Abstract
Compromised alveolar development and pulmonary vascular remodeling are hallmarks of pediatric lung diseases such as bronchopulmonary dysplasia (BPD) and alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV). Although advances in surfactant therapy, corticosteroids, and antiinflammatory drugs have improved clinical management of preterm infants, those who suffer with severe vascular complications still lack viable treatment options. Paucity of the alveolar capillary network in ACDMPV causes respiratory distress and leads to mortality in a vast majority of infants with ACDMPV. The discovery of endothelial progenitor cells (EPCs) in 1997 brought forth the paradigm of postnatal vasculogenesis and hope for promoting vascularization in fragile patient populations, such as those with BPD and ACDMPV. The identification of diverse EPC populations, both hematopoietic and nonhematopoietic in origin, provided a need to identify progenitor cell–selective markers that are linked to progenitor properties needed to develop cell-based therapies. Focusing on the future potential of EPCs for regenerative medicine, this review will discuss various aspects of EPC biology, beginning with the identification of hematopoietic, nonhematopoietic, and tissue-resident EPC populations. We will review knowledge related to cell surface markers, signature gene expression, and key transcriptional regulators and will explore the translational potential of EPCs for cell-based therapy for BPD and ACDMPV. The ability to produce pulmonary EPCs from patient-derived induced pluripotent stem cells in vitro holds promise for restoring vascular growth and function in the lungs of patients with pediatric pulmonary disorders.
Keywords: endothelial progenitor cells, pulmonary disease, bronchopulmonary dysplasia, alveolar capillary dysplasia with misalignment of pulmonary veins, directed differentiation of embryonic stem cells and induced pluripotent stem cells into endothelial progenitor cells
Advances in neonatal medicine have made it possible to provide lifesaving care for preterm infants (1). However, owing to the late maturation of the lung, the number of neonates born with respiratory complications, including respiratory distress syndrome and bronchopulmonary dysplasia (BPD), continues to increase (2). BPD is a multifactorial, developmental disease associated with alveolar simplification, inflammation, and variable fibrotic remodeling. Features of BPD include bronchial and pulmonary vascular abnormalities, the latter of which are seen in severe BPD and associated with increased morbidity and mortality (3, 4). As the BPD population ages, increasing numbers of patients with rapidly declining respiratory function are to be expected in the future. Novel therapies aimed at promoting pulmonary vascularization and improving lung function in patients with BPD are critically needed. Severe pulmonary vascular abnormalities are also hallmarks of alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV), a rare, usually fatal lung disorder that is linked to mutations in the FOXF1 (Forkhead box protein F1) gene locus, characterized by defective growth and morphogenesis of pulmonary capillaries and abnormal positioning of pulmonary veins (5–7). Most infants with ACDMPV experience respiratory insufficiency and pulmonary hypertension soon after birth, and most succumb to respiratory failure shortly thereafter (8, 9). Similarly, pulmonary vascular growth and functions are disrupted in congenital diaphragmatic hernia, a more common disorder associated with lung hypoplasia and pulmonary hypertension (10). A better understanding of pulmonary vascular formation, growth, and regeneration is critically needed to develop effective treatments for ACDMPV, BPD, and other disorders of lung growth and development.
Vasculogenesis and angiogenesis are two distinct biological processes through which blood vessels are formed. Vasculogenesis describes the de novo formation of the vascular network by means of differentiation of endothelial progenitor cells (EPCs) as they assemble blood vessels, whereas angiogenesis describes the process of vascular formation by sprouting from preexisting blood vessels. Although vascular remodeling in the mature lung was believed to be restricted to angiogenesis, Asahara and colleagues published the first paper describing putative adult EPCs in 1997, providing support to the feasibility of EPC-based therapy for vascular disease (11). There is continued controversy regarding the identities and defining characteristics of EPCs. This review will address discoveries and controversies surrounding the identification of both hematopoietic and nonhematopoietic EPC populations. We will review specific EPC markers, key transcriptional regulators, and various protocols developed to generate EPC populations from pluripotent embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Lastly, we will explore the therapeutic potential of EPCs for regenerative medicine for pulmonary disorders.
Development of Pulmonary Vasculature
Vasculogenesis and Angiogenesis in the Embryonic Lung
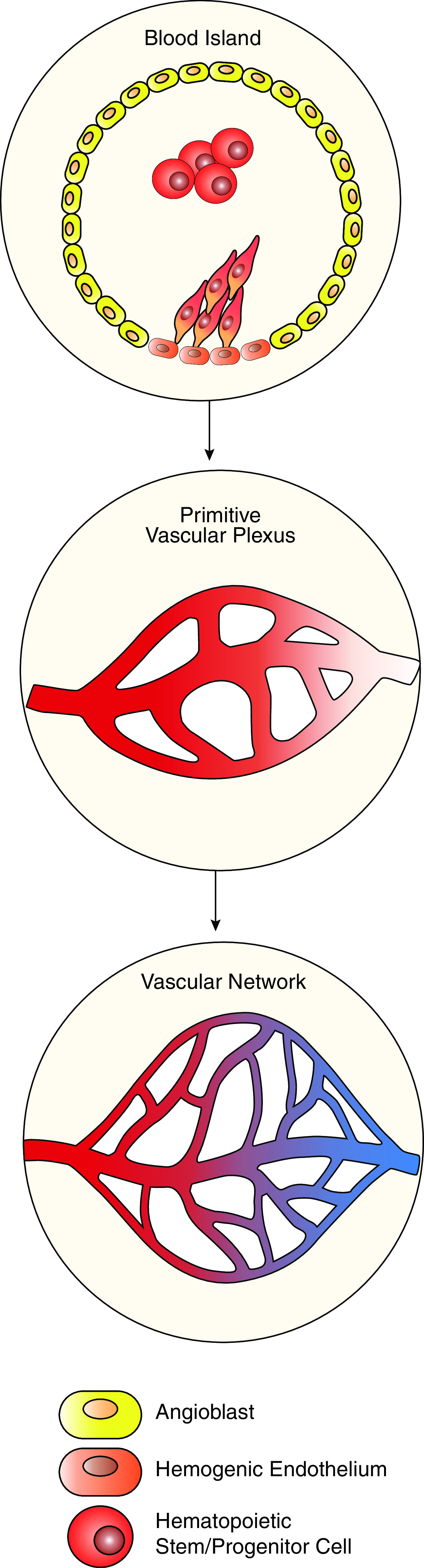
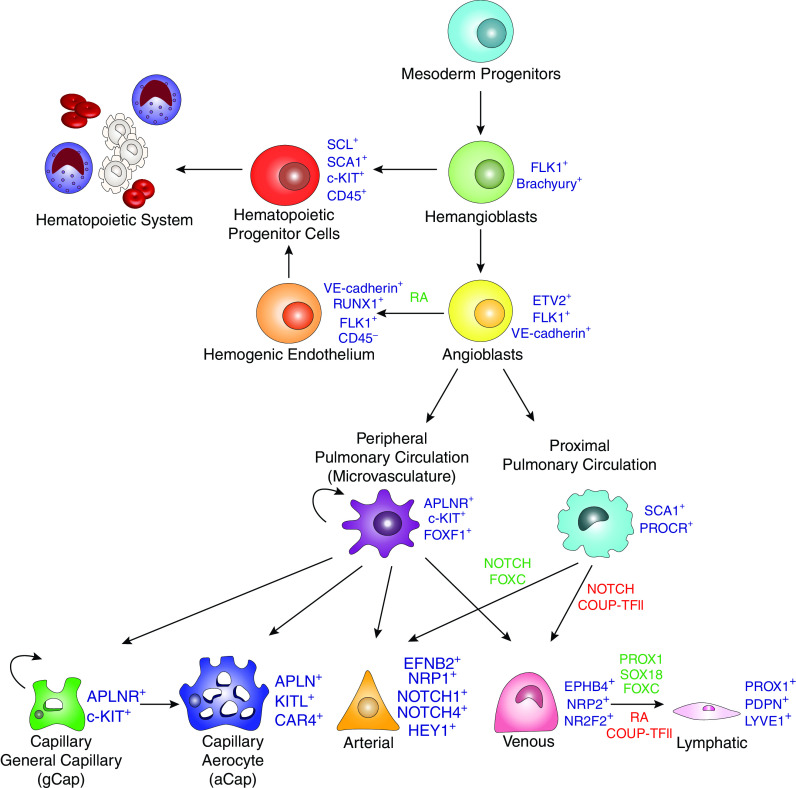
In mammals, vasculogenesis occurs in extraembryonic tissues (yolk sac, allantois, and placenta) and in the dorsal aorta, giving rise to primitive embryonic vasculature (12). Extraembryonic vasculogenesis is initiated by signaling from the visceral endoderm, which serves to direct the patterning of the underlying mesoderm in the yolk sac (13, 14). In the mouse embryo, this begins during gastrulation at embryonic Day 6.5 (E6.5) (15). Mesodermal-derived bipotent hemangioblasts serve as progenitors of hematopoietic and endothelial cell lineages. By E7.5, hemangioblast differentiation results in the formation of blood islands wherein primitive hematopoietic progenitors are located centrally and endothelial progenitors (angioblasts) are located at the periphery (Figure 1) (16, 17). Hemogenic endothelium, identified as a side population using FACS analysis, provides a unique source of precursors from which multipotent hematopoietic progenitors arise (18, 19). To meet the demands of the growing embryo, blood islands coalesce to form the primitive capillary plexus (E8.5), which subsequently undergoes rapid growth and remodeling by means of angiogenesis (14, 20). Proangiogenic signaling induces sprouting from the preexisting vasculature, vascular fusion, and intussusception, during which vascular lumen are further divided, resulting in the formation of vascular branching points. In the developing embryo, blood flow without vascular leak is seen as early as E11.5, whereas anastomoses of microvascular arteries and veins are first observed during the pseudoglandular stage of lung development at E13.5 (16, 21, 22). Extensive vascular remodeling occurs once blood flow is established and continues through the saccular and alveolar stages of development and the emergence of the complex lymphatic network (23, 24). Endothelial cells of blood and lymphatic vessels share the expression of CD31 (PECAM-1) but lack expression of hematopoietic marker CD45. Gene expression signatures and specific cell surface markers of fully differentiated arterial, venous, and capillary endothelial cells have been identified (reviewed in [12, 25]), yet there is a lack of consensus regarding gene expression signatures and specific markers of pulmonary EPC subsets, keeping us from fully understanding the process of endopoiesis. It is likely that endopoiesis represents a complex hierarchy of cell types, with restricted commitment to peripheral and proximal endothelial lineages, a concept analogous to that of the hematopoietic tree. As angioblasts move down the path of differentiation, their fate likely becomes restricted to either multipotent EPCs residing predominantly in the peripheral pulmonary microcirculation or proximal endothelial colony-forming cells residing in endothelium of large blood vessels (Figure 2). EPCs in the peripheral microvasculature express APLNR (apelin receptor), c-KIT, and FOXF1 and are capable of self-renewal and further differentiation into capillary, arterial, and venous but not lymphatic endothelial cells (26). Conversely, proximal endothelial progenitors express SCA1 and PROCR together with common endothelial markers and serve to replenish the arterial and venous endothelial cell lineages (Figure 2). A recent publication by Schupp and colleagues compiled a cellular transcriptomic atlas using more than 15,000 human lung endothelial cells from 73 subjects. Their analysis revealed two populations of previously indistinguishable venous cells, which they termed pulmonary–venous ECs and systemic–venous ECs. Pulmonary–venous ECs localize with the lung parenchyma, whereas systemic–venous ECs localize in airways and the visceral pleura. The functions of these cells and their relation to other pulmonary cells remains to be characterized (27).
