To the Editor:
Hypercoagulability may be a key factor leading to multiorgan failure and death in critically ill patients with coronavirus disease (COVID-19) (1). Extensive pulmonary microthrombi have been described in patients with acute respiratory distress syndrome from COVID-19 (2, 3). These observations have prompted some clinicians to advocate for the use of fibrinolytic therapy with recombinant tissue plasminogen activator (tPA) in select critically ill patients with COVID-19 (4, 5), yet sparse data on safety and efficacy are available (6, 7).
We used data from STOP-COVID (Study of the Treatment and Outcomes in Critically Ill Patients with COVID-19), a multicenter cohort study of critically ill adults with COVID-19 admitted to 68 geographically diverse hospitals across the United States, to examine the safety and efficacy of tPA in this setting (8). We included patients from the STOP-COVID registry admitted to intensive care units (ICUs) between March 1 and July 1, 2020, who received tPA for confirmed pulmonary embolism (PE) or suspected PE or pulmonary microthrombi within 14 days after ICU admission. Patients were followed until hospital discharge or death.
The primary safety endpoint was major bleeding within 7 days of tPA administration, defined as bleeding within a critical area or organ (e.g., intracranial, retroperitoneal, pericardial, or intramuscular bleeding with compartment syndrome) or bleeding requiring a procedural intervention (9). The primary efficacy endpoint was change in the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2:FiO2) before and after tPA administration. Secondary efficacy endpoints were the proportion of patients who required therapies for refractory hypoxemia (prone position, neuromuscular blockade, or inhaled pulmonary vasodilators), the proportion of patients with an increase in the PaO2:FiO2 ratio ⩾50% after tPA administration, and the change in the vasoactive-inotropic score (VIS) in the first 24 hours after tPA administration (10–12). We collected data on the PaO2:FiO2 ratio from the closest arterial blood gas obtained within 48 hours before tPA and the first three arterial blood gases within 48 hours after tPA receipt. We collected VIS data immediately before and at 6, 12, and 24 hours after tPA receipt.
Among 5,154 patients enrolled in STOP-COVID, 93 (1.8%) received tPA. We excluded patients who received tPA for intracatheter thrombosis (n = 28), peripheral arterial thrombosis (n = 2), ischemic stroke (n = 2), myocardial infarction (n = 1), or catheter-directed thrombolysis (n = 1), leaving a total of 59 patients (from 16 institutions) included in this analysis.
Baseline characteristics of these 59 patients (1.1% of the parent cohort) are shown in Table 1. The median age was 60 years (interquartile range [IQR], 50–67), 43 (72.9%) were male, and 19 (32.2%) were White. A total of 58 patients (98.3%) were receiving invasive mechanical ventilation at the time of tPA administration, 43 (72.9%) were receiving vasoactive medications, and 8 (13.6%) were receiving renal replacement therapy for acute kidney injury. The median PaO2:FiO2 ratio at the time of tPA receipt was 86 (IQR, 69–157), and 30 patients (50.8%) were receiving at least one therapy for refractory hypoxemia. PE was radiologically confirmed in 12 patients (20.3%); the remaining 47 patients (79.7%) had suspected PE or pulmonary microthrombi. Six patients (10.2%) received tPA during a cardiac arrest, and all six of these patients died. The median time from ICU admission to tPA administration was 4 days (IQR, 1–8), the median total dose of tPA was 50 mg (IQR, 20–100), and the median infusion time was 2 hours (IQR, 1–2). A total of 45 patients (76.3%) who received tPA died during hospitalization.
Table 1.
