Abstract
Rationale: A central component of septic shock treatment is the infusion of vasopressors, most commonly starting with norepinephrine. However, the optimal approach and practice patterns for initiating adjunctive vasopressors and corticosteroids are unknown.
Objectives: To characterize practice pattern variation in the norepinephrine dose at which secondary vasopressors and adjunctive corticosteroids are initiated and to identify factors associated with a treatment strategy favoring secondary vasopressors compared with factors associated with a treatment strategy favoring adjunctive corticosteroids among patients with septic shock on norepinephrine.
Methods: We used a multicenter intensive care unit (ICU) database to identify patients with septic shock who were started on norepinephrine followed by an additional vasopressor or corticosteroids. We used multilevel models to determine the hospital risk-adjusted norepinephrine dose at which additional vasopressors and corticosteroids were started, the percentage of variation in the norepinephrine dose at the time of adjunctive treatment associated with the hospital of admission, and the factors associated with choosing an “additional-vasopressor-first” strategy versus a “corticosteroid-first” strategy.
Results: Among 4,401 patients with septic shock on norepinephrine, 1,940 (44.0%) were started on adjuncts (1,357 received an additional-vasopressor-first strategy, and 583 received a corticosteroid-first strategy). The hospital risk-adjusted norepinephrine dose at which vasopressors were initiated ranged from 6.4 μg/min (95% confidence interval [CI], 5.9–7.0 μg/min) to 92.6 μg/min (95% CI, 72.8–113.0 μg/min). The hospital risk-adjusted norepinephrine dose at which corticosteroids were initiated ranged from 3.0 μg/min (95% CI, 2.4–3.8 μg/min) to 32.7 μg/min (95% CI, 24.9–43.0 μg/min). Of the variation in the norepinephrine dose at which additional vasopressors were initiated, 25.1% (intraclass correlation coefficient 95% CI, 24.8–25.5%) was explained by the hospital site after adjusting for all hospital- and patient-level covariates. The hospital of admission was strongly associated with receiving an additional-vasopressor-first strategy over a corticosteroid-first strategy (median odds ratio, 3.28 [95% CI, 2.81–3.83]).
Conclusions: Practice patterns for adjunctive therapies to norepinephrine during septic shock are variable and are determined in large part by the hospital of admission. These results inform several future studies seeking to improve septic shock management.
Keywords: norepinephrine, vasopressin, hydrocortisone, methylprednisolone, intensive care unit
Septic shock is the most severe form of sepsis, during which severe circulatory and metabolic abnormalities result in case fatality rates that approach 50% (1). A central component of septic shock treatment is the infusion of vasopressors, most commonly starting with norepinephrine (2). Although guidelines favor norepinephrine as the initial vasopressor in septic shock, the optimal approach to use of adjunctive treatments to norepinephrine (3) in patients with persistent or worsening shock is unclear. For example, guidelines do not specify the optimal norepinephrine dose at which administration of additional vasopressors or adjunctive corticosteroids should be considered for patients with worsening shock (4), and the optimal sequence to initiate adjunctive treatments is unknown. An understanding of usual care practices for secondary vasopressors and adjunctive corticosteroid use during septic shock is necessary for designing future comparative effectiveness trials with clinical equipoise. Thus, we sought to 1) characterize practice pattern variation in the norepinephrine dose at which secondary vasopressors and adjunctive corticosteroids are initiated during septic shock and 2) identify factors associated with a treatment strategy in which secondary vasopressors are added first to norepinephrine versus a treatment strategy in which corticosteroids are added first to norepinephrine. We hypothesized 1) that the norepinephrine doses at which secondary vasopressors and adjunctive corticosteroids are initiated would be highly variable and mostly driven by idiosyncratic hospital variation (e.g., infusion protocols, provider preference) rather than by measured hospital- and patient-level characteristics and 2) that the hospital of admission would be the strongest driver determining whether patients received a treatment strategy favoring secondary vasopressors or a treatment strategy favoring adjunctive corticosteroids.
Methods
Study Participants
We used the Philips eICU Collaborative Database (5, 6), a deidentified database composed of an ∼20% random sample of intensive care unit (ICU) patients admitted to each of 208 U.S. hospitals that participate in the Philips telehealth program from 2014 to 2015. Among patients admitted to hospitals that record infusions in the eICU database, we identified patients with sepsis by using previously validated International Classification of Diseases, Ninth Edition, codes (7), as the database has minimal culture data precluding the replication of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) criteria (8). We then identified the first instance in which patients with sepsis were started on norepinephrine followed by additional vasopressors or intravenous corticosteroids (hydrocortisone or methylprednisolone—the most frequently used corticosteroids during sepsis [9] and in trials of septic shock [10]). We excluded patients who received norepinephrine doses that could not be readily converted to units of micrograms per minute, patients admitted to hospitals that did not record infusions in the eICU database, and patients aged <18 years. We also excluded patients receiving nonnorepinephrine vasopressors or corticosteroids before initiating norepinephrine in the ICU. We chose to include patients with glucocorticoid use before ICU admission (e.g., glucocorticoids listed on home medications), as chronic glucocorticoid use may be an important factor in determining the use of corticosteroids during septic shock. For patients with multiple ICU admissions, we randomly selected a single ICU admission to evaluate. We excluded patients admitted to hospitals where fewer than 10 patients met inclusion and exclusion criteria to increase the likelihood that our models yielded stable estimates.