Figure 1.
Development of embryonic vasculature begins in blood islands. Blood islands contain angioblasts at the periphery. Angioblasts differentiate to endothelial cells surrounding the primitive hematopoietic stem/progenitors. Hemogenic endothelium serves as an additional source of vascular cells, which differentiate into the hematopoietic lineage. To meet the needs of the growing embryo, blood islands coalesce to form the primitive vascular plexus, which undergoes rapid remodeling to establish a mature vascular network.
Figure 2.
The hierarchy of endopoiesis begins with mesoderm-derived hemangioblasts. FLK1+ hemangioblasts serve as a branching point separating endothelial and hematopoietic cell lineages. Angioblasts are the first primitive cells committed to the endothelial lineage. Angioblasts serve as initial progenitors of committed peripheral and proximal endothelial progenitors. Multipotent endothelial progenitor cells (EPCs) (c-KIT+APLNR+FOXF1+) differentiate into capillary cell types, as well as arterial and venous endothelial cells. Conversely, endothelial colony-forming cells (ECFCs) (SCA1+PRORC+) are more restricted progenitors, producing only arterial and venous cell types. Lymphatic endothelial cells (PROX1+PDPN+LYVE1+) differentiate directly from venous cells.
Signaling Pathways Regulating Vascular Development in the Embryonic Lung
At approximately E9.5 in the mouse and 3 weeks after conception in the human, a complex signaling network initiates the process of lung morphogenesis. Anterior–ventral foregut endoderm progenitors become specified to pulmonary epithelium, marked by the expression of transcription factor NK2 homeobox 1 (NKX2-1, thyroid transcription factor 1 aka TTF-1) (12). Nkx2-1−/− mice die at birth because of respiratory insufficiency and lack of peripheral pulmonary structures (28–31). Other critical signaling pathways including wingless-type mouse mammary tumor virus integration site (WNT), BMPs (bone morphogenetic proteins), retinoic acid (RA), FGFs (fibroblast growth factors), and SHH (sonic hedgehog) have been implicated in lung development and were reviewed by Whitsett and colleagues (12) and others (12, 20, 32–34). Herein, we will focus attention to signaling pathways critical for pulmonary microvascular development.
Vascular endothelial growth factor-A
Formation of large pulmonary vessels in mouse models of lung agenesis demonstrate that the initial formation of the large pulmonary vessels occurs without signaling from the endoderm (35). Endoderm-derived respiratory epithelium produces VEGF-A (vascular endothelial growth factor-A), which is critical for angiogenesis and vasculogenesis in the embryonic lung. VEGF-A is a paracrine growth factor and member of the VEGF family consisting of VEGF-A, -B, -C, -D, and PGF (placenta growth factor). VEGF-A binds and activates tyrosine kinase receptors FLT1 (VEGF receptor type I, VEGFR1) and FLK1 (VEGF receptor type II, VEGFR2, KDR [human]), both of which are present in endothelial cells, to stimulate growth and maturation of pulmonary vasculature. In mice, heterozygous loss of Vegfa impairs development of the pulmonary vasculature, whereas homozygous deletion of Vegfa causes severe vascular abnormalities resulting in embryonic lethality at mid-gestation (36, 37). Inhibition of Vegfa after birth causes the loss of pulmonary microvasculature and alveolar simplification. Vegfa overexpression stimulates lung vascularization but disrupts lung function, demonstrating that precise regulation of VEGF-A is required for embryonic development of pulmonary vasculature (38, 39). Deletion of Flt1 or Flk1 blocks formation of primitive blood islands and mature blood vessels, resulting in embryonic lethality (40, 41).
SHH and FOXF1
SHH produced by endodermally derived epithelium is critical for pulmonary branching morphogenesis, mesenchymal proliferation, and vascular development in the growing embryo. Shh−/− mice form hypoplastic single-lobe lungs, fail to achieve proper separation between the esophagus and trachea, and lack branching of the distal airways (42, 43). SHH is required for normal expression of critical regulators of lung morphogenesis, including FGF10, BMP4, and WNTs (12, 30, 43–45). SHH transcriptionally activates GLI transcription factors in the pulmonary mesenchyme, in turn regulating expression of FOXF1, which is expressed in mesenchymal cells, pulmonary tissue–resident EPCs, and differentiated capillary endothelial cells (46–50). FOXF1 is a transcription factor from the FOX (Forkhead Box) family, members of which play important roles in cellular proliferation, angiogenesis, and tissue repair (51–57). FOXF1 gene is evolutionarily conserved (58) and is critical for embryonic development, carcinogenesis, and lung repair and regeneration (59–65). Foxf1−/− mice die in utero because of the lack of vascular development of the yolk sac and allantois (45). Heterozygous deletion of Foxf1 in mice increases mortality after birth, causing lung hypoplasia, aberrant inflammation, and impaired formation of the pulmonary microvasculature (66, 67). Genomic deletions and point mutations in the human FOXF1 gene cause ACDMPV, a severe and usually fatal lung disorder of neonates and infants (7, 68). FOXF1 cooperates with other transcription factors such as STAT3 and FOXM1 to stimulate organ morphogenesis (50, 69–71) and cell cycle progression (63, 72–74). Nanoparticle delivery of proangiogenic transcription factors, including FOXF1, STAT3, and FOXM1, stimulates neonatal lung angiogenesis in mouse models of ACDMPV and BPD (64, 68, 75, 76).
Angiopoietin 1 and ephrins
Multiple paracrine and juxtacrine interactions among epithelial, mesenchymal, and vascular progenitors direct the development of pulmonary vasculature. While embryonic respiratory epithelial cells produce VEGF-A, pericytes also contribute to angiogenesis by secreting ANG1 (angiopoietin 1), which binds to the TIE-2 receptor (CD202b, TEK), on the surface of endothelial cells (77). During embryogenesis, ANG1 promotes vascular growth and stimulates maturation of newly formed blood vessels. In the adult lung, ANG1 maintains quiescence of the mature vasculature (78). Tie2−/− and Ang1−/− mice display a simplified vascular network with reduced branching and fewer endothelial cells (79, 80). Pericytes also influence endothelial permeability and regulate blood flow via secretion of Ephs (ephrins). Deletion of EphrinB2 in mice disrupts angiogenesis and alveolarization, resulting in a simplified lung (81–83).
Hematopoietic EPCs
Myeloid Angiogenic Cells
To identify endothelial progenitor cells, Asahara and colleagues used temporal and spatial information regarding formation of blood islands in which angioblasts were identified at the periphery and hematopoietic progenitors at the center of the islands (11). The spatial relationships among these cells and the overlap in expression of specific cell surface antigens suggests the concept that these two cell types originated from a common precursor. Magnetic bead sorting was used to isolate CD34+ (human) or FLK1+ (murine) mononuclear cell (MNC) populations from peripheral blood (11). In vitro, CD34+ cells adhered to fibronectin-coated dishes and developed a spindle shape. Coculture of CD34+ and CD34− cells resulted in the formation of blood island–like clusters consisting of round cells at the center and sprouting, spindle-shaped cells at the periphery. CD34+ cells at the periphery uptake acLDL (Dil-labeled acetylated low-density lipoprotein), whereas cells located centrally failed to do so and subsequently detached from the fibronectin coating. The murine FLK1+ cell fraction behaved in a similar manner. Attached CD34+ (AT-CD34+) cells gradually lost expression of the hematopoietic marker CD45, expressed low amounts of monocyte–macrophage marker CD68, and increased amounts of endothelial markers, CD31, TIE-2, FLK1, E-selectin, and eNOS (endothelial nitric oxide synthase). The authors concluded that AT-CD34+ cells had differentiated into endothelial-like cells in culture. In vivo studies showed that these CD34+ and FLK1+ cells engrafted into sites of injury in mouse and rabbit models of hindlimb ischemia, where they integrated into capillary vessel walls and colocalized with CD31 and TIE-2. (11). These findings were the first to describe CD34+ (human) and FLK1+ (murine) cells as circulating adult EPCs, supporting the concept of postnatal vasculogenesis.
Tissue integration of circulating EPCs was further supported by experiments with embryoid bodies, chicken embryos, and canines (84–86). Angiostatin significantly inhibited EPC growth in vitro, whereas angiostatin had no effect on mature endothelial cells (87). Hill and colleagues measured the number of EPC colony-forming units (CFU-Hill) in the peripheral blood of men with varying degrees of cardiovascular risk but without prior history of vascular disease and demonstrated a correlation between the number of circulating EPCs and the Framingham risk factor score. EPCs from individuals with a high risk for cardiovascular events exhibited increased senescence in vitro, compared with low-risk individuals, suggesting that EPCs play a role in vascular homeostasis (88). These studies relied on either the colony-forming capacity or “blood island–like” morphology of EPCs in vitro, despite using distinct methods to achieve colony growth on fibronectin. Low proliferation rates and expression of monocyte and macrophage markers in EPCs derived in vitro led Rehman and colleagues to suggest that circulating angiogenic cell is an appropriate name for these EPC-like cell populations (89). Prolonged culture of these cells resulted in the formation of colonies that resembled “islands” in which hematopoietic cells at the center contained both myeloid and lymphoid cell lineages. Cells at the periphery were macrophage-like, which expressed cell surface markers shared with endothelial cells (90, 91). These findings demonstrated that the previously identified clusters were not blood islands containing “putative EPCs” but rather various cells of hematopoietic origin that gained endothelial-like phenotypes in culture. Through the release of proangiogenic growth factors, such as VEGF, these cells were capable of promoting angiogenesis in animal models of ischemia and served to predict cardiovascular outcomes in patients.
