Abstract
Rationale: Patients with idiopathic pulmonary fibrosis (IPF) and their caregivers experience stress, symptom burden, poor quality of life, and inadequate preparedness for end-of-life (EOL) care planning as the disease progresses. The hypothesis for this study was that the early introduction of palliative care in the course of IPF would improve knowledge and preparation for EOL, patient-reported outcomes, and advance care planning in patients with IPF and their caregivers.
Objectives: We sought to determine the feasibility, acceptability, and efficacy of a nurse-led early palliative care intervention entitled “A Program of SUPPORT” (Symptom management, Understanding the disease, Pulmonary rehabilitation, Palliative care, Oxygen therapy, Research participation, and Transplantation) in patients with IPF and their caregivers.
Methods: Patients with IPF (diagnosed in the year previous to their initial center visit) from the University of Pittsburgh Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease at University of Pittsburgh Medical Center—together with their caregivers—were randomized to receive the intervention “A Program of SUPPORT” or usual care. This included a total of three research visits aligned with their clinic visit over a period of 6 to 8 months. We measured feasibility, acceptability, and efficacy of this intervention.
Results: A total of 136 patient/caregiver dyads were eligible, and a total of 76 dyads were enrolled and participated. Participants were predominately White males >65 years old. Thirteen percent did not have an identified caregiver. Feasibility was limited; 56% of eligible dyads were enrolled. Eligible dyads (24%) were interested in participating but too fatigued to stay after their clinic visit. There was high attrition (20% of participants died before the study was completed). “A Program of SUPPORT” was acceptable to participants. Efficacy demonstrated a significant improvement in caregiver’s knowledge, disease preparedness, and confidence in caring for the patient as well as an improvement in knowledge and advance care planning completion in patient participants.
Conclusions: Patients with IPF and their caregivers have unmet needs regarding knowledge of their disease, self-management strategies, and preparedness for EOL planning. This nurse-led intervention demonstrated acceptability and efficacy in knowledge and advance care planning completion in patients and in knowledge, disease preparedness, and confidence in caregivers. Future research should identify additional strategies, including telemedicine resources to reach additional patients and their caregivers earlier in their disease course.
Clinical trial registered with clinicaltrials.gov (NCT02929017).
Keywords: palliative care, idiopathic pulmonary fibrosis, caregivers, quality of life, nurse-led intervention
Patients with idiopathic pulmonary fibrosis (IPF) and their caregivers experience stress, symptom burden, poor quality of life, and inadequate preparedness for end-of-life care planning as the disease progresses (1–5). IPF is a progressive, life-limiting lung disease that affects more than 200,000 people in the United States today, with approximately 50,000 new cases diagnosed each year (6). A disease of aging associated with intense medical and financial burden, IPF is expected to grow in incidence within the U.S. population (7, 8). The disease course is unpredictable, with a median survival from diagnosis of approximately 3.8 years, and many patients succumb to a rapid death within 6 months (7, 9). Antifibrotic therapies became available for patients in 2015 (10, 11). These medications are believed to slow the rate of deterioration of lung function but have no impact on quality of life (12). Lung transplantation is the only cure (13), but it is underutilized in patients with IPF, most often because of late referral (14). Patients have few treatment options and are predicted to experience a progressive course (15). Despite the fatal prognosis, patients and caregivers often lack knowledge about the disease and fail to understand the poor prognosis as the disease relentlessly progresses (2).
There is an extensive body of literature that supports the role of palliative care as standard of care in patients with life-limiting conditions (16–19), but the evidence reveals that referral to palliative care for patients with advanced lung disease commonly occurs late in the disease course or not at all (1, 20–22). In one study among patients managed at an IPF referral center between 2001 and 2016, only 14% of patients who died of IPF had a formal palliative care referral. The patients with IPF referred to palliative care compared with those who were not referred to palliative care were older at diagnosis, older at death, and had more severe comorbidities. Referred patients resided closer to the specialty referral center, had more total outpatient visits, and were more active participants in support groups. Referral to palliative care was associated with more in-home and hospice deaths (23).
On the basis of past observations, our hypothesis was that the early introduction of palliative care in the course of IPF would improve knowledge and preparation for end of life, patient-reported outcomes, and advance care planning in patients with IPF and their caregivers. The purpose of this randomized controlled trial was to determine the feasibility, acceptability, and efficacy of an early, nurse-led palliative care intervention—entitled “A Program of SUPPORT” (Symptom management, Understanding the disease, Pulmonary rehabilitation, Palliative care, Oxygen therapy, Research participation, and Transplantation)—in patients with IPF and their caregivers.
Methods
Design
A randomized controlled trial to test the feasibility, acceptability, and efficacy of the “A Program of SUPPORT” intervention compared with routine care in patients with IPF and their caregivers was conducted (24). The study was approved by the University of Pittsburgh Institutional Review Board Study 19060209.
Sample/Setting
Patient and family caregiver dyads were recruited by a clinician within 1 month after confirmation of the IPF diagnosis. The University of Pittsburgh Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease at University of Pittsburgh Medical Center was the setting for this study.
Intervention
“A Program of SUPPORT” is a multicomponent, nurse-led intervention booklet that was developed using focus group input from stakeholders (patients, family caregivers, and providers) and was tested and revised in an iterative manner to address the palliative care needs of patients and their families to maximize disease self-management (24). This intervention booklet and accompanying materials are copyright registered for intellectual property protection through the University of Pittsburgh Innovation Institute.
Study Format and Schedule
Participants were randomized to intervention or routine care. There were three study visits timed to coincide with clinical visits over a 6- to 8-month time period. To avoid intervention contamination, the patient/caregiver dyads were randomized to the intervention or control group (routine care) based on clinic-day visit. Physicians have assigned clinic-day slots, and patients return to clinic on the same day of the week as the original visit to see their assigned physician (e.g., Monday patients remain on Monday).
Once the patient/caregiver dyad was recruited and consent was completed, the nurse interventionist provided the intervention dyad with the SUPPORT booklet and read the booklet to the dyad; the dyad directed the pace of intervention delivery. Printed information and tablet content were addressed in four sections over the course of three research visits (Table 1): 1) overview of SUPPORT intervention goals and content (“What is IPF and how does it affect my lungs”) and explanation of tests used to diagnose and monitor progression; 2) self-management addressing the most common symptoms (cough, fatigue, and low blood oxygen saturation) and rationale for pulmonary rehabilitation and use of oxygen; 3) caring for the caregiver, with specific information about ways to support this individual; and 4) planning for the future, including a discussion of lung transplant as an option, research participation, and advance care planning (Table 1). The nurse interventionist introduced the standard patient education folder to participants in the control group for home review.
