), macrophages (anti-CD68 M814) and CD4+ cells (anti-CD4 M834, all from Dako Ltd.). BAL fluid cell counts (total cell counts, lymphocytes, neutrophils, macrophages, and eosinophils) were performed by the Hospital Central Laboratory, as previously described (22).
Statistical Analysis
Children’s characteristics were expressed by using the median and interquartile range (IQR) for continuous variables and counts and percentages for categorical variables. Comparisons among groups were evaluated with either the Student’s t test or the Mann-Whitney U test as appropriate. Distributions of categorical variables were compared by using the χ2 test or Fisher’s exact test when the sample size was small (n < 5). We performed analysis by using a generalized additive model with a Gamma in extenso family (23) to evaluate the association between air pollution and both inflammation and structural parameters measured in the biopsy specimen. We also analyzed the impact of outdoor air pollution on relevant clinical parameters (i.e., wheezing frequency, RTI frequency).
We used penalized cubic regression splines to account for the nonlinear relationship between the pollutant and the outcome. The possible delayed effect in time was analyzed by using the average concentrations of pollutants from 0 to 90 days before the bronchoscopy. Therefore, “lag 0–90” indicates the average concentration of the pollutant in the 90 days preceding the bronchoscopy, “lag 0–89” indicates the average concentration of the 89 days before, and so on. The model structure was chosen according to the lowest Akaike information criterion (24). All models were adjusted for temperature, atmospheric pressure, relative humidity, and wheezing. To assess the potential influence of crucial confounding factors on our study, such as atopy, secondhand smoke exposure, ICS treatment, and age of children at bronchoscopy, we included them in the model as possible covariates. However, these covariates did not change the fit of the models. A second analysis was performed separately on the two subpopulations of children with and without wheezing.
Results reported as rate ratios (RRs) and relative 95% confidence intervals (CI) were calculated for an increase in the IQR of the pollutant. The lag with the highest RR in absolute terms for each outcome is labeled as the “best RR.”
Missing environmental data (4%) were imputed by using multiple imputation with a expectation-maximization with bootstrapping algorithm (25). All analyses were performed by using R statistical software and the Amelia ad mgcv R package (R Foundation for Statistical Computing) (26, 27).
Results
Clinical and Demographic Characteristics
Initially, 121 children were enrolled, clinical data were recorded, and bronchial biopsy specimens were examined for all children. However, data on outdoor pollution exposure were available for 98 out of 120 children (81%). The clinical and pathological features of children excluded because of a lack of pollution data did not differ from those of children included in the study.
Clinical indications for bronchoscopy, according to international guidelines, are reported in Table E1. At the time of bronchoscopy, 53 children (54%) had history of repeated wheezing episodes even apart from colds and were responsive to bronchodilators, whereas 45 (46%) did not have wheezing reported in their clinical history. Clinical characteristics of the cohort are summarized in Table 1. The mean age at the onset of wheezing was 4.5 years, and children with wheezing had an age and sex distribution similar to those of children without wheezing. Children with wheezing showed higher levels of serum IgE (P = 0.04). ICS treatment tended to be more frequent in children with wheezing than in children without wheezing; only a minority of children were receiving oral corticosteroids in both groups. The two groups of children had comparable histories of RTIs and secondhand smoke exposure. Finally, no differences were observed between children with wheezing and children without wheezing in the blood total and differential cell counts, including those for blood eosinophils (Table 1).
Table 1.