Patient characteristics, tPA administration, and outcomes
| Characteristic | tPA Recipients (n = 59) |
|---|---|
| Demographics | |
| Age, yr, median (IQR) | 60 (50–67) |
| Sex, M, n (%) | 43 (72.9) |
| Race, n (%) | |
| White | 19 (32.2) |
| Black | 13 (22.0) |
| Other or unknown | 27 (45.8) |
| Hispanic, n (%) | 18 (30.5) |
| BMI, median (IQR) | 32.2 (27.6–36.8) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 21 (35.6) |
| Hypertension | 34 (57.6) |
| COPD | 4 (6.8) |
| Current or former smoker | 14 (23.4) |
| Coronary artery disease | 6 (10.2) |
| Atrial fibrillation or atrial flutter | 5 (8.5) |
| Congestive heart failure | 3 (5.1) |
| Chronic kidney disease | 3 (5.1) |
| Laboratory values at tPA receipt, median (IQR) | |
| Leukocyte count/mm3 | 11.6 (8.2–15.7) |
| Hemoglobin, g/dl | 13.1 (11.3–13.8) |
| Platelet count/mm3 | 254 (211–313) |
| D-dimer, ng/ml | 10,000 (3,382–25,966) |
| Lactate, mmol/L | 2.3 (1.6–3.4) |
| C-reactive protein, mg/L | 148 (74–258) |
| Ferritin, ng/ml | 1,094 (639–2,048) |
| Fibrinogen, mg/dl | 649 (484–796) |
| International normalized ratio | 1.3 (1.2–1.4) |
| Partial thromboplastin time, s | 36.5 (29.5-68.9) |
| Severity of illness at tPA receipt, n (%) | |
| Invasive mechanical ventilation | 58 (98.3) |
| Vasopressors | 43 (72.9) |
| Acute renal replacement therapy | 8 (13.6) |
| VV-ECMO | 1 (1.7) |
| Therapeutic-dose anticoagulation at tPA receipt, n (%) | |
| Any | 42 (71.2) |
| Heparin infusion | 25 (42.4) |
| Enoxaparin | 17 (28.8) |
| tPA indication, n (%) | |
| Suspected pulmonary embolism | 47 (79.7) |
| Confirmed pulmonary embolism | 12 (20.3) |
| tPA administration, median (IQR) | |
| Days from ICU admission to tPA receipt | 6 (0–14) |
| Cumulative dose, mg | 50 (50–100) |
| Initial bolus dose, mg | 50 (25–100) |
| Infusion time, h | 2 (1–2) |
| Administered during cardiac arrest, n (%) | 6 (10.2) |
| Major bleeding | |
| Major bleeding with 7 d after tPA, n (%) | 6 (10.2) |
| Characteristics of bleeding, n/n bleed (%) | |
| Site of major bleed | |
| Bronchopulmonary | 2/6 (33.3) |
| Central nervous system | 1/6 (16.7) |
| Gastrointestinal | 1/6 (16.7) |
| Mucocutaneous | 2/6 (33.3) |
| Invasive hemostatic intervention for bleeding | 2/6 (33.3) |
| Received red cell transfusion for bleeding | 2/6 (33.3) |
| Therapeutic anticoagulation at time of tPA | 5/6 (83.3) |
| Antiplatelet therapy at time of tPA | 0/6 (0) |
| Major bleed clearly fatal or important contributor to death | 3/6 (50.0) |
| Cumulative dose of tPA, mg, median (IQR) | 50 (50-88) |
| Oxygenation and ventilation pre-tPA/post-tPA* | |
| PaO2, mm Hg, median (IQR) | 84 (60–107) / 80 (56–103) |
| FiO2, median (IQR) | 0.9 (0.6–1.0) / 1.0 (0.7–1.0) |
| PaO2:FiO2, median (IQR) | 86 (69–157) / 102 (67–174) |
| PaCO2, mm Hg, median (IQR) | 52 (45–71) / 54 (44–71) |
| Tidal volume, ml, median (IQR) | 420 (385–500) / 440 (368–500) |
| Respiratory rate, breaths/min, median (IQR) | 29 (22–30) / 30 (25–33) |
| Minute ventilation, L, median (IQR) | 12.2 (10.0–14.6) / 12.8 (10.1–14.4) |
| Dead space, physiologic, ml, median (IQR) | 297 (229-411) / 308 (236-388) |
| Therapies for refractory hypoxemia pre-tPA/post-tPA, n (%)† | |
| Prone positioning | 22 (37.2) / 16 (27.1) |
| Neuromuscular blockade | 17 (28.8) / 20 (35.6) |
| Inhaled pulmonary vasodilators‡ | 12 (20.3) / 12 (20.3) |
| At least one therapy | 30 (50.8) / 30 (50.8) |
| At least two therapies | 14 (23.7) / 14 (23.7) |
| Number of vasopressors pre-tPA/post-tPA, n (%)† | |
| 0 | 23 (39.0) /14 (23.7) |