Table 1.
Intervention component delivery
| Intervention Component | Rationale and Which Component of “A Program of SUPPORT” | |
|---|---|---|
| 1. Education regarding disease, typical disease course, prognosis, treatment options, and futility of ICU | Rare disease, patients/CGs are often uninformed about disease course and prognosis and therefore unaware of likelihood of ICU hospitalization—Understanding the Disease | |
| 2. Self-management training for most common and distressing symptoms | Progressive escalation of incapacitating symptoms, e.g., cough, dyspnea, and hypoxemia—Symptom Management, Pulmonary Rehabilitation, and Oxygen Therapy | |
| 3. Caring for CG | Impacts family owing to rapid change in life status for previously healthy individual—CG often neglects own health | |
| 4. Planning for future and development of shared EOL goals | Rapid progression of disease and lack of discussion beforehand often leaves CG without adequate preparation for making EOL decisions—Palliative Care, Research Participation, and Transplantation |
Definition of abbreviations: CG = caregiver; EOL = end of life; ICU = intensive care unit; SUPPORT = Symptom management, Understanding the disease, Pulmonary rehabilitation, Palliative care, Oxygen therapy, Research participation, and Transplantation.
Data Collection
Demographics
Age, sex, race, income, and education level for patients and caregivers were collected at baseline, as well as additional information for patients: Neighborhood Deprivation Index (25); baseline pulmonary function tests; oxygen use; number of outpatient visits, inpatient visits, and emergency department visits; Charlson Comorbidity Index score; and time from first visit to the center to recruitment in the current study. The Neighborhood Deprivation Index allows for rankings of neighborhoods by socioeconomic status disadvantage in a region of interest (e.g., at the state or national level). It includes factors for the theoretical domains of income, education, employment, and housing quality and gives a broader perspective of demographics (25). A block group with a ranking of 1 indicates the lowest level of “disadvantage” within the nation, and an area deprivation index with a ranking of 100 indicates the highest level of “disadvantage.” A previous review of participants at this center revealed that 50% of patients traveled from surrounding rural areas >56 miles each way for their clinic visit.
Intervention feasibility
The number of dyads were recorded: 1) those eligible, 2) those who consented, 3) those who were enrolled, 4) those who completed the study, and 5) those who completed the SUPPORT intervention in the intervention arm, together with reasons for refusal or attrition.
Intervention acceptability
At the end of the intervention, participants (both patients and caregivers) were surveyed to rate their satisfaction with the “A Program of SUPPORT” intervention. Without a validated instrument available, a measurement tool was created to rank satisfaction (scale of 1 = not at all satisfied to 10 = very much satisfied) with the intervention content in its ability to meet educational needs, its materials, the appropriateness of the timing in their disease course, the degree to which the patients shared the booklet and website with their family, and whether they found the individual instruction with the nurse interventionist to be helpful.
Efficacy
We assessed the impact of the “A Program of SUPPORT” intervention on knowledge about IPF, disease preparedness and confidence, patient-reported outcomes, and the completion of advance care planning compared with usual care pre and post study. There is no standard measure of IPF knowledge, and therefore the literature and clinical experience were used to create the knowledge questionnaire. Regional interstitial lung disease (ILD) nurses were engaged for their assistance in the development of a 14-item questionnaire with yes/no responses regarding what they believed were important topics that patients and caregivers should know about their disease. To assess the perception of disease preparedness in patients with IPF, patients were asked to complete a numbered rating scale (from 1 [not at all prepared or not confident] to 10 [very well prepared or very confident]) when answering two questions: 1) How well do you feel prepared for this disease? 2) How confident are you that your loved ones and clinician understand your wishes regarding care as your disease progresses? To assess the perception of disease preparedness in caregivers, caregivers were asked to complete the same numbered rating scale with the two questions adapted to their role: 1) How well do you feel prepared for this disease? 2) How confident are you that you understand your loved one’s wishes regarding care as the disease progresses? Patient-reported outcomes (stress, quality of life, symptom burden) were measured using validated instruments, including the Perceived Stress Scale (26), A Tool to Assess Quality of Life in Idiopathic Pulmonary Fibrosis (ATAQ-IPF) (27), and Patient Reported Outcome Measurement Information System (PROMIS-29) (28).
Statistical Analysis
Previous work using the Perceived Stress Scale found that a similar intervention could decrease the Perceived Stress Scale score by 3 points (standard deviation = 3.6) among patients with IPF and their caregivers (2). No changes were expected in the Perceived Stress Scale score in the control arm. A sample size of 32 in each arm (a total of 64 new IPF patient/caregiver dyads) would provide 90% power (α = 0.05, two-sided test) to detect this difference. Considering a 20% drop-out, the number of dyads that needed to be approached to achieve the final desired sample size was 76.
In each study arm, the continuous variables were reported as median (interquartile range [IQR]) at baseline and last visit. In addition, categorical variables were reported as n (%) in each visit separately. The rate of retention was compared between the two arms by a Fisher exact test. The effect of the “A Program of SUPPORT” intervention was tested (compared with control group) for change in scores (between last visit and baseline visit) for knowledge, disease preparedness, confidence, and patient-reported outcomes (quality of life [for each dimension], symptom burden, stress) using a linear regression analysis (with robust variance estimator). In these models, the effect of the intervention on each outcome was adjusted for age, sex, and baseline forced vital capacity. All analyses were performed in Stata 16.2 (StataCorp).
Results
Baseline Characteristics
Baseline characteristics of dyad groups were comparable between intervention and control. The patients were predominantly male (80% in intervention arm vs. 85% in control arm), White (100%), and older than age 65 (median age of 70 in intervention arm vs. 73 in control arm) (Table 2). The caregivers (majority were spouses) were predominantly female (93% in intervention arm vs. 100% in control arm), White (95% in intervention arm vs. 100% in control arm), and older (median age of 67 in intervention arm vs. 68 in control arm) (Table 3). Of note, some patients presented without a caregiver (n = 10) and were allowed to participate in the study, and some presented with two caregivers (n = 3). In that case, one caregiver was randomly selected to complete questionnaires. Baseline area deprivation index characteristics for this cohort were comparable (median 56 in intervention vs. 55 in control arm).
Table 2.