Clinical features and blood cell counts in the whole cohort and in children with and without wheezing
| Whole Cohort | With Wheezing | Without Wheezing | P | |
|---|---|---|---|---|
| Subjects | 98 (100%) | 53 (54%) | 45 (46%) | — |
| Male | 53 (54%) | 27 (50%) | 26 (57%) | NS |
| Age, yr | 4.5 (4–6) | 5 (3–6.5) | 4 (4–5.5) | NS |
| Symptom onset, yr | 1.5 (0.5–3) | 1.5 (0.5–3) | N/A | — |
| Symptom frequency (scale of 0–6) | 0 (0–3) | 3 (2–4) | 0 | — |
| RTI during previous yr | 1 (0.5–1) | 0.5 (0.5–1) | 1 (0.5–1.5) | NS |
| ICS therapy at bronchoscopy | 23 (23%) | 16 (30%) | 7 (15%) | 0.06 |
| OCS therapy at bronchoscopy | 6 (6%) | 2 (3%) | 4 (6%) | NS |
| Secondhand smoke | 29 (41%) (n = 70) | 19 (48%) (n = 40) | 10 (33%) (n = 30) | NS |
| Atopy | 42 (43%) | 26 (50%) | 16 (34%) | NS |
| Rhinitis prevalence | 17 (22%) (n = 74) | 12 (28%) (n = 42) | 5 (15%) (n = 32) | NS |
| Dermatitis prevalence | 29 (30%) (n = 94) | 18 (35%) (n = 51) | 11 (25%) (n = 43) | NS |
| Serum IgE, kU/L | 57 (23–170) | 72 (32–526) | 50 (11–82) | 0.04 |
| Leukocytes, cells/μl (n = 78/98) | 7,350 (5,765–9,040) | 6,880 (5,640–8,617) | 7,919 (6,205–9,340) | NS |
| Neutrophils cells/μl (n = 78/98) | 2,855 (1,845–4,152) | 2,420 (1,810–3,750) | 3,215 (2,500–4,690) | NS |
| Lymphocytes, cells/μl (n = 78/98) | 3,135 (2,365–4,332) | 3,140 (2,365–3,997) | 3,050 (2,430–4,495) | NS |
| Monocytes, cells/μl (n = 78/98) | 560 (420–675) | 530 (427–660) | 580 (400–745) | NS |
| Eosinophils, cells/μl (n = 98/98) | 190 (120–310) | 220 (119–323) | 170 (117–300) | NS |
| Basophils, cells/μl (n = 78/98) | 30 (20–50) | 30 (20–50) | 30 (25–45) | NS |
Definition of abbreviations: ICS = inhaled corticosteroid; IgE = immunoglobulin E; kU/L = 2.5 ng/ml; OCS = oral corticosteroid; N/A = not applicable; NS = not significant; RTI = respiratory tract infection.
Data are expressed as n (%) or the median (interquartile range). The P values refer to the Mann-Whitney U test, Student’s t test, or chi-square test comparison between children with wheezing and children without wheezing.
The pathological features (structural and inflammatory) measured in bronchial biopsy specimens are shown in Table 2. As reported in our previous studies, the pathological features characteristic of adult asthma were already present in children with wheezing (3–5). Children with wheezing had an increased reticular BM thickness (P < 0.0001) and epithelial shedding (P = 0.008) compared with children without wheezing. Inflammatory infiltrates also differed between the two groups, with children with wheezing displaying more eosinophils (P = 0.0002) and more mast cells (P = 0.01) than children without wheezing. No differences were observed with regard to neutrophil, macrophage, and CD4+ T lymphocyte counts in biopsy specimens. BAL fluid inflammatory cell counts were similar in the two groups (Table 2).
Table 2.
Pathological features in the whole cohort and in children with and without wheezing
| Whole Cohort | With Wheezing | Without Wheezing | P | |
|---|---|---|---|---|
| Basement membrane, μm (n = 98/98) | 4.2 (3.4–5.1) | 4.8 (3.8–6) | 3.8 (3.1–4.4) | <0.0001 |
| Destroyed epithelium, % (n = 93/98) | 60 (35–88) | 67 (46–91) | 44 (18–85) | 0.008 |
| Eosinophils, cells/mm2 (n = 85/98) | 28 (8–86) | 62 (23–146) | 9 (0–35) | 0.0002 |
| Neutrophils, cells/mm2 (n = 85/98) | 135 (72–275) | 133 (65–342) | 137 (77–209) | NS |
| Mast cells, cells/mm2 (n = 80/98) | 128 (62–370) | 220 (78–450) | 100 (53–238) | 0.01 |
| CD4+ T lymphocytes, cells/mm2 (n = 70/98) | 315 (152–560) | 321 (84–690) | 314 (175–524) | NS |
| Macrophages, cells/mm2 (n = 81/98) | 101 (41–206) | 113 (44–215) | 82 (42–176) | NS |
| Total cellularity, cells × 103/ml (n = 88/98) | 200 (100–457) | 173 (75–376) | 315 (117–580) | NS |
| Eosinophils, cells × 103/ml (n = 86/98) | 0 (0–1.3) | 0 (0–2) | 0 (0–0) | NS |
| Neutrophils, cells × 103/ml (n = 86/98) | 29 (3.4–164) | 22 (4–76) | 46 (3.1–263) | NS |
| Lymphocytes, cells × 103/ml (n = 85/98) | 13 (4–25) | 13 (5–24) | 13 (3.7–28.5) | NS |
| Macrophages, cells × 103/ml (n = 85/98) | 142 (72–251) | 139 (61–382) | 149 (88–261) | NS |
Definition of abbreviation: NS = not significant.