Nonhematopoietic EPCs
Circulating Endothelial Colony-Forming Cells
Although endothelial cells found in peripheral blood were initially considered mature cells that had sloughed from the vascular wall and entered circulation (92), Lin and colleagues identified a distinct EPC population that they termed endothelial outgrowth cells, identified after sex-mismatched bone marrow transplantation (93). The authors observed that peripheral blood samples of transplant recipients contained a predominant population of circulating endothelial cells that matched the genotype of the recipient whereas only 5% matched the genotype of the donor, indicating that the latter population of cells originated from the donor. Endothelial cells derived from both recipient and donor genotypes were produced in vitro. Cells from the recipient expanded ∼17-fold in vitro, whereas cells from the donor, the endothelial outgrowth cells, expanded >1,000-fold. Endothelial outgrowth cells exhibited a cobblestone morphology, incorporated acLDL, and expressed endothelial markers KDR, vWF (von Willebrand factor), VE-cadherin, CD31, and CD34 and uniformly lacked CD14, distinguishing them from monocytes and macrophages. Taken together, the ability of circulating endothelial progenitors to undergo clonal expansion was matched with cell surface markers to identify EPC populations. A multidimensional approach to EPC identification, including cell surface markers as well as clonogenic and proliferative potentials in vitro, was used to establish the hierarchy of EPCs in adult peripheral and umbilical cord blood (UCB), analogous to the well-established hematopoietic tree (94). MNCs from adult peripheral blood and from UCB of full-term infants formed endothelial cell colonies in vitro; however, the colonies formed from UCB were larger and emerged earlier than those that arose from adult peripheral blood (95). Both adult and UCB endothelial cell colonies expressed endothelial markers CD31, CD141 (thrombomodulin, TM), CD105 (END [endoglin]), CD146 (MCAM [melanoma cell adhesion molecule]), VE-cadherin, vWF, and KDR but lacked expression of hematopoietic lineage markers CD14 and CD45. Serial passaging of endothelial cell colonies revealed that UCB-derived cells could undergo at least 100 cell doublings, whereas proliferation of adult-derived endothelial cell colonies ended as cells underwent senescence after 20–30 cell doublings. The majority of adult-derived colonies were small, consisting of 2–50 cells per cluster, together with the formation of few colonies of >500 cells. In contrast, a majority of UCB-derived colonies contained 2,000–10,000 cells per colony that had high telomerase activity and formed perfused blood vessels in vivo (95). Based on these studies, endothelial cell colonies were subdivided into high proliferative potential (HPP) and low proliferative potential (LPP) endothelial progenitors. HPP endothelial progenitor cells are now more commonly referred to as endothelial colony-forming cells (ECFCs).
Consensus on Nomenclature of Endothelial Progenitors
Since the first study describing EPCs in peripheral blood was published in 1997 (11), the ambiguity in nomenclature, origin, and function of EPC-like populations complicated the field. While recognizing the translational potential of EPCs, preparations and culturing methods produced distinct cell types capable of promoting angiogenesis by a diversity of mechanisms. A published consensus statement suggested that EPCs isolated in culture would be referred to as either myeloid angiogenic cells (MACs) or ECFCs (96). Early-outgrowth, proangiogenic cells of hematopoietic origin were termed MACs, whereas nonhematopoietic, late-outgrowth cells able to contribute to vascular repair and de novo vascular formation were termed ECFCs.
Resident Pulmonary EPCs
Adult endothelial cells typically reside in a state of quiescence with a low rate of proliferation (97); however, several groups identified variability in the rate of endothelial cell turnover. Another study demonstrated that endothelial tissues are composed of a heterogeneous pool of cells with varying proliferative potentials (98). The pulmonary vascular system can be subdivided into proximal and distal regions. The proximal region consists of veins and arteries, whereas the distal or gas exchange region is composed of microvascular (capillary) networks (12). Repair of the microvasculature is dependent on proliferation of mature capillary endothelial cells as well as resident tissue EPCs. Circulating MACs contribute to lung repair by promoting angiogenesis and providing signals that enhance proliferation of pulmonary parenchymal cells via release of paracrine factors, such as VEGF. MACs engraft poorly and do not contribute substantially to angiogenesis in a cell-autonomous manner (99). In contrast, pulmonary-resident EPC populations with both HPP and vasculogenic competence have been identified (100). Single-cell clonogenic assays, comparing rat pulmonary artery endothelial cells and pulmonary microvascular endothelial cells, demonstrated that the majority of pulmonary microvascular endothelial cells (75%) divided in culture and (50%) gave rise to large colonies (>2,000 cells/colony). Conversely, the majority of pulmonary artery endothelial cells (60%) were already fully differentiated (101). Taken together, Alvarez and colleagues reasoned that because of the large surface area of the microvascular bed and its enhanced growth potential, pulmonary microvascular endothelial cells are enriched with progenitors. “Side” population cells identified by Hoechst dye efflux (102, 103) are heterogeneous and highly enriched for cells with progenitor properties, making up 0.03–0.07% of adult pulmonary cells (102). EPCs capable of differentiating into both hematopoietic and lymphatic cell lineages, and able to establish the hierarchy of endovascular progenitor, transit-amplifying, and differentiated cells, have been isolated from lung tissue (104, 105). EPC subsets were identified using antibodies against CD157 and protein receptor C (106, 107). The following sections will describe the most recently identified pulmonary-resident EPC populations.
Tissue-Resident ECFCs
To determine the source of circulating ECFCs, Ingram and colleagues (100) compared vessel wall–derived human umbilical vein endothelial cells, human aortic endothelial cells, and UCB-derived ECFCs. Human umbilical vein endothelial cells and human aortic endothelial cells could be passaged in culture for at least 40 population doublings and shared cell surface markers CD31, CD141, CD105, CD146, VE-cadherin, vWF, and VEGFR2 (KDR) but not hematopoietic markers CD45 and CD14. In slight disagreement with the findings described by Alvarez and colleagues, Ingram and colleagues demonstrated that subsets of human umbilical vein endothelial cells and human aortic endothelial cells behaved similarly to UCB-derived ECFCs in clonogenic assays. All three cell populations formed small and large colonies, similar to those formed by HPP and LPP ECFCs (95). Exposure to hyperoxia in vitro or after isolation of lung ECFCs from hyperoxia-exposed rats impaired proliferation, decreased clonogenic capacity, and resulted in fewer capillary-like networks in vitro. Intrajugular administration of ECFCs isolated from human cord blood into hyperoxia-exposed neonatal rats attenuated pulmonary hypertension and restored colony-forming and capillary network–forming capabilities (108). Protective effects of ECFCs despite low engraftment demonstrate that ECFCs likely function through paracrine mechanisms. Taken together, these findings demonstrate that the intima of large pulmonary blood vessel walls is a source of both HPP and LPP ECFCs and that these cells likely act through paracrine mechanisms to improve pulmonary function after injury.
c-KIT+ Tissue-Resident EPCs
A number of cell selective markers distinguish one tissue-resident EPC population from another, including the c-KIT receptor (also known as CD117). C-KIT is a receptor tyrosine kinase that acts as a receptor for SCF (stem cell factor) and plays a critical role in hematopoiesis. C-KIT promotes endothelial cell proliferation and survival (47, 109, 110). Lineage tracing studies showed that c-KIT+ cells differentiate into endothelial but not epithelial cells (109). C-KIT+ EPCs were detected in the alveolar region of neonatal mouse and human tissues (47, 110). C-KIT+ EPCs expressed common endothelial markers CD31, CD102 (ICAM-2), and EMCN but lacked expression of pericyte (CD140b), fibroblast (CD140a), epithelial (CD326), and hematopoietic (CD45) cell markers (26, 47). Analysis of single-cell RNA sequencing (scRNA-seq) data from mouse and human newborn lungs identified a unique and conserved gene signature of c-KIT+ ECs that was enriched in expression of FOXF1 and its transcriptional target genes, including TIE-2, VE-cadherin, and VEGFR2 (47). Endothelial-specific deletion of Foxf1 or the inactivation of Kit in mice decreased the number of c-KIT+ EPCs in the lung and impaired postnatal lung angiogenesis and alveolarization (47). Conversely, adoptive transfer of c-KIT+ (but not c-KIT−) endothelial cells into hyperoxia-exposed neonatal mice resulted in engraftment of c-KIT+ cells into the alveolar microvasculature, and their proliferation and expansion after injury increased capillary density and reduced alveolar simplification (47). Interestingly, a recent study (26) demonstrated that the c-KIT+ EPC cell subset is also heterogeneous, consisting of FOXF1+c-KIT+ and FOXF1−c-KIT+ EPCs. Only FOXF1+c-KIT+ EPCs were capable of engraftment into the neonatal lung to stimulate angiogenesis and improve alveolarization in a mouse model of ACDMPV (26). C-KIT and FOXF1 identify a subset of pulmonary EPCs that are highly sensitive to hyperoxia exposure and are capable of engrafting and enhancing regeneration of alveolar tissue in mouse models of ACDMPV. Importantly, these data provide proof of principle that adoptive transfer of pulmonary FOXF1+c-KIT+ EPCs into the neonatal circulation can stimulate lung angiogenesis and prevent alveolar simplification.
Car4-High Tissue-Resident EPCs
Recently, Niethamer and colleagues described a new population of microvascular endothelial cells expressing high amounts of Carbonic Anhydrase 4 (Car4-high ECs) (111). Ellis and colleagues found that ∼15% of pulmonary endothelial cells express Car4 (112). These Car4-high ECs are critical for lung repair after injury and are strategically located in regeneration zones surrounding sites of alveolar damage caused by influenza or bleomycin. Importantly, Car4-high ECs are primed to respond to VEGF-A signaling from neighboring alveolar type 1 (AT1) cells to stimulate lung repair. During lung development, Car4+ ECs form close spatial relationships with AT1 cells and are separated from them by a thin basement membrane, without intervening pericytes. Formation and maintenance of Car4+ ECs required AT1-derived VEGF-A signaling (111, 112).
General Capillary Cells
Advances in scRNA-seq, lineage tracing, and confocal microscopy have made it possible to systematically identify and visualize heterogeneous cell populations. Gillich and colleagues demonstrated that alveolar capillaries consist of a mosaic comprising two intermingled cell types, which they termed general capillary cells (gCaps) and aerocytes (aCaps) (113). Lineage tracing demonstrated that these cells arise from common bipotent progenitors that differentiate to form the capillary network. The features and functions of gCap and aCap cells are strikingly distinct. Aerocytes appear during embryonic development at E17.5 and mature into large cells with extensions and pores spanning multiple alveoli. Aerocytes are located in thin regions of the alveolar walls and their elongated morphology is analogous to that of AT1 cells, supporting their specialized role in gas exchange. Conversely, gCaps are smaller, contain fewer pores, and rarely span multiple alveoli, where they are found in close association with stromal cells. Gene expression profiling predicted both their cooperative and distinct functions. Aerocytes express high amounts of Car4 and are likely involved in leukocyte trafficking. gCaps express c-KIT and MHC class II–associated genes and may be involved in antigen presentation. Based on scRNA-seq data, both cell types are predicted to signal to one another. Aerocytes produce ligands such as apelin (APLN) and SCF (also known as KIT ligand) recognized by the APLN and c-KIT receptors present on gCaps. Conversely, gCaps produce endothelin 1 (EDN1) and VEGF-A, which may activate EDNRB (endothelin B) and FLK1 receptors on aCaps. In a mouse model of emphysema, lineage-labeling studies demonstrated that gCap but not aCap cells proliferated after injury and served as progenitors of both gCap and aCap cells during alveolar repair.
EPCs and the Tree of Endopoiesis
The diversity of EPC populations with overlapping cell surface markers and gene expression profiles makes comparing these EPC populations a nontrivial task. Similarities in phenotypic and functional characteristics support the close relationships among these cells, which may represent cell states rather than distinct cell types. It is likely that proximal and distal pulmonary vasculature contain unique, heterogeneous populations of tissue-resident EPCs. We can begin to draw comparisons and hypothesize how these cell types fit together to establish a hierarchy of endopoiesis (Figure 2). Consistent with their expression of Sca1, it is likely that the ECFC signature overlaps with side population cells, residing in arterial and venous endothelium of large pulmonary vessels. ECFCs also express protein receptor C, suggesting overlap with the EPC population identified by Yu and colleagues (107). Car4+ cells and aCaps are located in close apposition to AT1 cells and express EDNRB and CAR4. gCaps and c-KIT+ EPCs express c-KIT and may represent the same population of lung-resident EPCs. Differences in cell markers may be related to technical differences in their isolation or to differences in cell “states” rather than distinct cell types. Further investigation of EPC heterogeneity in the lung, both at homeostasis and during vascular repair after injury, will advance our understanding of pulmonary endopoiesis and aid in identifying cells useful for treatment of pulmonary diseases.