Baseline characteristics by group: patients
| Intervention (n = 50) | Control (n = 26) | |
|---|---|---|
| Age, yr, median (IQR) | 70 (67–74) | 73 (68–76) |
| Male, n (%) | 40 (80) | 22 (85) |
| White, n (%) | 50 (100) | 26 (100) |
| Income group, n (%) | ||
| <$40,000 | 14 (29) | 10 (38) |
| $40,000–$79,000 | 22 (45) | 7 (27) |
| $80,000+ | 13 (27) | 9 (35) |
| Education group, n (%) | ||
| Less than college | 16 (32) | 12 (46) |
| College | 21 (42) | 10 (38) |
| Postgraduate | 13 (26) | 4 (15) |
| Neighborhood deprivation index, median (IQR) | 56 (37–69) | 55 (41–68) |
| Baseline FVC% predicted, median (IQR) | 69 (56–81) | 71 (59–84) |
| Baseline DlCO% predicted, median (IQR) | 46 (34–59) | 51 (39–65) |
| Use of oxygen, n (%) | 30 (60) | 18 (69) |
| Have any outpatient visit, n (%)* | 43 (86) | 24 (92) |
| Median (IQR) of outpatient visit* | 5 (1–9) | 5 (3–7) |
| Have any inpatient visit, n (%)* | 13 (16) | 4 (15) |
| Mean (SD) of inpatient visit* | 0.26 (0.69) | 0.19 (0.49) |
| Have any emergency visit, n (%)* | 8 (16) | 3 (12) |
| Mean (SD) of emergency visit* | 0.16 (0.37) | 0.12 (0.33) |
| Charlson comorbidity index, mean (SD) | 1.0 (1.4) | 1.1 (1.9) |
| Heart disease, n (%) | 39 (78) | 18 (69) |
| Emphysema, n (%) | 5 (10) | 4 (15) |
| GERD, n (%) | 32 (64) | 18 (69) |
| Cancer, n (%) | 6 (12) | 5 (19) |
| Time from first visit to recruitment, mo, median (IQR) | 6 (1–13) | 5 (3–9) |
Definition of abbreviations: DlCO = diffusing capacity of the lung for carbon monoxide; FVC = forced vital capacity; GERD = gastroesophageal reflux disease; IQR = interquartile range; SD = standard deviation.
During course of study.
Table 3.
Baseline characteristics by group: caregivers
| Intervention (n = 40) | Control (n = 22) | |
|---|---|---|
| Age, yr, median (IQR) | 67 (62–72) | 68 (55–73) |
| Female, n (%) | 37 (93) | 22 (100) |
| White, n (%) | 40 (100) | 21 (95) |
| Income group, n (%) | ||
| <$40,000 | 8 (22) | 4 (20) |
| $40,000–$79,000 | 18 (49) | 7 (35) |
| $80,000+ | 11 (30) | 9 (45) |
| Unknown | 3 | 2 |
| Education group, n (%) | ||
| Less than college | 15 (38) | 11 (55) |
| College | 18 (46) | 7 (35) |
| Postgraduate | 6 (15) | 2 (10) |
| Unknown | 1 | 2 |
Definition of abbreviation: IQR = interquartile range.
Feasibility of Intervention
Recruitment began in March 2017 and was completed in December 2020. A total of 136 dyads (patients with IPF and their designated caregivers) were eligible to participate (Figure 1). A total of 76 dyads (56%) were enrolled and participated in this study. An additional 60 dyads (44%) were eligible to participate. Of those, 28 dyads refused to participate, and 32 declined to participate (with the common reason being that they were too fatigued to stay) but expressed interest in participating at a future visit. Of the 76 dyads enrolled, 50 dyads were assigned to the “A Program of SUPPORT” intervention arm and 26 dyads to the control arm based on their clinic day. The total retention rate for the intervention arm was 35 out of 50 (70%) compared with 19 out of 26 (73%) for the control arm (P > 0.9, Figure 1). The most important reasons for not receiving the full dose of allocated intervention in the intervention arm were death (n = 11), lung transplant (n = 2), and drop-out (n = 4) before study completion. The most important reasons for not receiving the allocated intervention visits in the control arm were death (n = 4), lung transplant (n = 2), and drop-out (n = 1) before study completion.
Figure 1.
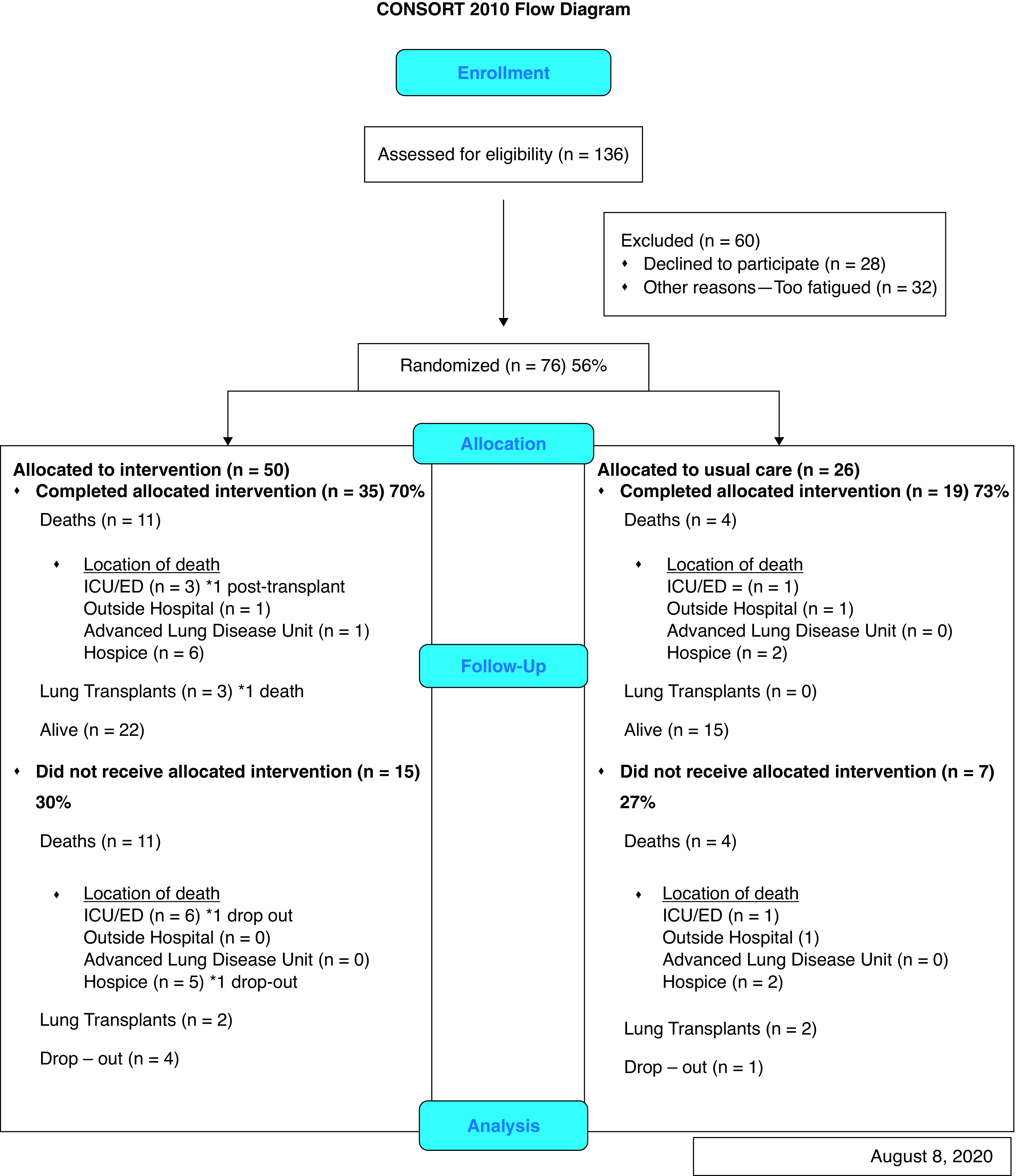
CONSORT flow diagram. CONSORT = Consolidated Standards of Reporting Trials; ED = emergency department; ICU = intensive care unit.