Data are expressed as the median (interquartile range). The P values refer to the Mann-Whitney U test comparison between children with wheezing and children without wheezing.
Outdoor Air Pollution Exposure
Data on average levels of outdoor air pollutants during the 90 days before bronchoscopy are summarized in Table 3. Of note, SO2 levels measured during the observation period were negligible across the whole region, so we did not consider this pollutant for further analyses. As shown in Table 3, all children in our cohort were exposed to high levels of pollutants. Exposure to NO2 exceeded the World Health Organization threshold for half of the days, whereas PM10 exceeded the threshold for 38% of the days. Of note, the levels of exposure to PM10 and NO2 did not differ among different districts or between children with wheezing and children without wheezing.
Table 3.
Pollutant exposure during the 90 days before bronchoscopy stratified for children’s district of residence for the whole cohort, children with wheezing, and children without wheezing
| n (%) | Whole Cohort |
With Wheezing |
Without Wheezing |
||||
|---|---|---|---|---|---|---|---|
| PM10 (μg/m3) | NO2 (μg/m3) | PM10 (μg/m3) | NO2 (μg/m3) | PM10 (μg/m3) | NO2 (μg/m3) | ||
| Whole region | 98 (100) | 40 (25–66) | 40 (27–53) | 37 (24–60) | 37 (25–50) | 45 (27–75) | 43 (30–57) |
| Padova | 35 (35) | 58 (41–68) | 45 (39–54) | 43 (35–66) | 42 (39–53) | 63 (58–74) | 49 (42–56) |
| Venezia | 34 (34) | 43 (32–54) | 32 (25–41) | 40 (31–54) | 31 (25–41) | 45 (37–54) | 39 (28–45) |
| Vicenza | 13 (14) | 45 (35–59) | 38 (32–47) | 42 (33–51) | 35 (31–39) | 54 (42–64) | 45 (38–50) |
| Treviso | 16 (17) | 36 (29–60) | 37 (32–50) | 31 (29–50) | 33 (30–44) | 46 (31–61) | 41 (33–51) |
Definition of abbreviations: NO2 = nitric dioxide; PM10 = particulate matter less than 10 μm in aerodynamic diameter.
Data are expressed as the median (interquartile range).
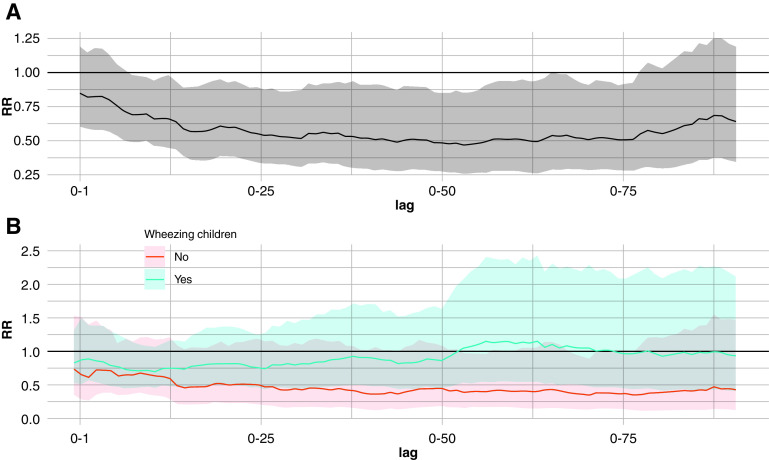
As shown in Figure 1A, in the whole cohort, BM thickness significantly increased with prolonged exposure to PM10. For each IQR increase in the PM10 concentration, we observed a significant enlargement of the BM of up to 30%, particularly from lag 0–15 to lag 0–90 (best RR at lag 0–63, 1.29; 95% CI, 1.09–1.52).
Figure 1.
The strength of the association between basement membrane thickness and exposure to particulate matter less than 10 μm in aerodynamic diameter. The membrane thickness rate ratio (RR) for each interquartile range increase of the moving average concentration of particulate matter less than 10 μm in aerodynamic diameter (PM10) from 0 to 90 days before bronchoscopy is shown. (A) Data for the whole cohort. (B) Data for children with wheezing (blue) and children without wheezing (red) shown separately. The line indicates the RR and its variation in each lag duration, and the shaded area indicates the confidence interval (CI). When the lowest (or the highest) limit of the CI (shaded area) does not include 1, the association is statistically significant.