Outcomes
The primary outcome was the norepinephrine dose at which additional vasopressors and corticosteroids were initiated. Secondary outcomes were the hours from norepinephrine initiation to 1) additional vasopressor administration and 2) corticosteroid administration, 3) the additional vasopressor type (vasopressin, epinephrine, phenylephrine, dopamine, or multiple vasopressors), 4) the additional corticosteroid type (hydrocortisone or methylprednisolone), and 5) the additional therapy treatment strategy, defined as “additional vasopressor first” if patients were started on vasopressors at the same time or before corticosteroids (or if patients were started on vasopressors but not corticosteroids) and as “corticosteroid first” for patients started on corticosteroids before additional vasopressors (or for patients started on corticosteroids but not vasopressors).
Covariates
Patient-level covariates included age, sex, race and ethnicity, ICU type, history of supraventricular tachycardia, history of renal insufficiency, use of glucocorticoids before ICU admission, Acute Physiology and Chronic Health Evaluation IV score (11), Sequential Organ Failure Assessment (SOFA) score (12) at the time of norepinephrine initiation, the net fluid balance in the 48 hours preceding norepinephrine initiation, albumin administration in the time from norepinephrine initiation to adjunct treatment initiation, and whether the norepinephrine dose was weight based (e.g., micrograms per kilogram per minute) before conversion to units of micrograms per minute. Hospital-level covariates included the U.S. region, hospital teaching status, hospital bed size, and hospital of admission (as a random effect). Covariate definitions are found in Table E1 the online supplement.
Missing Data
We assumed that missing covariates were missing at random and created 20 imputed data sets by using multiple imputation with chained equations to impute missing covariates from all nonmissing covariates, outcomes, and hospital discharge statuses. We then constructed models for each imputed data set separately and pooled the model results by using Rubin’s rules (13).
Statistical Analysis
Patient and hospital characteristics at the time of norepinephrine initiation were summarized by using means (standard deviations [SDs]) and medians (interquartile ranges [IQRs]) when appropriate for continuous variables and by using proportions for categorical variables. Among patients not started on adjuncts, we determined the range and median (IQR) of the maximum norepinephrine dose. We also determined the percentages of patients per hospital with septic shock on norepinephrine started on at least one adjunct. Among patients started on both adjunct vasopressors and corticosteroids, we determined the median time from adjunct vasopressor initiation to adjunct corticosteroid initiation and the proportion of patients who were initiated on both therapies within a 1-hour window.
Among patients treated with an additional-vasopressor-first strategy, we calculated the overall median norepinephrine dose and range at the time of additional vasopressor initiation and the median hours from norepinephrine initiation to additional vasopressor initiation and associated 95% confidence intervals (CIs) by using bootstrapping. We constructed multilevel linear models, adjusting for all patient- and hospital-level covariates, with the hospital site being used as a random effect (random intercept) for the log of the norepinephrine dose at which additional vasopressors are initiated. From these models we identified 1) the hospital case mix–adjusted average norepinephrine dose per hospital and 95% CI, 2) the association (β and 95% CI) between each covariate and the log of the norepinephrine dose, and 3) the intraclass correlation coefficient (the percentage of variation in the norepinephrine dose at the time of additional therapy explained by unidentified hospital factors [14]). We repeated these analyses for patients treated with a corticosteroid-first strategy and used additional analyses for the unadjusted median norepinephrine dose when we stratified by glucocorticoid use before ICU admission and when we restricted analysis to those patients initiated on each corticosteroid type (hydrocortisone or methylprednisolone). In addition, we calculated the percentage of hospitals that used hydrocortisone alone, methylprednisolone alone, or both. We then calculated the Spearman rank correlation coefficient between the hospital case mix–adjusted average norepinephrine dose at which additional vasopressors were initiated and the hospital case mix–adjusted average norepinephrine dose at which corticosteroids were initiated to assess whether hospitals that generally start vasopressors at higher norepinephrine doses also start corticosteroids at higher norepinephrine doses.
To determine whether the hospital site was a major determinant for choosing an additional vasopressor or corticosteroid first, we constructed multilevel logistic regression models, adjusting for all patient- and hospital-level factors, with the hospital site being used as a random effect (random intercept) for the additional therapy treatment strategy. We reported odds ratios (ORs) for each covariate such that ORs greater than 1 were associated with higher odds of an additional-vasopressor-first strategy and ORs less than 1 were associated with lower odds of an additional-vasopressor-first strategy (and were thus associated with higher odds of a corticosteroid-first strategy). We calculated the hospital median OR, interpreted as the median increased odds of starting an additional vasopressor rather than starting corticosteroids when moving from a hospital of lower to higher risk (15) to facilitate comparisons between hospital sites and covariates.
We conducted a sensitivity analysis to assess the robustness of our results in relation to a modified definition of Sepsis-3 septic shock (8) (excluding the need for culture data that are unavailable in eICU): administration of antibiotics, SOFA score ⩾2, and lactate >2 mmol/L within 72 hours of initiation of norepinephrine. R software (version 4.0.2; R Foundation for Statistical Computing) was used for analyses. This study was designated as “not human subjects research” by Boston University’s institutional review board.