Acceptability of the Intervention
Patients and caregivers were satisfied with the “A Program of SUPPORT” booklet (median, 9; IQR, 8–10), website (median, 8; IQR, 8–9), and timing (median, 8; IQR, 6–10), and they rated the nurse interventionist with the highest score (median, 10; IQR, 8–10). Approximately 50% of patients reviewed the accompanying website, and 41% shared with family. Approximately 35% of caregivers reviewed the accompanying website, and 27% shared with family (Table 4).
Table 4.
Intervention acceptability
| Aspect of Intervention | Intervention Group Patient (n = 32) | Intervention Group CG (n = 23) |
|---|---|---|
| Overall satisfaction, median (IQR) | 9 (8–10) | 9 (8–10) |
| Booklet, median (IQR) | 9 (8–10) | 9 (8–10) |
| Web help, median (IQR) | 8 (8–9) | 8 (5–8) |
| Timing, median (IQR) | 8 (6–10) | 8 (6–10) |
| Nurse, median (IQR) | 10 (8–10) | 10 (8–10) |
| Look at support website, n (%) | 17 (53) | 8 (35) |
| Share website with family, n (%) | 13 (41) | 6 (27) |
Definition of abbreviations: CG = caregiver; IQR = interquartile range.
Baseline Distribution of Efficacy Outcomes in Intervention and Control Group
Patients and caregivers in both groups were comparable at baseline for knowledge, disease preparedness, confidence, and advance care planning (Table 5 and Table 7). Patients were comparable at baseline in both groups for patient-reported outcomes (stress, quality of life, and PROMIS-29 variables) (Table 9).
Table 5.
Baseline efficacy outcomes: patients’ knowledge, preparedness, confidence, and advance care planning
| Baseline Variables Visit 1 | Arm 1 (n = 50) | Arm 2 (n = 26) |
|---|---|---|
| Knowledge, median (IQR) | 12 (11–13) | 12 (11–13) |
| Preparedness, median (IQR) | 7 (5–8) | 7 (5–8) |
| Confidence, median (IQR) | 8 (7–10) | 8 (7–9) |
Definition of abbreviation: IQR = interquartile range.
Table 7.
Baseline efficacy outcomes: caregivers’ knowledge, preparedness, confidence
| Baseline Variables | Arm 1 (n = 39) | Arm 2 (n = 22) |
|---|---|---|
| Knowledge, median (IQR) | 11 (11–13) | 12 (11–13) |
| Stress, median (IQR) | 17 (13–20) | 15 (12–20) |
| Preparedness, median IQR | 5 (4–5) | 4 (3–5) |
| Confidence, median IQR | 7 (5–8) | 8 (6–9) |
Definition of abbreviation: IQR = interquartile range.
Table 9.
Baseline outcomes: patient-reported outcomes
| Arm 1 (n = 50) [Mean, Median, IQR] | Arm 2 (n = 26) [Mean, Median, IQR] | |
|---|---|---|
| Stress | 16 (10–19) | 15 (12–18) |
| Total ATAQ | 46 (32–58) | 43 (35–54) |
| Symptom subscale | 46 (33–59) | 41 (36–54) |
| Impact subscale | 48 (32–59) | 43 (34–63) |
| PROMIS-29 | ||
| Anxiety/fear | 55 (40–60) | 52 (48–60) |
| Depression/sadness | 50 (41–58) | 41 (41–59) |
| Fatigue | 51 (49–59) | 53 (46–57) |
| Pain interference | 52 (42–57) | 42 (42–61) |
| Physical function | 35 (31–40) | 36 (32–37) |
| Satisfaction with social roles | 45 (40–52) | 43 (39–52) |
| Sleep disturbance | 53 (51–55) | 53 (52–55) |
Definition of abbreviations: ATAQ = A Tool to Assess Quality of Life; IQR = interquartile range; PROMIS = Patient-Reported Outcomes Measurement Information System.
Efficacy
Median knowledge scores were 13 in the intervention arm and 12 in the control arm (maximum score 14), median preparedness scores were 8 in the intervention arm and 7 in the control arm (maximum score 10), and median confidence scores were 9 in the intervention arm and 8 in the control arm (maximum score 10). Sixty-two percent of patients in the intervention arm completed advance care planning compared with 33% in the control arm (Table 6).
Table 6.
End-of-intervention efficacy outcomes and change of outcome from baseline visit by group: patients
| End of Intervention | Arm 1 (n = 34) [Median (IQR) or n (%)] | Arm 2 (n = 18) [Median (IQR) or n (%)] | Mean Difference (95% CI; P Value)* |
|---|---|---|---|
| Knowledge | 13 (12–13) | 12 (12–13) | 0.86 (−0.09 to 1.81; 0.075) |
| Preparedness | 8 (7–9) | 7 (7–8) | −0.42 (−1.85 to 1.02; 0.57) |
| Confidence | 9 (7–10) | 8 (7–10) | 0.13 (−1.36 to 1.61; 0.86) |
| Advance care planning | 21 (62) | 6 (33) | 3.28 (0.95 to 11.26; 0.059)† |
Definition of abbreviations: CI = confidence interval; IQR = interquartile range.