When stratifying our study population by the presence of wheezing (Figure 1B), a positive association was found in children with wheezing for an exposure to PM10 longer than 13 days (best RR at lag 0–80, 1.34; 95% CI, 1.09–1.66), whereas an association at earlier time points was detected in children without wheezing (best RR at lag 0–7, 1.23; 95% CI, 1.1–1.36). In our cohort, we did not observe any influence of NO2 on BM thickness (see Figure E1 in the online supplement) or of PM10 or NO2 on epithelial integrity. Raw data are reported in online supplement (Table E2).
With regard to the association between air pollution and inflammatory features (Figures 2 and 3), we observed different patterns in children with wheezing and in children without wheezing. In children with wheezing, there was a positive association between prolonged exposure to PM10 and tissue eosinophilic inflammation, as shown in Figure 2B. This association progressively increased with prolonged exposure, reaching its maximum value at lag 0–68, when the number of eosinophils tripled for each IQR increase in the PM10 concentration (RR, 3.16; 95% CI, 1.35–7.39).
Figure 2.
The strength of the association between eosinophils in bronchial biopsy specimens and exposure to particulate matter less than 10 μm in aerodynamic diameter (PM10). The eosinophil rate ratio (RR) for each interquartile range increase of the moving average concentration of particulate matter less than 10 μm in aerodynamic diameter from 0 to 90 days before bronchoscopy is shown. (A) Data for the whole cohort. (B) Data for children with wheezing (blue) and children without wheezing (red) shown separately. The line indicates the RR and its variation during each lag, and the shaded area indicates the confidence interval (CI). When the lowest (or the highest) limit of the CI (shaded area) does not include 1, the association is statistically significant.
Figure 3.
The strength of the association between neutrophils in bronchial biopsy specimens and exposure to particulate matter less than 10 μm in aerodynamic diameter. The neutrophil rate ratio (RR) for each interquartile range increase of the moving average concentration of particulate matter less than 10 μm in aerodynamic diameter (PM10) from 0 to 90 days before bronchoscopy is shown. (A) Data for the whole cohort. (B) Data for children with wheezing (blue) and children without wheezing (red) shown separately. The line indicates the RR and its variation during each lag, and the shaded area indicates the confidence interval (CI). When the lowest (or the highest) limit of the CI (shaded area) does not include 1, the association is statistically significant.
Conversely, in children without wheezing, prolonged exposure to PM10 was associated with reduced eosinophil numbers in bronchial biopsy specimens (Figure 2B) (best RR at lag 0–77, 0.12; 95% CI, 0.02–0.6). In children without wheezing, prolonged exposure to PM10 also reduced the number of neutrophils (best RR at lag 0–70, 0.36; 95% CI, 0.14–0.89; Figure 3B), although this association only trended toward statistical significance. No influence of PM10 exposure on neutrophils was found in children with wheezing.
Regarding NO2 exposure, we observed weak negative associations during longer lags with eosinophils (best RR at lag 0–55, 0.33; 95% CI, 0.14–0.77) and during shorter lags with neutrophils (best RR at lag 0–14, 0.57; 95% CI, 0.34–0.93) (Figures E2 and E3) in the whole cohort. Raw data are reported in the online supplement (Tables E3–E6). In our cohort, other inflammatory cell subtypes (lymphocytes, macrophages, mast cells) in bronchial biopsy specimens were not influenced by air pollution. Although exposure to PM10 and NO2 influences airway tissue inflammation, we did not observe any significant relationship between air pollution and BAL fluid or blood inflammatory cell counts (including blood eosinophils). Furthermore, there was no link with clinical parameters, such as the frequency of wheezing symptoms and frequency of RTIs.
Discussion
To assess whether outdoor air pollution might affect airway pathology in children with wheezing, we investigated the association between the pathological hallmarks of asthma in airway biopsy specimens and exposure to the major air pollutants detected by regional air quality sensors. Our study illustrates for the first time, in vivo, a significant association between chronic air pollution exposure and histopathological changes in children. In children with wheezing, we found that exposure to high levels of particulate matter is associated with airway structural changes and chronic eosinophilic inflammation, possibly contributing to the pathophysiological mechanisms leading to asthma. Conversely, in children without wheezing, exposure to outdoor air pollution is associated with decreased eosinophilic and neutrophilic infiltrate in tissue, possibly reflecting a general impairment of innate immunity.