Results
Among the 73,547 ICU admissions to 152 hospitals that record infusions in the eICU database, 34,157 admissions were for adults with sepsis, and 4,401 patients were started on norepinephrine before the initiation of other vasopressors or corticosteroids (see Figure E1 in the online supplement). Among patients with septic shock started on norepinephrine, 1,940 (44.0%)—from 69 hospitals—were started on an adjunct treatment (an additional vasopressor in 1,128, a corticosteroid in 457, and both in 355). A total of 1,357 patients received an additional-vasopressor-first strategy, and 583 received a corticosteroid-first strategy. Among patients administered both additional vasopressors and corticosteroids, 223 (62.8%) received the additional vasopressor before receiving corticosteroids, and 6 (1.7%) received the additional vasopressor and corticosteroids at the same time. The median time from vasopressor to corticosteroid initiation among patients started on both medications was 2 hours (95% CI, 0.7–3 h), and 43.0% (95% CI, 37.8–48.3%) of patients were started on both medications within 1 hour. The median number of patients started on an adjunct treatment per hospital was 24 (IQR, 12–51). The median percentage of patients with septic shock on norepinephrine started on adjuncts per hospital was 42.4% (IQR, 31.6–51.7%) and ranged from 14.3% to 84.6%. Among patients not started on adjunct medications, the median maximum norepinephrine dose was 10 μg/min (IQR, 6–20 μg/min) and ranged from 1 to 422 μg/min. Characteristics of the included patients and data missingness are shown in Table 1.
Table 1.
Characteristics of patients with septic shock on norepinephrine
| Characteristic | Corticosteroid Treatment Strategy (n = 583) | Vasopressor Treatment Strategy (n = 1,357) | No Adjuncts (n = 2,461) |
|---|---|---|---|
| Age, yr, mean (SD) | 66 (14) | 65 (15) | 66 (16) |
| Male, n (%) | 290 (49.7) | 736 (54.2) | 1,306 (53.1) |
| Race and ethnicity, n (%) | |||
| Black | 33 (5.7) | 129 (9.5) | 219 (8.9) |
| Hispanic | 25 (4.3) | 58 (4.3) | 131 (5.3) |
| White | 473 (81.1) | 1,051 (77.5) | 1,908 (77.5) |
| Other or unknown | 52 (8.9) | 119 (8.8) | 203 (8.2) |
| U.S. region,*n (%) | |||
| Midwest | 131 (25.3) | 394 (30.4) | 822 (35.6) |
| Northeast | 89 (17.2) | 210 (16.2) | 290 (12.6) |
| South | 108 (20.8) | 379 (29.3) | 656 (28.4) |
| West | 190 (36.7) | 311 (24.0) | 540 (23.4) |
| Hospital bed count,†n (%) | |||
| 100–249 | 88 (17.1) | 184 (14.7) | 418 (18.9) |
| 250–499 | 160 (31.1) | 329 (26.3) | 672 (30.4) |
| ⩾500 | 266 (51.8) | 738 (59.0) | 1,123 (50.7) |
| Teaching hospital, n (%) | 176 (30.2) | 539 (39.7) | 825 (33.5) |
| ICU type, n (%) | |||
| Medical | 73 (12.5) | 250 (18.4) | 382 (15.5) |
| Cardiac | 101 (17.3) | 215 (15.8) | 403 (16.4) |
| Medical–surgical | 370 (63.5) | 793 (58.4) | 1,492 (60.6) |
| Neurological | 11 (1.9) | 34 (2.5) | 75 (3.0) |
| Surgical | 28 (4.8) | 65 (4.8) | 109 (4.4) |
| ICU admission source, n (%) | |||
| Direct admit or transfer from another hospital | 71 (12.5) | 150 (11.1) | 224 (9.1) |
| Emergency room | 287 (49.2) | 682 (50.3) | 1,326 (53.9) |
| Hospital floors | 169 (29.0) | 399 (29.4) | 631 (25.7) |
| Operating room or recovery room | 21 (3.6) | 76 (5.6) | 175 (7.1) |
| Other ICU | 35 (6.0) | 50 (3.7) | 103 (4.2) |
| History of supraventricular tachycardia, n (%) | 79 (13.6) | 181 (13.3) | 326 (13.2) |
| History of renal insufficiency, n (%) | 20 (3.4) | 74 (5.5) | 126 (5.1) |
| Hours from ICU admission to norepinephrine initiation, median (IQR) | 1.4 (0.1–8.0) | 1.9 (0.2–7.0) | 2.9 (0.4–11.3) |
| APACHE-IV score,‡ mean (SD) | 85 (28) | 101 (32) | 81 (26) |
| SOFA score at the time of norepinephrine initiation, mean (SD) | 11 (3) | 13 (3) | 10 (3) |
| Glucocorticoid use before ICU, n (%) | 37 (6.3) | 15 (1.1) | 44 (1.8) |
| Net fluid balance in ml in the 48 h before norepinephrine initiation, mean (SD)§ | 831 (2,282) | 870 (2,271) | 846 (2,278) |
| Albumin administration in the time from norepinephrine onset to adjunct, n (%) | 23 (3.9) | 60 (4.4) | NA |
| Use of weight-based norepinephrine, n (%) | 87 (14.8) | 126 (9.5) | 183 (7.4) |
| Expired in hospital,|n (%) | 177 (30.8) | 749 (55.4) | 532 (21.8) |
Definition of abbreviations: APACHE-IV = Acute Physiology and Chronic Health Evaluation IV; ICU = intensive care unit; IQR = interquartile range; NA = not applicable; SOFA = Sequential Organ Failure Assessment; SD = standard deviation.
Sixty-five patients initiated on corticosteroids and 63 patients initiated on additional vasopressors were missing the U.S. region.
Sixty-nine patients initiated on corticosteroids and 106 patients initiated on additional vasopressors were missing the hospital bed number.
Seventy-nine patients initiated on corticosteroids and 131 patients initiated on additional vasopressors were missing the APACHE-IV score.
Seventy patients initiated on corticosteroids and 267 patients initiated on additional vasopressors were missing the net fluid balance.
Eight patients initiated on corticosteroids and six patients initiated on additional vasopressors were missing an expired-in-hospital status.