A positive mean shows that the intervention increases the score more than the control.
Effect of arm 1 on mean difference of score change from baseline adjusted for age, sex, and baseline FVC.
Odds ratio (P value) adjusted for age, sex, and baseline FVC.
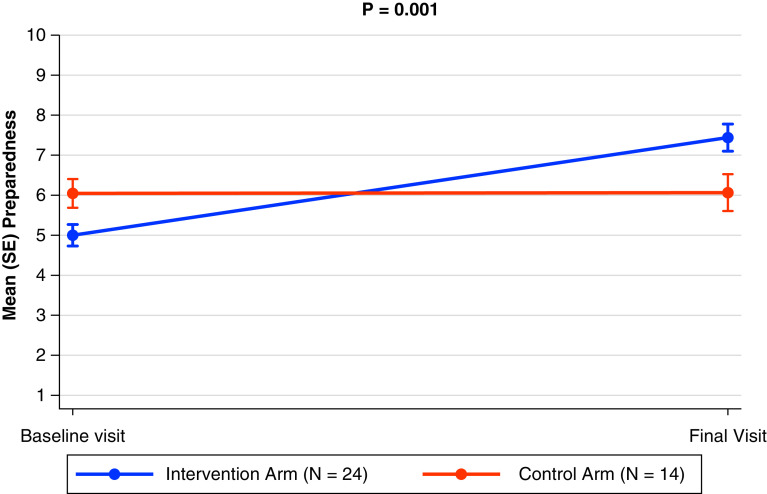
Outcomes for caregivers were measured in three areas: median knowledge scores were 12 in the intervention arm and 12 in the control arm (maximum score 14), median preparedness scores were 8 in the intervention arm and 6 in the control arm (maximum score 10), and median confidence scores were 9 in the intervention arm and 9 in the control arm (maximum score 10) (Table 8). Fifty-eight percent of caregivers in the intervention arm completed an advance care plan compared with 46% in the control arm. Caregivers in the intervention group exhibited significant improvements in their knowledge (mean, 1.34; 95% confidence interval [CI], 0.26–2.42; P = 0.016), preparedness (mean, 2.66; 95% CI, 1.21–4.11; P = 0.001), and confidence (mean, 1.33; 95% CI, 0.02–2.62; P = 0.046) (Figure 2). There were no significant changes in either arm for any of the patient-reported outcomes at the completion of the study (Table 10).
Table 8.
End-of-intervention efficacy outcomes and change of outcome from baseline visit by group: Caregivers
| End of Intervention | Arm 1 (n = 24) [Median (IQR) or n (%)] | Arm 2 (n = 13–14) [Median (IQR) or n (%)] | Mean Difference (95% CI; P Value)* |
|---|---|---|---|
| Knowledge | 12 (12–13) | 12 (12–12) | 1.34 (0.26 to 2.42; 0.016) |
| Stress | 16 (12–20) | 15 (12–19) | −1.00 (−4.53 to 2.52; 0.57) |
| Preparedness | 7 (5–9) | 6 (5–7) | 2.66 (1.21 to 4.11; 0.001) |
| Confidence | 9 (8–10) | 9 (8–10) | 1.33 (0.02 to 2.62; 0.046) |
| Advance care planning | 14 (58) | 6 (46) | 1.60 (0.37 to 1.69; 0.53)† |
Definition of abbreviations: CI = confidence interval; IQR = interquartile range.
A positive mean shows that the intervention increases the score more than the control.
Effect of arm 1 on mean difference of score change from baseline adjusted for age and sex.
Odds ratio (P value) adjusted for age.
Figure 2.
Preparedness score (pre-post): caregiver. This is recorded as the mean (standard error) for these scores.
Table 10.
End-of-intervention outcomes and change of outcome from baseline visit
| Arm 1 (n = 34) [Median (IQR)] | Arm 2 (n = 19–20) [Median (IQR)] | Mean Difference (95% CI; P Value)* | |
|---|---|---|---|
| Stress | 15 (10–18) | 15 (11–16) | 1.23 (−1.59 to 4.05; 0.39) |
| Total ATAQ (as earlier) | 46 (34–58) | 44 (36–54) | −0.93 (−8.57 to 6.71; 0.81) |
| Symptom subscale | 46 (34–57) | 43 (27–51) | −0.90 (−8.44 to 6.63; 0.81) |
| Impact subscale | 44 (32–58) | 49 (25–58) | −0.95 (−8.88 to 7.98; 0.83) |
| PROMIS-29 | |||
| Anxiety/fear | 51 (40–60) | 51 (40–56) | 0.01 (−5.55 to 5.57; 0.99) |
| Depression/sadness | 51 (41–56) | 45 (41–55) | −0.12 (−6.10 to 5.86; 0.97) |
| Fatigue | 51 (46–57) | 52 (49–58) | 1.38 (−5.37 to 2.62; 0.49) |
| Pain interference | 42 (42–57) | 50 (42–56) | −1.35 (−5.78 to 3.07; 0.54) |
| Physical function | 35 (30–39) | 35 (32–37) | −0.73 (−3.59 to 2.13; 0.61) |
| Satisfaction with social roles | 46 (42–52) | 44 (35–49) | 3.66 (1.02 to 8.34; 0.12) |
| Sleep disturbance | 53 (50–56) | 51 (48–54) | 2.63 (−0.53 to 5.79; 0.10) |
Definition of abbreviations: ATAQ = A Tool to Assess Quality of Life; CI = confidence interval; FVC = forced vital capacity; IQR = interquartile range; PROMIS =Patient-Reported Outcomes Measurement Information System.
A positive mean shows that the intervention increases the score more than the control.
Effect of arm 1 on mean difference of score change from baseline adjusted for age, sex, and baseline FVC.
Discussion
To the best of our knowledge, this is the first study to investigate the impact of a nurse-led early palliative care intervention to evaluate knowledge, disease preparedness, confidence, patient-reported outcomes, and advance care planning in patients with IPF and their caregivers. The study findings assert that “A Program of SUPPORT” was very acceptable and led to significant improvement in knowledge, disease preparedness, and confidence for caregivers as well as an improvement in patients’ knowledge and advance care planning. The nurse interventionist was actively involved throughout the entire study, and participants ranked the nurse interventionist with the highest score on the acceptability survey. Previous studies have demonstrated feasibility and acceptability in the delivery of nurse-led palliative care to patients with various cancers (29–31). Several factors contribute to the nurse-led delivery of palliative care. By nature of the education provided in nursing programs, the curriculum includes a bio-psycho-social theoretical model (32), a model also used in palliative care, and nurses in clinical practice spend more time in direct patient contact, building trust with the patient and caregiver.