Thickening of the reticular BM represents a key structural change in bronchial asthma. Its pathogenetic role has been increasingly appreciated in recent decades, as it has been identified as a marker of epithelial–mesenchymal cross-talk, which may even anticipate inflammatory changes (28). Of importance, BM thickening is detectable from the beginning of the natural history (3–5) and is associated with airway hyperresponsiveness and lung function decline (29). Moreover, our group has recently shown that, among the different inflammatory and structural changes present in the airways of young children, BM thickening is the one that best correlates with the persistence of asthma across adolescence (5).
Our study shows that children with wheezing who live in areas with poor air quality (i.e., those exposed to high levels of PM10) have a significant thickening of the reticular BM. This association was independent of potential confounding factors, including age, atopy, ICS treatment, and secondhand smoke exposure, suggesting that particulate matter might have a major role in promoting remodeling early in life. To our knowledge, this is the first study in vivo to suggest a potential contribution of air pollution to airway remodeling in children with wheezing, who are at high risk for asthma. Previously, only Churg and colleagues (30) had demonstrated similar in vivo results, showing greater amounts of airway fibrosis in the autoptic sections from 20 healthy women who were lifelong residents of a high-PM10 area. Our results expand previous studies that demonstrated in in vitro or animal models that PM enhances airway remodeling (11) and the production of proremodeling factors (VEGF, IL8, MUC5A) (31) or the activation of fibroblastic activation pathways (32, 33).
Eosinophilic inflammation represents the typical inflammatory trait of asthma, generally considered as the hallmark of an atopy-driven T-helper cell type 2 cytokine environment (2). However, it is increasingly recognized that, besides atopy, many other factors may enhance eosinophilic inflammation (2). The present study demonstrates that chronic exposure to air pollution might be one such factor. Indeed, children with wheezing exposed to PM10 for a long time show an increased number of eosinophils, increased by even more than three times, which is independent from the presence of atopy and ICS treatment.
It is conceivable that the airways of this high-risk population were more susceptible to various stimuli, thus supporting the hypothesis that particulate matter enhances existing mechanisms that amplify airway remodeling and inflammation (11). The eosinophil increase we observed in vivo is in line with the findings of many in vitro or animal model studies demonstrating that air pollution enhances the T-helper cell type 2 cytokine environment. Possible mechanisms involved can act through the release of IL-33 or IL-13 or through modulation of the antigen presentation process (34–38). Although there was a clear association between PM10 exposure and eosinophil amounts in airway biopsy specimens, no such association was detectable with peripheral blood eosinophils. These results confirm that blood eosinophils poorly represent the mechanisms regulating eosinophilic inflammation in the lung tissue (39).
Of interest, chronic exposure to air pollution was related to different patterns of tissue inflammation in children with and without wheezing in our cohort. Children without wheezing who live in more polluted areas show reduced numbers of eosinophils and neutrophils in bronchial biopsy specimens. Similar reductions in airway neutrophils were observed with prolonged exposure to PM10 but were also observed with short-term exposure to NO2. Our results in children in vivo are in line with recent evidence in vitro showing that air pollutants may impair innate immune responses to influenza virus infection, particularly by downregulating type 1 interferons, downregulating IL-6, and preventing NLRP3 inflammasome formation (40, 41). Conversely, other studies reported an enhancement of luminal airway inflammation (in sputum or BAL fluid), particularly after acute exposure (42, 43). Interestingly, Stenfors and colleagues (42), who found increased neutrophils in BAL fluid, reported a reduction in neutrophils in biopsy specimens of healthy subjects, suggesting a movement of cells from the airway wall into the airway lumen. Thus, it is conceivable that air pollution may weaken the normal innate immune mechanisms in the tissue, possibly promoting respiratory infections (44, 45)
Limitations
The retrospective design of this study is certainly a limitation; however, this is the only ethical way to study the effects of chronic outdoor air pollution exposure on the lung in vivo. Furthermore, we could not assess the levels of particulate matter ⩽2.5 μm in aerodynamic diameter in our cohort, which is another potential limitation of our study. We also acknowledge that the cohort of children who underwent a clinically indicated bronchoscopy may not be representative of the entire population and that the condition prompting the bronchoscopy might have influenced the results. However, these concomitant conditions were evenly present among the study groups and are unlikely to have affected the observed differences. Finally, the relatively low number of patients, especially when analyzing the two subgroups of children with and without wheezing, led to a widening of confidence intervals.