Among patients with the additional vasopressor-first treatment strategy, patients were most frequently initiated on vasopressin (n = 921, 67.9%), followed by phenylephrine (n = 203, 15.0%), dopamine (n = 69, 5.1%), and epinephrine (n = 66, 4.9%). For 98 (7.2%) patients, multiple additional vasopressors were initiated simultaneously. The median norepinephrine dose at the time of additional vasopressor initiation was 20 μg/min (95% CI, 19–20 μg/min) and ranged from 1 to 432 μg/min. The median time from norepinephrine initiation to additional vasopressor initiation was 7 hours (95% CI, 6–7.5 h).
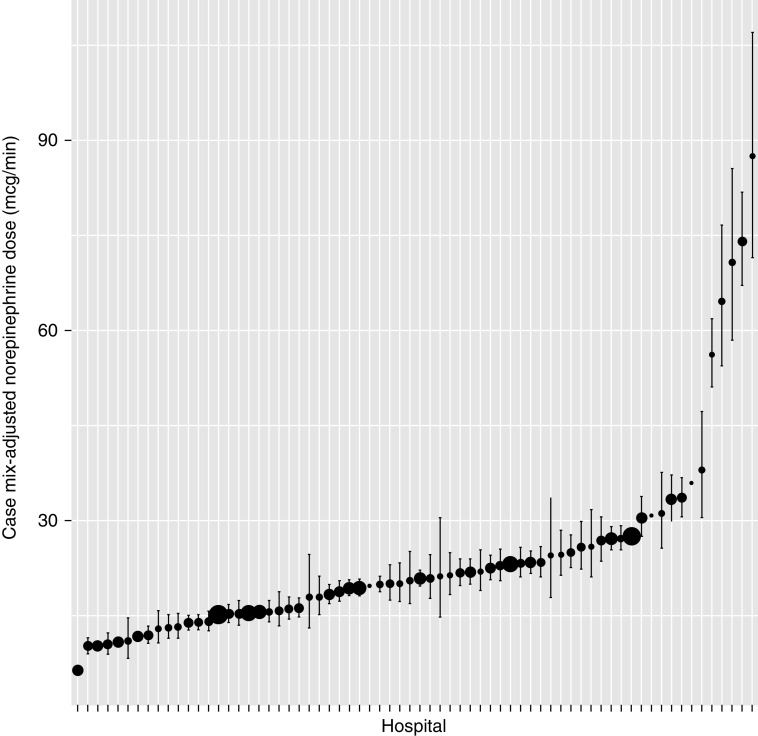
Among patients receiving an additional-vasopressor-first strategy, 25.1% (intraclass correlation coefficient 95% CI, 24.8% to 25.5%) of the variation in the norepinephrine dose at which additional vasopressors were initiated was explained by the hospital of admission. The hospital case mix–adjusted average norepinephrine dose at the time of second vasopressor initiation ranged from 6.4 μg/min (95% CI, 5.9 to 7.0 μg/min) to 92.6 μg/min (95% CI, 72.8 to 113.0 μg/min), with most hospitals having a case mix–adjusted average between 10 and 30 μg/min (Figure 1). Patients with higher SOFA scores (β per 1-SD increase, 0.18 [95% CI, 0.12 to 0.24]), those initiated on weight-based norepinephrine (β compared with non–weight-based norepinephrine, 0.76 [95% CI, 0.20 to 1.32]), and patients given albumin (β compared with no albumin, 0.22 [95% CI, −0.04 to 0.48]) were possibly started on additional vasopressors at higher norepinephrine doses (Table 2). Among patients with renal insufficiency, there was a trend toward starting additional vasopressors at lower norepinephrine doses (β compared with no renal insufficiency, −0.17 [95% CI, −0.39 to 0.05]).
Figure 1.
Case mix–adjusted norepinephrine dose per hospital at the time of additional vasopressors. Black dots show the case mix–adjusted norepinephrine dose per hospital at the time of additional vasopressor initiation and the associated 95% confidence intervals. The size of the dot corresponds to the number of patients at each hospital started on additional vasopressors.
Table 2.