Family caregivers are at a high risk for distress and poor quality of life (33–35). In one study looking at the benefits of palliative care in patients with advanced cancer and their caregivers, both patients and caregiver participants appreciated palliative care as ongoing care that improved quality of life (36). Another study reported that an interdisciplinary approach to palliative care in lung cancer resulted in statistically significant improvements in family caregivers’ social well-being and a lessening of psychological distress and caregiver burden (37). Gaps in the literature evaluating support needs for caregivers of patients with pulmonary fibrosis warrant further exploration.
Feasibility was impacted by the number of enrolled participants and the number of dyads that completed the study. Of the 56% of eligible participants who enrolled in the study, 71% of all dyads completed the study—35 out of 50 in the intervention arm and 19 out of 26 in the control arm. Participants in the intervention arm (n = 50) received the nurse-led delivery of the SUPPORT intervention at the first research visit. Following patient/caregiver lead for delivery and allowing time for breaks, each dyad opted to have the nurse read the entire book at the first visit and questions at follow-up visits. Of the 136 eligible participants, 32 dyads (24%) expressed interest in participating in the study but were too fatigued to stay after their clinical visit for the research visit. Alternate modes of delivery of the intervention could potentially reach this group. Lastly, 20% of all participants in the study died before the study was completed. This is not unusual considering the unpredictable disease course and life expectancy (15).
The efficacy of the “A Program of SUPPORT” intervention demonstrated a significant improvement in knowledge, disease preparedness, and confidence for caregivers in the intervention arm as noted earlier. This SUPPORT intervention did not significantly decrease stress, as in a previous study in patients or caregivers (2), and did not improve quality of life or symptom burden in patients, demonstrated by progressively worsened questionnaire scores throughout the study period.
Patients with IPF and their caregivers have unmet education needs (38) in the presence of an unpredictable disease course (39). Compelling literature reveals that patients and caregivers wanted clarity around what their future with IPF will look like (40). In another study, patients and caregivers wanted honest information about their future (41). Early palliative care provides an opportunity to provide patients and caregivers with information about their disease and address symptom burden and advance care planning (18). In a systematic review and meta-analysis of randomized controlled clinical trials of patients with primarily noncancer illness, palliative care—compared with usual care—was associated with less acute healthcare use and modestly lower symptom burden, but there was no significant difference in quality of life (42). Previous studies using nurse-led integration of palliative care in other lung diseases revealed that patients gained self-confidence and their coping behavior increased (43), there was an increase in advance care planning (44, 45), and there was improved symptom burden and family satisfaction in patients with lung cancer (29). For patients with chronic obstructive pulmonary disease, palliative care interventions demonstrated an effectiveness in decreasing hospital readmissions and emergency department visits and in improving exercise capacity, health-related quality of life, and satisfaction (46).
Patients with advanced lung disease, such as patients with IPF, prove to be a challenging population in whom to deliver palliative care (1, 20, 41, 47–50). This early palliative care intervention was acceptable to patients with IPF and their caregivers. Feasibility was affected by fatigue expressed by patients about a long clinic day and early attrition due to disease progression indicated by death, lung transplantation, or drop-out. The rapid rise of telemedicine use and acceptance as a means of healthcare delivery (51) may help to reduce these feasibility issues. The intervention had a significant impact on caregivers’ knowledge, preparedness, and confidence.
Recruitment for this study took longer than originally planned. It was anticipated that 76 dyads of patients with IPF and their caregivers would be recruited in a 2-year period. The recruitment period was 2 years and 9 months. We speculate that declining numbers of patients with IPF seen at our center is the result of IPF medications now available in the community, leading to increased disease management in their local area of residence. Our lung transplant program has a wider referral source and also noted decline in the referral of patients with IPF for lung transplant evaluation. The study was completed just before the clinic restrictions due to coronavirus disease (COVID-19).
Future research should evaluate multimodality availability of this intervention delivery via digital and telehealth options, which have now become widely available owing to the COVID-19 pandemic (52). This may be a solution to these limitations. Patient and caregiver input regarding what and how information should be received will be valuable to incorporate into future work (53–56). Findings from this study support future research that assesses alternative modes of delivery of early palliative care, including delivery of the intervention for review at home via various platforms for the patient with IPF and their caregiver.
Limitations
This study was conducted in an academic specialty ILD center and may not be reflective of the care or access to care that patients with IPF receive in the community. Demographics of this study population reveal a lack diversity, with a sample of White male (82%) and White female (18%) participants, reflective of national trends in lack of diversity in IPF (57). Fatigue, long intervals between clinic visits, and high attrition impacted the feasibility of the intervention delivery. While the nurse was a key component for delivery of this intervention, a nurse may not always be available where patients receive their care. In addition, the randomization of patients was based on clinic day to reduce contamination, but this resulted in a 2:1 unequal distribution between intervention and control arms, limiting statistical power. Lastly, the knowledge questionnaire was created by a thorough review of the literature and from input of local clinic nurses, yet the patients and caregivers had high scores before and after the study. It would be beneficial for further study to develop a psychometrically confirmed questionnaire to measure knowledge without this ceiling effect.
Conclusions
Patients with IPF and their caregivers have unmet needs regarding knowledge of their disease, self-management strategies, and preparedness for end-of-life planning. This nurse-led intervention demonstrated acceptability and initial impact on knowledge and advance care planning completion in patients and knowledge, disease preparedness, and confidence in caregivers. Future research should identify additional strategies, including how the presence of a nurse contributes to downstream healthcare use, telemedicine resources for delivery of early palliative care, and further exploration of the unmet needs of caregivers.
Acknowledgments
Acknowledgment
The authors thank Dr. Diane Angelini for editorial review and the research coordinators for their input and involvement: Michelle Meyers, B.S.N., R.N., Michelle MacPherson, M.A.T., and Morgan Carnahan, B.S.
Footnotes
Supported by the U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Nursing Research (1K23NR016276-01A1); Three Lakes Foundation, LLC; and the University of Pittsburgh Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease at UPMC.