EPCs: Bench to Bedside
Advances in cell biology are providing new insight into fundamental mechanisms underlying organogenesis, tissue repair, and oncogenesis. Mouse ESCs, human ESCs, mesenchymal stem cells (MSCs), and iPSCs provide useful tools for exploring the stages of vascular development and endothelial cell differentiation. Stem cells hold great promise for future cell-based therapies because they can be used to generate large quantities of specific cell types in vitro. Transplantation of MSCs (114), MSC conditioned media (115), and MSC-derived exosomes (116) have been extensively studied in both animal models of lung disease and in human clinical trials (reviewed in (117, 118)). After transplantation, MSCs are believed to serve primarily as sources of vasculogenic growth factors, supplying VEGF, FGF2, ANG1, and EGF (epidermal growth factor) to neighboring cells. MSCs alone have limited engraftment potential, whereas cotransplantation of MSCs with ECFCs increases vascular engraftment (117, 119). Risks of MSC transplantation include graft versus host disease and malignancy, indicating the need for further optimizations of MSC therapies for clinical use (120).
Directed Differentiation of ESCs and iPSCs into EPCs
Since the discovery of distinct EPC populations, considerable work has focused on development of protocols enabling directed differentiation of mouse and human stem cells to produce engraftable endothelial cells (Table 1). Early protocols seeking to develop EPCs relied either on the three-dimensional generation of embryoid bodies or on two-dimensional monolayer cell culture systems to isolate hemangioblasts, known to give rise to both hematopoietic and endothelial cell progenitors (121, 122). ESC-derived bipotent cells producing endothelial and mural cell lineages have also been described (123). Recently, differentiation protocols have evolved to a sophisticated multiphase system allowing for stepwise differentiation from ESCs/iPSCs to mesodermal precursors, hemangioblasts, angioblasts, and, finally, endothelial progenitors, which can be maintained or differentiated into mature endothelial cells (Figure 3A). In 2014, Prasain and colleagues published a robust protocol using a two-dimensional monolayer culture system of ESCs or iPSCs to generate large quantities of stable cord blood ECFCs (Figure 3B) (124). Cord blood ECFCs were expanded and maintained for up to 18 passages without loss of endothelial surface markers (124). ESC/iPSC-derived ECFCs are highly proliferative, form capillary-like structures in Matrigel, and contribute to vascular repair in both ischemic limbs and hyperoxia-injured retinas (124). Lian and colleagues demonstrated that canonical WNT/β-catenin signaling is required for generation of CD34+CD31+ EPCs from iPSCs (125). Directed EPC differentiation was achieved by activation of canonical WNT signaling using GSK3 inhibition (CHIR99021), in the absence of exogenous growth factors VEGF and FGF2 (Figure 3C). Functional VEGFR2+CD34+CD31+VE-cadherin+ EPCs were produced using laminins and chemically defined xeno-free conditions without Matrigel (Figure 4A) (126). Endothelial progenitor cells with high proliferative potential that express the progenitor cell marker CD157 or classical endothelial markers, CD31, VE-cadherin, CD34, KDR, and CXCR4, were produced from both ESCs and iPSCs (Figure 4B). Although questions remain regarding how ESC/iPSC-derived endothelial progenitors phenocopy their endogenous counterparts, ESCs and iPSCs share the potential to produce large quantities of EPCs required for cell-based therapies for pulmonary diseases.
Table 1.
Summary of Differentiation Protocols for the Generation of Various EPC Populations
| Starting Cell Type | Cell Type Generated | Protocol Summary | Key Contribution | Citation |
|---|---|---|---|---|
| mESC | FLK1+/E-cadherin− | Generation and isolation of FLK1+ cells after 4 d in culture Addition of VEGF165 to sorted cells results in sheets of CD31+ ECs |
mESC-derived Flk1+ cells give rise to endothelial and mural cell types VEGF165 promotes development of the endothelial lineage |
Yamashita et al., 2000 (123) |
| hESC Embryoid Body |
CD31+/KDR+/VE-Cadherin+/CD45− | Generation of EBs Treatment with SCF, Flt3L, IL-3, IL-6, G-CSF, and BMP4 FACS sorted CD45+ and CD45− populations Culture in EC media |
Role of cytokines and BMP4 in promoting hematopoietic differentiation Identification of CD45− bipotent cells with hematopoietic and endothelial capacity |
Chadwick et al., 2003 (121) |
| hESC Embryoid body |
SCL+/LMO2+/FLT+/CD31−/CD34−/KDR− | Generation of early-stage EBs with factors such as BMP4, VEGF, SCF, and Tpo Dissociation of EBs and plating single cells in semisolid blast colony growth medium |
Large-scale generation of hemangioblast cells under serum-free culture conditions | Lu et al., 2007 (122) |
| hESCs Embryoid body |
CD144+/KDR+/CD31− | Generation of EBs using the established protocol by Chadwick and colleagues (2003) with varying concentrations of BMP4 | BMP4 accelerates commitment of hESCs to the endothelial cell lineage while inhibiting commitment toward hematopoiesis | Goldman et al., 2009 (144) |
| hESC/iPSC Embryoid body + Monolayer |
CD31+/CD146+/KDR+/CD133+/CD34+ | Generation of EBs Plating of EBs on fibronectin-coated dishes in EGM-2 media supplemented with VEGF Expansion of vascular progenitor fraction in EGM-2 media |
Three-dimensional and two-dimensional culture system for the simultaneous generation of hematopoietic and vascular progenitors Use of minimal factors (VEGF, BMP4, FGF2) |
Park et al., 2013 (145) |
| iPSCs Embryoid body |
CD31+/VE-cadherin+/KDR+/eNOS+ | VE-cadherin+ cells are isolated from EBs and seeded on fibronectin-coated dishes | Characterization of humoral-, pharmacological-, and biomechanical-induced functional phenotypes of iPSC-derived ECs | Adams et al., 2013 (146) |
| iPSC Monolayer |
CD34+/CD31+ | Adherent culture system with small molecule GSK3 inhibition in the absence of exogenous growth factors | Temporal activation of WNT signaling, using small molecule CHIR, is sufficient to generate EPCs in the absence of factors VEGF and FGF2 | Lian et al., 2014 (125) |
| hESC/iPSC Monolayer |
NRP-1+/CD31+/CD144+/KDR+ (CB-ECFCs) | Adherent culture system with Activin A, BMP4, VEGF, and FGF2 in serum-free media | Two-dimensional system that does not require EB development, feeder cells, or TGF-β inhibition Protocol generates HPP-ECFCs with robust vessel-forming ability in vivo |
Prasain et al., 2014 (124) |
| hESC Embryoid body + Monolayer |
VE-cadherin+/CD31+/CD34+/CD14−/KDRhigh | Three-phase protocol including both suspension and adherent culture method | Controlled modulation of BMP, Wnt, VEGF, and Notch pathways results in rapid production of EPCs by Day 6 and mature ECs by Day 14 | Sahara et al., 2014 (147) |
| iPSC Monolayer |
CD144+/KDR+/CD31+/CD34+/CD105+ | Six-day differentiation protocol involving differentiation to mesodermal lineage with either CP21 or CHIR and/or BMP4 with subsequent exposure to VEGF-A to induce EC development | Efficient and quick production of endothelial cells with efficiencies between 61.8% and 88.8% | Patsch et al., 2015 (148) |
| hESC Monolayer |
KDR+/CD34+/CD31+/VE-cadherin+ | hESCs are cultured on stem cell niche LN matrices (LN521) This protocol involves the use of factors such as Activin A, BMP4, VEGF, and bFGF |
A chemically defined, xeno-free protocol using LN coating to generate roughly 95% functional EPCs | Nguyen et al., 2016 (126) |
| hESC/iPSC Monolayer |
CD157+/CD31+/CD144+/CD34+/KDR+/CXCR4+ (HPP-ECFCs) |
Synergistic three-phase protocol using unique media compositions Generation of primitive streak → KDR+ mesoderm → primitive endothelium |
Highly efficient, fully defined protocol for generation of EPCs with minimal variability HPP-ECFC–like cells with expression of EPC marker CD157 in addition to other standard EPC surface markers |
Farkas et al., 2020 (149) |
Definition of abbreviations: bFGF = basic fibroblast growth factor; BMP4 = bone morphogenetic protein 4; CB-ECFCs = cord blood endothelial colony-forming cells; EB = embryoid body; EGM-2 = endothelial growth medium-2; eNOS = endothelial nitric oxide synthase; EPCs = endothelial progenitor cells; FGF2 = fibroblast growth factor 2; hESC = human embryonic stem cell; HPP-ECFCs = high-proliferative-potential endothelial colony-forming cells; iPSC = induced pluripotent stem cell; LN = laminin; mESCs = murine embryonic stem cells; SCF = stem cell factor; TGF-β = transforming growth factor-β; VEGF = vascular endothelial growth factor.
Figure 3.