| 1 | 20 (33.9) / 11 (18.6) |
| 2 | 8 (13.6) / 11 (18.6) |
| ⩾2 | 8 (13.6) / 26 (39.0) |
| VIS, median (IQR) | |
| 0 h | 6.0 (0–20.0) |
| 6 h | 7.6 (0–20.3) |
| 12 h | 5.3 (0–21.0) |
| 24 h | 3.3 (0–14.9) |
| Outcomes | |
| In-hospital death, n (%) | 45 (76.3) |
| Discharged alive, n (%) | 14 (23.7) |
| Length of stay among survivors, d, median (IQR) | 13 (3–38) |
| Increase in PaO2:FiO2 ratio ⩾ 50%, n (%)* | 8/42 (19.0) |
| Causes of Death, n (%)§ | |
| Respiratory failure | 40 (88.9) |
| Septic shock | 25 (55.6) |
| Heart failure | 8 (17.8) |
| Renal failure | 15 (33.3) |
| Hepatic failure | 2 (4.4) |
| Other | 12 (26.7) |
| Previously reported, n (%)ǁ | 17 (28.8) |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; ICU = intensive care unit; IQR = interquartile range; PaCO2 = partial pressure of carbon dioxide; PaO2:FiO2 = ratio of partial pressure of arterial oxygen to fraction of inspired oxygen; tPA = tissue plasminogen activator; VIS = vasoactive-inotropic score; VV-ECMO = venovenous extracorporeal membrane oxygenation.
Oxygenation and ventilation values before and after tPA are derived from the closest arterial blood gas obtained within 48 hours before and after tPA receipt, respectively. These data were available for 42 of the 59 patients (71.2%) in the cohort.
Therapies for refractory hypoxemia and number of vasopressors before and after tPA were assessed within 24 hours before and 24 hours after tPA receipt, respectively.
Inhaled pulmonary vasodilators include epoprostenol and nitric oxide.
Patients may have had more than one cause of death.
Six patients (10.2%) experienced a major bleed within 7 days after tPA administration, four of whom (67%) bled within 2 days of tPA receipt (Table 2). Sites of major bleeding included the bronchopulmonary tree (n = 2), central nervous system (n = 1), gastrointestinal tract (n = 1), and mucocutaneous (n = 2). Among the six patients with a major bleed, five (83.3%) were receiving therapeutic anticoagulation at the time of tPA receipt (Table 2). All six patients with a major bleed died (median time from tPA receipt to death, 7 days [IQR, 5–16]).
Table 2.
Characteristics of patients who experienced a major bleed
| Age/Sex | Invasive Mechanical Ventilation at Bleed? | Vasopressors at Bleed? | Site of Bleed(s) | Days from tPA to Bleed | Days from tPA to Death | On Therapeutic Anticoagulation at Bleed? | PTT at Bleed (s) | INR at Bleed | Platelet Count at Bleed (109/L) | pRBC Units Administered* | Invasive Hemostatic Intervention Performed? | Fatal or Important Contributor to Death? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 57/M | Yes | Yes | NP | 2 | 21 | Yes, enoxaparin |
79 | 1.4 | 120 | 2 | Yes | No |
| 58/M | Yes | No | Bronchopulmonary | 1 | 8 | Yes, heparin infusion |
66 | 1.3 | 200 | 0 | Yes | No |
| 46/M | Yes | Yes | Bronchopulmonary and GI | 0 | 0 | No (receiving prophylactic-dose heparin) | 39 | 1.1 | 205 | 0 | No | Yes |
| 37/M | Yes | Yes | Intracranial | 6 | 6 | Yes, enoxaparin |
32 | 1.4 | 391 | 0 | No | Yes |
| 47/F | Yes | Yes | NP and OP | 0 | 19 | Yes, heparin infusion |
70 | 1.1 | 149 | 1 | No | No |
| 59/M | Yes | Yes | Intracranial | 4 | 4 | Yes, heparin infusion |
68 | 1.2 | 138 | 1 | No | Yes |
Definition of abbreviations: GI = gastrointestinal; INR = international normalized ratio; NP = nasopharyngeal; OP = oropharyngeal; pRBC = packed red blood cells; PTT = partial thromboplastin time; tPA = tissue plasminogen activator.
pRBC transfusion was assessed within 2 days after the bleed.