β Estimates for fixed effect covariates for the multilevel model of the log norepinephrine dose at the time of additional vasopressor initiation and corticosteroid administration among patients with septic shock
| Covariate | Corticosteroid Treatment Strategy [β Estimate (95% CI)] | Vasopressor Treatment Strategy [β Estimate (95% CI)] |
|---|---|---|
| Age per 1-SD increase | 0.04 (−0.05 to 0.12) | −0.02 (−0.07 to 0.03) |
| Male vs. female sex | 0.04 (−0.11 to 0.19) | 0.06 (−0.04 to 0.16) |
| Hispanic vs. Black | −0.30 (−0.82 to 0.23) | 0.13 (−0.22 to 0.47) |
| White vs. Black | −0.23 (−0.56 to 0.10) | −0.06 (−0.23 to 0.11) |
| Other or unknown vs. Black | −0.21 (−0.61 to 0.19) | −0.05 (−0.29 to 0.19) |
| Northeast vs. Midwest | −0.27 (−1.26 to 0.73) | −0.32 (−0.86 to 0.22) |
| South vs. Midwest | 0.00 (−0.43 to 0.43) | 0.15 (−0.21 to 0.50) |
| West vs. Midwest | 0.23 (−0.20 to 0.66) | 0.16 (−0.25 to 0.57) |
| 250–499 vs. 100–249 hospital beds | −0.16 (−0.60 to 0.28) | 0.15 (−0.24 to 0.54) |
| ⩾500 vs. 100–249 hospital beds | −0.45 (−0.89 to −0.004) | −0.23 (−0.65 to 0.19) |
| Teaching hospital | −0.27 (−0.81 to 0.27) | −0.08 (−0.51 to 0.36) |
| Cardiac vs. medical ICU | −0.11 (−0.47 to 0.24) | −0.04 (−0.25 to 0.17) |
| Medical–surgical vs. medical ICU | −0.24 (−0.60 to 0.13) | −0.02 (−0.23 to 0.19) |
| Neurological vs. medical ICU | 0.20 (−0.45 to 0.85) | 0.15 (−0.20 to 0.50) |
| Surgical vs. medical ICU | −0.66 (−1.13 to −0.19) | −0.16 (−0.44 to 0.12) |
| History of supraventricular tachycardia | −0.06 (−0.29 to 0.17) | −0.08 (−0.23 to 0.07) |
| History of renal insufficiency | 0.10 (−0.31 to 0.51) | −0.17 (−0.39 to 0.05) |
| APACHE-IV score per 1-SD increase | 0.10 (−0.002 to 0.21) | 0.05 (−0.01 to 0.11) |
| SOFA score per 1-SD increase | 0.21 (0.12 to 0.31) | 0.18 (0.12 to 0.24) |
| Glucocorticoid use before ICU admission | −0.07 (−0.39 to 0.26) | −0.29 (−0.76 to 0.18) |
| Net fluid balance | 0.09 (−0.004 to 0.18) | 0.01 (−0.05 to 0.07) |
| Albumin administration | −0.02 (−0.44 to 0.41) | 0.22 (−0.004 to 0.48) |
| Weight-based norepinephrine | 0.94 (0.05 to 1.84) | 0.76 (0.20 to 1.32) |
Definition of abbreviations: APACHE-IV = Acute Physiology and Chronic Health Evaluation IV; CI = confidence interval; ICU = intensive care unit; SOFA = sequential organ failure assessment; SD = standard deviation.
Among patients receiving the corticosteroid-first strategy, 324 (55.6%) received methylprednisolone, and 259 (44.4%) received hydrocortisone (7.5% of hospitals that initiated a corticosteroid initiated only hydrocortisone, 37.7% initiated both hydrocortisone and methylprednisolone, and 54.7% initiated only methylprednisolone). The median norepinephrine dose at the time of corticosteroid initiation was 6 μg/min (95% CI, 6–7 μg/min) (both when including [n = 583] and excluding [n = 546] patients who used glucocorticoids before ICU admission) and ranged from 1 to 110 μg/min. The median norepinephrine dose at the time of hydrocortisone initiation was 7 μg/min (95% CI, 6–8 μg/min), and the median norepinephrine dose at the time of methylprednisolone initiation was 5 μg/min (95% CI, 4–6 μg/min). The median time from norepinephrine initiation to corticosteroid administration was 17.5 hours (95% CI, 13.6–22 h)
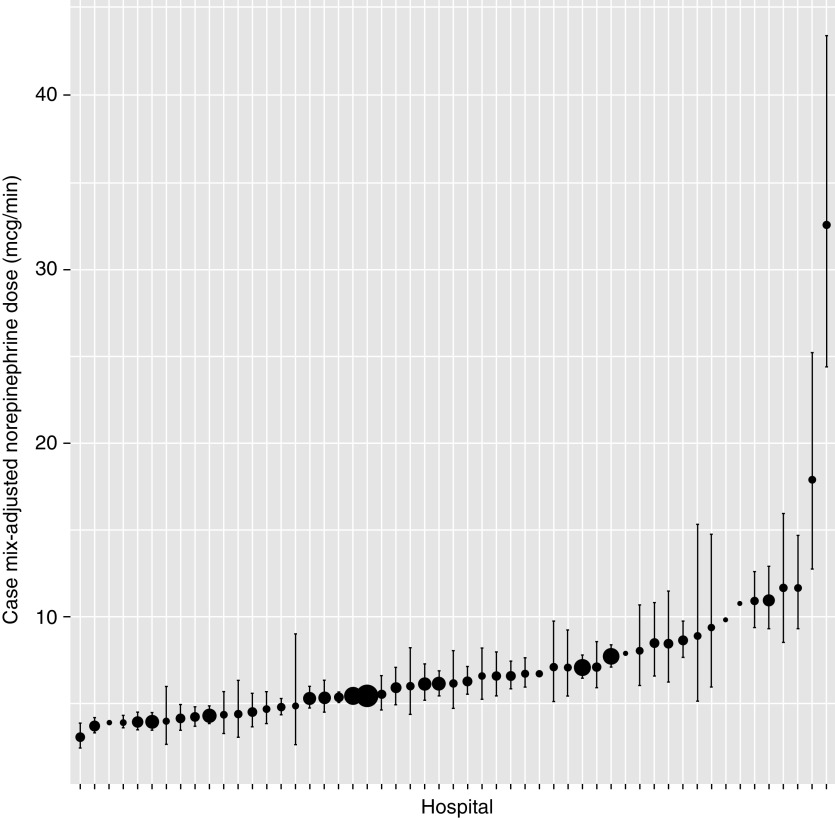
Among patients receiving a corticosteroid treatment strategy, 25.1% (intraclass correlation coefficient 95% CI, 24.6% to 25.5%) of the variation in the norepinephrine dose at which a corticosteroid was initiated was explained by the hospital to which a patient was admitted. The hospital case mix–adjusted average norepinephrine dose at the time of corticosteroid initiation ranged from 3.0 μg/min (95% CI, 2.4 to 3.8 μg/min) to 32.7 μg/min (95% CI, 24.9 to 43.0 μg/min) (Figure 2). Patients with higher SOFA scores (β per 1-SD increase, 0.21 [95% CI, 0.12 to 0.31]) and those initiated on weight-based norepinephrine (β compared with non–weight-based norepinephrine, 0.94 [95% CI, 0.05 to 1.84]) were started on corticosteroids at higher norepinephrine doses (Table 2). Patients admitted to surgical ICUs (β compared with medical ICUs, −0.66 [95% CI, −1.13 to −0.19]) and patients admitted to hospitals with ⩾500 beds (β compared with 100–249 beds, −0.45 [95% CI, −0.89 to −0.004]) were started on corticosteroids at lower norepinephrine doses. There was a moderate positive correlation between the hospital case mix–adjusted average norepinephrine dose at which additional vasopressors were added and the hospital case mix–adjusted average norepinephrine dose at which corticosteroids were added (Spearman rank correlation coefficient, 0.48 [95% CI, 0.24 to 0.67]).