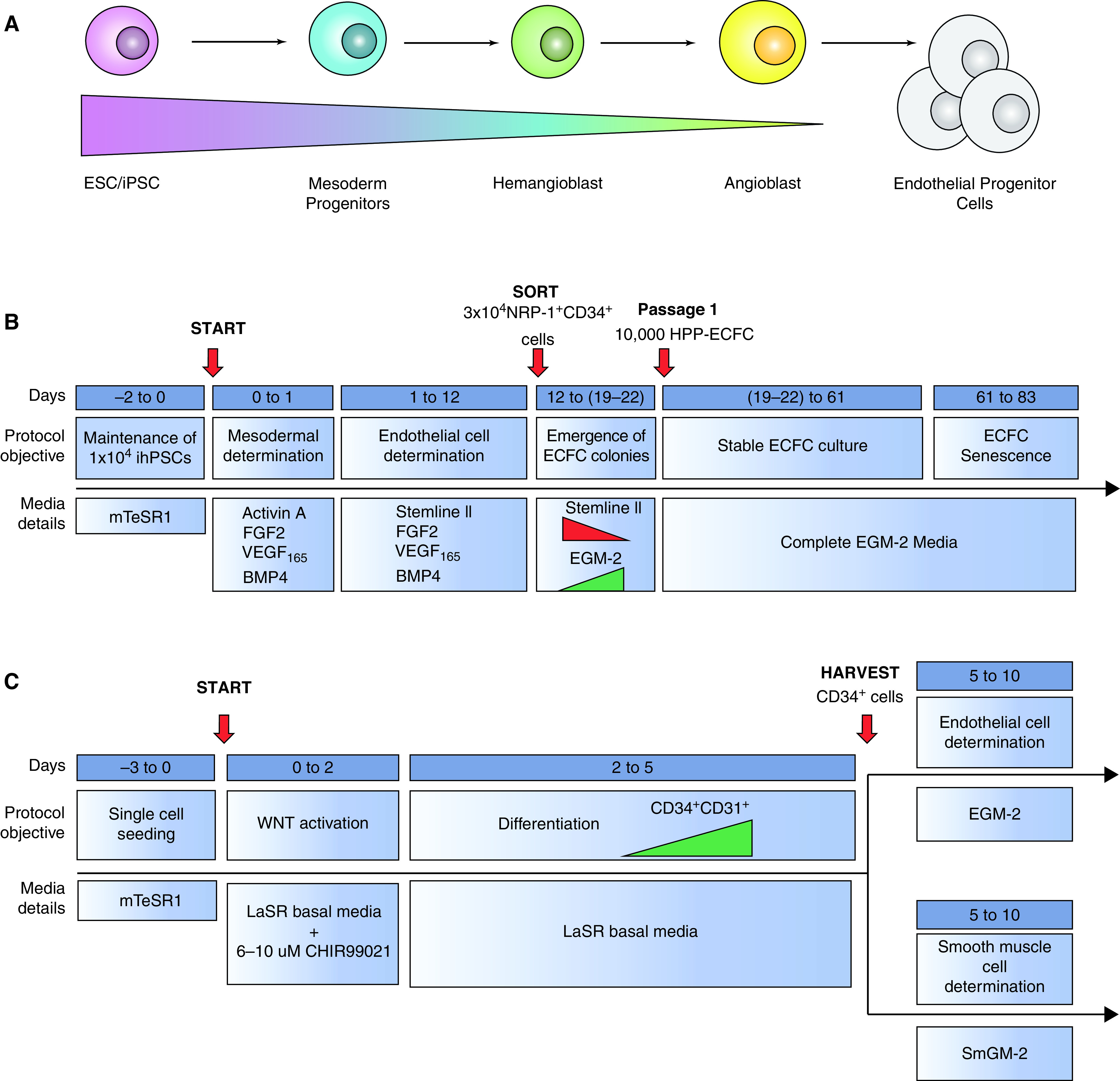
Directed differentiation protocols designed to produce EPCs. (A) Schematic of stepwise directed differentiation designed to generate EPCs from embryonic stem cells (ESCs)/induced pluripotent stem cells (iPSCs). (B) Directed differentiation of human iPSCs (hiPSCs) into cord blood ECFCs (Prasain and colleagues, 2014). (C) Differentiation of hiPSCs to CD34+CD31+ EPCs (Lian and colleagues, 2014). BMP = bone morphogenetic protein; EGM-2 = endothelial growth medium-2; FGF = fibroblast growth factor; HPP = high proliferative potential; VEGF = vascular endothelial growth factor.
Figure 4.
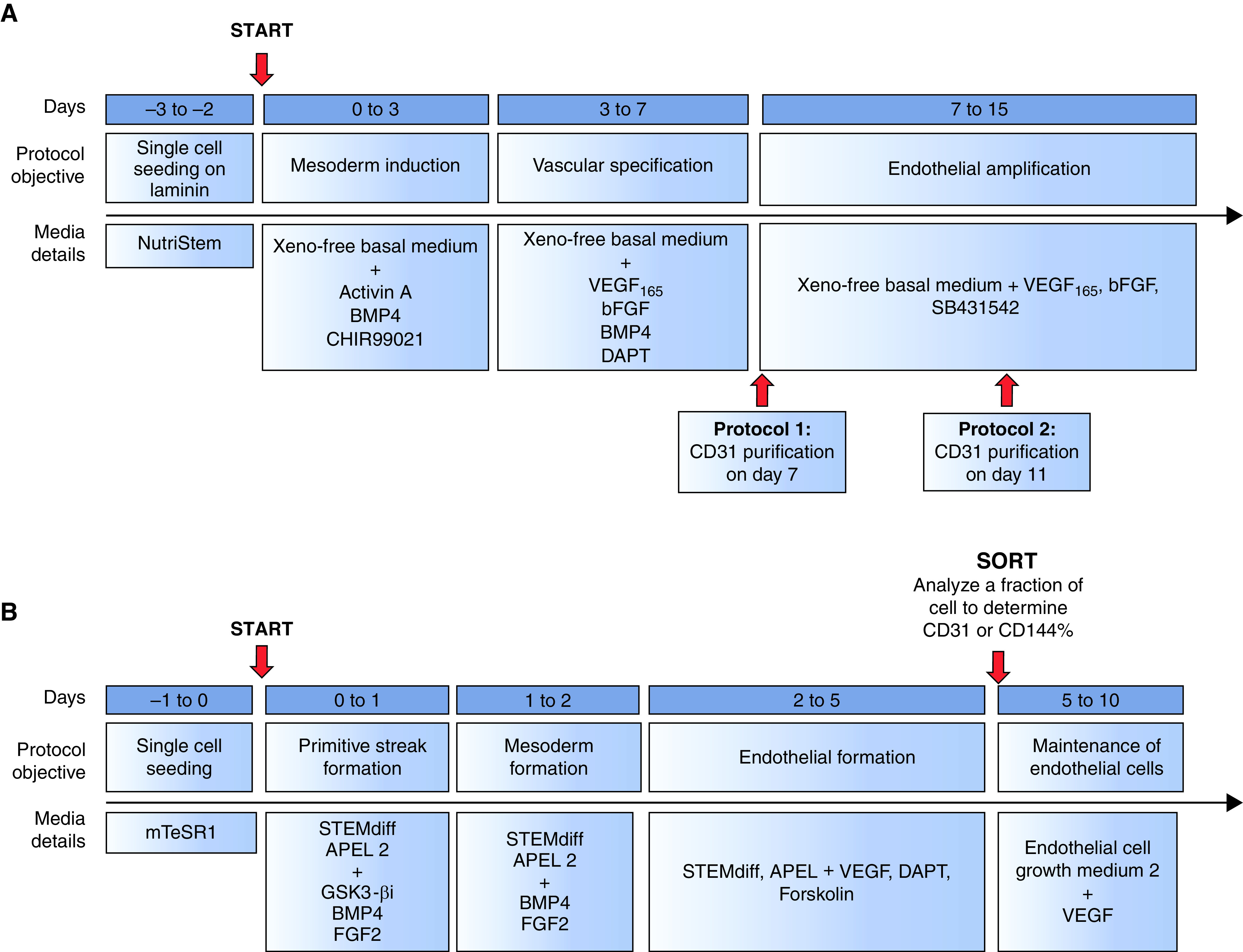
Additional directed differentiation protocols designed to produce EPCs. (A) Differentiation of human ESCs to EPCs in xeno-free culture conditions (126). (B) Differentiation of hiPSCs to CD157+ HPP-ECFCs (149).
EPCs in Regenerative Medicine
Discoveries such as surfactant, antiinflammatory drugs, and corticosteroids as well as gentle approaches to ventilation have made it possible to improve lung function and enhance survival of preterm infants with respiratory diseases. Nevertheless, debilitating pediatric lung diseases associated with decreased, dysfunctional, and damaged vasculature remain without effective treatments. In recent years, several approaches have been considered to improve the neonatal pulmonary vasculature. One such promising option is cell therapy with EPCs (99). Despite the controversies surrounding EPC classification, positive outcomes of EPC transplantation were reported in mice (26, 47, 127), rats (128, 129), rabbits (130), canines (131), and nonhuman primates (132–135). Studies in neonatal mice demonstrated that hyperoxia exposure reduced EPC numbers in the blood, bone marrow, and lung (136). Reduced numbers of EPCs were found in mouse models of ACDMPV (26, 47). Decreased numbers of EPCs were present in lungs from patients with BPD, pulmonary arterial hypertension (PAH), and chronic obstructive pulmonary disease (137–140). ECFC frequency in cord blood was reduced in infants with a gestational age <28 weeks compared with infants with a gestational age >28 weeks. Infants with reduced ECFC frequency subsequently developed BPD (4). Increased frequency of EPCs was associated with better survival after acute lung injury and reduced risks of developing BPD (4, 141). A search for “endothelial progenitor cells” in the National Institute of Health’s clinical trials database identified more than 180 completed trials and many more in “recruiting” and “active” stages; however, only 5 clinical trials are related to pulmonary disorders. Ongoing clinical trials explore the therapeutic potential of EPCs for cell therapies. Autologous bone marrow or peripheral blood samples are collected, manipulated or enriched ex vivo, and infused back into patients with vascular injury.
A prospective, randomized trial tested whether EPC transplantation together with conventional therapy improved clinical outcomes of patients with idiopathic PAH (142). Fifteen participants received a one-time intravenous infusion of EPCs (mean = 0.6 × 107 cells per infusion) in addition to conventional therapy and 16 participants received conventional therapy alone. Twelve weeks after treatment, the mean walking distance was increased in patients with PAH who received both the EPC infusion and conventional therapy compared with patients with PAH who received the conventional therapy alone. Autologous EPCs significantly improved pulmonary artery pressure, pulmonary vascular resistance, and overall cardiac output without severe adverse outcomes (142). A phase 1 clinical trial conducted by Granton and colleagues (143) used apheresis to isolate MNCs from peripheral blood of patients with severe PAH. Endothelial outgrowth cells were produced from MNCs from blood and transfected with a plasmid containing human eNOS cDNA and infused into patients with PAH. Participants were assigned to one of three escalating cell dose regimens consisting of 7, 25, and 50 million cells, respectively, administered over 3 consecutive days. Although no cell dose–effect relationship was reported, participants from all groups experienced short-term hemodynamic improvement. More clinical studies investigating cell dosing, efficacy, and timing of administration are critically required to establish a treatment that is both effective and best tolerated by patients. Several studies using EPCs in PAH, non-small cell lung cancer, and chronic obstructive pulmonary disease are listed on ClinicalTrials.gov; however, the results of these studies are not yet available. Autologous EPC transplantation has been used in clinical trials, testing its safety and efficacy for treatment of diseases affecting the respiratory system, heart, liver, and other organs (Table 2). EPC mobilization and EPC-coated stents provide additional uses for EPC delivery to targeted organs.
Table 2.
Completed Clinical Trials Involving Testing of EPCs as a Transplantation Cellular Therapeutic for Various Conditions, as Reported by ClinicalTrials.gov
| Disease | Identifier Number | Cell Type Used | Outcome | Citation |
|---|---|---|---|---|
| Pulmonary | ||||
| Idiopathic pulmonary arterial hypertension | NCT00641836 | Autologous transplantation of circulating EPCs | Preliminary study showed feasibility and safety. Might have beneficial effects |
Wang et al., 2007 (142) |
| Idiopathic pulmonary arterial hypertension | NCT00551408 | Not reported | No study results found | N/A |
| Pulmonary hypertension | NCT00469027 | Transplantation of autologous eNOS-overexpressing EPCs | Tolerated by patients Evidence of short-term improvement associated with long-term benefits in function and quality of life |
Granton et al., 2015 (143) |
| NSCLC COPD |
NCT00826683 | Identification of circulating EPCs in peripheral blood of patients with NCSLC compared with patients with COPD | No study results found | N/A |
| Hepatic | ||||
| Advanced liver cirrhosis | NCT01333228 | Bone marrow–derived EPCs expanded ex vivo vWF+/acLDL+ |
No adverse effects observed Higher levels of VEGFR2, vWF, and acLDL showed greater improvement in liver function |
D’Avola et al., 2016 (150) |
| Cardiac | ||||
| Coronary artery disease Refractory angina |
NCT00694642 | CliniMACS selection of autologous CD133+ cells | No study results found | N/A |
| Idiopathic dilated cardiomyopathy | NCT00629096 | Intracoronary infusion of autologous bone marrow–derived MNCs | No study results found | N/A |
| Other | ||||
| Critical limb ischemia | NCT01595776 | CliniMACS selection of autologous CD133+ cells | Six of eight (75%) patients experienced complete wound healing Two of eight (25%) patients experienced no benefit |
Arici et al., 2015 (151) |
| Leg ulcer/gangrene | NCT00221143 | CliniMACS selection of autologous CD34+ cells from mobilized donors | Improvement in Wong-Baker FACES pain rating, TBPI, transcutaneous partial oxygen pressure, total or pain-free walking distance, and ulcer size observed in all patients | Kawamoto et al., 2009 (152) |
| Lymphedema (after mastectomy) | NCT01112189 | Transplantation of autologous CD34+ cells (unsorted) from buffy coat samples | Significantly reduced volume of Lymphedema and improved associated symptoms | Maldonado et al., 2011 (153) |
Definition of abbreviations: acLDL = Dil-labeled acetylated low-density lipoprotein; COPD = chronic obstructive pulmonary disease; MNCs = mononuclear cells; N/A = not applicable; NSCLC = non-small cell lung cancer; TBPI = toe brachial pressure index; VEGFR2 = vascular endothelial growth factor receptor 2; vWF = von Willebrand factor.