Author Contributions: K.O.L., M.N., and M.Q.R. established the study design. S.J.K., M.S.V., K.F.G., and D.J.K. made substantial contributions to the acquisition of the work. K.O.L., S.J.K., K.F.G., D.J.K., M.N., and M.Q.R. interpreted the data, and wrote, revised, and approved the final version to be published and agree to be accountable for the accuracy and integrity of all aspects of the work.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Bajwah S, Higginson IJ, Ross JR, Wells AU, Birring SS, Riley J, et al. The palliative care needs for fibrotic interstitial lung disease: a qualitative study of patients, informal caregivers and health professionals. Palliat Med . 2013;27:869–876. doi: 10.1177/0269216313497226. [DOI] [PubMed] [Google Scholar]
- 2. Lindell KO, Olshansky E, Song MK, Zullo TG, Gibson KF, Kaminski N, et al. Impact of a disease-management program on symptom burden and health-related quality of life in patients with idiopathic pulmonary fibrosis and their care partners. Heart Lung . 2010;39:302–313. doi: 10.1016/j.hrtlng.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swigris JJ, Gould MK, Wilson SR. Health-related quality of life among patients with idiopathic pulmonary fibrosis. Chest . 2005;127:284–294. doi: 10.1378/chest.127.1.284. [DOI] [PubMed] [Google Scholar]
- 4. Wijsenbeek M, Kreuter M, Olson A, Fischer A, Bendstrup E, Wells CD, et al. Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin . 2019;35:2015–2024. doi: 10.1080/03007995.2019.1647040. [DOI] [PubMed] [Google Scholar]
- 5. Ryerson CJ, Donesky D, Pantilat SZ, Collard HR. Dyspnea in idiopathic pulmonary fibrosis: a systematic review. J Pain Symptom Manage . 2012;43:771–782. doi: 10.1016/j.jpainsymman.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Pulmonary Fibrosis Foundation. Chicago, IL: Pulmonary Fibrosis Foundation; 2021. https://www.pulmonaryfibrosis.org/life-with-pf/about-ipf [Google Scholar]
- 7. Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med . 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 8. Lederer DJMF, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med . 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 9. Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 11. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 12. Graney BAL, Lee JS. Impact of novel antifibrotic therapy on patient outcomes in idiopathic pulmonary fibrosis: patient selection and perspectives. Patient Relat Outcome Meas . 2018;9:321–328. doi: 10.2147/PROM.S144425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet . 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 14. Paoletti L, Palmer S, Yow E, Neely ML, Gamerman V, Whelan T. Underutilization of lung transplant referral among patients with newly diagnosed idiopathic pulmonary fibrosis (IPF) J Heart Lung Transplant . 2017;36:S115. [Google Scholar]
- 15. Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2011;183:431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 16.American Association of Colleges of Nursing. End-of-Life Nursing Education Consortium (ELNEC). History, statewide effort and recommendations for the future: advancing palliative nursing care. 2012https://www.aacnnursing.org/ELNEC/About.
- 17. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med . 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 18. Ferrell BR, Twaddle ML, Melnick A, Meier DE. National consensus project clinical practice guidelines for quality palliative care guidelines. J Palliat Med . 2018;21:1684–1689. doi: 10.1089/jpm.2018.0431. [DOI] [PubMed] [Google Scholar]
- 19. Smallwood N, Thompson M, Warrender-Sparkes M, Eastman P, Le B, Irving L, et al. Integrated respiratory and palliative care may improve outcomes in advanced lung disease. ERJ Open Res . 2018;4:00102-2017. doi: 10.1183/23120541.00102-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beernaert K, Cohen J, Deliens L, Devroey D, Vanthomme K, Pardon K, et al. Referral to palliative care in COPD and other chronic diseases: a population-based study. Respir Med . 2013;107:1731–1739. doi: 10.1016/j.rmed.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Bajwah S, Ross JR, Wells AU, Mohammed K, Oyebode C, Birring SS, et al. Palliative care for patients with advanced fibrotic lung disease: a randomised controlled phase II and feasibility trial of a community case conference intervention. Thorax. 2015;70:830–839. doi: 10.1136/thoraxjnl-2014-206583. [DOI] [PubMed] [Google Scholar]
- 22. Brown CE, Jecker NS, Curtis JR. Inadequate palliative care in chronic lung disease. an issue of health care inequality. Ann Am Thorac Soc . 2016;13:311–316. doi: 10.1513/AnnalsATS.201510-666PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou RH, Nouraie M, Chen X, Saul MI, Kaminski N, Gibson KF, et al. Assessing patterns of palliative care referral and location of death in patients with idiopathic pulmonary fibrosis: a 16-year single-center retrospective cohort study. J Palliat Med. 2019;22:538–544. doi: 10.1089/jpm.2018.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lindell KO, Nouraie M, Klesen MJ, Klein S, Gibson KF, Kass DJ, et al. Randomised clinical trial of an early palliative care intervention (SUPPORT) for patients with idiopathic pulmonary fibrosis (IPF) and their caregivers: protocol and key design considerations. BMJ Open Resp Res. 2018;5:e000272. doi: 10.1136/bmjresp-2017-000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.University of Wisconsin School of Medicine and Public Health Madison, WI: University of Wisconsin-Madison; https://www.neighborhoodatlas.medicine.wisc.edu. [Google Scholar]
- 26. Cohen S, Hoberman HM. Positive events and social support as buffers of life change stress. J Appl Soc Psychol . 1983;13:99–125. [Google Scholar]
- 27. Swigris JJ, Wilson SR, Green KE, Sprunger DB, Brown KK, Wamboldt FS. Development of the ATAQ-IPF: a tool to assess quality of life in IPF. Health Qual Life Outcomes . 2010;8:77. doi: 10.1186/1477-7525-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ader DN. Developing the patient-reported outcomes measurement information system (PROMIS) Med Care. 2007;45:S1–S2. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reinke LF, Vig EK, Tartaglione EV, Backhus LM, Gunnink E, Au DH. Protocol and pilot testing: the feasibility and acceptability of a nurse-led telephone-based palliative care intervention for patients newly diagnosed with lung cancer. Contemp Clin Trials . 2018;64:30–34. doi: 10.1016/j.cct.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 30. Schenker Y, White D, Rosenzweig M, Chu E, Moore C, Ellis P, et al. Care management by oncology nurses to address palliative care needs: a pilot trial to assess feasibility, acceptability, and perceived effectiveness of the CONNECT intervention. J Palliat Med . 2015;18:232–240. doi: 10.1089/jpm.2014.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA . 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry . 1980;137:535–544. doi: 10.1176/ajp.137.5.535. [DOI] [PubMed] [Google Scholar]