Conclusions
In conclusion, this study reports an association between air pollution and histopathological changes in the airways of children with wheezing. First, prolonged exposure to high levels of PM10 promotes BM thickening and enhances eosinophilic inflammation in children with wheezing, who are at high risk for asthma. Second, in subjects without wheezing, exposure to high levels of air pollution reduces the levels of eosinophils and neutrophils, suggesting an impairment of innate immunity. These results reveal for the first time in vivo in children the mechanisms linking air pollution and the pathological hallmarks of asthma, with important implications for improved understanding of the pathophysiology and mechanisms of progression in this disease being demonstrated. The importance of air quality control to promote lung health cannot be overemphasized.
Footnotes
Supported by University of Padova (BARA_BIRD2020_01 and BIRD194033).
Author Contributions: M.B., E.G., M.S., D.G., and S.B.: Conception of the study design and drafting and revision of the manuscript. M.B., E.G., E.B., G.M., L.Z., M.G.C., and A.B.: Acquisition and analysis of data (bronchoscopy, clinical examination, experimental analyses of bronchial biopsy specimens; air pollution data; and statistical analysis).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Global Initiative for Asthma. Fontana, WI: Global Initiative for Asthma; 2019. [Google Scholar]
- 2. Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers. 2015;1:15025. doi: 10.1038/nrdp.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turato G, Barbato A, Baraldo S, Zanin ME, Bazzan E, Lokar-Oliani K, et al. Nonatopic children with multitrigger wheezing have airway pathology comparable to atopic asthma Am J Respir Crit Care Med 2008178476–482.. [DOI] [PubMed] [Google Scholar]
- 4. Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Tura M, et al. Airway inflammation in childhood asthma. Am J Respir Crit Care Med. 2003;168:798–803. doi: 10.1164/rccm.200305-650OC. [DOI] [PubMed] [Google Scholar]
- 5. Bonato M, Bazzan E, Snijders D, Tinè M, Biondini D, Turato G, et al. Clinical and pathologic factors predicting future asthma in wheezing children: a longitudinal study. Am J Respir Cell Mol Biol. 2018;59:458–466. doi: 10.1165/rcmb.2018-0009OC. [DOI] [PubMed] [Google Scholar]
- 6. Ullmann N, Mirra V, Di Marco A, Pavone M, Porcaro F, Negro V, et al. Asthma: differential diagnosis and comorbidities. Front Pediatr. 2018;6:276. doi: 10.3389/fped.2018.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuchs O, Bahmer T, Rabe KF, von Mutius E. Asthma transition from childhood into adulthood. Lancet Respir Med. 2017;5:224–234. doi: 10.1016/S2213-2600(16)30187-4. [DOI] [PubMed] [Google Scholar]
- 8. Pénard-Morand C, Raherison C, Charpin D, Kopferschmitt C, Lavaud F, Caillaud D, et al. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J. 2010;36:33–40. doi: 10.1183/09031936.00116109. [DOI] [PubMed] [Google Scholar]
- 9. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thurston GD, Balmes JR, Garcia E, Gilliland FD, Rice MB, Schikowski T, et al. Outdoor air pollution and new-onset airway disease: an official American Thoracic Society workshop report. Ann Am Thorac Soc. 2020;17:387–398. doi: 10.1513/AnnalsATS.202001-046ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khreis H, Cirach M, Mueller N, de Hoogh K, Hoek G, Nieuwenhuijsen MJ, et al. Outdoor air pollution and the burden of childhood asthma across Europe. Eur Respir J. 2019;54:1802194. doi: 10.1183/13993003.02194-2018. [DOI] [PubMed] [Google Scholar]
- 12. Delfino RJ, Staimer N, Gillen D, Tjoa T, Sioutas C, Fung K, et al. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environ Health Perspect. 2006;114:1736–1743. doi: 10.1289/ehp.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dales R, Wheeler A, Mahmud M, Frescura AM, Smith-Doiron M, Nethery E, et al. The influence of living near roadways on spirometry and exhaled nitric oxide in elementary schoolchildren. Environ Health Perspect. 2008;116:1423–1427. doi: 10.1289/ehp.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 15. Cai Y, Hansell AL, Granell R, Blangiardo M, Zottoli M, Fecht D, et al. Prenatal, early-life, and childhood exposure to air pollution and lung function: the ALSPAC cohort. Am J Respir Crit Care Med. 2020;202:112–123. doi: 10.1164/rccm.201902-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonato M, Gallo E, Bazzan E, Cosio M, Barbato A, Saetta M, et al. European Respiratory Society Annual Meeting.