To achieve postnatal alveolarization in patients with pulmonary diseases, EPCs must properly engraft into regions of damaged tissue and be able to form functional relationships with various endogenous cell types to ensure that the vascular system is rebuilt properly and efficiently. Coordination between the vascular and lymphatic systems must also be demonstrated in an effective EPC-based therapy. These crucial points need to be examined and addressed as more trials are conducted and completed in this field. Advances in EPC isolation, methods of production in vitro, and knowledge regarding mechanisms of vascular regeneration will enable autologous and iPSC-derived EPC therapeutics for pulmonary diseases in the future.
Conclusions and Future Directions
In just two decades, great strides have been made in identifying, evaluating, and understanding the role of EPCs in development and disease, as well as their potential for translational medicine. Many similarities and differences can be drawn from the vast amount of work describing EPC populations, which lead to the conclusion that the hierarchy of endothelial cell development (endopoiesis) is a complex network with similarities to hematopoiesis. Endothelial development involves heterogeneous populations of progenitor cells with various differentiation and proliferative potentials. Advances in technology, such as imaging, lineage tracing, and scRNA-seq, are enabling identification of a diverse population of EPCs in the lung. The ability to derive EPC populations from patient-specific iPSCs and to modify them ex vivo is the first of many steps on a path toward discovering patient-tailored therapies. EPCs derived from patient-specific iPSCs reduce the need for donor matching or the risk of adverse effects of lung vascular repair for our most fragile patient populations. Future discoveries enabling the identification of distinct EPC populations and understanding their specific roles in endopoiesis are needed. EPCs generated in vitro require extensive testing to ensure their stability and ability to regenerate vasculature without causing off-target effects. As we begin to operationalize EPCs in therapies, it will be important to establish source methods for their collection and propagation ex vivo to provide patient-specific cells that can be used throughout a patient’s life.
Footnotes
Supported by National Heart, Lung, and Blood Institute grants HL141174, HL149631, and HL152973 (V.V.K.).
Author Contributions: O.A.K., J.A.W, T.V.K., and V.V.K. wrote the review.
Originally Published in Press as DOI: 10.1165/rcmb.2021-0152TR on July 22, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Baker CD, Abman SH. Impaired pulmonary vascular development in bronchopulmonary dysplasia. Neonatology. 2015;107:344–351. doi: 10.1159/000381129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davidson LM, Berkelhamer SK. Bronchopulmonary dysplasia: chronic lung disease of infancy and long-term pulmonary outcomes. J Clin Med. 2017;6:4. doi: 10.3390/jcm6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alvira CM. Aberrant pulmonary vascular growth and remodeling in bronchopulmonary dysplasia. Front Med (Lausanne) 2016;3:21. doi: 10.3389/fmed.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghesi A, Massa M, Campanelli R, Bollani L, Tzialla C, Figar TA, et al. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2009;180:540–546. doi: 10.1164/rccm.200812-1949OC. [DOI] [PubMed] [Google Scholar]
- 5. Dharmadhikari AV, Szafranski P, Kalinichenko VV, Stankiewicz P. Genomic and epigenetic complexity of the FOXF1 locus in 16q24.1: implications for development and disease. Curr Genomics. 2015;16:107–116. doi: 10.2174/1389202916666150122223252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sen P, Dharmadhikari AV, Majewski T, Mohammad MA, Kalin TV, Zabielska J, et al. Comparative analyses of lung transcriptomes in patients with alveolar capillary dysplasia with misalignment of pulmonary veins and in foxf1 heterozygous knockout mice. PLoS One. 2014;9:e94390. doi: 10.1371/journal.pone.0094390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009;84:780–791. doi: 10.1016/j.ajhg.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar capillary dysplasia. Am J Respir Crit Care Med. 2011;184:172–179. doi: 10.1164/rccm.201010-1697CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Towe CT, White FV, Grady RM, Sweet SC, Eghtesady P, Wegner DJ, et al. Infants with atypical presentations of alveolar capillary dysplasia with misalignment of the pulmonary veins who underwent bilateral lung transplantation. J Pediatr. 2018;194:158–164.e1. doi: 10.1016/j.jpeds.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ameis D, Khoshgoo N, Keijzer R. Abnormal lung development in congenital diaphragmatic hernia. Semin Pediatr Surg. 2017;26:123–128. doi: 10.1053/j.sempedsurg.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 11. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 12. Whitsett JA, Kalin TV, Xu Y, Kalinichenko VV. Building and regenerating the lung cell by cell. Physiol Rev. 2019;99:513–554. doi: 10.1152/physrev.00001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vokes SA, Krieg PA. Endoderm is required for vascular endothelial tube formation, but not for angioblast specification. Development. 2002;129:775–785. doi: 10.1242/dev.129.3.775. [DOI] [PubMed] [Google Scholar]
- 14. Marcelo KL, Goldie LC, Hirschi KK. Regulation of endothelial cell differentiation and specification. Circ Res. 2013;112:1272–1287. doi: 10.1161/CIRCRESAHA.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–1679. [PubMed] [Google Scholar]
- 16. deMello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol. 1997;16:568–581. doi: 10.1165/ajrcmb.16.5.9160839. [DOI] [PubMed] [Google Scholar]
- 17. Dyer LA, Patterson C. Development of the endothelium: an emphasis on heterogeneity. Semin Thromb Hemost. 2010;36:227–235. doi: 10.1055/s-0030-1253446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldie LC, Lucitti JL, Dickinson ME, Hirschi KK. Cell signaling directing the formation and function of hemogenic endothelium during murine embryogenesis. Blood. 2008;112:3194–3204. doi: 10.1182/blood-2008-02-139055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gritz E, Hirschi KK. Specification and function of hemogenic endothelium during embryogenesis. Cell Mol Life Sci. 2016;73:1547–1567. doi: 10.1007/s00018-016-2134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schittny JC. Development of the lung. Cell Tissue Res. 2017;367:427–444. doi: 10.1007/s00441-016-2545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao Y, Cornfield DN, Stenmark KR, Thébaud B, Abman SH, Raj JU. Unique aspects of the developing lung circulation: structural development and regulation of vasomotor tone. Pulm Circ. 2016;6:407–425. doi: 10.1086/688890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwarz MA, Caldwell L, Cafasso D, Zheng H. Emerging pulmonary vasculature lacks fate specification. Am J Physiol Lung Cell Mol Physiol. 2009;296:L71–L81. doi: 10.1152/ajplung.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bolte C, Whitsett JA, Kalin TV, Kalinichenko VV. Transcription factors regulating embryonic development of pulmonary vasculature. Adv Anat Embryol Cell Biol. 2018;228:1–20. doi: 10.1007/978-3-319-68483-3_1. [DOI] [PubMed] [Google Scholar]
- 24. Bolte C, Kalin TV, Kalinichenko VV. Molecular, cellular, and bioengineering approaches to stimulate lung regeneration after injury. Semin Cell Dev Biol. 2020;100:101–108. doi: 10.1016/j.semcdb.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 25. Corada M, Morini MF, Dejana E. Signaling pathways in the specification of arteries and veins. Arterioscler Thromb Vasc Biol. 2014;34:2372–2377. doi: 10.1161/ATVBAHA.114.303218. [DOI] [PubMed] [Google Scholar]
- 26. Wang G, Wen B, Ren X, Li E, Zhang Y, Guo M, et al. Generation of pulmonary endothelial progenitor cells for cell-based therapy using interspecies mouse-rat chimeras. Am J Respir Crit Care Med. 2021;204:326–338. doi: 10.1164/rccm.202003-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schupp JC, Adams TS, Cosme C, Jr, Raredon MSB, Yuan Y, Omote N, et al. Integrated single cell atlas of endothelial cells of the human lung. Circulation. 2021;144:286–302. doi: 10.1161/CIRCULATIONAHA.120.052318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 29. Yuan B, Li C, Kimura S, Engelhardt RT, Smith BR, Minoo P. Inhibition of distal lung morphogenesis in Nkx2.1(-/-) embryos. Dev Dyn. 2000;217:180–190. doi: 10.1002/(SICI)1097-0177(200002)217:2<180::AID-DVDY5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 30. Minoo P. Transcriptional regulation of lung development: emergence of specificity. Respir Res. 2000;1:109–115. doi: 10.1186/rr20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wen B, Li E, Ustiyan V, Wang G, Guo M, Na CL, et al. In vivo generation of lung and thyroid tissues from embryonic stem cells using blastocyst complementation. Am J Respir Crit Care Med. 2021;203:471–483. doi: 10.1164/rccm.201909-1836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ornitz DM, Yin Y. Signaling networks regulating development of the lower respiratory tract. Cold Spring Harb Perspect Biol. 2012;4:a008318. doi: 10.1101/cshperspect.a008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ustiyan V, Zhang Y, Perl AK, Whitsett JA, Kalin TV, Kalinichenko VV. β-catenin and Kras/Foxm1 signaling pathway are critical to restrict Sox9 in basal cells during pulmonary branching morphogenesis. Dev Dyn. 2016;245:590–604. doi: 10.1002/dvdy.24393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li E, Ustiyan V, Wen B, Kalin GT, Whitsett JA, Kalin TV, et al. Blastocyst complementation reveals that NKX2-1 establishes the proximal-peripheral boundary of the airway epithelium. Dev Dyn. 2021;250:1001–1020. doi: 10.1002/dvdy.298. [DOI] [PubMed] [Google Scholar]
- 35. Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, et al. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013;500:589–592. doi: 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 37. Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999;56:794–814. doi: 10.1046/j.1523-1755.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 38. Zeng X, Wert SE, Federici R, Peters KG, Whitsett JA. VEGF enhances pulmonary vasculogenesis and disrupts lung morphogenesis in vivo. Dev Dyn. 1998;211:215–227. doi: 10.1002/(SICI)1097-0177(199803)211:3<215::AID-AJA3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 39. Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 40. Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 41. Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 42. Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- 43. Fernandes-Silva H, Correia-Pinto J, Moura RS. Canonical sonic hedgehog signaling in early lung development. J Dev Biol. 2017;5:3. doi: 10.3390/jdb5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- 45. Mahlapuu M, Enerbäck S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. doi: 10.1242/dev.128.12.2397. [DOI] [PubMed] [Google Scholar]