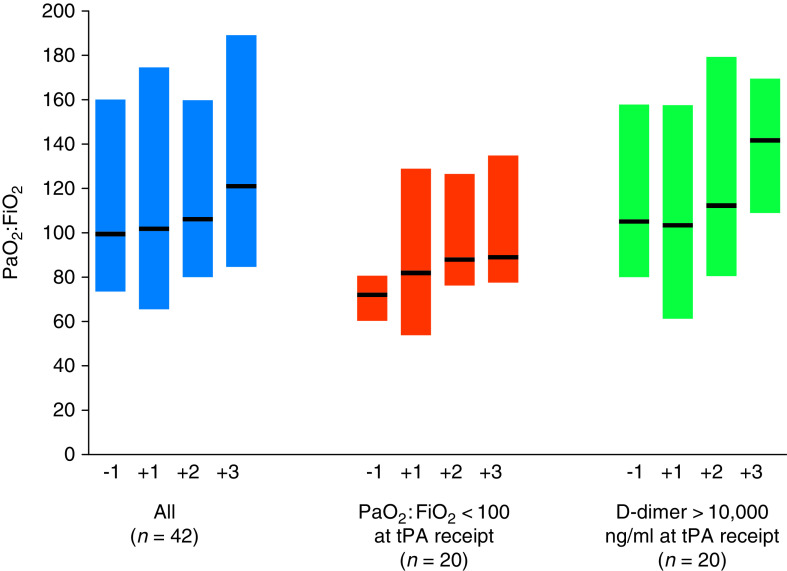
Oxygenation and ventilation parameters were unchanged after tPA receipt, as was the proportion of patients who required therapies for refractory hypoxemia (Table 1). Similarly, tPA did not affect the PaO2:FiO2 ratio longitudinally, either overall or in two prespecified subgroups: those with severe hypoxemia (PaO2:FiO2 <100) and D-dimer >10,000 ng/ml (13) at the time of tPA receipt (Figure 1). Results were similar when patients who received tPA during cardiac arrest were excluded. PaO2:FiO2 ratio increased by ⩾50% in only 8 of 42 patients (19.0%) with data available (Table 1). tPA also did not affect the amount of hemodynamic support as quantified by the VIS (Table 1).
Figure 1.
Effect of tissue plasminogen activator (tPA) on hypoxemia. Bars represent median (interquartile range [IQR]) values for the ratio of the partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2:FiO2) before and after tPA receipt. “−1” represents the closest PaO2:FiO2 ratio obtained within 48 hours before tPA receipt. “+1,” “+2,” and “+3” represent the first three PaO2:FiO2 ratios obtained within 48 hours after tPA receipt. The median time from the “−1” PaO2:FiO2 ratio to tPA administration was 2.4 hours (IQR, 1.0–6.2). The median time from tPA administration to the “+1,” “+2,” and “+3” PaO2:FiO2 ratios was 3.0 (IQR, 1.3–9.1), 11.3 (IQR, 6.0–23.0), and 17.5 (IQR, 10.0–32.6) hours, respectively. The number of patients in the “All” category (n = 42) is less than 59 because 17 patients (28.8%) did not have arterial blood gas measurements obtained within 48 hours before and after tPA receipt.
In summary, we report the outcomes of 59 critically ill patients with COVID-19 who received fibrinolytic therapy with tPA. Despite the promising theoretical benefits of fibrinolytic therapy in COVID-19, administration of tPA did not improve oxygenation or hemodynamics in the patients in our cohort. We found that major bleeding occurred in 6.8% of patients within 2 days of tPA administration and in 10.2% of patients within 7 days, all of whom died. Although a control arm was not available to directly compare bleeding risk in patients with similar disease severity who did or did not receive tPA, rates of major bleeding in this study were similar to those previously reported with tPA (14). Interestingly, five of the six patients who experienced a major bleed were receiving therapeutic anticoagulation at the time of tPA receipt, suggesting the combination of these two therapies may result in a particularly high risk of bleeding.