- 17.Midulla F, de Blic J, Barbato A, Bush A, Eber E, Kotecha S, et al. ERS Task Force Flexible endoscopy of paediatric airways Eur Respir J 200322698–708.. [DOI] [PubMed] [Google Scholar]
- 18. Faro A, Wood RE, Schechter MS, Leong AB, Wittkugel E, Abode K, et al. American Thoracic Society Ad Hoc Committee on Flexible Airway Endoscopy in Children. Official American Thoracic Society technical standards: flexible airway endoscopy in children. Am J Respir Crit Care Med. 2015;191:1066–1080. doi: 10.1164/rccm.201503-0474ST. [DOI] [PubMed] [Google Scholar]
- 19. Gallo E, Folino F, Buja G, Zanotto G, Bottigliengo D, Comoretto R, et al. Daily exposure to air pollution particulate matter is associated with atrial fibrillation in high-risk patients. Int J Environ Res Public Health. 2020;17:6017. doi: 10.3390/ijerph17176017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Parliament and the Council of the European Union Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe Off J Eur Union 2008L 1521–44.. [Google Scholar]
- 21.Air quality guidelines: global update 2005. Particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Geneva, Switzerland: World Health Organization; 2005https://www.euro.who.int/en/health-topics/environment-and-health/Housing-and-health/publications/pre-2009/air-quality-guidelines.-global-update-2005.-particulate-matter,-ozone,-nitrogen-dioxide-and-sulfur-dioxide [PubMed] [Google Scholar]
- 22. Snijders D, Agostini S, Bertuola F, Panizzolo C, Baraldo S, Turato G, et al. Markers of eosinophilic and neutrophilic inflammation in bronchoalveolar lavage of asthmatic and atopic children. Allergy. 2010;65:978–985. doi: 10.1111/j.1398-9995.2009.02282.x. [DOI] [PubMed] [Google Scholar]
- 23.Hastie TJ, Tibshirani RJ. Generalized additive models. 1st ed. Boca Raton, FL: Chapman and Hall/CRC; 1990. [Google Scholar]
- 24.Burnham KP, Anderson DR, editors. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 25. Zhang Z. Multiple imputation for time series data with Amelia package. Ann Transl Med. 2016;4:56. doi: 10.3978/j.issn.2305-5839.2015.12.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Honaker J, King G, Blackwell M. Amelia: a program for missing data. J Stat Softw. 2019;45:1–47. [Google Scholar]
- 27.Wood S.mgcv: Mixed GAM computation vehicle with automatic smoothness estimation. Vienna, Austria: R Foundation for Statistical Computing; 2019https://CRAN.R-project.org/package=mgcv. [Google Scholar]
- 28.Hackett TL.Epithelial-mesenchymal transition in the pathophysiology of airway remodelling in asthma Curr Opin Allergy Clin Immunol 20121253–59.. [DOI] [PubMed] [Google Scholar]
- 29. Chetta A, Foresi A, Del Donno M, Bertorelli G, Pesci A, Olivieri D. Airways remodeling is a distinctive feature of asthma and is related to severity of disease. Chest. 1997;111:852–857. doi: 10.1378/chest.111.4.852. [DOI] [PubMed] [Google Scholar]
- 30. Churg A, Brauer M, del Carmen Avila-Casado M, Fortoul TI, Wright JL. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ Health Perspect. 2003;111:714–718. doi: 10.1289/ehp.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iwanaga K, Elliott MS, Vedal S, Debley JS. Urban particulate matter induces pro-remodeling factors by airway epithelial cells from healthy and asthmatic children. Inhal Toxicol. 2013;25:653–660. doi: 10.3109/08958378.2013.827283. [DOI] [PubMed] [Google Scholar]