- 46. Cai Y, Bolte C, Le T, Goda C, Xu Y, Kalin TV, et al. FOXF1 maintains endothelial barrier function and prevents edema after lung injury. Sci Signal. 2016;9:ra40. doi: 10.1126/scisignal.aad1899. [DOI] [PubMed] [Google Scholar]
- 47. Ren X, Ustiyan V, Guo M, Wang G, Bolte C, Zhang Y, et al. Postnatal alveologenesis depends on FOXF1 signaling in c-KIT+ endothelial progenitor cells. Am J Respir Crit Care Med. 2019;200:1164–1176. doi: 10.1164/rccm.201812-2312OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kalinichenko VV, Gusarova GA, Shin B, Costa RH. The forkhead box F1 transcription factor is expressed in brain and head mesenchyme during mouse embryonic development. Gene Expr Patterns. 2003;3:153–158. doi: 10.1016/s1567-133x(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 49. Hoggatt AM, Kim JR, Ustiyan V, Ren X, Kalin TV, Kalinichenko VV, et al. The transcription factor Foxf1 binds to serum response factor and myocardin to regulate gene transcription in visceral smooth muscle cells. J Biol Chem. 2013;288:28477–28487. doi: 10.1074/jbc.M113.478974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ustiyan V, Bolte C, Zhang Y, Han L, Xu Y, Yutzey KE, et al. FOXF1 transcription factor promotes lung morphogenesis by inducing cellular proliferation in fetal lung mesenchyme. Dev Biol. 2018;443:50–63. doi: 10.1016/j.ydbio.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kalinichenko VV, Zhou Y, Shin B, Stolz DB, Watkins SC, Whitsett JA, et al. Wild-type levels of the mouse Forkhead Box f1 gene are essential for lung repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1253–L1265. doi: 10.1152/ajplung.00463.2001. [DOI] [PubMed] [Google Scholar]
- 52. Ramakrishna S, Kim IM, Petrovic V, Malin D, Wang IC, Kalin TV, et al. Myocardium defects and ventricular hypoplasia in mice homozygous null for the Forkhead Box M1 transcription factor. Dev Dyn. 2007;236:1000–1013. doi: 10.1002/dvdy.21113. [DOI] [PubMed] [Google Scholar]
- 53. Wang X, Bhattacharyya D, Dennewitz MB, Kalinichenko VV, Zhou Y, Lepe R, et al. Rapid hepatocyte nuclear translocation of the Forkhead Box M1B (FoxM1B) transcription factor caused a transient increase in size of regenerating transgenic hepatocytes. Gene Expr. 2003;11:149–162. doi: 10.3727/000000003108749044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ren X, Zhang Y, Snyder J, Cross ER, Shah TA, Kalin TV, et al. Forkhead box M1 transcription factor is required for macrophage recruitment during liver repair. Mol Cell Biol. 2010;30:5381–5393. doi: 10.1128/MCB.00876-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bolte C, Ren X, Tomley T, Ustiyan V, Pradhan A, Hoggatt A, et al. Forkhead box F2 regulation of platelet-derived growth factor and myocardin/serum response factor signaling is essential for intestinal development. J Biol Chem. 2015;290:7563–7575. doi: 10.1074/jbc.M114.609487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Milewski D, Pradhan A, Wang X, Cai Y, Le T, Turpin B, et al. FoxF1 and FoxF2 transcription factors synergistically promote rhabdomyosarcoma carcinogenesis by repressing transcription of p21Cip1 CDK inhibitor. Oncogene. 2017;36:850–862. doi: 10.1038/onc.2016.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xia H, Ren X, Bolte CS, Ustiyan V, Zhang Y, Shah TA, et al. Foxm1 regulates resolution of hyperoxic lung injury in newborns. Am J Respir Cell Mol Biol. 2015;52:611–621. doi: 10.1165/rcmb.2014-0091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim IM, Zhou Y, Ramakrishna S, Hughes DE, Solway J, Costa RH, et al. Functional characterization of evolutionarily conserved DNA regions in forkhead box f1 gene locus. J Biol Chem. 2005;280:37908–37916. doi: 10.1074/jbc.M506531200. [DOI] [PubMed] [Google Scholar]
- 59. Xu J, Liu H, Lan Y, Aronow BJ, Kalinichenko VV, Jiang R. A Shh-Foxf-Fgf18-Shh molecular circuit regulating palate development. PLoS Genet. 2016;12:e1005769. doi: 10.1371/journal.pgen.1005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hoffmann AD, Yang XH, Burnicka-Turek O, Bosman JD, Ren X, Steimle JD, et al. Foxf genes integrate tbx5 and hedgehog pathways in the second heart field for cardiac septation. PLoS Genet. 2014;10:e1004604. doi: 10.1371/journal.pgen.1004604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Flood HM, Bolte C, Dasgupta N, Sharma A, Zhang Y, Gandhi CR, et al. The Forkhead box F1 transcription factor inhibits collagen deposition and accumulation of myofibroblasts during liver fibrosis. Biol Open. 2019;8:bio039800. doi: 10.1242/bio.039800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Black M, Milewski D, Le T, Ren X, Xu Y, Kalinichenko VV, et al. FOXF1 inhibits pulmonary fibrosis by preventing CDH2-CDH11 cadherin switch in myofibroblasts. Cell Rep. 2018;23:442–458. doi: 10.1016/j.celrep.2018.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bolte C, Flood HM, Ren X, Jagannathan S, Barski A, Kalin TV, et al. FOXF1 transcription factor promotes lung regeneration after partial pneumonectomy. Sci Rep. 2017;7:10690. doi: 10.1038/s41598-017-11175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bolte C, Ustiyan V, Ren X, Dunn AW, Pradhan A, Wang G, et al. Nanoparticle delivery of proangiogenic transcription factors into the neonatal circulation inhibits alveolar simplification caused by hyperoxia. Am J Respir Crit Care Med. 2020;202:100–111. doi: 10.1164/rccm.201906-1232OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Milewski D, Shukla S, Gryder BE, Pradhan A, Donovan J, Sudha P, et al. FOXF1 is required for the oncogenic properties of PAX3-FOXO1 in rhabdomyosarcoma. Oncogene. 2021;40:2182–2199. doi: 10.1038/s41388-021-01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kalinichenko VV, Lim L, Stolz DB, Shin B, Rausa FM, Clark J, et al. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev Biol. 2001;235:489–506. doi: 10.1006/dbio.2001.0322. [DOI] [PubMed] [Google Scholar]
- 67. Kalin TV, Meliton L, Meliton AY, Zhu X, Whitsett JA, Kalinichenko VV. Pulmonary mastocytosis and enhanced lung inflammation in mice heterozygous null for the Foxf1 gene. Am J Respir Cell Mol Biol. 2008;39:390–399. doi: 10.1165/rcmb.2008-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pradhan A, Dunn A, Ustiyan V, Bolte C, Wang G, Whitsett JA, et al. The S52F FOXF1 mutation inhibits STAT3 signaling and causes alveolar capillary dysplasia. Am J Respir Crit Care Med. 2019;200:1045–1056. doi: 10.1164/rccm.201810-1897OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bolte C, Zhang Y, Wang IC, Kalin TV, Molkentin JD, Kalinichenko VV. Expression of Foxm1 transcription factor in cardiomyocytes is required for myocardial development. PLoS One. 2011;6:e22217. doi: 10.1371/journal.pone.0022217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bolte C, Zhang Y, York A, Kalin TV, Schultz JelJ, Molkentin JD, et al. Postnatal ablation of Foxm1 from cardiomyocytes causes late onset cardiac hypertrophy and fibrosis without exacerbating pressure overload-induced cardiac remodeling. PLoS One. 2012;7:e48713. doi: 10.1371/journal.pone.0048713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ustiyan V, Wert SE, Ikegami M, Wang IC, Kalin TV, Whitsett JA, et al. Foxm1 transcription factor is critical for proliferation and differentiation of Clara cells during development of conducting airways. Dev Biol. 2012;370:198–212. doi: 10.1016/j.ydbio.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Milewski D, Balli D, Ustiyan V, Le T, Dienemann H, Warth A, et al. FOXM1 activates AGR2 and causes progression of lung adenomas into invasive mucinous adenocarcinomas. PLoS Genet. 2017;13:e1007097. doi: 10.1371/journal.pgen.1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sun L, Ren X, Wang IC, Pradhan A, Zhang Y, Flood HM, et al. The FOXM1 inhibitor RCM-1 suppresses goblet cell metaplasia and prevents IL-13 and STAT6 signaling in allergen-exposed mice. Sci Signal. 2017;10:eaai8583. doi: 10.1126/scisignal.aai8583. [DOI] [PubMed] [Google Scholar]
- 74. Shukla S, Milewski D, Pradhan A, Rama N, Rice K, Le T, et al. The FOXM1 inhibitor RCM-1 decreases carcinogenesis and nuclear β-catenin. Mol Cancer Ther. 2019;18:1217–1229. doi: 10.1158/1535-7163.MCT-18-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sun F, Wang G, Pradhan A, Xu K, Gomez-Arroyo J, Zhang Y, et al. Nanoparticle delivery of STAT3 alleviates pulmonary hypertension in a mouse model of alveolar capillary dysplasia. Circulation. 2021;144:539–555. doi: 10.1161/CIRCULATIONAHA.121.053980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dunn AW, Kalinichenko VV, Shi D. Highly efficient in vivo targeting of the pulmonary endothelium using novel modifications of polyethylenimine: an importance of charge. Adv Healthc Mater. 2018;7:e1800876. doi: 10.1002/adhm.201800876. [DOI] [PubMed] [Google Scholar]
- 77. Partanen J, Puri MC, Schwartz L, Fischer KD, Bernstein A, Rossant J. Cell autonomous functions of the receptor tyrosine kinase TIE in a late phase of angiogenic capillary growth and endothelial cell survival during murine development. Development. 1996;122:3013–3021. doi: 10.1242/dev.122.10.3013. [DOI] [PubMed] [Google Scholar]
- 78. Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006;98:1014–1023. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 80. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 81. Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 82. Vadivel A, van Haaften T, Alphonse RS, Rey-Parra GJ, Ionescu L, Haromy A, et al. Critical role of the axonal guidance cue EphrinB2 in lung growth, angiogenesis, and repair. Am J Respir Crit Care Med. 2012;185:564–574. doi: 10.1164/rccm.201103-0545OC. [DOI] [PubMed] [Google Scholar]
- 83. Wilkinson GA, Schittny JC, Reinhardt DP, Klein R. Role for ephrinB2 in postnatal lung alveolar development and elastic matrix integrity. Dev Dyn. 2008;237:2220–2234. doi: 10.1002/dvdy.21643. [DOI] [PubMed] [Google Scholar]
- 84. Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 85. Hatzopoulos AK, Folkman J, Vasile E, Eiselen GK, Rosenberg RD. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–1468. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- 86. Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 87. Ito H, Rovira II, Bloom ML, Takeda K, Ferrans VJ, Quyyumi AA, et al. Endothelial progenitor cells as putative targets for angiostatin. Cancer Res. 1999;59:5875–5877. [PubMed] [Google Scholar]
- 88. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 89. Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 90. Rohde E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, et al. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–367. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 91. Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, et al. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007;25:1746–1752. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 92. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 93. Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 96. Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med. 2017;6:1316–1320. doi: 10.1002/sctm.16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 98. McDonald AI, Shirali AS, Aragón R, Ma F, Hernandez G, Vaughn DA, et al. Endothelial regeneration of large vessels is a biphasic process driven by local cells with distinct proliferative capacities. Cell Stem Cell. 2018;23:210–225.e6. doi: 10.1016/j.stem.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang M, Malik AB, Rehman J. Endothelial progenitor cells and vascular repair. Curr Opin Hematol. 2014;21:224–228. doi: 10.1097/MOH.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 101. Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol. 2008;294:L419–L430. doi: 10.1152/ajplung.00314.2007. [DOI] [PubMed] [Google Scholar]