Figure 2.
Case mix–adjusted norepinephrine dose per hospital at the time of adjunctive corticosteroids. Black dots show the case mix–adjusted norepinephrine dose per hospital at the time of corticosteroid initiation and the associated 95% confidence intervals. The size of the dot corresponds to the number of patients at each hospital started on corticosteroids.
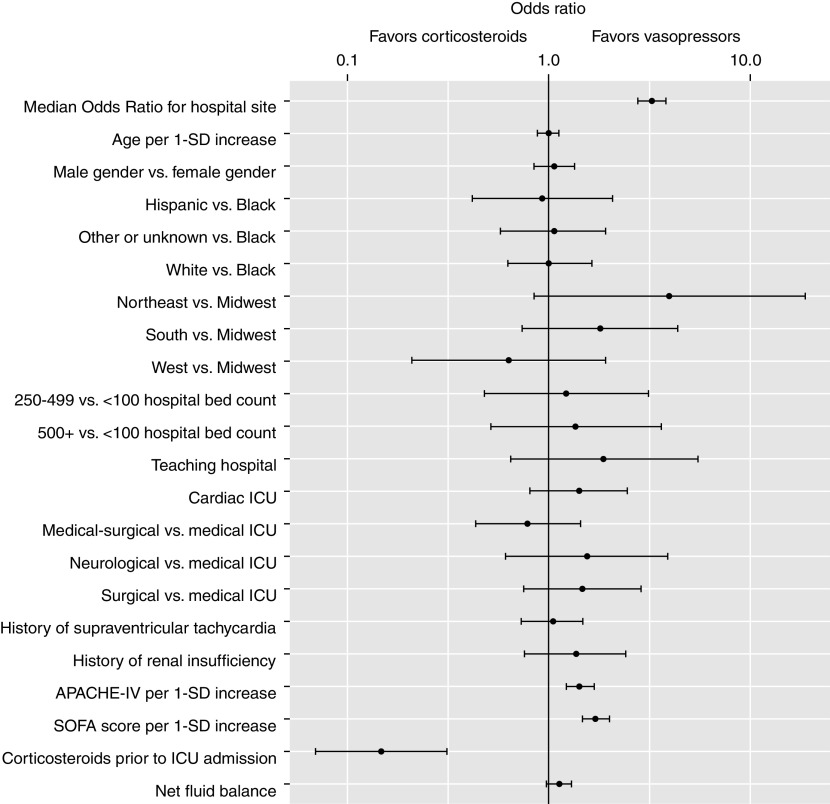
In the multilevel logistic regression model for the treatment strategy, factors associated with increased odds of an additional vasopressor-first strategy being used were the SOFA score (OR per 1-SD increase, 1.71 [95% CI, 1.46–1.99]) and the Acute Physiology and Chronic Health Evaluation IV score (OR, 1.43 [95% CI, 1.22–1.69]), whereas the use of glucocorticoids before ICU admission was associated with lower odds (OR, 0.15 [95% CI, 0.07–0.32]) of the additional-vasopressor-first strategy being used. The median OR for the hospital site associated with an additional vasopressor-first strategy was 3.28 (95% CI, 2.81–3.83), an association stronger than all but one of the identified hospital- and patient-level factor ORs (Figure 3).
Figure 3.
Association between hospital- and patient-level factors and an “additional-vasopressor-first” treatment strategy among patients with septic shock on norepinephrine. Black dots show odds ratios and 95% confidence intervals. Odds ratios greater than 1 correspond to increased odds of an additional-vasopressor-first treatment strategy. Odds ratios less than 1 correspond to decreased odds of an additional-vasopressor-first treatment strategy (and thus increased odds of a “corticosteroid-treatment-first” strategy). APACHE-IV = Acute Physiology and Chronic Health Evaluation IV; ICU = intensive care unit; SOFA = Sequential Organ Failure Assessment; SD = standard deviation.
Results from our sensitivity analysis (n = 1,200 in the additional vasopressor-first analysis, n = 335 in the corticosteroid-first analysis) obtained by using a modified Sepsis-3 septic shock definition did not substantively change our findings (Tables E2 and E3 and Figures E2, E3, and E4).
Discussion
We examined practice pattern variation associated with the norepinephrine dose at which additional vasopressors and corticosteroids were initiated during septic shock. We found that 1) the hospital to which a patient was admitted was a major driver of practice variation in the timing and choice of additional therapies, 2) hospitals varied in the risk-adjusted doses of norepinephrine at which additional vasopressors (∼10–30 μg/min) and corticosteroids (∼5–15 μg/min) were added, and 3) corticosteroids were started at lower doses of norepinephrine, but at later time periods, compared with additional vasopressors.