- 32. Mei M, Song H, Chen L, Hu B, Bai R, Xu D, et al. Early-life exposure to three size-fractionated ultrafine and fine atmospheric particulates in Beijing exacerbates asthma development in mature mice. Part Fibre Toxicol. 2018;15:13. doi: 10.1186/s12989-018-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tien CP, Chen CH, Lin WY, Liu CS, Liu KJ, Hsiao M, et al. Ambient particulate matter attenuates Sirtuin1 and augments SREBP1-PIR axis to induce human pulmonary fibroblast inflammation: molecular mechanism of microenvironment associated with COPD. Aging (Albany NY) 2019;11:4654–4671. doi: 10.18632/aging.102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Grove KC, Provoost S, Braun H, Blomme EE, Teufelberger AR, Krysko O, et al. IL-33 signalling contributes to pollutant-induced allergic airway inflammation. Clin Exp Allergy. 2018;48:1665–1675. doi: 10.1111/cea.13261. [DOI] [PubMed] [Google Scholar]
- 35. Herbert C, Siegle JS, Shadie AM, Nikolaysen S, Garthwaite L, Hansbro NG, et al. Development of asthmatic inflammation in mice following early-life exposure to ambient environmental particulates and chronic allergen challenge. Dis Model Mech. 2013;6:479–488. doi: 10.1242/dmm.010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pourazar J, Frew AJ, Blomberg A, Helleday R, Kelly FJ, Wilson S, et al. Diesel exhaust exposure enhances the expression of IL-13 in the bronchial epithelium of healthy subjects. Respir Med. 2004;98:821–825. doi: 10.1016/j.rmed.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 37. Huang KL, Liu SY, Chou CC, Lee YH, Cheng TJ. The effect of size-segregated ambient particulate matter on Th1/Th2-like immune responses in mice. PLoS One. 2017;12:e0173158. doi: 10.1371/journal.pone.0173158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muñoz X, Barreiro E, Bustamante V, Lopez-Campos JL, González-Barcala FJ, Cruz MJ. Diesel exhausts particles: Their role in increasing the incidence of asthma: reviewing the evidence of a causal link. Sci Total Environ. 2019;652:1129–1138. doi: 10.1016/j.scitotenv.2018.10.188. [DOI] [PubMed] [Google Scholar]
- 39. Bonato M, Bazzan E, Snijders D, Turato G, Biondini D, Tinè M, et al. Blood eosinophils relate to atopy and not to tissue eosinophils in wheezing children. Allergy. 2020;75:1497–1501. doi: 10.1111/all.14170. [DOI] [PubMed] [Google Scholar]
- 40. Tao RJ, Cao WJ, Li MH, Yang L, Dai RX, Luo XL, et al. PM2.5 compromises antiviral immunity in influenza infection by inhibiting activation of NLRP3 inflammasome and expression of interferon-β. Mol Immunol. 2020;125:178–186. doi: 10.1016/j.molimm.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 41. Mishra R, Krishnamoorthy P, Gangamma S, Raut AA, Kumar H. Particulate matter (PM10) enhances RNA virus infection through modulation of innate immune responses. Environ Pollut. 2020;266:115148. doi: 10.1016/j.envpol.2020.115148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stenfors N, Nordenhäll C, Salvi SS, Mudway I, Söderberg M, Blomberg A, et al. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur Respir J. 2004;23:82–86. doi: 10.1183/09031936.03.00004603. [DOI] [PubMed] [Google Scholar]
- 43. Nightingale JA, Maggs R, Cullinan P, Donnelly LE, Rogers DF, Kinnersley R, et al. Airway inflammation after controlled exposure to diesel exhaust particulates. Am J Respir Crit Care Med. 2000;162:161–166. doi: 10.1164/ajrccm.162.1.9908092. [DOI] [PubMed] [Google Scholar]
- 44. Nhung NTT, Amini H, Schindler C, Kutlar Joss M, Dien TM, Probst-Hensch N, et al. Short-term association between ambient air pollution and pneumonia in children: a systematic review and meta-analysis of time-series and case-crossover studies. Environ Pollut. 2017;230:1000–1008. doi: 10.1016/j.envpol.2017.07.063. [DOI] [PubMed] [Google Scholar]
- 45. Baraldo S, Contoli M, Bonato M, Snijders D, Biondini D, Bazzan E, et al. Deficient immune response to viral infections in children predicts later asthma persistence. Am J Respir Crit Care Med. 2018;197:673–675. doi: 10.1164/rccm.201706-1249LE. [DOI] [PubMed] [Google Scholar]