- 33. Dionne-Odom JN, Ejem DB, Wells R, Azuero A, Stockdill ML, Keebler K, et al. Effects of a telehealth early palliative care intervention for family caregivers of persons with advanced heart failure: the ENABLE CHF-PC randomized clinical trial. JAMA Netw Open . 2020;3:e202583. doi: 10.1001/jamanetworkopen.2020.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Covinsky KE, Goldman L, Cook EF, Oye R, Desbiens N, Reding D, et al. The impact of serious illness on patients’ families. SUPPORT investigators. Study to understand prognoses and preferences for outcomes and risks of treatment. JAMA. 1994;272:1839–1844. doi: 10.1001/jama.272.23.1839. [DOI] [PubMed] [Google Scholar]
- 35. Hebert RS, Arnold RM, Schulz R. Improving well-being in caregivers of terminally ill patients. Making the case for patient suffering as a focus for intervention research. J Pain Symptom Manage . 2007;34:539–546. doi: 10.1016/j.jpainsymman.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zimmermann C, Swami N, Krzyzanowska M, Leighl N, Rydall A, Rodin G, et al. Perceptions of palliative care among patients with advanced cancer and their caregivers. CMAJ . 2016;188:E217–E227. doi: 10.1503/cmaj.151171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun V, Grant M, Koczywas M, Freeman B, Zachariah F, Fujinami R, et al. Effectiveness of an interdisciplinary palliative care intervention for family caregivers in lung cancer. Cancer . 2015;121:3737–3745. doi: 10.1002/cncr.29567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morisset J, Dubé BP, Garvey C, Bourbeau J, Collard HR, Swigris JJ, et al. The unmet educational needs of interstitial lung disease patients. Setting the stage for tailored pulmonary rehabilitation. Ann Am Thorac Soc . 2016;13:1026–1033. doi: 10.1513/AnnalsATS.201512-836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ley B, Collard HR. Risk prediction in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2012;185:6–7. doi: 10.1164/rccm.201111-1960ED. [DOI] [PubMed] [Google Scholar]
- 40. Ramadurai D, Corder S, Churney T, Graney B, Harshman A, Meadows S, et al. Idiopathic pulmonary fibrosis: educational needs of health-care providers, patients, and caregivers. Chron Respir Dis . 2019;16:1479973119858961. doi: 10.1177/1479973119858961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holland AE, Fiore JF, Jr, Goh N, Symons K, Dowman L, Westall G, et al. Be honest and help me prepare for the future: what people with interstitial lung disease want from education in pulmonary rehabilitation. Chron Respir Dis . 2015;12:93–101. doi: 10.1177/1479972315571925. [DOI] [PubMed] [Google Scholar]
- 42. Quinn KL, Shurrab M, Gitau K, Kavalieratos D, Isenberg SR, Stall NM, et al. Association of receipt of palliative care interventions with health care use, quality of life, and symptom burden among adults with chronic noncancer illness: a systematic review and meta-analysis. JAMA . 2020;324:1439–1450. doi: 10.1001/jama.2020.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baker E, Fatoye F. Patient perceived impact of nurse-led self-management interventions for COPD: a systematic review of qualitative research. Int J Nurs Stud . 2019;91:22–34. doi: 10.1016/j.ijnurstu.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 44. Sinclair C, Auret KA, Evans SF, Williamson F, Dormer S, Wilkinson A, et al. Advance care planning uptake among patients with severe lung disease: a randomised patient preference trial of a nurse-led, facilitated advance care planning intervention. BMJ Open. 2017;7:e013415. doi: 10.1136/bmjopen-2016-013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Houben CHM, Spruit MA, Luyten H, Pennings HJ, van den Boogaart VEM, Creemers JPHM, et al. Cluster-randomised trial of a nurse-led advance care planning session in patients with COPD and their loved ones. Thorax . 2019;74:328–336. doi: 10.1136/thoraxjnl-2018-211943. [DOI] [PubMed] [Google Scholar]
- 46. Wang LHZY, Zhao Y, Chen LY, Zhang L, Zhang YM. The effect of a nurse-led self-management program on outcomes of patients with chronic obstructive pulmonary disease. Clin Respir J . 2020;14:148–157. doi: 10.1111/crj.13112. [DOI] [PubMed] [Google Scholar]
- 47. Wijsenbeek M, Bendstrup E, Ross J, Wells A. Cultural difference in palliative care in patients with idiopathic pulmonary fibrosis. Chest . 2015;148:e56. doi: 10.1378/chest.15-0705. [DOI] [PubMed] [Google Scholar]
- 48. Sampson C, Gill BH, Harrison NK, Nelson A, Byrne A. The care needs of patients with idiopathic pulmonary fibrosis and their carers (CaNoPy): results of a qualitative study. BMC Pulm Med . 2015; 15:155. doi: 10.1186/s12890-015-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lindell KO, Liang Z, Hoffman LA, Rosenzweig MQ, Saul MI, Pilewski JM, et al. Palliative care and location of death in decedents with idiopathic pulmonary fibrosis. Chest . 2015;147:423–429. doi: 10.1378/chest.14-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang Z, Hoffman LA, Nouraie SM, Donahoe MP, Saul MI, Donahoe M, Kass DJ, et al. 201720134–140.. [DOI] [PubMed] [Google Scholar]
- 51.Center to Advance Palliative Care New York, NY: Center to Advance Palliative Care; https://www.capc.org/covid-19/. [Google Scholar]
- 52. Ritchey KC, Foy A, McArdel E, Gruenewald DA. Reinventing palliative care delivery in the era of COVID-19: how telemedicine can support end of life care. Am J Hosp Palliat Care . 2020; 37:992–997. doi: 10.1177/1049909120948235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Senanayake S, Harrison K, Lewis M, McNarry M, Hudson J. Patients’ experiences of coping with Idiopathic Pulmonary Fibrosis and their recommendations for its clinical management. PLoS One . 2018;13:e0197660. doi: 10.1371/journal.pone.0197660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Swigris JJ, Stewart AL, Gould MK, Wilson SR. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes . 2005;3:61. doi: 10.1186/1477-7525-3-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Russell AM, Sprangers MAG, Wibberley S, Snell N, Rose DM, Swigris JJ. The need for patient-centred clinical research in idiopathic pulmonary fibrosis. BMC Med . 2015;13:240. doi: 10.1186/s12916-015-0475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kalluri M, Luppi F, Ferrara G. What patients with idiopathic pulmonary fibrosis and caregivers want: filling the gaps with patient reported outcomes and experience measures. Am J Med . 2020;133:281–289. doi: 10.1016/j.amjmed.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 57. Raghu G, Chen SY, Hou Q, Yeh WS, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18–64 years old. Eur Respir J. 2016;48:179–186. doi: 10.1183/13993003.01653-2015. [DOI] [PubMed] [Google Scholar]