Our results should be considered in the context of prior studies examining the use of vasopressors during septic shock. The risk-adjusted norepinephrine doses at which additional vasopressors were added in our study were consistent with the average enrollment doses (∼20 μg/min) of norepinephrine in trials studying the addition of adjunct vasopressors to norepinephrine (16). In contrast, the doses of norepinephrine at which corticosteroids were initiated in our study were lower than those at enrollment in large clinical trials studying the addition of corticosteroids (17, 18), even when excluding patients who received glucocorticoids before ICU admission. Patients receiving norepinephrine may have improvements in hemodynamic measures up to norepinephrine doses of ∼140 μg/min (19). However, we found that most patients in our United States–based cohort who received adjuncts treatments were started on additional therapies at much lower doses of norepinephrine. Our finding that the use of weight-based norepinephrine was associated with starting adjunctive vasopressors and corticosteroids at higher norepinephrine doses is consistent with prior research (20) that found that weight-based norepinephrine was associated with higher total cumulative norepinephrine doses.
We previously described practice pattern variation in the use of norepinephrine and dopamine as initial vasopressors during septic shock (21) and found that several hospital-level characteristics (location, teaching hospital status) and patient-level characteristics (treating physician specialty, age, history of arrhythmia and renal impairment, severity of illness) were associated with the choice of the first vasopressor. In our current study, we identified relatively few hospital-level and patient-level characteristics that were associated with the choice of adjunctive therapies after norepinephrine was started. In addition, the hospital of admission was a strong driver of both the variation in the norepinephrine dose at which adjuncts are initiated and the choice of first adjunct treatment. Thus, unlike the initial vasopressor choice, the choice of adjunctive therapy for patients with septic shock on norepinephrine may be driven primarily by idiosyncratic hospital practice variation rather than by measured characteristics. We speculate that variable use of ICU protocols (22) and protocol differences in the allowed upper limit of the norepinephrine dose may partially explain the between-hospital variation identified in our study. In addition, the moderate positive correlation between the hospital norepinephrine dose at which corticosteroids are added and the hospital norepinephrine dose at which additional vasopressors are added supports a hypothesis that hospitals are either “early adjunctive starters” (i.e., initiating both additional vasopressors and corticosteroids at low norepinephrine doses) or “late adjunctive starters” (i.e., initiating both additional vasopressors and corticosteroids at high norepinephrine doses).
The observed practice patterns of additional vasopressors and corticosteroids suggest that these two therapies are perhaps being used for distinct reasons in septic shock. Corticosteroids were started at lower norepinephrine doses at later time periods in lower-acuity patients, whereas additional vasopressors were started at higher norepinephrine doses at earlier time periods in higher-acuity patients. We speculate that these patterns suggest that clinicians add additional vasopressors to norepinephrine when circulatory parameters are not met despite moderate-dose norepinephrine use and that clinicians add corticosteroids to norepinephrine as an adrenergic-sparing therapy for alternative etiologies (e.g., serum cortisol thresholds). These findings may limit the ability for observational data to be used to study the comparative effectiveness of adjunctive corticosteroids versus secondary vasopressors in septic shock because of selection bias. In addition, the strong association between a greater severity of illness and higher doses of norepinephrine at which adjunct treatments were started may limit the use of observational data to determine the optimal norepinephrine dose at which to add vasopressin (i.e., patients started on vasopressin at higher norepinephrine doses are inherently sicker than patients started on vasopressin at lower norepinephrine doses, increasing the risk of unmeasured confounding) (23). Furthermore, the association between albumin use and the dose of norepinephrine at which adjunct vasopressors are added suggests that providers may be using volume-expanding fluids (e.g., albumin and crystalloid) as alternative adjuncts to vasopressors. Future studies should evaluate practices related to the use of volume-expanding fluids for patients with septic shock on vasopressors.
Our results help to inform myriad future studies necessary for determining the optimal practice regarding treatment of patients with septic shock. In the context of prior studies showing that the addition of vasopressin to norepinephrine decreases the risk of arrhythmia (24), our study provides guidance regarding the range (10–30 μg/min) of norepinephrine thresholds typically used in practice that could be tested in future randomized controlled trials examining the optimal timing of adjunctive vasopressors. In addition, overlap in the adjusted hospital average norepinephrine doses at which either additional vasopressors or corticosteroids are added (10–15 μg/min) may provide a target norepinephrine dose inclusion criterion to compare the effectiveness of adjunctive vasopressor-first shock treatment strategy with the corticosteroid-first shock treatment strategy. Future qualitative studies should examine potential reasons to explain the large practice pattern variation that is unexplained by the observed variables identified in our study. Future observational studies should examine whether hospitals outside the United States also initiate adjunctive therapies at the low-to-moderate doses of norepinephrine identified in our United States–based cohort. Lastly, we were unable to ascertain whether patients were maintained on norepinephrine alone because of an active decision by a clinician to avoid adjunctive therapies, and we thus chose to exclude patients on norepinephrine alone from our analysis. Future studies are needed to compare a treatment strategy using high-dose norepinephrine alone with treatment strategies using a secondary vasopressor and adjunctive corticosteroids.
Strengths and Limitations
Our study has several strengths. We used a large database to examine variation across the spectrum of norepinephrine doses generally used in clinical practice across ICU types in the United States. We incorporated granular patient-level factors that have previously been difficult to estimate when using less granular databases. In addition, we strengthened the external validity of our results by including patients from both medical and surgical ICUs and by conducting a sensitivity analysis using a cohort of patients with septic shock that aligned with the Sepsis-3 definition (8).