- 102. Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97–L104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 103. Summer R, Kotton DN, Liang S, Fitzsimmons K, Sun X, Fine A. Embryonic lung side population cells are hematopoietic and vascular precursors. Am J Respir Cell Mol Biol. 2005;33:32–40. doi: 10.1165/rcmb.2005-0024OC. [DOI] [PubMed] [Google Scholar]
- 104. Schniedermann J, Rennecke M, Buttler K, Richter G, Städtler AM, Norgall S, et al. Mouse lung contains endothelial progenitors with high capacity to form blood and lymphatic vessels. BMC Cell Biol. 2010;11:50. doi: 10.1186/1471-2121-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Patel J, Seppanen EJ, Rodero MP, Wong HY, Donovan P, Neufeld Z, et al. functional definition of progenitors versus mature endothelial cells reveals key SoxF-dependent differentiation process. Circulation. 2017;135:786–805. doi: 10.1161/CIRCULATIONAHA.116.024754. [DOI] [PubMed] [Google Scholar]
- 106. Wakabayashi T, Naito H, Suehiro JI, Lin Y, Kawaji H, Iba T, et al. CD157 marks tissue-resident endothelial stem cells with homeostatic and regenerative properties. Cell Stem Cell. 2018;22:384–397.e6. doi: 10.1016/j.stem.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 107. Yu QC, Song W, Wang D, Zeng YA. Identification of blood vascular endothelial stem cells by the expression of protein C receptor. Cell Res. 2016;26:1079–1098. doi: 10.1038/cr.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Alphonse RS, Vadivel A, Fung M, Shelley WC, Critser PJ, Ionescu L, et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation. 2014;129:2144–2157. doi: 10.1161/CIRCULATIONAHA.114.009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liu Q, Huang X, Zhang H, Tian X, He L, Yang R, et al. c-kit(+) cells adopt vascular endothelial but not epithelial cell fates during lung maintenance and repair. Nat Med. 2015;21:866–868. doi: 10.1038/nm.3888. [DOI] [PubMed] [Google Scholar]
- 110. Fang S, Wei J, Pentinmikko N, Leinonen H, Salven P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 2012;10:e1001407. doi: 10.1371/journal.pbio.1001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Niethamer TK, Stabler CT, Leach JP, Zepp JA, Morley MP, Babu A, et al. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. eLife. 2020;9:e53072. doi: 10.7554/eLife.53072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vila Ellis L, Cain MP, Hutchison V, Flodby P, Crandall ED, Borok Z, et al. Epithelial Vegfa specifies a distinct endothelial population in the mouse lung. Dev Cell. 2020;52:617–630.e6. doi: 10.1016/j.devcel.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gillich A, Zhang F, Farmer CG, Travaglini KJ, Tan SY, Gu M, et al. Capillary cell-type specialization in the alveolus. Nature. 2020;586:785–789. doi: 10.1038/s41586-020-2822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am J Respir Crit Care Med. 2017;196:266–273. doi: 10.1164/rccm.201701-0107CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pill K, Hofmann S, Redl H, Holnthoner W. Vascularization mediated by mesenchymal stem cells from bone marrow and adipose tissue: a comparison. Cell Regen (Lond) 2015;4:8. doi: 10.1186/s13619-015-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Saeedi P, Halabian R, Imani Fooladi AA. A revealing review of mesenchymal stem cells therapy, clinical perspectives and modification strategies. Stem Cell Investig. 2019;6:34. doi: 10.21037/sci.2019.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 122. Lu SJ, Feng Q, Caballero S, Chen Y, Moore MA, Grant MB, et al. Generation of functional hemangioblasts from human embryonic stem cells. Nat Methods. 2007;4:501–509. doi: 10.1038/nmeth1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 124. Prasain N, Lee MR, Vemula S, Meador JL, Yoshimoto M, Ferkowicz MJ, et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat Biotechnol. 2014;32:1151–1157. doi: 10.1038/nbt.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lian X, Bao X, Al-Ahmad A, Liu J, Wu Y, Dong W, et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Reports. 2014;3:804–816. doi: 10.1016/j.stemcr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nguyen MTX, Okina E, Chai X, Tan KH, Hovatta O, Ghosh S, et al. Differentiation of human embryonic stem cells to endothelial progenitor cells on laminins in defined and xeno-free systems. Stem Cell Reports. 2016;7:802–816. doi: 10.1016/j.stemcr.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Keighron C, Lyons CJ, Creane M, O’Brien T, Liew A. Recent advances in endothelial progenitor cells toward their use in clinical translation. Front Med (Lausanne) 2018;5:354. doi: 10.3389/fmed.2018.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nagaya N, Kangawa K, Kanda M, Uematsu M, Horio T, Fukuyama N, et al. Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation. 2003;108:889–895. doi: 10.1161/01.CIR.0000079161.56080.22. [DOI] [PubMed] [Google Scholar]
- 129. Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 130. He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke. 2004;35:2378–2384. doi: 10.1161/01.STR.0000141893.33677.5d. [DOI] [PubMed] [Google Scholar]
- 131. Takahashi M, Nakamura T, Toba T, Kajiwara N, Kato H, Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng. 2004;10:771–779. doi: 10.1089/1076327041348563. [DOI] [PubMed] [Google Scholar]
- 132. Shelley WC, Leapley AC, Huang L, Critser PJ, Zeng P, Prater D, et al. Changes in the frequency and in vivo vessel-forming ability of rhesus monkey circulating endothelial colony-forming cells across the lifespan (birth to aged) Pediatr Res. 2012;71:156–161. doi: 10.1038/pr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shi Q, Cox LA, Hodara V, Wang XL, VandeBerg JL. Repertoire of endothelial progenitor cells mobilized by femoral artery ligation: a nonhuman primate study. J Cell Mol Med. 2012;16:2060–2073. doi: 10.1111/j.1582-4934.2011.01501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sun W, Zheng L, Han P, Kang YJ. Isolation and characterization of endothelial progenitor cells from Rhesus monkeys. Regen Med Res. 2014;2:5. doi: 10.1186/2050-490X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Qin M, Guan X, Zhang Y, Shen B, Liu F, Zhang Q, et al. Evaluation of ex vivo produced endothelial progenitor cells for autologous transplantation in primates. Stem Cell Res Ther. 2018;9:14. doi: 10.1186/s13287-018-0769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–L1084. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- 137. Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, et al. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells. 2006;24:1806–1813. doi: 10.1634/stemcells.2005-0440. [DOI] [PubMed] [Google Scholar]
- 138. Baker CD, Balasubramaniam V, Mourani PM, Sontag MK, Black CP, Ryan SL, et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J. 2012;40:1516–1522. doi: 10.1183/09031936.00017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hansmann G, Plouffe BD, Hatch A, von Gise A, Sallmon H, Zamanian RT, et al. Design and validation of an endothelial progenitor cell capture chip and its application in patients with pulmonary arterial hypertension. J Mol Med (Berl) 2011;89:971–983. doi: 10.1007/s00109-011-0779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Diller GP, van Eijl S, Okonko DO, Howard LS, Ali O, Thum T, et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation. 2008;117:3020–3030. doi: 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- 141. Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172:854–860. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 142. Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 143. Granton J, Langleben D, Kutryk MB, Camack N, Galipeau J, Courtman DW, et al. Endothelial NO-synthase gene-enhanced progenitor cell therapy for pulmonary arterial hypertension: the PHACeT trial. Circ Res. 2015;117:645–654. doi: 10.1161/CIRCRESAHA.114.305951. [DOI] [PubMed] [Google Scholar]
- 144. Goldman O, Feraud O, Boyer-Di Ponio J, Driancourt C, Clay D, Le Bousse-Kerdiles MC, et al. A boost of BMP4 accelerates the commitment of human embryonic stem cells to the endothelial lineage. Stem Cells. 2009;27:1750–1759. doi: 10.1002/stem.100. [DOI] [PubMed] [Google Scholar]
- 145. Park TS, Zimmerlin L, Zambidis ET. Efficient and simultaneous generation of hematopoietic and vascular progenitors from human induced pluripotent stem cells. Cytometry A. 2013;83:114–126. doi: 10.1002/cyto.a.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Adams WJ, Zhang Y, Cloutier J, Kuchimanchi P, Newton G, Sehrawat S, et al. Functional vascular endothelium derived from human induced pluripotent stem cells. Stem Cell Reports. 2013;1:105–113. doi: 10.1016/j.stemcr.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sahara M, Hansson EM, Wernet O, Lui KO, Später D, Chien KR. Manipulation of a VEGF-Notch signaling circuit drives formation of functional vascular endothelial progenitors from human pluripotent stem cells. Cell Res. 2014;24:820–841. doi: 10.1038/cr.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol. 2015;17:994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Farkas S, Simara P, Rehakova D, Veverkova L, Koutna I. Endothelial progenitor cells produced from human pluripotent stem cells by a synergistic combination of cytokines, small compounds, and serum-free medium. Front Cell Dev Biol. 2020;8:309. doi: 10.3389/fcell.2020.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. D’Avola D, Fernández-Ruiz V, Carmona-Torre F, Méndez M, Pérez-Calvo J, Prósper F, et al. Phase 1-2 pilot clinical trial in patients with decompensated liver cirrhosis treated with bone marrow-derived endothelial progenitor cells. Transl Res. 2017;188:80–91.e2. doi: 10.1016/j.trsl.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 151. Arici V, Perotti C, Fabrizio C, Del Fante C, Ragni F, Alessandrino F, et al. Autologous immuno magnetically selected CD133+ stem cells in the treatment of no-option critical limb ischemia: clinical and contrast enhanced ultrasound assessed results in eight patients. J Transl Med. 2015;13:342. doi: 10.1186/s12967-015-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kawamoto A, Katayama M, Handa N, Kinoshita M, Takano H, Horii M, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27:2857–2864. doi: 10.1002/stem.207. [DOI] [PubMed] [Google Scholar]
- 153. Maldonado GE, Pérez CA, Covarrubias EE, Cabriales SA, Leyva LA, Pérez JC, et al. Autologous stem cells for the treatment of post-mastectomy lymphedema: a pilot study. Cytotherapy. 2011;13:1249–1255. doi: 10.3109/14653249.2011.594791. [DOI] [PubMed] [Google Scholar]