We acknowledge several limitations, including observational design, absence of a control group, and heterogeneity of patients and tPA dosing regimens. Patients in this cohort were severely ill, with a median PaO2:FiO2 ratio < 100 at the time of tPA receipt, a substantial number who required therapies for refractory hypoxemia, and a 76% in-hospital mortality rate. Indications for tPA administration were assessed via manual chart review; however, documentation of precise clinical decision-making was not always reliably captured. Arterial blood gases were not obtained at standardized time points, and factors such as changes in positioning (i.e., prone vs. supine) could have had an important influence of the PaO2:FiO2 ratio in ways that were not captured by our data set. Many clinicians may have administered fibrinolytic therapy as an emergency treatment to save patients who were already dying. In fact, 10% of patients received tPA during a cardiac arrest. It is likely that some of the major bleeding events, particularly those detected more than 2 days after tPA administration, may have been due to other factors, particularly given the short half-life of tPA.
The possibility that tPA may confer benefits to select patients with COVID-19, perhaps if administered earlier in the disease course or to a more homogeneous group of patients, remains unknown. Randomized clinical trials of tPA in severely ill patients with COVID-19 (NCT04357730) will shed much needed light on the efficacy and safety of tPA in this setting.
Footnotes
Supported by the following grants from the National Institutes of Health: R01HL144566 and R01DK125786 (D.E.L.), K08GM134220 and R03AG060179 (S.S.), K23HL130648 (K.S.M.), K23DK124645 (L.C.), and K08ES031678 (J.R.).
Author Contributions: D.J.D., S.S., J.R., and D.E.L. conceived the study, had full access to the data in the study, and take responsibility for the integrity of the data and accuracy of the analyses. D.J.D. and D.E.L. wrote the manuscript. D.J.D., I.P., and D.E.L. designed the table and figure. D.J.D., S.S., S.K.B., and D.E.L. acquired the data. All authors provided feedback on the protocol and critically revised and approved the final version of the manuscript.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost . 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med . 2020;18:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo W, Yu H, Gou J, Li X, Sun Y, Li J.Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19) [preprint] Preprints . 2020. https://www.preprints.org/manuscript/202002.0407/v4
- 4. Barrett CD, Moore HB, Moore EE, McIntyre RC, Moore PK, Burke J, et al. Fibrinolytic therapy for refractory COVID-19 acute respiratory distress syndrome: scientific rationale and review. Res Pract Thromb Haemost . 2020;18:524–531. doi: 10.1002/rth2.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore HB, Barrett CD, Moore EE, Jhunjhnuwala R, McIntyre RC, Moore PK, et al. Study of alteplase for respiratory failure in SARS-Cov2/COVID-19: study design of the phase IIa STARS trial. Res Pract Thromb Haemost . 2020;18:984–996. doi: 10.1002/rth2.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orfanos S, El Husseini I, Nahass T, Radbel J, Hussain S.Observational study of the use of recombinant tissue-type plasminogen activator in COVID-19 shows a decrease in physiological dead space ERJ Open Res 20201800455–2020.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barrett CD, Oren-Grinberg A, Chao E, Moraco AH, Martin MJ, Reddy SH, et al. Rescue therapy for severe COVID-19-associated acute respiratory distress syndrome with tissue plasminogen activator: a case series. J Trauma Acute Care Surg . 2020;18:453–457. doi: 10.1097/TA.0000000000002786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. STOP-COVID Investigators. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med . 2020;18:1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, et al. ANNEXA-4 Investigators. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med . 2019;18:1326–1335. doi: 10.1056/NEJMoa1814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med . 2010;18:234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 11. Han J, Pinsino A, Sanchez J, Takayama H, Garan AR, Topkara VK, et al. Prognostic value of vasoactive-inotropic score following continuous flow left ventricular assist device implantation. J Heart Lung Transplant . 2019;18:930–938. doi: 10.1016/j.healun.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McIntosh AM, Tong S, Deakyne SJ, Davidson JA, Scott HF. Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr Crit Care Med . 2017;18:750–757. doi: 10.1097/PCC.0000000000001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schutte T, Thijs A, Smulders YM. Never ignore extremely elevated D-dimer levels: they are specific for serious illness. Neth J Med . 2016;18:443–448. [PubMed] [Google Scholar]
- 14. Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA . 2014;18:2414–2421. doi: 10.1001/jama.2014.5990. [DOI] [PubMed] [Google Scholar]