Our study also has several limitations. The eICU database includes hospitals participating in telehealth, which may have resulted in a narrower range of practice variation due to a single clinician overseeing multiple ICUs. In addition, only 152 of the 208 hospitals in the eICU database recorded infusion use in the database. It is unclear whether excluding hospitals that did not record infusion practices in the Phillips electronic medication record introduced selection bias. Because the eICU database contains information from 2014 to 2015 and admission dates are deidentified, it is unclear whether the practice patterns identified in our study have remained stable over time. Future studies should examine temporal changes in septic shock adjunct medication practices in response to future guideline changes and clinical trial data.
Conclusions
The norepinephrine dose at the time of adjunct treatments during septic shock was generally between 5 and 30 μg/min when adjusted for the hospital case mix, and the hospital of admission was a major driver of practice variation when adding adjunctive medications to norepinephrine. These results inform future studies seeking to optimize the treatment of patients with septic shock.
Footnotes
Supported by the following National Institutes of Health grants: National Institute of General Medical Sciences grant 1F32GM133061-01, National Heart, Lung, and Blood Institute grant 5R01HL139751-02, and 5R01HL136660-03.
Author Contributions: N.A.B. takes responsibility for the integrity of the work as a whole, from inception to the published article. N.A.B., B.T., H.W., and A.J.W. substantially contributed to the conception and design of this study. N.A.B. acquired the data. All authors were involved in the interpretation of data. N.A.B. drafted the manuscript, and all authors revised it critically for important intellectual content. All authors read and approved the final version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Sepsis Definitions Task Force. Developing a new definition and assessing new clinical criteria for septic shock: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vail E, Gershengorn HB, Hua M, Walkey AJ, Rubenfeld G, Wunsch H. Association between US norepinephrine shortage and mortality among patients with septic shock. JAMA. 2017;317:1433–1442. doi: 10.1001/jama.2017.2841. [DOI] [PubMed] [Google Scholar]
- 3. Scheeren TWL, Bakker J, De Backer D, Annane D, Asfar P, Boerma EC, et al. Current use of vasopressors in septic shock. Ann Intensive Care. 2019;9:20. doi: 10.1186/s13613-019-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign. International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 5. Pollard TJ, Johnson AEW, Raffa JD, Celi LA, Mark RG, Badawi O. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci Data. 2018;5:180178. doi: 10.1038/sdata.2018.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101:E215–E220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 7. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 8. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamontagne F, Rochwerg B, Lytvyn L, Guyatt GH, Møller MH, Annane D, et al. Corticosteroid therapy for sepsis: a clinical practice guideline. BMJ. 2018;362:k3284. doi: 10.1136/bmj.k3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y, et al. Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst Rev. 2019;12:CD002243. doi: 10.1002/14651858.CD002243.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 12. Vincent J-L, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 13.Rubin RB. Multiple imputation for nonresponse in surveys. Hoboken, NJ: John Wiley & Sons; 1987. [Google Scholar]
- 14. Merlo J, Chaix B, Yang M, Lynch J, Råstam L. A brief conceptual tutorial of multilevel analysis in social epidemiology: linking the statistical concept of clustering to the idea of contextual phenomenon. J Epidemiol Community Health. 2005;59:443–449. doi: 10.1136/jech.2004.023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Russell JA, Walley KR, Singer J, Gordon AC, Hébert PC, Cooper DJ, et al. VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 17. Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot J-P, Siami S, et al. CRICS-TRIGGERSEP Network. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378:809–818. doi: 10.1056/NEJMoa1705716. [DOI] [PubMed] [Google Scholar]
- 18. Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. ADRENAL Trial Investigators; Australian–New Zealand Intensive Care Society Clinical Trials Group. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378:797–808. doi: 10.1056/NEJMoa1705835. [DOI] [PubMed] [Google Scholar]
- 19. Redl-Wenzl EM, Armbruster C, Edelmann G, Fischl E, Kolacny M, Wechsler-Fördös A, et al. The effects of norepinephrine on hemodynamics and renal function in severe septic shock states. Intensive Care Med. 1993;19:151–154. doi: 10.1007/BF01720530. [DOI] [PubMed] [Google Scholar]
- 20. Vadiei N, Daley MJ, Murthy MS, Shuman CS. Impact of norepinephrine weight-based dosing compared with non-weight-based dosing in achieving time to goal mean arterial pressure in obese patients with septic shock. Ann Pharmacother. 2017;51:194–202. doi: 10.1177/1060028016682030. [DOI] [PubMed] [Google Scholar]
- 21. Fawzy A, Evans SR, Walkey AJ. Practice patterns and outcomes associated with choice of initial vasopressor therapy for septic shock. Crit Care Med. 2015;43:2141–2146. doi: 10.1097/CCM.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prasad M, Christie JD, Bellamy SL, Rubenfeld GD, Kahn JM. The availability of clinical protocols in US teaching intensive care units. J Crit Care. 2010;25:610–619. doi: 10.1016/j.jcrc.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Grooth H-J, Girbes ARJ, van der Ven F, Oudemans-van Straaten HM, Tuinman PR, de Man AME. Observational research for therapies titrated to effect and associated with severity of illness: misleading results from commonly used statistical methods. Crit Care Med. 2020;48:1720–1728. doi: 10.1097/CCM.0000000000004612. [DOI] [PubMed] [Google Scholar]
- 24. McIntyre WF, Um KJ, Alhazzani W, Lengyel AP, Hajjar L, Gordon AC, et al. Association of vasopressin plus catecholamine vasopressors vs catecholamines alone with atrial fibrillation in patients with distributive shock: a systematic review and meta-analysis. JAMA. 2018;319:1889–1900. doi: 10.1001/jama.2018.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]